Abstract
Introduction
Targeting pathogenetic mechanisms rather than essential processes represents a very attractive alternative for the development of new antibiotics. This may be particularly important in the case of antimycotics, due to the urgent need for novel antifungal drugs and the paucity of selective fungal targets. The opportunistic pathogenic fungus Candida albicans is the main etiological agent of candidiasis, the most common human fungal infection. These infections carry unacceptably high mortality rates, a clear reflection of the many shortcomings of current antifungal therapy, including the limited armamentarium of antifungal agents, their toxicity, and the emergence of resistance. Moreover the antifungal pipeline is mostly dry.
Areas covered
This review covers some of the most recent progress towards understanding C. albicans pathogenetic processes and how to harness this information for the development of anti-virulence agents. The two principal areas covered are filamentation and biofilm formation, as C. albicans pathogenicity is intimately linked to its ability to undergo morphogenetic conversions between yeast and filamentous morphologies and to its ability to form biofilms.
Expert opinion
We argue that filamentation and biofilm formation represent high value targets, yet clinically unexploited, for the development of novel anti-virulence approaches against candidiasis. Although this has proved a difficult task despite increasing understanding at the molecular level of C. albicans virulence, we highlight new opportunities and prospects for antifungal drug development targeting these two important biological processes.
Keywords: Antifungals, candidiasis, Candida albicans, virulence factors, targeting virulence, filamentation, biofilms
1. Introduction: Candida and candidiasis
In the last few decades fungal infections are being increasingly recognized as a major health threat to an ever-expanding population of compromised patients 1. Of these, and without question, infections caused by Candida species represent the main cause of opportunistic fungal infections worldwide, leading to significant morbidity and mortality, and Candida albicans remains the most common etiological agent of candidiasis 2. C. albicans is part of the normal human microbiota; it is usually acquired early in neonatal life and as a normal commensal that colonizes mucosal surfaces, particularly those of the skin, gastrointestinal tract (including the oral cavity) and genitourinary tract, produces little to no damage to the host 3. However, in immune- or otherwise compromised patients, and depending on the underlying host defect, it is able to cause a variety of infections that range from mucosal to life-threatening invasive candidiasis, having the ability to infect virtually every organ in the host 2. The potential list of predisposing factors for candidiasis is extensive and includes immunosuppressive therapy, broad spectrum antibiotics, mucosal or cutaneous barrier disruption, cytotoxic therapy, intravenous catheters and indwelling devices, parenteral nutrition, very low birth weight, aging, AIDS, diabetes, transplantation medicine and drug abuse, among others 2. For example, oropharyngeal candidiasis remains the most common oral manifestation of human immunodeficiency virus (HIV) infection, and it is also frequent in head and neck cancer patients as well as transiently in individuals treated with antibiotics or corticosteroids 4–6. Vulvovaginal candidiasis (and also recurrent vulvovaginal candidiasis) affects a significant number of women during their childbearing age 7. But perhaps most importantly, Candida species are now the third-fourth most common organism to be isolated from the bloodstream of hospitalized patients 8. The incidence of systemic candidiasis in the US is approximately 20 cases per 100,000 people (or about 60,000 cases per year) and in high risk hospitalized patients this incidence increases by a factor of 50. Of note, these rates represent a 20-fold increase compared with just two decades ago. The seriousness of this problem is heightened by the fact that, even with treatment using available antifungal agents, mortality rates lie in the 30–50% range for these infected patients 2, 9, 10. In fact, in a study designed specifically to identify microbiological factors influencing the outcome of infection, among the top ten pathogens Candida was associated with the overall highest attributable mortality and was the only pathogen identified as an independent determinant of the risk of death 11. Of course, together with the high mortality, these devastating infections also come accompanied by a significant added cost to our health care system. For example, in pediatric patients, candidiasis is associated with a 10.0% increase in mortality, a mean 21.1-day increase in length of stay, and a mean increase in total per-patient hospital charges of $92,266. Similarly, in adult patients candidiasis is associated with a 14.5% increase in mortality, a mean 10.1-day increase in length of stay, and a mean increase in hospital charges of $39,331 12. The total estimated direct cost of candidiasis to the US health care system was approximately $2–4 billion annually in the year 2000 13, and it is probably much higher now.
2. Antifungal drug therapy and antifungal drug resistance
Clearly, the unacceptably high morbidity and mortality rates associated with candidiasis indicate that current antifungal therapy to combat candidiasis is still ineffective. In stark contrast with antibacterial antibiotics, the current arsenal of antifungal drugs is exceedingly short. Moreover, there are no new effective drugs in sight, and the antifungal pipeline is mostly dry, so that no new antifungal drugs are expected to reach the market any time soon 14. The future seems even more pessimistic as big pharmaceutical companies are mostly focusing their efforts on drugs treating chronic conditions typically associated with the sedentary lifestyle, at the expense of the much less “profitable” antibiotics. Antifungal drug development is further complicated by the fact that fungal cells are eukaryotic and thus it is much more difficult to identify selective pathogen-specific targets for drug discovery and development. Interestingly, this is also the main reason for the elevated toxicity of some of the current therapies. Strictly from a clinical point of view to combat candidiasis, the current options are limited to three classes of antifungal agents: polyenes, azoles and echinocandins 15. For example, amphotericin B, a broad-spectrum polyene that binds to ergosterol and compromises membrane integrity, remained the “gold standard” of antifungal therapy during decades after its introduction in the 1950s; but its efficacy is severely limited by its inherent toxicity, particularly nephrotoxicity. Azoles, and more specifically triazoles (i.e. fluconazole), which are fungistatic drugs that inhibit ergosterol biosynthesis, were developed in the 1980s and 1990s; however a major problem has been the emergence of resistance (including cross-resistance against multiple azole derivatives), mostly through the development of point mutations on the target enzyme (lanosterol demethylase) or by overexpression of efflux pumps 16. Also, formation of biofilms, associated with a high percentage of candidiasis (see below), renders azoles completely ineffective, as sessile cells within these biofilms display high levels of resistance (up to 1,000 times higher) against azole antifungal agents 17, 18. Finally, the new millennium brought a novel class of antifungals to the market, the echinocandins (i.e. caspofungin). These semisynthetic lipopeptide antibiotics inhibit the synthesis of 1,3-β-D-glucan, a key structural component of the fungal cell wall, and are therefore the first class of antifungal drugs that act against a specific component of the fungal organisms not present in mammalian (host) cells. Although they represent a welcome addition to the antifungal arsenal due to their excellent safety profile, emergence of resistance, mostly due to mutation in the FKS1 gene encoding the target enzyme, glucan synthase 19, 20, is becoming a major problem despite their relatively recent introduction into the clinics. Unfortunately, echinocandins are only available as intravenous formulations so that their use in normally limited to the treatment of the most serious invasive forms of candidiasis.
3. Targeting virulence: a new paradigm, yet mostly unexploited, for antibiotic drug development
Conventional antibiotics, including antifungal agents, target cell viability, either by killing (-cidal) or by inhibiting growth (-static). These modes of action impose a high degree of selective pressure that ultimately fosters the growth of antibiotic-resistant strains 21. Indeed, since the introduction of penicillin, clinically significant antibiotic resistance has evolved against literally every single antibiotic ever deployed. Thus, there is an urgent need for antibiotics with novel modes of action. An attractive alternative approach, aided by our increased understanding of microbial pathogenesis, is to target functions essential for infection such as virulence factors required to cause damage to the host 21, 22. Virulence is generally defined as the ability of a pathogen to cause overt disease, and virulence determinants as microbial factors or processes that actively cause damage to host tissues 23, 24. The main advantages of targeting virulence are: i) the expansion of the number of potential targets, which is of particular interest for the development of novel antifungals as mentioned above, ii) the preservation of the host microbiome, of critical importance in the case of normal commensals such as C. albicans, and iii) exerting weaker selective pressure for the development of antibiotic resistance 21. Although the use of such approaches is still unclear, anti-virulence agents could potentially be used as monotherapy, as combination therapy (with conventional antibiotics) or as prophylactic agents. One consideration is that, since virulence is often pathogen-specific, anti-virulence drugs would normally display narrow spectrum of action against the single pathogen against which they were developed and would necessitate an accurate and rapid diagnosis prior to their use 21, which at the moment could be problematic for candidiasis due to the lack of rapid and accurate diagnostic tests.
4. Filamentation and biofilm formation are important virulence factors in C. albicans and represent high value targets for the development of novel antifungal agents
The pathogenicity of C. albicans is multifactorial and, as an opportunistic pathogen, results from a very delicate balance between its intrinsic virulence attributes and mechanisms of host immunity 23–27. This gives rise to the highly complex nature of host-fungus interactions which ultimately determines the outcome of infection. In the past, C. albicans virulence has been associated with production of extracellular hydrolytic enzymes, phenotypic switching, antigenic variability and molecular mimicry 28–31. However, currently filamentation and the ability to form biofilms are being increasingly recognized as the main virulence factors contributing to the pathogenesis of candidiasis. Thus, the following sections of this review will focus on these two important biological processes, their role in the pathogenesis of C. albicans infections, and potential strategies to target them for the development of new therapies against candidiasis.
4.1. Targeting C. albicans filamentation for antifungal drug development
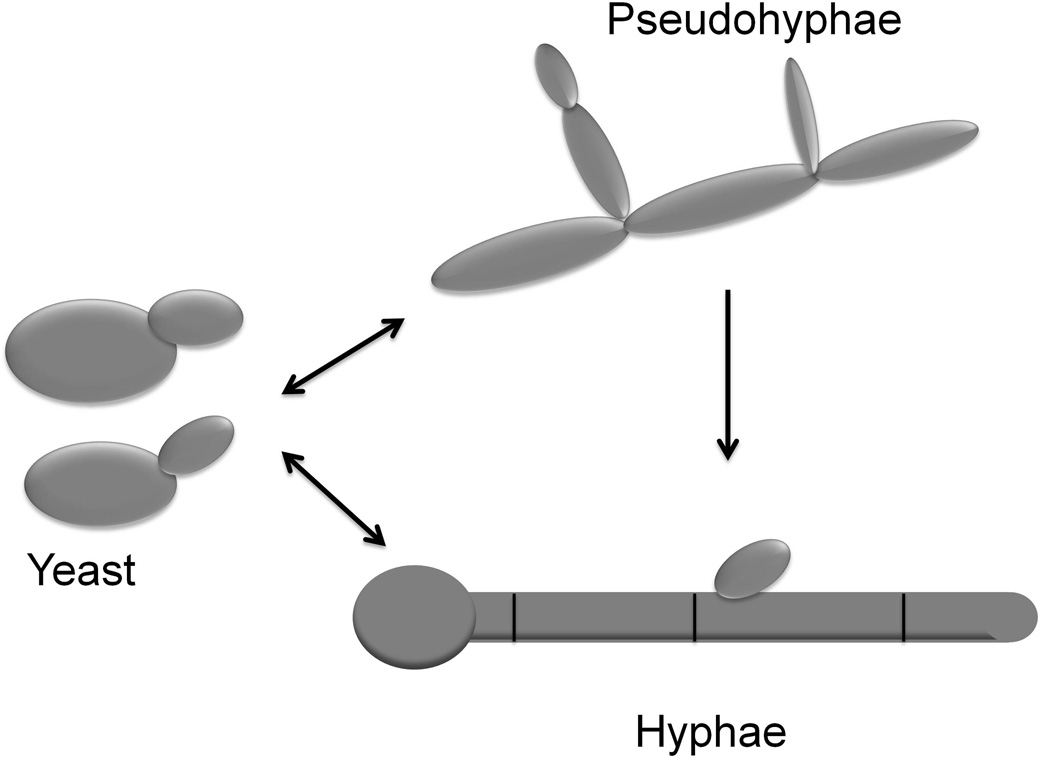
C. albicans is a polymorphic fungus and is able to undergo reversible morphological transitions between yeast and filamentous forms 28, 32, 33 (Figure 1). Round budding-yeast cells can be induced to form true hyphae, which grow by continuous apical extension followed by septation, whereas pseudohyphae grow by unipolar budding where buds develop into elongated cells, which remain attached to mother cells. Filamentation not only represents a virulence trait per se, but it is also coordinately regulated with other virulence factors, which are associated with cellular morphology 29, 31, 34. Morphogenetic transitions occur in response to external stimuli including body temperature (37°C), serum, neutral pH, amino acids, nutrients such as N-Acetyl glucosamine, embedded/microaerophilic conditions, and certain human hormones: these factors presumably reflect the variety of signals detected by the fungus in the different microenvironments it encounters within the human host. Thus, the activity of signaling pathways, including key transcriptional regulators, have been investigated to elucidate the molecular mechanisms involved in morphogenetic conversions. From studies to date, it is clear that this is a complex and highly orchestrated process, as signaling pathways may converge on separate or identical transcription factors and transcription factors may converge themselves on common target genes to trigger expression of hypha-specific genes 35. Filamentation is subject to both positive and negative regulation. Key C. albicans genes involved in positive regulation of filamentation include CPH1, EFG1, HGC1, and UME6, induced by multiple host environmental cues and involved in hyphal extension. Negative regulation of filamentation is mainly dependent upon C. albicans TUP1, a general transcriptional repressor molecule. Also, CaNrg1p is a DNA-binding protein containing a zinc-finger domain which also functions as a negative regulator of filamentation together with Tup1p. 36–44.
Figure 1.
Schematic diagram representing C. albicans morphologies and morphogenetic conversions.
These reversible morphological transitions contribute to disease establishment and progression. While yeast cells are probably disseminated more effectively, filamentous forms are better adapted to penetrate and damage tissue, ultimately causing death 43, 45, 46. Early evidence for the major role of filamentation in C. albicans virulence came primarily from experiments using genetically defined mutant strains, constructed by gene disruption, that are locked in the yeast morphology (i.e. Δefg1 and Δefg1/Δcph1), – these strains are unable to filament and displayed attenuated virulence in murine models of disseminated candidiasis 47. More recently, further evidence for the important role of morphogenetic conversions in virulence has been provided by the use of genetically engineered strains using the tetracycline regulatable promoter system 48, 49. In these strains, morphogenetic conversions can be easily manipulated within the animals, and using the C. albicans tet-UME6 and tet-NRG1 strains it was demonstrated both increased virulence associated with increased filamentation, a well as decreased mortality (100% survival) when filamentation is not allowed to occur within infected tissues 40, 43. Moreover, the authors took full advantage of the manipulability of the tet-NRG1 regulatable strain (cells of this strain are not “locked” in one form but rather morphology is controlled by the presence or absence of doxycycline in the animals’ drinking water) to provide “proof of concept” that inhibition of filamentation represents an attractive target for the development of new antifungal drugs. To this end, mice receiving doxycycline were injected with the C. albicans tet-NRG1 strain, leading to the establishment of infection. The antibiotic was either administered throughout the experiment or discontinued at 24 or 48 hours post-infection to mimic a therapeutic intervention with an “anti-filamentation agent”. Results indicated that doxycycline removal resulted in the conversion of the fungal cells infecting the tissues into a yeast morphology, as compared to mostly filamentous morphology in control animals continuously kept on doxycycline. Moreover, removal of the antibiotic also led a dramatic increase in survival 50. However, as with conventional antifungal drug therapy, the mortality increased markedly the longer this intervention (removal of doxycyline) was delayed. Importantly, this timeline of treatment initiation and resulting survival rates closely mirrors those seen in animal models using any of the three major classes of anti-fungal drugs currently available. Thus, these results represented compelling evidence for the importance of filamentation in the progression to active disease, and provided genetic validation of this pivotal physiological/cellular process as a target for the development of a novel class of antifungal agents for the prevention and treatment of candidiasis.
However, as mentioned above and despite this increased knowledge of C. albicans filamentation at the molecular level, until now it has not been possible to harness all this information for the development of new drugs for the treatment of candidiasis (or any other fungal infection). Certainly, an increasing number of small molecules are being reported that are able to modulate morphogenetic conversions and inhibit filamentation. These include, among others, bacterial and fungal regulators of the C. albicans yeast-to-hyphae transition (such as phenazines and homoserine lactones from Pseudomonas aeruginosa, mutanobactins from Streptococcus mutans, and capric acid secreted by Saccharomyces boulardii), farnesol and other autoregulatory alcohols which act as quorum sensing molecules produced by C. albicans itself, retigeric acid, bisbibenzyls 51–58. Using a small molecule screen the Jonhson group identified up to 21 different inhibitors of C. albicans filamentation and subsequently demonstrated that some of these inhibitors act through different signaling pathways 59, 60. However, it needs to be noted that the great majority of this work has been in vitro and there is an overall lack of evidence for the effects of the same compounds in vivo, using relevant models of infection 61, also with some general concerns about potency and potential toxicity for their eventual development as antifungal agents. Moreover, in the very rare instances that some of these compounds have been tested in vivo, results to date have been mostly marginal or negative 61. For example exogenous administration of farnesol, which in vitro prevents the C. albicans yeast-to-mycelium conversion, surprisingly increased virulence in the murine model of hematogenously disseminated candidiasis 62. On the other hand, mice injected intraperitoneally with a cocktail solution simulating the composition of alcohols present in a C. albicans culture supernatant (which includes farnesol) demonstrated increased survival and decreased organ fungal burden in a similar model of candidiasis 63.
One additional advantage of inhibiting filamentation in C. albicans is the ensuing modulation of host immune responses, which may be critical for resolution of infection. A picture has emerged in the last few years indicating that different cells of the immune system (particularly dendritic cells, monocytes, macrophages and neutrophils) and also epithelial cells sense yeast and filamentous cells in distinct ways, resulting in induction of qualitatively and quantitatively different innate and adaptive immune responses: in general exposure to the yeast form results in a protective response, whereas exposure to filamentous forms results in non-protective immunity, even contributing to pathogenicity 64, 65. For example, mucosal epithelial cells are able to discriminate between the yeast and filamentous forms, and an exacerbated inflammatory response is associated with C. albicans filamentation which contributes to the pathology of vaginal and oral candidiasis 66, 67. At the systemic level, filamentation is of critical importance for escape from phagocytes and overall immune evasion, and is associated with the triggering of an acute phase response leading to an overly inflammatory state which is deleterious to the host 68, 69. Thus, one very intriguing possibility is that treatment with an anti-filamentation compound will also benefit the host by modulating immune responses. In fact, from an immunological point of view, treatment with an anti-virulence compound may even result in a very similar scenario to that observed when using live attenuated vaccines.
4.2. Targeting C. albicans biofilm formation for antifungal drug development
Many microbes in their natural habitats are found as attached to surfaces and not as free-floating (planktonic) organisms, and as such biofilms represent one of the evolving paradigms of modern Microbiology 70, 71. Biofilms are defined as structured microbial communities that are attached to a surface and encased in a matrix of exopolymeric material. These communities of cells have the potential to initiate or prolong infections by providing a safe haven from which cells can invade local tissue, seed new infection sites and resist eradication efforts. It is now estimated that 60–80% of all human microbial infections involve biofilm formation 72–74. C. albicans remains the fungal species most commonly associated with the formation of biofilms and indeed, most clinical manifestations of candidiasis are linked to biofilm formation 75–79. Indeed, the increase in candidiasis in the last few decades has virtually paralleled the increase and widespread use of a broad range of medical implant devices, such as stents, shunts, prostheses, implants, endotracheal tubes, pacemakers and various types of catheters, mainly in populations with impaired host defenses. Strikingly, yeasts (mainly C. albicans) are the third leading cause of catheter-related infections 80. Studies of catheter-related candidiasis have unequivocally shown that retention of vascular catheters is associated with prolonged fungemia, high antifungal therapy failure rates, increased risk of metastatic complications and death 73, 77, 81, 82. Other manifestations, such as oropharyngeal candidiasis, denture stomatitis and even vaginal candidiasis, are also associated with C. albicans biofilm formation. From the clinical perspective, the most notable feature of C. albicans biofilms is their high levels of resistance to conventional antifungal therapy. Several groups have unequivocally demonstrated that the Candida biofilm life-style leads to dramatically increased levels of resistance (up to 1,000 times) to most clinically used antifungal agents, particularly azoles and polyenes 76, 78. The net effect is that Candida biofilms adversely impact the health of these patients, with increasing frequency and severity, and with soaring economic sequelae 17, 18, 75, 77.
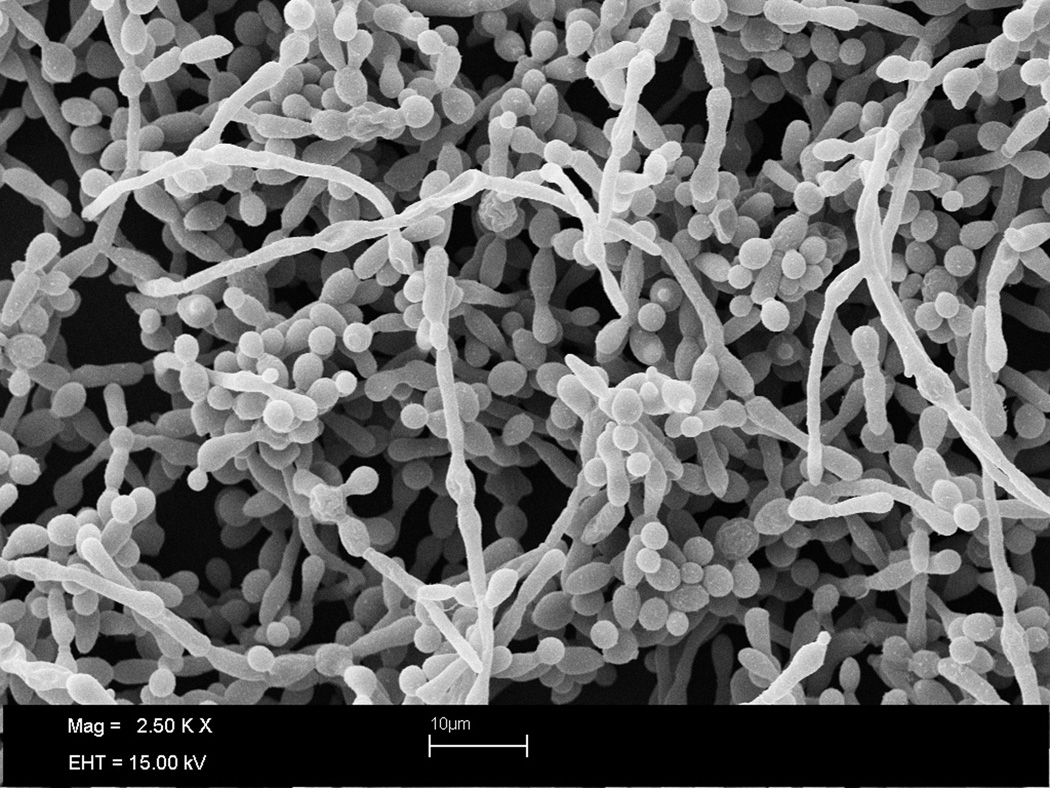
Biofilm formation in C. albicans is a complex multistep process that occurs through a series of developmental stages 83, 84. These include initial attachment to a biological or inert surface, closely followed by cell division, proliferation, and biofilm maturation with production of exopolymeric matrix. Also, cells are dispersed from biofilms, which represents the return of detached microorganisms to a planktonic mode of growth allowing re-colonization of new surfaces, thereby completing the biofilm life-cycle 85. Filamentation and cell-cell communication (quorum sensing) play pivotal roles in the C. albicans biofilm mode of growth 86–88. Similar to filamentation, biofilm development occurs in response to distinct environmental cues and is controlled, at the molecular level, by a complex regulatory network 84, 89. Figure 2 shows a mature C. albicans biofilm.
Figure 2.
Scanning electron microphotograph depicting a mature C. albicans biofilm.
As mentioned above, C. albicans cells within biofilms display high levels of resistance to conventional antifungal therapy, which clearly indicates the urgent medical, but also economical, need for new antifungal agents and strategies. These include both preventative (i.e. inhibition of biofilm formation) as well as therapeutic (i.e. against pre-formed biofilms) approaches to combat biofilm-associated infections. These strategies may be as diverse as development of biomaterials which do not support C. albicans biofilm growth, catheter coatings and lock solutions (use of suprapharmacological concentrations of antifungals locally inside the catheter) 90, 91; but also involve the search for new molecules active against cells within biofilms. Indeed this is currently an area of very active research, which has been greatly facilitated by the development of simple, inexpensive, accurate, robust and reliable microtiter plate-based models for the formation and antifungal susceptibility testing of Candida biofilms 92. These efforts include the use of calcineurin inhibitors (i.e. cyclosporine, FK506) and hsp90 inhibitors (i.e. geldanamycin) to overcome biofilm drug resistance 93, 94, the examination of anti-biofilm activity of a variety of natural products (reviewed in 95), as well as screening of small molecule compounds present in different chemical libraries. For example, LaFleur and colleagues performed primary screens for potentiators of the anti-biofilm activity of clotrimazole (an azole antifungal which per se is not effective against C. albicans biofilms) 96. Most recently our group performed a comprehensive screen of a small molecule library consisting of 1,200 off-patent drugs already approved by the Food and Drug Administration (FDA), the Prestwick Chemical Library, to search for inhibitors of C. albicans biofilm formation: identification of several inhibitory compounds without previously characterized antifungal activity indicates that repurposing FDA-approved drugs may open up a valuable new avenue for identification and rapid development of antifungal agents 97. It can also be possible to target the biofilm matrix; for example Martins and colleagues demonstrated that addition of DNase (as extracellular DNA is a component of the C. albicans biofilm matrix) improves the anti-biofilm activity of some antifungal drugs 98, 99. Another possible strategy, particularly in the case of catheter-associated biofilm infections, is to target dispersion, as cells dispersed from the biofilms are responsible for dissemination, extravasation and establishment of deep-seated candidiasis at distal organs 85. We also note here that because the intimate link between filamentation and biofilms, drugs which modulate C. albicans morphogenetic conversions (as discussed above) have also the potential to inhibit biofilm development; and the same is true for modulators of quorum sensing mechanisms.
One of the major bottlenecks in the development of newer antibiotics, including antifungals, has been the fact that conventional microbiological culture techniques are mostly incompatible with modern methodologies for drug discovery that are dominated by HTS and its “hunger for speed”. Thus, to overcome this impediment Srinivasan et al. recently described the development of a high-throughput microarray based technology for the formation of C. albicans biofilms 100. The resulting chip, called CaBChip (for Candida albicans Biofilm Chip), consisted of 768 spatially distinct and equivalent nano-biofilms, each with a volume of approximately 50 nanoliters, on a standard microscope glass slide. Despite a near 2,000-fold miniaturization, the nanoscale biofilms on the CaBChip displayed phenotypic properties (i.e. morphological and architectural characteristics and increased drug resistance) comparable to biofilms formed using the conventional 96-well microtiter plate model. The nanobiofilm chip enables rapid and easy handling, is amenable to automation and is fully compatible with standard microarray technology and equipment. Moreover, it minimizes manual labor, cuts reagents use and drastically reduces assay costs. As such, this new technology platform for C. albicans biofilms should allow for true high-throughput screening in search for new anti-biofilm drugs, and should accelerate the antifungal drug discovery process by enabling rapid, convenient and inexpensive screening of hundreds-to-thousands of compounds simultaneously.
5. Conclusion
The high morbidity and mortality rates associated with candidiasis, the most common fungal infection, clearly indicate that there is an urgent and unmet need for the development of new antifungal agents; but this is complicated by the paucity of selective targets. Rather than inhibiting growth or killing the microorganism, which exerts high selective pressure and may lead to the emergence of resistance, targeting virulence represents a prosperous alternative for the development of new antifungals with novel modes of action, which may be used alone and/or in combination with current antimycotics. C. albicans filamentation and biofilm formation are inextricably linked to the pathogenesis of candidiasis. As we gain a better understanding of these two complex biological processes, it is conceivable that they could eventually be targeted pharmacologically for the prevention and treatment of these fastidious infections.
6. Expert opinion
The unacceptably high morbidity and mortality rates associated with candidiasis, the most frequent human fungal infection, point to the fact that current antifungal therapy is still ineffective, as on a daily basis clinicians are confronted with almost unsurmountable obstacles such as the exceedingly short armamentarium of antifungal agents, the toxicity displayed by some of the current therapies and the emergence of resistance to most classes of antifungals. These problems point to an urgent need for the development of novel antifungals against these devastating infections; however, the antifungal pipeline is eminently dry as pharmaceutical companies are focusing their attention to most “profitable” types of drugs to combat chronic diseases associated with the sedentary life style. The fact that fungi are eukaryotic (similar to our own cells) translates into a paucity of potential targets and poses difficult challenges to antifungal drug development. Rather than killing the pathogen or arresting its growth, targeting virulence mechanisms associated with the pathogenesis of infection represents a very attractive and auspicious alternative for the development of new antifungal agents. In the case of candidiasis, and particularly C. albicans, this should be facilitated by the increasing knowledge of its pathogenetic mechanisms, including at the molecular level. For example, in the last decade the completion of the C. albicans genome sequencing project, and the subsequent entry into the post-genome era, have revolutionized the studies on the basic biology of this organism: from comparative and functional genomics and large scale mutant generation, to proteomics and implementation of new and powerful molecular approaches to dissect the biology and pathogenicity of C. albicans; or the so called “explosion of molecular biology” in the field of candidiasis 84, 101, 102. However, it has been difficult to translate basic science discoveries into new treatments for candidiasis and the paradox here is that during the same time infection rates for candidiasis have increased substantially and morbidity and mortality rates have remained basically unchanged. Thus, as it has happened in virtually every other scientific discipline, in the case of candidiasis the accompanying hope of new antifungals based on the promise of genomics and the increased knowledge at the molecular level has not yet materialized into any new drug. For example, a majority of journal articles and grant submissions on C. albicans basic research almost invariably claim that the work could lead to the identification of new targets and the development of new antifungals: not to rain on the parade but none, in fact, has done so. Indeed, the only new class of antifungals introduced during this time has been the echinocandins, which originated from “classical” screens by pharmaceutical companies of natural products from soil microorganisms 103.
From the point of view of future development of C. albicans anti-virulence agents, perhaps targeting filamentation and biofilm formation holds the most promise; as these two complex biological processes are inextricably linked to the pathogenesis of candidiasis (including during both mucosal and invasive disease). Certainly, these are also two areas of very active research, with literally hundreds of groups around the globe studying these processes, both at the molecular level but also with clear translational impetus. Hopefully these efforts may crystallize, in a not so distant future, in the development of novel classes of antifungal agents to add and/or complement the current antifungal arsenal. To conquer this formidable challenge, and because of time and budget considerations, without any question this will necessitate a concerted effort and the establishment of partnerships between basic and clinical scientists, public health specialists, governmental and other funding agencies, as well as biotechnology and pharmaceutical companies. This should ultimately save the lives of countless number of patients, while at the same time reduce associated costs to our health care system.
ARTICLE HIGHLIGHTS BOX.
Candidiasis is the most frequent fungal infection affecting an increasing number of compromised patients. Candida albicans remains the main causative of candidiasis and these infections are associated with unacceptably high morbidity and mortality rates.
Current antifungal therapy is limited by the short arsenal of antifungal drugs, toxicity problems and the emergence of resistance. Moreover, the antifungal drug pipeline is mostly dry.
Since fungal cells are eukaryotic, there is a paucity of targets that can be used for antifungal drug development. Thus, rather than killing or inhibiting growth, an attractive alternative is to target virulence.
In C. albicans, filamentation and biofilm formation are being increasingly recognized as two major virulence factors inextricably linked to the pathogenesis of candidiasis. As such, they represent high value targets for the development of novel antifungal agents.
Successful development of new antifungals will necessitate a concerted effort and the establishment of partnerships between basic researchers and clinicians, funding and governmental agencies, biotechnology and pharmaceutical companies.
Acknowledgements
Biofilm-related work in the laboratory is funded by Grant numbered 1R01DE023510 from the National Institute of Dental & Craniofacial Research (to J.L.L.-R.), and by the Army Research Office of the Department of Defense under Contract No. W911NF-11-1-0136. CGP acknowledges the receipt of a predoctoral fellowship from American Heart Association, numbered 51PRE30004. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCR, the NIH, AHA or the DoD.
Bibliography
- 1. Brown GD, Denning DW, Gow NA, et al. Hidden killers: human fungal infections. Sci Transl Med. 2012 Dec 19;4(165):165rv13. doi: 10.1126/scitranslmed.3004404. **Highly contemporary review to try to bring increased attention to the often disregarded problem of fungal infections
- 2. Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007 Jan;20(1):133–163. doi: 10.1128/CMR.00029-06. * An excellent overview of the epidemiology of candidiasis
- 3.Calderone RA. Candida and Candidiasis. Washington: ASM Press; 2002. [Google Scholar]
- 4.Lopez-Martinez R. Candidosis, a new challenge. Clin Dermatol. 2010 Mar 4;28(2):178–184. doi: 10.1016/j.clindermatol.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Redding SW, Zellars RC, Kirkpatrick WR, et al. Epidemiology of oropharyngeal Candida colonization and infection in patients receiving radiation for head and neck cancer. J Clin Microbiol. 1999 Dec;37(12):3896–3900. doi: 10.1128/jcm.37.12.3896-3900.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson GR, 3rd, Patel PK, Kirkpatrick WR, et al. Oropharyngeal candidiasis in the era of antiretroviral therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010 Apr;109(4):488–495. doi: 10.1016/j.tripleo.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fidel PL., Jr Vaginal candidiasis: review and role of local mucosal immunity. AIDS Patient Care STDS. 1998 May;12(5):359–366. doi: 10.1089/apc.1998.12.359. [DOI] [PubMed] [Google Scholar]
- 8.Edmond MB, Wallace SE, McClish DK, et al. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin Infect Dis. 1999 Aug;29(2):239–244. doi: 10.1086/520192. [DOI] [PubMed] [Google Scholar]
- 9.Gudlaugsson O, Gillespie S, Lee K, et al. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis. 2003 Nov 1;37(9):1172–1177. doi: 10.1086/378745. [DOI] [PubMed] [Google Scholar]
- 10.Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009 Mar 1;48(5):503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pittet D, Li N, Woolson RF, et al. Microbiological factors influencing the outcome of nosocomial bloodstream infections: a 6-year validated, population-based model. Clin Infect Dis. 1997 Jun;24(6):1068–1078. doi: 10.1086/513640. [DOI] [PubMed] [Google Scholar]
- 12.Zaoutis TE, Argon J, Chu J, et al. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis. 2005 Nov 1;41(9):1232–1239. doi: 10.1086/496922. [DOI] [PubMed] [Google Scholar]
- 13.Wilson LS, Reyes CM, Stolpman M, et al. The direct cost and incidence of systemic fungal infections. Value Health. 2002 Jan-Feb;5(1):26–34. doi: 10.1046/j.1524-4733.2002.51108.x. [DOI] [PubMed] [Google Scholar]
- 14.Ostrosky-Zeichner L, Casadevall A, Galgiani JN, et al. An insight into the antifungal pipeline: selected new molecules and beyond. Nat Rev Drug Discov. 2010 Sep;9(9):719–727. doi: 10.1038/nrd3074. [DOI] [PubMed] [Google Scholar]
- 15.Odds FC, Brown AJ, Gow NA. Antifungal agents: mechanisms of action. Trends Microbiol. 2003 Jun;11(6):272–279. doi: 10.1016/s0966-842x(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 16.Perea S, Lopez-Ribot JL, Kirkpatrick WR, et al. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2001 Oct;45(10):2676–2684. doi: 10.1128/AAC.45.10.2676-2684.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhn DM, Ghannoum MA. Candida biofilms: antifungal resistance and emerging therapeutic options. Curr Opin Investig Drugs. 2004 Feb;5(2):186–197. [PubMed] [Google Scholar]
- 18.Ramage G, Vande Walle K, Wickes BL, et al. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother. 2001 Sep;45(9):2475–2479. doi: 10.1128/AAC.45.9.2475-2479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez S, Lopez-Ribot JL, Najvar LK, et al. Caspofungin resistance in Candida albicans: correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive Candida esophagitis. Antimicrob Agents Chemother. 2004 Apr;48(4):1382–1383. doi: 10.1128/AAC.48.4.1382-1383.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiederhold NP, Grabinski JL, Garcia-Effron G, et al. Pyrosequencing to detect mutations in FKS1 that confer reduced echinocandin susceptibility in Candida albicans. Antimicrob Agents Chemother. 2008 Nov;52(11):4145–4148. doi: 10.1128/AAC.00959-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clatworthy AE, Pierson E, Hung DT. Targeting virulence: a new paradigm for antimicrobial therapy. Nat Chem Biol. 2007 Sep;3(9):541–548. doi: 10.1038/nchembio.2007.24. ** Exquisitely well written review and an excellent resource on the topic of targeting virulence; with a major focus on antibacterial antibiotics
- 22.Gauwerky K, Borelli C, Korting HC. Targeting virulence: a new paradigm for antifungals. Drug Discov Today. 2009 Feb;14(3–4):214–222. doi: 10.1016/j.drudis.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 23. Casadevall A, Pirofski LA. The damage-response framework of microbial pathogenesis. Nat Rev Microbiol. 2003;1(1):17–24. doi: 10.1038/nrmicro732. ** A well thought and somewhat provocative theory of microbial pathogenesis that takes into account both microbial virulence and host response
- 24.Casadevall A, Pirofski LA. Microbial virulence results from the interaction between host and microorganism. Trends Microbiol. 2003;11(4):157–158. doi: 10.1016/s0966-842x(03)00008-8. author reply 58-9. [DOI] [PubMed] [Google Scholar]
- 25.Casadevall A, Pirofski L. Host-pathogen interactions: the attributes of virulence. J Infect Dis. 2001;184(3):337–344. doi: 10.1086/322044. Epub 2001 Jun 27. [DOI] [PubMed] [Google Scholar]
- 26.Casadevall A, Pirofski LA. Host-pathogen interactions: basic concepts of microbial commensalism, colonization, infection, and disease. Infect Immun. 2000;68(12):6511–6518. doi: 10.1128/iai.68.12.6511-6518.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casadevall A, Pirofski LA. What is a pathogen? Ann Med. 2002;34(1):2–4. doi: 10.1080/078538902317338580. [DOI] [PubMed] [Google Scholar]
- 28.Calderone RA, Cihlar RL. Principles and Clinical Applications. Marcel Decker: New York; 2002. Fungal pathogenesis. [Google Scholar]
- 29.Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol. 2001;9(7):327–335. doi: 10.1016/s0966-842x(01)02094-7. [DOI] [PubMed] [Google Scholar]
- 30.Davis D. Adaptation to environmental pH in Candida albicans and its relation to pathogenesis. Curr Genet. 2003;44(1):1–7. doi: 10.1007/s00294-003-0415-2. Epub 2003 Jun 18. [DOI] [PubMed] [Google Scholar]
- 31.Soll DR. Candida commensalism and virulence: the evolution of phenotypic plasticity. Acta Trop. 2002;81(2):101–110. doi: 10.1016/s0001-706x(01)00200-5. [DOI] [PubMed] [Google Scholar]
- 32.Chaffin WL, Lopez-Ribot JL, Casanova M, et al. Cell wall and secreted proteins of Candida albicans: identification, function, and expression. Microbiol Mol Biol Rev. 1998 Mar;62(1):130–180. doi: 10.1128/mmbr.62.1.130-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cutler JE. Putative virulence factors of Candida albicans. Annu Rev Microbiol. 1991;45:187–218. doi: 10.1146/annurev.mi.45.100191.001155. [DOI] [PubMed] [Google Scholar]
- 34.Odds FC, Gow NA, Brown AJ. Fungal virulence studies come of age. Genome Biol. 2001;2:1009.1–1009.4. doi: 10.1186/gb-2001-2-3-reviews1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sudbery PE. Growth of Candida albicans hyphae. Nat Rev Microbiol. 2011 Oct;9(10):737–748. doi: 10.1038/nrmicro2636. * An excellent and comprehensive review on molecular mechanisms involved in C. albicans filamentation
- 36.Banerjee M, Thompson DS, Lazzell A, et al. UME6, a novel filament-specific regulator of Candida albicans hyphal extension and virulence. Mol Biol Cell. 2008 Apr;19(4):1354–1365. doi: 10.1091/mbc.E07-11-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braun BR, Johnson AD. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science. 1997;277(5322):105–109. doi: 10.1126/science.277.5322.105. [DOI] [PubMed] [Google Scholar]
- 38.Braun BR, Johnson AD. TUP1, CPH1 and EFG1 make independent contributions to filamentation in candida albicans. Genetics. 2000;155(1):57–67. doi: 10.1093/genetics/155.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braun BR, Kadosh D, Johnson AD. NRG1, a repressor of filamentous growth in C.albicans, is down-regulated during filament induction. Embo J. 2001;20(17):4753–4761. doi: 10.1093/emboj/20.17.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carlisle PL, Banerjee M, Lazzell A, et al. Expression levels of a filament-specific transcriptional regulator are sufficient to determine Candida albicans morphology and virulence. Proc Natl Acad Sci U S A. 2009 Jan 13;106(2):599–604. doi: 10.1073/pnas.0804061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ernst JF. Transcription factors in Candida albicans - environmental control of morphogenesis. Microbiology. 2000;146(Pt 8):1763–1774. doi: 10.1099/00221287-146-8-1763. [DOI] [PubMed] [Google Scholar]
- 42.Murad AM, Leng P, Straffon M, et al. NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans. Embo J. 2001;20(17):4742–4752. doi: 10.1093/emboj/20.17.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saville SP, Lazzell AL, Monteagudo C, et al. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot Cell. 2003 Oct;2(5):1053–1060. doi: 10.1128/EC.2.5.1053-1060.2003. * The authors use the tetracycline regulatable gene expression system to dissect the contributions of yeast and filements during invasive candidiasis, challenging some of the current paradigms in the field
- 44.Zheng X, Wang Y. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. Embo J. 2004 Apr 21;23(8):1845–1856. doi: 10.1038/sj.emboj.7600195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacCallum DM, Odds FC. Temporal events in the intravenous challenge model for experimental Candida albicans infections in female mice. Mycoses. 2005 May;48(3):151–161. doi: 10.1111/j.1439-0507.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- 46.Phan QT, Belanger PH, Filler SG. Role of hyphal formation in interactions of Candida albicans with endothelial cells. Infect Immun. 2000;68(6):3485–3490. doi: 10.1128/iai.68.6.3485-3490.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lo HJ, Kohler JR, DiDomenico B, et al. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90(5):939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 48.Chaturvedi AK, Lazzell AL, Saville SP, et al. Validation of the tetracycline regulatable gene expression system for the study of the pathogenesis of infectious disease. PLoS One. 2011;6(5):e20449. doi: 10.1371/journal.pone.0020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakayama H, Mio T, Nagahashi S, et al. Tetracycline-regulatable system to tightly control gene expression in the pathogenic fungus Candida albicans. Infect Immun. 2000;68(12):6712–6719. doi: 10.1128/iai.68.12.6712-6719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saville SP, Lazzell AL, Bryant AP, et al. Inhibition of filamentation can be used to treat disseminated candidiasis. Antimicrob Agents Chemother. 2006 Oct;50(10):3312–3316. doi: 10.1128/AAC.00628-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang W, Li Y, Zhang L, et al. Retigeric acid B attenuates the virulence of Candida albicans via inhibiting adenylyl cyclase activity targeted by enhanced farnesol production. PLoS One. 2012;7(7):e41624. doi: 10.1371/journal.pone.0041624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gibson J, Sood A, Hogan DA. Pseudomonas aeruginosa-Candida albicans interactions: localization and fungal toxicity of a phenazine derivative. Appl Environ Microbiol. 2009 Jan;75(2):504–513. doi: 10.1128/AEM.01037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hogan DA, Vik A, Kolter R. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol Microbiol. 2004 Dec;54(5):1212–1223. doi: 10.1111/j.1365-2958.2004.04349.x. [DOI] [PubMed] [Google Scholar]
- 54. Hornby JM, Jensen EC, Lisec AD, et al. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol. 2001 Jul;67(7):2982–2992. doi: 10.1128/AEM.67.7.2982-2992.2001. * First report in the literature of quorum sensing in eukaryotes
- 55.Joyner PM, Liu J, Zhang Z, et al. Mutanobactin A from the human oral pathogen Streptococcus mutans is a cross-kingdom regulator of the yeast-mycelium transition. Org Biomol Chem. 2010 Dec 21;8(24):5486–5489. doi: 10.1039/c0ob00579g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martins M, Henriques M, Azeredo J, et al. Morphogenesis control in Candida albicans and Candida dubliniensis through signaling molecules produced by planktonic and biofilm cells. Eukaryot Cell. 2007 Dec;6(12):2429–2436. doi: 10.1128/EC.00252-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murzyn A, Krasowska A, Stefanowicz P, et al. Capric acid secreted by S. boulardii inhibits C. albicans filamentous growth, adhesion and biofilm formation. PLoS One. 2010;5(8):e12050. doi: 10.1371/journal.pone.0012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang L, Chang W, Sun B, et al. Bisbibenzyls, a new type of antifungal agent, inhibit morphogenesis switch and biofilm formation through upregulation of DPP3 in Candida albicans. PLoS One. 2011;6(12):e28953. doi: 10.1371/journal.pone.0028953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Midkiff J, Borochoff-Porte N, White D, et al. Small molecule inhibitors of the Candida albicans budded-to-hyphal transition act through multiple signaling pathways. PLoS One. 2011;6(9):e25395. doi: 10.1371/journal.pone.0025395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toenjes KA, Munsee SM, Ibrahim AS, et al. Small-molecule inhibitors of the budded-to-hyphal-form transition in the pathogenic yeast Candida albicans. Antimicrob Agents Chemother. 2005 Mar;49(3):963–972. doi: 10.1128/AAC.49.3.963-972.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shareck J, Belhumeur P. Modulation of morphogenesis in Candida albicans by various small molecules. Eukaryot Cell. 2011 Jun 3;3:3. doi: 10.1128/EC.05030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Navarathna DH, Hornby JM, Krishnan N, et al. Effect of farnesol on a mouse model of systemic candidiasis, determined by use of a DPP3 knockout mutant of Candida albicans. Infect Immun. 2007 Apr;75(4):1609–1618. doi: 10.1128/IAI.01182-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martins M, Lazzell AL, Lopez-Ribot JL, et al. Effect of exogenous administration of Candida albicans autoregulatory alcohols in a murine model of hematogenously disseminated candidiasis. J Basic Microbiol. 2012 Aug;52(4):487–491. doi: 10.1002/jobm.201100158. [DOI] [PubMed] [Google Scholar]
- 64.Cheng SC, Joosten LA, Kullberg BJ, et al. Interplay between Candida albicans and the mammalian innate host defense. Infect Immun. 2012 Apr;80(4):1304–1313. doi: 10.1128/IAI.06146-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Netea MG, Brown GD, Kullberg BJ, et al. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008 Jan;6(1):67–78. doi: 10.1038/nrmicro1815. * Informative and authoritative review on host defenses against C. albicans
- 66.Fidel PL, Jr, Barousse M, Espinosa T, et al. An intravaginal live Candida challenge in humans leads to new hypotheses for the immunopathogenesis of vulvovaginal candidiasis. Infect Immun. 2004 May;72(5):2939–2946. doi: 10.1128/IAI.72.5.2939-2946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moyes DL, Runglall M, Murciano C, et al. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe. 2010 Sep 16;8(3):225–235. doi: 10.1016/j.chom.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lorenz MC, Fink GR. The glyoxylate cycle is required for fungal virulence. Nature. 2001 Jul 5;412(6842):83–86. doi: 10.1038/35083594. [DOI] [PubMed] [Google Scholar]
- 69.MacCallum DM, Castillo L, Brown AJ, et al. Early-expressed chemokines predict kidney immunopathology in experimental disseminated Candida albicans infections. PLoS One. 2009;4(7):e6420. doi: 10.1371/journal.pone.0006420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Costerton JW, Cheng KJ, Geesey GG, et al. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 71.Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002 Sep;8(9):881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999 May 21;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 73.Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004 Apr 1;350(14):1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 74.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002 Apr;15(2):167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev. 2004 Apr;17(2):255–267. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nett J, Andes D. Candida albicans biofilm development, modeling a host-pathogen interaction. Curr Opin Microbiol. 2006 Aug;9(4):340–345. doi: 10.1016/j.mib.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 77.Ramage G, Martinez JP, Lopez-Ribot JL. Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res. 2006 Nov;6(7):979–986. doi: 10.1111/j.1567-1364.2006.00117.x. [DOI] [PubMed] [Google Scholar]
- 78.Ramage G, Mowat E, Jones B, et al. Our current understanding of fungal biofilms. Crit Rev Microbiol. 2009;35(4):340–355. doi: 10.3109/10408410903241436. [DOI] [PubMed] [Google Scholar]
- 79.Ramage G, Saville SP, Thomas DP, et al. Candida biofilms: an update. Eukaryot Cell. 2005 Apr;4(4):633–638. doi: 10.1128/EC.4.4.633-638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Crump JA, Collignon PJ. Intravascular catheter-associated infections. Eur J Clin Microbiol Infect Dis. 2000 Jan;19(1):1–8. doi: 10.1007/s100960050001. [DOI] [PubMed] [Google Scholar]
- 81.Raad I. Intravascular-catheter-related infections. Lancet. 1998 Mar 21;351(9106):893–898. doi: 10.1016/S0140-6736(97)10006-X. [DOI] [PubMed] [Google Scholar]
- 82.Viudes A, Peman J, Canton E, et al. Candidemia at a tertiary-care hospital: epidemiology, treatment, clinical outcome and risk factors for death. Eur J Clin Microbiol Infect Dis. 2002 Nov;21(11):767–774. doi: 10.1007/s10096-002-0822-1. [DOI] [PubMed] [Google Scholar]
- 83.Chandra J, Kuhn DM, Mukherjee PK, et al. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001 Sep;183(18):5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nobile CJ, Mitchell AP. Genetics and genomics of Candida albicans biofilm formation. Cell Microbiol. 2006 Sep;8(9):1382–1391. doi: 10.1111/j.1462-5822.2006.00761.x. * Excellent review on molecular mechanisms involved in C. albicans biofilm formation
- 85.Uppuluri P, Chaturvedi AK, Srinivasan A, et al. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog. 2010 Mar;6(3):e1000828. doi: 10.1371/journal.ppat.1000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ramage G, Saville SP, Wickes BL, et al. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol. 2002 Nov;68(11):5459–5463. doi: 10.1128/AEM.68.11.5459-5463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ramage G, VandeWalle K, Lopez-Ribot JL, et al. The filamentation pathway controlled by the Efg1 regulator protein is required for normal biofilm formation and development in Candida albicans. FEMS Microbiol Lett. 2002 Aug 27;214(1):95–100. doi: 10.1111/j.1574-6968.2002.tb11330.x. [DOI] [PubMed] [Google Scholar]
- 88.Lopez-Ribot JL. Candida albicans biofilms: more than filamentation. Curr Biol. 2005 Jun 21;15(12):R453–R455. doi: 10.1016/j.cub.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 89. Nobile CJ, Fox EP, Nett JE, et al. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell. 2012 Jan 20;148(1–2):126–138. doi: 10.1016/j.cell.2011.10.048. **This manuscript provides important insights into how biofilm development is controlled in C. albicans
- 90.Cateau E, Rodier MH, Imbert C. In vitro efficacies of caspofungin or micafungin catheter lock solutions on Candida albicans biofilm growth. J Antimicrob Chemother. 2008 Jul;62(1):153–155. doi: 10.1093/jac/dkn160. [DOI] [PubMed] [Google Scholar]
- 91.Redding S, Bhatt B, Rawls HR, et al. Inhibition of Candida albicans biofilm formation on denture material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009 May;107(5):669–672. doi: 10.1016/j.tripleo.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 92. Pierce CG, Uppuluri P, Tristan AR, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3(9):1494–1500. doi: 10.1038/nport.2008.141. *The authors describe a simple and reproducible model of C. albicans biofilm formation using 96-well microtiter plates, and its adaptation to antifungal susceptibility testing. This technique has been adopted by hundreds of laboratories working on fungal biofilms
- 93.Robbins N, Uppuluri P, Nett J, et al. Hsp90 governs dispersion and drug resistance of fungal biofilms. PLoS Pathog. 2011 Sep;7(9):e1002257. doi: 10.1371/journal.ppat.1002257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Uppuluri P, Nett J, Heitman J, et al. Synergistic effect of calcineurin inhibitors and fluconazole against Candida albicans biofilms. Antimicrob Agents Chemother. 2008 Mar;52(3):1127–1132. doi: 10.1128/AAC.01397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sardi JC, Scorzoni L, Bernardi T, et al. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol. 2013 Jan;62(Pt 1):10–24. doi: 10.1099/jmm.0.045054-0. ** A comprehensive and up to date review on these topics
- 96.LaFleur MD, Lucumi E, Napper AD, et al. Novel high-throughput screen against Candida albicans identifies antifungal potentiators and agents effective against biofilms. J Antimicrob Chemother. 2011 Apr;66(4):820–826. doi: 10.1093/jac/dkq530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Siles S, Srinivasan A, Pierce CP, et al. High-throughput screening of a collection of known pharmacologically active small compounds for the identification of Candida albicans biofilm inhibitors. Antimicrob Agents Chemother. 2013 doi: 10.1128/AAC.00680-13. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martins M, Henriques M, Lopez-Ribot JL, et al. Addition of DNase improves the in vitro activity of antifungal drugs against Candida albicans biofilms. Mycoses. 2012 Jan;55(1):80–85. doi: 10.1111/j.1439-0507.2011.02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martins M, Uppuluri P, Thomas DP, et al. Presence of extracellular DNA in the Candida albicans biofilm matrix and its contribution to biofilms. Mycopathologia. 2010 May;169(5):323–331. doi: 10.1007/s11046-009-9264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Srinivasan A, Uppuluri P, Lopez-Ribot J, et al. Development of a High-Throughput Candida albicans Biofilm Chip. PLoS One. 2011;6(4):e19036. doi: 10.1371/journal.pone.0019036. ** The authors report on the development of cellular chips for C. albicans biofilms as a new technology platform for microbial culture and high throughput screning
- 101.Braun BR, van Het Hoog M, d'Enfert C, et al. A human-curated annotation of the Candida albicans genome. PLoS Genet. 2005 Jul;1(1):36–57. doi: 10.1371/journal.pgen.0010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saville SP, Thomas DP, Lopez-Ribot JL. Use of genome information for the study of the pathogenesis of fungal infections and the development of diagnostic tools. Rev Iberoam Micol. 2005 Dec;22(4):238–241. doi: 10.1016/s1130-1406(05)70049-8. [DOI] [PubMed] [Google Scholar]
- 103.Letscher-Bru V, Herbrecht R. Caspofungin: the first representative of a new antifungal class. J Antimicrob Chemother. 2003 Mar;51(3):513–521. doi: 10.1093/jac/dkg117. [DOI] [PubMed] [Google Scholar]