Abstract
Bile acids have historically been considered to mainly function in cholesterol homeostasis and facilitate fat digestion in the gastrointestinal tract. Recent discoveries show that bile acids also function as signaling molecules that exert diverse endocrine and metabolic actions by activating G protein-coupled bile acid receptor 1 (GPBAR1/G-protein-coupled bile acid receptor 1 or TGR5), a membrane G protein-coupled receptor, and farnesoid × receptor (FXR), a member of the nuclear hormone receptor superfamily. These bile acid sensing receptors are expressed in intestinal epithelial cells, TGR5 in enteroendocrine cells and FXR in enterocytes, which line the mucosa of gut lumen. A dominant effect of intestinal FXR activation by bile acids is secretion of fibroblast growth factor (FGF) 19, a novel enterokine that functions as a central enterohepatic signal to maintain bile acid homeostasis in the liver. Activation of TGR5 on enteroendocrine cells stimulates secretion of glucagon-like peptides (GLP)-1 and -2, which function, respectively, as the major incretin hormone involved in glucose homeostasis and key trophic hormone in intestinal adaptation and growth in response to food ingestion. The biological actions induced by bile acid activation of intestinal FXR and TGR5 have important therapeutic implications for the pathogenesis and treatment of several metabolic diseases, such as cholestasis and diabetes. This review highlights these new developments in the biology of intestinal bile acid sensing and metabolic function and discusses the potential implications for the health and agricultural production of domestic swine.
Keywords: enteroendocrine, farnesoid × receptor, fibroblast growth factor 19, glucagon-like peptide-1, glucagon-like peptide-2, G protein-coupled bile acid receptor 1 TGR5
INTRODUCTION
Nutrient sensing in the gastrointestinal (GI) tract is accomplished by specialized, differentiated epithelial cells that sense the presence of nutrients in the stomach and intestinal lumen after they are ingested and processed after a meal. The process of nutrient sensing also is referred to as “tasting” because it actually refers to the chemosensation of ingested nutrients by the host that begins in the oral cavity by gustatory taste cells in mammals. In fact, some of the same molecular mechanisms that mediate taste sensory function in the oral cavity also function in the small intestine. The specialized intestinal cells, called enteroendocrine (EE) cells, are one of four different epithelial cell lineages derived from stem cells that reside in the inner layer of the mucosal lining referred to as the crypt (May and Kaestner, 2010; Potthoff et al., 2013). The EE cells represent less than 1% of the total epithelial cell population in the mucosal lining of the intestine yet collectively represent the largest endocrine “organ” or system in the body. The cells are specialized in structure as well as function, in that they possess an open-type morphology with an apical brush border surface that extends into the gut lumen and comes in contact with nutrients (Fig. 1). The basolateral side of these cells faces the plasma membrane into which gut peptide hormones are released from intracellular secretory granules into the bloodstream when cells are activated.
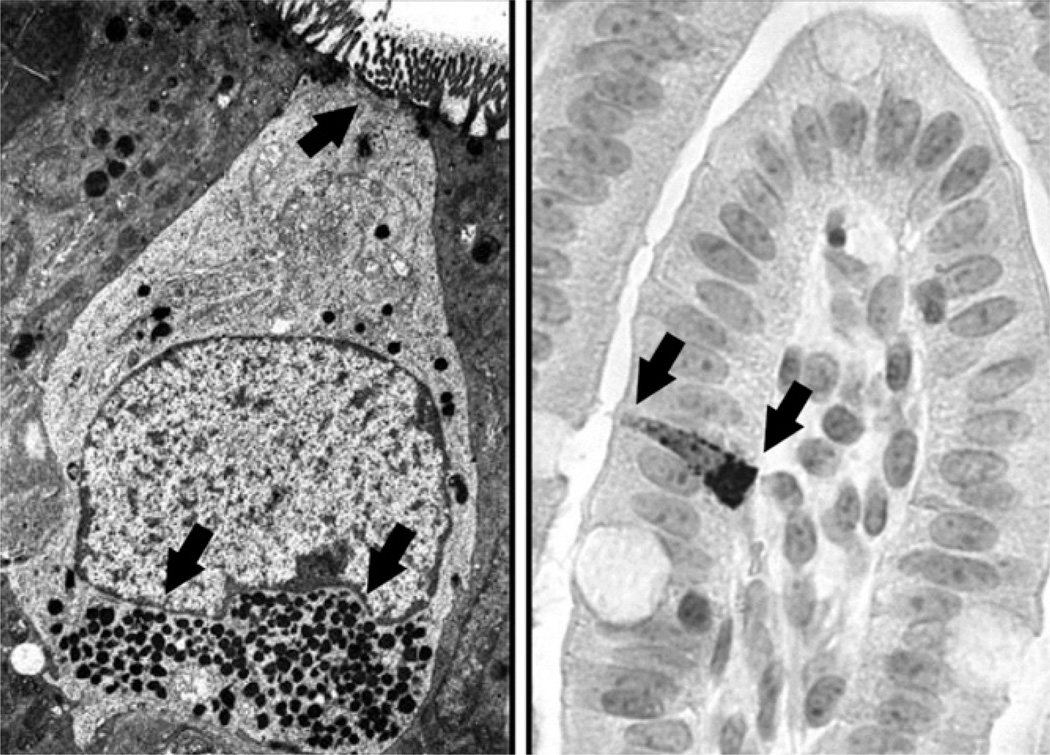
Figure 1.
Enteroendocrine cell morphology. The left panel shows an electron micrograph image of an enteroendocrine cell. Note the top apical brush border and lower electron-dense secretory granules marked with black arrows. The right panel shows staining of an enteroendocrine cell in neonatal pig ileum tissue using immunohistochemical labeling with a polyclonal antibody directed against the glucagon-like peptides (GLP)-2 (Image courtesy of C. Bauchart-Thevret, USDA Children’s Nutrition Research Center, and Baylor College of Medicine, Houston, TX). Note the apical brush border to left and basolateral surface on right marked with black arrows. Electron micrograph image was reproduced from (Meschere, 2010) with permission.
The EE cells function as key nutrient sensors within the mucosal wall that recognize sugars, fatty acids, and amino acids and peptides in the gut lumen. Therefore, the EE cells have a central role to coordinate the recognition of luminal nutrients with secretion of hormones and neurotransmitters that, in turn, regulate gut physiological functions, such as motility, fluid secretion, and blood flow. There are more than 20 different EE cells types that differ in their gut location and type of hormones secreted (Raybould, 2010). The EE cells differ from most other epithelial cells, such as parietal cells or enterocytes that are programmed to produce acid and digestive enzymes to digest food components into their simplest, constituent units, namely sugars, fatty acids, and amino acids, and transport them into the bloodstream. An important physiological element of the intestinal nutrient sensing process is activation of afferent nerve terminals that are part of the intrinsic and extrinsic neural circuits. These nerve terminals located in the mucosal wall express receptors for a variety of gut hormones [e.g., cholecystokinin, glucagon-like peptide (GLP)-1, peptide YY] and neurotransmitters [i.e., 5-hydroxytryptamine (5-HT)] forming a paracrine link between EE cells and physiological functions, such as blood flow and secretion.
A specific example of nutrient sensing in intestinal EE cells is the response to luminal glucose. Glucose absorption from the intestine occurs via the sodium-glucose co-transporter 1 (SGLT1) expressed mainly on absorptive enterocytes. In vivo animal studies show that the SGLT1 transporter is upregulated by the presence of glucose as well as nonmetabolizable glucose analogs in the intestinal lumen (Dyer et al., 2007; Raybould, 2010). The mechanism by which glucose increases the expression of SGLT1 in enterocytes is thought to involve EE cells because they also express the same taste receptors (T1R2+T1R3) that are present in taste buds. Glucose also activates the EE cell release of incretin hormones involved in insulin secretion and peripheral glucose uptake, namely glucose-dependent insulinotropic peptide (GIP), GLP-1, and GLP-2. The current theory is that glucose-dependent activation of T1R in EE cells triggers the release of hormones that eventually lead to increased SGLT1 expression and glucose uptake. Luminal glucose also can activate EE cell release of 5-HT or serotonin, which regulates gastric emptying and pancreatic exocrine and intestinal fluid secretion by interaction with vagal afferent neural circuits (Raybould et al., 2006; Raybould, 2010).
GLUCAGON-LIKE PEPTIDE SECRETION
Proglucagon: One Gene Many Peptides
This review focuses on GLP-1 and GLP-2, both of which are members of a family of GLP that are derived from one proglucagon gene. There are more in-depth reviews describing the biology of GLP and other peptide products of the proglucagon gene (Burrin et al., 2003; Drucker, 2005; Estall and Drucker, 2006; Rowland and Brubaker, 2011). The proglucagon gene is expressed as a 2-kb transcript containing 6 exons and 5 introns. There are 3 primary sites of proglucagon gene expression, namely the α-cell of the pancreatic islets, the enteroendocrine “L” cells located in the distal ileum and colon, and in the nucleus tractus solitarius regions of the brain. A key feature of GLP secretion is the tissue-specific, posttranslational processing of the proglucagon precursor peptide, which is composed of 160 AA. The tissue-specific regulation of proglucagon processing and secretion is evident after a meal in that intestinal secretion of GLP-1 and GLP-2 is stimulated whereas pancreatic glucagon secretion is effectively suppressed. In the pancreatic α-cells, the proglucagon peptide is processed primarily to glucagon whereas in the intestinal L-cells and regions of the brain, the proglucagon peptide is processed primarily to GLP-1 and GLP-2. The constituent proglucagon-like peptide fragments within the precursor peptide are flanked by basic amino acids that serve as cleavage sites for intracellular endopeptidases, termed prohormone convertases (PC). Thus, the differential secretion of end products (i.e., GLP-1, GLP-2, and glucagon) of this proglucagon-like peptide fragment is accomplished by the fact that the prohormone convertases are differentially expressed in selected cell populations of the brain and neuroendocrine system, and 2 of these enzymes, PC1/3 and PC2, are expressed in intestinal L-cells and pancreatic α-cells, respectively. The PC1/3 is responsible for cleavage of the C-terminal region of proglucagon forming GLP-1 and GLP-2 in L-cells whereas PC2 is responsible for N-terminal cleavage of proglucagon to form glucagon.
Glucagon-Like Peptide Secretion
Oral feeding or enteral nutrient ingestion is the primary stimulus for GLP-1 and GLP-2 secretion (Baggio and Drucker, 2007). The critical role of enteral feeding for the stimulation of GLP secretion was first shown in dogs (Unger et al., 1968). These studies showed that intraduodenal glucose infusion more than doubled the circulating concentration of glucagon-like immunoreactivity whereas intravenous glucose infusion had no effect. This observation led to the concept of the incretin effect, which describes the gut-associated potentiation of insulin secretion due to humoral factors, termed incretins, after oral or enteral food ingestion. Subsequent reports in parenterally and enterally fed animals (Burrin et al., 2000a; van Goudoever et al., 2001; Stoll et al., 2012) and humans (Xiao et al., 1999) have confirmed the importance of enteral nutrient stimulation for GLP-1 and GLP-2 secretion. Our studies in neonatal piglets indicate that an enteral intake of at least 40% of the total nutrient intake is required to maintain physiological plasma GLP-2 concentrations. Studies also indicate that liquid diets are more stimulatory than solid diets, yet there is no difference between bolus versus continuous feeding. In vivo studies in humans indicate that carbohydrate and fat are more potent secretagogues of GLP than protein. In pigs, GLP-1 secretion was increased more by fat than glucose. Studies with perfused ileal segments indicate that GLP-1 secretion is stimulated by sugars transported via the SGLT1 transporter, including glucose, galactose, and 3-O-methylglucose, but not by fructose, mannose, xylose, or 2-deoxyglucose. The results with 3-O-methylglucose indicate that sugar metabolism is not required to stimulate GLP-1 secretion. Studies in vivo and with cultured cells indicate that monounsaturated fatty acids are a more potent stimulus of GLP-1 secretion than saturated fatty acids. Although in vivo studies indicate that protein and AA are poor secretagogues, peptones have been shown to stimulate GLP-1 secretion from the perfused ileum in rodents. Other endogenous luminal factors have been shown to induce GLP-1 secretion, including bile acids, yet the molecular mechanisms were poorly understood until recently.
In addition to nutritional regulation, GLP secretion is also mediated by endocrine and neural control (Dube and Brubaker, 2004). Studies in adult humans demonstrated a rapid increase in circulating GLP-1 and GLP-2 concentrations after a meal suggesting that humoral signals from the proximal bowel activate L-cell secretion before direct nutrient exposure in the distal intestine. This observation prompted a series of studies that demonstrated that rapid nutrient-mediated secretion of GLP-1 and likely GLP-2 involves both endocrine stimulation, via GIP released from enteroendocrine K-cells in the duodenum, and neural reflexes involving gastrin-releasing peptide. Both GLP-1 and GIP are often referred to collectively as incretin hormones because of the dual cooperation in augmenting insulin secretion.
BILE ACIDS AS SIGNALING MOLECULES
Bile Acid Physiology
The concept that bile acids function as signaling molecules is a relatively recent development because historically bile acids have been viewed mainly for their key physiological role in dietary fat absorption and cholesterol excretion (Hofmann and Hagey, 2008; Lefebvre et al., 2009). Bile acids are amphipathic molecules synthesized from cholesterol in hepatocytes. Chenodeoxycholic acid (CDCA) and cholic acid are primary bile acids in many species, including humans and pigs. Intrahepatic bile acids undergo further conjugation with taurine and glycine to form negatively charged molecules that are fully ionized and highly soluble at pH of the small intestine during digestion. Once synthesized and conjugated in the hepatocyte, bile acids are transported into the biliary tract and represent the major constituent of bile. The intraluminal concentration of conjugated bile acids is high in biliary tract and small intestine because they are impermeable to the apical membrane and paracellular junctions of biliary tract cholangiocytes and intestinal enterocytes. Once secreted into the intestine, the central function of bile acids is the solubilization of dietary lipids and promotion of their digestion and absorption in the digestive tract.
An important aspect of bile acid metabolism is that most bile acids secreted into the intestine are reabsorbed in the distal small intestine or ileum and recirculate back to the liver via the portal venous blood by a process termed enterohepatic circulation. In adult humans, the bile acid pool is approximately 2 g and is recycled via the enterohepatic circulation 12 times/d. Approximately 95% of bile acids secreted into the gut are recycled per day whereas the remaining 5% are lost in feces and represent the main route of cholesterol excretion. During passage along the small intestine, conjugated bile acids undergo deconjugation and conversion to secondary bile acids by luminal gut bacteria. Bile acid deconjugation begins in the distal small intestine so that the terminal ileum is presented with a mixture of conjugated and unconjugated bile acids. Conjugated bile acids are poorly absorbed in the proximal small intestine but efficiently taken up by the apical sodium-dependent bile acid transporter in the ileum. Once taken up by the ileal enterocyte, bile acids bind to the cytosolic ileal lipid binding protein and exported across the basolateral membrane into the portal circulation by the organic solute transporter (OST), OSTα-OSTβ (Dawson et al., 2010).
Bile Acid Receptors and Signaling Pathways: Farnesoid × receptor and TGR5
In 1999, the idea that bile acids could function as signaling molecules was established with the discovery that bile acids were natural ligands of the nuclear receptor, farnesoid × receptor (FXR; Parks et al., 1999; Wang et al., 1999). The FXR is a member of the nuclear receptor superfamily and is highly expressed in liver, intestine, kidney, and adrenal glands. The most potent natural FXR ligand is CDCA (concentration of a drug that gives half-maximal response, EC50 at 10 µM), but other bile acids (i.e., cholic, lithocholic, and deoxycholic) also activate FXR. Numerous reports during the past decade show that FXR is the primary sensor of bile acids and are involved in every aspect of bile acid metabolism, including bile acid synthesis, transport, detoxification, and excretion in the liver and intestine. Bile acid synthesis is tightly regulated because disruption results in metabolic disorder of the liver and biliary tract, such as cholestasis and fibrosis. Therefore, regulation of bile acid synthesis by direct FXR activation is a central control mechanism and occurs by bile acid synthesized within the hepatocyte or by bile acids taken up from portal blood as part of the enterohepatic circulation. Activation of FXR potently inhibits the expression of cholesterol 7α-hydroxylase (CYP7A1), a rate-limiting enzyme in bile acid synthesis, and maintains hepatocyte bile acid homeostasis by regulating the expression of genes involved in synthesis (i.e., CYP7A1), uptake (i.e., Na+-taurocholate co-transporter polypeptide and organic anion-transporting peptide 2/8), and export of bile acid (i.e., bile salt export pump and multidrug resistance protein 2).
A second FXR signaling pathway that functions as negative feedback mechanism to suppress hepatic bile acid synthesis involves fibroblast growth factor (FGF) 19. Studies in mice showed that FXR stimulates the transcription of intestinal FGF15 and its human ortholog FGF19 (Kim et al., 2007; Potthoff et al., 2013). Before discovery of FXR, it was known that intestinal administration of bile acids suppresses hepatic CYP7A1, implicating a secreted intestinal factor that acts to suppress bile acid synthesis. The first evidence that FGF19 was the secreted factor came from studies showing that FGF19 repressed CYP7A1 expression in both isolated hepatocytes and mice. However, it was not until the studies by Kim et al. (2007) showed that tissue-specific FXR knockout in either the liver (FxrL) or intestine (FxrIE) increases the bile acid pool size. However, treatment with GW4064 (a FXR-selective agonist) significantly repressed CYP7A1 in FxrL mice but not in FxrIE mice. This indicated that CYP7A1 repression is mediated primarily by FXR activation in the intestine and not in the liver. More recent evidence confirms this showing that selective activation of intestinal FGF15 in mice protects against hepatic cholestasis (Modica et al., 2012). The induction of intestinal FGF19 secretion via FXR is thought to occur primarily in epithelial cells in the distal region of the ileum because this is where the bile acid transporters are most highly expressed (Dawson et al., 2010; see Fig. 2). The tissue-specific localization and molecular regulation of the FXR–FGF19 axis in intestinal epithelial cells is poorly understood and warrants further detailed studies. The receptor for FGF19 is FGF receptor 4 (FGFR4), which is abundant in the liver, and mice lacking FGFR4 have an increased bile acid pool. Activation of FGF19 signaling in cells via FGFR4 also requires the coreceptor β-Klotho and tissue-specific expression of this co-receptor is an important determinant of FGF19 responsiveness (Potthoff et al., 2012). The significance of FGF19 in human bile metabolism has been shown in subjects treated with cholestyramine and the FXR ligand CDCA. Treatment with cholestyramine led to an increase in serum 7α-hydroxycholest-4-en-3-one (C4; i.e., a marker for CYP7A1 activity) and a reduction in FGF19 levels whereas CDCA treatment increased plasma FGF19 and decreased serum C4 (Lundasen et al., 2006). Our recent study demonstrated the importance of luminal bile acid stimulation for maintenance of circulating FGF19 secretion in total parenterally fed (TPN) neonatal piglets (Jain et al., 2012). We showed that TPN markedly decreased concentrations of circulating FGF19 whereas duodenal infusion of CDCA significantly induced FGF19. We also found that liver pathologies associated with TPN were markedly improved with CDCA infusion. The finding that TPN results in reduced FGF19 secretion is novel and may provide a mechanism to explain the cholestasis and steatosis observed with parenteral nutrition-associated liver disease. Because FGF19 production in the small intestine causes suppression of CYP7A1 in hepatocytes, diminished FGF19 concentrations in TPN could increase CYP7A1 expression in the liver, resulting in persistent activation of bile acid synthesis and cholestasis. These studies showed that FGF19 is a novel enterokine that is induced via intestinal FXR bile acid activation and functions as an enterohepatic signal in the feedback suppression of bile acid synthesis.
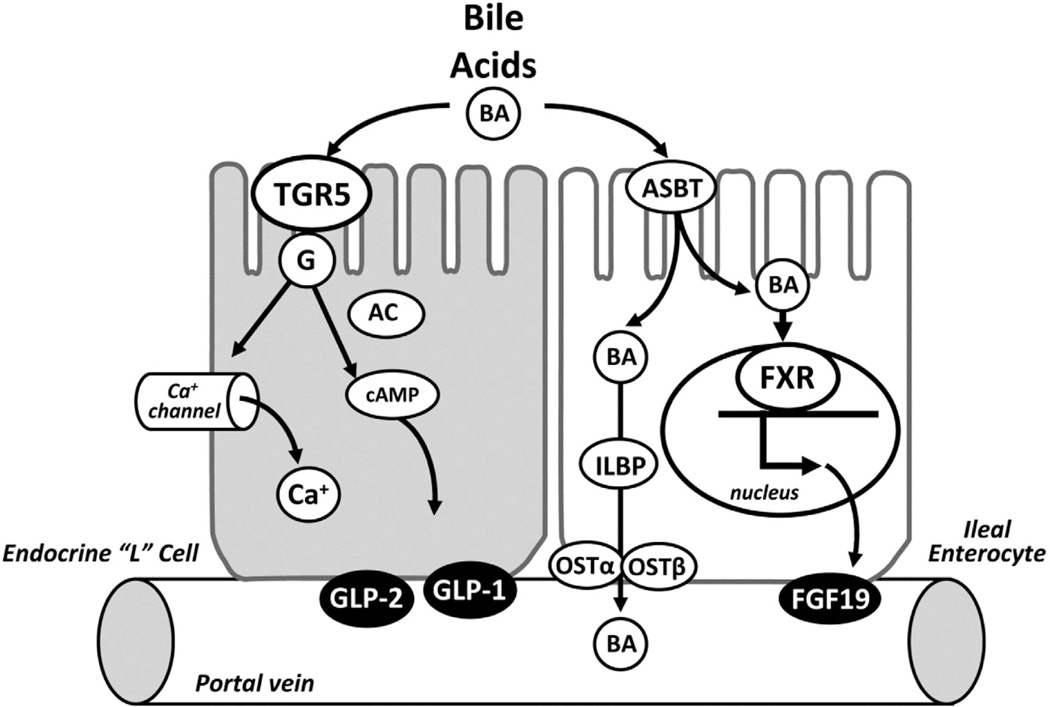
Figure 2.
Intestinal epithelial cell bile acid (BA) sensing. Illustration shows TGR5 receptor signaling in enteroendocrine cells and farnesoid × receptor (FXR) signaling in enterocytes. Signaling mechanisms for apical membrane-associated TGR5 receptor in enteroendocrine cells involve activation of G protein coupled pathways with adenylate cyclase (AC) and cyclic adenosine monophosphate (cAMP), and calcium channels that trigger secretion of glucagon-like peptides (GLP)-1 and GLP-2 into the portal vein. Signaling mechanism for FXR located in the nucleus of enterocytes triggers secretion of fibroblast growth factor (FGF) 19 into the portal vein. Conjugated bile acids are transported into ileal enterocytes by the apical sodium-dependent bile acid transporter (ASBT) and bind intracellular ileal lipid binding protein (ILBP) before export into the portal vein by the organic solute transporter (OST)..
The second major bile acid receptor characterized in recent years is the G protein coupled membrane receptor (GPCR), TGR5, also known as G protein-coupled bile acid receptor 1 (GPBAR 1), or GPR131 (Kawamata et al., 2003; Thomas et al., 2008). The TGR5 receptor is a cell-surface bile acid receptor is a member of the rhodopsin-like subfamily of GPCR (class A). Originally considered an orphan GPCR, TGR5 was recently reclassified as the founder of the bile-acid receptor subclass of GPCRs41. Human TGR5 is activated by multiple bile acids, with lithocholic acid being the most potent natural agonist (EC50 of 0.53 µM), yet other conjugated and unconjugated bile acids activate TGR5 including deoxycholic acid, CDCA, and cholic acid. The TGR5 receptor mRNA is ubiquitously transcribed with the greatest transcription in the gall bladder and less transcription in brown adipose tissue, liver, and intestine. The broad tissue expression profile and the fact that it is highly conserved among mammals indicates that TGR5 has an important role in physiology and metabolism. The relatively high expression of TGR5 within the biliary tract, gallbladder, and gastrointestinal tissues signals its importance in bile acid signaling and gut function. Additional evidence shows that TGR5 has an important role in gallbladder filling and contraction (Li et al., 2011). It was recently demonstrated that TGR5 is localized in primary enteroendocrine cells within the intestinal epithelium and highly expressed in enteroendocrine cell lines. Moreover, these studies showed that bile acids activate cyclic adenosine monophosphate (cAMP)and calcium signaling and trigger secretion of GLP-1 in these enteroendocrine cells (Parker et al., 2012; Reimann et al., 2008; see Fig. 2). These observations were of interest given that previous studies showed that bile acids were capable of stimulating GLP-1 secretion from isolated perfused ileum. The importance of the TGR5 receptor as the molecular mechanism to explain bile acid-induced GLP-1 secretion was shown in a series of in vivo and cell culture studies by Thomas et al. (2009) using mice with targeted disruption and overexpression of the TGR5 gene. These studies also demonstrated the important metabolic effect of bile acids on glucose intolerance and metabolic dysfunction in mice fed high-fat diets.
The importance of bile acids as signaling molecules via these 2 classes of receptors has led to the pharmacological development and screening of bile acid analogs (i.e., “designer bile acids”) designed to increase potency and selectivity for the specific receptors. Several of these bile acid analogs have been developed and tested in preclinical models and are now in clinical trials for treatment of various metabolic and liver diseases (Pellicciari et al., 2004, 2009; Fiorucci et al., 2009; Baghdasaryan et al., 2011). An analog has been developed that selectively targets FXR, called obeticholic acid (6-ethyl chenodeoxycholic acid; INT-747), that has been shown to protect against cholestatic liver injury and fibrosis, experimental colitis, and augment innate immunity in mice (Fiorucci et al., 2004, 2005; Mencarelli et al., 2009; Cipriani et al., 2011). Another analog selectively targets TGR5 [6a-ethyl-23(S)-methyl-cholic acid; INT-777] and was used to show the importance of bile acid-induced GLP-1 secretion in protection against high-fat diet induced metabolic dysfunction (Thomas et al., 2009; Genet et al., 2010). An additional analog targets both FXR and TGR5 receptors (semisynthetic 23-sulfate derivative of INT-747; INT-767) and has been shown to protect against chronic cholangiopathy in mice (Rizzo et al., 2010; Baghdasaryan et al., 2011). A few of these analogs are in clinical trials for treatment of primary biliary cirrhosis, diabetes with nonalcoholic fatty liver disease, and bile acid malabsorption diarrhea (see http://www.clinicaltrials.gov/).
METABOLIC ACTIONS OF BILE ACIDS
The bile-acid stimulated secretion of FGF19 and GLP-1 by different epithelial cells in the intestine generates an endocrine signal that has a variety of metabolic actions in the body (Fig. 3). As discussed previously, the dominant effect of intestinal FGF19 secretion is to modulate hepatic bile acid homeostasis, but the function of FGF19 goes well beyond bile acid homeostasis. Fibroblast growth factor 19 exerts important regulatory effects on glucose, protein, and lipid metabolism (Kuro-O, 2008; Thomas et al., 2008; Potthoff et al., 2012). Overexpression or infusion of FGF19 in mice reduced adiposity and serum triglycerides and increased metabolic rate and glycemic control (Tomlinson et al., 2002; Fu et al., 2004). Likewise, FGFR4 knockout mice exhibit glucose intolerance, dyslipidemia, and increased adiposity, but these phenotypes appear to be determined by hepatic FGFR4 expression (Huang et al., 2007). More recent studies in mice have led to the concept that FGF19 functions as a parallel yet independent endocrine hormone along with insulin to control hepatic glucose and protein metabolism in response to feeding (Kir et al., 2011). These studies showed that FGF19 induced hepatic glycogen and protein synthesis while suppressing gluconeogenesis. Importantly, these metabolic effects appear to be mediated via intracellular signaling pathways that are separate from those controlled by insulin. The effect of FGF19 on hepatic lipid metabolism is poorly understood but likely increases fatty acid oxidation and suppresses triglyceride synthesis; both outcomes could reduce hepatic steatosis.
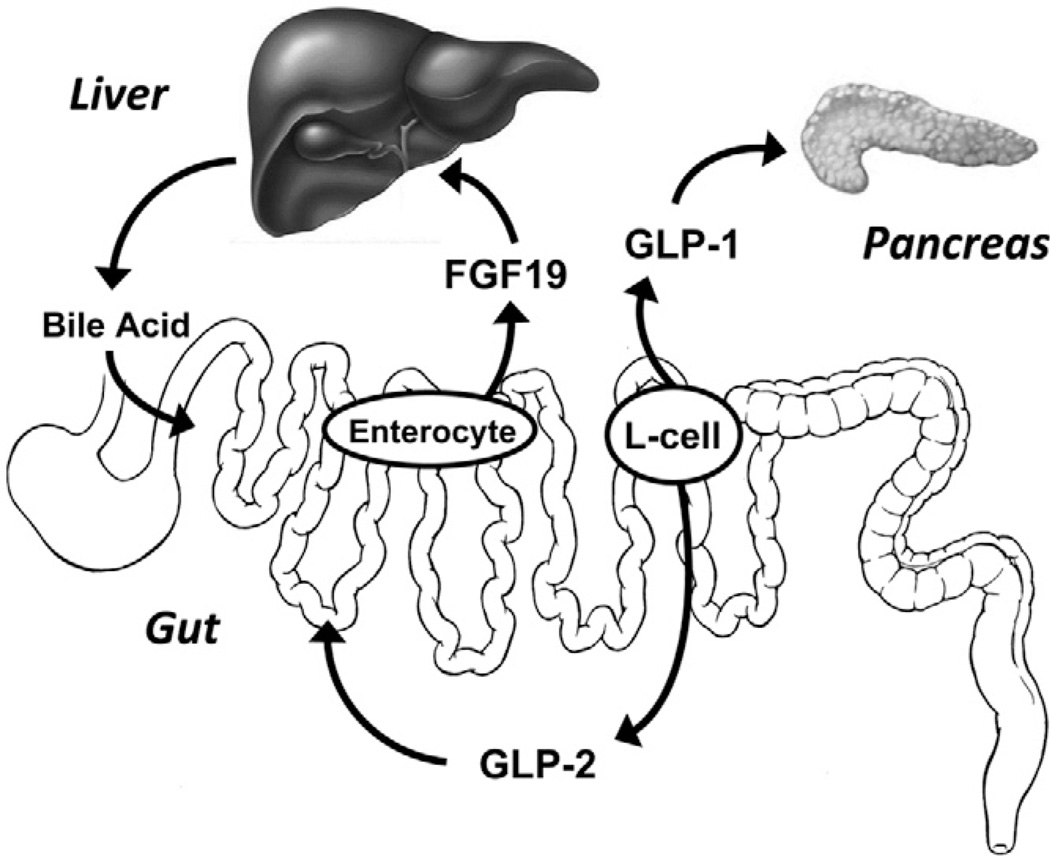
Figure 3.
Bile acid-induced gut hormone secretion. Illustration shows differential activation of enterocyte fibroblast growth factor (FGF) 19 secretion and enteroendocrine glucagon-like peptides (GLP)-1 and GLP-2 secretion by luminal bile acids in the small intestine. The target for FGF19 action is mainly the liver and negative feedback of enterohepatic bile acid secretion. The targets for GLP-1 and GLP-2 action are mainly the pancreas β cell and the gastrointestinal mucosa, respectively.
The bile acid induction of intestinal endocrine cell GLP section also has multiple metabolic actions. Most of the attention, however, has focused on bile acid-induced GLP-1 secretion because this has major implications for treatment of diabetes and obesity. Glucagon-like peptide 1 functions as the dominant incretin hormone after a meal to stimulate insulin secretion, increase blood glucose clearance and insulin sensitivity, reduce glucagon secretion and hepatic gluconeogenesis, and reduce gastric emptying and appetite (Drucker, 2006; Barrera et al., 2011). The molecular mechanism that mediates most of the metabolic effects is mediated by the GLP-1 receptor localized in cells within these tissues (i.e., liver, pancreas, and gut mucosa) but also in nerves that may function in neural reflexes between the gut and brain. The ability to augment GLP-1 secretion and biological function has become a central drug target of the pharmaceutical industry for treatment and glycemic control of type 2 diabetic patients. The therapeutic use of GLP-1 has spawned a new class of drugs termed “incretin mimetics” that are designed to increase both GLP-1 and GIP secretion and activity (e.g., exenatide and liraglutide; Lovshin and Drucker, 2009). Moreover, because the biological activity of these gut hormones is controlled by the activity of dipeptidyl peptidase-4 (DPP-4), a separate class of drugs has evolved to inhibit this proteolytic enzyme, DPP-4 inhibitors (e.g., sitagliptin, saxagliptin).
Given the potential impact of augmented incretin function on treatment of diabetes and obesity, the report by Thomas et al. (2009) was significant because it demonstrated the proof-of-concept that bile acids could function as an orally active modality to increase GLP-1 secretion and protect against metabolic dysfunction and glucose intolerance. The link between intestinal bile acid sensing and GLP-1 secretion has also revealed a previously unrecognized metabolic effect resulting from modulating intestinal bile acid physiology. Bile acid sequestrants were developed to limit bile acid-mediated lipid and cholesterol absorption in the gut; these include agents such as cholestyramine and colesevelam (Beysen et al., 2012; Holst and McGill, 2012). Studies in animals and human diabetic patients show that bile acid sequestrants alone and in combination with DDP-4 inhibitors improve glycemia and insulin sensitivity, augment pancreatic β-cell mass, and increase GLP-1 secretion (Shang et al., 2010, 2012; Beysen et al., 2012). The emerging evidence that bile acid sequestrant treatment improved glycemia appears to be mediated by bile acid induction of TGR5, especially in the colon, and increased GLP-1 secretion (Potthoff et al., 2013).
The connection between bile acid physiology and GLP-1 secretion is also being explored with respect to the metabolic effects observed after bariatric surgery in obese patients. The improvement in glycemia and insulin sensitivity and suppression of appetite after bariatric surgery in obese humans or in rodents after Roux-en-Y gastric bypass (RYGB) or sleeve gastrectomy has been linked to increased secretion of incretin hormones, namely, GLP-1 (Kindel et al., 2009; Rubino et al., 2010; Chambers et al., 2011; Gaitonde et al., 2012; Mingrone et al., 2012; Potthoff et al., 2013). These observations are consistent with the idea that bypassing the proximal GI tract resulting in increased distal intestine delivery and exposure to bile acids produces a greater stimulation of TGR5 receptor and GLP-1 secretion. It remains unclear whether the metabolic effects of bariatric surgery also involve activation of the intestinal FXR–FGF19 axis, but recent evidence would support this mechanism because serum FGF19 concentrations are increased soon after RYGB surgery in obese patients and central FGF19 suppresses food intake in rats (Pournaras et al., 2012; Ryan et al., 2013).
LOCAL GASTROINTESTINAL EFFECTS OF BILE ACID-INDUCED SIGNALS
Much of the preceding discussion has focused on the actions of bile acid-induced endocrine effects of FGF19 and GLP-1 on metabolism in the liver and peripheral tissues beyond the gastrointestinal tract. However, there are a variety of physiological actions triggered by bile acids in the gut lumen that may involve not only FGF19 or GLP-1 but also other gut hormones secreted by enteroendocrine cells, including GLP-2 and peptide YY. This is especially true for GLP-2 because it is co-secreted with GLP-1. There is a longstanding literature describing the effects of biliary tract diversion and bile acids on intestinal adaptation and cell proliferation that was performed before the discovery of FXR and TGR5 and their role in the gut (Al-Mukhtar et al., 1983; Rainey et al., 1983, 1986; Savage et al., 1988). The induction of GLP-2 secretion is a likely factor involved in the trophic actions of luminal bile acids. Recent studies have observed increased plasma GLP-2 concentrations associated with intestinal growth after RYGB surgery in rodent and enteral bile acid infusion in parenterally fed pigs (le Roux et al., 2010; Jain et al., 2012). Consistent with this is the observation that disruption of intestinal bile acid transporter (OSTα) leads to augmented intestinal length, weight, and villus morphology and is not lost in mice with dual knockout of OSTα and FXR (Lan et al., 2012). These observations indicate that loss of enterohepatic circulation and fecal accumulation of bile acids may trigger trophic actions in the small intestine that could be explained by increased GLP-2 secretion. Whether the trophic effects of bile acids are explained by TGR5-mediated GLP-2 secretion warrants further study. Furthermore, it remains to be seen if bile acids can be used therapeutically to augments the desirable actions associated with GLP-2, GLP-1 and peptide YY related to gastrointestinal motility, fluid absorption, and intestinal function.
SUMMARY AND CONCLUSIONS
The field of digestive physiology has expanded rapidly in recent years as the molecular basis of nutrient sensing has come of age. The discovery of new cellular receptor sensing mechanisms for specific nutrients and secreted factors in the gut has become an active area of research. This review has highlighted the physiology and molecular mechanisms of the FXR and TGR5 bile sensing pathways. The characterization and development of the intestinal FXR-FGF19 signaling pathway has revealed a novel enterokine (i.e., FGF19) that functions in enterohepatic regulation of bile acid homeostasis and the control of hepatic glucose and lipid metabolism. The finding that TGR5 is a molecular link between luminal bile acid sensing in enteroendocrine cells to incretin hormone secretion (i.e., GLP-1) puts bile acids on the growing list of nutrients and other naturally occurring compounds that trigger gut hormone secretion.
Most of the information that has emerged about the biology of these signaling pathways has been derived from studies in rodents and humans. Yet, the biological function of these pathways has potential implications for health and growth of domestic pigs. Our research has used pigs as a model for human infant nutrition and gastroenterology to show the importance of GLP-1 and GLP-2 as enteral nutrient-mediated gut signals involved in TPN-related intestinal atrophy and metabolic dysfunction (Burrin et al., 2000a,b, 2003; Stoll et al., 2010, 2012). Despite this and additional reports describing the function of these hormones in pigs, the further investigation and application for domestic pig production has yet to be realized. There would appear to be many potential applications of these concepts in the management and nutrition of young neonatal and weanling pigs. This is because the period of birth through weaning in pigs is marked by profound changes in the composition and nutritional quality of the diet as well as maturation and development of the GI physiology. The extent to which the young pig can adapt physiologically to these changes determines its survival, health, and subsequent growth rate. The development of strategies to enhance GLP-2 secretion and bioactivity during the weaning phase of production offers several advantages to augment intestinal growth and digestive capacity and limit the fluid secretory and accelerated intestinal motility associated with diarrhea. The parenteral delivery of the GLP-2 peptide to piglets with sustained bioactivity presents a logistical challenge in the setting of commercial swine production. Therefore, attempts to identify the most potent orally active agents, such as specific nutrients, bile acids, or DPP-4 inhibitors, offers an attractive option. Recent studies by I. Ipharraguerre and others (Lucta S. A., unpublished data) show preliminary evidence that oral delivery of bile acids by gavage or diet can boost GLP-2 secretion in weanling pigs fed standard cereal-based diet. In these studies, bile acid-increased GLP-2 secretion was not associated with improved intestinal growth or function, yet they provide important proof of concept that a natural TGR5 agonist can modulate GLP-2 secretion in weanling pigs. These recent studies should serve to stimulate further research as to whether the ability to therapeutically manipulate intestinal nutrient sensing is commercially viable in pig production.
Footnotes
Based on a presentation at the preconference symposium titled “Gut Chemosensing: Integrating nutrition, gut function and metabolism in pigs” preceding the 12th International Symposium on Digestive Physiology of Pigs in Keystone, Colorado, May 29–June 1, 2012, with publication sponsored by Lucta S.A., the American Society of Animal Science, and the Journal of Animal Science.
This work is a publication of the USDA-ARS Children’s Nutrition Research Center, and Department of Pediatrics, Baylor College of Medicine and Texas Children’s Hospital, Houston, TX. This work was supported by federal funds from the USDA, ARS under Cooperative Agreement Number 58-6250-6-00. The contents of this publication do not necessarily reflect the views or policies of the USDA or mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
LITERATURE CITED
- Al-Mukhtar MY, Sagor GR, Ghatei MA, Bloom SR, Wright NA. The role of pancreatico-biliary secretions in intestinal adaptation after resection, and its relationship to plasma enteroglucagon. Br. J. Surg. 1983;70:398–400. doi: 10.1002/bjs.1800700703. [DOI] [PubMed] [Google Scholar]
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Baghdasaryan A, Claudel T, Gumhold J, Silbert D, Adorini L, Roda A, Vecchiotti S, Gonzalez FJ, Schoonjans K, Strazzabosco M, Fickert P, Trauner M. Dual farnesoid × receptor/TGR5 agonist INT-767 reduces liver injury in the Mdr2−/−(Abcb4−/−) mouse cholangiopathy model by promoting biliary HCO(−)(3) output. Hepatology. 2011;54:1303–1312. doi: 10.1002/hep.24537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera JG, Sandoval DA, D’Alessio DA, Seeley RJ. GLP-1 and energy balance: An integrated model of short-term and long-term control. Nat. Rev. Endocrinol. 2011;7:507–516. doi: 10.1038/nrendo.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beysen C, Murphy EJ, Deines K, Chan M, Tsang E, Glass A, Turner SM, Protasio J, Riiff T, Hellerstein MK. Effect of bile acid sequestrants on glucose metabolism, hepatic de novo lipogenesis, and cholesterol and bile acid kinetics in type 2 diabetes: A randomised controlled study. Diabetologia. 2012;55:432–442. doi: 10.1007/s00125-011-2382-3. [DOI] [PubMed] [Google Scholar]
- Burrin DG, Stoll B, Guan X. Glucagon-like peptide 2 function in domestic animals. Domest. Anim. Endocrinol. 2003;24:103–122. doi: 10.1016/s0739-7240(02)00210-2. [DOI] [PubMed] [Google Scholar]
- Burrin DG, Stoll B, Jiang R, Chang X, Hartmann B, Holst JJ, Greeley GH, Jr, Reeds PJ. Minimal enteral nutrient requirements for intestinal growth in neonatal piglets: How much is enough? Am. J. Clin. Nutr. 2000a;71:1603–1610. doi: 10.1093/ajcn/71.6.1603. [DOI] [PubMed] [Google Scholar]
- Burrin DG, Stoll B, Jiang R, Petersen Y, Elnif J, Buddington RK, Schmidt M, Holst JJ, Hartmann B, Sangild PT. GLP-2 stimulates intestinal growth in premature TPN-fed pigs by suppressing proteolysis and apoptosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2000b;279:G1249–G1256. doi: 10.1152/ajpgi.2000.279.6.G1249. [DOI] [PubMed] [Google Scholar]
- Chambers AP, Stefater MA, Wilson-Perez HE, Jessen L, Sisley S, Ryan KK, Gaitonde S, Sorrell JE, Toure M, Berger J, D’Alessio DA, Sandoval DA, Seeley RJ, Woods SC. Similar effects of roux-en-Y gastric bypass and vertical sleeve gastrectomy on glucose regulation in rats. Physiol. Behav. 2011;105:120–123. doi: 10.1016/j.physbeh.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani S, Mencarelli A, Chini MG, Distrutti E, Renga B, Bifulco G, Baldelli F, Donini A, Fiorucci S. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PLoS. One. 2011;6:e25637. doi: 10.1371/journal.pone.0025637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson PA, Hubbert ML, Rao A. Getting the mOST from OST: Role of organic solute transporter, OSTalpha-OSTbeta, in bile acid and steroid metabolism. Biochim. Biophys. Acta. 2010;1801:994–1004. doi: 10.1016/j.bbalip.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ. Biologic actions and therapeutic potential of the proglucagon-derived peptides. Nat. Clin. Pract. Endocrinol. Metab. 2005;1:22–31. doi: 10.1038/ncpendmet0017. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Dube PE, Brubaker PL. Nutrient, neural and endocrine control of glucagon-like peptide secretion. Horm. Metab. Res. 2004;36:755–760. doi: 10.1055/s-2004-826159. [DOI] [PubMed] [Google Scholar]
- Dyer J, Daly K, Salmon KS, Arora DK, Kokrashvili Z, Margolskee RF, Shirazi-Beechey SP. Intestinal glucose sensing and regulation of intestinal glucose absorption. Biochem. Soc. Trans. 2007;35:1191–1194. doi: 10.1042/BST0351191. [DOI] [PubMed] [Google Scholar]
- Estall JL, Drucker DJ. Glucagon-like peptide-2. Annu. Rev. Nutr. 2006;26:391–411. doi: 10.1146/annurev.nutr.26.061505.111223. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Antonelli E, Rizzo G, Renga B, Mencarelli A, Riccardi L, Orlandi S, Pellicciari R, Morelli A. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 2004;127:1497–1512. doi: 10.1053/j.gastro.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Clerici C, Antonelli E, Orlandi S, Goodwin B, Sadeghpour BM, Sabatino G, Russo G, Castellani D, Willson TM, Pruzanski M, Pellicciari R, Morelli A. Protective effects of 6-ethyl chenodeoxycholic acid, a farnesoid × receptor ligand, in estrogen-induced cholestasis. J. Pharmacol. Exp. Ther. 2005;313:604–612. doi: 10.1124/jpet.104.079665. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Mencarelli A, Palladino G, Cipriani S. Bileacid-activated receptors: Targeting TGR5 and farnesoid-×-receptor in lipid and glucose disorders. Trends Pharmacol. Sci. 2009;30:570–580. doi: 10.1016/j.tips.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Fu L, John LM, Adams SH, Yu XX, Tomlinson E, Renz M, Williams PM, Soriano R, Corpuz R, Moffat B, Vandlen R, Simmons L, Foster J, Stephan JP, Tsai SP, Stewart TA. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145:2594–2603. doi: 10.1210/en.2003-1671. [DOI] [PubMed] [Google Scholar]
- Gaitonde S, Kohli R, Seeley R. The role of the gut hormone GLP-1 in the metabolic improvements caused by ileal transposition. J. Surg. Res. 2012;178:33–39. doi: 10.1016/j.jss.2011.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genet C, Strehle A, Schmidt C, Boudjelal G, Lobstein A, Schoonjans K, Souchet M, Auwerx J, Saladin R, Wagner A. Structure-activity relationship study of betulinic acid, a novel and selective TGR5 agonist, and its synthetic derivatives: Potential impact in diabetes. J. Med. Chem. 2010;53:178–190. doi: 10.1021/jm900872z. [DOI] [PubMed] [Google Scholar]
- Hofmann AF, Hagey LR. Bile acids: Chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol. Life Sci. 2008;65:2461–2483. doi: 10.1007/s00018-008-7568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst JJ, McGill MA. Potential new approaches to modifying intestinal GLP-1 secretion in patients with type 2 diabetes mellitus: Focus on bile acid sequestrants. Clin. Drug Investig. 2012;32:1–14. doi: 10.2165/11595370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Huang X, Yang C, Luo Y, Jin C, Wang F, McKeehan WL. FGFR4 prevents hyperlipidemia and insulin resistance but underlies high-fat diet induced fatty liver. Diabetes. 2007;56:2501–2510. doi: 10.2337/db07-0648. [DOI] [PubMed] [Google Scholar]
- Jain AK, Stoll B, Burrin DG, Holst JJ, Moore DD. Enteral bile acid treatment improves parenteral nutrition-related liver disease and intestinal mucosal atrophy in neonatal pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302:G218–G224. doi: 10.1152/ajpgi.00280.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL, Kliewer SA, Gonzalez FJ. Differential regulation of bile acid homeostasis by the farnesoid × receptor in liver and intestine. J. Lipid Res. 2007;48:2664–2672. doi: 10.1194/jlr.M700330-JLR200. [DOI] [PubMed] [Google Scholar]
- Kindel TL, Yoder SM, Seeley RJ, D’Alessio DA, Tso P. Duodenal-jejunal exclusion improves glucose tolerance in the diabetic, Goto-Kakizaki rat by a GLP-1 receptor-mediated mechanism. J. Gastrointest. Surg. 2009;13:1762–1772. doi: 10.1007/s11605-009-0912-9. [DOI] [PubMed] [Google Scholar]
- Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, Xu HE, Shulman GI, Kliewer SA, Mangelsdorf DJ. FGF19 as a postprandial, insulinin-dependent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuro-O M. Endocrine FGFs and Klothos: Emerging concepts. Trends Endocrinol. Metab. 2008;19:239–245. doi: 10.1016/j.tem.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Lan T, Rao A, Haywood J, Kock ND, Dawson PA. Mouse organic solute transporter alpha deficiency alters FGF15 expression and bile acid metabolism. J. Hepatol. 2012;57:359–365. doi: 10.1016/j.jhep.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Roux CW, Borg C, Wallis K, Vincent RP, Bueter M, Goodlad R, Ghatei MA, Patel A, Bloom SR, Aylwin SJ. Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Ann. Surg. 2010;252:50–56. doi: 10.1097/SLA.0b013e3181d3d21f. [DOI] [PubMed] [Google Scholar]
- Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- Li T, Holmstrom SR, Kir S, Umetani M, Schmidt DR, Kliewer SA, Mangelsdorf DJ. The G protein-coupled bile acid receptor, TGR5, stimulates gallbladder filling. Mol. Endocrinol. 2011;25:1066–1071. doi: 10.1210/me.2010-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2009;5:262–269. doi: 10.1038/nrendo.2009.48. [DOI] [PubMed] [Google Scholar]
- Lundasen T, Galman C, Angelin B, Rudling M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J. Intern. Med. 2006;260:530–536. doi: 10.1111/j.1365-2796.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- May CL, Kaestner KH. Gut endocrine cell development. Mol. Cell. Endocrinol. 2010;323:70–75. doi: 10.1016/j.mce.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencarelli A, Renga B, Migliorati M, Cipriani S, Distrutti E, Santucci L, Fiorucci S. The bile acid sensor farnesoid × receptor is a modulator of liver immunity in a rodent model of acute hepatitis. J. Immunol. 2009;183:6657–6666. doi: 10.4049/jimmunol.0901347. [DOI] [PubMed] [Google Scholar]
- Meschere AL. Junqueira’s basic histology: Text and atlas. 12th Edition. Columbus, OH: McGraw-Hill Companies, Inc.; 2010. [Google Scholar]
- Mingrone G, Panunzi S, De GA, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, Rubino F. Bariatric surgery versus conventional medical therapy for type 2 diabetes. New Engl. J. Med. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- Modica S, Petruzzelli M, Bellafante E, Murzilli S, Salvatore L, Celli N, Di TG, Palasciano G, Moustafa T, Halilbasic E, Trauner M, Moschetta A. Selective activation of nuclear bile acid receptor FXR in the intestine protects mice against cholestasis. Gastroenterology. 2012;142:355–365. doi: 10.1053/j.gastro.2011.10.028. [DOI] [PubMed] [Google Scholar]
- Parker HE, Wallis K, le Roux CW, Wong KY, Reimann F, Gribble FM. Molecular mechanisms underlying bile acid-stimulated glucagon-like peptide-1 secretion. Br. J. Pharmacol. 2012;165:414–423. doi: 10.1111/j.1476-5381.2011.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Bile acids: Natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- Pellicciari R, Costantino G, Camaioni E, Sadeghpour BM, Entrena A, Willson TM, Fiorucci S, Clerici C, Gioiello A. Bile acid derivatives as ligands of the farnesoid × receptor. Synthesis, evaluation, and structure-activity relationship of a series of body and side chain modified analogues of chenodeoxycholic acid. J. Med. Chem. 2004;47:4559–4569. doi: 10.1021/jm049904b. [DOI] [PubMed] [Google Scholar]
- Pellicciari R, Gioiello A, Macchiarulo A, Thomas C, Rosatelli E, Natalini B, Sardella R, Pruzanski M, Roda A, Pastorini E, Schoonjans K, Auwerx J. Discovery of 6alpha-ethyl-23(S)-methylcholic acid (S-EMCA, INT-777) as a potent and selective agonist for the TGR5 receptor, a novel target for diabesity. J. Med. Chem. 2009;52:7958–7961. doi: 10.1021/jm901390p. [DOI] [PubMed] [Google Scholar]
- Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: From feast to famine. Genes Dev. 2012;26:312–324. doi: 10.1101/gad.184788.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff MJ, Potts A, He T, Duarte JA, Taussig R, Mangelsdorf DJ, Kliewer SA, Burgess SC. Colesevelam suppresses hepatic glycogenolysis by TGR5-mediated induction of GLP-1 action in DIO mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;304:G371–G380. doi: 10.1152/ajpgi.00400.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D, Bekker JH, Ghatei MA, Bloom SR, Walters JR, Welbourn R, le Roux CW. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153:3613–3619. doi: 10.1210/en.2011-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey JB, Davies PW, Bristol JB, Williamson RC. Adaptation and carcinogenesis in defunctioned rat colon: Divergent effects of faeces and bile acids. Br. J. Cancer. 1983;48:477–484. doi: 10.1038/bjc.1983.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey JB, Maeda M, Williamson RC. The tropic effect of intrarectal deoxycholate on rat colorectum is unaffected by oral metronidazole. Cell Tissue Kinet. 1986;19:485–490. doi: 10.1111/j.1365-2184.1986.tb00747.x. [DOI] [PubMed] [Google Scholar]
- Raybould HE. Gut chemosensing: Interactions between gut endocrine cells and visceral afferents. Auton. Neurosci. 2010;153:41–46. doi: 10.1016/j.autneu.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybould HE, Glatzle J, Freeman SL, Whited K, Darcel N, Liou A, Bohan D. Detection of macronutrients in the intestinal wall. Auton. Neurosci. 2006;125:28–33. doi: 10.1016/j.autneu.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: A primary cell study. Cell Metab. 2008;8:532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo G, Passeri D, De FF, Ciaccioli G, Donadio L, Rizzo G, Orlandi S, Sadeghpour B, Wang XX, Jiang T, Levi M, Pruzanski M, Adorini L. Functional characterization of the semisynthetic bile acid derivative INT-767, a dual farnesoid × receptor and TGR5 agonist. Mol. Pharmacol. 2010;78:617–630. doi: 10.1124/mol.110.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland KJ, Brubaker PL. The “cryptic” mechanism of action of glucagon-like peptide-2. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;301:G1–G8. doi: 10.1152/ajpgi.00039.2011. [DOI] [PubMed] [Google Scholar]
- Rubino F, R’bibo SL, del Genio F, Mazumdar M, McGraw TE. Metabolic surgery: The role of the gastrointestinal tract in diabetes mellitus. Nat. Rev. Endocrinol. 2010;6:102–109. doi: 10.1038/nrendo.2009.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KK, Kohli R, Gutierrez-Aguilar R, Gaitonde SG, Woods SC, Seeley RJ. Fibroblast growth factor-19 action in the brain reduces food intake and body weight and improves glucose tolerance in male rats. Endocrinology. 2013;154:9–15. doi: 10.1210/en.2012-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage AP, Sian MS, Matthews JL, Bloom SR, Cooke T. Experimental colonic carcinogenesis: Changes in faecal bile acids after promotion of intestinal tumours by small bowel resection in the rat. Gut. 1988;29:495–502. doi: 10.1136/gut.29.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Q, Liu MK, Saumoy M, Holst JJ, Salen G, Xu G. The combination of colesevelam with sitagliptin enhances glycemic control in diabetic ZDF rat model. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302:G815–G823. doi: 10.1152/ajpgi.00295.2011. [DOI] [PubMed] [Google Scholar]
- Shang Q, Saumoy M, Holst JJ, Salen G, Xu G. Colesevelam improves insulin resistance in a diet-induced obesity (F-DIO) rat model by increasing the release of GLP-1. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298:G419–G424. doi: 10.1152/ajpgi.00362.2009. [DOI] [PubMed] [Google Scholar]
- Stoll B, Horst DA, Cui L, Chang X, Ellis KJ, Hadsell DL, Suryawan A, Kurundkar A, Maheshwari A, Davis TA, Burrin DG. Chronic parenteral nutrition induces hepatic inflammation, steatosis, and insulin resistance in neonatal pigs. J. Nutr. 2010;140:2193–2200. doi: 10.3945/jn.110.125799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll B, Puiman PJ, Cui L, Chang X, Benight NM, Bauchart-Thevret C, Hartmann B, Holst JJ, Burrin DG. Continuous parenteral and enteral nutrition induces metabolic dysfunction in neonatal pigs. JPEN J. Parenter. Enteral Nutr. 2012;36:538–550. doi: 10.1177/0148607112444756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 2008;7:678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- Tomlinson E, Fu L, John L, Hultgren B, Huang X, Renz M, Stephan JP, Tsai SP, Powell-Braxton L, French D, Stewart TA. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology. 2002;143:1741–1747. doi: 10.1210/endo.143.5.8850. [DOI] [PubMed] [Google Scholar]
- Unger RH, Ohneda A, Valverde I, Eisentraut AM, Exton J. Characterization of the responses of circulating glucagon-like immunoreactivity to intraduodenal and intravenous administration of glucose. J. Clin. Invest. 1968;47:48–65. doi: 10.1172/JCI105714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Goudoever JB, Stoll B, Hartmann B, Holst JJ, Reeds PJ, Burrin DG. Secretion of trophic gut peptides is not different in bolus- and continuously fed piglets. J. Nutr. 2001;131:729–732. doi: 10.1093/jn/131.3.729. [DOI] [PubMed] [Google Scholar]
- Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- Xiao Q, Boushey RP, Drucker DJ, Brubaker PL. Secretion of the intestinotropic hormone glucagon-like peptide 2 is differentially regulated by nutrients in humans. Gastroenterology. 1999;117:99–105. doi: 10.1016/s0016-5085(99)70555-x. [DOI] [PubMed] [Google Scholar]