Abstract
Disorganized behavior is a key symptom of schizophrenia. The objective assessment of disorganized behavior is particularly challenging. Actigraphy has enabled the objective assessment of motor behavior in various settings. Reduced motor activity was associated with negative syndrome scores, but simple motor activity analyses were not informative on other symptom dimensions. The analysis of movement patterns, however, could be more informative for assessing schizophrenia symptom dimensions. Here, we use time series analyses on actigraphic data of 100 schizophrenia spectrum disorder patients. Actigraphy recording intervals were set at 2 s. Data from 2 defined 60-min periods were analyzed, and partial autocorrelations of the actigraphy time series indicated predictability of movements in each individual. Increased positive syndrome scores were associated with reduced predictability of movements but not with the overall amount of movement. Negative syndrome scores were associated with low activity levels but unrelated with predictability of movement. The factors disorganization and excitement were related to movement predictability but emotional distress was not. Thus, the predictability of objectively assessed motor behavior may be a marker of positive symptoms and disorganized behavior. This behavior could become relevant for translational research.
Key words: schizophrenia, actigraphy, behavior, time series analysis, positive symptoms, negative symptoms
Introduction
Irregular and peculiar movement patterns have been noted in schizophrenia by Kraepelin1 (pp59–62) as well as in case notes from the 19th century.2 Bleuler described irregular movement patterns as “ambivalence of will” (ambitendence) or disturbance of behavior, both of which he considered as resulting from the disturbance of association.3 Disorganization of behavior is still conceived as one of the hallmarks of schizophrenia.4 The observation of spontaneous motor behavior remains an important contribution to psychopathological rating scales. In addition to spontaneous behavior, the behavior of schizophrenia patients in experimental settings has been found to be less predictable.5
Motor behavior is altered in schizophrenia, including specific motor disturbances and general motor activity levels.6,7 Indeed, schizophrenia patients engage less frequently in sports,8,9 but more in sedentary behaviors,9 and have less activity during leisure time than the average population.10 Activity monitoring using actigraphy allows an objective assessment of schizophrenia motor behavior.6,11 Reduced activity levels were repeatedly reported in patients using actigraphy.12–15 However, few studies have aimed at investigating associations of objective motor behavior and psychopathological phenomena. We have previously demonstrated a moderate association of actigraphically assessed motor activity and the negative syndrome as assessed by the positive and negative syndrome scale (PANSS).16,17 Higher negative syndrome scores were associated with reduced motor activity.15,16 However, the associations between positive symptoms and motor activity remain inconsistent.14–16,18
Besides the analysis of average motor activity, one could also investigate the movement patterns over time, which may provide additional insight into the structure of behavior. Investigations of large-scale rhythms such as circadian rhythm disturbances, however, found no associations with psychopathology in schizophrenia.19,20 Still, detailed analyses of movement patterns in smaller intervals are more likely to produce relevant and informative results for objective assessments of psychopathology. Within shorter time periods, behavioral patterns of schizophrenia patients in an experimental setting were associated with psychopathology.5 Thus, patterns of spontaneous motor behavior within 1h might be related to the severity of schizophrenia symptoms.
In the present study we used time-series analyses of actigraphic data to evaluate the predictability of movement behavior in “schizophrenia spectrum patients” and its association with psychopathological phenomena. We expected that the predictability of motor behavior would largely vary in a group of 100 schizophrenia spectrum patients. Furthermore, we hypothesized that the predictability of motor behavior was associated with schizophrenia symptom dimensions, most likely with the positive and negative syndrome scores. Positive symptoms were expected to lead to more disrupted patterns, and negative symptoms were expected to lead to more stable patterns of motor behavior.
Methods
Subjects
Participants were recruited at the inpatient units of the University Hospital of Psychiatry, Bern, Switzerland. Inclusion was restricted to patients with schizophrenia, schizophreniform or schizoaffective disorder according to DSM-IV. Exclusion criteria involved substance dependence other than nicotine, abuse of illegal substances within the last week and medical conditions with impaired motor function, eg weakness or hemiplegia. Diagnoses were given following clinical psychiatric examination and chart review. All but 3 patients were on antipsychotic medication at the time of study. The study sample consists of data that have been included in previous studies of our group with different aims and data analyses.12,16,21–23
Procedures
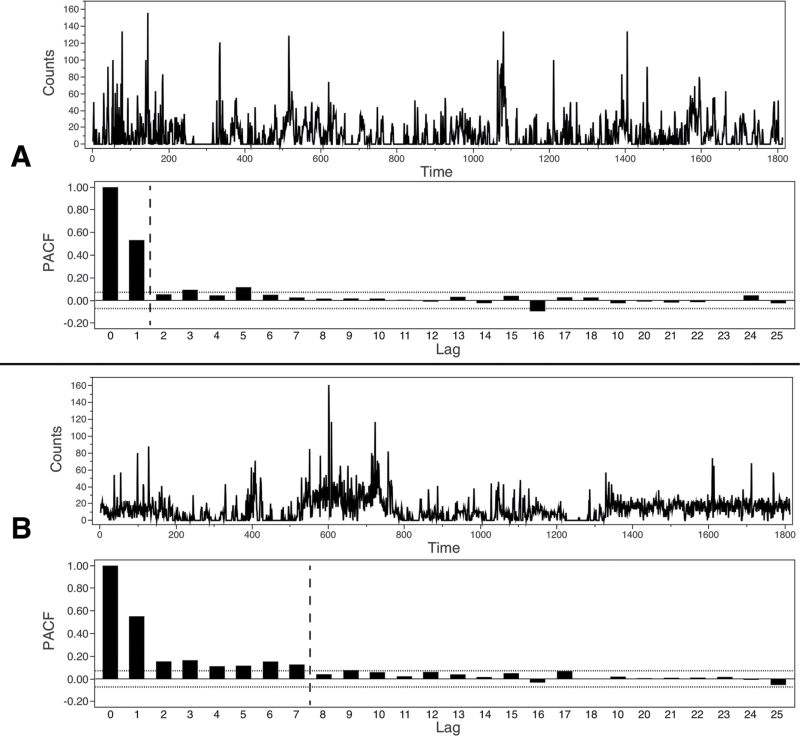
Symptom severity was assessed using the PANSS administered by a trained psychiatrist (S.W.). Following the PANSS interview, patients wore an actigraph (Actiwatch, Cambridge Neurotechnology, UK) at the wrist of the nondominant arm for 24 consecutive hours. Activity monitoring at the nondominant arm was found to reflect whole body activity.24 A piezoelectric sensor integrates accelerations into movement counts that were stored at 2-s intervals. Thus, we have continuous 2-s-wise data on the number of movements for each participant (the activity data over 1h is plotted in figure 1 for 2 subjects). Patients provided sleep log information, and activity monitoring was paused for bathing or showering. The study protocol was approved by the local ethics committee, and all participants provided written informed consent.
Fig. 1.
Time series for 2 patients (A, B) with respective autocorrelation plots. Note: PACF, Partial Autocorrelation; Vertical dashed reference lines in autocorrelation plots: Maximal lag where PACF is above ±2 standard errors of an approximate 95% confidence limit.
Data Analysis
Two 60-min periods were chosen for analysis from the total recording of 24h, 10:00–11:00 am and 3:00–4:00 pm. Circadian social rhythms are highly normalized in psychiatric hospitals, eg meals are served at the same time period. The 2 time periods were chosen for analysis because the first includes various single or group activities before lunchtime and the second supervised group activities, which are attended by the vast majority of patients. Patients are asked to choose 1 of the activities of the weekly schedule but may also withdraw. Morning group activities are predominantly indoors and include neurocognitive training, arts, basic occupational therapy, music therapy, psychoeducation, or sessions with the treating psychiatrist. The afternoon activities may additionally include outdoor activities such as shopping, walking, and training of public transportation use. The nursing staff is to encourage participation and to reduce sedentary behaviors. However, patients are not forced into activities. Therefore, we may conclude that the social context and rhythm are comparable across 100 participants and thus constitute the most standardized periods during the day. At other periods of the day there is less structured guidance and therefore far more variation in the patients’ activities.
Data were inspected for recording pauses or inconsistencies with the patients’ report. Of the 100 patients’ data sets, 88 included complete and consistent data between 10:00–11:00 am and 95 between 3:00–4:00 pm. All data available were entered in the analyses. The mean activity level (AL) is given as the movement counts per 2 s.
In a first step, we analyzed the 60-min motor activity data as a time series of movement counts. The aim was to establish whether the amount of movement at one time point would be associated with the amount of movement at subsequent time points. Therefore, the partial autocorrelation function (PACF) was computed for each 60-min time series with a total of 1800 bins resulting from the 2-s intervals. The PACF consists of all partial autocorrelations and indicates how much the time series is correlated to itself. The partial autocorrelation at lag p is the correlation of the movement count of a patient with his or her movement count p lags (ie, 2 p seconds) earlier, partialling out the effect of the p-1 previous intervals. Correlation coefficients for 2-s intervals are depicted in ascending order (see figure 1). Generally, correlations are predominantly positive and decrease with increasing P; the predictability of the movement time series tends towards 0 with elapsing time. For each data set we extracted the longest lag with partial autocorrelation still significant at P < .05 (with n = 1800, the threshold is approximately r = .046), indicating the window of predictability of motor behavior. Higher lag numbers indicate a longer time period in which movement counts correlate significantly. Therefore, if 1 subject had a short lag, eg, 1 (see figure 1A), we suspect less structured behavioral patterns than in a subject with a longer lag, eg, 7 (see figure 1B). In the first case movement counts have an autoregressive memory of only 2 s, in the second case of 14 s. Therefore, predictability of movement is superior in the second case, as partial autocorrelations cover a longer time period.
In a second step, we computed a linear regression model for each PANSS subscale as dependent variable with the predictors lag and AL, to explore the impact of partial autocorrelation lags on psychopathological dimensions. Because recording period (10:00–11:00 am or 3:00–4:00 pm) did not influence the predictors (cf. table 2), we used the mean values of lag and AL in the regression models. Furthermore, the PANSS 5-factor structure according to van der Gaag et al.25 was tested. We chose the 5-factor solution that included all 30 PANSS items, as promoted by the authors. We derived scores for the factors “positive symptoms,” “negative symptoms,” “disorganization symptoms,” “excitement,” and “emotional stress.” The factor disorganization includes the PANSS items “conceptual disorganization,” “difficulty in abstract thinking,” “stereotyped thinking,” “mannerisms and posturing,” “unusual thought content,” “disorientation,” “poor attention,” “lack of judgement and insight,” “disturbance of volition,” and “preoccupation.”25 Each model’s parameter estimates are reported in terms of standardized betas (b).
Table 2.
Autocorrelation Values and Motor Activity
| Parameter | Mean (SD) | Range |
|---|---|---|
| Number of Lags | 3.91 (1.82) | 1–10 |
| 10:00–11:00 am | 3.85 (1.87) | 1–10 |
| 3:00–4:00 pm | 3.96 (1.79) | 1–10 |
| Mean Motor Activity | 9.73 (7.88) | 0.85–54.01 |
| 10:00–11:00 am | 10.48 (8.11) | 0.86–51.96 |
| 3:00–4:00 pm | 9.07 (7.65) | 0.85–54.01 |
Note: Mean motor activity given as average 2-s movement counts. Values for 10:00–11:00 am and 3:00–4:00 pm not statistically different for mean motor activity (pairwise t[79] = −1.29, P = .20) and number of lags (pairwise t[79] = 0.69, P = .49).
Results
Descriptive and clinical characteristics are given in table 1. Assessments were conducted in acute and chronic conditions, for most patients within 1–4 weeks following admission (23% in the first week, 18% in the second, 10% in the third week, and 13% in the fourth week). A proportion of 36% of the participants were assessed after 2–6 months post admission. The mean and ranges of autocorrelation lags and AL are given in table 2. Neither cumulative chlorpromazine equivalents (F[1, 98] = 0.04, P = .850) and type of antipsychotic administered (none, first generation, second generation or both; F[3, 96] = 0.16, P = .924) nor sedative use (no sedatives vs sedatives; F[1, 98] = 0.24, P = .625]) had an effect on the number of lags with significant autocorrelation. No difference in movement parameters was found for schizophrenia spectrum diagnoses in terms of significant autocorrelations (F[5, 94] = 0.33, P = .893), or in terms of activity levels (F[5, 94] = 0.46, P = .779]).
Table 1.
Demographic and Clinical Information
| Age (y) | 39.2 (11.1), range 18–65 |
| Male | 57% |
| Duration of illness (y) | 10.5 (9.9), range 0–40 |
| Chlorpromazine equivalents (mg) | 585.1 (450.0), range 0–2060 |
| Antipsychotic treatment (%) | No antipsychotic (3) |
| FGA (7) | |
| SGA (72) | |
| Both FGA and SGA (18) | |
| Sedative use (%) | None (63) |
| Zopiclone or zoldipem (8) | |
| Benzodiazepines (29) | |
| Mood stabilizers (%) | None (75) |
| Mood stabilizer use (25) | |
| Diagnoses (%) | Paranoid schizophrenia (58) |
| Disorganized schizophrenia (12) | |
| Catatonic schizophrenia (10) | |
| Undifferentiated schizophrenia (1) | |
| Schizophreniform disorder (14) | |
| Schizoaffective disorder (5) | |
| PANSS positive (SD) | 15.3 (5.7), range 7–32 |
| PANSS negative (SD) | 17.5 (6.5), range 7–31 |
| PANSS general psychopathology (SD) | 33.5 (9.4), range 16–58 |
| PANSS total score (SD) | 66.3 (17.8), range 32 – 111 |
| PANSS DIS (SD) | 22.7 (6.9), range 10–41 |
| PANSS EXC (SD) | 15.1 (5.5), range 8–34 |
| PANSS EMO (SD) | 18.0 (6.4), range 8–34 |
Note: FGA, First Generation Antipsychotic; SGA, Second Generation Antipsychotic; PANSS, Positive and Negative Syndrome Scale; DIS, Disorganization Factor; EXC, Excitement Factor; EMO, Emotional Distress Factor.
Classic PANSS Subscales
PANSS positive syndrome scores were predicted (F[2, 97] = 3.10, P = .049]) by the number of lags (b = −.25, P = .015) but not by the mean motor activity (b = .02, P = .834). Thus, reduced number of lags was associated with more severe positive symptoms. In contrast, PANSS negative syndrome scores were predicted (F[2, 97] = 3.48, P = .035) by the mean motor activity (b = −.22, P = .027) but not by the number of lags (b = −.09, P = .362). Therefore, reduced motor activity levels were associated with increased negative syndrome severity. The models for the PANSS general symptom score and PANSS total score were similar to the PANSS positive scores. PANSS general psychopathology scores were predicted (F[2, 97] = 3.19, P = .046]) by the number of lags (b = −.24, P = .020) but not by the mean motor activity (b = −.04, P = .671). PANSS total score was predicted (F[2, 97] = 4.02, P = .021]) by the number of lags (b = −.24, P = .018) but not by the mean motor activity (b = −.10, P = .317). When investigating single items instead of PANSS factors or subscales, we found only weak correlations with mean motor activity and autocorrelation lags (see supplementary table 1).
Alternative PANSS Dimensions
When taking into account the PANSS factor solutions of van der Gaag et al., we found a statistical trend in the model of DIS, the PANSS disorganization factor (F[2, 97] = 2.76, P = .069; number of lags b = −.23, P = .022; mean motor activity b = .02, P = .855). The emotional distress factor EMO (F[2, 97] = 1.48, P = .233; number of lags b = −.17, P = .104; mean motor activity b = −.02, P = .850]) was unrelated to patients’ movement. Again, van der Gaag’s PANSS positive factor POS and the negative factor NEG demonstrated significant results as in the classic PANSS subscales. POS was associated with the number of lags (F[2, 97] = 3.26, P = .043; number of lags b = −.25, P = .014; mean motor activity b = .00, P = .979), and NEG with the mean motor activity (F[2, 97] = 3.17, P = .046; number of lags b = −.05, P = .603; mean motor activity b = −.23, P = .023]). Additionally, the excitement factor EXC (F[2, 97] = 3.28, P = .042; number of lags b = −.23, P = .027; mean motor activity b = −.07, P = .449]) was statistically linked to movement predictors. Thus, the predictability of movement patterns is associated with the positive, disorganization, and excitement syndrome scores. In contrast, the negative syndrome was predicted by the mean activity level.
Discussion
The present study investigated the internal structure of movements over time in relation to severity of psychopathology. The main findings are as follows: Positive syndrome and general psychopathology severity may be predicted by less structured movement patterns irrespective of the overall level of motor activity. The same findings apply to the PANSS factors excitement and at trend level to disorganization. In contrast, negative syndrome severity is predicted by reduced motor activity but not by the structure of movement patterns.
Disorganized behavior is one of the key symptoms in schizophrenia.4 Self-report and observer ratings are of limited value for the assessment of behavioral disorganization. Thus, objective assessment methods such as actigraphy are preferred. At the broadest level of rest-activity patterns, disrupted circadian rhythms have been frequently reported in schizophrenia.19,20,26 Disrupted circadian rhythms may impair cognitive performance in schizophrenia but are unrelated to psychopathological dimensions.20 A recent actigraphy study investigated the distribution of rest and activity, ie the persistency of resting and active periods. The authors reported less variable behavioral movement patterns in schizophrenia patients.27 This study did not find associations of behavioral patterns with psychopathology, probably due to the small sample size and the inclusion of data collected during sleep. In our study, the pattern of locomotor behavior in 100 schizophrenia spectrum patients was analyzed in 2 60-min periods at very small time scales, ie 2-s intervals.
Pattern of Motor Activity
The association of the PANSS positive syndrome score and reduced predictability of spontaneous motor behavior had been hypothesized. Conceptually, one would expect items such as “conceptual disorganization” and “excitement” to contribute to patterns of motor behavior. Our findings parallel those of an experimental study reporting less predictable movement patterns with symptoms of excitability and agitation in schizophrenia.5 Interestingly, in our study the activity level was unrelated to the predictability of movement. Thus, the association of disrupted movement patterns and positive symptoms was not solely driven by agitation. Most previous reports on actigraphic movement assessment and symptom severity found no clear evidence for an association of motor activity and positive syndrome scores.16,18,19 One study in patients of an rehabilitation ward with very low PANSS scores (mean PANSS total score of 40) found a negative correlation of PANSS positive syndrome scores and activity levels, ie lower activity was associated with more severe positive symptoms.15 This seems contradictory to our findings, however, because the majority of patients had no or only mild symptoms, patients with persistent symptoms may have had lower motor activity and thus contributed to the inverse correlation. Taken together, our findings suggest that positive symptoms are not associated with the amount of motor activity but with its temporal organization in windows covering 10–15 s.
The positive syndrome includes symptom domains of reality distortion (delusions and hallucinations) and disorganization (conceptual disorganization and bizarre behavior), the latter being related also to poor neurocognitive performance.28 Indeed, some authors have attributed disorganized behavior to conceptual disorganization.3,29 One could imagine that conceptual disorganization prevents proper action planning, which in turn leads to less predictable and disorganized motor behavior. In addition, disorganized behavior may also be related to symptoms associated with the motor dimension of schizophrenia, such as stereotypies or ambitendency.4,6 This view is supported by 2 large studies in first episode medication naïve patients as well as admissions with schizophrenia spectrum disorders demonstrating positive correlations of disorganization and catatonia symptoms such as echophenomena, stereotypies, mannerisms, and agitation.30,31 Likewise, the PANSS disorganization factor of van der Gaag et al.25 was associated with less predictable movement patterns. Irrespective to the overall motor activity level, the same association was observed for the excitement factor25 in our study.
Finally, positive symptoms were suggested to be associated with aberrant sense of causality in schizophrenia.32 The study reported the failure to predict the effect of one’s own actions to be associated with positive symptoms.32 In a gestalt perception paradigm, the positive factor was linked to reduced gestalt perception stability in schizophrenia.33 In line with that, our findings suggest that positive symptoms reduced the temporal organization of behavior; in other words, positive symptoms reduced the timeframe in which actions may be predicted. We may speculate that the reduced predictability of movements and the altered sense of agency are both related to the same deficit of internal action monitoring in schizophrenia.34
Amount of Motor Activity
Our finding of an association between negative syndrome severity and reduced motor activity parallels previous results of actigraphy studies,15,16,18 studies using video-analyses35 or comprehensive motor rating scales.30,31 Consistently, psychomotor slowing was correlated with the negative syndrome, particularly with avolition and emotional withdrawal.6,11,16,36 According to our findings, negative symptoms are unrelated to the predictability of movements. One could have hypothesized that motor slowing and avolition would lead to increased predictability of movements, ie higher probability of succeeding resting periods. However, also in our previous work, negative symptoms were unrelated with the average duration of immobility.16 Thus, negative symptoms seem to reduce overall activity but do not affect the pattern of motor behavior.
Implications for Translational Research
In subjects with a current schizophrenia episode, positive symptom severity was not related to static measures of motor activity16,18,19 or circadian rhythm disturbances.19,20,26 However, in our study and an experimental study, the aberrant patterns of motor behavior were linked to positive symptom severity.5 The disruption of behavioral patterns has been suggested to be valuable for translational research.5,27 Particularly, the spatial distribution of movement was useful to separate hyperactivity as seen in mania and schizophrenia from exploratory behavior in mania.5 Indeed, locomotor hyperactivity has widely been used to model positive symptoms of schizophrenia in rodents because of its association with hyperdopaminergic states.37 Our findings and those of Sano and colleagues27 call for the exploration of temporal patterns of animal motor behavior, which might become an interesting model for positive symptoms in schizophrenia. For larger scale rest-activity patterns, this has been already attempted. Mutants of blind-drunk mice displayed behavioral alterations similar to schizophrenia: reduced overall locomotor activity and disrupted circadian rhythms. This mouse model links abnormal neurotransmitter signaling with circadian rhythm disturbances in schizophrenia.38
Strengths and Limitations
The strengths of the current study are the large amount of objectively assessed continuous motor activity data and the selection of time periods for the analyses in which social interaction and social rhythms were well comparable across the 100 schizophrenia spectrum patients. Some limitations, however, require discussion. Actigraphy was used to record motor behavior, but it has to be kept in mind that the method is neither a valid assessment of particular movement disorders in the schizophrenia spectrum nor does it distinguish goal directed from random motor behavior. But with the method applied we still derived important information on the distribution of motor activity. Diagnoses were given after thorough clinical psychiatric examination and chart review; however, structured interviews such as SCID were not applied. All of the patients had been exposed to antipsychotic medication, and 97% were on antipsychotics during the recording period. Even though autocorrelation lags were not correlated with chlorpromazine equivalents and did not differ between antipsychotic substance classes, we cannot completely rule out medication effects. The effect of antipsychotics on motor behavior in schizophrenia spectrum disorders is a complex problem. Motor abnormalities including psychomotor slowing may already be present before the onset of medication; antipsychotics may ameliorate, deteriorate or induce motor abnormalities.6,11,30 Furthermore, we did not systematically assess movement disorders in this study. Therefore, a proportion of patients may have experienced specific catatonic behavior. Still, because the sample size is quite large, we expect that the positive and negative effects of medication on the actigraphy data were balanced within the whole group. The PANSS was assessed only once, assuming symptom stability over 24h. However, in a proportion of participants modest changes of symptom severity may have occurred. Symptoms of depression may have influenced results but were not specifically assessed. Still, only 18% of the patients had a score of 4 or more on the PANSS item depression (G6), which was also not correlated to motor behavior data (see supplementary table 1). Another limitation is the selection of the time periods examined. We could not establish an effect of the time period (morning vs afternoon) on the autocorrelation lags. However, it might be possible that this is not true for every hour of the day. Our study was conducted in a large group of schizophrenia spectrum patients during inpatient treatment. Therefore, field studies have to determine whether the findings may also hold true for outpatients. The methodological problem of the effects of multiple heterogeneous external inputs on motor behavior, however, then needs consideration.
Conclusion
In a large group of inpatients with schizophrenia spectrum disorders, positive syndrome severity was related to disrupted motor behavior patterns. The behavioral pattern observed may represent an expression of conceptual disorganization and impaired action monitoring. In contrast, negative syndrome severity was unrelated to the pattern of movements but associated with reduced motor activity levels. Objective assessments of motor behavioral patterns may become a useful tool for translational studies of schizophrenia dimensions.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Kraepelin E. Einführung in die Psychiatrische Klinik. Leipzig, Germany: Verlag von Johann Ambrosius Barth; 1921. [Google Scholar]
- 2. Turner T. Rich and mad in Victorian England. Psychol Med. 1989; 19: 29–44 [DOI] [PubMed] [Google Scholar]
- 3. Bleuler E. Dementia praecox oder Gruppe der Schizophrenien. Leipzig, Germany, and Wien, Austria: Franz Deuticke; 1911. [Google Scholar]
- 4. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009; 110: 1–23 [DOI] [PubMed] [Google Scholar]
- 5. Perry W, Minassian A, Paulus MP, et al. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Arch Gen Psychiatry. 2009; 66: 1072–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walther S, Strik W. Motor symptoms and schizophrenia. Neuropsychobiology. 2012; 66: 77–92 [DOI] [PubMed] [Google Scholar]
- 7. Whitty PF, Owoeye O, Waddington JL. Neurological signs and involuntary movements in schizophrenia: intrinsic to and informative on systems pathobiology. Schizophr Bull. 2009; 35: 415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McLeod HJ, Jaques S, Deane FP. Base rates of physical activity in Australians with schizophrenia. Psychiatr Rehabil J. 2009; 32: 269–275 [DOI] [PubMed] [Google Scholar]
- 9. Brown S, Birtwistle J, Roe L, Thompson C. The unhealthy lifestyle of people with schizophrenia. Psychol Med. 1999; 29: 697–701 [DOI] [PubMed] [Google Scholar]
- 10. Vancampfort D, Probst M, Sweers K, Maurissen K, Knapen J, De Hert M. Relationships between obesity, functional exercise capacity, physical activity participation and physical self-perception in people with schizophrenia. Acta Psychiatr Scand. 2011; 123: 423–430 [DOI] [PubMed] [Google Scholar]
- 11. Morrens M, Hulstijn W, Sabbe B. Psychomotor slowing in schizophrenia. Schizophr Bull. 2007; 33: 1038–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bracht T, Schnell S, Federspiel A, et al. Altered cortico-basal ganglia motor pathways reflect reduced volitional motor activity in schizophrenia. Schizophr Res. 2013; 143: 269–276 [DOI] [PubMed] [Google Scholar]
- 13. Berle JO, Hauge ER, Oedegaard KJ, Holsten F, Fasmer OB. Actigraphic registration of motor activity reveals a more structured behavioural pattern in schizophrenia than in major depression. BMC Res Notes. 2010; 3: 149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walther S, Federspiel A, Horn H, et al. Alterations of white matter integrity related to motor activity in schizophrenia. Neurobiol Dis. 2011; 42: 276–283 [DOI] [PubMed] [Google Scholar]
- 15. Wichniak A, Skowerska A, Chojnacka-Wójtowicz J, et al. Actigraphic monitoring of activity and rest in schizophrenic patients treated with olanzapine or risperidone. J Psychiatr Res. 2011; 45: 1381–1386 [DOI] [PubMed] [Google Scholar]
- 16. Walther S, Koschorke P, Horn H, Strik W. Objectively measured motor activity in schizophrenia challenges the validity of expert ratings. Psychiatry Res. 2009; 169: 187–190 [DOI] [PubMed] [Google Scholar]
- 17. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987; 13: 261–276 [DOI] [PubMed] [Google Scholar]
- 18. Minassian A, Henry BL, Geyer MA, Paulus MP, Young JW, Perry W. The quantitative assessment of motor activity in mania and schizophrenia. J Affect Disord. 2010; 120: 200–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Afonso P, Brissos S, Figueira ML, Paiva T. Schizophrenia patients with predominantly positive symptoms have more disturbed sleep-wake cycles measured by actigraphy. Psychiatry Res. 2011; 189: 62–66 [DOI] [PubMed] [Google Scholar]
- 20. Bromundt V, Köster M, Georgiev-Kill A, et al. Sleep-wake cycles and cognitive functioning in schizophrenia. Br J Psychiatry. 2011; 198: 269–276 [DOI] [PubMed] [Google Scholar]
- 21. Bracht T, Heidemeyer K, Koschorke P, et al. Comparison of objectively measured motor behavior with ratings of the motor behavior domain of the Bern Psychopathology Scale (BPS) in schizophrenia. Psychiatry Res. 2012; 198: 224–229 [DOI] [PubMed] [Google Scholar]
- 22. Walther S, Horn H, Koschorke P, Müller TJ, Strik W. Increased motor activity in cycloid psychosis compared to schizophrenia. World J Biol Psychiatry. 2009; 10: 746–751 [DOI] [PubMed] [Google Scholar]
- 23. Walther S, Horn H, Razavi N, Koschorke P, Müller TJ, Strik W. Quantitative motor activity differentiates schizophrenia subtypes. Neuropsychobiology. 2009; 60: 80–86 [DOI] [PubMed] [Google Scholar]
- 24. Middelkoop HA, van Dam EM, Smilde-van den Doel DA, Van Dijk G. 45-hour continuous quintuple-site actimetry: relations between trunk and limb movements and effects of circadian sleep-wake rhythmicity. Psychophysiology. 1997; 34: 199–203 [DOI] [PubMed] [Google Scholar]
- 25. van der Gaag M, Hoffman T, Remijsen M, et al. The five-factor model of the Positive and Negative Syndrome Scale II: a ten-fold cross-validation of a revised model. Schizophr Res. 2006; 85: 280–287 [DOI] [PubMed] [Google Scholar]
- 26. Wulff K, Dijk DJ, Middleton B, Foster RG, Joyce EM. Sleep and circadian rhythm disruption in schizophrenia. Br J Psychiatry. 2012; 200: 308–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sano W, Nakamura T, Yoshiuchi K, et al. Enhanced persistency of resting and active periods of locomotor activity in schizophrenia. PLoS ONE. 2012; 7: e43539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ventura J, Thames AD, Wood RC, Guzik LH, Hellemann GS. Disorganization and reality distortion in schizophrenia: a meta-analysis of the relationship between positive symptoms and neurocognitive deficits. Schizophr Res. 2010; 121: 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Andreasen NC. A unitary model of schizophrenia: Bleuler’s “fragmented phrene” as schizencephaly. Arch Gen Psychiatry. 1999; 56: 781–787 [DOI] [PubMed] [Google Scholar]
- 30. Peralta V, Campos MS, De Jalón EG, Cuesta MJ. Motor behavior abnormalities in drug-naïve patients with schizophrenia spectrum disorders. Mov Disord. 2010; 25: 1068–1076 [DOI] [PubMed] [Google Scholar]
- 31. Peralta V, Cuesta MJ. Motor features in psychotic disorders. I. Factor structure and clinical correlates. Schizophr Res. 2001; 47: 107–116 [DOI] [PubMed] [Google Scholar]
- 32. Voss M, Moore J, Hauser M, Gallinat J, Heinz A, Haggard P. Altered awareness of action in schizophrenia: a specific deficit in predicting action consequences. Brain. 2010; 133: 3104–3112 [DOI] [PubMed] [Google Scholar]
- 33. Tschacher W, Bergomi C. Cognitive binding in schizophrenia: weakened integration of temporal intersensory information. Schizophr Bull. 2011; 37 (Suppl 2):S13–S22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Turken AU, Vuilleumier P, Mathalon DH, Swick D, Ford JM. Are impairments of action monitoring and executive control true dissociative dysfunctions in patients with schizophrenia? Am J Psychiatry. 2003; 160: 1881–1883 [DOI] [PubMed] [Google Scholar]
- 35. Kupper Z, Ramseyer F, Hoffmann H, Kalbermatten S, Tschacher W. Video-based quantification of body movement during social interaction indicates the severity of negative symptoms in patients with schizophrenia. Schizophr Res. 2010; 121: 90–100 [DOI] [PubMed] [Google Scholar]
- 36. Docx L, Morrens M, Bervoets C, et al. Parsing the components of the psychomotor syndrome in schizophrenia. Acta Psychiatr Scand. 2012; 126: 256–265 [DOI] [PubMed] [Google Scholar]
- 37. van den Buuse M. Modeling the positive symptoms of schizophrenia in genetically modified mice: pharmacology and methodology aspects. Schizophr Bull. 2010; 36: 246–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oliver PL, Sobczyk MV, Maywood ES, et al. Disrupted circadian rhythms in a mouse model of schizophrenia. Curr Biol. 2012; 22: 314–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.