Abstract
To investigate the interconnection of apoptosis and autophagy in trophoblastic cells, we treated JEG-3 cells with tumor necrosis factor α (TNF-α) after transfecting LC3 or Beclin 1 or calpain small interfering RNA (siRNA), which blocks cleavage of autophagy-related gene 5 (Atg5) into N-terminal truncated Atg5 (tAtg5), a mediator between apoptosis and autophagy, and assessed the changes in LC3-II, caspase 9, caspase 3, and tAtg5. We also assessed the TNF-α-induced changes in LC3-II, caspase 9, and caspase 3 in primary trophoblasts from term placentae after transfecting siRNA for LC3 or Beclin 1. In both types of cells, transfection of LC3 or Beclin 1 siRNA significantly attenuated TNF-α-induced increases in LC3-II and activations of caspase 9 and caspase 3. There was significant abrogation of TNF-α-induced expression of tAtg5 after transfection with LC3 or Beclin 1 siRNA. Moreover, transfection with calpain siRNA significantly decreased TNF-α-induced changes in caspase 3 and caspase 9 in addition to tAtg5 in JEG-3 cells. Our data suggest that TNF-α-induced autophagy mediates intrinsic apoptosis, probably through tAtg5, in trophoblastic cells.
Keywords: apoptosis, Atg5, autophagy, trophoblasts
Introduction
In normal pregnancies, apoptosis is involved in implantation of the blastocyst, trophoblastic invasion, tissue remodeling, and normal placental aging process.1,2 In contrast, increased trophoblastic apoptosis has been reported in pregnancies complicated by fetal growth restriction (FGR) or preeclampsia (PE),3–5 suggesting that altered regulation of trophoblastic apoptosis may contribute to the pathophysiology of FGR or PE.6
Autophagy has been implicated in pathophysiologic processes including cellular differentiation, cancer, metabolic or neurodegenerative diseases, and cell death.7,8 However, autophagic function in the human placenta is not fully understood. Our previous study showed increased autophagy in placentae from pregnancies complicated by severe PE,9 suggesting that autophagy plays a role in placental dysfunction. In addition, autophagic activity, observed in trophoblasts from term placental villi, was significantly higher in placentae from the marginal area or cesarean section than those from the central area or vaginal delivery.10 It was also hypothesized that autophagy in trophectoderm might be a surviving mechanism of dormant blastocysts in an experimentally delayed implantation model.11 These findings imply that autophagy is involved in the placental pathophysiology in addition to apoptosis.
Recently, cellular machineries involved in the cross-talk between apoptosis and autophagy including Bcl-2/Bcl-xL/Beclin 1 protein complex (Beclin 1 is a Bcl-2-related protein forming a multiprotein complex and known to be an autophagy inducer in initial stage)12 and N-terminal truncated autophagy-related gene 5 (tAtg5)13 have been deciphered at the molecular level. These interconnections of apoptosis and autophagy may be synergistic or antagonistic in the context of cell type and stimuli.14,15
We previously showed that tumor necrosis factor α (TNF-α) increased autophagy in JEG-3 cells.9 In this study, we analyzed the relationship between apoptosis and autophagy in JEG-3 cells and primary trophoblasts cultured from human term placentae under TNF-α stimulation in conjunction with transfection of small interfering RNA (siRNA) for LC3 or Beclin 1 in order to inhibit autophagy. We also analyzed the changes in tAtg5 to assess the possible role of tAtg5 as a link between apoptosis and autophagy in trophoblastic cells.
Materials and Methods
Cell Culture and Chemical Treatment
The human choriocarcinoma JEG-3 cell line was obtained from the American Type Culture Collection (ATCC, Rockville, Maryland). The cell line was maintained in Dulbecco modified Eagle medium (DMEM; GIBCO-BRL, Grand Island, New York) supplemented with 10% fetal bovine serum (FBS; GIBCO-BRL) containing an antibiotic mixture at 37°C under a humidified atmosphere containing 5% CO2/95% air.
Primary human cytotrophoblasts were isolated from term placenta of 6 uncomplicated pregnancies. This study was approved by institutional review board for Clinical Research at Samsung Medical Center, and informed consent was obtained from all participating pregnant women. Isolation of trophoblasts from placenta was performed using the trypsin-DNAse-dispase/Percoll method16 with previously published modifications.17 In brief, fetal membranes and maternal decidua were removed, and villous tissue was minced into fragments and washed extensively with saline to remove blood within 30 minutes after delivery. The tissue was then digested in Hank buffered salt solution containing trypsin-DNase-dispase at 37°C. Trophoblasts were isolated using a continuous Percoll gradient, washed, and plated at a density of 300 000 cells/cm2 in DMEM containing 10% FBS, 20 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.4; Sigma-Aldrich, St Louis, Missouri), 0.5 mmol/L l-glutamine, penicillin (10 U/mL), streptomycin (10 g/mL), and fungizone (0.25 g/mL). Trophoblast cultures were maintained under standard conditions in 20% O2 and 5% CO2 atmosphere at 37° C in order to avoid the possible influence of hypoxia on autophagy. The cell culture medium was changed every 24 hours.
To examine the regulation of cytokine expression in JEG-3 cells and primary trophoblasts, the cells were stimulated with 30 ng/mL TNF-α (Biosource International Inc, Camarillo, California) for up to 48 hours. Three independent cultures were performed for each experiment.
Inhibition of Autophagy
Transfection was carried out using Lipofectamine RNAiMAX (Life Technologies, Carlsbad, California) according to the manufacturer’s protocol. One day before transfection, approximately 1 × 106 JEG-3 cells were put in a 60-mm2 culture plate and grown in DMEM medium supplemented with 10% FBS at 37°C under a humidified 5% CO2 atmosphere. Primary trophoblasts were used for transfection 4 hours after plating. The culture medium was changed with Minimum Essential Media (MEM; GIBCO-BRL) containing 10% FBS approximately 1 to 2 hours before transfection, and the cells were placed in a 5% CO2 incubator. To inhibit autophagy, cells were transfected with 60 pmol of siRNA for Beclin 1 (5′-CAGUUUGGCACAAUCAAUA-3′), LC3 (5′-GAAGGCGCUUACAGCUCAA-3′), or the small unit of calpain (5′-CGCACACAUUACUCCAACAUU-3′). The transfection mixture was added to the cells and incubated at 37°C under a 5% CO2 atmosphere in order to avoid the possible influence of hypoxia on autophagy in trophoblastic cells. After 16 hours of siRNA transfection, the medium containing the transfection mixture was changed with MEM, and the siRNAs were maintained with cells. If necessary, TNF-α was added to the medium. The culture medium was changed every 24 hours.
Western Blotting Analysis
For immunoblot analysis of the cellular proteins, cell lysates were prepared as follows. JEG-3 cells were washed twice with cold phosphate-buffered saline and then lysed in a radioimmunoprecipitation assay buffer (50 mmol/L Tris-Cl, 150 mmol/L NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate [SDS], pH 7.5) containing 1 mmol/L phenylmethylsulfonyl fluoride. To facilitate complete solubilization of cellular proteins, cell lysates were incubated in ice for 30 minutes and then centrifuged. Protein concentrations for each sample were determined using the Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, California). Protein (20 µg) was separated by 10% to 13.5% SDS-polyacrylamide gel electrophoresis under reducing conditions and then electrotransferred to a polyvinylidene difluoride (Millipore Corporation, Billerica, Massachusetts) membrane. Blots were blocked for 1 hour with 5% nonfat dry milk in Tween-Tris-buffered saline (0.1% Tween-20 in 100 mmol/L Tris-Cl [pH 7.5], 0.9% NaCl) and then the membranes were incubated with polyclonal antibodies to LC3 (PM 036; Medical & Biological Laboratories Co, Nagoya, Japan), Beclin 1 (CST 3738; Cell Signal Technology, Beverly, Massachusetts), calpain (MAB3083; Chemicon International, Temecula, California), caspase 3 (CST 9502; Cell Signal Technology), caspase 9 (CST 9662; Cell Signal Technology), Atg5 (NB110-53818; Novus Biologicals, Littleton, Colorado), and tAtg5 (AP1812a; Abgent, San Diego) at a dilution of 1:200 to 1:1000 overnight at 4°C. Immunoreactive bands were detected by incubation with goat antirabbit or antimouse IgG conjugated to horseradish peroxidase (diluted 1:5000; Santa Cruz Biotechnology, Santa Cruz, California) at room temperature for 1 hour. Peroxidase activity was visualized with the enhanced chemiluminescence detection system (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, UK) and captured on X-ray film. Blots were stripped with Restore Western blot stripping buffer (Thermo Scientific, Rockford, Illinois) and reprobed, when necessary, with β-actin (Sigma-Aldrich) antibody as a control.
LC3 Flux Assay Using Bafilomycin A1
Bafilomycin A1 inhibits degradation of autolysosomes, and increased LC3-II expression in cells treated with bafilomycin A1 indicates accumulation of LC3-II-positive autophagosomes.18 To verify that changes in the expression of LC3-II after transfection of LC3 siRNA were associated with changes in autophagosome synthesis (autophagic activity) and not with autophagosome turnover (delayed trafficking to the lysosomes or impaired lysosomal proteolytic activity), we performed LC3 flux assays using 0.5 µmol/L bafilomycin A1 (B1793; Sigma-Aldrich).
Statistical Analysis
The densitometry results of Western blots were analyzed using the Mann-Whitney U test and Kruskal-Wallis test. The results were considered statistically significant when P values were less than .05. All analyses were performed using SPSS 19.0 version 9.1 (SPSS Inc, Chicago, Illinois).
Results
Inhibition of Basal Autophagy by siRNA for LC3 or Beclin 1 in JEG-3 Cells
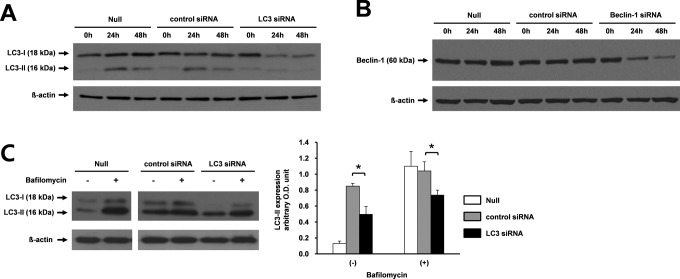
Under naive conditions, we assessed whether LC3 or Beclin 1 siRNA efficiently inhibited autophagy in JEG-3 cells. When JEG-3 cells were transfected with LC3 siRNA, the expression of LC3 decreased by about 80% compared with the control siRNA-transfected cells and nontransfected cells at 24 hours (Figure 1A). The expression of 16 kDa LC3-II, a marker of autophagosome formation, was evident up to 24 hours in JEG-3 cells, reflecting basal autophagy. As expected, the expression of 18 kDa LC3-I profoundly decreased when cells were transfected with LC3 siRNA, indicating inhibition of LC3 synthesis. The expression of Beclin 1 also decreased in JEG-3 cells by transfection of Beclin 1 siRNA by 50% to 60% compared to control siRNA-transfected cells and nontransfected cells at 48 hours (Figure 1B).
Figure 1.
Knockdown of LC3 and Beclin 1 by LC3 or Beclin 1 siRNA, respectively, and LC3 flux assay in the presence of bafilomycin A1 in JEG-3 cells. A, LC3 expression was determined by Western blot analysis after transfection with LC3 siRNA into JEG-3 cells. The expression of LC3 was determined at 24 hours and 48 hours after transfection with LC3 siRNA, control siRNA, or without transfection. This experiment was repeated twice, and the representative figure is shown. B, Western blot analysis was performed after transfection with Beclin 1 siRNA into JEG-3 cells. The expression of Beclin 1 was analyzed at 24 hours and 48 hours after transfection with Beclin 1 siRNA compared with control siRNA-transfected cells and nontransfected cells. This experiment was repeated twice, and the representative figure is shown. C, LC3 flux assay was performed in the presence and absence of bafilomycin A1, which inhibits degradation of autolysosomes. JEG-3 cells were transfected with LC3 siRNA, or control siRNA. Bafilomycin A1 was treated for 24 hours. Representative blots from 3 independent experiments are shown. Western blotting for β-actin was done for the loading control. Null indicates nontransfected cells; siRNA, small interfering RNA.
In LC3 flux assays using bafilomycin A1, which inhibits degradation of autolysosomes, JEG-3 cells were transfected with LC3 siRNA and then treated with 0.5 µmol/L bafilomycin A1. As shown in Figure 1C, the expression of LC3-II (in the presence and absence of bafilomycin A1) decreased at 24 hours after transfection with LC3 siRNA compared with control siRNA. This result indicated that the accumulation of LC3-II decreased after transfection with LC3 siRNA.
Increased Autophagic and Apoptotic Activity by TNF-α Treatment in JEG-3 Cells
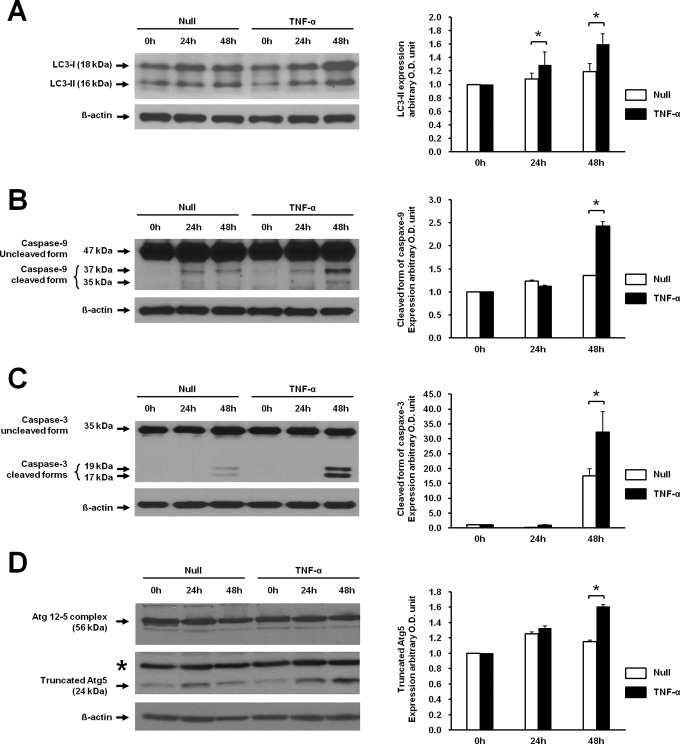
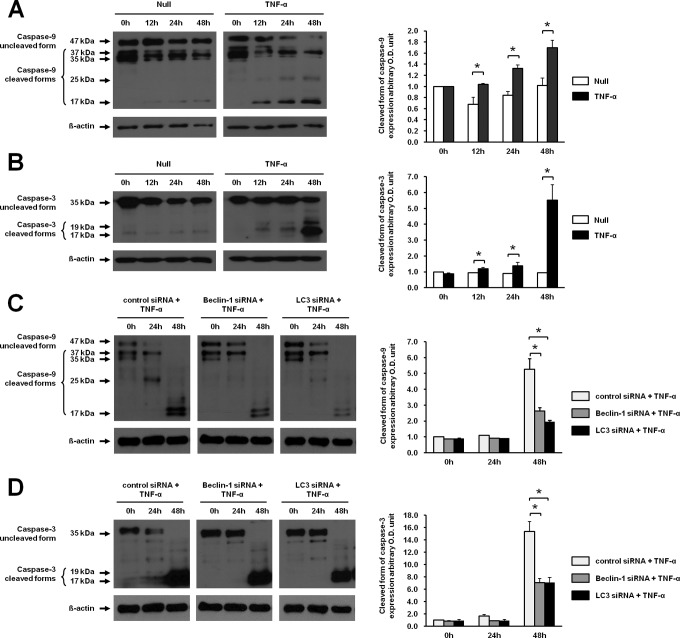
We previously demonstrated that TNF-α induced autophagy in JEG-3 cells.9 In order to study the correlation between autophagy and apoptosis, apoptotic activity was assessed in JEG-3 cells treated with TNF-α. Consistent with the previous study, the expression of LC3-II in JEG-3 cells significantly increased from 24 hours to 48 hours after stimulation with TNF-α compared with nontreated control cultures (Figure 2A). Likewise, the cleaved forms of caspase 9 (35 kDa and 37 kDa) and caspase 3 (17 kDa and 19 kDa) were significantly increased in JEG-3 cells at 48 hours after stimulation with TNF-α compared with nontreated control cultures (Figure 2B and C). Interestingly, tAtg5, which is known as a switch molecule from autophagy and apoptosis, was also increased in response to TNF-α treatment (Figure 2D). These results demonstrated that TNF-α induced both autophagy and apoptosis in JEG-3 cells.
Linkage Between Autophagy and Apoptosis in JEG-3 Cells
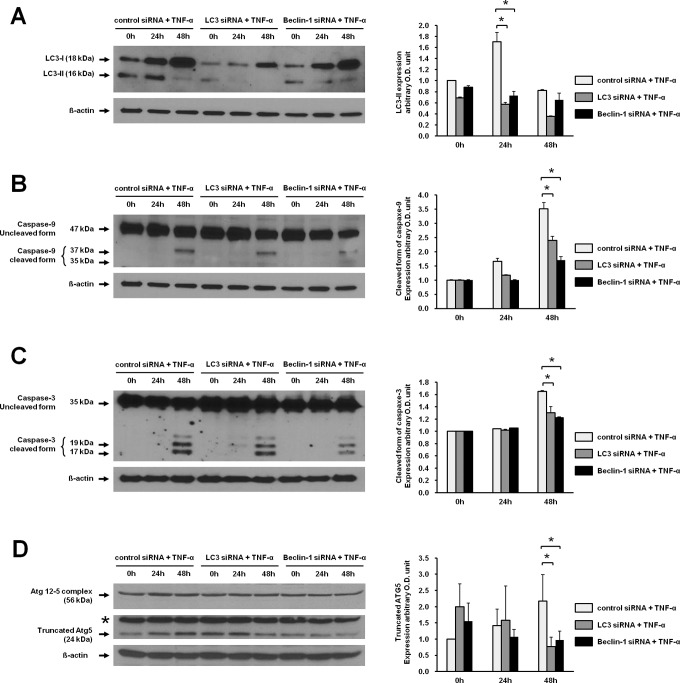
To determine whether apoptosis is mediated by autophagy in the presence of TNF-α, apoptosis was measured under inhibition of autophagy using siRNAs for LC3 and Beclin 1. When JEG-3 cells were transfected with siRNA for LC3 or Beclin 1, the increased expression of LC3-II induced by TNF-α was significantly attenuated at 24 hours compared to control siRNA (Figure 3A). Similarly, knockdown of LC3 or Beclin 1 by siRNA in JEG-3 cells decreased TNF-α-induced expression of the cleaved forms of caspase 9 and caspase 3 at 48 hours compared to transfection with control siRNA (Figure 3B and C). These results demonstrated that autophagy induced by TNF-α might mediate apoptosis.
Figure 2.
Effect of TNF-α on LC3-II, caspase 9, caspase 3 activation, and tAtg5 in JEG-3 cells. A, The expression of LC3-II was assessed in the presence of TNF-α. TNF-α was treated for 24 hours and 48 hours in JEG-3 cells. B, Activation of caspase 9 (cleavage of 47 kDa proform into its cleaved forms, 35 kDa and 37 kDa) by TNF-α was determined at the indicated time points by Western blot analysis. C, Activation of caspase 3 (cleavage of 35 kDa proform to 17 kDa and 19 kDa) was also determined at the indicated time points by Western blot analysis. D, Expression of tAtg5 was assessed in JEG-3 cells treated with TNF-α. Right panels demonstrate the expression level of each protein detected by Western blotting after normalization with β-actin. The data are presented as the mean ± SEM of 3 independent experiments (*P < .05). Representative blots are shown. *indicates nonspecific band; Null, nontreated cells; TNF-α, tumor necrosis factor α; SEM, standard error of the mean; tAtg5, truncated autophagy-related gene 5.
In order to explore the cross talk between autophagy and apoptosis in trophoblastic cells, we evaluated the change in Atg5 after inhibition of autophagy in JEG-3 cells. When JEG-3 cells were transfected with control siRNA, TNF-α stimulation induced expression of the 24 kDa tAtg5 up to 48 hours. After transfection with LC3 or Beclin 1 siRNA, the expression of tAtg5 by TNF-α stimulation was profoundly decreased from 24 hours to 48 hours (Figure 3D).
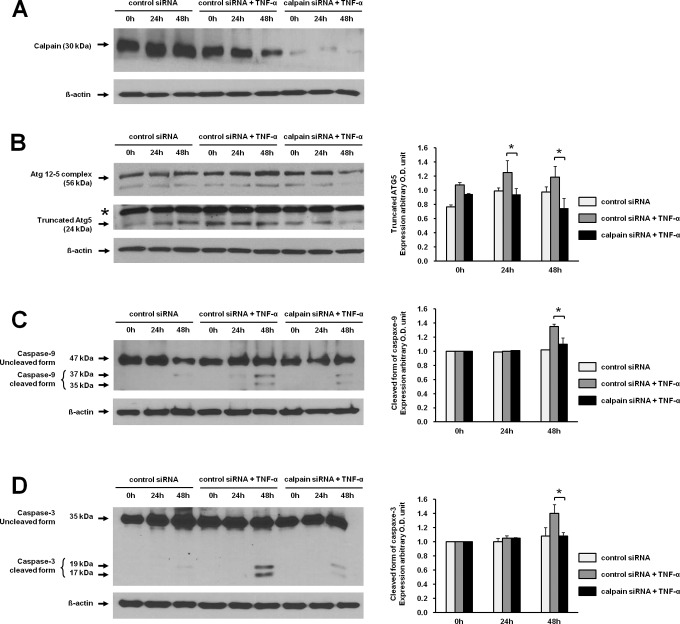
Inhibition of Calpain Decreased Apoptosis in JEG-3 Cells
When JEG-3 cells were transfected with siRNA against calpain, which cleaves Atg5 into tAtg5, the expression of calpain was significantly decreased compared with control siRNA (Figure 4A). Transfection with calpain siRNA attenuated the TNF-α-induced tAtg5 (Figure 4B). The increased expressions of the cleaved forms of caspase 9 and caspase 3 induced by TNF-α were also significantly decreased at 48 hours in JEG-3 cells transfected with calpain siRNA (Figure 4C and D).
Figure 3.
Inhibition of TNF-α-induced expression of LC3-II, caspase 9, caspase 3, and tAtg5 by transfection with LC3 or Beclin 1 siRNA in JEG-3 cells. A, The expression of LC3-II was analyzed by Western blotting after treatment with TNF-α at different time points. siRNAs for LC3, Beclin 1, and control were transfected into JEG-3 cells. After 16 hours of transfection, TNF-α was treated for 24 hours and 48 hours. B, Activation of caspase 9 was analyzed in cells transfected with LC3 or Beclin 1 siRNA after treatment with TNF-α by Western blotting using the antibody to detect the cleaved forms (35 and 37 kDa). C, Activation of caspase 3 was measured in cells transfected with LC3 or Beclin 1 siRNA in the presence of TNF-α by Western blotting using the antibody detecting the cleaved forms, 17 and 19 kDa. D, The expression of tAtg5 was analyzed in cells transfected with LC3 or Beclin 1 siRNA by Western blotting using the antibody against Atg5. The 24 and 56 kDa bands correspond to the tAtg5 and Atg12-5 complex, respectively. Western blot analysis for β-actin was used as a loading control. Right panels demonstrate the expression level of each protein detected by Western blotting after normalization with β-actin. The data are presented as the mean ± SEM of 3 independent experiments (*P < .05), and the representative blots are shown. Atg5 indicates autophagy-related gene 5; tAtg5, truncated autophagy-related gene 5; *, nonspecific band; 0 hour, before treatment of TNF-α 24 hours after transfection with siRNAs; TNF-α, tumor necrosis factor α; SEM, standard error of the mean; siRNA, small interfering RNA.
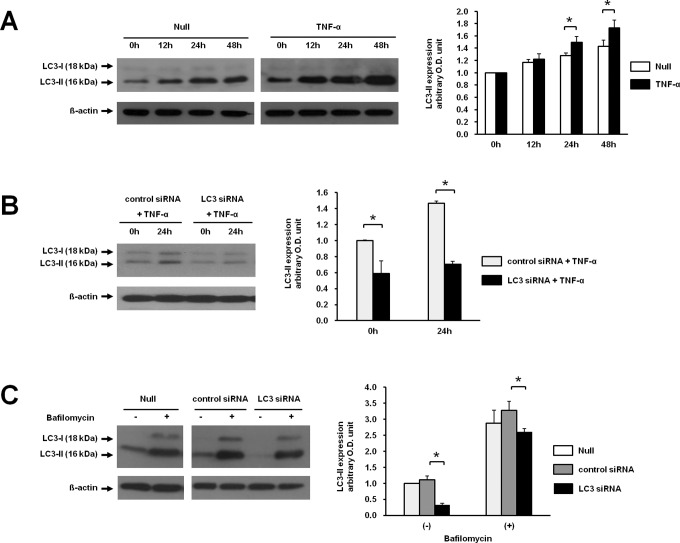
Inhibition of TNF-α-Induced Autophagy by LC3 or Beclin 1 siRNA in Primary Trophoblasts
To investigate whether TNF-α can induce autophagy in primary trophoblasts or not, primary trophoblasts were prepared from term placentae, and TNF-α was applied to the primary cells. Consistent with our observations in JEG-3 cells, the expression of LC3-II in primary trophoblasts was significantly increased from 24 to 48 hours after treatment with TNF-α compared with the nontreated controls (Figure 5A). When primary trophoblasts were transfected with LC3 siRNA, the expression of TNF-α-induced LC3-II was significantly attenuated at 24 hours compared with the control siRNA (Figure 5B). Although the transfection efficiency of primary trophoblasts is very low, the expression of LC3 was reduced by 46% by LC3 siRNA compared to the control siRNA-transfected primary culture at 0 hour (Figure 5B, right panel). As demonstrated in the JEG-3 cells, the expression of Beclin 1 was also inhibited in primary trophoblasts by transfection with Beclin 1 siRNA (data not shown).
Figure 5.
Induced expression of LC3-II by TNF-α and LC3 flux assay in the presence of bafilomycin A1 in primary trophoblasts. A, The expression of LC3-II was measured in primary trophoblasts after treatment with TNF-α for the indicated time. B, The expression of LC3-II was measured in primary trophoblasts transfected with siRNA for either LC3 or control followed by treatment with TNF-α for 24 hours by Western blotting. C, The expression of LC3-II was measured in primary trophoblasts transfected with siRNAs for either LC3 or control in the absence and presence of bafilomycin A1 by Western blotting. Right panels demonstrate the expression level of LC3-II detected by Western blotting after normalization with β-actin. The data are presented as the mean ± SEM from 3 different cell preparations (placentas) for each independent experiment (*P < .05) and representative blots are shown. TNF-α indicates tumor necrosis factor α; SEM, standard error of the mean; siRNA, small interfering RNA.
In LC3 flux assays using bafilomycin A1, primary trophoblasts were transfected with LC3 siRNA and treated with bafilomycin A1. As shown in Figure 5C, expression of LC3-II was decreased at 24 hours after transfection with LC3 siRNA compared with control siRNA, indicating decreased flux of LC3-II.
Figure 4.
Inhibition of apoptosis via inhibition of tAtg5 production by calpain siRNA in JEG-3 cells. A, The expression of calpain was analyzed in cells transfected with siRNA for calpain followed by treatment of TNF-α by Western blotting using the antibody against calpain. siRNAs either for calpain or control were transfected into JEG-3 cells. After 24 hours of transfection, cells were incubated with TNF-α for 24 and 48 hours. B, The expression of tAtg5 was analyzed in JEG-3 cells transfected with calpain siRNA by Western blotting using the antibody against Atg5. C, The cleaved forms of caspase 9 were measured in the absence and presence of TNF-α in JEG-3 cells transfected with siRNAs for either calpain or control by Western blotting. D, The 17 and 19 cleaved forms of caspase 3 were detected in the absence and presence of TNF-α in JEG-3 cells transfected with siRNA for either calpain or control by Western blotting. Western blot analysis for β-actin was used as a loading control. Right panels demonstrate the expression level of each protein detected by Western blotting after normalization with β-actin. The data are presented as the mean ± SEM of 3 independent experiments (* P < .05) and the representative blots are shown. * indicates nonspecific band; 0 hour, before treatment of TNF-α 24 hours after transfection with siRNAs; TNF-α, tumor necrosis factor α; SEM, standard error of the mean; siRNA, small interfering RNA.
Inhibition of TNF-α-Induced Apoptosis in Primary Trophoblasts by Inhibition of Autophagy
The activation and cleavage of caspase 9 and caspase 3 were also significantly increased from 12 hours to 48 hours in primary trophoblasts after stimulation with TNF-α compared with nontreated controls (Figure 6A and B). In order to inhibit autophagy, primary trophoblasts were transfected with LC3 or Beclin 1 siRNA. After 48 hours of TNF-α stimulation, the activation and cleavage of caspase 9 and caspase 3 were significantly decreased at 48 hours compared with the siRNA control group (Figure 6C and D).
Figure 6.
Inhibition of TNF-α-induced apoptosis by LC3 or Beclin 1 siRNA in primary trophoblasts cultured from human term placentae. A, Activation of caspase 9 by TNF-α was determined in primary trophoblasts by detecting its cleaved forms by Western blotting using antibody against caspase 9 at the indicated time points. B, Activation of caspase 3 (35 kDa proform) into its cleaved forms (17 kDa and 19 kDa) was detected in primary trophoblasts treated with TNF-α for the indicated times by Western blotting. C, Activation of caspase 9 was determined in primary trophoblasts transfected with siRNA for the control, Beclin 1, or LC3 followed by treatment with TNF-α at different time points by Western blotting. D, Activation of caspase 3 was determined in primary trophoblasts transfected with siRNA for the control, Beclin 1, or LC3 followed by treatment with TNF-α at different time points by Western blotting. Right panels demonstrate the amount of cleaved forms of each protein detected by Western blotting after normalization with β-actin. The data are presented as the mean ± SEM from 3 different cell preparations (placentas) for each independent experiment (*P < .05). Representative blots are shown. TNF-α indicates tumor necrosis factor α; SEM, standard error of the mean; siRNA, small interfering RNA.
Discussion
In this study, we observed that treatment with TNF-α increased autophagic and apoptotic activity in JEG-3 cells and primary trophoblasts. An increase in LC3-II was observed earlier than increases in cleaved caspase 9 and caspase 3, suggesting that increased autophagy might have preceded apoptosis in JEG-3 cells. In addition, we observed that inhibition of autophagy by knockdown of LC3 or Beclin 1 attenuated TNF-α-induced apoptosis with decreased expression of tAtg5. Moreover, inhibition of cleavage of Atg5 into tAtg5 by transfection with calpain siRNA resulted in reduction of TNF-α-induced apoptosis. Therefore, we hypothesized that inhibition of autophagy would attenuate TNF-α-induced apoptosis, and the cross talk between autophagy and apoptosis might be regulated by calpain-mediated production of tAtg5. In our experience, in trophoblastic cells, autophagy was induced mainly under apoptotic stimuli (data not shown). With this background, we investigated the interconnections of apoptosis and autophagy under TNF-α stimulation in JEG-3 and primary trophoblasts.
Studies of the relationship between apoptosis and autophagy have shown variable interactions depending on the types of cell lines and the environment of cells.19–23 First, autophagy may be required for apoptosis. In T-lymphoblastic leukemia cell lines, TNF-α-induced apoptosis is completely inhibited by 3-methyladenine, which inhibits the formation of autophagosomes, implying that the early stages of autophagy are required for apoptosis.24 In addition, apoptotic stimuli increase the number of autophagic particles, and treatment with an antiautophagic drug delays apoptosis by inhibiting the release of cytochrome c from mitochondria and preventing caspase activation in isolated sympathetic neurons.25 Second, autophagy and apoptosis may antagonize each other. In contrast with our result, a recent study reported that the expression of LC3-II and cleaved poly(adenosine diphosphate ribose) polymerase was inversely related in cultured trophoblasts.23 Inhibition of caspase 8 leads to autophagic cell death which requires the genes ATG7 and beclin 1.26 Interestingly, cell death by a caspase inhibitor is associated with autophagy-mediated depletion of the reactive oxygen species detoxifying enzyme catalase and is inhibited by depletion of ATG7 and Beclin 1.26,27 Moreover, HeLa cells are sensitized to starvation-induced cell death when the early stages of autophagy are inhibited either genetically or pharmacologically.28 In addition to these interactions, experiments using mouse embryonic fibroblasts from the Bax−/− Bak−/− double knockout revealed that BAX and BAK, which are required for apoptotic cell death, might have roles in regulating autophagy as well as in modulating apoptosis.15 These 2 types of cell death also manifest themselves in a mutually exclusive manner, as inhibition of autophagy may switch the cellular response to death signals from autophagic type II cell death to apoptotic type I cell death.27 Consistent with previous findings,24 the results of the present study suggest that autophagy induced by TNF-α might mediate apoptosis in trophoblastic cells.
The role of tATG5 in cross talk between autophagy and apoptosis has been reported by several studies. In prostate cancer cells, induction of autophagy by adenovirus expressing melanoma differentiation–associated gene 7 leads to calpain-mediated cleavage of Atg5 in a manner that could promote switching to apoptosis.19 Overexpression of Atg5 can induce autophagy and also enhance the susceptibility of tumor cells to activation of the intrinsic cell death pathway.12,13 Moreover, Atg5 cleavage is independent of the cell type and apoptotic stimuli.13 In our study, the induction of tAtg5 by TNF-α was decreased after transfection with LC3 or Beclin 1 siRNA in JEG-3 cells. We also demonstrated that the increased expressions of tAtg5, caspase 9, and caspase 3 by TNF-α were decreased by knockdown of calpain using siRNA, which cleaves Atg5 into tAtg5. We cannot discard the possibility that knockdown of calpain by siRNA could have effects on its substrates that might have functions in autophagy and apoptosis. However, in this study, inhibition of autophagic activity by knockdown of LC3 and Beclin 1 decreased the expression level of tAtg5 and inhibited the intrinsic apoptotic pathway in JEG-3 cells. Moreover, activation of caspase 9 and caspase 3 induced by TNF-α was also attenuated when autophagy was inhibited by transfection with Beclin 1 or LC3 siRNA in both JEG-3 cells and primary trophoblasts. This finding suggests that tAtg5 mediates the cross talk between apoptosis and autophagy in primary trophoblasts under TNF-α stimulation.
In addition to tAtg5, a previous report29 suggested that the balance between apoptosis and autophagy in cells is finely tuned through autophagic degradation of caspase 8 in apoptosis-resistant tumor cells. Caspase can cleave Beclin 1, thereby destroying its proautophagic activity, and the resulting C-terminal fragment of Beclin 1 is able to amplify intrinsic apoptosis.30 In addition, the Bcl-2/Bcl-xL/Beclin 1 complex is a nexus for both apoptosis and autophagy.31 A previous report32 showed that Bcl-2 overexpression blocks Beclin 1-dependent autophagy under nutrient deprivation, but Beclin 1 overexpression does not increase apoptosis and fails to neutralize the antiapoptotic action of Bcl-2, suggesting unidirectional rather than mutual interaction between Bcl-2 and Beclin 1.
In conclusion, our results suggest that autophagy mediates the intrinsic apoptotic pathway in trophoblastic cells. Additional studies of the regulation of autophagy in placentae are needed in order to unveil its role in normal and complicated pregnancies.33
Acknowledgments
The authors are grateful to Dr Chel-Hun Choi for his review of the manuscript and Dr Lindsay Seong for editing the language in the revised manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Research Foundation of Korea (NRF) funded by the Korea government (Ministry of Education, Science and Technology) [2011-0016004] and Samsung Biomedical Research Institute [#SBRI C-A8-212-2] in part.
References
- 1. Abumaree MH, Stone PR, Chamley LW. An in vitro model of human placental trophoblast deportation/shedding. Mol Hum Reprod. 2006;12(11):687–694. [DOI] [PubMed] [Google Scholar]
- 2. Straszewski-Chavez SL, Abrahams VM, Mor G. The role of apoptosis in the regulation of trophoblast survival and differentiation during pregnancy. Endocr Rev. 2005;26(7):877–897. [DOI] [PubMed] [Google Scholar]
- 3. Crocker IP, Cooper S, Ong SC, Baker PN. Differences in apoptotic susceptibility of cytotrophoblasts and syncytiotrophoblasts in normal pregnancy to those complicated with preeclampsia and intrauterine growth restriction. Am J Pathol. 2003;162(2):637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huppertz B, Kadyrov M, Kingdom JC. Apoptosis and its role in the trophoblast. Am J Obstet Gynecol. 2006;195(1):29–39. [DOI] [PubMed] [Google Scholar]
- 5. Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol. 2002;186(1):158–166. [DOI] [PubMed] [Google Scholar]
- 6. Tuuli MG, Longtine MS, Nelson DM. Review: Oxygen and trophoblast biology—a source of controversy. Placenta. 2011;32(suppl 2):S109–S118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choi AM, Ryter SW, Levine B. Autophagy in human health and diasease. N Engl J Med. 2013;368(7):651–662. [DOI] [PubMed] [Google Scholar]
- 8. Liang C. Negative regulation of autophagy. Cell Death Differ. 2010;17(12):1807–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oh SY, Choi SJ, Kim KH, Cho EY, Kim JH, Roh CR. Autophagy-related proteins, LC3 and Beclin-1, in placentas from pregnancies complicated by preeclampsia. Reprod Sci. 2008;15(9):912–920. [DOI] [PubMed] [Google Scholar]
- 10. Signorelli P, Avagliano L, Virgili E, et al. Autophagy in term normal human placentas. Placenta. 2011;32(6):482–485. [DOI] [PubMed] [Google Scholar]
- 11. Lee JE, Oh HA, Song H, et al. Autophagy regulates embryonic survival during delayed implantation. Endocrinology. 2011;152(5):2067–2075. [DOI] [PubMed] [Google Scholar]
- 12. Luo S, Rubinsztein DC. Atg5 and Bcl-2 provide novel insights into the interplay between apoptosis and autophagy. Cell Death Differ. 2007;14(7):1247–1250. [DOI] [PubMed] [Google Scholar]
- 13. Yousefi S, Perozzo R, Schmid I, et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006;8(10):1124–1132. [DOI] [PubMed] [Google Scholar]
- 14. Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23(16):2891–2906. [DOI] [PubMed] [Google Scholar]
- 15. Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8(9):741–752. [DOI] [PubMed] [Google Scholar]
- 16. Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF III. Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118(4):1567–1582. [DOI] [PubMed] [Google Scholar]
- 17. Anteby EY, Johnson RD, Huang X, Nelson DM, Sadovsky Y. Transcriptional regulation of prostaglandin-H synthase-2 gene in human trophoblasts. J Clin Endocrinol Metab. 1997;82(7):2289–2293. [DOI] [PubMed] [Google Scholar]
- 18. Shacka JJ, Klocke BJ, Roth KA. Autophagy, bafilomycin and cell death: the “a-B-cs” of plecomacrolide-induced neuroprotection. Autophagy. 2006;2(3):228–230. [DOI] [PubMed] [Google Scholar]
- 19. Bhutia SK, Dash R, Das SK, et al. Mechanism of autophagy to apoptosis switch triggered in prostate cancer cells by antitumor cytokine melanoma differentiation-associated gene 7/interleukin-24. Cancer Res. 2010;70(9):3667–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lian J, Wu X, He F, et al. A natural BH3 mimetic induces autophagy in apoptosis-resistant prostate cancer via modulating Bcl-2-Beclin1 interaction at endoplasmic reticulum. Cell Death Differ. 2011;18(1):60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsuda H, Ochiai K, Suzuki N, Otsuka K. Butyrate, a bacterial metabolite, induces apoptosis and autophagic cell death in gingival epithelial cells. J Periodontal Res. 2010;45(5):626–634. [DOI] [PubMed] [Google Scholar]
- 22. Voss V, Senft C, Lang V, et al. The pan-Bcl-2 inhibitor (-)-gossypol triggers autophagic cell death in malignant glioma. Mol Cancer Res. 2010;8(7):1002–1016. [DOI] [PubMed] [Google Scholar]
- 23. Chen B, Longtine MS, Nelson DM. Hypoxia induces autophagy in primary human trophoblasts. Endocrinology. 2012;153(10):4946–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jia L, Dourmashkin RR, Allen PD, Gray AB, Newland AC, Kelsey SM. Inhibition of autophagy abrogates tumour necrosis factor alpha induced apoptosis in human T-lymphoblastic leukaemic cells. Br J Haematol. 1997;98(3):673–685. [DOI] [PubMed] [Google Scholar]
- 25. Xue L, Fletcher GC, Tolkovsky AM. Autophagy is activated by apoptotic signalling in sympathetic neurons: an alternative mechanism of death execution. Mol Cell Neurosci. 1999;14(3):180–198. [DOI] [PubMed] [Google Scholar]
- 26. Yu L, Alva A, Su H, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304(5676):1500–1502. [DOI] [PubMed] [Google Scholar]
- 27. Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 2003;63(9):2103–2108. [PubMed] [Google Scholar]
- 28. Boya P, Gonzalez-Polo RA, Casares N, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25(3):1025–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hou W, Han J, Lu C, Goldstein LA, Rabinowich H. Autophagic degradation of active caspase-8: a crosstalk mechanism between autophagy and apoptosis. Autophagy. 2010;6(7):891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Djavaheri-Mergny M, Maiuri MC, Kroemer G. Cross talk between apoptosis and autophagy by caspase-mediated cleavage of Beclin 1. Oncogene. 2010;29(12):1717–1719. [DOI] [PubMed] [Google Scholar]
- 31. Zhou F, Yang Y, Xing D. Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis. FEBS J. 2011;278(3):403–413. [DOI] [PubMed] [Google Scholar]
- 32. Ciechomska IA, Goemans GC, Skepper JN, Tolkovsky AM. Bcl-2 complexed with Beclin-1 maintains full anti-apoptotic function. Oncogene. 2009;28(21):2128–2141. [DOI] [PubMed] [Google Scholar]
- 33. Bildirici I, Longine MS, Chen B, Nelson DM. Survival by self-destruction: a role for autophagy in the placenta? Placenta. 2012;33(8):591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]