Abstract
Objective
Although combination pharmacotherapy is common in child/adolescent psychiatry, there has been little research evaluating it. We tested the value of adding risperidone to concurrent psychostimulant and parent training (PT) in behavior management for children with severe aggression
Method
We randomized 168 children age 6–12 years (mean 8.89 ±2.01) with severe physical aggression to a 9-week trial of PT, stimulant, and placebo (Basic treatment; n=84) or PT, stimulant, and risperidone (Augmented treatment; n=84). All had diagnoses of attention-deficit/ hyperactivity disorder (ADHD) and either oppositional defiant (n= 124) or conduct disorder (n= 44). Children received psychostimulant (usually OROS methylphenidate) for 3 weeks, titrated for optimal effect, while parents received PT. If there was room for improvement at the end of Week 3, either placebo or risperidone was added. Assessments included parent ratings on the Nisonger Child Behavior Rating Form (NCBRF; Disruptive-Total subscale = Primary outcome) and Antisocial Behavior Scale (ABS); blinded clinicians rated change on the Clinical Global Impressions (CGI) scale.
Results
Compared to Basic treatment (PT + stimulant[STIM][44.8±14.6 mg/day] + placebo [1.88±0.72]), Augmented treatment (PT + STIM[46.1±16.8 mg/day] + risperidone[1.65±0.75]) showed statistically significant improvement on the NCBRF Disruptive–Total subscale (treatment-by-time interaction p= 0.0016), the NCBRF Social Competence subscale (p= 0.0049), and ABS Reactive Aggression (p= 0.01). CGI scores were substantially improved for both groups but did not discriminate between treatments (CGI-I ≤ 2, 70% for Basic treatment vs. 79% for Augmented treatment). Prolactin elevations and gastrointestinal upset occurred more with Augmented; other adverse events differed modestly from Basic treatment; weight gain within the Augmented treatment group was minor.
Conclusions
Risperidone provided moderate but variable improvement in aggressive and other seriously disruptive child behavior when added to PT and optimized stimulant treatment. Clinical trial registration information—Treatment of Severe Childhood Aggression (The TOSCA Study); http://clinicaltrials.gov/; NCT00796302.
Keywords: disruptive behavior disorders, parent training, physical aggression, psychostimulants, risperidone
Physical aggression in childhood is associated with serious negative consequences later in life. Many longitudinal studies have followed children from early childhood to evaluate the impact of early disruptive behavior disorders (DBDs) and/or aggressive behavior.1–4 Such DBD and aggression have been linked to (a) adult smoking, alcohol use, hard drug use, physical aggression, and risky sexual behavior;1 (b) frequent occurrences on police registers, repeated/serious crimes, involvement in confrontational and destructive offenses;2 (c) presence of generalized anxiety disorder (35%), social phobia (20%), obsessive compulsive disorder (21%), depression (23%), bipolar disorder (46%), attention-deficit/hyperactivity disorder (ADHD) (62%), physical aggression, and substance abuse;3 (d) nonviolent offending, and violent delinquency.4 Thus, DBD and aggressive behaviors are not only problematic at the time of first occurrence in the child’s life, but they are also important early warning signs of potential deleterious consequences later in life. Therefore, evidence-based attempts to attenuate these problems early in life are clearly warranted.
Concomitant pharmacotherapy is rising quickly in child and adolescent psychiatry, and this is especially the case for children with DBDs. For example, in a nationally representative sample of child psychiatric patients, 50% of the children with ADHD and 61% of the children with DBDs were taking combination pharmacotherapy.5 In another nationally representative sample of 3,466 youth, subsuming 27,979 visits to U.S. physicians, the most commonly reported diagnostic categories were DBDs and ADHD (49%).6 Multiclass prescriptions rose from 14% (1996–1999) to 20% (2004–2007) of patient visits that included psychotropic medication (57% increase in combination therapy over 8 years).6 When risperidone was prescribed, concomitant psychotropic prescribing occurred 62% of the time.6 The combination of ADHD medication plus antipsychotic medication was about 6 times more likely to occur than other multiclass combinations. Thus, augmented pharmacotherapy has become a fact of life in child psychiatry, despite the lack of controlled studies of combined pharmacotherapy.5–7 One of the most contentious issues in the treatment of children with disruptive behavior problems is the increasing use of multiple concurrent medications, especially the addition of atypical antipsychotic agents, because little is known about the safety and efficacy of such regimens.6
Aggressive behavior is one of the most prominent targets for the use of augmented pharmacotherapy in children. Of the medicines that have been assessed for managing aggression in youth, there is abundant evidence that psychostimulants, such as methylphenidate, can be helpful.8,9 This is not surprising given that ADHD and aggression often co-occur in children. Connor et al.8 conducted a meta-analysis of 28 stimulant studies involving aggressive behavior in children with ADHD. They reported a wide range of effect sizes (ES) for overt aggression (CI= 0.70–1.02; ES range= 0.24–2.12). Furthermore, the presence of ODD or CD led to significantly diminished ES in managing overt aggression. This raises the possibility that DBDs may be linked to diminished psychostimulant effect on aggression and poses the question of what to do when children show unsatisfactory psychostimulant response.
Risperidone has been shown consistently to reduce disruptive behavior in children. Findling et al.10 reported less aggression after 10 weeks of risperidone versus placebo for 20 children with CD. Two large trials (Ns=110, 118) of risperidone in children with subaverage IQs (IQ <85) and high scores on the Conduct Problem subscale of the Nisonger Child Behavior Rating Form (NCBRF) showed highly significant reductions on the Conduct Problem subscale; an approximately 45% decline accompanied risperidone compared to about a 15% decline with placebo.11, 12 Large follow-up studies (Ns=107, 504) showed maintained improvements over a year in previously medicated children, new gains in previously unmedicated children, and generally good tolerability (although weight gain was a problem for some).13, 14
Hence, psychostimulants and atypical antipsychotics are a commonly used form of augmented pharmacotherapy, and each effectively reduces DBD symptoms. However, very little research has tested their combined efficacy despite their common joint use in the community for children with DBDs. In a pilot study of risperidone versus placebo for 25 aggressive children with ADHD, Armenteros et al.15 studied 25 children with ADHD and overt aggression; only children with affective/impulsive aggression were enrolled. They added risperidone or placebo to constant doses of stimulants that were begun 3 weeks before study entrance. No significant differences in parent or teacher ratings of aggression were found after 4 weeks of combined risperidone or placebo augmentation. Although the paper reported a statistically significant difference in response rate (≥30% improvement on the parent-rated aggression scale) favoring risperidone, our re-analysis of that finding was not statistically significant (Fisher’s Exact Test, p=0.22; Yates’ chi-square, p=0.24). It is possible that, with only 25 subjects, the study was under powered.
Other studies of atypical antipsychotics added to stimulants lacked proper controls. Kronenberger et al.16 assessed the effects of open-label quetiapine over 9 weeks among 24 youth whose aggression was not adequately controlled after 3 weeks of OROS methylphenidate. All measures of aggression showed marked reductions from baseline to the end of methylphenidate monotherapy and further substantial statistically significant reductions when quetiapine was added. However, with this design, it is impossible to separate the effects of quetiapine alone from the placebo effect and the passage of time. Thus, there is a dearth of properly controlled and well-powered studies of combined stimulant and antipsychotic treatment in children with severe physical aggression and comorbid DBDs.
In this study, we evaluated risperidone’s contribution to the control of childhood DBDs (specifically, CD or ODD) with severe aggression and comorbid ADHD, when combined with ongoing psychostimulant (STIM) therapy and parent training (PT) in behavior management. We included PT due to its well-established efficacy in reducing childhood disruptive behaviors, 17 particularly in combination with stimulant treatment in ADHD.18 Thus the design of the study was closely aligned with recommendations from an expert panel’s Treatment Recommendations for Antipsychotics for Aggressive Youth (TRAAY).19 We predicted immediate reduction of DBD behaviors with initial stimulant treatment. Further, we predicted that the combination of PT and psychostimulant + risperidone (PT + STIM + RIS) would produce significant improvement, exceeding that of PT + psychostimulant + placebo (PT + STIM + PBO).
Method
Design
As both psychostimulants (STIM) and parent training (PT) are established treatments for ADHD and aggressive behavior, we termed this Basic treatment. We attempted to augment these effects by adding risperidone (RIS) for half of the participants. This was a four-site, randomized, double-blind, placebo-controlled study of PT and STIM (Basic treatment) compared with PT, STIM, and RIS (called “Augmented” hereafter) for the treatment of disruptive behavior in children with ADHD and ODD or CD. PT + STIM were initiated at the end of baseline (BL) assessment. If subjects failed to show a sufficient clinical response (defined below) at 3 weeks or if they deteriorated between 4 and 6 weeks, the second agent (RIS or PBO) was added to the treatment package. Subjects were randomized in a 1:1 ratio at baseline and the randomization was stratified by site and balanced for comorbid disruptive-disorder diagnosis (CD vs. ODD) through a web-based centralized randomization system. The clinical sites were Ohio State University, Case Western University, the University of Pittsburgh, and Stony Brook University. More details regarding the background, methods, design, and variables are provided by Farmer et al.9
Ratings of global behavioral response were made by blinded evaluators who were not permitted to ask about adverse events (AEs) or to know treatment assignment. Primary clinicians rated AEs, made titration adjustments, and were responsible for breaking the blind if subjects, due to nonresponse, exited study treatment at the end of the acute trial (9 weeks). During Weeks 3 to 8, a clinical responder was defined a priori as having a blinded evaluator-determined Clinical Global Impressions–Improvement (CGI-I) score20 of 1 (very much improved)and a parentrated NCBRF–Typical IQ (TIQ) Disruptive Behavior total (NCBRF D-Total) score of ≤15 (within 0.5 sd of the normative mean).21 This stringent definition of responder was used to make sure that any youngster who had room for further improvement was given the chance to receive the second medication. This provided a rigorous test of the added value of combined pharmacotherapy and encouraged the ideal target of behavioral normalization, especially in the case of serious childhood aggression. For the purposes of statistical evaluation at study endpoint, a clinical responder was defined in the more usual way, as having a reduction to the NCBRF D-Total of ≥25% and a CGI–I of 1 (very much improved) or 2 (much improved).
Subjects
Inclusion criteria were as follows
(i) ages 6–12 years, inclusive; (ii) DSM-IV DBD diagnosis (CD or ODD); (iii) DSM-IV diagnosis of ADHD (any subtype); (iv) evidence of serious physical aggression as rated on the Overt Aggression Scale–M22 (score ≥3 on assaults against other people, objects, or self); and (v) evidence of seriously disruptive behavior as determined by parent or guardian rating ≥ 27 (90th %ile) on the NCBRF D–Total. In addition, a CGI–Severity score of ≥4 (“Moderately ill” or higher) for aggression was required by blinded clinicians. Participants needed to be free of psychotropic medicines for 2 weeks for most drugs (such as most antidepressants, alpha agonists, beta blockers, anxiolytics, mood stabilizers, oral antipsychotics, and antihistamines), and 4 weeks for depot antipsychotics or fluoxetine. This rule was occasionally relaxed (to as little as 3–7 days) for extreme cases who could not tolerated being unmedicated the full time, as approved by the cross-site steering committee.
Exclusion criteria included
(i) full-scale IQ below 71; (ii) pregnancy or a history of seizure disorder, other neurological or medical disorder for which medication may present a considerable risk; (iii) abnormal liver function; (iv) pervasive developmental disorder, schizophrenia, other psychotic disorders, or eating disorders; (v) hypomanic/biphasic score of ≥36 as rated by child’s parent on the General Behavior Inventory (see below) and, if positive, confirmed by clinician as indication of mood disorder; (vi) current or history of major depressive disorder or diagnosis of bipolar disorder; (vii) current use of psychotropic medications from which discontinuation would present a significant risk; (viii) active substance use disorder; (ix) evidence of current child abuse or neglect; (x) history of suicide attempt in the past year or current suicidal ideation; and (xi) family history of Type II Diabetes in 2 or more first-degree relatives (owing to potential weight gain with risperidone).
The study was approved by the IRB of each investigative site; parents/guardians signed consent forms, and study participants gave assent before enrollment.
Procedure
Thereafter, families were involved in the following visits and assessments. A schedule of measures appears in Farmer et al.9
1. Screening visit(s)
occurred within 4 weeks of BL. During screening, we completed a physical exam, conducted clinical laboratory tests and an ECG, and interviewed parents regarding the child’s medical history. IQ was assessed with the Kaufman Brief Intelligence Test–2;23 and clinicians interviewed both child and parent using the Schedule for Affective Disorders and Schizophrenia for school-aged children (K-SADS-PL)24 to establish the presence of ADHD and ODD or CD and to rule out bipolar disorder, schizophrenia, and other exclusionary conditions. Parents rated their child on the General Behavior Inventory25 to ensure further that children with bipolar disorder were detected.
Clinicians completed the Overt Aggression Scale–Modified (OAS-M) a 7-item instrument from both parent and child report.22 Questions assess aggression on the dimensions of assaults against (a) objects, (b) others, and (c) self, on rating subscales ranging from 0 (no events) to 5 (severe events). Children receiving a score of at least 3, both at screen and at BL, qualified for inclusion. Providing some notion of initial severity, a score of 3 for assaults against objects is anchored with “Breaks objects, smashes windows,” whereas a score of 3 for assaults against others is characterized as “Attacks others, causing mild injury (bruises, sprains, welts, etc.).”
Parents also rated their child on the NCBRF–TIQ (“TIQ” referring to “Typical IQ version;” called NCBRF hereafter) at screen, BL, and Weeks 3 through 9.21 The NCBRF provides one prosocial subscale (Positive/Social) and six problem behavior subscales: (a) Conduct Problem, (b) Oppositional Behavior, (c) Hyperactive, (d) Inattentive, (e) Overly Sensitive, and (f) Withdrawn/Dysphoric.21 The NCBRF has excellent internal consistency, distinguishes between controls and subjects with DBDs, and its predecessor (for children with developmental disabilities) was highly drug-sensitive. Conduct Problem and Oppositional Behavior map closely to DSM-IV-TR symptoms of CD and ODD; they were scored together to form a variable called the D(isruptive)-Total. The D-Total was the primary outcome measure for this study. The Hyperactive and Inattentive subscale scores were combined to form an ADHD-Total.
In order to qualify, a child also required a Clinical Global Impressions–Severity (CGI-S)20 score of at least 4, reflecting the presence of consistent ADHD, disruptive, and physically aggressive/destructive behavior. Anger, defiance, and aggressive speech were not enough to qualify children for the study. Subjects had to display behavior that was physically harmful to others, themselves, or the environment around them. We established interrater reliability on the CGI-S and CGI-I subscales by discussion at Investigators Meetings, subsequent “gold standard” test vignettes, and repeated recertifications of blinded evaluators.
The subjects were assessed on the following safety measures at screening and all visits thereafter: (a) Barnes Akathisia Scale,26 a clinician-completed scale utilizing both objective observation/clinical judgment and the patient’s subjective experience of restlessness; (b) The Simpson-Angus Rating Scale checks for extrapyramidal side effects (rigidity, dystonia, and abnormal glabellar reflex) of antipsychotics;27 (c) The Abnormal Involuntary Movement Scale (AIMS),28 a standardized clinician-rated review of tremor, dyskinesia, and other possible antipsychotic neuromotor side effects.
2. Baseline (BL) and subsequent visits
At BL and thereafter, we collected the following assessments: vital signs, height, weight, open-ended elicited AEs and specific side effects ratings by parents and primary clinicians, plus concomitant medications. The CGI-I was obtained at all visits, whereas CGI-S was completed at end of Weeks 3 and 9. Finally, parents completed the Antisocial Behavior Scale (ABS)29 at BL and Week 9 or end-point. The 28-item ABS has a Proactive Aggression subscale (5 proactive items and 5 covert antisocial items) and a Reactive Aggression subscale (6 items).29 This instrument was used to differentiate affective and proactive subtypes of aggression and to assess treatment effects on both.
Beginning with BL, we administered a 9-session course of PT with up to 2 optional booster sessions using an empirically established program for children (the Community Parent Education Program [COPE]).30 Fidelity was monitored through audio tapes, reviewed by the Pittsburgh site, and by regular conference calls. The COPE’s focus on strategies for managing impulsive behavior, including reactive aggression, made it a good fit for this protocol.
From BL through Week 3, primary clinicians openly titrated psychostimulant medication to optimal effect balancing benefit and side effects, usually in the form of Osmotic Release Oral System (OROS) methylphenidate (Concerta). For smaller children (<25 kg), dosage was titrated clinically using the following daily doses: 18 mg (7 days), 36 mg, 54 mg (maximum maintenance dose). For larger children (>25 kg), dosage was increased every 3–4 days using the following daily doses: 18, 36, 54, 72 mg.9 Subjects unable to tolerate OROS methylphenidate or unable to swallow pills were offered an alternative (at comparable doses) from the following, of which the capsule contents could be sprinkled onto food: mixed amphetamine salts (Adderall), dextromethylphenidate extended release (Focalin XR), or lisdexamphetamine dimesylate (Vyvanse).
If residual symptoms remained, randomized placebo or risperidone was then added to treatment at Weeks 4 through 6. For children < 25 kg, risperidone was dosed between 0.5 to 2.5 mg/day; for children >25 kg, dosing ranged from 0.5 to 3.5 mg/day.9 The risperidone titration schemes allowed for dose increases every 3–7 days, following a schedule that specified maximum dose increases over 29 days of titration; doses could always be held constant or reduced if satisfactory clinical response occurred or if indicated by AEs.
Statistical Analysis
Primary/Secondary analyses
For the primary outcome NCBRF D-Total score, a constrained longitudinal data analysis (cLDA) model, in which both BL and post-BL values were treated as dependent variables, was used in the intention-to-treat (ITT) population (all randomized subjects).31 The outcome was square root transformed to accommodate the assumption of normality. Fixed effects in the final model included those for time, group, time × group interaction, site, and disorder type. Other interaction effects such as effects for site × time × group, site × time and site × group were explored and none of them was statistically significant. An unstructured variance covariance matrix was assumed for the correlated measures within each subject. Empirically-based sandwich estimators were obtained to assess the group differences at Week 9.32 Although mixed models can be used in the presence of missing data, the missing at random assumption is not directly testable. Thus, various sensitivity analyses were conducted to examine the robustness of results. Secondary longitudinal outcome variables, such as subscales other than the D-Total of the NCBRF and weight, were modeled in a similar fashion to the D-Total. Dichotomous rates of response to treatments were compared between groups by Fisher’s exact tests. For the ABS proactive and reactive aggression subscales rated by parents, BL and post-BL measures were analyzed by cLDA model with fixed effects for time, group, site, and disorder. As their distribution was non-normal, prolactin levels were compared using Wilcoxon ranked sum test. The secondary outcome variables were corrected for multiplicity by using Bonferroni corrections at the scale level (α= 0.0125 for NCBRF [4 subscales]; α =0.025 for CGI [2 subscales]; α =0.025 for ABS [2 subscales]). We used Cohen’s d to estimate effect size (ES); we calculated ES using complete cases, a subset of the ITT. All analyses were conducted in SAS (version 9.2, SAS Institute Inc., Cary, NC).
Power Analysis
With a two-sided α of 0.05, we had 80% power to detect an ES of 0.5 with complete data from 128 subjects (64 per group). To allow for average attrition of 20%, we concluded that 160 patients (80 per group) were needed, although the ITT analysis includes all subjects.
Data Management
The Data Coordinating Center used Teleform® (HP Autonomy) to generate data collection instruments and a secure SQLserver database to provide storage and access services. SharePoint was used as the study collaboration portal. A patient recruitment website was developed using Drupal.
Results
Subjects, Failure To Use Second Medication (RIS), and Dosage
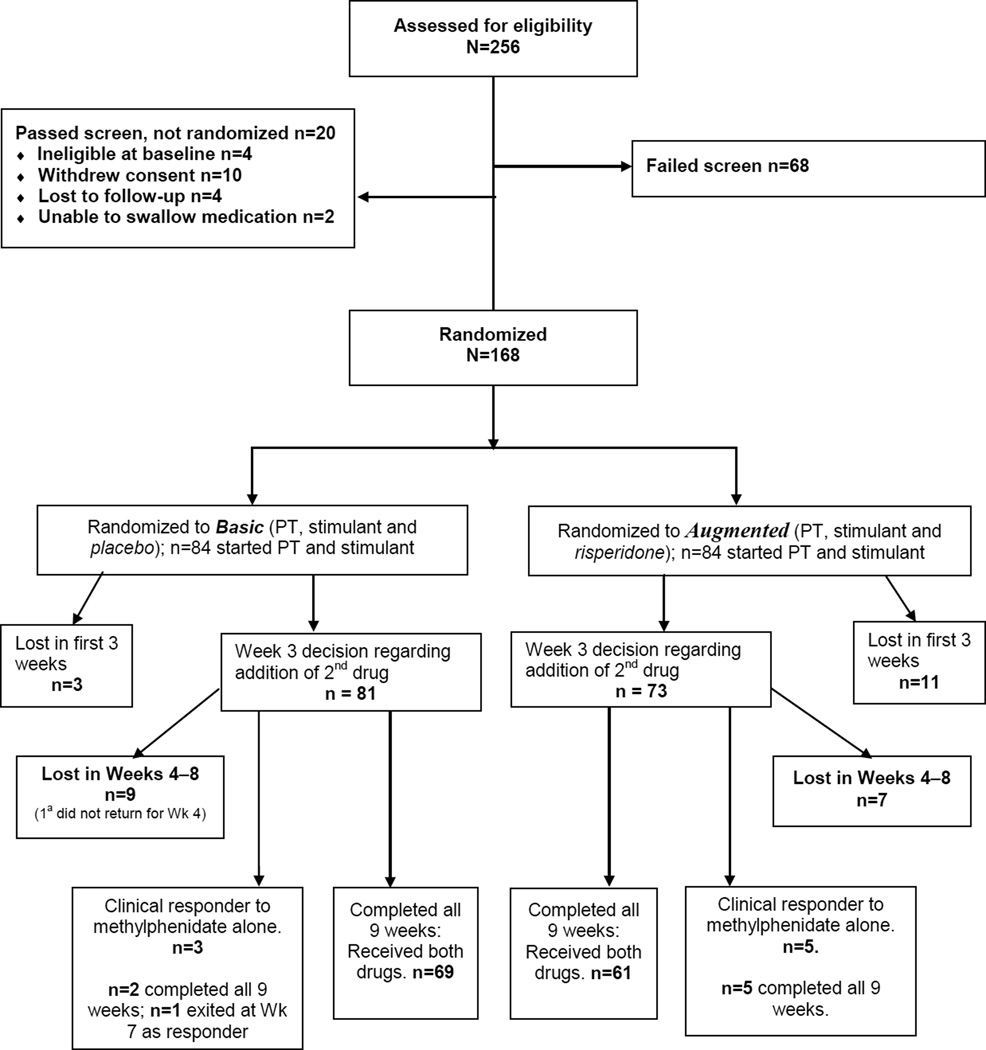
One hundred sixty-eight subjects (84 per treatment group) were randomized before starting Basic (STIM + PT). Figure 1 contains the CONSORT diagram showing disposition of subjects. Subject characteristics appear in Table 1. As would be expected for youth with DBDs, about 75% of the sample were boys.33 The large majority of the sample (73.8%) had ODD; the others had CD but would have qualified for ODD except for CD pre-emption of diagnosis. IQ was virtually identical for the two groups, and most demographic variables were similar between groups. Household incomes were relatively low, and parental education (especially for fathers) was also low. Thus, this sample largely comprised lower socioeconomic status families, although there was also a significant minority from the middle class (Table 1).
Figure 1.
CONSORT diagram showing subject allocation and subject attrition. Note: All 168 subjects were retained in the mixed model analysis regardless of whether they exited the study before Week 9, failed to respond, or were clinical responders to Basic (parent training [PT] + stimulant [STIM]). a Although 2nd medication was dispensed, subject was lost to follow-up with no Week 4 assessments.
Table 1.
Demographic Features of Participants
| Overall | Basic | Augmented | |
|---|---|---|---|
| Characteristic | (N=168) | (n=84) | (n=84) |
| Gender, male, n (%) | 129 (76.8) | 64 (76.2) | 65 (77.4) |
| Disorder, n (%) | |||
| Conduct Disorder | 44 (26.2) | 22 (26.2) | 22 (26.2) |
| Oppositional Defiant Disorder | 124 (73.8) | 62 (73.8) | 62 (73.8) |
| Age, years at screening, m (SD) | 8.89 (2.01) | 8.75(1.98) | 9.03 (2.05) |
| IQat screening, m (SD) | 97.1(14.1) | 97.0(13.9) | 97.2 (14.4) |
| Race/Ethnicity, n, (%) | |||
| African American | 58 (34.5) | 30 (35.7) | 28 (33.3) |
| Multiracial | 17(10.1) | 10(11.9) | 7 (8.3) |
| White | 89 (53.0) | 41 (48.8) | 48 (57.1) |
| Othera | 4 (2.4) | 3 (3.6) | 1(1.2) |
| Hispanic Origin | 9 (5.4) | 5 (6.0) | 4 (4.8) |
| Non-Hispanic Origin | 157 (93.5) | 78 (92.9) | 79 (94.0) |
| Unknown | 2(1.2) | 1 (1.2) | 1(1.2) |
| Child's Type of School | |||
| Regular Public (or private parochial) | 145 (86.3) | 73 (86.90) | 72 (85.7) |
| Otherb | 23(13.7) 1 | 11(13.1) | 12 (14.3) |
| Mother's Employment | |||
| Homemaker | 21(12.5) | 10(11.9) | 11(13.1) |
| Otherc | 59(35.1) | 28 (33.3) | 31 (36.9) |
| Working full/part time | 88 (52.4) | 46 (54.8) | 42 (50.0) |
| Father's Employment | |||
| Homemaker | 1 (0.6) | 1 (1.2) | 0 (0.0) |
| Otherc | 75 (44.6) | 38 (45.2) | 37 (44.0) |
| Working full/part time | 89 (53.0) | 42 (50.0) | 47 (56.0) |
| Unknown | 3(1.8) | 3 (3.6) | 0 (0.0) |
| Mother's Education | |||
| Some High School or less | 16(9.5) | 4 (4.8) | 12 (14.3) |
| High School Graduate orGED | 40 (23.8) | 16(19.0) | 24 (28.6) |
| Some College or More | 111(66.1) | 63 (75.0) | 48 (57.1) |
| Not in Household/Unknown | 1 (0.6) | 1(1.2) | 0 (0.0) |
| Father's Education | |||
| Some High School or Less | 7 (4.2) | 3 (3.6) | 4 (4.8) |
| High School Graduate orGED | 49 (29.2) | 23 (27.4) | 26(31.0) |
| Some College or More | 59(35.1) | 29 (34.5) | 30(35.7) |
| Not in Household/Unknown | 53 (31.5) | 29 (34.5) | 24 (28.6) |
| Household Income, $ | |||
| <20,000 | 61 (36.3) | 33 (39.3) | 28 (33.3) |
| 20,001–40,000 | 35 (20.8) | 16(19.0) | 19 (22.6) |
| 40,001–60,000 | 24 (14.3) | 9 (10.7) | 15(17.9) |
| 60,000–90,000 | 21(12.5) | 12 (14.3) | 9 (10.7) |
| >90,000 | 21(12.5) | 10(11.9) | 11(13.1) |
| Unknown | 6 (3.6) | 4 (4.8) | 2 (2.4) |
Note: Basic = parent training + stimulant + placebo ; Augmented = parent training + stimulant + risperidone . All comparisons were nonsignificant, except Mother's Education, where mothers in the Basic treatment had more schooling (Fisher's Exact test, p=0.021).
Other category for Race included: Asian, American Indian and Alaska Native, and Unknown.
Other category included: Home school, special class for children with learning disabilities, special class for children with emotional disabilities, or Other (open-ended response).
Other category included: Unemployed, disabled, retired, student, not in household, or Other (openended response).
Fourteen subjects (3 from the Basic treatment group and 11 from the Augmented group) dropped out before completing the third week of the study, before the opportunity for adding the second drug. With our analytic model, these participants did not appreciably affect the statistical outcomes. Eight subjects were clinical responders by the end of Week 3 and did not take the second medication (3 from the Basic treatment group and 5 from the Augmented group). Thus, 22 subjects either dropped out before they had an opportunity to benefit from Augmentation or were deemed not to need it. Treatment dropouts were generally lost to further follow-up.
The mean Week 9 methylphenidate dose for the Basic group was 44.8±14.6 mg/day, as compared with 46.1 ±16.8 mg/day for the Augmented group (p=0.88). For the second drug, the subjects receiving PBO had a mean Week 9 “dose” of 1.9±0.72 mg/day, as compared with 1.7 + 0.75 mg/day of risperidone for those taking active drug (Augmented) (p=0.07).
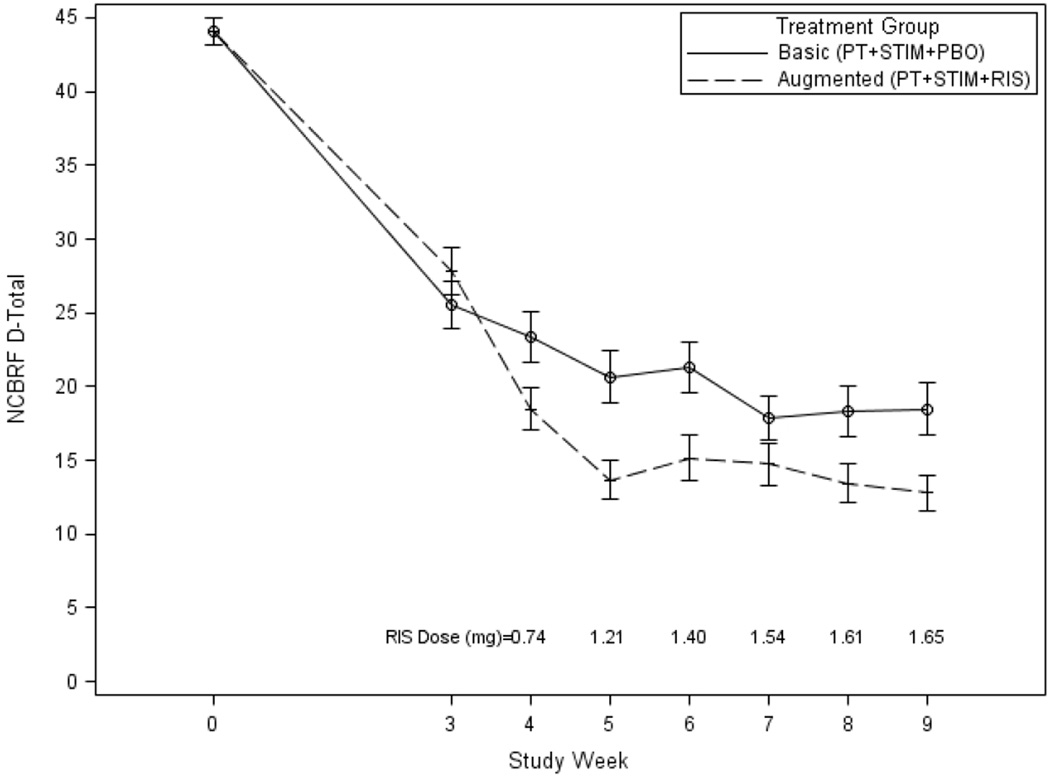
Primary Outcome
The results for the NCBRF D-Total appear in Table 2 and Figure 2. As determined by the linear mixed effects model, the group-by-time interaction was statistically significant (p= 0.0016), indicating that the Augmented group D-Total scores decreased more over time than the Basic treatment group. At Week 9, the difference between groups was statistically significant (p= 0.0143), with an ES of 0.43. Using change from Week 3 to Week 9, the ES was 0.50 (most ESs appear in Table 2). We conducted a sensitivity analysis for ES by excluding those who never experienced the second drug (14 pre–Week-3 dropouts and 8 PT + STIM responders). ES relative to Baseline was 0.51 and relative to Week 3 was 0.62.
Table 2.
Results for Comparisons on Nisonger Child Behavior Rating Form (NCBRF)-Typical IQ (TIQ) and Antisocial Behavior Scale
| Baseline | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Week 9 | p valuea | Estimate | Effect | Effect | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | m(SD) | m(SD) | m(SD) | m(SD) | m(SD) | m(SD) | m(SD) | m(SD) | (Cl)a | Sizeb | Sizec | |
| NCBRF | ||||||||||||
| D-Total | ||||||||||||
| n's: Basic | 84 | 82 | 80 | 75 | 74 | 73 | 69 | 71 | ||||
| n's: Augmented | 84 | 75 | 71 | 69 | 70 | 68 | 67 | 66 | ||||
| Basic | 43.5 (10.3) | 24.9(15.3) | 22.4(15.1) | 20.1(15.7) | 20.7(14.6) | 16.8(12.8) | 17.8(15.0) | 17.8(15.4) | 0.0143d | 0.67 | 0.43 | 0.50 |
| Augmented | 42.1(10.4) | 25.9(15.2) | 17.1(12.5) | 12.1(10.1) | 13.8(12.1) | 13.0(11.2) | 11.7(10.2) | 10.7(9.0) | (0.14, 1.20) | |||
| Overly Sensitive | ||||||||||||
| Basic | 6.2 (3.4) | 4.6(3.1) | 4.1 (3.4) | 3.5(3.3) | 3.4(2.9) | 2.9(2.5) | 2.8(3.0) | 2.5(2.9) | 0.1977 | 0.51 | 0.15 | 0.16 |
| Augmented | 6.3(3.2) | 3.9(3.1) | 2.9 (2.6) | 2.2(2.5) | 2.2(2.1) | 2.4(2.3) | 2.0(2.4) | 1.9(2.2) | (−0.27,1.30) | |||
| Positive Social | ||||||||||||
| Basic | 9.1(4.2) | 13.6(4.7) | 13.9(5.6) | 14.4(5.9) | 15.2(6.5) | 15.4(5.9) | 15.0(6.2) | 15.5(6.6) | 0.0112 | −2.82 | 0.35 | 0.46 |
| Augmented | 10.0(3.7) | 13.8(5.6) | 16.0(6.0) | 18.0(6.5) | 17.1(6.9) | 17.6(6.9) | 17.7(7.4) | 18.7(6.8) | (−4.99,−0.65) | |||
| ADHD | ||||||||||||
| Basic | 25.3 (6.0) | 13.7(8.8) | 12.7(8.6) | 11.4(8.2) | 11.2(7.5) | 10.1 (8.0) | 10.1 (7.7) | 9.5 (7.8) | 0.1557 | 1.61 | 0.19 | 0.26 |
| Augmented | 24.7(6.1) | 14.2 (7.7) | 10.6(7.5) | 7.8(6.7) | 8.4(7.3) | 7.4(5.7) | 6.8 (5.4) | 7.0(6.1) | (−0.62,3.83) | |||
| Withdrawn-Dysphoric | ||||||||||||
| Basic | 13.3(8.0) | 8.3 (6.7) | 7.1(7.1) | 7.0 (8.2) | 6.5 (6.3) | 5.5(6.1) | 5.6(6.3) | 5.0(6.5) | 0.3565d | 1.19 | 0.13 | 0.003 |
| Augmented | 13.7(8.0) | 7.8(5.8) | 5.4(5.8) | 4.0 (5.0) | 4.6 (4.8) | 4.4(4.6) | 3.7(4.3) | 3.6 (4.4) | (−0.21,0.58) | |||
| ABS | ||||||||||||
| n's: Basic | 84 | — | — | — | — | — | — | 77 | ||||
| n's: Augmented | 84 | — | — | — | — | — | — | 75 | ||||
| Proactive Behavior | ||||||||||||
| Basic | 20.2(4.6) | — | — | — | — | — | — | 15.1(4.3) | 0.0935 | 0.87 | 0.16 | |
| Augmented | 19.6(4.3) | — | — | — | — | — | — | 14.0(3.4) | (−0.15,1.88) | |||
| Reactive Behavior | ||||||||||||
| Basic | 15.9(1.8) | — | — | — | — | — | — | 12.3(3.1) | 0.0105 | 1.16 | 0.29 | |
| Augmented | 15.5 (2.4) | — | — | — | — | — | — | 11.0(2.7) | (0.27,2.04) |
Notes: Basic = parent training + stimulant + placebo ; Augmented = parent training + stimulant + risperidone; ABS= Antisocial Behavior Scale.
p values and estimates (CI) represent results of linear mixed model for comparison of therapies at Week 9
Effect size for Baseline to Week 9.
Effect size for Week 3 to Week 9.
p values, estimate (CI), and effect sizes represent results from square root transformation on specified NCBRF measures.
Figure 2.
Nisonger Child Behavior Rating Form (NCBRF) Disruptive Behavior Total (D-Total) score as a function of treatment condition and study visit. Note: Mean doses for risperidone are provided above the X axis.
Other NCBRF and ABS Outcomes
Findings for the other NCBRF and ABS variables also appear in Table 2. The results for the Positive-Social subscale of the NCBRF revealed a significant group-by-time interaction (p= 0.005): parents rated Augmented children as increasingly more socially competent than those with Basic treatment, ES = 0.35 (ES=0.46 from Week 3 to Week 9). Analyses of the ABS showed no significant treatment effect for the Proactive subscale and a significant treatment-by-time effect for the Reactive subscale (p= 0.0105, ES = 0.29). Thus, Augmented reduced reactive (“hot”) aggression more than did Basic treatment, whereas treatment effects on proactive (“cold”) aggression were not significantly different.
CGI and Responder Status
End-point CGI-I scores
No significant difference was observed between groups on CGI-I scores at endpoint; within the Basic group, 58 (70%) were “much or very much improved,” 22 (26%) were minimally improved, and three (4%) as unchanged or worse. Within the Augmented group, 63 (79%) were much/very much improved, 11 (14%) were minimally improved, and six (7%) were unchanged/worse (p= 0.09). Similarly, no significant effect of risperidone augmentation was observed on CGI-S. At end-point, for those receiving Basic treatment, 49 (59%) were rated as “normal/borderline/mildly ill,” and 34 (41%) were “moderately/markedly/severely ill.” For Augmented treatment, the figures were 56 (72%) and 22 (28%), respectively (p= 0.10). Our a priori definition of responder (reduction on D-Total of ≥25% and CGI-I of 1 or 2) was met by 70% of Basic treatment subjects and 79% of Augmented therapy subjects. This difference failed to reach significance (Fisher’s exact test, p= 0.09). The following percentages of children met the stringent definition of responder (parent-rated D-Total score of ≤15 and CGI-I of 1: [a] Weeks 4-8 means were 19.8% for Basic treatment and 32.9% for Augmented; [b] end-point 33.7% for Basic and 34.2% for Augmented [N.S.]).
Adverse Events (AEs)
No serious AEs related to study treatments were noted. We report only AEs for Weeks 4– 9, (when the second medication was used) in Table 3. After subtracting the 22 subjects who were not given the second medication, AE data were available for 80 Basic treatment and 73 Augmented participants. AEs occurring in 9 or more subjects per group are presented in Table 3. Trouble falling asleep (p= 0.02) was more common in the Basic treatment, whereas gastrointestinal upset (p= 0.03) occurred more commonly with augmentation.
Table 3.
Adverse Events Reported In Weeks 4 to 9 for Nine or More Subjects Per Group
| basic (n=80) |
Augmented (n=73) |
||||
|---|---|---|---|---|---|
| Variable | n | % | n | % | p valuea |
| Trouble falling asleep | 29 | 36.3 | 14 | 19.2 | 0.0205 |
| Rhinorrhea | 14 | 17.5 | 11 | 15.0 | 0.8273 |
| Cough | 20 | 25.0 | 14 | 19.2 | 0.4394 |
| Appetite decrease | 19 | 23.8 | 9 | 12.3 | 0.0935 |
| Appetite increase | 7 | 8.8 | 10 | 13.7 | 0.4412 |
| Diarrhea | 9 | 11.3 | 5 | 6.8 | 0.4088 |
| Gastrointestinal discomfort | 4 | 5.0 | 12 | 16.4 | 0.0322 |
| Vomiting | 6 | 7.5 | 10 | 13.7 | 0.2910 |
| Sedation | 20 | 25.0 | 16 | 21.9 | 0.7504 |
| Headache | 17 | 21.3 | 16 | 21.9 | 1.0000 |
Notes: Basic = parent training + stimulant + placebo ; Augmented = parent training + stimulant + risperidone. Sample size for each group excludes those who dropped out at or before Week 3. One subject within Basic treatment took medication between Week 3 and 4 but did not return for assessment, resulting in n=80.
Fisher's Exact Test used to compare incidence of adverse events between groups
Abnormal Laboratory Tests
There were four clinically significant abnormal lab values, 2 with risperidone (triglyceride of 389 and prolactin of 112 [we adopted convention of reporting any prolactin >100 as abnormal]) and 2 with placebo (fasting glucose of 144 and fasting insulin of 24). We analyzed prolactin concentrations at screen and endpoint for 77 children assigned to Basic treatment and 75 children assigned to Augmented treatment. Although the values were very similar at screen (5.7 ± 3.9 and 5.9 ± 3.0 μg/L, respectively), the values at endpoint were significantly different (Basic treatment, 7.1 ± 9.3; Augmented: 36.0 ± 27.5; Wilcoxon Ranked Sum test, p <0.0001). Using upper limits of >18.0 ng/mL for boys and >30 ng/mL for girls, we found that 46 of 68 children (68%) assigned to Augmented had elevated prolactins, compared with 4 of 73 (5%) assigned to Basic treatment. None were considered to be causing sexual or other AEs.
Weight
Mean (±SD) kg weights for the two groups were as follows: Basic treatment: BL, 33.2±12.9; Week 9, 32.0±10.9; Augmented: BL, 36.0±14.5; Week 9, 37.8±15.5; F (8, 1311) p= <.0001. Body mass index [BMI] percentiles were: Basic treatment, BL=67.2%ile; Week 9=56.5%ile; Augmented, BL=66.6%ile; Week 9=67.0%ile (p=<.0001). Thus, analysis of BMI data suggested that the weight gain in the Augmented group was associated with overall growth, as BMI percentile did not change appreciably over the course of the trial.
Discussion
Three positive clinical findings resulted from this study. First, the primary outcome, NCBRF D-Total score, improved more with Augmented than with Basic treatment (ES = 0.43 relative to baseline, 0.50 relative to Week 3, when need for further clinical improvement was determined and risperidone or PBO was added). Second, there was a significant interaction on the ABS, with scores improving more on the Reactive subscale for Augmented therapy than for Basic, but not so on the proactive scale. Third, improvement on the Social Competence subscale of the NCBRF was significantly better with Augmented treatment. Conversely, clinician ratings of improvement on the CGI-I did not show a statistically significant advantage with Augmented treatment. Together, the findings indicate that risperidone, when added to optimized stimulant treatment and parent training, provides a moderate advantage in parental ratings of disruptive behavior for children with serious aggression and additional disruptive behaviors.
Our primary finding was not consistent with that of Armenteros et al.,15 who found no significant advantage for parent-rated aggression when risperidone was added to stimulant. The inconsistency might be explained by differences in study design, choice of primary outcome measure (ours did not emphasize aggression per se), or severity of DBDs in study samples. The ESs (0.43 and 0.50) for Augmentedf treatment in this study were more modest than ESs of 0.82 and 0.75 reported in Aman et al. and Snyder et al., respectively.11,12 This study extends well beyond those investigations in terms of total exposure to intervention because all participants in the present study received 2 evidence-based treatments, PT + STIM, before commencing placebo or risperidone. In these earlier studies, any STIM was maintained, but there was no effort to titrate it to optimal effect; no PT was provided; and STIM effect was included in the baseline score.
It is also interesting to compare CGI findings across studies. Aman et al.11 reported 8% of the placebo group and 54% of the risperidone group were much/very much improved. Snyder et al.12 reported 6% of placebo and 48% of risperidone subjects as much/very much improved. Conversely, in this trial, 70% of Basic treatment and 79% of Augmented treatment subjects were rated with CGI-I scores of 1 (very much improved) or 2 (much improved) with far more children benefiting from the overall treatment package provided in the current study. Indeed, the entry criteria in the current study were more exclusive (in terms of greater severity of aggressive behavior), as compared with previous studies, which increased the opportunity for improvement for all children. In addition, we observed marked benefits conferred by Basic (PT + STIM), even before risperidone was added. Despite nominal differential impact on the CGI, we observed statistically significant improvement on NCBRF D-Total, NCBRF Social Competence, and ABS Reactive Aggression. Hence, from the perspective of parent ratings, risperidone produced better results than placebo.
This is one of the first augmented treatment studies in child psychiatry even though augmented treatment has become widespread in practice.5,9 The question that naturally arises is whether such co-therapy is worth the added expense, inconvenience, and potential risks that may accompany use of more than one drug. In attempting to answer this, it is important to keep in mind the following: First, risperidone was added only after stimulant therapy was optimized and after PT was begun. Second, problem behavior, as assessed by the NCBRF D-Total, had declined by about 42% by Week 3. Indeed, the pre–post effect size at Week 3 for all participants was 1.25 for NCBRF D-Total and 0.81 for Social Competence, creating very substantial reductions overall before introducing augmentation. In a study of methylphenidate monotherapy in children with CD,34 clinician ratings of improvement reached 68% (similar to our control treatments) showing that stimulants often have robust effects on antisocial behavior. Third, even compared to continuance of stimulant and PT for the last 6 weeks, Augmented therapy was of further benefit (about 21% additional reduction on the NCBRF D-Total [ES= 0.50]). Fourth, a meta analysis of 45 randomized clinical trials targeting aggression in children35 found a mean ES of 0.56 for monotherapy. As previously noted, our ESs for the ITT sample when risperidone was added to 2 other treatments were 0.43 (relative to Baseline) and 0.50 (relative to Week 3), and our sensitivity ESs were 0.51 and 0.62, respectively. Finally, DBDs, especially when accompanied by aggressive behavior, are often associated with severe later psychosocial problems and impairments such as substance abuse, sexual promiscuity, violent crime, accidents, depression, suicide attempts, spousal abuse, abusive parenting, and incarcerations,36 making the price of ineffective treatment potentially very high (although continued treatment through childhood and adolescence is likely needed to affect long-term outcomes). Hopefully, these findings will help to inform clinical decisions regarding the utility of short-term augmented treatment. They do not speak to the issue of medium- and long-term usefulness. We have exploratory moderator analyses planned for these data, which may better identify prime candidates for augmented treatment. We already have some indication that the risk-benefit ratio is more favorable for “hot” reactive aggression than for “cold” proactive aggression.
The finding of enhanced Social Competence is reassuring, suggesting that our participants were engaging in more socially appropriate behavior with Augmented treatment and that the treatment was not simply suppressing all behaviors, including those that are considered adaptive. Further, the observation of Augmented therapy having significantly greater effect on the ABS Reactive subscale than Basic treatment is in line with prevailing thought in the field.37 This suggests that a principal mechanism of drug effect is on the impulsive, unplanned disruptive actions of these children rather than on callous, planned aggression. As such, the findings help to verify a widely held clinical belief in the field for which there has been little empirical evidence one way or the other.38
Figure 2 shows a trend for D-Total scores to converge after Week 5 and diverge again by Week 9. We were unable to find a potential cause for this observation, including attrition or any tendency of children receiving higher risperidone doses to benefit less. PT was completed by Week 9, and it may have contributed to the regained improvement at Week 9.
Children receiving Augmented treatment gained more weight than those receiving Basic treatment. This appeared to be due more to Basic subjects losing weight than to Augmented subjects gaining weight. Indeed, the mean weight gain for the Augmented group was only 0.38 BMI percentile, using CDC norms. As expected, there was a significant increase in prolactin concentrations with Augmented therapy (above the threshold of normal in 65% of cases); those increases were not associated with sexual side effects in this short study. Finally, gastrointestinal upset was significantly greater with Augmented treatment (16.4% vs. 5.0%). We are preparing a separate paper to evaluate AEs in detail.
There were several limitations in this study. First, we were assessing a treatment strategy rather than a treatment combination per se. Consequently, when subjects dropped out or were found to be clinical responders in Weeks 1–3, they did not contribute to the medication signal for participants assigned to Augmented treatment. Second, to alleviate subject burden, we did not require parent ratings on the NCBRF in Weeks 1 and 2. This proved to be unfortunate because it prevented us from modeling the trajectory for participants who dropped out before Week 3, and hence, we did not fully benefit from the mixed effects regression model. An additional concern was the absence of a statistically significant advantage on CGI-I responder analysis. This finding might be explained by a lag or difference in clinician appreciation of improvements in overall functioning, versus parent ratings of specific behaviors, during a fairly short interval. It is also possible that group differences in dimensional scores of disruptive behavior are not translated into differences in overall functioning, perhaps diminishing any perceived added clinical benefit from risperidone. Finally, we did not measure or control for parental psychopathology, which might have influenced the effect of parent training; however, with 168 subjects, randomization should have distributed the effect of parental psychopathology evenly across groups.
Some of these limitations could obscure the signal from addition of risperidone, which showed a moderate statistical advantage when compared to Basic treatment. Although many children with severe aggressive behavior benefit sufficiently from stimulant coupled with PT, an important subgroup continues to experience a degree of behavioral dysregulation. The results of this study provide some reassurance that further behavioral benefit may be obtained from addition of risperidone in cases where there is a suboptimal response to first line PT + STIM, as per TRAAY guidelines.19 It is important to note that these results were obtained with children selected for severity of aggression and disruptive behavior, and the risk–benefit ratio may well be diminished for children with milder disruptive behavior or no physical aggression.
The discrepancies between our a priori selected primary outcome variable (parent ratings of disruptive behavior), and the blinded clinician ratings of improvement, warrant consideration by clinicians and clinical researchers. A common standard for clinical improvement is a CGI-I of 1 or 2, which in this study did not distinguish significantly between the groups. Moreover, based on our rigorous criteria for clinical responder (CGI-I=1 and parent-rated D-Total ≤15) in Week 3, equal percentages of children in each group benefited from treatment at end-point (33.7% vs. 34.2%), although there was a mean 13% advantage for Combined treatment for the intermediate Weeks 4 through 8. The discrepancy in outcomes between the primary NCBRF D-Total and secondary CGI scores suggest that the blinded clinicians may have missed some important evidence of clinical benefit noted by parents. These discrepant results, observed in many studies, are difficult to interpret due to complete confounding of reporter with measure type. However, they do underscore the potential importance of not relying on one source of information when evaluating response to treatment in the clinical setting. Consistent with our results, TRAAY guidelines,19 recommend the use of standardized assessment instruments to help clinicians make more systematic and objective clinical decisions, especially when combined treatment is being considered. Our findings suggest that practicing clinicians may need to use similar rating scales in order to detect improvement.
In conclusion, the findings suggest that after experiencing marked improvements from behavior therapy and optimized STIM, children with DBD and ADHD who have continuing aggression and other disruptive behaviors can experience further improvement with added risperidone. Given the clinical severity of this child population, these findings provide evidence for an additional treatment should it be deemed necessary. It is important to emphasize, however, that these results are based on a relatively brief 6-week trial with possible subsequent waning of efficacy of risperidone or the emergence of problematic adverse events (e.g., weight gain and metabolic disturbance) and that the true implications of this study for informing clinical practice will be more fully realized when analyses of our 3-month follow-up assessment are completed.
Supplementary Material
Acknowledgments
This study was supported by grants from National Institute of Mental Health (NIMH) to Ohio State University (R01 MH077907), Case Western University (R01 MH077750), the University of Pittsburgh (R01 MH077676), and Stony Brook University (R01 MH 077997).
The project was supported a National Institutes of Health (NIH) General Clinical Research Center grant M01RR10710 (State University of New York [SUNY] Stony Brook) and Clinical and Translational Science Awards (CTSA) from the National Center For Advancing Translational Sciences (NCATS): Grant 8UL1TR000090-05 (O.S.U.); UL1 RR024153 and UL1TR000005 (University of Pittsburgh). The content is solely the responsibility of the authors and does not necessarily represent the official views of the respective National Centers For Advancing Translational Sciences or the NIH.
The authors gratefully acknowledge guidance and supervision of the Data and Safety and Monitoring Board, comprising Drs. Daniel Connor (University of Connecticut Medical School), Walter Meyer, III (University of Texas, Galveston), Carson Reider (Ohio State University), and Wesley Thompson (University of California, San Diego). Dr. Connor receives grant support from Shire Pharmaceuticals and is an attention-deficit/hyperactivity disorder (ADHD) consultant for Shire and Rhodes Pharmaceuticals. Drs. Reider, Meyer, and Thompson have no disclosures to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Dr. Aman has received research contracts, consulted with, or served on advisory boards of Biomarin Pharmaceuticals, Bristol-Myers Squibb, CogState, Confluence Pharmaceutica, Coronado Biosciences, Forest Research, Hoffman LaRoche, Johnson and Johnson, Novartis, and Supernus Pharmaceutica. Dr. Bukstein has received royalties from Routledge Press and acted as a consultant for Ezra Innovations and PRIME CME. Dr. Arnold has received research funding from CureMark, Forest, Lilly, and Shire; advisory board honoraria from Biomarin, Novartis, Noven, Roche, Seaside Therapeutics, and Shire; consulting fees from Tris Pharma; and travel support from Noven. Dr. McNamara has received research support from Forest Research, GlaxoSmithKline, Eli Lilly and Co., Lundbeck, Merck, NIH, Novartis, Otsuka, Pfizer, Rhodes Pharmaceuticals, Roche, Shire, Stanley Medical Research Institute, Sunovion, and Supernus Pharmaceuticals. Dr. Rundberg-Rivera has received research support from GlaxoSmithKline, Merck/Schering Plough, National Inst. Of Mental Health, Covance/Otsuka, and Pfizer. Dr. Bangalore has received research support from Supernus. Dr. Hurt has received research support from Bristol-Myers Squibb. Dr. Findling has received research support, acted as a consultant and/or served on a speaker's bureau for Alexza Pharmaceuticals, American Psychiatric Press, AstraZeneca, Bracket, Bristol-Myers Squibb, Clinsys, Cognition Group, Forest, GlaxoSmithKline, Guilford Press, Johns Hopkins University Press, Johnson and Johnson, KemPharm, Eli Lilly and Co., Lundbeck, Merck, NIH, Novartis, Noven, Otsuka, Pfizer, Physicians Postgraduate Press, Rhodes Pharmaceuticals, Roche, Sage, Seaside Pharmaceuticals, Shire, Stanley Medical Research Institute, Sunovion, Supernus Pharmaceuticals, Transcept Pharmaceuticals, Validus, and WebMD. Ms. Kipp has received research support from Supernus. Ms. Baker has received research support from Amicus, BioMarin, Enobia, Genzyme, GlaxoSmithKline, Hyperion, Shire, Supernus, and Ultragenyx. Drs. Gadow, Molina, Li, Schneider, Butter, Sprafkin, Rice, and Farmer; and Ms. Austin, Ms. Buchan-Page, Ms. Arradaza, and Ms. Grondhuis report no biomedical financial interests or potential conflicts of interest.
References
- 1.Timmermans M, van Lier PA, Koot HM. Which forms of child/adolescent externalizing behaviors account for late adolescent risky sexual behavior and substance use? J Child Psychol Psychiatry. 2008;49:386–394. doi: 10.1111/j.1469-7610.2007.01842.x. [DOI] [PubMed] [Google Scholar]
- 2.Stattin H, Magnussson D. The role of early aggressive behavior in the frequency, seriousness, and types of later crime. J Consult Clin Psychol. 1989;57:710–718. doi: 10.1037//0022-006x.57.6.710. [DOI] [PubMed] [Google Scholar]
- 3.Masi G, Milone A, Manfredi A, Pari C, Paziente A, Millepiedi S. Conduct disorder in referred children and adolescents: Clinical and therapeutic issues. Compr Psychiatry. 2008;49:146–153. doi: 10.1016/j.comppsych.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Broidy LM, Nagin DS, Tremblay RE, et al. Developmental trajectories of childhood disruptive behaviors and adolescent delinquecy: A six-site, cross-national study. Dev Psychol. 2003;39(2):222–245. doi: 10.1037//0012-1649.39.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffy FF, Narrow WE, Rae DS, et al. Concomitant pharmacotherapy among youths treated in routine psychiatric practice. J Child Adolesc Psychopharmacol. 2005;15:12–25. doi: 10.1089/cap.2005.15.12. (2005) [DOI] [PubMed] [Google Scholar]
- 6.Comer JS, Olfson M, Mojtabai R. National trends in child and adolescent psychotropic polypharmacy in office-based practice, 1996–2007. J Am Acad Child Adolesc Psychiarty. 2010;49:1001–1010. doi: 10.1016/j.jaac.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bussing R, Winterstein AG. Polypharmacy in attention deficit hyperactivity disorder treatment: Current status, challenges and next steps. Curr Psychiatry Rep. 2012;14:447–449. doi: 10.1007/s11920-012-0295-6. [DOI] [PubMed] [Google Scholar]
- 8.Connor DF, Glatt SJ, Lopez ID, Jackson D, Melloni RH., Jr Psychopharmacology and aggression. I: A meta-analysis of stimulant effects on overt/covert aggression-related behaviors in ADHD. J Am Acad Child Adolesc Psychiatry. 2002;41:253–261. doi: 10.1097/00004583-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Farmer C, Arnold L, Bukstein O, et al. The treatment of severe child aggression (TOSCA) study: Trial design challenges. Child Adolesc Psychiatry Ment Health. 2011;5(36):1–11. doi: 10.1186/1753-2000-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Findling RL, McNamara NK, Branicky LA, Schluchter MD, Lemon E, Blumer JL. A double-blind pilot study of risperidone in the treatment of conduct disorder. J Am Acad Child Adolesc Psychiatry. 2000;39:509–516. doi: 10.1097/00004583-200004000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Aman MG, De Smedt G, Derivan A, Lyons B, Findling RL The Risperidone Disruptive Behavior Study Group. Risperidone treatment of children with disruptive behavior disorders and subaverage IQ: A double-blind, placebo-controlled study. Am J Psychiatry. 2002;159:1337–1346. doi: 10.1176/appi.ajp.159.8.1337. [DOI] [PubMed] [Google Scholar]
- 12.Snyder R, Turgay A, Aman MG, Binder C, Fisman S, Carroll A The Risperidone Conduct Study Group. Effects of risperidone on conduct and disruptive behavior disorders in children with subaverage IQs. J Am Acad Child Adolesc Psychiatry. 2002;41:1026–1036. doi: 10.1097/00004583-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Croonenberghs J, Fegert JM, Findling RL, De Smedt G, Van Dongen S the Risperidone Disruptive Behavior Study Group. Risperidone in children with disruptive behavior disorders and subaverage intelligence: A 1-year, open-label study of 504 patients. J Am Acad Child Adolesc Psychiatry. 2005;44:64–72. doi: 10.1097/01.chi.0000145805.24274.09. [DOI] [PubMed] [Google Scholar]
- 14.Findling RL, Aman MG, Eerdekens M, Derivan A, Lyons B the Risperidone Disruptive Behavior Study Group. Long-term, open-label study of risperidone in children with severe disruptive behaviors and below-average IQ. Am J Psychiatry. 2004;161:677–684. doi: 10.1176/appi.ajp.161.4.677. [DOI] [PubMed] [Google Scholar]
- 15.Armenteros JL, Lewis JE, Davalos M. Risperidone augmentation for treatment-resistant aggression in Attention-Deficit/Hyperactivity Disorder : A placebo-controlled pilot study. J Am Acad Child Adolesc Psychiatry. 2007;46:558–565. doi: 10.1097/chi.0b013e3180323354. [DOI] [PubMed] [Google Scholar]
- 16.Kronenberger WG, Giauque AL, Lafata DE, Bohnstedt BN, Maxey LE, Dunn DW. Quetiapine addition in methylphenidate treatment-resistant adolescents with comorbid attention-deficit/hyperactivity disorder, conduct/oppositional-defiant disorder, and aggression: A prospective, open-label study. J Child Adolesc Psychopharm, 2007;17:334–347. doi: 10.1089/cap.2006.0012. [DOI] [PubMed] [Google Scholar]
- 17.Chorpita BF, Daleiden EL, Ebesutani C, et al. Evidence-Based Treatments for Children and Adolescents: An Updated Review of Indicators of Efficacy and Effectiveness. Clin Psychol: Sci Pract. 2011;18:154–172. [Google Scholar]
- 18.Swanson JM, Kraemer HC, Hinshaw SP, et al. Clinical relevance of the primary findings of the MTA: Success rates based on severity of ADHD and ODD symptoms at the end of treatment. J Am Acad Child Adolesc Psychiatry. 2001;40(2):168–179. doi: 10.1097/00004583-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Pappadopulos E, MacIntre JC, Crismon ML, et al. Treatment Recommendations for the use of Antipsychotics for Aggressive Youth (TRAAY). Part II. J Am Acad Child Adolesc Psychiatry. 2003;42(2):145–161. doi: 10.1097/00004583-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Rapoport J, Conners CK, Reatig N. CGI (Clinical Global Impression) scale – NIMH. Psychopharmacol Bull [Special Issue] 1985;21(4):839–843. [Google Scholar]
- 21.Aman MG, Leone S, Lecavalier L, Park L, Buican B, Coury D. The Nisonger child behavior rating form — Typical IQ version, for children with typical IQ. Int Clin Psychopharmacol. 2008;23:232–242. doi: 10.1097/YIC.0b013e3282f94ad0. [DOI] [PubMed] [Google Scholar]
- 22.Coccaro EF, Harvey PH, Kupshaw-Lawrence E, Herbert JL, Bernstein DP. Development of neuropharmacologically based behavioral assessments of impulsive aggressive behavior. J Neuropsychiatry Clin Neurosci. 1991;3(suppl 2):44–51. [PubMed] [Google Scholar]
- 23.Kaufman AS, Kaufman NL. Kaufman Assessment Battery for Children Second Edition. Circle Pines, MN: American Guidance Service; 2004. [Google Scholar]
- 24.Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school age children: Present and lifetime version (KSADS—PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Findling RL, Youngstrom EA, Danielson CK, et al. Clinical decision-making using the General Behavior Inventory in juvenile bipolarity. Bipolar Disord. 2002;4:34–42. doi: 10.1034/j.1399-5618.2002.40102.x. [DOI] [PubMed] [Google Scholar]
- 26.Barnes TRE. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- 27.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 28.Rapoport J, Connors CK, Reatig N. AIMS (Abnormal Involuntary Movement Scale) – NIMH. Psychopharmacol Bull [Special Issue] 1985;21(4):839–843. [Google Scholar]
- 29.Brown K, Atkins MS, Osborne ML, Milnamow M. A revised teacher rating scale for reactive and proactive aggression. J Abnorml Child Psychol. 1996;24(4):473–480. doi: 10.1007/BF01441569. [DOI] [PubMed] [Google Scholar]
- 30.Cunningham CE. Large group, community based, parenting courses. In: Barkley RA, editor. Attention Deficit Hyperactivity: A Handbook for Diagnosis and Treatment. New York: Guilford Press; 1998. [Google Scholar]
- 31.Lu K. On efficiency of constrained longitudinal data analysis versus longitudinal analysis of covariance. Biometrics. 2010;66(3):891–896. doi: 10.1111/j.1541-0420.2009.01332.x. [DOI] [PubMed] [Google Scholar]
- 32.Gurka M, Edwards LJ, Muller KE. Avoiding bias in mixed model inference for fixed effects. Stat Med. 2011;30(22):2696–2707. doi: 10.1002/sim.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Psychiatric Association. Text Revision (DSM-IV-TR) Fourth Edition. Washington, DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 34.Klein RG, Abikoff H, Klass E, et al. Clinical efficacy of methylphenidate in conduct disorder with and without attention deficit hyperactivity disorder. Arch Gen Psychiatry. 1997;54:1073–1080. doi: 10.1001/archpsyc.1997.01830240023003. [DOI] [PubMed] [Google Scholar]
- 35.Pappadopulos E, Woolston S, Chait A, et al. Pharmacotherapy of aggression in children and adolescents: Efficacy and effect size. J Cdn Child Adolesc Psychiatry. 2003;15(1):27–39. [PMC free article] [PubMed] [Google Scholar]
- 36.Tremblay RE, Nagin DS, Seguin JR, et al. Physical aggression during early childhood: trajectories and predictors. Pediatr. 2004;114(1):43–50. doi: 10.1542/peds.114.1.e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kempes M, Matthys W, de Vries H, van Engeland H. Reactive and proactive aggression in children - A review of theory, findings and the relevance for child and adolescent psychiatry. Eur Child Adolesc Psychiatry. 2005;14(1):11–19. doi: 10.1007/s00787-005-0432-4. [DOI] [PubMed] [Google Scholar]
- 38.Barratt ES, Stanford MS, Felthous AR, Kent TA. The effects of phenytoin on impulsive and premeditated aggression: a controlled study. J Clin Psychopharmacol. 1997;17:341–349. doi: 10.1097/00004714-199710000-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.