Abstract
Recurrent miscarriage (RM) is defined as 3 or more miscarriages before 20 weeks’ pregnancy. In recent years, interest has been focused on chronic endometritis (CE), a subtle inflammation thought to be associated with RM. We aimed to evaluate the relationships between CE and RM. The records of 360 women with unexplained RM were retrospectively analyzed. Data from hysteroscopy, endometrial histology, endometrial culture, and polymerase chain reaction for chlamydia, performed before and after antibiotic treatment for CE, were analyzed. The occurrence of successful pregnancies within 1 year after treatment was also evaluated. Results showed that 208 (57.8%) women with RM showed CE at hysteroscopy; 190 (91.3%), positive at hysteroscopy, were also positive at histology, and 142 (68.3%) had positive cultures. Common bacteria were found in 110 (77.5%) patients. Mycoplasma and Ureaplasma were found in 36 (25.3%) patients and Chlamydia in 18 patients (12.7%). In 102 (71%) women, antibiogram-based antibiotic treatment normalized hysteroscopy, histology, and cultures (group 1); while in 40 (28.2%) patients, CE was still present at hysteroscopy (group 2). In 16 of the 66 patients positive at hysteroscopy, but not at cultures, the hysteroscopy becomes normal (group 3) after a Centers for Disease Control and Prevention-based therapy; while in 50 women, CE was still present (group 4). One year after treatment, group 1 showed a significantly higher number of pregnancies (78.4%) compared to group 2 (17.5%; P < .001) and group 4 (15.3%; P = .005). The CE is frequent in women with RM. Antibiotic treatment seems to be associated with an improved reproductive outcome.
Keywords: chronic endometritis, repeated miscarriage, hysteroscopy, antibiotic treatment, endometrial culture
Introduction
Recurrent miscarriage (RM), defined as 3 or more miscarriages before 20 weeks of pregnancy, affects ∼3% of all couples.1 Genetic abnormalities, antiphospholipid syndrome, endocrine disorders, and uterine abnormalities are detected in ∼50% of these couples. The other 50% are diagnosed as couples with unexplained RM.
Today, the adequacy of the uterine environment is an issue of great interest. Conventional infertility investigations like ultrasounds and hysterosalpingography (HSG) are not able to detect small intrauterine lesions, so at hysteroscopy the prevalence of unsuspected intrauterine abnormalities has been demonstrated to range between 11% and 45%.2–8 In recent years, a growing interest has been focused on chronic endometritis (CE), a subtle and often asymptomatic inflammation of the endometrial lining. Traditionally, histological identification of plasma cells in the endometrial stroma is considered the gold standard for the diagnosis9; however, since inflammatory cells are normally present in the endometrium, even histological examination may miss the presence of CE. We previously demonstrated that fluid hysteroscopy is a reliable technique for diagnosing CE based on demonstrating specific signs such as micro polyps, stromal edema, and focal or diffuse hyperemia.10–13
It is conceivable that CE may hamper endometrial receptivity and may cause infertility, since it has been demonstrated that, in the event of CE, the endometrium is characterized by an abnormal pattern of lymphocyte subsets and, consequently, an aberrant endometrial microenvironment.14,15 Accordingly, CE was detected in patients undergoing hysteroscopy for unexplained infertility,13 and it was identified in 30.3% of the patients with repeated implantation failure at in vitro fertilization (IVF).16 In the Johnston-Mac Ananny study, women diagnosed with CE had lower implantation rates (11.5%) after IVF cycle.16 On the contrary, in a recent article, Kasius and coworkers reported that the clinical implication of CE seems minimal, since it can be rarely diagnosed in a population of asymptomatic infertile patients with a normal transvaginal ultrasound examination (TVS) and that the reproductive outcome after IVF/intracytoplasmic sperm injection cycles was not negatively affected by CE.17 All the above-mentioned data in the literature suggest that the role of CE in infertility and, more in general, in reproduction is controversial. Considering the difficulty in diagnosing CE and the need for expertise in the hysteroscopy and histological field, in this study we analyzed the prevalence of CE at hysteroscopy, histology, and endometrial cultures, in a large population of women with unexplained RM, in order to evaluate the role of CE in infertile women complaining of RM and the effects of the antibiotic treatment based on antibiogram results. Moreover, we compared the time to first successful pregnancy and live birth in women in whom CE was successfully treated with those women in whom treatment was not effective in restoring normal endometrium.
Materials and Methods
We retrospectively analyzed the records of 360 women (age 30.5 ± 4.9 years, mean ± standard deviation) referred in the period January 2008 to January 2011 for unexplained RM.
This retrospective study was approved by our local ethical committee. All women referred to our department gave their informed consent to use, anonymously, their data for research purposes. Inclusion criteria were age < 40 years and 3 or more miscarriages before 20 weeks’ pregnancy. Exclusion criteria were as follows: family history of RM, uterine abnormalities, hormonal or metabolic disorders, severe male factor infertility, previous surgery for myoma and/or endometriosis, clinical or ultrasound diagnosis of endometriosis, known clinical autoimmune disease, antiphospholipid syndrome, thrombophilic condition requiring anticoagulant therapy, and unwilling to give informed consent. Women underwent diagnostic mini-hysteroscopy in the follicular phase of menstrual cycle. Mini-hysteroscopy was performed using a lens-based 3-mm optical density (OD) mini telescope, 105° angle of visual field equipped with a 3.5-mm OD single-flow diagnostic sheath (Karl Storz, Tuttlingen, Germany) as described previously.10–11 Saline was used to distend the uterine cavity. A 300-W light source with a xenon bulb, a digital camera (Karl Storz, Tuttlingen, Germany), and a 21-in color screen video were used. The exploration of the uterine cavity consisted of a panoramic view of the cavity followed by a thorough evaluation of the endometrial mucosa as described previously. All hysteroscopies were performed by 2 of the authors (E.C. and M.M.).
Diagnosis of CE was based on the demonstration of micropolyps that fluctuate in the cavity, stromal edema, and focal or diffuse hyperhemia, as previously published.11–13
All women without evidence of CE at hysteroscopy underwent endometrial biopsy for histological examination. In women diagnosed with CE at hysteroscopy in the follicular phase of the subsequent cycle, an endometrial biopsy was performed using a 3-mm Novak curette connected to a 20-mL syringe for cultural and histological purposes. As regards infectious agents, common bacteria, Neisseria gonorrhea, Chlamydia trachomatis, Mycoplasma species, Ureaplasma urealyticum, and yeast were looked for at endometrial levels. In order to minimize the risk of endometrial cultures being contaminated in the vagina, after placing a vaginal speculum and cleaning external uterine ostium using a gauze soaked in iodine solution, the Novak’s cannula was inserted under visual control into the uterine cavity, taking care to avoid any contact with vaginal walls. Endometrial samples were diluted in 2 mL of saline and divided into 2 aliquots, one for cultures and the other placed in formalin for histological examination. Based on the infectious agent detected and on the antibiogram result, an appropriate antibiotic treatment was prescribed. In the follicular phase of the cycle, following the therapy, all women were asked to refer again to reevaluate uterine cavity at hysteroscopy as regards the presence of signs of CE and to collect endometrial samples for histology and culture. Treatment was repeated until the negativity of the cultures and normalization of both hysteroscopic and histological findings were obtained. At the end of treatment, women were invited to try to conceive.
Microbiological Analysis
Specimens for N gonorrhea were immediately placed in Stuart transport medium and transported to the laboratory. In the laboratory, endometrial specimens were Gram stained; then the endometrial specimens were plated into appropriate agar medium, 5% sheep blood Columbia Agar Base, Chocolate Agar, Mannitol Salt Agar and Mac Conkey Agar (Bio Merieux, Rome, Italy), and the presence of microorganisms was evaluated. The plates were incubated for 48 hours in air or 5% CO2. The identification of bacteria was made using the published criteria (Dade International Inc, Milan, Italy). Genital Mycoplasmas were quantitatively detected by immunoassay (Mycoplasma-IST, Biomerieux, Rome, Italy). Chlamydia was tested by reverse transcriptase–polymerase chain reaction (RT-PCR).
For yeast isolation, specimens were plated into Saboraud Chloramphenicol Agar and identification was performed using commercial kits (API-C System, Biomerieux, Rome, Italy).
Histological Analysis
Endometrial samples were fixed in neutral formalin and later embedded in paraffin for histological analysis. Five microsections were stained with hematoxyline and eosin. The histological examinations were performed by a single operator (L.R.) who was unaware of the hysteroscopic findings. Histological diagnosis of CE was based on the criteria previously described.10–13 Attention was paid to the following features: superficial stromal edema, increased stromal density, pleo-morphic stromal inflammatory infiltrate dominated by lymphocytes, and plasma cells.
Reproductive Parameter Calculation
In August 2012, all women positive at hysteroscopy, histology, and cultures were contacted by telephone and asked the reproductive outcome regarding a period no longer than 1 year after therapy. The questions were about the time of the first successful pregnancy and live birth in the first year after therapy. All women confirmed their consent to use their data, anonymously, for research purposes.
Data Analysis
Clinical features were compared using Dunnett test and chi-square test. Chi-square test and Fisher exact test, where appropriate, were used to compare number of pregnancies and miscarriages. Slopes of regression lines calculated on cumulative frequencies of pregnancy for each trimester were employed to compare the reproductive outcomes in different groups. Statistical analysis was performed using Epi Info 6.04 (Centers for Disease Control and Prevention, Atlanta, Georgia); a P < .05 was considered as limit for significance.
Results
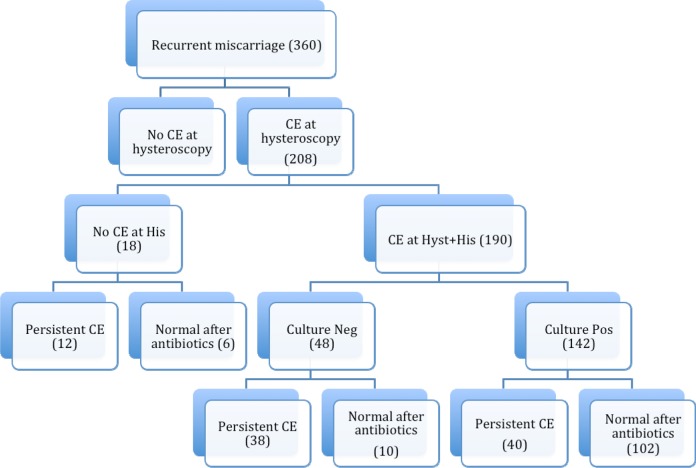
In 152 women, no evidence of CE was found at hysteroscopy; accordingly, histology was also negative for CE. Notably, 208 (57.8%) of the 360 women with RM were diagnosed with CE at hysteroscopy; 190 (91.3%)of the 208 were also positive at histology, while 18 (8.6%) of the 208 were negative at histology (Figure 1); 142 (68.3%) of the 208 patients with signs of CE both at hysteroscopy and at histology were also positive at the cultures, and 22 patients were with multiple positivity at first evaluation (Figure 1). In 110 (77.5%) of the 142 patients common bacteria (Escherichia coli, Enterococcus faecalis, Streptococcus agalactiae, etc) were found. Mycoplasma and U urealyticum were found in 36 (25.3%) patients and Chlamydia in 18 (12.7%) patients (Table 1). Mycoplasmas in 20 patients and Chlamydia in 2 patients coexisted with common bacteria. In 102 (71.8%) women, the control hysteroscopy as well as histology and cultures were normal (group 1) after antibiogram-guided antibiotic treatment, while in the other 40 (28.2%) patients, signs of CE were still present at last hysteroscopy (group 2). In 66 patients that, at first evaluation, were positive at hysteroscopy but not at cultures, a broad-spectrum antibiotic therapy based on the CDC guidelines was proposed.18 After therapy, hysteroscopy becomes normal (group 3) in 16 (24.2%) of the 66 women, while in the remaining 50 (75.7%) women signs of CE were still present (group 4; Figure 1). The clinical data of women in the 4 groups are shown in Table 2. The groups were homogeneous regarding age, partner’s age, body mass index, and number of miscarriages. Based on antibiogram results, in most of cases of gram-negative bacteria, ciprofloxacin 500 mg twice a day for 10 days was used as first-line therapy. In the event of gram-positive bacteria, amoxicillin + clavulanate 1 g twice a day for 8 days was prescribed. Mycoplasma and U urealyticum were treated with josamicine 1 g twice a day for 12 days, while minocycline 100 mg twice a day for 12 days was used in cases of persistence.
Figure 1.
Illustration of the study and distribution of the population investigated. CE indicates chronic endometritis.
Table 1.
Infectious Agents Found at 164 Endometrial Cultures (142 Patients Positive at a Single Agent and 22 Patients With Multiple Positivity at First Evaluation) in Women With Recurrent Miscarriage and the Relative Negativization Rate.
| No. | % | Negativization Rate, % | P | |
|---|---|---|---|---|
| Staphylococcus hemolyticum | 4 | 2.8 | 100 | NS |
| Enterococcus faecalis | 32 | 22.9 | 56.2 | .009 |
| Staphylococcus epidermidis | 1 | 0.7 | 100 | NS |
| Staphylococcus aureus | 3 | 2.1 | 100 | NS |
| Streptococcus bovis | 7 | 4.9 | 100 | NS |
| Streptococcus viridans | 4 | 2.8 | 10 | NS |
| Streptococcus agalactiae | 17 | 11.9 | 64.7 | NS |
| Streptococcus mitis | 7 | 4.9 | 100 | NS |
| Streptococcus milleri | 6 | 4.2 | 100 | NS |
| Escherichia coli | 24 | 16.9 | 54.2 | .017 |
| Candida | 1 | 0.7 | 100 | NS |
| Klebsiella pneumoniae | 4 | 2.8 | 75 | NS |
| Mycoplasmas/Ureaplasma | 36 | 25.3 | 83.3 | NS |
| Chlamydia | 18 | 12.7 | 88.9 | NS |
Abbreviation: NS, not significant.
Table 2.
Clinical Characteristics of Women in Groups 1, 2, 3, and 4.
| Group 1 (n = 102) | Group 2 (n = 40) | Group 3 (n = 16) | Group 4 (n = 50) | ||
|---|---|---|---|---|---|
| Age, years | 32.1 ± 4.0 | 31.3 ± 4.2 | 32.7 ± 3.9 | 31.3 ± 4.1 | NS |
| Partner’s age, years | 33.5 ± 3.6 | 34.1 ± 3.3 | 34.3 ± 4.5 | 34.3 ± 3.3 | NS |
| BMI, kg/m2 | 25.1 ± 2.5 | 24.1 ± 3.2 | 24.9 ± 2.3 | 24.8 ± 3.0 | NS |
| Miscarriages, n | 2.4 ± 0.6 | 2.3 ± 0.5 | 2.2 ± 0.4 | 2.4 ± 0.6 | NS |
Abbreviations: CE, chronic endometritis; NS, not significant.
In women with negative cultures, a treatment based on ceftriaxone 250 mg intramuscularly in a single dose plus doxycycline 100 mg orally twice a day for 14 days with metronidazole 500 mg orally twice a day for 14 days was administered according to the CDC guidelines.18
In the event of the persistence of signs of CE at control hysteroscopy, the protocol was repeated up to 3 times. The persistence rate after the first cycle of treatment was different depending on the infectious agent detected, as shown in Table 3. In particular, in the event of positivity for E faecalis, E coli, and S agalactiae, a repeated treatment was needed in 43.7%, 45.8%, and 35.3% of the cases, respectively.
Table 3.
Persistence of Infectious Agent Positivity at Endometrial Cultures After Antibiogram-Guided Antibiotic Therapy in 142 Women (22 Women With Multiple Positivity at First Evaluation) With RM Affected by Chronic Endometritis.
| No. | % | |
|---|---|---|
| Staphylococcus hemolyticum | 0/4 | 0 |
| Enterococcus faecalis | 14/32 | 43.7 |
| Staphylococcus epidermidis | 0/1 | 0 |
| Staphylococcus aureus | 0/3 | 0 |
| Streptococcus bovis | 0/7 | 0 |
| Streptococcus viridans | 0/4 | 0 |
| Streptococcus agalactiae | 6/17 | 35.3 |
| Streptococcus mitis | 0/7 | 0 |
| Streptococcus milleri | 0/6 | 0 |
| Escherichia coli | 11/24 | 45.8 |
| Candida | 0/1 | 0 |
| Klebsiella pneumoniae | 1/4 | 25 |
| Mycoplasmas/Ureaplasma | 6/36 | 16.7 |
| Chlamydia | 2/18 | 11.1 |
Abbreviation: RM, recurrent miscarriage.
The 208 women who were positive for CE at baseline at hysteroscopy were contacted by telephone and asked about reproductive outcome. After treatment, a higher number of successful pregnancies were reported in groups 1 and 3 compared to groups 2 and 4 (n = 80 [78.4%] and 8 [50%] vs n = 7 [17.5%] and 15 [30%], respectively). More specifically, the number of successful pregnancies in group 1 women was significantly higher than in group 2 since the second trimester (6th month P < .003; Table 4). When compared to group 4, group 1 showed a higher, but not significant, number of pregnancies since the second trimester, reaching statistical significance only at 9th month (9th month P < .001; Table 4). As regards group 3, the difference among the trend of the pregnancy rates over the months was significantly different when compared with the trend of groups 2 and 4 (P < .001), although the number of pregnancies did not differ significantly (Table 4). The percentage distribution of time to start the first successful pregnancy in the 4 groups is shown in Figure 2.
Table 4.
Multiple Comparisons of Cumulated Frequencies of Pregnancy Per Trimester of Treatment in the 4 Groups.
| Group 1 vs Group 2 | Group 1 vs Group 3 | Group 1 vs Group 4 | Group 2 vs Group 3 | Group 2 vs Group 4 | Group 3 vs Group 4 | |
|---|---|---|---|---|---|---|
| 3rd Month | 0.669 | 0.933 | 0.835 | 0.656 | 0.903 | 1.000 |
| 6th Month | 0.003a | 0.520 | 0.064 | 0.170 | 0.167 | 1.000 |
| 9th Month | <0.001a | 0.326 | 0.007a | 0.271 | 0.438 | 0.729 |
| 12th Month | <0.001a | 0.442 | 0.005a | 0.135 | 0.404 | 0.479 |
| Trends | <0.001a | <0.001a | <0.001a | <0.001a | <0.001a | <0.001a |
aIndicates significant values.
Figure 2.
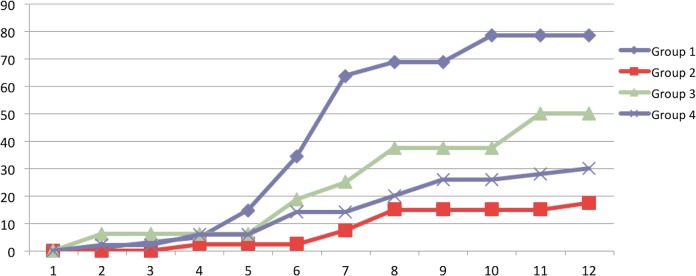
The percentage distribution of time, in months, in the beginning of the first successful pregnancy with live birth in the 4 groups of women with recurrent miscarriage and diagnosed with CE. CE indicates chronic endometritis.
Comparison of regression lines slope calculated on cumulative frequencies showed that the pregnancy rate seems to increase in a similar way in groups 1 and 3.
Groups 2 and 4 showed the worst trend compared to the other groups (Figure 2).
Discussion
The results of this retrospective pilot study indicate a relationship between RM and chronic endometrial inflammation and could have several clinical implications. First, they demonstrate that CE is a common finding in women complaining of RM. Second, this study indicates that, at least in our population, common bacteria and Mycoplasmas are the most frequently involved infectious agents in CE. Third, this study confirms that hysteroscopy is a reliable diagnostic technique for CE and that the previously described endometrial lesions10–11 such as micropolyps, stromal edema, and focal or diffuse hyperemia are actually related to the inflammatory state of the endometrium. Moreover, results showed that a normal hysteroscopic pattern after an appropriate antibiotic treatment seems to be associated with an improved reproductive outcome. Fourth, the results demonstrated that in women with CE fertility was restored after appropriate antibiotic treatment, suggesting the suitability of performing a hysteroscopy in women with RM. Fifth, the persistence of hysteroscopic signs of CE after treatment, even in case of negativity of endometrial cultures, is related to a worse reproductive outcome.
As regards the first point, we detected signs of CE in about 60% of the women with unexplained RM. The finding that CE may have a role in RM etiology agrees with many experimental and clinical articles.14–16,19 We found that common bacteria and Mycoplasma are the most frequent etiological agents for CE. Chlamydia was demonstrated in only about 13% of the positive endometrial cultures. No single case of N gonorrhea was found, and this was probably due to the specific characteristics of the population studied. Our results are in accordance with those of the PID Evaluation and Clinical Health (PEACH) study, which showed that approximately 60% of women with pelvic inflammatory disease have nongonococcal non-Chlamydia infection.20 These data are not surprising considering the high prevalence of bacterial vaginosis and the knowledge that ascending bacteria can colonize the uterine cavity.21–24 Indeed bacterial vaginosis have been associated with pelvic inflammatory disease25 and, in IVF, may decrease conception rates, increase early pregnancy losses, and may also increase the risk of preterm birth.26–29
The results of this study suggest that in approximately 24% of the cases, Mycoplasma and U urealyticum may be responsible for CE. The pathogenic role of Mycoplasma agrees with an article by Haggerty and coworkers who found Mycoplasma genitalium in 12% of the cervical specimens and in 8% of endometrial specimens from women affected by nongonococcal, non-Chlamydia endometritis.30 At the endometrial level, Chlamydia was found in only 2.7% of the cases, with a prevalence lower than that, about 14%, reported in the PEACH study20; only in 1 case Chlamydia was detected in the vagina. Ethnic differences and characteristic of the population enrolled in the study may account for this discrepancy. On the other hand, our data are in full accordance with Stern and coworkers who detected the presence of Chlamydia by PCR in only 1 of the 43 specimens of histologically diagnosed CE, concluding for a limited, if any, role of Chlamydia in the origin of CE.31 Our study has the intrinsic limitations of all studies that rely on bacteriological cultures of the endometrial cavity, using traditional culture techniques and trans-cervical sampling. Only microorganisms able to grow under conventional microbiology laboratory conditions can be recovered and may, therefore, yield biased microbial findings. It, however, does not exclude that other microorganisms (anaerobic bacteria, viruses, etc) might also coexist and play a role. Notably, in our series of women with RM and diagnosed with CE at hysteroscopy, in about 31.7% of the cases, the endometrial cultures were negative, probably due to the presence of noncultivable bacteria. Accordingly, even women with negative cultures showed a significant improvement in the reproductive outcomes after a broad spectrum of antibiotic treatment used according to the CCD guidelines, so that about 50% of these women had a live birth at 1 year. In our previous article, we observed a significant discordance between the infectious agents found at vaginal and endometrial levels, concluding that the possibility that endometrial results could be biased by contamination was low.32 Moreover, extreme care was taken during endometrial sampling in order to avoid any contact between the curette and the vaginal walls. Finally, the fact the antibiotic treatment against infectious agents detected at endometrial culture resulted in a normalization of hysteroscopic findings and in a significant improvement in pregnancy rate speaks in favor of the actual endometrial origin of infectious agents found in endometrial specimens. The data in this article are not in disagreement with the recent view that the uterine cavity is normally not sterile and that presence of microorganisms does not mean inflammation.23 Hence, it is not just the presence of infectious agents within the internal genital tract but rather the interactions between the infectious agents and the endometrial environment that is seen today as the most critical issue that determines the presence of pathology.26
Third, this study strongly confirms that hysteroscopy is a reliable diagnostic technique for CE and that the previously described lesions are actually related to the inflammatory state of the endometrium; indeed, in most positive cases signs of CE disappeared after antibiogram-based antibiotic treatment.
The results suggest the advantage of performing a hysteroscopy in women with RM, in accordance with the previous articles.33,34 Fluid mini-hysteroscopy is a minimally invasive technique that can be performed in an office setting without anesthesia,35 so the advantages in terms of diagnosis and treatment amply overcome the costs of hysteroscopy.
The importance of the hysteroscopic evaluation in women with RM is supported by the improved reproductive outcome that we found when treatment was able to normalize the hysteroscopic endometrial pattern, both after the antibiogram-guided antibiotic treatment and after treatment based on the CDC guidelines. Significantly better results were obtained with the approach based on antibiogram results. Indeed, our data showed that at 1 year, the percentage of live births in group 1 was higher compared to the group treated with standard CDC (78.4% vs 50%). Anyway a comparative trial is required to confirm the superiority of the antibiogram-oriented antibiotic treatment versus the standard CDC treatment in women with CE. In the event of persistent CE, no more than 3 cycles of treatment were performed, so although we can’t exclude the possibility to generate antibiotic resistance, the potential benefit for patients outweighs the risk.
Another important issue emerging from our data is that in the presence of a negative endometrial culture, patients with persistence of CE at hysteroscopy (group 4) showed a poorer reproductive outcome compared to patients without hysteroscopic signs of CE after treatment (group 3). This allows speculation that the hysteroscopic evaluation of the endometrial inflammatory disease could have a higher sensitivity than the endometrial cultures and that a normal endometrial pattern at hysteroscopy could be more accurate in predicting the possibility of a successful pregnancy after therapy.
In conclusion, even with potential limitations related to retrospective studies, the results of this study, based on analyzing a large number of patients, demonstrate that CE is a condition frequently associated with RM. In our population, the most prevalent infectious agents are common bacteria and Mycoplasma. In women with RM, hysteroscopy reliably detects the existence of CE. The normalization of the hysteroscopic endometrial pattern seems to be associated with a significant improvement in the reproductive outcome.
Although further prospective clinical randomized trials, in progress, are needed to confirm our findings, we can conclude that hysteroscopy should be a part of the diagnostic workup of infertile women complaining of unexplained RM. Moreover, we can speculate that an appropriate antibiotic treatment, based on the antibiogram results, may improve the reproductive outcome in these patients.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the University personal research grants of Ettore Cicinelli .
References
- 1. Rai R, Regan L. Recurrent miscarriage. Lancet. 2006;368(9535):601–611. [DOI] [PubMed] [Google Scholar]
- 2. La Sala GB, Montanari R, Dessanti L, Cigarini C, Sartori F. The role of diagnostic hysteroscopy and endometrial biopsy in assisted reproductive technologies. Fertil Steril. 1998;70(2):378–380. [DOI] [PubMed] [Google Scholar]
- 3. Oliveira FG, Abdelmassih VG, Diamond MP, Dozortsev D, Nagy ZP, Abdelmassih R. Uterine cavity findings and hysteroscopic interventions in patients undergoing in vitro fertilization-embryo transfer who repeatedly cannot conceive. Fertil Steril. 2003;80(6):1371–1375. [DOI] [PubMed] [Google Scholar]
- 4. Demirol A, Gurgan T. Effect of treatment of intrauterine pathologies with office hysteroscopy in patients with recurrent IVF failure. Reprod Biomed Online. 2004;8(5):590–594. [DOI] [PubMed] [Google Scholar]
- 5. Rama Raju GA, Shashi Kumari G, Krishna KM, Prakash GJ, Madan K. Assessment of uterine cavity by hysteroscopy in assisted reproduction programme and its influence on pregnancy outcome. Arch Gynecol Obstet. 2006;274(3):160–164. [DOI] [PubMed] [Google Scholar]
- 6. Bosteels J, Weyers S, Puttemans P, et al. The effectiveness of hysteroscopy in improving pregnancy rates in subfertile women without other gynaecological symptoms: a systematic review. Hum Reprod Update. 2010;16(1):1–11. [DOI] [PubMed] [Google Scholar]
- 7. Fatemi HM, Kasius JC, Timmermans A, et al. Prevalence of unsuspected uterine cavity abnormalities diagnosed by office hysteroscopy prior to in vitro fertilization. Hum Reprod. 2010;25(8):1959–1965. [DOI] [PubMed] [Google Scholar]
- 8. Makrakis E, Pantos K. The outcomes of hysteroscopy in women with implantation failures after in-vitro fertilization: findings and effect on subsequent pregnancy rates. Curr Opin Obstet Gynecol. 2010;22(4):339–343. [DOI] [PubMed] [Google Scholar]
- 9. Greenwood SM, Moran JJ. Chronic endometritis: morphologic and clinical observations. Obstet Gynecol. 1981;58(2):176–184. [PubMed] [Google Scholar]
- 10. Resta L, Palumbo M, Rossi R, Piscitelli D, Grazia Fiore M, Cicinelli E. Histology of micro polyps in chronic endometritis. Histopathology. 2012;60(4):670–674. [DOI] [PubMed] [Google Scholar]
- 11. Cicinelli E, Resta L, Nicoletti R, Zappimbulso V, Tartagni M, Saliani N. Endometrial micropolyps at fluid hysteroscopy suggest the existence of chronic endometritis. Hum Reprod. 2005;20(5):1386–1389. [DOI] [PubMed] [Google Scholar]
- 12. Cicinelli E, Resta L, Nicoletti R, et al. Detection of chronic endometritis at fluid hysteroscopy. J Minim Invasive Gynecol. 2005;12(6):514–518. [DOI] [PubMed] [Google Scholar]
- 13. Cicinelli E, De Ziegler D, Nicoletti R, et al. Chronic endometritis: correlation among hysteroscopic, histologic, and bacteriologic findings in a prospective trial with 2190 consecutive office hysteroscopies. Fertil Steril. 2008;89(3):677–684. [DOI] [PubMed] [Google Scholar]
- 14. Matteo M, Cicinelli E, Greco P, et al. Abnormal pattern of lymphocyte subpopulations in the endometrium of infertile women with chronic endometritis. Am J Reprod Immunol. 2009;61(5):322–329. [DOI] [PubMed] [Google Scholar]
- 15. Kitaya K, Yasuo T. Aberrant expression of selectin E, CXCL1, and CXCL13 in chronic endometritis. Mod Pathol. 2010;23(8):1136–1146. [DOI] [PubMed] [Google Scholar]
- 16. Johnston-MacAnanny EB, Hartnett J, Engmann LL, Nulsen JC, Sanders MM, Benadiva CA. Chronic endometritis is a frequent finding in women with recurrent implantation failure after in vitro fertilization. Fertil Steril. 2010;93(2):437–441. [DOI] [PubMed] [Google Scholar]
- 17. Kasius JC, Fatemi HM, Bourgain C, et al. The impact of chronic endometritis on reproductive outcome. Fertil Steril. 2011;96(6):1451–1456. [DOI] [PubMed] [Google Scholar]
- 18. Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1–110. [PubMed] [Google Scholar]
- 19. Nigro G, Mazzocco M, Mattia E, Di Renzo GC, Carta G, Anceschi MM. Role of the infections in recurrent spontaneous abortion. J Matern Fetal Neonatal Med. 2011;24(8):983–989. [DOI] [PubMed] [Google Scholar]
- 20. Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) randomized trial. Am J Obstet Gynecol. 2002;186(5):929–937. [DOI] [PubMed] [Google Scholar]
- 21. Espinoza J, Erez O, Romero R. Preconceptional antibiotic treatment to prevent preterm birth in women with a previous preterm delivery. Am J Obstet Gynecol. 2006;194(3):630–637. [DOI] [PubMed] [Google Scholar]
- 22. Kamiyama S, Teruya Y, Nohara M, Kanazawa K. Impact of detection of bacterial endotoxin in menstrual effluent on the pregnancy rate in in vitro fertilization and embryo transfer. Fertil Steril. 2004;82(4):788–792. [DOI] [PubMed] [Google Scholar]
- 23. Cowling P, McCoy DR, Marshall RJ, Padfield CJ, Reeves DS. Bacterial colonization of the non-pregnant uterus: a study of pre-menopausal abdominal hysterectomy specimens. Eur J Clin Microbiol Infect Dis. 1992;11(2):204–205. [DOI] [PubMed] [Google Scholar]
- 24. Cicinelli E, Ballini A, Marinaccio M, et al. Microbiological findings in endometrial specimen: our experience. Arch Gynecol Obstet. 2012;285(5):1325–1329. [DOI] [PubMed] [Google Scholar]
- 25. Korn AP, Bolan G, Padian N, Ohm-Smith M, Schachter J, Landers DV. Plasma cell endometritis in women with symptomatic bacterial vaginosis. Obstet Gynecol. 1995;85(3):387–390. [DOI] [PubMed] [Google Scholar]
- 26. Eckert LO, Moore DE, Patton DL, Agnew KJ, Eschenbach DA. Relationship of vaginal bacteria and inflammation with conception and early pregnancy loss following in-vitro fertilization. Infect Dis Obstet Gynecol. 2003;11(1):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Romero R, Espinoza J, Mazor M. Can endometrial infection/inflammation explain implantation failure, spontaneous abortion, and preterm birth after in vitro fertilization? Fertil Steril. 2004;82(4):799–804. [DOI] [PubMed] [Google Scholar]
- 28. Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31(3):553–584. [DOI] [PubMed] [Google Scholar]
- 29. Salim R, Ben-Shlomo I, Colodner R, Keness Y, Shalev E. Bacterial colonization of the uterine cervix and success rate in assisted reproduction: results of a prospective survey. Hum Reprod. 2002;17(2):337–340. [DOI] [PubMed] [Google Scholar]
- 30. Haggerty CL, Totten PA, Astete SG, Ness RB. Mycoplasma genitalium among women with nongonococcal, nonchlamydial pelvic inflammatory disease. Infect Dis Obstet Gynecol. 2006;2006:30184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stern RA, Svoboda-Newman SM, Frank TS. Analysis of chronic endometritis for Chlamydia trachomatis by polymerase chain reaction. Hum Pathol. 1996;27(10):1085–1088. [DOI] [PubMed] [Google Scholar]
- 32. Cicinelli E, De Ziegler D, Nicoletti R, et al. Poor reliability of vaginal and endocervical cultures for evaluating microbiology of endometrial cavity in women with chronic endometritis. Gynecol Obstet Invest. 2009;68(2):108–115. [DOI] [PubMed] [Google Scholar]
- 33. Valli E, Zupi E, Marconi D, et al. Hysteroscopic findings in 344 women with recurrent spontaneous abortion. J Am Assoc Gynecol Laparosc. 2001;8(3):398–401. [DOI] [PubMed] [Google Scholar]
- 34. Bohlmann MK, von Wolff M, Luedders DW, et al. Hysteroscopic findings in women with two and with more than two first-trimester miscarriages are not significantly different. Reprod Biomed Online. 2010;21(2):230–236. [DOI] [PubMed] [Google Scholar]
- 35. Cicinelli E, Parisi C, Galantino P, Pinto V, Barba B, Schonauer S. Reliability, feasibility, and safety of minihysteroscopy with a vaginoscopic approach: experience with 6,000 cases. Fertil Steril. 2003;80(1):199–202. [DOI] [PubMed] [Google Scholar]