Abstract
Reproductive surgeries leave women more susceptible to postoperative hypervolemic hyponatremia because during this period women can retain water at an accelerated pace and much faster than they do sodium. This review proposes that estrogen and progestogen exposure play an important role in the increased risk of hyponatremia in menopausal women. Estrogen and progesterone exposure have important effects on both body fluid regulation and cardiovascular function and both of these reproductive hormones impact blood pressure responses to sodium loads. This article provides information on the effects of female reproductive hormones and hormone therapy (HT) on fluid regulation and cardiovascular function during menopause. Thirst- and fluid-regulating hormones respond to both osmotic and volume stimuli. Aging women maintain thirst sensitivity to osmotic stimuli but lose some thirst sensitivity to changes in central body fluid volume. Thus, older adults are more at risk of dehydration because they may replenish fluids at a slower rate. Estrogen therapy increases osmotic sensitivity for mechanisms to retain body water so may help menopausal women control body fluids and avoid dehydration. Some progestogens can mitigate estradiol effects on water and sodium retention through competition with aldosterone for the mineralocorticoid receptor and attenuating aldosterone-mediated sodium retention in the distal tubule. However, some progestogens can increase cardiovascular risks. Appropriate balance of these hormones within HT is important to avoid the negative consequences of body fluid and sodium retention, including edema and hypertension.
Keywords: hormone therapy, estrogen, progesterone, body fluid regulation, arginine vasopressin
Reproductive surgeries leave women more susceptible to postoperative hypervolemic hyponatremia because women retain water at a faster rate than sodium during this period,1–5 especially after reproductive surgeries.4,7 Indeed, women are at high risk of postoperative hyponatremia even after relatively low-risk surgeries such as operative hysteroscopy. In such surgeries, glycine solution (lacking electrolytes) is used as a distension medium and can cause intravascular volume retention, increasing the risk of hyponatremia, cerebral and/or pulmonary edema, psychological disturbances, and even death.4,8–9 A combination of anesthesia, nausea, and the postsurgical experience tends to increase arginine vasopressin (AVP). This hormone, the primary hormone involved in free water retention, is also associated with altered astroglia volume regulation in both pre- and postmenopausal (PM) women.3,5,10,11 Studies in animals support an important role for estradiol in increasing astroglia volume in women with hyponatremia, suggesting a role for female reproductive hormones in the dire consequences that can be associated with postoperative hyponatremia in both young and PM women.6,12
Hormones During Menopause
The stages of the menopause transition are classified by changes in menstrual bleeding, concomitant with changes in the pituitary gonadotropin follicle-stimulating hormone (FSH). The menopause transition is characterized by wide variability in both FSH and estradiol in the blood, so changes in the circulating levels of these hormones are not consistent indicators of menopausal status during perimenopause. As women advance through menopause, the ovaries produce less estrogen, and these changes in estrogen exposure have important effects on most tissues in the body. Although PM women have depleted their supply of follicles capable of developing into mature ovum, the pituitary gland continues to produce FSH, and this production continues throughout life. Interestingly, both estrogen exposure and gonadotropin levels in menopause can be elevated by conditions such as obesity and insulin resistance, which are both related to inactivity. This review will focus on the effects of lower estrogen and progesterone exposure on fluid regulation.
Synthetic versus naturally produced estrogens and progestogens
Hormone therapy (HT) is a primary treatment for vasomotor symptoms and other symptoms related to menopause. A progestogen is given to women with an intact uterus to avoid endometrial hyperplasia that can progress to endometrial carcinoma. It is important to distinguish between the physiological effects of synthetic estrogens and progestogens compared to endogenously produced estradiol and progesterone. Within the context of water regulation, for example, progesterone has a high affinity for the mineralocorticoid receptor and competes with aldosterone for this receptor. It is primarily through this mechanism that progesterone increases sodium excretion in men13–17 and can prevent water retention associated with estrogens.14 Most progestins do not possess this antimineralocorticoid effect so have little impact on the water and sodium retention properties of estrogens. Our studies in young women have demonstrated this contrast comparing osmotic regulation of AVP as well as sodium and water regulation during combined oral contraceptives containing norethindrone versus the mid-luteal phase of the menstrual cycle.15 Thus, it has been suggested that this property of progestins may contribute to the weight gain associated with HT. Newer synthetic progestogens, such as drospirenone (combined with ethinylestradiol for HT), have similar pharmacodynamics to progesterone and may be effective aldosterone antagonists.14 The route of administration of these hormones may also impact physiological responses. Estrogens are administered orally, as a subcutaneous implant, a vaginal gel, or a transdermal patch. The impact of the different delivery routes has important physiological and metabolic and cardiovascular effects in PM women,16 although the effects of the different modes of administration on water regulation have not been studied. For an excellent review on the different modes of delivery and impact on these physiological systems, see Khalil.16
Overview of Water Regulation
Water is the largest component of the human body representing 60% to 70% of a healthy, young individual’s body weight. In a 60-kg healthy PM woman, in whom lean mass is reduced, approximately 33 to 34 L of body is composed of water, or about 55%. Of the body’s water, approximately 65% is contained in the cells (intracellular water), and approximately 5% of the remaining extracellular water is in the bloodstream (blood or plasma volume). It is this small percentage of water (from 3.0 to 4.0 liters) that is used by the body’s fluid regulatory and cardiovascular systems to control fluid intake and output, thirst, and blood pressure.
Integrated neural and hormonal systems have evolved to control thirst and sodium appetite in order to ensure the precise regulation of volume and osmolality of body fluids. These systems are sensitive to stimuli arising from deficits in the intracellular and extracellular fluid compartments or to changes in plasma osmolality (or “tonicity” of the blood because plasma osmolality is primarily a function of plasma sodium concentration in humans). Arginine vasopressin, the primary hormone controlling renal free water clearance, is synthesized in the cell bodies of the paraventricular nuclei (PVN) and supraoptic nuclei (SON) located in the anterior hypothalamus. Axons from these nuclei project into the posterior pituitary where AVP is stored and released in response to stimulation of central osmoreceptors. Osmotic stimuli are sensed by Osmo-Na+ receptor neurons located in the PVN and SON. This system is described in detail in Figure 1. Thirst and AVP are sensitive to increases in POsm as slight as 5 mOsmol (2%-3%); following this threshold for osmotic AVP and thirst stimulation, these variables increase linearly with POsm.19,21 Early studies using arterial injections of hypertonic saline directly to rat brains were the first to demonstrate the existence of these so-called “osmoreceptors”22 and that these receptors possessed a direct control over AVP22; studies that followed indicated the anterior/preoptic hypothalamus was central to the regulation of thirst and drinking (see Figure 1). In addition to osmoreceptors, nerves responsible for detecting changes in plasma or blood volume, called volume–pressure receptors, exist in the superior and inferior vena cava and the atria of the heart. These baroreceptors detect stretch that occurs during changes in central blood volume, which are initiated with intravascular fluid volume decrements of approximately 10% or greater. Thus, an acute change in central volume or plasma osmolality can initiate a global response including thirst sensation, sodium appetite, sympathetic nervous system activity, renin–angiotensin–aldosterone system activity, and atrial natriuretic peptide secretion,23 in addition to AVP secretion. McKinley et al23 and Antunes Rodrigues et al22 are suggested for excellent in-depth reviews on central thirst mechanisms and body fluid regulation.
Figure 1.
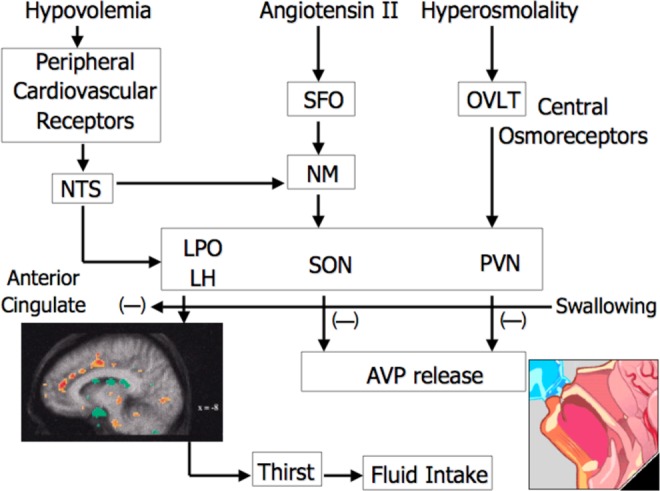
Schematic of central regulation of body fluid regulation in response to acute changes in sodium and volume. The PET scan of the anterior cingulate cortex is from Denton et al,20 reprinted . Copyright (1999) National Academy of Sciences, USA. The organum vasculosum of the lamina terminalis (OVLT) is a circumventricular organ located outside the blood–brain barrier (BBB), in the anteroventral part of the third ventricle that is an essential component of the osmotic thirst sensation pathway. Osmotic information from the OVLT is transmitted neurally to the hypothalamus and ultimately results in thirst, drinking, and arginine vasopressin (AVP) release. The median preoptic nucleus (NM) is responsible for initiating drinking in response to volumetric and angiotensinergic thirst stimulation. Through these mechanisms, the NM is utilized by both the subfornical organ (SFO) and the nucleus of the solitary tract (NTS), structures at the center of sodium appetite and thirst regulation. Changes in volume are initiated by the kidneys and stimulated by angiotensin outside the BBB. The SFO sends a message across the BBB to the NM, which then initiates volumetrically controlled thirst and drinking responses. Atrial baroreceptors also send a signal to the NTS. In addition to stimulating thirst, angiotensin stimulates the SFO and causes fluid-regulating hormones to be secreted by the pituitary and adrenal glands, increases blood pressure, and eventually causes the kidney to stop secreting sodium and water, which, in turn, decreases both salt appetite and water intake. The paraventricular (PVN) and supraoptic nuclei (SON), both located in the hypothalamus, signal the release of AVP by the posterior pituitary. Thus, both the PVN and the SON also represent important structures involved in the control of water and sodium regulation because AVP has powerful effects on both blood pressure and free water retention. Finally, the anterior cingulate cortex is involved in the relay of neural signals between the right and the left hemispheres of the brain. This part of the brain is important for decision making and plays an important role in sensing thirst and initiating drinking.17 Adapted with permission from G. W. Mack17.
Ovarian Hormones and Water Regulation
Estrogen and progesterone effects on the osmotic regulation of AVP
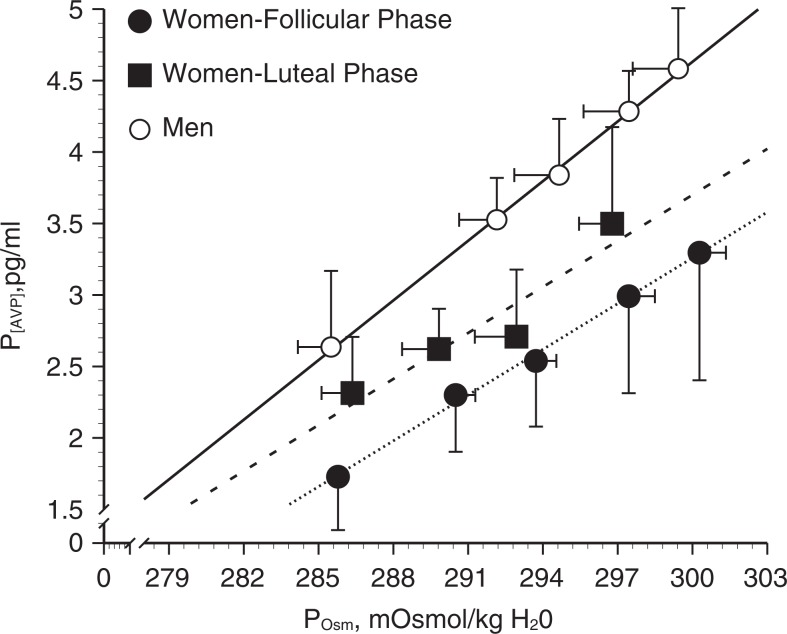
Both estrogens and progestogens can influence the neural and hormonal systems described earlier, which control thirst, fluid intake, sodium appetite and renal fluid, and sodium regulation (Figure 2). Estrogen receptors (ERs) are present in the PVN and SON of animals,26,27 and there are sex differences in the AVP neuron activity and size in these nuclei.27 To assess reproductive hormone effects on osmotic regulation of AVP, we began our studies in young (19- to 35-year-old) women (Figure 3). We examined the slope and intercept of the P[AVP]: osmolality (POsm) and thirst: POsm relationships during exercise-induced dehydration and hypertonic saline infusion under different levels of reproductive hormone exposure, including different phases of the menstrual cycle, oral contraceptive pills, and hormone suppression.21 In a series of studies, we demonstrated an estrogen-associated shift to an earlier abscissal intercept or threshold for osmotic sensation of thirst and the release of AVP, with no change in the slope or sensitivity of this relationship (Figure 3).19,28 In other words, a smaller increase in POsm was required to trigger AVP release and thirst in the brain. These shifts persisted during progestin and combined ethinyl estradiol–norethindrone oral contraceptive treatments and were supported by our subsequent hormone suppression studies.28 However, in these young women, the earlier osmotic AVP release was not associated with greater renal water retention indicating a shift in the osmotic operating point for body fluid to a lower POsm during estradiol exposure.
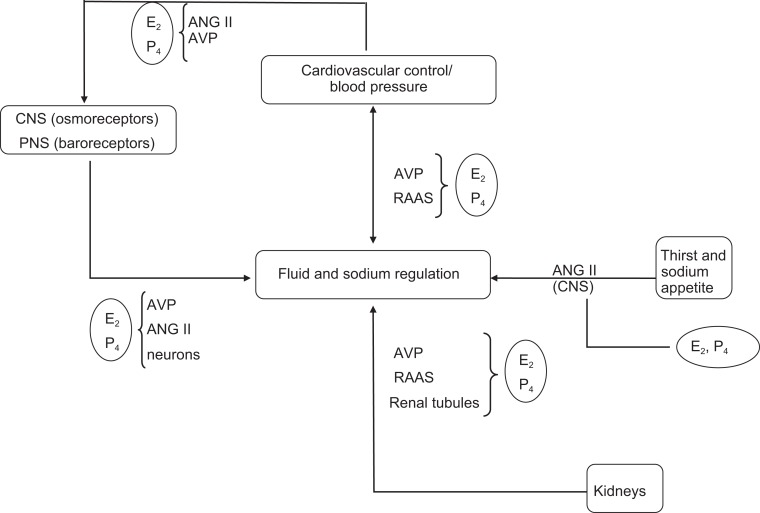
Figure 2.
Schematic to illustrate the complex control of fluid and sodium balance and the multiple ways in which estradiol (E2) and progesterone (P4) may influence these processes. Adapted with permission from Stachenfeld.24 Fluid and sodium regulation are controlled by a number of complex systems, all of which are influenced by estrogens and progesterone. Both the central nervous system (CNS) and the peripheral nervous system (PNS) contribute to fluid regulation; estrogens and progesterones can influence fluid regulation directly via the brain or indirectly by influencing the actions of angtiotensin II (ANG II) and arginine vasopressin (AVP) blood pressure changes and changes in the sodium-regulating hormones (aldosterone and renin [RAAS]). Estradiol and P4 can also increase brain ANG II mediated in the brain and increase this hormone’s important stimulating effect on thirst and fluid intake. Finally, both E2 and P4 influence sodium and water regulation in the distal tubules of the kidney. This impact can occur directly on the tubules or through both AVP and the RAAS and contribute to water retention.
Figure 3.
Osmotic regulation of P[AVP] in women during the follicular and luteal phases of the menstrual cycle and in men.19 Women (follicular phase): f(x) = 0.14*x + −273, R2 = .97; women (luteal phase): f(x) = 0.09*x + −263, R2 = .89; Men: f(x) = 0.24*x + −270, R2 = .99. Data are expressed as means ± standard error of the mean. Adapted with permission from Stachenfeld et al.19
Menopause and Hydration
Hydration and aging
Independent of menopause, aging has important effects on fluid balance. Aging is associated with higher basal POsm, and there is an age-related blunting of thirst sensation during exercise and water deprivation.18 Most important, body fluid homeostasis restoration following dehydration or water loading is slower relative to younger individuals,18 most likely due to slower kidney function. We demonstrated that osmoreceptor signaling triggering thirst sensation remains intact in older adults, but thirst sensitivity to extracellular volume change is reduced in older adults,29,30 indicating volume-sensing mechanisms are impaired in older adults. Conversely, older individuals are slower to excrete water relative to their younger counter parts, so aging also increases the risk of hyponatremia. There are no reports of sex differences in fluid regulation in older adults, and we did not detect any trends within our own data.29,30
Estrogen and progesterone effects on the regulation of body water and electrolytes in menopause
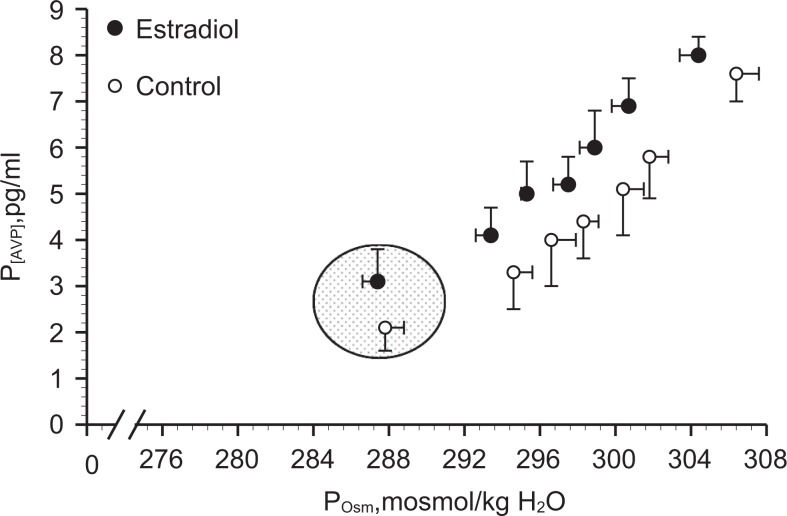
In PM women, estradiol administration is associated with increased basal P[AVP], plasma volume expansion, and an earlier osmotic threshold for AVP release (280 vs 285 mOsmol/kg H2O18; Figure 4). Unlike in younger women, the estrogen-related earlier osmotic AVP threshold is associated with water and sodium retention in PM women.18–19 Specifically, although thirst and drinking appear unaffected by estradiol exposure in the older women, urine output is reduced resulting in greater overall fluid retention (Figure 5). The mechanism for the estradiol effects within PM women has not been determined, but estradiol influences the central regulation of AVP synthesis and release (see Figure 2). Estrogens readily cross the blood–brain barrier and gain access to the hypothalamic nuclei that control AVP synthesis and release.27,31–33 The impact of estrogens could be mediated by either ER-α or ER-β as both are expressed in hypothalamic–pituitary system. Estrogen receptor-α is expressed in the organum vasculosum of the lamina terminalis, the subfornical organ, and the Median preoptic nucleus (MPO) (brain regions that connect with the PVN and SON; Figure 1) so may mediate osmotic regulation of AVP. Evidence also suggests a role for the ER-β, as this receptor is expressed in magnocellular vasopressin neurons in the PVN and SON.34–36 Finally, it is well established that progesterone plays an important role in the hypothalamic regulation of oxytocin via the progesterone metabolite, allopregnanalone, and the progesterone receptor is not expressed in this region.37 An interesting study also linked allopregnanalone to nitric oxide production and plasma volume expansion.38 Taken together, these studies suggest a role of progesterone as well as estrogen in the central regulation of body fluids and that water regulation oxytocin may be the relevant hormone. Although the evidence in animals has clearly demonstrated that chronic hyperosmolality inhibits ER-β messenger RNA expression, hypo-osolality can enhance the expression, and ER-β can induce AVP secretion under hypo-osmotic conditions, there are cross-species differences and these ER effects have not been directly tied to water or electrolyte homeostasis and have not been identified in humans. For a full review on the specific roles of the ER subtypes, progesterone, and androgens in the neurohypophysial neurons, see Sladek and Somponpun.34
Figure 4.
Osmotic regulation of P[AVP] in postmenopausal women taking 0.1 mg/d 17-β-estradiol (patch) compared to placebo control. Shaded area is baseline. Mean linear regression equations were P[AVP] = 0.33 (POsm − 280) and P[AVP] = 0.36 (POsm − 285) for estrogen and placebo control treatments, respectively. Data are expressed as means ± standard error of the mean. Adapted with permission from Stachenfeld et al.18
Figure 5.
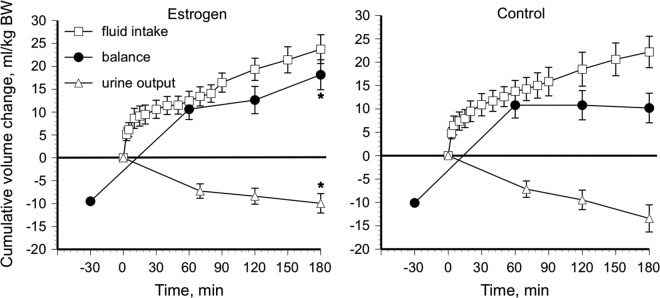
Fluid balance during estrogen administration and placebo control after hypertonic (3.0% NaCl) saline infusion in postmenopausal women. *Different from placebo control, P < .05. Data are expressed as means ± standard error of the mean. Adapted with permission from Stachenfeld et al.19
Estrogens may also impact AVP regulation indirectly by acting on catecholaminergic neurons25 that bind estrogen and project to the PVN and SON; early evidence indicated estradiol-binding sites in the nuclei of catecholamine cell bodies in the PVN and SON. Investigations have also revealed parallel changes in brain norepinephrine and AVP in female rats and that ovarian steroids modulated norepinephrine turnover in the PVN,39 supporting a role for estradiol in the osmoregulatory system through catecholamines. There is also evidence for cholinergic and angiotensinergic innervation of vasopressinergic cells in the PVN and SON, both of which are modulated by sex steroids.40 Finally, in older women, estrogen effects on osmoregulation may be related to follicle-stimulating hormone through an adenylate cyclase mechanism, although in PM women gonadotropins are reduced after estrogen administration as reported by Studd et al.41
Ovarian hormone effects on the renin–angiotensin–aldosterone system in PM women
Both estrogens and progesterones play important roles in whole-body water and sodium regulation as well as in the brain. Moreover, the impact of estrogen and progesterone exposure on systems that regulate water and sodium could be especially profound in PM women. Estradiol stimulates the liver to synthesize angiotensinogen, a substrate to the kidney hormone renin. Renin is necessary to form angiotensin I that is subsequently converted to angiotensin II (ANG II) by angiotensin-converting enzyme. Angiotensin II, one of the most powerful vasoconstrictors in the body, can increase blood pressure and also stimulate the adrenal gland to release aldosterone. Aldosterone is a primary hormone involved in tubular-regulated sodium retention by the kidney, and this greater sodium retention usually results in water retention. In our studies during hypertonic saline infusions in older women, we found that the primary cause of the estrogen-related water retention was a reduction in sodium and total osmol excretion, consistent with other studies in PM women during long-term estrogen therapy.42 Thus, the primary method of water retention in the PM women was via sodium retention rather than AVP-mediated free water retention (we did not give our participants progesterone or progestins). There is evidence that estrogen-related sodium retention is mediated through aldosterone42 and that the estrogen-associated changes in electrolyte handling are a consequence of changes in aldosterone distal tubule binding sites or a direct estrogen effect on the proximal tubule. Specifically, the effects of estrogens on fluid regulation in older women are mediated in the kidney. However, short-term estradiol administration does not alter plasma aldosterone concentration at rest or in response to hypertonic saline infusion.18 Moreover, estrogen-related sodium retention appears independent of changes in potassium excretion, which would also argue against an aldosterone-dependent mechanism.
Although much of the research in HT in PM women has focused on estrogens, progesterone is the primary steroid involved in blood pressure changes around menopause43 and is important for fluid regulation as well.42 For example, progesterone and drospirenone (the progestin in Yasmin) antagonizes the aldosterone effects on the cardiovascular system, and progesterone competes with the mineralocorticoid receptor in the distal tubule of the kidney. In contract, progestogens that upregulate androgen receptors may increase hypertension risk in susceptible women.44 Moreover, such progestogens may upregulate the thrombin receptor in the vessel wall, increasing atherosclerotic risk and thromboembolic disease.45 In young women, progesterone can inhibit aldosterone-dependent sodium reabsorption at distal sites in the nephron and produce a transient natriuresis followed by a compensatory stimulation of the renin–aldosterone system. However, while the impact of progesterone on the renin–angiotensin–aldosterone system effects on blood pressure changes have been studied in PM women, fluid and sodium regulation in response to the renin–angiotensin–aldosterone system has not been directly examined.
Summary
Estrogen and progesterone exposure have important effects on both body fluid regulation and cardiovascular function. It is clear that both of these reproductive hormones impact osmotic regulation of AVP and mediate sex differences in blood pressure responses to sodium loads. It is also likely that because of these sex effects young women are at greater risk of the negative outcomes of hyponatremia, just as young men are at greater risk of hypertension. As women age, they not only lose the hormone-mediated protection for cardiovascular disease, but HT often used for bone metabolism and treatment of vasomotor symptoms may change body fluid and sodium regulation in PM women.
Suggestions for Further Lines of Research
Although much research has focused on estrogen effects in women as they age, progesterone exposure has been largely ignored. Considering that progesterone is the primary mediator of blood pressure changes as well as sodium regulation, the study of progesterone in menopause is an important area of study. The renin–angiotensin–aldosterone system is the most important fluid- and sodium-regulating system in the body, has profound effects on cardiovascular function, and is sensitive to progesterone. Hormone therapy in PM women most often includes progesterone, and some women whose health preclude them from using estrogens during the perimenopausal transition exclusively use progesterone to treat vasomotor symptoms. Despite the widespread use of progestogens in older women and the clear impact of these hormones on hypertension, cardiovascular disease, and fluid regulation, the progesterone effects on the regulation of sodium and water retention have not been studied.
Acknowledgments
The author acknowledges the intellectual contributions of the late Ethan Nadel, PhD, Gary W. Mack, PhD, and Wendy Calzone, MS; the technical assistance of Cheryl Leone, MA, and Andrew Grabarek, BS; the clinical support of Drs Hugh Taylor and Celso Silva; and the cooperation of the volunteers.
Author’s Note: This work was completed at the John B. Pierce Laboratory and The Yale School of Medicine, Department of Obstetrics, Gynecology and Reproductive Sciences and supported, in part, by the Department of Defense. The views, opinions, and findings contained in this report are those of the author and should not be construed as an official Department of Defense position, policy, or decision unless so designated by other documentation. In conduct of research where humans are the subjects, the investigators adhered to the policies regarding the protection of human subjects as prescribed by 45 CFR 46 and 32 CFR 219 (Protection of Human Subjects). The Human Investigation Committee at Yale School of Medicine also approved all protocols. The preparation of this manuscript was supported, in part, by PepsiCo Inc. The views expressed in this article are those of the author and do not necessarily reflect the position or policy of PepsiCo, Inc.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported, in part, by National Heart Lung Blood Institute R01 HL62240 and R01 HL71159. This work was also supported, in part, by the U.S. Army Medical and Research and Materiel Command under contract DAMD17-96-C-6093. Presentation and manuscript preparation were supported by PepsiCo, Inc, Purchase, NY 10577.
References
- 1. Arieff AI, Ayus JC. Pathogenesis of hyponatremic encephalopathy. Curr Concepts. Chest. 1993;103(2):607–610. [DOI] [PubMed] [Google Scholar]
- 2. Ayus JC, Arieff AI. Brain damage and postoperative hyponatremia: the role of gender. Neurology. 1996;46(2):323–328. [DOI] [PubMed] [Google Scholar]
- 3. Ayus JC, Wheeler JM, Arieff AI. Postoperative hyponatremic encephalopathy in menstruent women. An Intern Med. 1992;117(11):891–897. [DOI] [PubMed] [Google Scholar]
- 4. Bergeron ME, Ouellet P, Bujold E, et al. The impact of anesthesia on glycine absorption in operative hysteroscopy: a randomized controlled trial. Anesth Analg. 2011;113(4):723–728. 10.1213/ANE.0b013e31822649d4. [DOI] [PubMed] [Google Scholar]
- 5. Fraser CL, Arieff AI. Epidemiology, pathophysiology, and management of hyponatremic encephalopathy. Am J Med. 1997;102(1):67–77. [DOI] [PubMed] [Google Scholar]
- 6. Fraser CL, Swanson RA. Female sex hormones inhibit volume regulation in rat brain astrocyte culture. Am J Physiol. 1994;267(4 pt 1):C909–C914. [DOI] [PubMed] [Google Scholar]
- 7. Amede FJ, James KA, Michelis MF, Gleim GW. Changes in serum sodium, sodium balance, water balance, and plasma hormone levels as the result of pelvic surgery in women. Int Urol Nephrol. 2002;34(4):545–550. [DOI] [PubMed] [Google Scholar]
- 8. Arieff AI, Ayus JC. Endometiral ablation complicated by fatal hyponatremic encephalopathy. J Amer Med Assoc. 1993;270(10):1230–1232. [PubMed] [Google Scholar]
- 9. Fieldman NR, Forsling ML, Le Quesne LP. The effect of vasopressin on solute and water excretion during and after surgical operations. Ann Surg. 1985;201(3):383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Agraharkar M, Agraharkar A. Posthysteroscopic hyponatremia: evidence for a multifactorial cause. Am J Kidney Dis. 1997;30(5):717–719. [DOI] [PubMed] [Google Scholar]
- 11. Arieff AI. Hyponatremia, convulsions, respiratory arrest, and permanent brain damage after elective surgery in healthy women. N Engl J Med. 1986;314(24):1529–1535. [DOI] [PubMed] [Google Scholar]
- 12. Fraser CL, Sarnacki P. Na+-K+-ATPase pump function in rat brain synaptosomes is different in males and females. Am J Physiol. 1989;257(2):E284–E289. [DOI] [PubMed] [Google Scholar]
- 13. Oelkers W, Schoneshofer M, Blumel A. Effects of progesterone and four synthetic progestagens on sodium balance and the renin-aldosterone system in man. J Clin Endocrinol Metab. 1974;39(5):882–890. [DOI] [PubMed] [Google Scholar]
- 14. Oelkers WHK. Drospirenone in combination with estrogens: for contraception and hormone replacement therapy. Climacteric. 2005;8(s3):19–27. [DOI] [PubMed] [Google Scholar]
- 15. Stachenfeld NS, Silva CS, Keefe DL, Kokoszka CA, Nadel ER. Effects of oral contraceptives on body fluid regulation. J Appl Physiol. 1999;87(3):1016–1025. [DOI] [PubMed] [Google Scholar]
- 16. Khalil RA. Estrogen, vascular estrogen receptor and hormone therapy in postmenopausal vascular disease. Biochem Pharmacol. 2013;86(12):1627–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stachenfeld NS. Acute effects of sodium ingestion on thirst and cardiovascular function. Curr Sports Med Rep. 2008;7(4 suppl):S7–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stachenfeld NS, DiPietro L, Palter SF, Nadel ER. Estrogen influences osmotic secretion of AVP and body water balance in postmenopausal women. Am J Physiol. 1998;274(1):R187–R195. [DOI] [PubMed] [Google Scholar]
- 19. Stachenfeld NS, Splenser AE, Calzone WL, Taylor MP, Keefe DL. Sex differences in osmotic regulation of AVP and renal sodium handling. J Appl Physiol. 2001;91(4):1893–1901. [DOI] [PubMed] [Google Scholar]
- 20. Denton D, Shade R, Zamarippa F, Egan G, Blair-West J, McKinley M, Fox P. Correlation of regional cerebral blood flow and change of plasma sodium concentration during genesis and satiation of thirst. Proc. Natl. Acad. Sci. USA; 1991, 96, 2532–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Verney E. The antidiuretic hormone and the factors which determine its release. Proc Soc Lond B Biol. 1947;135(878):25–105. [PubMed] [Google Scholar]
- 22. Antunes Rodrigues J, de Castro M, Elias LL, Valenca MM, McCann SM. Neuroendocrine control of body fluid metabolism. Physiol Rev. 2004;84(1):169–208. [DOI] [PubMed] [Google Scholar]
- 23. McKinley MJ, Cairns MJ, Denton DA, et al. Physiological and pathophysiological influences on thirst. Physiol Behav. 2004;81(5):795–803. [DOI] [PubMed] [Google Scholar]
- 24. Stachenfeld NS. Sex hormone effects on body fluid regulation. Exercise Sport Sci Rev. 2008;36(3):152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heritage AS, Stumpf WE, Sar M, Grant LD. Brainstem catecholamine neurons are target sites for sex steroid hormones. Science. 1980;207(4437):1377–1379. [DOI] [PubMed] [Google Scholar]
- 26. Sar M, Stumpf WE. Simultaneous localization of [3H]estradiol and neurophysin I or arginine vasopressin in hypothalamic neurons demonstrated by a combined technique of dry-mount autoradiography and immunohistochemistry. Neurosci Lett. 1980;17(1-2):179–184. [DOI] [PubMed] [Google Scholar]
- 27. Ishunina TA, Swaab DF. Vasopressin and oxytocin neurons of the human supraoptic and paraventricular nucleus; size changes in relation to sex and age. J Clin Endocrinol Metab. 1999;84(12):4637–4644. [DOI] [PubMed] [Google Scholar]
- 28. Stachenfeld NS, Keefe DL. Estrogen effects on osmotic regulation of AVP and fluid balance. Am J Physiol. 2002;283(4):E711–E721. [DOI] [PubMed] [Google Scholar]
- 29. Mack GW, Weseman CA, Langhans GW, Scherzer H, Gillen CM, Nadel ER. Body fluid balance in dehydrated healthy older men: thirst and renal osmoregulation. J Appl Physiol. 1994;76(4):1615–1623. [DOI] [PubMed] [Google Scholar]
- 30. Stachenfeld NS, Mack GW, DiPietro L, Nadel ER. Mechanism for attenuated thirst in aging: role of central volume receptors. Am J Physiol. 1997;272(1):R148–R157. [DOI] [PubMed] [Google Scholar]
- 31. Akaishi T, Sakuma Y. Estrogen-induced modulation of hypothalamic osmoregulation in female rats. Am J Physiol. 1990;258(4):R924–R929. [DOI] [PubMed] [Google Scholar]
- 32. Barron WM, Schreiber J, Lindheimer MD. Effect of ovarian sex steroids on osmoregulation and vasopressin secretion in the rat. Am J Physiol. 1986;250(4):E352–E361. [DOI] [PubMed] [Google Scholar]
- 33. Crowley WR, O'Donohue TL, George JM, Jacobowitz DM. Changes in pituitary oxytocin and vasopressin during the estrous cycle and after ovarian hormones: evidence for mediation by norepinephrine. Life Sci. 1978;23(26):2579–2786. [DOI] [PubMed] [Google Scholar]
- 34. Sladek CD, Somponpun SJ, Oestrogen Receptor β: Role in Neurohypophyseal Neurones. J Neuroendocrinol. 2004;16(4):365–371. [DOI] [PubMed] [Google Scholar]
- 35. Somponpun SJ, Sladek CD. Osmotic regulation of estrogen receptor-beta in rat vasopressin and oxytocin neurons. J Neurosci. 2003;23(10):4261–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Somponpun SJ, Sladek CD. Depletion of oestrogen receptor-β expression in magnocellular arginine vasopressin neurones by hypovolaemia and dehydration. J Neuroendocrinol. 2004;16(6):544–549. [DOI] [PubMed] [Google Scholar]
- 37. Blyth BJ, Hauger RL, Purdy RH, Amico JA. The neurosteroid allopregnanolone modulates oxytocin expression in the hypo-halamic paraventricular nucleus. Am J Physiol. 2000;278(3):R684–R691. [DOI] [PubMed] [Google Scholar]
- 38. Lo F, Kaufman S. Effect of 5 alpha-pregnan-3 alpha-ol-20-one on nitric oxide biosynthesis and plasma volume in rats. Am J Physiol Regul Integr Comp Physiol. 2001;280(6):R1902–R1905. [DOI] [PubMed] [Google Scholar]
- 39. Crowley RS, Amico JA. Gonadal steroid modulation of oxytocin and vasopressin gene expression in the hypothalamus of the osmotically stimulated rat. Endocrinol. 1993;133(6):2711–2718. [DOI] [PubMed] [Google Scholar]
- 40. Stone JD, Crofton JT, Share L. Sex differences in central cholinergic and angiotensinergic control of vasopressin release. Am J Physiol. 1992;263(5):R1030–R1034. [DOI] [PubMed] [Google Scholar]
- 41. Studd J, Magos A. Hormone pellet implantation for the menopause and premenstrual syndrome. In: RD Gambrell, editor. Obstetrics Gynecol Clinics North America: The menopause. Philadelphia: W.B. Saunders Co;1987:229–249. [PubMed] [Google Scholar]
- 42. Boschitsch E, Mayerhofer S, Magometschnigg D. Hypertension in women: the role of progesterone and aldosterone. Climacteric. 2010;13(4):307–313. [DOI] [PubMed] [Google Scholar]
- 43. Cifkova R, Pitha J, Lejskova M, Lanska V, Zecova S. Blood pressure around the menopause: a population study. J Hypertens. 2008;26(10):1976–1982. [DOI] [PubMed] [Google Scholar]
- 44. Hapgood JP, Africander D, Louw R, Ray RM, Rohwer JM. Potency of progestogens used in hormonal therapy: toward understanding differential actions [published online August 14, 2013]. J Steroid bioch Mol Biol. 2013. [DOI] [PubMed] [Google Scholar]
- 45. Kuhl H. Mechanisms of sex steroids: future developments. Maturitas. 2004;47(4):285–291. [DOI] [PubMed] [Google Scholar]