Abstract
Background:
This study examined smooth pursuit eye movement (SPEM), prepulse inhibition (PPI), and auditory event-related potentials (ERP) to paired stimuli as putative endophenotypes of psychosis across the schizophrenia-bipolar disorder dimension.
Methods:
Sixty-four schizophrenia probands (SZP), 40 psychotic bipolar I disorder probands (BDP), 31 relatives of SZP (SZR), 26 relatives of BDP (BDR), and 53 healthy controls (HC) were tested. Standard clinical characterization, SPEM, PPI, and ERP measures were administered.
Results:
There were no differences between either SZP and BDP or SZR and BDR on any of the SPEM, PPI, or ERP measure. Compared with HC, SZP and BDP had lower SPEM maintenance and predictive pursuit gain and ERP theta/alpha and beta magnitudes to the initial stimulus. PPI did not differ between the psychosis probands and HC. Compared with HC, SZR and BDR had lower predictive pursuit gain and ERP theta/alpha and beta magnitudes to the first stimulus with differences ranging from a significant to a trend level. Neither active symptoms severity nor concomitant medications were associated with neurophysiological outcomes. SPEM, PPI, and ERP scores had low intercorrelations.
Conclusion:
These findings support SPEM predictive pursuit and lower frequency auditory ERP activity in a paired stimuli paradigm as putative endophenotypes of psychosis common to SZ and BD probands and relatives. PPI did not differ between the psychosis probands and HC. Future studies in larger scale psychosis family samples targeting putative psychosis endophenotypes and underlying molecular and genetic mediators may aid in the development of biology-based diagnostic definitions.
Key words: psychosis, schizophrenia, bipolar disorder, smooth pursuit eye movement, prepulse inhibition, auditory ERP
Introduction
Accumulating evidence indicates that dimensional characterization of psychosis captures several important aspects of severe mental illness including neurophysiologic, cognitive, and genetic manifestations that cut across Diagnostic and Statistical Manual of Mental Disorders (DSM) diagnoses.1 This is opposed to the traditional view of the 2 most prominent psychotic disorders, schizophrenia and bipolar disorder, being categorical entities.2 Optimal methods for categorizing psychotic illnesses remain uncertain given the lack of biology-based diagnostic criteria. A promising strategy that seeks to identify valid diagnostic markers is the study of endophenotypes: the heritable characteristics of brain structure/function that are theoretically interposed between genes and behavior and may provide more efficient routes to the discovery of molecular underpinnings of disease definition than clinical syndromes.3
Putative endophenotypes of psychosis have been developed within schizophrenia, including alterations in smooth pursuit eye movement (SPEM), prepulse inhibition (PPI), and event-related potential (ERP) measures of auditory processing, and, more recently, extended to bipolar disorder. Similar SPEM abnormalities have been reported in schizophrenia probands (SZP) and bipolar disorder (BDP) and in their biological relatives (SZR and BDR)4 although in bipolar disorder these data are less extensive with results sometimes attributed to effects of lithium.5 PPI deficits have been reported in SZP and SZR6,7; Wynn et al8 found impaired prepulse facilitation (PPF) but normal PPI in SZP and their siblings. Studies of BDP largely indicate similar PPI deficits during mania with normalization in euthymic phases.9,10 Auditory ERPs in paired stimuli (S1–S2) tasks have indicated processing abnormalities in both SZP and BDP11,12 and their relatives.13,14 These abnormalities typically manifest as a larger difference between S1 and S2 responses for healthy controls (HC) than for psychosis groups caused by larger ERPs to S2 and/or an attenuated response to S1 and/or an attenuated response to S1.15,16 Quantification of these abnormalities can be efficiently captured using frequency domain rather than ERP voltage analyses.12
In this study, we examined 3 neurophysiological paradigms (SPEM, PPI, and auditory ERPs in a paired stimuli task) as putative psychosis endophenotypes, hypothesizing common alterations across the schizophrenia-bipolar disorder psychosis dimension. In accordance with the classic endophenotype conceptualization,3 at first, we examined these measures in SZP and psychotic BDP and contrasted with HC. Subsequently, to test their manifestation in biological relatives, we conducted analyses in SZR and BDR and contrasted with HC. We hypothesized that (1) SZP and BDP will show similar and abnormal performance on SPEM, PPI, and ERP measures, and (2) SZR and BDR will show similar and abnormal performance on these measures, albeit SPEM, PPI, and ERP alterations will be milder than those found in probands.
Methods
Characteristics of the Study Sample
The study included probands who met DSM-IV criteria for schizophrenia or bipolar disorder-type I, with lifetime history of psychosis; their first-degree relatives with and without lifetime psychiatric diagnoses; and community HC. Probands were recruited through advertising and by referrals from outpatient mental health centers; relatives were recruited with the probands’ consent. Individuals with a history of major neurological or decompensated medical illness, mental retardation, traumatic brain injury, substance abuse within the last month or substance dependence within 3 months were excluded. All volunteers were clinically screened for on-going excessive drinking and signs of alcohol intoxication/withdrawal and received illicit drug urine screen prior to the laboratory data acquisition. In addition, all volunteers abstained from nicotine and caffeine for a minimum of 30 min before SPEM, PPI, and ERP testing. The study was approved by UT southwestern institutional review board and was consistent with standard for the ethical conduct of human research. All volunteers provided written informed consent after the study procedures had been fully explained.
A total of 214 volunteers were recruited, including 100 probands (62 SZP and 38 BDP), 61 relatives (32 SZR and 29 BDR), and 53 HC. The few relatives who had a diagnosis of schizophrenia (n = 2: one SZR and one BDR) or psychotic bipolar disorder (n = 2: both BDR) were used as probands in all analyses. Therefore, the overall sample included 64 SZP, 40 BDP, 31 SZR, 26 BDR, and 53 HC although not all volunteers completed all measures. The sample sizes for each endophenotype are indicated in table 2. Demographic and clinical characteristics of the study sample are presented in table 1; the characteristics of the subsamples for each endophenotype were not different from an overall sample. There was a significant between-group difference in age accounted for by older age of SZP than for BDR. There was a higher proportion of males among SZP compared with HC, and a higher proportion of African-Americans among SZP and SZR compared with BDP and BDR groups. Therefore, age and gender were included as covariates in the relevant logistic regression analyses. There were no between-group differences in ethnicity, years of education, or the Wechsler Test of Adult Reading IQ estimates.
Table 2.
Sample Sizes, Group Means, and Standard Deviations for the SPEM, PPI, and Auditory ERP Outcomes in the Schizophrenia and Psychotic Bipolar I Disorder Probands, Their Relatives, and Healthy Controls
| SZP | BDP | SZR | BDR | HC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measures | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) |
| SPEM (gain) | ||||||||||
| MPG_9.9 | 57 | 0.86 (0.10) | 39 | 0.85 (0.13) | 29 | 0.88 (0.10) | 24 | 0.89 (0.10) | 39 | 0.91 (0.08) |
| MPG_18.7 | 57 | 0.82 (0.12) | 39 | 0.79 (0.16) | 29 | 0.84 (0.12) | 23 | 0.85 (0.12) | 39 | 0.86 (0.11) |
| MPG_25.0 | 57 | 0.73 (0.16) | 39 | 0.71 (0.17) | 29 | 0.77 (0.11) | 24 | 0.76 (0.17) | 39 | 0.81 (0.13) |
| PPG_9.9 | 57 | 0.66 (0.10) | 39 | 0.65 (0.12) | 29 | 0.69 (0.09) | 24 | 0.68 (0.10) | 39 | 0.73 (0.10) |
| PPG_18.7 | 57 | 0.52 (0.13) | 39 | 0.52 (0.11) | 29 | 0.55 (0.11) | 24 | 0.57 (0.13) | 39 | 0.62 (0.11) |
| PPG_25.0 | 55 | 0.48 (0.13) | 38 | 0.46 (0.16) | 29 | 0.48 (0.12) | 24 | 0.48 (0.13) | 38 | 0.57 (0.14) |
| PPI and PPF (Δ%) | ||||||||||
| PPI | 34 | 43.58 (34.41) | 26 | 46.82 (29.48) | 24 | 55.16 (24.53) | 21 | 68.98 (18.95) | 22 | 49.93 (20.61) |
| PPF | 34 | −10.9 (34.57) | 26 | −6.39 (35.79) | 23 | −2.11 (36.81) | 21 | −17.41 (41.44) | 22 | −9.52 (48.08) |
| Auditory ERP (dB) | ||||||||||
| Beta | 40 | −0.62 (5.34) | 33 | −1.04 (6.07) | 26 | 0.93 (6.58) | 22 | 0.85 (4.95) | 13 | 4.03 (4.58) |
| Theta/alpha | 40 | 8.85 (6.11) | 33 | 9.47 (6.02) | 26 | 9.75 (5.91) | 22 | 9.99 (7.73) | 13 | 14.63 (6.47) |
Note: SPEM, smooth pursuit eye movement; ERP, event-related potentials; HC, healthy controls; MPG, maintenance pursuit gain (at 9.9°/s, 18.7°/s, and 25.0°/s velocity); PPG, predicative pursuit gain (at 9.9°/s, 18.7°/s, and 25.0°/s velocity); PPI, prepulse inhibition at 120 ms interstimulus interval; PPF, prepulse facilitation at 4500 ms interstimulus interval; beta, ERP beta frequency score; theta/alpha, ERP theta/alpha frequency score.
Table 1.
Demographic and Clinical Characteristics of Study Sample
| Sociodemographic Characteristics | SZP (n = 64) | BDP (n = 40) | SZR (n = 31) | BDR (n = 26) | HC (n = 53) |
|---|---|---|---|---|---|
| Age (y); mean (SD)a | 40.58 (10.73) | 36.18 (10.37) | 41.97 (10.84) | 32.19 (14.98) | 36.84 (11.35) |
| Gender/male; nb | 36 | 15 | 13 | 12 | 18 |
| Ethnicity, Hispanic; n | 4 | 3 | 2 | 2 | 5 |
| Race/ Black; nc | 20 | 3 | 8 | 1 | 12 |
| Education (y); mean (SD) | 13.66 (2.55) | 13.95 (2.61) | 14.93 (2.4) | 13.62 (3.02) | 13.7 (1.83) |
| WTAR IQ; Mean (SD) | 99.18 (13.57) | 102.24 (9.99) | 100.38 (12.59) | 101.50 (12.57) | 105.19 (8.91) |
| BPRS total scores; mean (SD)d | 48.29 (12.21) | 41.89 (10.61) | 31.36 (8.94) | 28.38(5.93) | n/a |
| BPRS psychosis scores; mean (SD) | 13.7 (5.98) | 8.71 (3.7) | 5.29 (2.07) | 5.35 (1.49) | n/a |
| BPRS affective scores; mean (SD) | 7.87 (2.55) | 9.47 (2.89) | 5.82 (1.89) | 5.54 (1.75) | — |
| Psychiatric diagnoses in relativese | n/a | n/a | 7 (22.58) | 6 (23.08) | — |
| No DSM-IV Axis I/II diagnoses; n (%) | — | — | — | 1 (3.85) | — |
| Substance-induced psychosis; n (%) | — | — | — | 1 (3.85) | — |
| Psychosis NOS; n (%) | — | — | 5 (15.13) | 1 (3.85) | — |
| Psychotic MDD; n (%) | — | — | 1 (3.23) | 1 (3.85) | — |
| Nonpsychotic bipolar disorder; n (%) | — | — | 8 (25.81) | 8 (30.8) | — |
| Nonpsychotic MDD; n (%) | — | — | 6 (19.35) | 3 (11.54) | — |
| Anxiety Disorders; n (%) | — | — | 9 (29.03) | 13 (50.0) | — |
| Substance abuse/dependence; n (%) | — | — | 6 (19.35) | 3 (11.54) | — |
| Cluster A personality disorder; n (%) | — | — | — | 2 (7.69) | — |
| Cluster B personality disorder; n (%) | — | — | 4 (12.90) | 5 (19.23) | — |
| Cluster C personality disorder; n (%) | — | — | 14 (45.16) | 13 (50.0) | — |
| Comorbid Axis I/II diagnoses; n (%) | — | — | — | — | — |
| Concomitant medications | |||||
| Off medications; n (%) | 1 (1.56) | 2 (5.00) | 19 (61.29) | 19 (73.08) | — |
| Typical antipsychotics; n (%) | 11 (17.19) | 1 (2.5) | 0 | 0 | — |
| Atypical antipsychotics; n (%) | 35 (54.69) | 20 (50.00) | 2 (6.45) | 2 (7.69) | — |
| Antidepressants; n (%) | 24 (37.5) | 17 (42.5) | 9 (29.03) | 4 (15.38) | — |
| Lithium; n (%) | — | 15 (37.5) | — | — | — |
| Other mood stabilizers; n (%) | 7 (10.94) | 22 (55.0) | 3 (9.68) | 1 (3.85) | — |
| Anxiolytics/hypnotics; n (%) | 13 (20.31) | 15 (37.5) | 5 (16.13) | 4 (15.38) | — |
| Combined medications; n (%) | 34 (53.13) | 29 (72.5) | 5 (16.13) | 2 (7.69) | — |
Note: SZP, probands with schizophrenia; BDP, probands with psychotic bipolar I disorder; SZR, relatives of SZP; BDR, relatives of BDP; WTAR IQ, Wechsler Test of Adult Reading general intelligence estimate; BPRS, the Brief Psychiatric Rating Scale; NOS, not otherwise specified; MDD, major depressive disorder; n/a, not available/not collected.
aAge: F (4, 199) = 4.131, P = .007; SZP vs BDR, P = .02.
bGender: SZP vs HC, χ2(1, N = 117) = 4.93, P = .026.
cRace: SZP vs BDP, χ2(1, N = 100) = 7.10, P = .008; SZP vs BDR, χ2(1, N = 87) = 6.83, P = .009; SZR vs BDR, χ2(1, N = 55) = 4.04, P = .044.
dBPRS scores: total score [F(3, 134) = 28.55, P < .001; SZP vs BDP, P = .022; SZP vs SZR, P < .001; SZP vs BDR, P < .001; BDP vs SZR, P < .001; BDP vs BDR, P < .001]; psychosis subscale score [F(3, 135) = 34.38, P < .001; SZP vs BDP, P < .001; SZP vs SZR, P < .001; SZP vs BDR, P < .001; BDP vs SZR, P = .005; BDP vs BDR, P = .008]; affective subscale score [F(3, 135) = 19.19, P < .001; SZP vs BDP, P = .01; SZP vs SZR, P = .002; SZP vs BDR, P < .001; BDP vs SZR, P < .001; BDP vs BDR, P < .001].
eEach Axis I–II diagnosis in relatives is reported separately. The number of relatives who had more than one Axis I/II diagnosis is indicated under “Comorbid Axis I/II diagnoses; n(%).”
The Structured Clinical Interviews for DSM-IV Axis I17 and Axis II18 disorders were used to determine lifetime diagnoses in probands and relatives. The probands were clinically stable medicated outpatients with active psychosis and/or mood symptoms severity varying from remission/euthymic state to mild symptoms. Approximately 23% of SZR and BDR had no lifetime Axis I/II diagnoses. The remaining 77% of relatives with lifetime psychiatric diagnosis were clinically stable and mildly symptomatic/asymptomatic at the time of testing. The Brief Psychiatric Rating Scale (BPRS)19 was used to evaluate active symptom severity. SZP had higher BPRS total and psychosis scores compared with BDP, whereas BDP had higher BPRS affective scores than SZP. Both proband groups had higher BPRS total, psychosis, and affective scores than relatives.
Based on self-reports, most probands (34/64 SZP and 29/40 BDP) were treated with a combination of psychotropic agents at the time of study, including antipsychotics (46/64 SZP and 21/40 BDP), mood stabilizers (7/64 SZP and 31/40 BDP), and other agents, among which antidepressants and anxiolytics were most common. Approximately one-third of relatives (12/31 SZR and 7/26 BDR) were treated with various psychotropic medications.
Laboratory Measures
Smooth Pursuit Eye Movement.
Horizontal eye movements were recorded using a video camera-based system (EyeLink II eyetracker, SR Research) sampling at 500 Hz in a room with controlled illuminance of 2 lux. A target (a cross in a 0.25° × 0.25° box with a photometric contrast of 2.1 log units) was presented on a 22-inch flat screen monitor (ViewSonic, P225f Professional System) set to 150 Hz, placed 60 cm in front of the volunteer. The digital data were filtered off-line using a low-pass filter with data acquisition and analysis software (AcqKnowledge 3.7.3 and IGOR Pro 5.0, Wavemetrics, Inc.). Data were inspected visually to eliminate artifacts (blinks) and saccades. Saccades were identified based on velocity (>35°/s) and acceleration (>600°/s)2 criteria. All saccades and blink artifacts were identified as missing data points.
A Ramp-Mask-Ramp SPEM task20 was administered. The task consisted of three 4-min sessions. Each session included 12 trials at velocities of 9.9°/s, 18.7°/s, and 25.0°/s. A trial started with calibration steps at –12, 0° and +12° of visual angle until the error between the target and the eye was less than 0.1°, followed by 1–3 s of center fixation. The target traversed horizontally across the screen from +12° to −12° of visual angle relative to the central fixation position at a steady velocity (a ramp). Each trial consisted of 1.5–2.5 cycles (ramps) of back-and-forth target motion and included a brief mask of 500 ms during which the target was unpredictably masked. The volunteers were told that the target would disappear briefly but would keep moving and were instructed to continue to follow the target. The mask occurred at the change in ramp direction and during the ramp.
Two primary outcome SPEM measures, maintenance pursuit gain (from ramp section) and predictive pursuit gain (from mask section), were computed at 3 target velocities: 9.9°/s, 18.7°/s, and 25.0°/s. Predictive pursuit gain was calculated using the average eye velocity in the direction of the expected ramp divided by the expected target velocity from 175 ms after the start of the mask to 175 ms before the end of the mask. This window for predictive pursuit was chosen similar to Thaker et al,21 given that the eye motion within 130–170 ms after the occurrence of the mask is considered to be a “residual” closed-loop response. The same window was chosen for the maintenance pursuit gain to match the predictive pursuit window. Thus, maintenance pursuit gain was calculated using average eye velocity divided by the target velocity from 175 ms after the start of the ramp to 175 ms before the end of the ramp. Scoring of the maintenance and predictive pursuit measures was fully automated and blind to the volunteer’s diagnoses.
Prepulse Inhibition.
Standard PPI paradigm was used similar to Hong et al.22 The orbicularis oculi electromyographic activity (EMG) was recorded from the right eye via two 20-mm disk Ag/AgCl electrodes positioned below and lateral to the right eye. A 40 × 50 mm ground electrode was placed on the right forearm. EMG activity was filtered (1- to 1000-Hz and 60-Hz notch filter), and digitized at a 1-kHz rate. The acoustic stimuli were generated by a Psylab Stand Alone monitor and a tone generator (both from Contact Precision Instruments) and delivered via headphones. The EMG was directed through a Grass A.C. Amplifier (model 1CP511, Astro-Med., Inc.) and was acquired by using commercially available hardware/software (BioPac). The sound intensity was measured under the same settings as above by using a headphone coupler (Model EC-9A, Quest Technologies), with a standard 1-lb weight strapped on the headphone during measurement.
During the test volunteers were instructed to relax and keep their eyes open. A session started with a 3-min acclimation period with 70 dB white noise. Startle pulse-alone trials contained 116 dB white noise lasting 40 ms. The prepulse-pulse trials contained a 20 ms, 80 dB white noise prepulse, or 10 dB above the background noise. The first 3 startle responses were discarded. Following the first 3 trials, the test included 18 pulse-alone trials; 12 prepulse-pulse trials with 120 ms interstimulus interval (ISI) for PPI, the ISI shown to detect the largest PPI effect in schizophrenia samples22,23; and 12 prepulse-pulse trials with 4500 ms ISI for PPF.8 Intertrial intervals varied from 12 to 20 s. These trials were evenly divided into 2 blocks and were randomized within each block.
The EMG recording was processed off-line with a 100-Hz high-pass filter and baseline correction by using a 100 ms prestimulus baseline. Response onset was defined by the first crossing from baseline within a 20–120 ms window after stimulus onset. Peak response amplitude was calculated by the difference of the most positive peak and most negative trough in a 20–150 ms window after pulse onset. Two primary outcome measures, PPI and PPF, were calculated for 120 and 4500 ms ISI conditions, respectively, as percent change in response amplitudes [PPI/PPF(Δ%) = (startle alone—prepulse-pulse condition)/startle alone × 100]; a positive value refers to PPI, and a negative value refers to PPF. Of the 176 subjects with PPI data (52 SZP, 36 BDP, 28 SZR, 26 BDR, and 34 HC), 49 were nonresponders (18 SZP, 10 BDP, 4 SZR, 5 BDR, and 12 HC), which was defined as response to less than 50% of the first 8 pulse-alone trials. These subjects were not included in the analyses. The rates of nonresponders were comparable with those reported elsewhere.22
Auditory ERP.
Electroencephalography (EEG) was recorded in a dimly lighted, sound-attenuated room. A standard paired stimuli paradigm was used.12 Participants passively listened to 150 binaural pairs of click stimuli (1 ms duration at 74 dB; 500 ms interclick interval; and 10 000 ms intertrial interval) delivered through STIM2 10 Ohm insert earphones (Compumedics, NeuroMedical Supplies). Recordings were obtained using a Neuroscan NuAmp running under Acquire, Scan4.3 software (Compumedics, Neuroscan), with a sampling rate of 1000 Hz and 0.1- to 100-Hz analog bandpass filter. EEG data consisted of 8 scalp locations (FZ, FCZ, CZ, CPZ, PZ, OZ, C3, and C4) with a linked mastoids reference; electro-oculogram was obtained from sensors placed at the outer canthi of both eyes. Skin impedance was <10 Kohms in all sensors at the onset of data collection.
Data were preprocessed similar to previous studies.12 Cardiac, ocular, and muscle artifacts were identified with Independent Components Analysis and removed (EEGLAB 9.0)24; EEG data on each trial were then segmented into 1250 ms epochs, extending from 250 ms pre-S1 to 1000 ms post-S1 (500 ms post-S2). Trials containing signals above 75 uV were excluded (no more than 20% in any subject). Data were digitally bandpass filtered from 0.5–55 Hz (zero phase filter; roll off: 6 and 48 dB/octave, respectively). The 100 ms before S1 was used for baseline adjustment over the remaining epoch, and data were averaged across trials. Spatial principal components analysis was conducted on the grand average EEG waveform to reduce the 8 scalp sensors to 1 virtual sensor (figure 1a).12,25 Virtual sensor data for each subject were then decomposed into time/frequency domain using modified morlet wavelets applied in 1-Hz steps.12 There were 3 statistically distinguishable frequency bands across all subjects: theta/alpha (4–16 Hz), beta (17–34 Hz), and gamma (35–60 Hz; see online supplementary Methods and Materials, Auditory ERP for details).
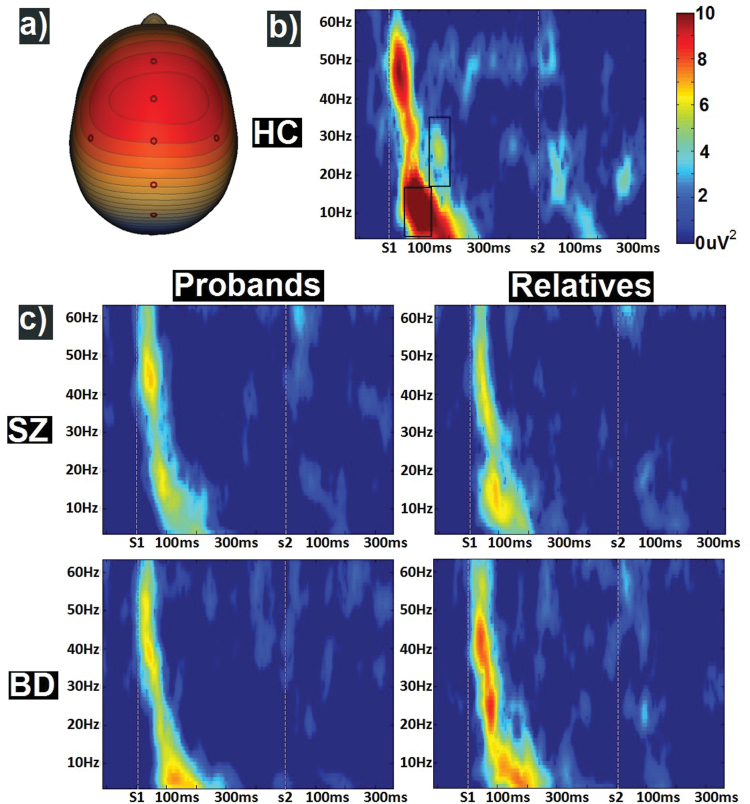
Fig. 1.
ERP analyses. Spatial principal components analysis-identified 1 component with a topography (a) with an FCz maximum. Time frequency plots of nonbaseline adjusted evoked power (b) for healthy control, and (c) proband (left) and relative (right) groups are presented with means across all groups and timepoints removed for each frequency or ease of viewing. Boxes in (b) indicate the time-frequency regions of interest for early theta/alpha and mid-latency beta scores used in the primary analysis.
To identify the time-frequency regions of interest, data were grouped into 25 ms time bins from 50 ms pre-S1 to 750 ms post-S2 and ANOVAs on group membership using only HC, and probands were completed for each frequency bin. Two effects spanning at least 2 consecutive time bins at P < .05 were present: (1) theta/alpha activity from 50 to 150 ms after S1 and (2) beta activity from 150 to 200 ms after S1 was greater in HC than probands and were used in the primary analyses.
Statistical Analyses
All statistical analyses were performed using SAS9.2. A one-way ANOVA with a subsequent post hoc Tukey honestly significant difference test, and Yates corrected chi-square test was used as appropriate for sociodemographic and clinical characteristics. To test the a priori hypotheses, (1) SPEM (maintenance pursuit gain and predictive pursuit gain at 9.9, 18.7, and 25.0°/s), (2) PPI/120 ms ISI and PPF/4500 ms ISI, and (3) ERP early theta/alpha and mid latency beta frequency magnitudes to S1 (values highlighted in figure 1b) measures were compared between the psychosis probands and HC (SZP vs BDP vs HC). Subsequently, the outcomes that were abnormal in probands were tested in relatives and HC (SZR vs BDR vs HC). Because we were interested in comparisons between SZP vs BDP and probands vs HC, as well as between SZR vs BDR and relatives vs HC, omnibus tests were not meaningful on their own. Therefore, the primary analyses were carried out using logistic regression with the effect sizes described in terms of the logistic regression parameter estimates (λ). Additionally, Cohen’s d were calculated to make these outcomes easily comparable to other endophenotype studies.
In addition, series of exploratory correlational analyses were conducted to test for effects of active symptoms severity and psychotropic medications on laboratory measures. For the symptom severity analyses, Pearson correlations between the BPRS scores and all SPEM, PPI, and ERP measures in probands and between the BPRS scores and SPEM and ERP measures in relatives were computed. For the medication effect analyses, each proband/relative was coded either “on” or “off” medication from 4 medication classes: antipsychotics, mood stabilizers, antidepressants, or anxiolytics/sedatives. Correlations between the dichotomized medication status and all SPEM, PPI, and ERP measures in probands and between the medication status and SPEM and ERP measures in relatives were calculated.
Finally, to examine associations among various endophenotypic measures, we conducted correlational analysis in the probands who completed all SPEM, PPI, and ERP tasks (n = 49, including 26 SZP and 23 BDP). Relative groups were not included in this analysis due to known variability of endophenotype manifestations in such samples. Because the primary analyses showed that predictive pursuit gain at 25.0°/s was the most sensitive measure in both probands and relatives, we included this SPEM measure, as well as PPI/PPF and ERP theta/alpha and beta scores, in this analysis.
Results
SPEM, PPI, and ERP Outcomes in Probands
The group means, standard deviations, and sample sizes for the SPEM, PPI/PPF, and ERP outcomes in probands, relatives, and HC are presented in table 2.
The chi-square values, associated P-values, and logistic regression parameter estimates for the primary outcomes in probands and HC are presented in table 3. No differences on any of the SPEM, PPI/PPF, or ERP measures were found to distinguish SZP and BDP. SZP had significantly lower maintenance pursuit gain at 9.9°/s and 25.0°/s velocities (P = .05 for 18.7°/s trials) and predictive pursuit gain at all tested velocities and lower ERP theta/alpha and beta frequency magnitudes compared with HC. Likewise, BDP had lower maintenance pursuit gain and predictive pursuit gain at all velocities and lower ERP (beta, theta/alpha) magnitudes compared with HC. No differences were found in either PPI or PPF between SZP vs HC and BDP vs HC.
Table 3.
Primary Outcomes (SPEM, PPI, Auditory ERP) in the Schizophrenia and Psychotic Bipolar I Disorder Probands, and Healthy Controls
| SZP vs BDP | SZP vs HC | BDP vs HC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | χ 2 | P-Value | λ | D | χ2 | P-Value | λ | d | χ 2 | P-Value | λ | d |
| SPEM (gain) | ||||||||||||
| MPG_9.9 | 0.00 | .98 | 0.05 | 0.09 | 5.91 | .02 | −6.93 | −0.56 | 5.49 | .02 | −6.00 | −0.57 |
| MPG_18.7 | 0.61 | .43 | 1.24 | 0.22 | 3.83 | .05 | −3.95 | −0.33 | 6.09 | .01 | −4.70 | −0.52 |
| MPG_25.0 | 0.04 | .84 | 0.28 | 0.13 | 6.49 | .01 | −4.22 | −0.53 | 7.25 | .01 | −4.59 | −0.67 |
| PPG_9.9 | 0.04 | .84 | 0.40 | 0.09 | 9.41 | .00 | −7.34 | −0.70 | 9.02 | .00 | −7.08 | −0.73 |
| PPG_18.7 | 0.00 | .99 | 0.02 | 0.00 | 12.44 | .00 | −7.27 | −0.83 | 12.83 | .00 | −9.49 | −0.91 |
| PPG_25.0 | 0.07 | .79 | 0.44 | 0.14 | 9.61 | .00 | −6.36 | −0.69 | 9.12 | .00 | −5.47 | −0.73 |
| PPI and PPF (Δ%)a | ||||||||||||
| PPI | 0.00 | .97 | 0.00 | −0.10 | 0.43 | .51 | −0.01 | −0.22 | 0.21 | .65 | −0.01 | −0.12 |
| PPF | 1.30 | .25 | −0.01 | −0.13 | 0.00 | .98 | 0.00 | −0.03 | 0.06 | .80 | 0.00 | 0.08 |
| Auditory ERP (dB) | ||||||||||||
| Beta | 0.00 | .96 | 0.00 | 0.07 | 5.81 | .02 | −0.17 | −0.90 | 5.37 | .02 | −0.19 | −0.90 |
| Theta/alpha | 0.19 | .66 | −0.02 | −0.10 | 7.21 | .01 | −0.17 | −0.93 | 5.24 | .02 | −0.15 | −0.84 |
aGender comparisons (ordinary regression): (1) SZP: PPI, t = 0.37, P = .71, λ = 4.85; PPF, t = 1.19, P = .24, λ = 14.80; (2) BDP: PPI, t = −0.89, P = .38, λ = −11.16; PPF, t = 0.62, P = .54, λ = 9.46; (3) HC: PPI, t = −0.05, P = .96, λ = −0.44; PPF, t = −2.51, P = .02, λ =−48.75.
SPEM and ERP Outcomes in Relatives
Because SPEM and ERP measures were found to be abnormal in both SZP and BDP, we pursued further analyses for these 2 putative endophenotypes in the relative groups. The chi-square values, associated P-values and λ values for these outcomes in relatives and HC are presented in table 4. No differences on any of the SPEM or ERP measures were found for SZR vs BDR. SZR were deficient compared with HC on predictive pursuit gain at higher velocities (18.7°/s and 25.0°/s) but not on maintenance pursuit gain. In addition, SZR had numerically lower ERP theta/alpha and beta frequency scores compared with HC although only the difference for theta/alpha reached statistical significance (theta/alpha, P = .03; beta, P = .11). BDR differed from HC on predictive pursuit gain at 18.7°/s and 25.0°/s velocities, but not on the rest of the SPEM measures. In addition, BDR showed lower ERP theta/alpha and beta magnitudes (theta/alpha, P = .09; beta, P = .04).
Table 4.
Primary Outcomes (SPEM and Auditory ERP) in the Relatives of Schizophrenia and Psychotic Bipolar I Disorder Probands, and Healthy Controls
| SZR vs BDR | SZR vs HC | BDR vs HC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | χ2 | P-Value | Λ | D | χ2 | P-Value | λ | d | χ2 | P-Value | λ | d |
| SPEM (gain) | ||||||||||||
| MPG_9.9 | 0.09 | .76 | 0.90 | −0.10 | 1.22 | .27 | −3.18 | −0.33 | 1.31 | .25 | −3.63 | −0.22 |
| MPG_18.7 | 0.01 | .90 | −0.33 | −0.08 | 0.31 | .57 | −1.30 | −0.18 | 0.30 | .58 | −1.41 | −0.09 |
| MPG_25.0 | 0.33 | .56 | 1.32 | 0.07 | 0.99 | .32 | −2.12 | −0.33 | 2.33 | .13 | −2.95 | −0.34 |
| PPG_9.9 | 0.29 | .59 | 1.75 | 0.11 | 2.28 | .13 | −4.11 | −0.42 | 3.60 | .06 | −5.70 | −0.50 |
| PPG_18.7 | 0.25 | .62 | −1.41 | −0.17 | 5.30 | .02 | −5.79 | −0.64 | 4.51 | .03 | −5.35 | −0.42 |
| PPG_25.0 | 0.59 | .44 | 2.07 | 0.00 | 6.43 | .01 | −5.73 | −0.69 | 6.71 | .01 | −6.54 | −0.64 |
| Auditory ERP (dB) | ||||||||||||
| Beta | 0.38 | .54 | 0.03 | 0.01 | 2.52 | .11 | −0.10 | −0.52 | 4.32 | .04 | −0.18 | −0.66 |
| Theta/alpha | 0.00 | .95 | 0.00 | −0.04 | 4.72 | .03 | −0.15 | −0.51 | 2.94 | .09 | −0.11 | −0.64 |
Effect of Symptom Severity and Medication on SPEM, PPI, and ERP in Probands and Relatives
No significant correlations were obtained between any of the BPRS scores (total, psychosis or affective) and any of the SPEM, PPI, or ERP measures in either probands or relatives. Likewise, no significant correlations between any of the neurophysiological measures and active medication status, including lithium use in BDP, were found in probands. In relatives, maintenance pursuit gain at 25.0°/s correlated weakly with anxiolytics/sedatives use (r = −0.48, P = .04), a correlation expected by chance alone.
Exploratory Associations Between the SPEM, PPI, and ERP Measures in Probands
The exploratory correlational analyses among the selected SPEM (maintenance pursuit gain and predictive pursuit gain at 25.0°/s), PPI/PPF, and ERP measures in probands with complete endophenotype tasks revealed 2 significant intercorrelations: r = 0.46, P < .001 for maintenance and predictive pursuit gain, and r = −0.38, P < .01 for PPI and ERP beta. The rest of correlations were nonsignificant (see online supplementary table 1).
Discussion
This study examined 3 putative neurophysiological endophenotypes for psychosis (SPEM, PPI, and auditory ERP) in probands with schizophrenia or psychotic bipolar disorder, and in their first-degree relatives. Although these measures along with other putative endophenotype-defining approaches (eg, P300 ERP, mismatch negativity, anatomical brain imaging, and cognitive function) have been extensively studied in schizophrenia, they have not been tested together and compared across the psychosis dimension including SZP, psychotic BDP, and those at genetic risk. Here, we tested for common heritable biological signatures of psychosis independent of the categorical diagnoses. We hypothesized that SZP and BDP would show similar and abnormal performance on SPEM, PPI, and ERP measures. Further, we hypothesized that SZR and BDR would have similar and abnormal performance on these measures, albeit milder, than those seen in probands. We found no differences on any of the SPEM (maintenance pursuit gain and predictive pursuit gain), PPI (inhibition, facilitation), or ERP (theta/alpha, beta magnitudes to S1) measures between SZP and BDP. Both SZP and BDP had lower maintenance and predictive pursuit gain at all tested velocities (9.9°/s, 18.7°/s, and 25.0°/s) compared with HC, with the effect sizes comparable with prior meta-analysis.26 In addition, both SZP and BDP had deficient ERP theta/alpha and beta frequency magnitudes to S1 compared with HC. In contrast, neither PPI nor PPF outcomes were different from HC in either SZP or BDP.
Because SPEM and ERP outcomes were abnormal in probands, we further tested these putative endophenotypes in relatives. There were no significant differences between SZR and BDR on any of the measures. Compared with HC, both SZR and BDR had normal maintenance gain but lower predictive pursuit gain at faster velocities (significantly different at 18.7°/s and 25.0°/s in both SZR and BDR vs HC). In addition, SZR and BDR had lower ERP theta/alpha and beta frequency scores compared with HC (significantly different for theta/alpha in SZR vs HC and beta in BDR vs HC).
The outcomes of this endophenotypes examination across the schizophrenia-bipolar psychosis dimension support SPEM predictive pursuit gain as a putative endophenotype of psychosis, overlapping both schizophrenia and psychotic bipolar I disorder. The data also support lower frequency auditory ERP activity in a paired stimuli paradigm as putative endophenotype of psychosis (significant alterations in SZP and BDP; lower scores in SZR and BDR ranging between significant and a trend level), yet this needs to be confirmed in larger samples. Finally, our results do not support either PPI or PPF as putative endophenotypes of psychosis.
Our findings of impaired maintenance pursuit and predictive pursuit gain in SZP and BDP are consistent with several,27–30 but not all,31 reports. Although prior studies suggested maintenance (closed-loop) gain as one of the SPEM components distinguishing SZR from HC (see Calkins et al32 for review and meta-analysis), we found normal maintenance gain but selectively impaired predictive pursuit gain in both SZR and BDR. This finding may be interpreted in the light of Thaker’s et al20 model of differential contributions of pursuit initiation (driven by retinal motion) and predictive pursuit (driven by extraretinal motion processing) to maintenance pursuit. SZR, especially those with schizotypy, have been found to differ from HC on the contributions from these 2 components toward maintaining SPEM such that maintenance pursuit in SZR is dependent more on the retinal and less on extraretinal processing.20 Such increased dependence on the retinal motion information may be due to compensation for impaired extraretinal processing (as found here in both SZR and BDR). It is possible that relatives are able to normally pursue the moving target (which is reflected in normal maintenance gain) using this compensatory mechanism.20 Furthermore, Avila et al27 reported higher sensitivity/predictive accuracy of predictive pursuit compared with traditional maintenance gain in SZP and SZR that may also contribute to our differential SPEM findings in relatives. These findings point out at high complexity of the SPEM system and suggest that there may be more than 1 abnormality within this system associated with schizophrenia liability. Our results support predictive pursuit gain as a putative endophenotype of psychosis thought to reflect a selective deficit in processing and/or utilizing extraretinal motion signals during SPEM.20 The time-frequency effects in auditory ERPs observed here replicate well-established findings in the literature.11,12,15,33 Importantly, beta power in the S1 time range has been shown to mediate “P50 gating” effects and has been theorized to reflect stimulus encoding,33,34 while theta/alpha power has been demonstrated to be the most heritable measure in this paradigm,35
Our finding that PPI was not altered in the psychosis probands compared with HC is inconsistent with several prior reports6,9,22 and with our a priori prediction. Nevertheless, a number of studies have failed to detect deficient PPI in either SZP36 or BDP.10 Wynn et al8 reported impaired PPF in the face of normal PPI in both SZP and their siblings; however, our results did not confirm this observation. Similar to previous studies that failed to detect PPI differences in SZP vs HC (see Hamm et al23 for review), our experiment produced approximately 44%–47% and 50% PPI in probands and HC, respectively. A considerable PPI variability in both control and clinical populations has been emphasized.23 The selection criteria for HC, in particular, may contribute. Here, we included community controls matched with the psychosis groups in age and years of education. Given previously reported effect of gender (higher PPI in males)23 and gender differences characteristic of this sample (more males among SZP vs HC), all primary analyses were adjusted for gender, as well as age. Furthermore, we conducted male vs female comparisons for PPI in HC and probands and found no differences in any of the groups (table 3, footnote). In addition to the sample selection criteria, differences in PPI acquisition across studies may contribute to the discrepant PPI/PPF findings. Here we used a standardized PPI paradigm similar to Hong et al,22 where the primary PPI outcomes were computed at 120 ms ISI, the lead condition found to detect the largest effect in schizophrenia samples.22 The subjects were not instructed to selectively attend to the prepulse thus excluding attentional PPI modulation at this lead condition as a potential confounding factor.18 Based on prior reports suggesting that PPI may be influenced by symptom state,9,10 another possible explanation for our negative PPI findings may be related to the overall moderate symptom severity and possible attenuation of PPI deficits in these SZP/BDP compared with more acutely ill samples. Nevertheless, we did not find correlations between the BPRS and PPI/PPF outcomes. Finally, the medication status could have contributed to these results. Although some studies reported no effect of psychotropic medications on PPI,37 others suggested that antipsychotics may temporarily improve PPI deficits23 and mood stabilizers tend to be associated with lower PPI.10 Because the majority of probands in this sample reported chronic treatment with a variety of psychotropic medications, we cannot rule out that “primary” disease-associated PPI/PPF alterations were modulated by chronic medication use. This study was not designed to specifically test for a medication effect on any of the neurophysiological measures, yet we did not find any correlations between the active treatment status and PPI/PPF performance in either proband or relative groups. Overall, this discussion points to important methodological issues related to PPI studies in both healthy and psychiatric populations. Future research exploring “normal” PPI variability and its disease-relevant characteristics is necessary.
The question of whether various psychosis endophenotyopes co-occur in the same individual and, presumably, rely on common neural circuitries and shared heritability markers remains debatable. Here, we found an expected significant correlation between the 2 SPEM measures (maintenance gain and predictive pursuit gain at 25.0°/s velocity, r = 0.46, P < .001) but limited intercorrelations across the 3 neurophysiologic paradigms (ie, all correlations between SPEM, PPI, and ERP measures were nonsignificant, except for a negative correlation between PPI and ERP/beta, r = −0.38, P < .01). These observations are in line with previous studies that reported no correlation between eye tracking (antisaccade) deficits, PPI,38,39 and P5040 in both SZP and HC. Although the neural circuits underlying SPEM, PPI, and ERP still require precise characterization, previous data implicated partially divergent brain regions: prefrontal cortex/frontal eye fields and temporal-parietal cortex for SPEM20; hippocampus, prefrontal and temporal-parietal cortices for paired stimuli ERP41; and a number of forebrain and other brain structures including medial prefrontal cortex, hippocampus, amygdala, nucleus accumbens, striatum, and pontine startle circuitry for PPI.42 In addition, several genetic studies have provided support for independent associations. SPEM has been linked to 6p21 in 2 schizophrenia samples.43,44 PPI has shown associations with NRG1 rs3924999,45 COMT Val(158)/Met,46 and PRODH47 in both SZP and HC. Disrupted P50 suppression has been linked to 15q13–14 alpha-7-nicotinic acetylcholine receptor48 and 22qD22s31549 markers in SZP. Overall, our findings taken together with earlier reports suggest that SPEM, PPI, and auditory ERP may represent independent measures modulated by at least partially distinct neuronal pathways, as well as unique sets of genetic markers, involved in various components of the multifactorial disease processes in psychosis.
Several limitations to this study should be noted. The modest sample size warrants cautious interpretation of these findings. Because the majority of probands and a proportion of relatives reported a long-term history of various psychotropic medication use, it was not possible to fully examine “pure” neurophysiological endophenotypes free of chronic treatment effects although the active medication effects were tested here and were nonsignificant. Finally, our sample was delimited by the diagnostic boundaries of schizophrenia and psychotic bipolar disorder and thus does not include a fuller spectrum of psychotic disorders. As endophenotypes of psychosis become better characterized, it will be necessary to extend these measures to other psychoses.
In conclusion, this study directly compared a broad panel of neurophysiological measures in probands and relatives across the schizophrenia-bipolar disorder psychosis dimension. Our findings support SPEM predictive pursuit and S1-associated ERP in beta and theta/alpha frequencies as putative endophenotypes of psychosis common to both schizophrenia and bipolar disorder although the ERP measures require confirmation in larger studies. Our results do not support either PPI or PPF as putative endophenotypes of psychosis. Neither active symptoms severity, nor concomitant treatments had an effect on SPEM, PPI, or ERP, supporting these measures as “trait” biological markers. SPEM, PPI/PPF, and ERP were found to be independent measures, possibly mediated by divergent biological systems. Future research examining heritability and molecular underpinnings of the psychosis endophenotypes may further the understanding of the biology-driven mechanisms of psychosis and aid in the development of biology-based diagnostic definitions and novel treatments.
Funding
National Institute of Mental Health (MH077851-01A1) and NARSAD/Brain & Behavior Research Foundation John Kennedy Harrison Young Investigator Award (17801).
Supplementary Material
Acknowledgments
We would like to thank Bradley Witte, Thomas Carmody, and Dorothy Denton at UT Southwestern Medical Center, and Amie Elliott at Maryland Psychiatric Research Center, University of Maryland School of Medicine for assistance with data management, analysis, and the manuscript preparation; we would like to thank all clinicians for patients referral and patients and their families that took part in this study.
References
- 1. Ivleva EI, Morris DW, Moates AF, Suppes T, Thaker GK, Tamminga CA. Genetics and intermediate phenotypes of the schizophrenia–bipolar disorder boundary. Neurosci Biobehav Rev. 2010;34:897–921 [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), 4thed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 3.Gottesman II, Shields J. Genetic theorizing and schizophrenia. Br J Psychiatry. 1973; 122: 15–30 [DOI] [PubMed] [Google Scholar]
- 4. Thaker GK. Neurophysiological endophenotypes across bipolar and schizophrenia psychosis. Schizophr Bull. 2008; 34: 760–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gooding DC, Iacono WG, Katsanis J, Beiser M, Grove WM. The association between lithium carbonate and smooth pursuit eye tracking among first-episode patients with psychotic affective disorders. Psychophysiology. 1993; 30: 3–9 [DOI] [PubMed] [Google Scholar]
- 6. Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl). 2001; 156: 234–258 [DOI] [PubMed] [Google Scholar]
- 7. Kumari V, Das M, Zachariah E, Ettinger U, Sharma T. Reduced prepulse inhibition in unaffected siblings of schizophrenia patients. Psychophysiology. 2005; 42: 588–594 [DOI] [PubMed] [Google Scholar]
- 8. Wynn JK, Dawson ME, Schell AM, McGee M, Salveson D, Green MF. Prepulse facilitation and prepulse inhibition in schizophrenia patients and their unaffected siblings. Biol Psychiatry. 2004;55:518–523 [DOI] [PubMed] [Google Scholar]
- 9. Perry W, Minassian A, Feifel D, Braff DL. Sensorimotor gating deficits in bipolar disorder patients with acute psychotic mania. Biol Psychiatry. 2001; 50: 418–424 [DOI] [PubMed] [Google Scholar]
- 10. Barrett SL, Kelly C, Watson DR, Bell R, King DJ. Normal levels of prepulse inhibition in the euthymic phase of bipolar disorder. Psychol Med. 2005; 35: 1737–1746 [DOI] [PubMed] [Google Scholar]
- 11. Brockhaus-Dumke A, Schultze-Lutter F, Mueller R, et al. Sensory gating in schizophrenia: P50 and N100 gating in antipsychotic-free subjects at risk, first-episode, and chronic patients. Biol Psychiatry. 2008; 64: 376–384 [DOI] [PubMed] [Google Scholar]
- 12. Hamm JP, Ethridge LE, Shapiro JR, et al. Spatiotemporal and frequency domain analysis of auditory paired stimuli processing in schizophrenia and bipolar disorder with psychosis. Psychophysiology. 2012; 49: 522–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clementz BA, Geyer MA, Braff DL. Poor P50 suppression among schizophrenia patients and their first-degree biological relatives. Am J Psychiatry. 1998; 155: 1691–1694 [DOI] [PubMed] [Google Scholar]
- 14. Schulze KK, Hall MH, McDonald C, et al. P50 auditory evoked potential suppression in bipolar disorder patients with psychotic features and their unaffected relatives. Biol Psychiatry. 2007; 62: 121–128 [DOI] [PubMed] [Google Scholar]
- 15. Clementz BA, Blumenfeld LD. Multichannel electroencephalographic assessment of auditory evoked response suppression in schizophrenia. Exp Brain Res. 2001; 139: 377–390 [DOI] [PubMed] [Google Scholar]
- 16. Chang WP, Arfken CL, Sangal MP, Boutros NN. Probing the relative contribution of the first and second responses to sensory gating indices: a meta-analysis. Psychophysiology. 2011; 48: 980–992 [DOI] [PubMed] [Google Scholar]
- 17. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders/Patient Edition (SCID-I/P). New York: New York State Psychiatric Institute, Biometrics Research Department; 1996. [Google Scholar]
- 18. Zanarini MC, Frankenburg FR, Sickel AE, Yong L. The Diagnostic Interview for DSM-IV Personality Disorders (DIP DIV). Belmont: McLean Hospital; 1996. [Google Scholar]
- 19. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962; 10: 799–812 [Google Scholar]
- 20. Thaker GK, Avila MT, Hong EL, Medoff DR, Ross DE, Adami HM. A model of smooth pursuit eye movement deficit associated with the schizophrenia phenotype. Psychophysiology. 2003;40:277–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thaker GK, Ross DE, Cassady SL, et al. Smooth pursuit eye movements to extraretinal motion signals: deficits in relatives of patients with schizophrenia. Arch Gen Psychiatry. 1998; 55: 830–836 [DOI] [PubMed] [Google Scholar]
- 22. Hong LE, Summerfelt A, Wonodi I, Adami H, Buchanan RW, Thaker GK. Independent domains of inhibitory gating in schizophrenia and the effect of stimulus interval. Am J Psychiatry. 2007;164:61–65 [DOI] [PubMed] [Google Scholar]
- 23. Hamm AO, Weike AI, Schupp HT. The effect of neuroleptic medication on prepulse inhibition in schizophrenia patients: current status and future issues. Psychopharmacology (Berl). 2001; 156: 259–265 [DOI] [PubMed] [Google Scholar]
- 24. Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004; 134: 9–21 [DOI] [PubMed] [Google Scholar]
- 25. Ethridge LE, Hamm JP, Shapiro JR, et al. Neural activations during auditory oddball processing discriminating schizophrenia and psychotic bipolar disorder. Biol Psychiatry. 2012;72:766–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O’Driscoll GA, Callahan BL. Smooth pursuit in schizophrenia: a meta-analytic review of research since 1993. Brain Cogn. 2008; 68: 359–370 [DOI] [PubMed] [Google Scholar]
- 27. Avila MT, McMahon RP, Elliott AR, Thaker GK. Neurophysiological markers of vulnerability to schizophrenia: sensitivity and specificity of specific quantitative eye movement measures. J Abnorm Psychol. 2002; 111: 259–267 [PubMed] [Google Scholar]
- 28. Kathmann N, Hochrein A, Uwer R, Bondy B. Deficits in gain of smooth pursuit eye movements in schizophrenia and affective disorder patients and their unaffected relatives. Am J Psychiatry. 2003; 160: 696–702 [DOI] [PubMed] [Google Scholar]
- 29. Hong LE, Avila MT, Adami H, Elliot A, Thaker GK. Components of the smooth pursuit function in deficit and nondeficit schizophrenia. Schizophr Res. 2003; 63: 39–48 [DOI] [PubMed] [Google Scholar]
- 30. Moates AF, Ivleva EI, O’Neill HB, et al. Predictive pursuit association with deficits in working memory in psychosis. Biol Psychiatry. 2012;72:752–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lencer R, Reilly JL, Harris MS, Sprenger A, Keshavan MS, Sweeney JA. Sensorimotor transformation deficits for smooth pursuit in first-episode affective psychoses and schizophrenia. Biol Psychiatry. 2010;67:217–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Calkins ME, Iacono WG, Ones DS. Eye movement dysfunction in first-degree relatives of patients with schizophrenia: a meta-analytic evaluation of candidate endophenotypes. Brain Cogn. 2008; 68: 436–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brenner CA, Kieffaber PD, Clementz BA, et al. Event-related potential abnormalities in schizophrenia: a failure to “gate in” salient information? Schizophr Res. 2009; 113: 332–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hong LE, Summerfelt A, McMahon RP, Thaker GK, Buchanan RW. Gamma/beta oscillation and sensory gating deficit in schizophrenia. Neuroreport. 2004; 15: 155–159 [DOI] [PubMed] [Google Scholar]
- 35. Hong LE, Summerfelt A, Mitchell BD, et al. Sensory gating endophenotype based on its neural oscillatory pattern and heritability estimate. Arch Gen Psychiatry. 2008; 65: 1008–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ford JM, Roth WT, Menon V, Pfefferbaum A. Failures of automatic and strategic processing in schizophrenia: comparisons of event-related brain potential and startle blink modification. Schizophr Res. 1999; 37: 149–163 [DOI] [PubMed] [Google Scholar]
- 37. Mackeprang T, Kristiansen KT, Glenthoj BY. Effects of antipsychotics on prepulse inhibition of the startle response in drug-naïve schizophrenic patients. Biol Psychiatry. 2002; 52: 863–873 [DOI] [PubMed] [Google Scholar]
- 38. Kumari V, Ettinger U, Crawford TJ, Zachariah E, Sharma T. Lack of association between prepulse inhibition and antisaccadic deficits in chronic schizophrenia: implications for identification of schizophrenia endophenotypes. J Psychiatr Res. 2005; 39: 227–240 [DOI] [PubMed] [Google Scholar]
- 39. Ettinger U, Hejda S, Flak V, Corr PJ. Prepulse inhibition of the acoustic startle reflex and oculomotor control. Psychophysiology. 2005; 42: 473–482 [DOI] [PubMed] [Google Scholar]
- 40. Price GW, Michie PT, Johnston J, et al. A multivariate electrophysiological endophenotype, from a unitary cohort, shows greater research utility than any single feature in the Western Australian family study of schizophrenia. Biol Psychiatry. 2006; 60: 1–10 [DOI] [PubMed] [Google Scholar]
- 41. Grunwald T, Boutros NN, Pezer N, et al. Neuronal substrates of sensory gating within the human brain. Biol Psychiatry. 2003; 53: 511–519 [DOI] [PubMed] [Google Scholar]
- 42. Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl). 2001; 156: 194–215 [DOI] [PubMed] [Google Scholar]
- 43. Arolt V, Lencer R, Purmann S, et al. Testing for linkage of eye tracking dysfunction and schizophrenia to markers on chromosomes 6, 8, 9, 20, and 22 in families multiply affected with schizophrenia. Am J Med Genet. 1999; 88: 603–606 [PubMed] [Google Scholar]
- 44. Matthysse S, Holzman PS, Gusella JF, et al. Linkage of eye movement dysfunction to chromosome 6p in schizophrenia: additional evidence. Am J Med Genet B Neuropsychiatr Genet. 2004; 128B: 30–36 [DOI] [PubMed] [Google Scholar]
- 45. Hong LE, Wonodi I, Stine OC, Mitchell BD, Thaker GK. Evidence of missense mutations on the neuregulin 1 gene affecting function of prepulse inhibition. Biol Psychiatry. 2008; 63: 17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Roussos P, Giakoumaki SG, Rogdaki M, Pavlakis S, Frangou S, Bitsios P. Prepulse inhibition of the startle reflex depends on the catechol O-methyltransferase Val158Met gene polymorphism. Psychol Med. 2008;38:1651–1658 [DOI] [PubMed] [Google Scholar]
- 47. Roussos P, Giakoumaki SG, Bitsios P. A risk PRODH haplotype affects sensorimotor gating, memory, schizotypy, and anxiety in healthy male subjects. Biol Psychiatry. 2009; 65: 1063–1070 [DOI] [PubMed] [Google Scholar]
- 48. Leonard S, Gault J, Hopkins J, et al. Association of promoter variants in the alpha7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry. 2002; 59: 1085–1096 [DOI] [PubMed] [Google Scholar]
- 49. Myles-Worsley M, Coon H, McDowell J, et al. Linkage of a composite inhibitory phenotype to a chromosome 22q locus in eight Utah families. Am J Med Genet. 1999; 88: 544–550 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.