Abstract
Objective:
To quantify the number of follicles in patients with ovarian pathologies, benign and malignant, in pregnant and nonpregnant states and to determine how the presence of ovarian masses and BRCA status affects follicular counts.
Materials and Methods:
Slides from 134 reproductive-aged women undergoing oophorectomy were examined using light microscopy by 3 independent counters blinded to the diagnosis. In all, 20 patients had cancer, 69 had benign conditions, and 35 patients were BRCA+ or had a strong family history of breast and/or ovarian cancer. In all, 10 women were either pregnant or immediately postpartum.
Results:
Patients undergoing risk-reducing surgery had significantly decreased follicle count compared to physiologic control. Patients with cancer had significantly decreased counts compared to all other groups. There were no differences within the benign cohort.
Conclusions:
When compared to benign masses, the cortex surrounding an ovarian malignancy has decreased follicle density. The stretch impact may minimize any impact on total follicle numbers. Furthermore, there may be a proliferation of ovarian stroma, with the same number of follicles spread over a larger surface area. This information is important when counseling women with ovarian masses regarding the use of ovarian tissue cryopreservation.
Keywords: follicle density, ovarian cancer, endometriosis, ovarian tissue cryopreservation, ovarian tissue transplantation, fertility preservation
Introduction
Advances in the diagnosis and treatment of childhood, adolescent, and adult cancers have greatly increased the life expectancy of women diagnosed with cancer. Unfortunately, these therapies have simultaneously contributed to a growing population of women with premature ovarian insufficiency.1–3 Aggressive chemotherapy, radiotherapy, and bone marrow transplantations effectively battle cancers but cause damage to the ovary, accelerating the normal age-related oocyte depletion and leading to infertility and early menopause.4 Several options have been used to preserve fertility in patients with cancer including embryo cryopreservation, oocyte cryopreservation, treatment with a gonadotropin analog, and ovarian tissue cryopreservation (OTC).4–6 The most suitable strategy for preserving fertility depends on various parameters including the type of cancer, the chemotherapeutic strategy, the patient’s age, and the partner status.1,4,6–9
According to the American Society for Reproductive Medicine, the only established method for fertility preservation is embryo cryopreservation.10 However, cryopreservation of ovarian tissue is emerging as a promising alternative to ovarian hyperstimulation, especially in patients with prepubertal cancer, for whom ovarian hyperstimulation with subsequent oocyte or embryo cryopreservation is contraindicated, or where the time necessary to undergo ovarian stimulation is not feasible.4,11 The advantages of using cryopreserved ovarian tissue include the possibility of future transplantation to temporarily restore hormonal function and fertility.4,12–16 However, tissue transplantation in patients with certain types of malignancies carries a theoretical risk of reintroducing cancerous cells.17–25 To avoid this risk, ongoing research is focused on isolating follicles from cryopreserved tissues to be grown using in vitro culture systems.26,27
In order to obtain the tissue for cryopreservation, a partial or total oophorectomy is required. It is currently unclear whether ovarian lesions, either benign or malignant, can adversely affect the surrounding cortex and follicles within this cortex, thus limiting the likelihood that this tissue could be used for further fertility preservation strategies.28–30 Preliminary studies have suggested that there is normal cortex surrounding most benign ovarian cysts.28 It has also been suggested that BRCA mutations may be associated with occult primary ovarian insufficiency.31 When counseling patients interested in OTC, it is important to have information about the likelihood that tissue obtained will contain follicles. However, the density of follicles in such regions could be simply reduced by the stretching and thinning of cortex over any cystic area, even around an enlarging follicle.
Although the size of the follicular pool has not been widely studied, cryopreservation and reimplantation of ovarian tissue from these patients is not likely to be feasible because of the risk of residual malignant cells in the tissue. However, screening of the tissue for malignant cells may also be an option for enhancing the safety of ovarian tissue transplantation.23,32,33
The ability to histologically assess the ovarian cortex relies on quantifying follicles at various states of folliculogenesis. Most believe that women are born with their complete oocyte pool,34–36 although some have suggested that the ovarian pool is renewable.37,38 The ovarian pool is made up of several classes of follicles (Figure 1). Primordial follicles, which make up the majority of the ovarian follicular pool, contain oocytes arrested in meiotic prophase I, which remain inactive until recruited for follicle maturation.39,40 When examined microscopically, these follicles contain a single layer of squamous, or flattened, granulosa cells. The granulosa cells of these primordial follicles change from a flat to a cuboidal structure, marking the beginning of the primary follicle. Primary follicles develop receptors to follicle-stimulating hormone at this time but are still gonadotropin independent. The next stage of follicle development, secondary follicles, contain hormone-producing theca cells as well as multiple layers of granulosa cells. The formation of a fluid-filled cavity adjacent to the oocyte called the antrum designates the follicle as an antral follicle. Further development after the antral state is dependent on gonadotropins.41,42 The duration required for transition from primordial to primary follicle stage is unknown.42 Greater than 120 days are required for the primary follicles to reach the secondary follicle stage, and 71 days are needed to grow from the secondary to the early antral follicle stage.42 However, ovary transplantation studies suggest that only 140 days are required for resting follicles to reach the ovulatory stage.11–16
Figure 1.
Histologic examples of follicle classes. A, Example of a primordial follicle (arrow) is shown with surrounding columnar epithelium seen, magnification ×40. B, An example of a primary follicle is shown (arrow) with cuboidal epithelium evident, magnification ×20. C, On the left, a primary follicle is shown and on the right a multilayer secondary (arrow), magnification ×20. D, An antral follicle with an oocyte is shown, magnification ×10.
At our institution, women undergoing oophorectomy for either a cancerous or a benign ovarian lesion are candidates for OTC.4 Prior to cryopreservation of tissue, there is an attempt to isolate follicles to preserve separately. Our anecdotal evidence suggested that certain ovarian masses had limited healthy cortical tissue available for OTC, and thus few follicles were isolated. This finding suggested that there may be a mass-driven effect on follicle numbers extending beyond the ovarian lesion. A better understanding of the likelihood of finding follicles and cryopreserving healthy ovarian tissue is critical for counseling women with ovarian masses regarding the use of OTC. Thus, we sought to quantify the number of follicles in patients with various ovarian pathologies, both benign and malignant, in pregnant and nonpregnant states to determine how the presence of various ovarian masses and BRCA status affects the follicular reserve.
Materials and Methods
Patients
Premenopausal patients who had undergone oophorectomy, aged 18 to 51 from 1996 to 2010, for any indication were identified, and their pathologic slides were initially screened by a single surgical pathologist. Slides of the ovary were identified and examined by 3 authors independently. The study was approved by the Northwestern Institutional Review Board for Human Research.
Follicle Counting and Analysis
All slides were examined by light microscopy by 3 independent counters who were blinded to the pathologic diagnosis. Primordial and primary follicles were examined under a 20×, objective while secondary and antral follicles were examined under a 10× objective. Follicles were classified as primordial, primary, secondary, or antral as defined by Gougeon.29,43 Follicles were classified as primordial when they contained a single layer of squamous granulosa cells, primary when they contained a single layer of cuboidal granulosa cells, secondary when multiple granulosa cell layers were seen, or antral when follicles with multiple granulosa cell layers and a distinct antrum were seen (Figure 1). Follicles were classified as unhealthy and excluded from follicular counts if they had abnormally shaped oocytes or disrupted granulosa cells. The total number of follicles per patient was divided by the number of slides obtained for that patient to determine the ovarian follicular density. The follicular density was then log transformed, because previous studies suggest that the decline in the follicular pool with age is exponential.35,36 This also allowed for better analysis of quantifiable changes in relation to age for each of the ovarian malignancies.
Clinical Data
The pathology reports of all patients included in this study were reviewed. In addition, history and physical and operative reports were reviewed when available. Extracted data included preoperative diagnosis, medical and surgical history, family history, and procedure performed. We were also able to extract whether the women were pregnant at the time of oophorectomy, and when during their pregnancy, the oophorectomy was performed. Information regarding the use of hormonal therapy and phase of the menstrual cycle were not available. All patients with malignant lesions had oophorectomy prior to initiating any treatments.
Statistics
Follicle density data were analyzed by descriptive statistical analysis. Log transformation was then performed, as the data were not normally distributed. Subsequently, analysis of variance with post hoc tests was done to control for multiple comparisons. Correlation between the follicular pool size and the age was performed by linear regression analysis. Statistical significance was defined as P <.05. All statistical analyses were performed using GraphPad Prism 4 (GraphPad Software Inc, San Diego, California).
Results
A total of 186 patients with available archived ovarian specimens were identified. Of these, we analyzed 134 patients based on the availability of clinical data. Of the patients studied, 28 had benign physiological findings (ie, follicular cysts, hemorrhagic cysts, and/or corpus luteum; ), 20 had cancer involving the ovary (n = 11 cancers involving the ovary , n = 9 borderline ovarian cancers , 18 patients had histologically confirmed endometriosis , 12 patients had mature cystic teratomas , and 11 patients had cystadenomas . None of the patients with cancer involving the ovary had been exposed to potentially gonadotoxic therapies. In addition, there were 35 patients who were undergoing risk-reducing surgery, either because they were found to be BRCA+ (n = 15) or because of a strong family history of breast or ovarian cancer . Within this cohort, only 15 patients were tested for BRCA. None of the patients included were tested and found to be BRCA negative. Occult ovarian cancer was found in 4 of these patients. We also identified 10 women with ovarian masses who were either pregnant or within 6 weeks of delivery (Tables 1 and 2).
Table 1.
Data Describing Slides Which Were Examined, Including Medical Confounding Variables.a
| Number of Patients | Pathology | Confounding Medical Factors |
|---|---|---|
| 35 | Physiological findings (absence of other pathologies and findings including follicular cysts, corpus luteum) | 8 patients pregnant or within 6 weeks of delivery |
| 10 | Invasive ovarian cancer | |
| 11 | Borderline ovarian cancer | 2 patients pregnant or within 6 weeks of delivery |
| 18 | Endometriosis | |
| 12 | Mature cystic teratoma | |
| 11 | Cystadenoma | |
| 35 | Strong family history of breast/ovarian cancer | 4 patients with occult ovarian cancer |
a Clinical patient data (n = 134).
Table 2.
Data Describing Slides Which Were Examined.
| Diagnosis | # Specimens | Age, Mean (Std Deviation) | # Slides Examined, Mean | Follicles/Section, Mean |
|---|---|---|---|---|
| Physiologic findings | 28 | 36.5 (±3.4) | 3.4 | 23.3 |
| Risk-reducing surgery | 35 | 37.3 (±3.5) | 8.4 | 15.4 |
| Cancer involving the ovary | 11 | 34.1 (±5.1) | 10.0 | 6.3 |
| Borderline cancer of the ovary | 9 | 38.8 (±1.6) | 15.7 | 3.3 |
| Mature cystic teratoma | 12 | 35.8 (±7.3) | 7.8 | 21.5 |
| Cystadenoma | 11 | 35.4 (±6.3) | 7.2 | 8.6 |
| Endometriosis | 18 | 37.7 (±4.3) | 4.9 | 14.6 |
| Pregnant | 10 | 34.6 (±3.0) | 2.9 | 17.2 |
Women with physiologic findings were undergoing hysterectomy with bilateral oophorectomy. Most of these women were having surgery because of symptomatic leiomyomata. The second most common reason was chronic pelvic pain without histologic evidence of endometriosis. Patients included in the category of physiological findings were deemed controls, because their ovaries were devoid of benign and malignant lesions. Of the women with cancer involving the ovary, 9 were found to have a borderline malignancy and 10 had invasive disease. Of the women undergoing oophorectomy in the setting of pregnancy, 4 were within their first trimester and 6 were done at the time of delivery (≥34 weeks of pregnancy).
There was no statistical difference in age between women studied (Table 1). The distribution of follicle classes between the various histologies was also examined. The overall distribution was found to be similar between the various histologies, with the primordial follicle class comprising the greatest percentage, as expected (Figure 2). We were able to find follicles of all classes in the various histologies.
Figure 2.
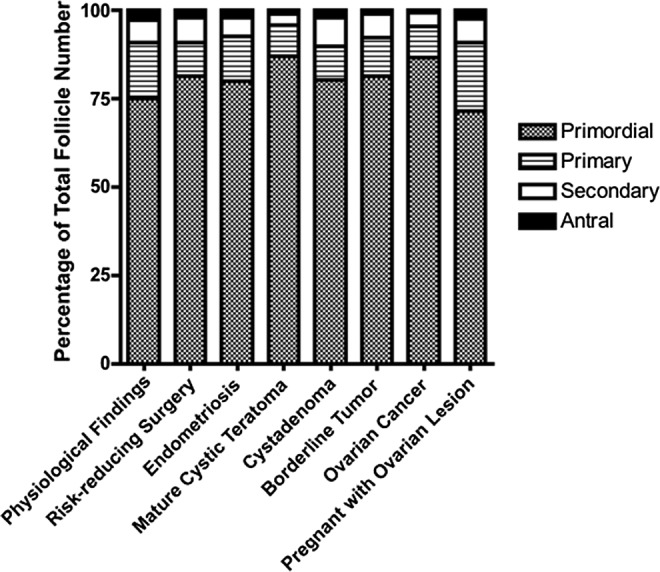
Ditrubution of follicle classes. The distribution of follicle classes is shown here. As evident, the overall distribution is similar between the various histologies, with the primordial follicle class comprising the greatest percentage, as expected. There was a trend in the secondary and antral follicle counts, with greater numbers seen in physiologic versus other lesions.
We then examined follicles/section in benign and cancerous histologies. We were able to identify follicles in all stages in most, but not all, ovarian cancer lesions. We found that patients with cancer had significantly fewer total follicles per section with significantly decreased follicle density at all stages compared to the physiologic findings controls (Figure 3). Patients undergoing risk-reducing surgery because of BRCA+ status or a strong family history of breast or ovarian cancer had statistically fewer follicles per section compared to those with physiologic findings. This is consistent with the finding that patients with the BRCA mutation may have decreased ovarian reserve.
Figure 3.
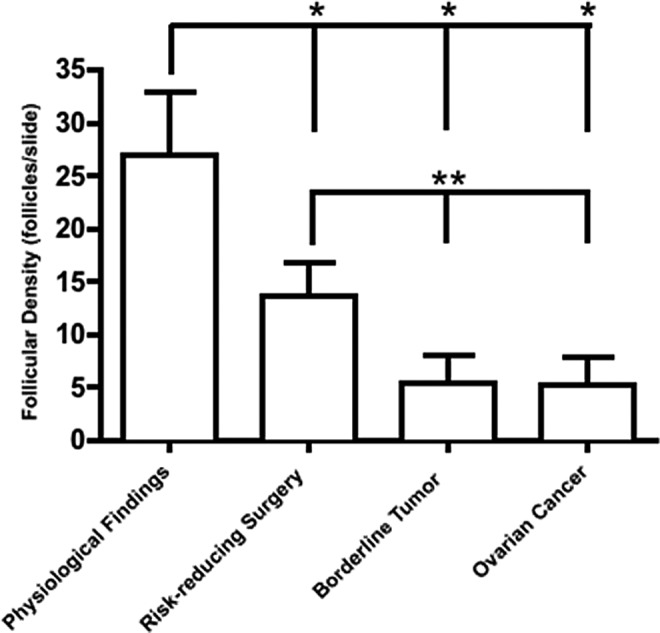
Follicle density by diagnosis: comparing cancerous and precancerous lesions. Patients with a cancerous ovarian lesion, either a borderline or an ovarian cancer, had significantly fewer total follicles per section with significantly decreased follicle density at all stages compared to controls with physiologic findings (*). Patients undergoing risk-reducing oophorectomy had statistically fewer follicles per section compared to those with physiologic findings (**). This is consistent with the finding that patients with the BRCA mutation may have decreased ovarian reserve. In addition, ovarian cancer and borderline lesions had significantly fewer follicles compared to women undergoing risk-reducing surgery (**).
We then examined benign ovarian histologies and ovaries removed in the setting of pregnancy. We were able to identify follicles of all stages in each of the benign histologies as well in the ovaries removed during pregnancy. We found that women with endometriosis involving the ovary had the highest number of follicles per section while women with cystadenomas had the fewest follicles per section. Although a trend was seen with decreased number of follicles compared to normal ovaries, this did not reach statistical significance (Figure 4). With benign ovarian lesions, there may be a proliferation of ovarian stroma, with the same number of follicles spread over a larger surface area.
Figure 4.
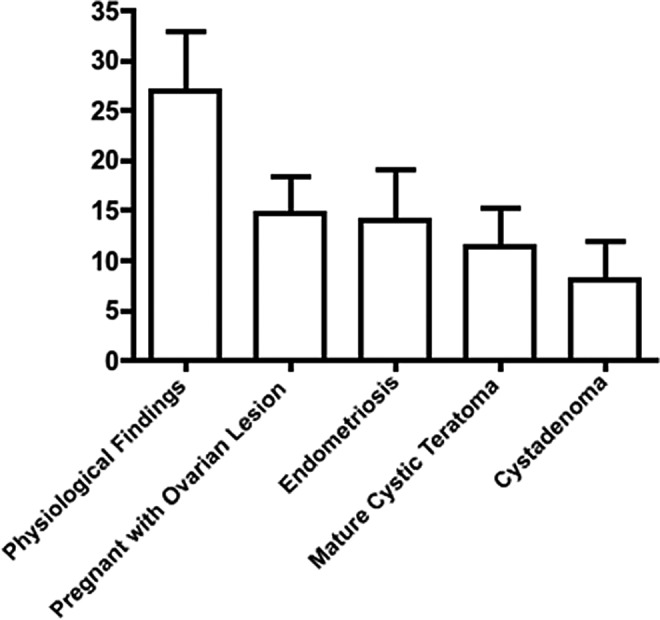
Follicle density by diagnosis, comparing benign lesions. Women with endometriosis involving the ovary had the highest number of follicles per section while women with cystadenomas had the fewest follicles per section. There was a trend seen in patients with benign lesions having decreased follicular density, but this did not reach statistical significance. With benign ovarian lesions, there may be a proliferation of ovarian stroma, with the same number of follicles spread over a larger surface area.
Discussion
In this study, we found that the cortex surrounding benign ovarian lesions contained follicles at all stages of development, as did borderline ovarian tumors, although to a lesser extent. However, tissue from ovaries with ovarian cancers did not contain many follicles, suggesting that this tissue may be unsuitable for follicle isolation and in vitro follicle maturation. Yet, with benign lesions, there may be a proliferation of ovarian stroma, with the same number of follicles spread over a larger surface area.
There is a well-established, age-dependent depletion of ovarian follicles that occurs over a woman’s reproductive years.36,44–47 The patients in our study were in their later reproductive years which may have negatively impacted their overall follicle counts.43–47 But, the ages were similar among women of various pathologies and thus controlled for in comparisons between pathologies. Furthermore, woman diagnosed with epithelial ovarian cancer tend to be diagnosed following reproductive time with average age of diagnosis being 53 years.48 Therefore, having an older reproductive cohort is of value for accurate patient counseling when dealing with ovarian malignancies. An extension of this work in younger patients would further enhance our ability to individualize counseling regarding in vitro follicle maturation.
Interestingly, consistent with the observations from Oktay et al, patients with the BRCA mutation or with a strong family history of breast and/or ovarian cancer had a lower total follicle count when compared to normal controls.31 This finding suggests that patients with the BRCA mutation may, indeed, be at risk of occult ovarian insufficiency. This finding may be biologically plausible, given the suggestion that BRCA genes may be involved in the repair and maintenance of chromosome telomere integrity, which is important during reproduction.49–53 Telomeres are DNA repeats at the end of chromosomes which protect the chromosome ends from degradation and fusion.54 The presence of short telomeres can result in programmed cell death (apoptosis)through telomere shortening, spindle disruption, or decreased formation of chiasmata during meiosis. Telomeres, which shorten during DNA replication, have been implicated as one of the determinants of a person’s reproductive lifespan. In addition to telomere length, factors affecting cell cycle and division and DNA repair may also affect reproductive lifespan.54 Given the range of functions of the BRCA proteins, it is reasonable to believe that defects in these genes may affect reproduction.
When large benign ovarian cysts are encountered, many gynecologists are tempted to remove the entire ovary, with the idea that there is little remaining normal ovarian cortex. The ovarian cortex surrounding a cyst may appear thin and stretched, and there is concern about whether these structural changes could lead to histologic irregularities and impaired function. The current study has found, however, that most benign ovarian lesions will have at least some normal cortex which is consistent with the findings of other investigators.28–30 Even when the ovarian cortex is thin and stretched by the growth of a benign tumor, there still appear to be follicles in all functional classes (Figure 2). Thus, whenever possible, conservative ovarian cystectomy should be the surgery of choice in women desiring future fertility. However, in cases where this cannot be safely accomplished, patients can be counseled to consider OTC.
This study found that follicular densities in women with endometriosis were similar to the densities found in other benign lesions. Other studies have suggested that ovaries from women affected with endometriosis may have a lower density.28,29 The study by Maneschi et al examined functional morphologic features of the ovarian cortex surrounding mature teratomas, benign cystadenomas, and endometriomas. This study found that follicular maturation to the antral stage was seen much more frequently in the cortical tissue surrounding mature teratomas and cystadenomas than in surrounding endometriomas (19%). They found a reduction in the overall number of follicles, aberrant vascularity, and alterations in the cortical stroma, including fibrosis. This group concluded that endometriomas may greatly affect the morphology of the ovarian cortex, and that this, in turn, could alter its functional potential.28 Another study by Schubert et al also found that follicular densities in the ovarian cortex surrounding ovaries affected by endometriosis were also lower than follicular densities of other benign lesions. This same trend was noted after cryopreserving and thawing the tissues.29 The findings from our study may differ, because we included all women with histologic evidence of endometriosis and did not limit analysis to those women who had ovarian endometriomas. In our study, the precise mechanism by which endometriosis may negatively impact follicular density is not well established and substantiated. Therefore, although the presence of an endometrioma may decrease follicular densities, the diagnosis of endometriosis on its own should not be a contraindication to performing OTC.
Few studies have examined the effects of ovarian cancer on the follicular pool. We found cancerous ovarian lesions to have significantly decreased follicle densities when compared to controls with physiological findings. The low number of follicles in these patients is consistent with the results from Abir et al, in which the ovaries of one 8-year-old patient with ovarian cancer was almost completely depleted of follicles.55 Because of the low follicular reserve in patients with ovarian cancer and the potential for residual malignant cells in the tissue, reimplantation of ovarian tissue from patients with ovarian cancer is generally not recommended.
This is the first study to examine these many different types of ovarian lesions. Relatively few other studies have been performed examining ovarian cortical tissue surrounding ovarian masses. The major strength of this study is in the large numbers of pathologic specimens inspected, and the fact that both benign and malignant conditions were examined. In addition, the slides were independently counted by 3 people who were blinded to the diagnosis, lessening the chance of bias. There were also a relatively large number of ovarian specimens without lesions that were examined for which comparisons could be made. Weaknesses of this study includes its retrospective design and the fact that the entire ovary was not sectioned and examined. The inspected slides were reflective of what the pathologist felt was important for diagnosis and may represent more of the cortex surrounding the lesion. Furthermore, lesions that required more sections had more slides available for counting, such as the ovarian cancers. This is an inevitable bias of retrospectively using ovarian tissue. However, we consistently observed that the cortex surrounding ovarian cancer lesions had far fewer follicles than the cortex surrounding benign lesions. This was true despite the fact that more slides were examined for patients with malignancy. Additionally, the primordial follicle class was similar throughout the various lesions. This served as a positive control and leads us to believe that we examined a representative portion of each ovary; our findings are consistent with those of other studies.44–47 In addition, the study by Schubert et al suggested that there was no correlation between the size of the cyst and the follicular density observed, leading us to believe that even if more of the ovarian mass was examined, the follicle count would still not differ.29 Additionally, to examine the entire human ovary from all these patients would be cost prohibitive.
It is not clear how the ovarian environment influences the persistence of the primordial follicle pool, but we suspect that the relatively lower follicle counts seen around malignant lesions may be caused by elevated cytokines or other structural changes within the physical environment, including the neovascularization and rerouting of tumor vasculature that is seen in neoplasms, alterations in the rigidity of the tissue and/or changes in physical pressure caused by the expanding neoplasm are all possible mechanisms.56,57 With benign ovarian lesions, there may be a proliferation of ovarian stroma, with the same number of follicles spread over a larger surface area. Future studies would explore each of these options. In addition, serum antimullerian hormone (AMH) levels, a sensitive marker of ovarian reserve, may assist in understanding whether lesions are causing an increase in follicular cell death or an alteration in ovarian volume resulting in decreased follicle density. We speculate that a lower AMH level would be seen if ovarian lesions are causing cell death. If this is true, simply collecting more ovarian cortex would not increase the likelihood of preserving follicles. If, however, serum AMH levels are found to be normal, collecting more tissue could increase the chance of preserving follicles. Moreover, prospective studies are planned in order to rigorously determine what impact gynecologic cancers and benign masses have on the ovary itself and serum AMH levels in order to better enable patient care. Finally, it is unknown what lasting impact a lesion, either benign or malignant, would have on a follicle obtained from the surrounding tissue. Furthermore, these lesions could impact the follicle growth characteristics or egg parameters of these follicles even in the in vitro follicle growth environment.
Clinically, this study has shown that women with benign ovarian lesions should undergo conservative surgery whenever possible, because although there is a reduction in follicle number in these patients, a significant number of follicles still exist. When an oophorectomy is done for benign lesions, these patients may be counseled about the option of OTC.20,58,59 When retransplantation is not an option because of residual disease, primary follicles may be matured in an in vitro system in the future.59,60 However, patients diagnosed with either a borderline or an invasive ovarian cancer may not be ideal candidates for either procedure given the overall low number of follicles seen. It appears that ovarian cancers do significantly reduce the numbers of follicles present in the ovarian cortex. Interestingly, patients with a BRCA mutation or a strong family history of breast and ovarian cancer may have reduced follicular pools compared to normal controls. Future prospective studies need to be done to verify these results as well as to examine serum markers of ovarian reserve in these patients.
Footnotes
Authors’ Note: This article was presented, in part, at the 2011 American Society for Reproductive Medicine meeting in Orlando, FL and at the 2010 Society for Gynecologic Investigations meeting in Orlando, FL. All authors played key roles in conception and design, data collection, analysis and interpretation, and manuscript writing. All authors approved the final document.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: Supported by K12HD050121, ASRM career development award (to MEP), and U54 HD076188 (to TKW).
References
- 1. Oktay K, Rodriguez-Wallberg K, Schover L. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360(25):2681; author reply 2682–2683. [PubMed] [Google Scholar]
- 2. Schover LR. Patient attitudes toward fertility preservation. Pediatr Blood Cancer. 200. 9;5 3(2):281–284. [DOI] [PubMed] [Google Scholar]
- 3. Partridge AH, Gelber S, Peppercorn J, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22(20):4174–4183. [DOI] [PubMed] [Google Scholar]
- 4. Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360(9):902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim SS. Fertility preservation in female cancer patients: current developments and future directions. Fertil Steril. 2006;85(1):1–11. [DOI] [PubMed] [Google Scholar]
- 6. Sonmezer M, Oktay K. Fertility preservation in female patients. Hum Reprod Update. 2004;10(3):251–266. [DOI] [PubMed] [Google Scholar]
- 7. Hirshfeld-Cytron J, Gracia C, Woodruff TK. Nonmalignant diseases and treatments associated with primary ovarian failure: an expanded role for fertility preservation. J Womens Health (Larchmt). 2011;20(10):1467–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roberts JE, Oktay K. Fertility preservation: a comprehensive approach to the young woman with cancer. J Natl Cancer Inst Monogr. 2005(34):57–59. [DOI] [PubMed] [Google Scholar]
- 9. Poirot C, Vacher-Lavenu MC, Helardot P, Guibert J, Brugieres L, Jouannet P. Human ovarian tissue cryopreservation: indications and feasibility. Hum Reprod. 2002;17(6):1447–1452. [DOI] [PubMed] [Google Scholar]
- 10. Fertility preservation and reproduction in cancer patients. Fertil Steril. 2005;83(6):1622–1628. [DOI] [PubMed] [Google Scholar]
- 11. Silber S, Kagawa N, Kuwayama M, Gosden R. Duration of fertility after fresh and frozen ovary transplantation. Fertil Steril. 2010;94(6):2191–2196. [DOI] [PubMed] [Google Scholar]
- 12. Silber SJ. Fresh ovarian tissue and whole ovary transplantation. Semin Reprod Med. 2009;27(6):479–485. [DOI] [PubMed] [Google Scholar]
- 13. Silber SJ, DeRosa M, Pineda J, et al. A series of monozygotic twins discordant for ovarian failure: ovary transplantation (cortical versus microvascular) and cryopreservation. Hum Reprod. 2008;23(7):1531–1537. [DOI] [PubMed] [Google Scholar]
- 14. Silber SJ, Gosden RG. Ovarian transplantation in a series of monozygotic twins discordant for ovarian failure. N Engl J Med. 2007;356(13):1382–1384. [DOI] [PubMed] [Google Scholar]
- 15. Silber SJ, Grudzinskas G, Gosden RG. Successful pregnancy after microsurgical transplantation of an intact ovary. N Engl J Med. 2008;359(24):2617–2618. [DOI] [PubMed] [Google Scholar]
- 16. Silber SJ, Lenahan KM, Levine DJ, et al. Ovarian transplantation between monozygotic twins discordant for premature ovarian failure. N Engl J Med. 2005;353(1):58–63. [DOI] [PubMed] [Google Scholar]
- 17. Sanchez-Serrano M, Novella-Maestre E, Rosello-Sastre E, Camarasa N, Teruel J, Pellicer A. Malignant cells are not found in ovarian cortex from breast cancer patients undergoing ovarian cortex cryopreservation. Hum Reprod. 2009;24(9):2238–2243. [DOI] [PubMed] [Google Scholar]
- 18. Radford JA, Lieberman BA, Brison DR, et al. Orthotopic reimplantation of cryopreserved ovarian cortical strips after high-dose chemotherapy for Hodgkin's lymphoma. Lancet. 2001;357(9263):1172–1175. [DOI] [PubMed] [Google Scholar]
- 19. Radford J. Autotransplantation of ovarian tissue and the risk of disease transmission. Eur J Obstet Gynecol Reprod Biol. 2004;113(suppl 1):S48–S49. [DOI] [PubMed] [Google Scholar]
- 20. Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364(9443):1405–1410. [DOI] [PubMed] [Google Scholar]
- 21. Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin's disease. Oncologist. 2007;12(12):1437–1442. [DOI] [PubMed] [Google Scholar]
- 22. Andersen CY, Rosendahl M, Byskov AG, et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23(10):2266–2272. [DOI] [PubMed] [Google Scholar]
- 23. Meirow D, Hardan I, Dor J, et al. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum Reprod. 2008;23(5):1007–1013. [DOI] [PubMed] [Google Scholar]
- 24. Meirow D. Fertility preservation in cancer patients using stored ovarian tissue: clinical aspects. Curr Opin Endocrinol Diabetes Obes. 2008;15(6):536–547. [DOI] [PubMed] [Google Scholar]
- 25. Oktay K, Buyuk E, Veeck L, et al. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;363(9412):837–840. [DOI] [PubMed] [Google Scholar]
- 26. Hirshfeld-Cytron J, Grobman WA, Milad MP. Fertility preservation for social indications: a cost-based decision analysis. Fertil Steril. 2012;97(3):665–670. [DOI] [PubMed] [Google Scholar]
- 27. Hirshfeld-Cytron JE, Duncan FE, Xu M, Jozefik JK, Shea LD, Woodruff TK. Animal age, weight and estrus cycle stage impact the quality of in vitro grown follicles. Hum Reprod. 2011;26(9):2473–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maneschi F, Marasa L, Incandela S, Mazzarese M, Zupi E. Ovarian cortex surrounding benign neoplasms: a histologic study. Am J Obstet Gynecol. 1993;169(2 pt 1):388–393. [DOI] [PubMed] [Google Scholar]
- 29. Schubert B, Canis M, Darcha C, et al. Human ovarian tissue from cortex surrounding benign cysts: a model to study ovarian tissue cryopreservation. Hum Reprod. 2005;20(7):1786–1792. [DOI] [PubMed] [Google Scholar]
- 30. Donnez J, Nisolle M, Gillet N, Smets M, Bassil S, Casanas-Roux F. Large ovarian endometriomas. Hum Reprod. 1996;11(3):641–646. [DOI] [PubMed] [Google Scholar]
- 31. Oktay K, Kim JY, Barad D, Babayev SN. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol. 2010;28(2):240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bockstaele L, Tsepelidis S, Dechene J, Englert Y, Demeestere I. Safety of ovarian tissue autotransplantation for cancer patients. Obstet Gynecol Int. 2012;2012:495142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim SS. Ovarian tissue banking for cancer patients. To do or not to do? Hum Reprod. 2003;18(9):1759–1761. [DOI] [PubMed] [Google Scholar]
- 34. McLaughlin EA, McIver SC. Awakening the oocyte: controlling primordial follicle development. Reproduction. 2009;137(1):1–11. [DOI] [PubMed] [Google Scholar]
- 35. Bristol-Gould SK, Kreeger PK, Selkirk CG, et al. Postnatal regulation of germ cells by activin: the establishment of the initial follicle pool. Dev Biol. 2006;298(1):132–148. [DOI] [PubMed] [Google Scholar]
- 36. Bristol-Gould SK, Kreeger PK, Selkirk CG, et al. Fate of the initial follicle pool: empirical and mathematical evidence supporting its sufficiency for adult fertility. Dev Biol. 2006;298(1):149–154. [DOI] [PubMed] [Google Scholar]
- 37. Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428(6979):145–150. [DOI] [PubMed] [Google Scholar]
- 38. White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18(3):413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Coccia ME, Rizzello F. Ovarian reserve. Ann N Y Acad Sci. 2008;1127:27–30. [DOI] [PubMed] [Google Scholar]
- 40. Gleicher N, Weghofer A, Barad DH. Defining ovarian reserve to better understand ovarian aging. Reprod Biol Endocrinol. 2011;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baerwald AR, Adams GP, Pierson RA. Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum Reprod Update. 2012;18(1):73–91. [DOI] [PubMed] [Google Scholar]
- 42. McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21(2):200–214. [DOI] [PubMed] [Google Scholar]
- 43. Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod. 1986;1(2):81–87. [DOI] [PubMed] [Google Scholar]
- 44. Faddy MJ, Gosden RG. A model conforming the decline in follicle numbers to the age of menopause in women. Hum Reprod. 1996;11(7):1484–1486. [DOI] [PubMed] [Google Scholar]
- 45. Gougeon A, Ecochard R, Thalabard JC. Age-related changes of the population of human ovarian follicles: increase in the disappearance rate of non-growing and early-growing follicles in aging women. Biol Reprod. 1994;50(3):653–663. [DOI] [PubMed] [Google Scholar]
- 46. Gougeon A, Chainy GB. Morphometric studies of small follicles in ovaries of women at different ages. J Reprod Fertil. 1987;81(2):433–442. [DOI] [PubMed] [Google Scholar]
- 47. Gougeon A. Ovarian follicular growth in humans: ovarian ageing and population of growing follicles. Maturitas. 1998;30(2):137–142. [DOI] [PubMed] [Google Scholar]
- 48. http://seer.cancer.gov/statfacts/html/ovary.html. Accessed November 1, 2012.
- 49. Chen J, Silver DP, Walpita D, et al. Stable interaction between the products of the BRCA1 and BRCA2 tumor suppressor genes in mitotic and meiotic cells. Mol Cell. 1998;2(3):317–328. [DOI] [PubMed] [Google Scholar]
- 50. Cressman VL, Backlund DC, Avrutskaya AV, Leadon SA, Godfrey V, Koller BH. Growth retardation, DNA repair defects, and lack of spermatogenesis in BRCA1-deficient mice. Mol Cell Biol. 1999;19(10):7061–7075. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 51. Cressman VL, Backlund DC, Hicks EM, Gowen LC, Godfrey V, Koller BH. Mammary tumor formation in p53- and BRCA1-deficient mice. Cell Growth Differ. 1999;10(1):1–10. [PubMed] [Google Scholar]
- 52. French JD, Dunn J, Smart CE, Manning N, Brown MA. Disruption of BRCA1 function results in telomere lengthening and increased anaphase bridge formation in immortalized cell lines. Genes Chromosomes Cancer. 2006;45(3):277–289. [DOI] [PubMed] [Google Scholar]
- 53. Xiong J, Fan S, Meng Q, et al. BRCA1 inhibition of telomerase activity in cultured cells. Mol Cell Biol. 2003;23(23):8668–8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hassold T, Hunt P. Maternal age and chromosomally abnormal pregnancies: what we know and what we wish we knew. Curr Opin Pediatr. 2009;21(6):703–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Abir R, Ben-Haroush A, Felz C, et al. Selection of patients before and after anticancer treatment for ovarian cryopreservation. Hum Reprod. 2008;23(4):869–877. [DOI] [PubMed] [Google Scholar]
- 56. Lee D. Ovarian Tissue Cryopreservation and Transplantation: Banking Reproductive Potential for the Future. In: Woodruff TK, Snyder KA, eds. Oncofertility. New York: Springer; 2007. [DOI] [PubMed] [Google Scholar]
- 57. Ko SY, Barengo N, Ladanyi A, et al. HOXA9 promotes ovarian cancer growth by stimulating cancer-associated fibroblasts. J Clin Invest. 2012;122(10):3603–3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Donnez J, Silber S, Andersen CY, et al. Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births. Ann Med. 2011;43(6):437–450. [DOI] [PubMed] [Google Scholar]
- 59. Vanacker J, Luyckx V, Dolmans MM, et al. Transplantation of an alginate-matrigel matrix containing isolated ovarian cells: first step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials. 2012;33(26):6079–6085. [DOI] [PubMed] [Google Scholar]
- 60. Hornick J, Duncan FE, Shea L, Woodruff TK. Multiple follicle culture supports primary follicle growth through paracrine-acting signals. Reproduction. 2013;145(1):19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]



