Abstract
Maternal obesity is associated with an increased risk of a number of pregnancy complications, including fetal demise, which may be linked to impaired placental development as a result of altered trophoblast invasion and vessel remodeling. Therefore, we examined these parameters in pregnant rats fed a control (normal weight) or high fat (HF) diet (obese) at 2 critical times of rat placental development. Early trophoblast invasion was increased by approximately 2-fold in HF-fed dams with a concomitant increase in the expression of matrix metalloproteinase 9 protein, a mediator of tissue remodeling and invasion. Furthermore, we observed significantly higher levels of smooth muscle actin surrounding the placental spiral arteries of HF-fed dams, suggesting impaired spiral artery remodeling. Taken together, the results of this study suggest that altered placental development is an important contributor to the poor pregnancy outcomes and increased fetal demise in our model of lifelong maternal obesity.
Keywords: placenta, obesity, trophoblast invasion, vascular remodeling
Introduction
Obese women have an increased risk of a number of pregnancy complications, including preeclampsia, miscarriage, stillbirth, as well as delivering both large-for-gestational-age and small-for-gestational-age (SGA) babies.1–3 The SGA or growth-restricted fetuses are at higher risk of fetal demise, poor neonatal health, and of adult onset diseases.4 At this time, the mechanisms involved in the development of these complications in the context of maternal obesity are not well understood. Utilizing a previously characterized rat model of lifelong maternal obesity, which mimics many of the adverse pregnancy outcomes, observed in human populations, we can begin to elucidate the link between obesity and these pregnancy complications. The adverse outcomes in our animal model include increased fetal death and impaired fetal growth in association with altered placental development.5
Altered trophoblast invasion and aberrant spiral artery remodeling have been implicated in many of the pregnancy complications associated with obesity, including preeclampsia, fetal growth restriction, and stillbirth.6,7 Trophoblast cells are stimulated to invade into the maternal decidua/myometrium through a complex interaction of cytokines, growth factors, and other signaling molecules in the local environment.8 In the rat, the onset of such trophoblast invasion occurs at approximately gestational day (GD) 15 and peaks at approximately GD18.9 The extent of this invasion into the mesometrial compartment follows a similar pattern as that observed in the human myometrium,10 making the rat an ideal model to study this process. The invasive trophoblasts migrate/invade into maternal uterine tissue to reach the spiral arteries through either endovascular trophoblast (ENVT) or interstitial trophoblast (IST) migratory pathways and contribute to the remodeling of these arteries.11,12 This remodeling process is essential for converting the spiral arteries into high-capacity, low-resistance vessels to increase blood flow to the placenta.13 In addition to trophoblasts, recent evidence demonstrates that decidua natural killer (dNK) cells may be important for the remodeling process.14 In normal pregnancy, the progressive disruption of smooth muscle cell layer surrounding the spiral arteries is an important component in the remodeling of these arteries. Although it is accepted that the failure to disrupt the vascular smooth muscle cell (VSMC) layer surrounding the spiral arteries can lead to a number of pregnancy-related pathologies,15 the mechanisms by which this process may be affected in obese pregnancies are not well understood. Importantly, the disorganization of the smooth muscle cell layer surrounding the spiral arteries plays an important role in the remodeling of these arteries in both rats and humans.16
Obesity is associated with a state of chronic systemic inflammation.17 Obesity during pregnancy can contribute to altered levels of circulating proinflammatory cytokines in the maternal circulation as well as in placental tissues.18 Interestingly, in vitro trophoblast invasion has been shown to be affected by a number of these proinflammatory cytokines. Some attenuate invasion (eg, tumor necrosis factor α and 8-isoprostaglandin F2α) and others increase invasion (eg, interleukin [IL]-6, IL-8).19–23 In addition, adipocyte-derived factors have been shown to affect VSMC proliferation and inflammation.24 Although experiments detailing the effects of individual cytokines and adipokines on these processes have been documented in vitro, the effects of obesity on trophoblast invasion and spiral remodeling are yet to be determined in vivo.
Therefore, the goal of these experiments was to assess changes in trophoblast invasion and vascular smooth muscle remodeling associated with maternal obesity in the established rat model of lifelong obesity.5
Materials and Methods
Animal Experiments
All animal procedures for this study were approved by the McMaster University Animal Research Ethics Board (AUP # 070740). A total of 50 female Sprague-Dawley rats, aged 21 days (84-100 g), were purchased from Charles River Laboratories (Wilmington, Massachusetts). Rats were maintained under controlled lighting (12-hour light–dark cycle) and temperature (22°C) with ad libitum access to food and water. Dams were randomly assigned to receive either standard rat chow (control [CON]; 16% kcal fat, 3.82 kcal/g; Harlan Teklad, Madison, Wisconsin) or a high fat (HF) diet (45% kcal fat, 4.70 kcal/g; Research Diets, New Brunswick, New Jersey). In all, 20 dams were assigned to the CON group and 30 to the HF-fed group (due to a diet-induced reduction in fertility5). Dams were maintained on their respective diets for 16 weeks before being mated with age-matched Sprague-Dawley males fed the CON diet. One female and 1 male per cage were paired until copulation was confirmed by visualization of sperm in a vaginal flush. The presence of sperm indicated day 0 of pregnancy (GD0). The HF and CON-fed dams underwent laparotomy at either GD15 (early trophoblast invasion) or GD18 (invasion completed, spiral artery remodeling completed). These time points were selected based on the work of Pijnenborg and colleagues25 who demonstrated that invasive ENVTs, in the rat, are observed at GD15, while maximal invasion of ISTs can be seen at approximately GD18.9 Blood sampling was performed by cardiac puncture prior to euthanasia. Two entire conceptuses from the second position from the cervix, in each uterine horn, were removed and preserved in 10% neutral-buffered formalin for immunohistological analysis. The remaining placentae were removed from the uterus, and the underlying mesometrial triangles were excised and frozen at −80°C for Western blot analysis.
Immunohistochemistry
Placental tissues were fixed in formalin and embedded in paraffin. Placentae were embedded upright with the fetal side of the placental disc perpendicular to the cross-sectional plane. Sections of 5 µm from the central area of the placenta containing a main spiral artery were cut and mounted on Superfrost Plus microslides (VWR, Mississauga, Ontario). Immunohistochemistry was performed to determine localization and expression of the protein or antigen of interest within placental and uterine tissue. Slides were deparaffinized in xylene and rehydrated in graded alcohol solutions. Endogenous peroxidase activity was inhibited with 3% hydrogen peroxide in methanol for 15 minutes at room temperature (RT). Retrieval of smooth muscle actin (SMA) antigen was achieved by immersing slides in 10 mmol/L citrate buffer at 90°C for 12 minutes. Antigen retrieval for cytokeratin analysis was achieved using microwaving slides just until boiling in a commercial citrate-based antigen unmasking solution (Vector Laboratories, Burlingame, California). Tissues were blocked using 1% (wt/vol) bovine serum albumin (BSA) with 10% (vol/vol) normal donkey serum for 1 hour at RT followed by incubation with antipan cytokeratin (a trophoblast marker; 1:300 dilution; Sigma-Aldrich, St Louis, Missouri) or anti-α-SMA (a VSMC marker; 1:200 dilution; Sigma-Aldrich) overnight at 4°C in a humidified chamber. Sections were washed in phosphate buffered saline, and immunostaining was identified using antimouse biotinylated secondary antibody (Millipore, Billerica, Massachusetts) and the Vectastain kit (Vector Laboratories, Burlingame, California) with diaminobenzidine (Sigma-Aldrich Canada Ltd, Oakville, Ontario) as the chromogen. Tissue sections were counterstained with Harris hematoxylin (Sigma-Aldrich), dehydrated, and mounted with Permount (Fisher Scientific, Fair Lawn, New Jersey).
Analysis of IST Trophoblast Invasion
Analysis of IST invasion was carried out on cytokeratin-stained sections containing a cross-section of the central maternal artery by a single investigator who was blinded to the treatment groups, as described previously.9,26 The entire mesometrial triangle was scanned at 4× magnification, and cytokeratin-positive cells within the mesometrial triangle were selected using a threshold analysis after elimination of nonspecific binding. The area covered by the mesometrial triangle was manually delineated and quantified, and the extent of IST invasion (ie, percentage of the mesometrial triangle with cytokeratin-positive cells) was reported as a percentage of the total area of the mesometrial triangle.
Analysis of ENVT Invasion
Analysis of ENVT invasion was carried out on sections containing a cross-section of the central maternal artery, as described previously.27 The entire mesometrial triangle was imaged at 4× magnification, and the arteries within the mesometrial triangle were counted and classified as (1) fully invaded, (2) partially invaded, or (3) not invaded. Values were reported as the percentage of arteries in the mesometrial triangle that were invaded by ENVTs.27–29
Analysis of Spiral Artery Remodeling
Pijnenborg and colleagues25 report that spiral artery remodeling in rats occurs primarily between GD17 to GD22. Therefore, we evaluated maternal spiral artery remodeling at GD18 on fetal–placental unit cross sections containing a central maternal artery, as described previously.29 Serial tissue sections were stained for α-SMA (Sigma-Aldrich) and scanned using the 4× objective. Spiral arteries in adjacent tissue sections were identified and analyzed for the presence of ENVTs and SMA-positive cells.
The length of the blood vessel contour with SMA-positive cells was traced and quantified using NIS Elements Software (Nikon Canada, Mississauga, Ontario), and the percentage of SMA coverage for each invaded (full and partially invaded) artery in the mesometrial triangle was calculated based on the total contour length of the same vessels. A minimum of 3 arteries per dam were quantified, and the values were averaged to obtain the amount of SMA coverage for each animal in the CON and HF groups.
Western Blotting
Decidual tissue samples were homogenized in a 1:20 ratio of tissue to homogenization buffer (5 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 100 mmol/L KCl, 70 mmol/L sucrose, 220 mmol/L mannitol, 1 mmol/L ethylene glycol tetraacetic acid; pH 7.2) containing protease inhibitor cocktail tablets (Roche Diagnostics, Indianapolis, Indiana). Levels of matrix metalloproteinase 2 (MMP-2) and metalloproteinase 9 (MMP-9) were quantified using Western blotting, as described previously.30 Briefly, 10 µg of decidual homogenate was separated by 10% (for the analysis of MMP-2 and MMP-9) sodium dodecyl sulfate–polyacrylamide gel and transferred to nitrocellulose membranes. Membranes were blocked in 5% BSA (MMP-2; Roche) or 5% milk (MMP-9) in Tris-buffered saline (TBST: 137 mmol/L NaCl, 2.7 mmol/L KCl, 25 mmol/L Tris-Cl, pH 8.0) supplemented with 0.1% Tween-20 overnight at 4°C. Membranes were then incubated with anti-MMP-2 (1:5000 in 3% BSA in TBST; Abcam Inc, Cambridge, Massachusetts), anti-MMP-9 (1:20 000 in 3% milk in TBST; Abcam Inc) for 2 hours, and washed with TBST before incubation with antimouse immunoglobulin G secondary antibody (1:5000; GE Healthcare, Mississauga, Ontario) in 3% BSA or 3% milk in TBST. Blots were developed using enhanced chemiluminescence (Millipore), and densitometric quantification was carried out using ImageJ software (ImageJ, Version 1.37; NIH, Bethesda, Maryland).
Monocyte Chemoattractant Protein 1 Enzyme-Linked Immunosorbent Assay
Total monocyte chemoattractant protein 1 (MCP-1) in serum, from CON- and HF-fed dams, collected during sacrifice at GD15 and GD18 was quantified using a rat MCP-1 enzyme-linked immunosorbent assay kit (Pierce, Rockford, Illinois), according to the manufacturer’s instructions. Serum samples were diluted 1:25 for the assay.
Statistics
Statistical analyses were performed in GraphPad Prism v4.0 (GraphPad Software Inc, La Jolla, California). The MCP-1 levels, IST invasion, and ENVT invasion were analyzed using a 2-way analysis of variance (ANOVA) with diet and age as the independent variables; Bonferroni’s post hoc testing was performed if a significant interaction effect of these variables was observed. The extent of spiral artery remodeling and Western blot data were analyzed using a 2-tailed Student t test. Statistics were performed using each dam as a single statistical unit. Results with P values of <.05 were considered to be statistically significant.
Results
Invasion of IST and ENVT Cells Is Increased Early in Gestation in HF-fed Dams
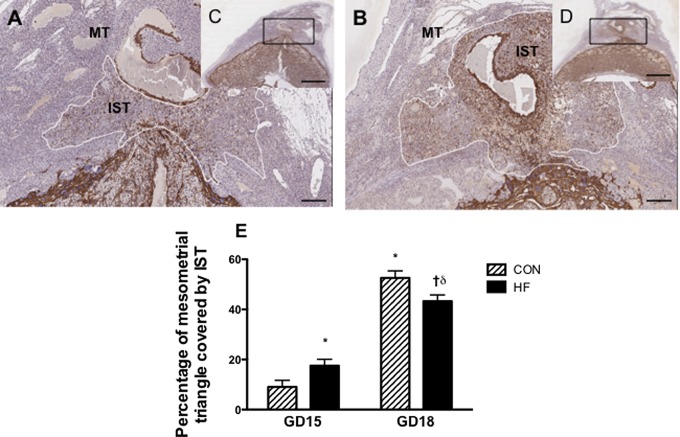
We assessed trophoblast invasion through both the IST and ENVT pathways at GD15 and GD18. Gestational day 15 is thought to represent the initial stages of invasion,25 and we observed approximately 2-fold greater invasion by IST cells at this stage in implantation sites from HF-fed dams (Figure 1; P < .05) compared to the CON-fed dams. When comparing the progression of invasion from GD15 to GD18 in CON-fed dams, the mesometrial triangle of GD18 dams exhibited approximately 43% greater cross-sectional surface area coverage by ISTs (P < .05). However, when comparing GD15 to GD18 in HF-fed dams, the mesometrial triangle of GD18 dams exhibited only approximately 26% more cross-sectional surface area coverage. Interestingly, at GD18, the peak of the trophoblast invasion process, the HF-fed dams had reduced cross-sectional surface area relative to CON-fed dams (P < .05).
Figure 1.
Percentage of cross-sectional surface area covered by IST cells in the MT is increased at GD15 and reduced at GD18 in HF-fed dams. Central slides (5 μm thick) were stained with antipan cytokeratin. Cytokeratin staining (brown) indicates the presence of trophoblast cells. Representative images of GD15 uteroplacental unit from CON-fed (C) and HF-fed dams (D) are shown; scale bar = 600 µm. Panels A and B are magnified images of boxed region of panels C and D. respectively; scale bar = 150 µm. E, Graphical representation of the percentage of the mesometrial triangle invaded by IST cells at GD15 and GD18. P < .05 was calculated by 2-way ANOVA; * indicates significantly different from the GD15 CON-fed group; †, significantly different from the HF-fed GD15 group; δ, significantly different from the CON-fed GD18 group, 1 implantation site was examined per dam, with a minimum of 5 dams per group; IST, interstitial trophoblast; MT, mesometrial triangle; GD, gestational day; HF, high fed; CON, control; ANOVA, analysis of variance.
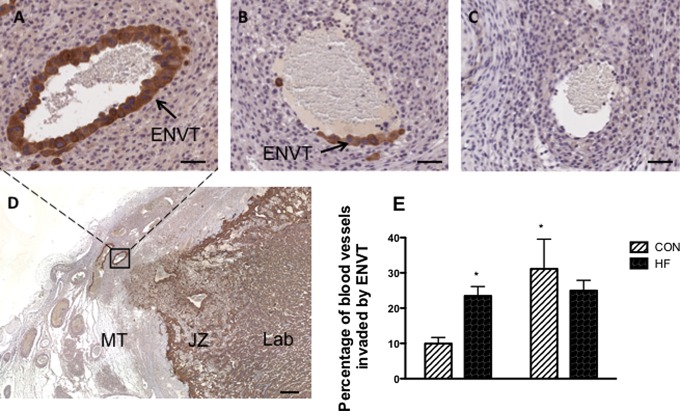
Trophoblast invasion through the ENVT pathway was also quantified by assessing the percentage of blood vessels in the mesometrial triangle invaded either partially or fully by ENVT cells. Examples of fully, partially, and invaded blood vessels within the mesometrial triangle are illustrated in Figure 2A and B and compared to blood vessels that have not been invaded (Figure 2C). Analysis by 2-way ANOVA demonstrated that the presence of ENVT in spiral arteries was significantly altered by gestational age (main effect: P < .05), but there was no main effect of diet (P > .05). There was, however, a significant interaction between gestational age and diet. The percentage of vessels invaded by ENVT was more than 2-fold higher in implantation sites from HF-fed dams compared to CON-fed dams (Figure 2; P < .05) at GD15. However, no significant difference in the percentage of vessels covered by ENVT was observed at GD18 between diet groups. In addition, there was a significant effect of gestational age within the CON-fed group (P < .05); an effect that was absent in the HF-fed group.
Figure 2.
The percentage of blood vessels invaded by endovascular trophoblast cells in the entire MT is higher in HF-fed dams at GD15 but not at GD18. Central slides (5 μm thick) were stained with antipan cytokeratin. All blood vessels in the MT were traced and vessels fully invaded (A), partially invaded (B), and not invaded (C) by endovascular trophoblast (brown positive staining) were counted. Scale bars = 50 μm. Panel D demonstrates the zoomed out image of the MT from which the image of the fully invaded spiral artery (A) was taken; scale bar = 250 µm. Panel E shows graphical representation of the percentage of total blood vessels that were fully or partially invaded, which was calculated for each implantation site before being averaged. P < .05 was calculated by 2-way ANOVA. * indicates significantly different from the CON-fed group, one implantation site was examined per dam, with a minimum of 4 dams per group; JZ, junctional zone; Lab, labyrinth; MT, mesometrial triangle; GD, gestational day; CON, control; ANOVA, analysis of variance.
The increased invasion early in gestation in HF-fed dams is associated with an increase in MMP-9 levels in the mesometrial triangle.
We measured the protein expression of MMP-2 and MMP-9 in decidua tissue homogenates, as these proteins are known to be important mediators of invasive trophoblast cells.31 We observed a 2-fold increase in MMP-9 levels in decidual tissue homogenates from HF-fed dams at GD15 (P < .05; Figure 3B). The mean MMP-2 level was also higher at GD15, although this did not reach statistical significance (P = .21; Figure 3A). Both MMP-9 and MMP-2 protein expression levels at GD18 were not significantly different (data not shown).
Figure 3.
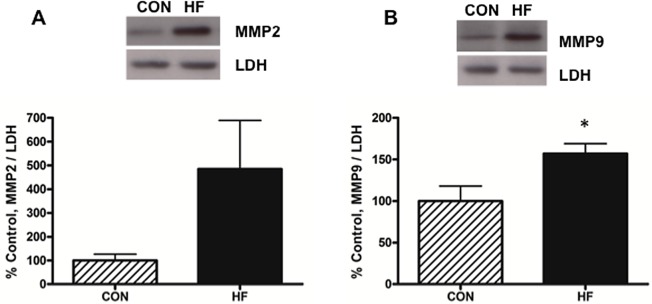
MMP-9 levels are elevated in the mesometrial triangle of HF-fed rats at GD15. MMP-2 (panel A) and MMP-9 (panel B) protein levels were measured by Western blot and normalized to cellular levels of LDH. Mesometrial triangle homogenates, 10 μg, were separated on a 10% polyacrylamide gel, transferred to nitrocellulose, and incubated in 3% milk with anti-MMP-9 monoclonal antibody or anti-MMP-2 monoclonal antibody; n = 7 per group. All values represent mean ± SEM; P values were calculated using 2-tailed Student t test; *P < .05. MMP indicates metalloproteinase; HF, high fed; GD, gestational day; LDH, lactate dehydrogenase; SEM, standard error of the mean.
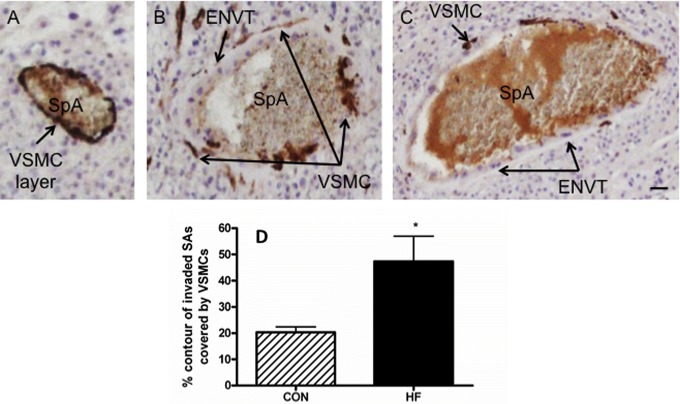
Remodeling of Spiral Arteries Is Compromised in Implantation Sites From HF-Fed Dams at GD18
The SMA-positive VSMCs, situated around the periphery of the spiral arteries, are lost when these vessels are converted to high-capacity, low-resistance vessels. In addition, the proportion of the spiral artery that is SMA positive decreases with the normal remodeling seen during pregnancy.25,32 Therefore, we assessed spiral artery remodeling by examining the loss of SMA-positive VSMCs in invaded spiral arteries at GD18 (Figure 4). The percentage of the total contour of partially and fully invaded arteries that were covered by VSMCs was analyzed (Figure 4A-C). At GD18, the arteries in the mesometrial triangle of HF-fed dams were covered by significantly greater amounts of VSMCs compared to that seen in CON-fed dams (Figure 4D; P < .05).
Figure 4.
Vascular smooth muscle cell layer remodeling in invaded spiral arteries is compromised in HF-fed dams at GD18. A-C, Representative images of slides stained with anti-SMA. The vessels that were partially or fully invaded by ENVT were analyzed. A representative blood vessel of a nonremodeled SpA (A), a partially remodeled artery (B), and an almost fully remodeled artery (C); scale bar = 50 µm. D, The percentage of contour length covered by SMA-positive VSMCs was quantified in relation to the entire artery contour of fully or partially invaded arteries. Sections containing 3 to 6 spiral arteries were analyzed in each animal, and an average value per animal was obtained; 2-tailed Student t tests were performed on these averages. n = at least 5 dams per group; *P < .05. HF, high fed; GD, gestational day; SMA, smooth muscle actin; ENVT, endovascular trophoblast; SpA, spiral artery; VSMCs, vascular smooth muscle cells.
HF-Fed Dams Show Evidence of Systemic Inflammation During Pregnancy
Since increased inflammatory stimuli are known to impact trophoblast invasion,20,23 we assessed levels of MCP-1, as a marker of systemic inflammation, in serum from CON- and HF-fed dams (Figure 5). At GD15, MCP-1 levels were 1.4-fold higher in plasma from HF-fed dams; while at GD18, the MCP-1 levels were 1.7-fold higher in plasma from HF-fed dams (all Ps < .01).
Figure 5.
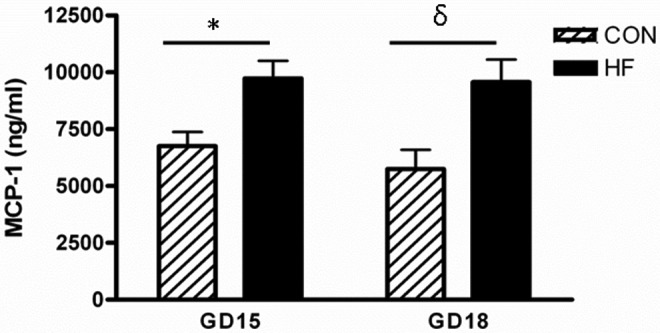
MCP-1 is elevated in the serum of HF-fed dams at GD15 and GD18. MCP-1 was measured using a commercially available ELISA according to the manufacturer’s instructions. P < .01 was calculated by 2-way ANOVA. * indicates significantly different from the GD15 CON-fed group; δ, significantly different from the GD18 CON-fed group; HF, high fed; GD, gestational day; MCP-1, monocyte chemoattractant protein 1; ELISA, enzyme-linked immunosorbent assay; ANOVA, analysis of variance.
Discussion
We developed a rat model of lifelong maternal obesity that results in increased fetal and neonatal death as well as in reduced birth weight.5 In the human population, obese women are more likely to develop complications including preeclampsia as well as delivering SGA babies.1,2 These complications have been linked with reduced trophoblast invasion and incomplete spiral artery remodeling,7,33 and the current literature suggests that this may be partially mediated by inflammatory signals.34 The parallels in outcome between our model and what is observed in the human population prompted a focus on the consequences of obesity during pregnancy and its impact on pregnancy-induced uterine modifications.
In HF-fed dams, we observed significantly increased IST and ENVT invasion at GD15. By GD18, these dams exhibited decreased IST invasion while there were no significant differences in the ENVT invasion between CON- and HF-fed animals. Taken together, this demonstrates a dramatically altered progression of trophoblast invasion in the placenta of HF-fed dams.
Since our study design focused on the analysis of 2 time points, GD15 (initiation) and GD18 (peak), we cannot delineate between 2 possible trajectories for the progression of invasion as a consequence of lifelong HF diet. The first is a complete shift in the temporal regulation of trophoblast invasion (ie, early initiation of invasion and an early retraction); and the second is a situation where early invasion is dramatically increased and is followed by halted progression (ie, greater levels of initial trophoblast invasion into the mesometrial compartment that is inhibited before reaching the peak). In both of these scenarios, the increased invasion at an earlier gestational time point results in an early arrival of an abundance of trophoblasts within the proximity of the uterine spiral arteries. Comparison of the temporal relationship in trophoblast invasion profile for the CON-fed and HF-fed dams demonstrated that while HF-fed dams exhibited increased early invasion at GD15, there is no significant difference in the extent of invasion between GD15 and GD18. In contrast, CON-fed dams exhibited significant effect of gestational age for both ENVT and IST invasion, as demonstrated by a dramatic increase in the area occupied by ENVT and IST at GD18 compared to GD15.
We observed significantly greater staining for α-smooth muscle actin around the spiral arteries that had been invaded by ENVT in mesometrial triangle of HF-fed dams. The increased levels of α-SMA staining, characteristic of VSMCs, suggest a deficiency associated with the normal loss of VSMCs, which is important for the progression of vascular remodeling in pregnancy.15 Much of the early evidence suggested that the remodeling of the spiral arteries might have been the result of trophoblast-mediated stimulation of apoptosis of the VSMCs.32,35,36 More recent findings implicate VSMC migration, rather than apoptosis, as having a major contributory role in remodeling the spiral arteries.37 The mechanisms underpinning the removal of the VSMCs surrounding the spiral artery require further investigation. Our data demonstrate that in the HF-fed dams, a premature increase in the presence of trophoblasts within the mesometrial triangle along with the deficiencies in the spiral artery remodeling process (ie, the disruption of the uterine VSMCs layer surrounding the spiral arteries) is associated with an increased rate of stillbirths and a reduction in birth weight (P = .05).
Increased trophoblast invasion in association with reduced fetal growth has also been observed in a rat model of pree-clampsia27 as well as following maternal exposure to hypoxia during gestation.38 Indeed, we previously reported evidence of increased placental hypoxia in placentas from HF-fed dams at GD15.5 This was associated with reduced fetal growth and increased stillbirth in our model. This reduced level of oxygen may be the consequence of altered trophoblast invasion and poor remodeling of the spiral arteries. The low-oxygen tension is likely an important contributor to the observed fetal outcomes5 that are similar to what has been reported in rat models, which directly evaluate the consequences of exposure to reduced levels of oxygen during pregnancy on birth outcomes.39 These observations are consistent with a recent report by Rosario et al, suggesting that hypoxia-inducible factor 1α , a well-accepted marker of hypoxia, is an important regulator of trophoblast invasion.26
Coinciding with the increase in trophoblast invasion in mesometrial triangles from HF-fed dams at GD15, we observed a 2-fold higher level of MMP-9 protein expression (Figure 3). This protease is involved in extracellular matrix cleavage and is important for trophoblast invasion; it is secreted by invasive trophoblast cells and also by various cells of the decidua including dNK cells.40–42 The important role of both MMP-2 and MMP-9 in placental development is supported by the observation that the inhibition of these gelatinases is associated with a reduction in trophoblast invasion.29 Additionally, the upregulation of MMP-9 (and MMP-2) has also been associated with obesity and may contribute to the increased invasiveness of cancers such as oesophagal adenocarcinoma.43 The increased MMP levels in obesity may be a consequence of increased dietary fat consumption, including oleate,44 or of increased levels of adipocytokines such as resistin.45 In addition, the expression of these enzymes is also known to be potentiated under hypoxia conditions.10,46 Under short-term hypoxic conditions (ie, 24 hours), Lash et al report that HTR8/SVneo cells (immortalized and invasive first trimester human trophoblasts47) exhibit increased invasion in vitro48; a result that in part supports our findings at GD15. In contrast to the increased expression of MMP-9 at GD15, levels of both MMP-9 and MMP-2 were not significantly different at GD18, suggesting that the decreased trophoblast invasion observed at this time point maybe the result of HF diet-induced alterations in regulatory factors other than MMPs. Interestingly, prolonged exposure to reduced levels of oxygen (72 hours) results in reduced trophoblast invasion.48 These seemingly divergent observations regarding the effects of hypoxia on invasion may be due, in part, to the role of paracrine factors such as vascular endothelial growth factor VEGF-A165 whose increased release as a consequence of prolonged exposure to hypoxia has been shown to inhibit HTR8/SVneo invasion.49 These in vitro studies provide a potential explanation to the altered invasion patterns observed at both gestational ages in our model. It should be borne in mind that while in vitro models provide valuable mechanistic information on individual factors affecting trophoblast function, they do not accurately address the influences of multiple cell types present in the mesometrial triangle during the process of spiral artery remodeling. One such cell type is the dNK cells.
The dNK cells are thought to play a central role in spiral artery remodeling. Indeed, Robson et al demonstrated that conditioned culture medium from dNK cells induced vascular smooth muscle disorganization in human myometrial arteries.14 This disorganization process is important in that it provides the invading IST cells access to the underlying spiral artery endothelium layer. Furthermore, in vitro studies have demonstrated that the interaction between trophoblasts and dNK cells may attenuate the vascular remodeling capability of the dNK cells.14 Additionally, factors secreted from dNK cells have also been shown to impact the ability of trophoblasts to form a network of vascular tubes and exhibit endothelium-like markers such as VEGF-C.50 The importance of the interaction between dNK cells and trophoblasts is also supported by in vivo evidence. Fraser et al demonstrated that dNK cells isolated from pregnancies with poor spiral artery remodeling secreted reduced levels of proinvasive factors and could not induce remodeling changes in VSMC in culture.51 Although the full extent of the interaction between dNK cells and trophoblasts is yet to be appreciated, this may be an important regulatory point that has impacted obese dams. The changes in the temporal progression of the trophoblast invasion process observed in the HF dams at GD15 may impact this important intercellular relationship and alter time-sensitive cellular functions.
Although the exact mechanism behind the altered progression of trophoblast invasion remains elusive, it is possible that cytokines may play an important role in this process. Cytokine levels are known to be altered in obese individuals52 and such changes may impact both the progression of trophoblast invasion and the interaction between the trophoblasts and dNK cells and ultimately impact spiral artery remodeling. Cytokines such as IL-1553 and macrophage inflammatory protein 1β44 may be important for recruiting dNK cells during the initial stages of placentation. We have confirmed that obesity during pregnancy significantly increases systemic levels of the proinflammatory cytokine MCP-1. Although we did not directly quantify placental inflammation, Frias et al have reported significantly elevated levels of placental MCP-1 and IL-1β in their nonhuman primate model of maternal obesity.54 In addition, Bar et al have also reported increased inflammatory placental lesions, which are characterized by multiple sites of neutrophil infiltration on the chorionic plate and extraplacental membrane,55 in association with obesity during pregnancy and have attributed these lesions to proinflammatory cytokines.56 While in vitro experiments assessing the effects of inflammatory cytokines on trophoblast invasion have shown both stimulatory and inhibitory effects,19,20,23 the mechanisms underlying these effects in the context of obesity are less clear.
Failure to remodel the uterine spiral arteries can lead to increased contractile function that can result in decreased placental perfusion and adverse pregnancy outcomes. In humans, a decreased luminal area of the arteries feeding the placenta can lead to increased blood pressure entering the exchange surface; this can cause rupture of the delicate placental villous tissue and may contribute to reduced fetal growth57 or fetal death. We have previously reported5 that our HF-fed dams have increased blood pressure at GD18; while Frias et al have demonstrated that maternal obesity and the resulting increase in placental inflammation lead to reduced placental blood flow.54 In both cases, the changes in blood pressure/blood flow were associated with increased incidence of stillbirth.
Impaired vascular remodeling and relaxation are often associated with obesity in humans58 and such impairments can complicate pregnancy.59 In the placenta, poor vascular function may be associated with increased placental inflammation.54 Furthermore, cytokines are also thought to play an important role in the remodeling of the vascular smooth muscle around the spiral arteries.34,60 In addition, immune cells within the decidua can also influence the remodeling process. Recent evidence suggests that the dNK cells may impact vascular remodeling, and these cells isolated from pregnancies that are complicated by hypertension are less able to affect the remodeling process.51 Such observations may help elucidate the connection between altered immune response and poor vascular function. Furthermore, understanding the defects in regulating the release of pro- and anti-inflammatory signaling molecules may be central to placental dysfunction in a variety of pathologies including those arising from maternal obesity.
Taken together, our results suggest that lifelong exposure to a HF diet alters the progression of trophoblast invasion into rat mesometrial triangle during pregnancy, and this change may be an important contributor to the reduced remodeling of the vascular smooth muscle surrounding the spiral arteries. We propose that altered trophoblast invasion in this model may be responsible, in part, for the increased fetal death and decreased birth weight as reported previously. Understanding the mechanisms by which maternal obesity can impact placental development will have profound implications for designing intervention strategies to improve pregnancy outcomes in obese women.
Acknowledgments
The authors extend their thanks to the staff of the McMaster University Central Animal Facility for their technical support and guidance.
Footnotes
Authors’ Note: Emily K. Hayes and Daniel R. Tessier contributed equally to this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The author(s) received support from Hospital for Sick Children Foundation (Grant No. XG09-045R), the Canadian Institutes of Health Research (Grant No. 94331) (SR), and the W. Garfield Weston Foundation Award in Obesity and Reproductive Health (AG).
References
- 1. Norman JE, Reynolds RM. The consequences of obesity and excess weight gain in pregnancy. Proc Nutr Soc. 2011;70(4):450–456. [DOI] [PubMed] [Google Scholar]
- 2. Perlow JH, Morgan MA, Montgomery D, Towers CV, Porto M. Perinatal outcome in pregnancy complicated by massive obesity. Am J Obstet Gynecol. 1992;167(4 pt 1):958–962. [DOI] [PubMed] [Google Scholar]
- 3. Rajasingam D, Seed PT, Briley AL, Shennan AH, Poston L. A prospective study of pregnancy outcome and biomarkers of oxidative stress in nulliparous obese women. Am J Obstet Gynecol. 2009;200(4):395 e391–e399. [DOI] [PubMed] [Google Scholar]
- 4. Wu G, Imhoff-Kunsch B, Girard AW. Biological mechanisms for nutritional regulation of maternal health and fetal development. Paediatr Perinat Epidemiol 2012;26(suppl 1):4–26. [DOI] [PubMed] [Google Scholar]
- 5. Hayes EK, Lechowicz A, Petrik JJ, et al. Adverse fetal and neonatal outcomes associated with a life-long high fat diet: role of altered development of the placental vasculature. PLoS One. 2012;7(3):e33370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93(10):1049–1059. [DOI] [PubMed] [Google Scholar]
- 7. Ball E, Bulmer JN, Ayis S, Lyall F, Robson SC. Late sporadic miscarriage is associated with abnormalities in spiral artery transformation and trophoblast invasion. J Pathol. 2006;208(4):535–542. [DOI] [PubMed] [Google Scholar]
- 8. Knofler M. Critical growth factors and signalling pathways controlling human trophoblast invasion. Int J Dev Biol. 2010;54(2-3):269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vercruysse L, Caluwaerts S, Luyten C, Pijnenborg R. Interstitial trophoblast invasion in the decidua and mesometrial triangle during the last third of pregnancy in the rat. Placenta. 2006;27(1):22–33. [DOI] [PubMed] [Google Scholar]
- 10. Soares MJ, Chakraborty D, Renaud SJ, et al. Regulatory pathways controlling the endovascular invasive trophoblast cell lineage. J Reprod Dev. 2012;58(3):283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wallace AE, Fraser R, Cartwright JE. Extravillous trophoblast and decidual natural killer cells: a remodelling partnership. Hum Reprod Update. 2012;18(4):458–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cartwright JE, Kenny LC, Dash PR, et al. Trophoblast invasion of spiral arteries: a novel in vitro model. Placenta. 2002;23(2-3):232–235. [DOI] [PubMed] [Google Scholar]
- 13. Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27(9-10):939–958. [DOI] [PubMed] [Google Scholar]
- 14. Robson A, Harris LK, Innes BA, et al. Uterine natural killer cells initiate spiral artery remodeling in human pregnancy. FASEB J. 2012;26(12):4876–4885. [DOI] [PubMed] [Google Scholar]
- 15. Helwig JJ, Le Bouteiller P. Physiological smooth muscle cell apoptosis contributes to the uterine vascular remodeling in human early pregnancy. Circ Res. 2007;100 (6):754–756. [DOI] [PubMed] [Google Scholar]
- 16. Soares MJ, Chakraborty D, Karim Rumi MA, Konno T, Renaud SJ. Rat placentation: an experimental model for investigating the hemochorial maternal-fetal interface. Placenta. 2012;33(4):233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schmatz M, Madan J, Marino T, Davis J. Maternal obesity: the interplay between inflammation, mother and fetus. J Perinatol. 2010;30(7):441–446. [DOI] [PubMed] [Google Scholar]
- 18. Roberts KA, Riley SC, Reynolds RM, et al. Placental structure and inflammation in pregnancies associated with obesity. Placenta. 2011;32(3):247–254. [DOI] [PubMed] [Google Scholar]
- 19. Bauer S, Pollheimer J, Hartmann J, Husslein P, Aplin JD, Knofler M. Tumor necrosis factor-alpha inhibits trophoblast migration through elevation of plasminogen activator inhibitor-1 in first-trimester villous explant cultures. J Clin Endocrinol Metab. 2004;89(2):812–822. [DOI] [PubMed] [Google Scholar]
- 20. Jovanovic M, Stefanoska I, Radojcic L, Vicovac L. Interleukin-8 (CXCL8) stimulates trophoblast cell migration and invasion by increasing levels of matrix metalloproteinase (MMP) 2 and MMP9 and integrins alpha5 and beta1. Reproduction. 2010;139(4):789–798. [DOI] [PubMed] [Google Scholar]
- 21. Pavan L, Hermouet A, Tsatsaris V, et al. Lipids from oxidized low-density lipoprotein modulate human trophoblast invasion: involvement of nuclear liver X receptors. Endocrinology. 2004;145(10):4583–4591. [DOI] [PubMed] [Google Scholar]
- 22. Staff AC, Ranheim T, Henriksen T, Halvorsen B. 8-Iso-prostaglandin f(2alpha) reduces trophoblast invasion and matrix metalloproteinase activity. Hypertension. 2000;35(6):1307–1313. [DOI] [PubMed] [Google Scholar]
- 23. Jovanovic M, Vicovac L. Interleukin-6 stimulates cell migration, invasion and integrin expression in HTR-8/SVneo cell line. Placenta. 2009;30(4):320–328. [DOI] [PubMed] [Google Scholar]
- 24. Nguyen Dinh Cat A, Briones AM, Callera GE, et al. Adipocyte-derived factors regulate vascular smooth muscle cells through mineralocorticoid and glucocorticoid receptors. Hypertension. 2011;58(3):479–488. [DOI] [PubMed] [Google Scholar]
- 25. Caluwaerts S, Vercruysse L, Luyten C, Pijnenborg R. Endovascular trophoblast invasion and associated structural changes in uterine spiral arteries of the pregnant rat. Placenta. 2005;26(7):574–584. [DOI] [PubMed] [Google Scholar]
- 26. Rosario GX, Konno T, Soares MJ. Maternal hypoxia activates endovascular trophoblast cell invasion. Dev Biol. 2008;314(2):362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Geusens N, Verlohren S, Luyten C, et al. Endovascular trophoblast invasion, spiral artery remodelling and uteroplacental haemodynamics in a transgenic rat model of pre-eclampsia. Placenta. 2008;29(7):614–623. [DOI] [PubMed] [Google Scholar]
- 28. Geusens N, Hering L, Verlohren S, et al. Changes in endovascular trophoblast invasion and spiral artery remodelling at term in a transgenic preeclamptic rat model. Placenta. 2010;31(4):320–326. [DOI] [PubMed] [Google Scholar]
- 29. Verlohren S, Geusens N, Morton J, et al. Inhibition of trophoblast-induced spiral artery remodeling reduces placental perfusion in rat pregnancy. Hypertension. 2010;56(2):304–310. [DOI] [PubMed] [Google Scholar]
- 30. Robertshaw HA, Raha S, Kaczor JJ, Tarnopolsky MA. Increased PFK activity and GLUT4 protein content in McArdle's disease. Muscle Nerve. 2008;37(4):431–437. [DOI] [PubMed] [Google Scholar]
- 31. Luo J, Qiao F, Yin X. Impact of silencing MMP9 gene on the biological behaviors of trophoblasts. J Huazhong Univ Sci Technolog Med Sci. 2011;31(2):241–245. [DOI] [PubMed] [Google Scholar]
- 32. Harris LK, Keogh RJ, Wareing M, et al. Invasive trophoblasts stimulate vascular smooth muscle cell apoptosis by a fas ligand-dependent mechanism. Am J Pathol. 2006;169(5):1863–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McCarthy NJ, Bennett MR. The regulation of vascular smooth muscle cell apoptosis. Cardiovasc Res. 2000;45(3):747–755. [DOI] [PubMed] [Google Scholar]
- 34. Whitley GS, Cartwright JE. Cellular and molecular regulation of spiral artery remodelling: lessons from the cardiovascular field. Placenta. 2010;31(6):465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harris LK, Keogh RJ, Wareing M, et al. BeWo cells stimulate smooth muscle cell apoptosis and elastin breakdown in a model of spiral artery transformation. Hum Reprod. 2007;22(11):2834–2841. [DOI] [PubMed] [Google Scholar]
- 36. Wood IS, Stezhka T, Trayhurn P. Modulation of adipokine production, glucose uptake and lactate release in human adipocytes by small changes in oxygen tension. Pflugers Arch. 2011;462(3):469–477. [DOI] [PubMed] [Google Scholar]
- 37. Bulmer JN, Innes BA, Levey J, Robson SC, Lash GE. The role of vascular smooth muscle cell apoptosis and migration during uterine spiral artery remodeling in normal human pregnancy. FASEB J. 2012;26(7):2975–2985. [DOI] [PubMed] [Google Scholar]
- 38. Chakraborty D, Rumi MA, Konno T, Soares MJ. Natural killer cells direct hemochorial placentation by regulating hypoxia-inducible factor dependent trophoblast lineage decisions. Proc Natl Acad Sci U S A. 2011;108(39):16295–16300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bourque SL, Dolinsky VW, Dyck JR, Davidge ST. Maternal resveratrol treatment during pregnancy improves adverse fetal outcomes in a rat model of severe hypoxia. Placenta. 2012;33(5):449–452. [DOI] [PubMed] [Google Scholar]
- 40. Staun-Ram E, Goldman S, Gabarin D, Shalev E. Expression and importance of matrix metalloproteinase 2 and 9 (MMP-2 and -9) in human trophoblast invasion. Reprod Biol Endocrinol. 2004;2:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Isaka K, Usuda S, Ito H, et al. Expression and activity of matrix metalloproteinase 2 and 9 in human trophoblasts. Placenta. 2003;24(1):53–64. [DOI] [PubMed] [Google Scholar]
- 42. Naruse K, Lash GE, Innes BA, et al. Localization of matrix metalloproteinase (MMP)-2, MMP-9 and tissue inhibitors for MMPs (TIMPs) in uterine natural killer cells in early human pregnancy. Hum Reprod. 2009;24(3):553–561. [DOI] [PubMed] [Google Scholar]
- 43. Allott EH, Lysaght J, Cathcart MC, et al. MMP9 expression in oesophageal adenocarcinoma is upregulated with visceral obesity and is associated with poor tumour differentiation. Mol Carcinog. 2011;52(2):144–154. [DOI] [PubMed] [Google Scholar]
- 44. Soto-Guzman A, Navarro-Tito N, Castro-Sanchez L, Martinez-Orozco R, Salazar EP. Oleic acid promotes MMP-9 secretion and invasion in breast cancer cells. Clin Exp Metastasis. 2010;27(7):505–515. [DOI] [PubMed] [Google Scholar]
- 45. Mu H, Ohashi R, Yan S, et al. Adipokine resistin promotes in vitro angiogenesis of human endothelial cells. Cardiovasc Res. 2006;70(1):146–157. [DOI] [PubMed] [Google Scholar]
- 46. Na KH, Lee HJ, Choi JH, et al. Dynamic alterations in integrin alpha4 expression by hypoxia are involved in trophoblast invasion during early implantation. J Cell Biochem. 2012;113(2):685–694. [DOI] [PubMed] [Google Scholar]
- 47. Graham CH, Hawley TS, Hawley RG, et al. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206(2):204–211. [DOI] [PubMed] [Google Scholar]
- 48. Lash GE, Hornbuckle J, Brunt A, et al. Effect of low oxygen concentrations on trophoblast-like cell line invasion. Placenta. 2007;28(5-6):390–398. [DOI] [PubMed] [Google Scholar]
- 49. Lash GE, Cartwright JE, Whitley GS, Trew AJ, Baker PN. The effects of angiogenic growth factors on extravillous trophoblast invasion and motility. Placenta. 1999;20(8):661–667. [DOI] [PubMed] [Google Scholar]
- 50. Hu Y, Eastabrook G, Tan R, MacCalman CD, Dutz JP, von Dadelszen P. Decidual NK cell-derived conditioned medium enhances capillary tube and network organization in an extravillous cytotrophoblast cell line. Placenta. 2010;31(3):213–221. [DOI] [PubMed] [Google Scholar]
- 51. Fraser R, Whitley GS, Johnstone AP, et al. Impaired decidual natural killer cell regulation of vascular remodelling in early human pregnancies with high uterine artery resistance. J Pathol. 2012;228(3):322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lolmede K, Durand de Saint Front V, Galitzky J, Lafontan M, Bouloumie A. Effects of hypoxia on the expression of proangiogenic factors in differentiated 3T3-F442A adipocytes. Int J Obes Relat Metab Disord. 2003;27(10):1187–1195. [DOI] [PubMed] [Google Scholar]
- 53. Kim CS, Park HS, Kawada T, et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes (Lond). 2006;30(9):1347–1355. [DOI] [PubMed] [Google Scholar]
- 54. Frias AE, Morgan TK, Evans AE, et al. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology. 2011;152(6):2456–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kovo M, Schreiber L, Ben-Haroush A, Shor S, Golan A, Bar J. Intrapartum fever at term: clinical characteristics and placental pathology. J Matern Fetal Neonatal Med. 2012;25(8):1273–1277. [DOI] [PubMed] [Google Scholar]
- 56. Bar J, Schreiber L, Saruhanov E, Ben-Haroush A, Golan A, Kovo M. Placental histopathological findings in obese and nonobese women with complicated and uncomplicated pregnancies. Arch Gynecol Obstet. 2012;286(6):1343–1347. [DOI] [PubMed] [Google Scholar]
- 57. Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30(6):473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sivitz WI, Wayson SM, Bayless ML, Sinkey CA, Haynes WG. Obesity impairs vascular relaxation in human subjects: hyperglycemia exaggerates adrenergic vasoconstriction arterial dysfunction in obesity and diabetes. J Diabetes Complications. 2007;21(3):149–157. [DOI] [PubMed] [Google Scholar]
- 59. Myers J, Hall C, Wareing M, Gillham J, Baker P. The effect of maternal characteristics on endothelial-dependent relaxation of myometrial arteries. Eur J Obstet Gynecol Reprod Biol. 2006;124(2):158–163. [DOI] [PubMed] [Google Scholar]
- 60. Whitley GS, Cartwright JE. Trophoblast-mediated spiral artery remodelling: a role for apoptosis. J Anat. 2009;215(1):21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]