Abstract
Mentalizing has been examined both in autism spectrum disorder (ASD) and schizophrenia (SCZ) primarily by either cognitive-linguistic (referred to as verbal) or emotion recognition from eyes (referred to as visual) mentalizing tasks. Each type of task is thought to measure different aspects of mentalizing. Differences in clinical features and developmental courses of each disorder may predict distinct patterns of mentalizing performance across dis orders on each type of task. To test this, a meta-analysis was conducted using 37 studies that assessed mentalizing either verbally or visually in adults with SCZ or ASD. We found that the estimated effect sizes of impairments in verbal and visual mentalizing tasks for both clinical groups were statistically large and at a similar level (overall Hedges’ g = 0.73-1.05). For each disorder, adults with SCZ showed a trend towards larger impairments on verbal (overall Hedges’ g = 0.99) than on visual mentalizing task (overall Hedges’ g = 0.73; Qbet = 3.45, p =.06, df =1). Adults with ASD did not show different levels of impairment on the verbal versus visual tasks (Qbet = 0.08, p =.78, df =1). These results suggest that both clinical groups share, at least in part, some common cognitive processing deficits associated with mentalizing impairments.
Key words: mentalizing, schizophrenia, autism/social cognition
Introduction
Mentalizing is a high-level component of social cognition defined as the essential ability that allows us to infer the mental states of others, such as beliefs, thoughts, and emotions.1,2 The development of successful mentalizing proceeds through different stages from childhood to adolescence into adulthood.3,4 Given that schizophrenia (SCZ) and autism spectrum disorders (ASD) are characterized by impaired social functioning,5,6 mentalizing has been frequently examined both for SCZ and ASD using identical mentalizing tasks in independent studies. However, mentalizing impairments commonly observed in the 2 disorders may reflect different causal factors. Comparing mentalizing impairments across the 2 disorders may help us understand whether there are common mentalizing impairments that cut across diagnostic categories7 or could help differentiate each disorder in a way that might enhance the identification of disorder-specific endophenotypic markers. We speculate that there might be disorder-specific patterns of mentalizing performance deficits on visually based measures of emotion recognition from eyes vs cognitive linguistic–based measures of intention and/or belief inference due to different clinical features and developmental courses for each disorder, as described in more detail below. Thus, the goal of the current meta-analysis was to determine if adults with SCZ vs ASD show similar or different levels of impairments on emotion recognition from eyes vs intention/belief inference tasks.
ASD is a neurodevelopmental disorder with an early onset of symptoms and disrupted development of cognitive and social communication skills.8 Core features of ASD are characterized by social impairments due to the failure of coordinating visual social cues on the basis of information from eyes.9,10 A body of literature has found that individuals with ASD show deficits of eye-gaze processing at different levels such as eye contact,11 gaze following,12,13 and joint attention,12,14–16 which provide crucial information about emotions and mental states for successful mentalizing.17 Eye-gaze processing is an early-developing mentalizing skill found in infancy and early childhood before the acquisition of vocal language.17,18 It allows one to learn social signals as early as the first month after birth.19 At 12–18 months of age, a typically developing infant attends to the object of another’s attention and is able to infer another’s mental state from an eye gaze at 36–48 months.17,20 The typical function of eye-gaze processing in an early stage of development is an essential precursor to the development of language and high-order mentalizing abilities in later life.21 For example, there is consistent evidence showing that the ability to initiate joint attention among preschool-aged children with ASD predicted later language ability at age 3–4 years.22 Similarly, early ability to understand and infer emotion in others predicts later high-order mentalizing abilities.23–25
Although successful processing of eye gaze in early development is critical, it is not sufficient for the development of advanced intention and/or belief inference, which usually emerges around 8–9 years of age.26 Intention and/or belief inference also requires the ability to recognize that another’s knowledge state is different from one’s own and integrate this information with situational context and advanced language understanding to determine “real” intentions and meanings beyond the literal meaning.27 Given this sequence of mentalizing development, early developmental disruptions in inferring others’ emotion from eyes in childhood may contribute to a cascade of later mentalizing deficits in adulthood of ASD.9
In contrast to ASD, SCZ has been frequently considered an adulthood disorder, with the median ages of 20–35 years at onset of the first psychotic symptoms (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; DSM-IV)8 although some subgroup of patients with SCZ presents their psychotic symptoms in early life.28 Some impairments in social and nonsocial cognition can precede the onset of psychotic symptoms in many individuals,29 leading some to think of SCZ as a neurodevelopmental disorder. A body of social cognitive literature suggests that mentalizing impairments occur at or prior to the onset of psychosis and continue throughout the course of SCZ, with evidence of verbally measured mentalizing deficits even in high-risk populations (though these may be less severe).6,30 Nonetheless, different from the ASD literature, there is no compelling evidence suggesting abnormal precursors of mentalizing, especially, emotion inference on the basis of information gained from eyes in early childhood or prodromal phase of SCZ.31,32
Interestingly, emerging evidence suggests that context processing, which has been long considered a core cognitive function thought to be supported by the dorsolateral prefrontal cortex (PFC), mediates, at least partly, mentalizing impairments in SCZ.33,34 Here, context processing refers to the ability to actively represent task- or situation-relevant information in working memory to guide ongoing processing.35 It should be noted that context processing in SCZ may reflect different psychological or neurobiological mechanisms from that of the ASD literature, which indicates “weak central coherence” on perceptual context processing tasks. In the SCZ literature, an increasing number of studies have shown the relevance of context processing to impairments on cognitive- linguistic mentalizing tasks in SCZ. For example, context processing, as measured by the AX-continuous performance test,36 partially mediated mental state inferences in patients with SCZ.37 This line of research suggests that mentalizing impairments in SCZ may be due to inefficient or failed context processing.38 As such, we speculate that at least some mentalizing impairments in SCZ may be secondary to other cognitive deficits, such as context processing deficits, and may emerge later in development, potentially providing a relative sparing of earlier developing mentalizing abilities such as inferring others’ emotions from eyes.
As described above, the different developmental courses of ASD and SCZ may predict different patterns of mentalizing impairments on tasks that measure emotion recognition from eyes vs cognitive-linguistic functions as measured by intention/belief inference tasks. Considering abnormal precursors of mentalizing in early life such as eye contact and gaze orientation for ASD,39 it is predicted that in the case of ASD, abnormal precursors to mentalizing in early life may lead to a cascade of later mentalizing deficits in adulthood such as intention/belief inference that may build upon these more basic functions. In contrast, mentalizing impairments in SCZ may stem from domain-general deficits, such as context processing, rather than from basic function in early development. Thus, we hypothesized that adults with ASD should show similar levels of impairment on emotion recognition from eyes vs intention/belief inference tasks, whereas individuals with SCZ should show greater impairments on intention/belief inference tasks than on emotion recognition from eyes. Further, we hypothesized that adults with ASD will show greater impairments on emotion recognition from eyes compared with those with SCZ. However, only a few studies have directly addressed these questions, with mixed results. For example, patients with ASD showed worse performance on a facial recognition test than did patients with SCZ,40 which is consistent with the hypothesis outlined above. In contrast, other work found that adults with SCZ and those with Asperger’s syndrome performed worse on the Hinting test41 and the “Reading the Mind in the Eyes” test (Eyes)14 relative to healthy controls (HCs), but that performance was not significantly different from each other on either task.42 Other research43 has shown that children with high-functioning ASD were more impaired compared with those with SCZ on a deception task44 though not on a false-belief task.45
The purpose of this study was to compare less cognitive-linguistic (referred to as visual) vs cognitive-linguistic (referred to as verbal) aspects of mentalizing across disorders by conducting a meta-analysis of studies with SCZ and ASD samples. This meta-analysis will provide a synthesized effect size estimate that has more power than individual studies. The verbal tasks included the Strange Stories46 and Faux Pas tests26, and the visual task was the Eyes task,47 which is hypothesized to require less demand on cognitive-linguistic abilities. We predicted that adults with ASD would show similar impairments in both types of tasks, but that adults with SCZ would show greater impairments in verbal tasks rather than the visual task. Further, we predicted that adults with ASD would show greater impairments in visual task compared with adults with SCZ but potentially similar deficits in the cognitive-linguistic tasks. Despite previous meta-analyses,6,48 mixed findings exist regarding the influence of clinical and demographic characteristics on mentalizing in SCZ. For example, it is still not clear whether mentalizing deficits are moderated by general intelligence deficits, antipsychotic medications, positive/negative symptom severity and other demographic features such as age and gender. Similarly, in the ASD literature, despite early theory suggesting female superiority of emphasizing in ASD,49 there is no empirical evidence whether gender moderates mentalizing performance for adults with ASD. Therefore, the second aim of this meta-analysis was to examine moderating effects of positive and negative symptom severity of SCZ, IQ, antipsychotic mediations, and other demographic characteristics including age and gender on mentalizing impairments in the 2 disorders.
Methods
Data Sources and Literature Search
The flowchart of the present meta-analysis is presented in figure 1.
Fig. 1.
Schematic procedure for studies included and excluded in meta-analysis.
ASD, autism spectrum disorders; HC, healthy controls; SCZ, schizophrenia.
aA literature search covered published papers up to December 2011 using the databases Pubmed, Medline, EMBASE, or Sciencedirect to identify the relevant studies.
bOf these 37 studies, 2 included both individuals with SCZ and those with ASD.
cThe total sample included 634 adults with SCZ (80% male), 362 ASD (82% male), and 926 HC (65% male).
Inclusion Criteria and Coding of the Variables
In terms of SCZ studies, consistent with prior review studies,6 we limited the sample to studies with SCZ disorder participants, including schizoaffective disorder or first-episode psychosis, but excluding any personality-related disorder. Studies involving the contrast between individuals with SCZ and HCs and/or between ASD and HC were included. The details of inclusion criteria and coding variables are presented in table 1.
Table 1.
Inclusion Criteria and Coding Variables
| Inclusion Criteria |
| 1. Studies should be published in peer-reviewed journals in English. |
| 2. Studies should report mentalizing abilities in adults with diagnoses of schizophrenia, schizoaffective disorder or first-episode psychosis (SCZ) or ASD according to Research Diagnostic Criteria, DSM-III-R, DSM-IV or the International Classification of Diseases criteria. |
| 3. Mentalizing tasks should be originally developed in autism and adopted to SCZ literature and used at least in 5 independent studies either for SCZ or for ASD. |
| 4. Studies should also include healthy subjects as a comparison group. |
| 5. For the comparison between adults with SCZ and ASD, the age of participants who performed those tasks should be between 18 and 65 years. |
| 6. Studies should report means and standard deviations, or F or t values, or exact P value so that standardized mean differences could be calculated. Measures with dichotomous outcomes were excluded. |
| Coding of the Variables |
| 1. Name of the first author and year of the publication. |
| 2. Number of participants and percentage of males in patients and healthy control groups (eg, schizophrenia and autism spectrum disorder). |
| 3. Mean and SDs for demographic variables (eg, age, duration of education), patients variables (eg, age of onset, duration of illness), percentage of medicated patients, and IQ assessment. |
| 4. Mean, SDs and calculated effect sizes of the individual tasks. A total mentalizing score that was calculated by averaging effect sizes of individual tasks. |
Note: ASD, autism spectrum disorders; SCZ, schizophrenia.
Mentalizing Tasks
Cognitive-Linguistic Tasks.
The typical cognitive- linguistic mentalizing task includes verbal passages in which participants have to infer a character’s mental state with given specific situational details. Without considering situational details, a person cannot infer the character’s mental states appropriately. As such, the correct responses in these tasks require participants to make explicit use of cognitive reasoning to provide situational explanations that go beyond the literal meaning about an interaction that has been described in verbal passages.46 Performance on cognitive-linguistic mentalizing tasks demand language understanding and may also require some aspects of executive function, as evidenced by prior research showing correlations among verbal-type mentalizing performance, impaired metaphor and/or proverb understanding, and executive function.50,51 We chose the Strange Stories46 and Faux Pas tasks26,52 because they have both been used in a sufficient number of studies with either SCZ or ASD (or both, see online supplementary methods). Although a few other studies used additional tasks that might measure similar constructs, we did not combine these tasks so as to minimize potential confounds arising from combining tasks with potentially different psychometric properties.
Emotion Recognition From Eyes (Less Cognitive-Linguistic Tasks). “Reading the Mind in the Eyes test” (the Eyes)47,53 measures recognition of emotions and mental state in others from eyes.54 Unlike the cognitive- linguistic tasks described above, the Eyes test does not include any situational details that should be integrated for correct responses. Therefore, relative to the cognitive-linguistic mentalizing tasks, performance on the Eyes test may depend on less cognitive function and language understanding.53
Statistical Analyses
Meta-Analyses Procedure.
The meta-analysis software, Comprehensive Meta-analysis,55 was used to analyze the data. The mean and standard deviation for each comparison (between adults with SCZ and HC: HC-SCZ, between adults with ASD and HC: HC-ASD) were used as a measure of the effect size. Homogeneity of the resulting mean-weighted effect sizes was tested with the Q test. We used a random-effects model for the meta-analyses. Publication bias was investigated using funnel plots, Egger’s test, and the Fail Safe number (see online supplementary methods for details).
Effect Size Estimation.
We estimated effect sizes using Hedges’ g. 56 For individual studies, we computed a standardized effect size by subtracting the mean of the patient group from that of the HC (eg, HC-SCZ or HC-ASD comparison). We then divided the results by the pooled standard deviation of the 2 groups with Hedges’ g correction for bias56 in small samples. We reported between-group differences as positive effect sizes when patient groups performed poorer than the HC. For studies that reported more than 1 mentalizing task, a pooled effect size was calculated.
Selecting Moderator Variables.
The list of moderating variables and summary data is reported in tables 5 and 6. The effects of demographic, clinical variables including symptoms severity, and general intelligence on group differences in mentalizing impairments were examined using a meta-regression method.
Table 5.
Meta-Regression of Potential Moderators of Effect Sizes in Schizophrenia
| Family of Variables | Descriptive | ||||
|---|---|---|---|---|---|
| Task Type | Variable Name | Ka | M (SD) | Unit | P-value |
| Verbal | Patient status (all outpatient=1, all inpatients=0) | 7 | 0.78 (0.39) | Mean proportion | .15 |
| BPRS or PANSS overall psychopathology | 7 | 0.32 (0.22) | Severity index 0–1 | .71 | |
| Positive symptoms | 11 | 0.14 (0.08) | Severity index 0–1 | .07 | |
| Negative symptoms | 11 | 0.22 (0.13) | Severity index 0–1 | .43 | |
| Age of onset | 7 | 24.08 (1.34) | Years | .88 | |
| Illness of duration | 9 | 11.76 (6.57) | Years | .14 | |
| Proportion of medicated patients | 8 | 0.99 (0.03) | Mean proportion | .09 | |
| Difference in male proportion | 11 | −0.09 (0.11) | Difference of mean proportion | .54 | |
| Difference in age | 11 | −0.08 (0.28) | Hedges’ g | .47 | |
| Difference in FIQ reported by NART or WAISa | 6 | 0.85 (0.40) | Hedges’ g | .49 | |
| Visual | Variable name | Ka | M (SD) | Unit | P-value |
| Patient status (all outpatient =1, all inpatients=0) | 8 | 0.94 (0.17) | Mean proportion | >.05 | |
| BPRS or PANSS overall psychopathology | 8 | 0.26 (0.21) | Severity index 0–1 | .85 | |
| Positive symptoms | 11 | 0.14 (0.09) | Severity index 0–1 | .24 | |
| Negative symptoms | 11 | 0.23 (0.11) | Severity index 0–1 | .26 | |
| Illness of duration | 11 | 12.48 (3.80) | Years | .21 | |
| Proportion of medicated patients | 10 | 0.983 (0.05) | Mean proportion | .96 | |
| Difference in male proportion | 15 | −0.06 (0.08) | Difference in mean proportion | .59 | |
| Difference in age | 16 | −0.14 (0.35) | Hedges’ g | .75 | |
| Difference in FIQ reported by NART or WAIS | 8 | 0.73 (0.31) | Hedges’ g | .44 | |
| Difference in education | 12 | 0.54 (0.55) | Hedges’ g | .69 | |
Note: PANSS, Positive and Negative Syndrome Scale;
Table 6.
Meta-Regression Analyses of Potential Moderators of Effect Sizes in Autism Spectrum Disorders
| Task Type | Family of Variables | Descriptive | |||
|---|---|---|---|---|---|
| Verbal | Variable Name | Ka | M (SD) | Unit | P Value |
| Difference in male proportion | 10 | −0.08 (0.14) | Difference of mean proportion | .08 | |
| Difference in age | 9 | −0.11 (0.23) | Hedges’ g | .67 | |
| Difference in FIQ reported by NART or WAIS | 7 | 0.47 (0.51) | Hedges’ g | .96 | |
| Visual | Variable Name | Ka | M (SD) | Unit | P Value |
| Difference in male proportion | 10 | −0.08 (0.11) | Difference of mean proportion | .71 | |
| Difference in age | 11 | −0.08 (0.50) | Hedges’ g | .33 | |
| Difference in FIQ reported by NART or WAIS | 8 | 0.26 (0.34) | Hedges’ g | .18 | |
Note: FIQ, full IQ; NART, National Adult Reading Test; WAIS, Wechsler Adult Intelligence Scale.
Results
Descriptive Information
The characteristics and effect sizes of studies included in the analysis are presented in figure 1 and tables 2 and 3.
Table 2.
Characteristics and Effect Sizes of Studies of Schizophrenia Included in the Meta-Analysis
| Cognitive-Linguistic Mentalizing Tasks | ||||
|---|---|---|---|---|
| Study | Sample (Male) | Matched For | Age (M) | Hedges’ g |
| de Achával et al. (2010) | 20 (13) SCZ | Age, sex | 30.9 | 0.84 |
| 20 (13) HC | 28.2 | |||
| Gavilan et al. (2011) | 22 (18) SCZ | Age, education, sex, premorbid IQ | 42.82 | 1.80 |
| 22 (18) HC | 41.95 | |||
| Herold et al. (2009) | 18 (11) SCZ | Age,sex | 28.7 | 0.82 |
| 21 (11) HC | 27.4 | |||
| Hooker et al. (2011) | 21 (17) SCZ | Age, sex | 44.33 | 1.46 |
| 17 (13) HC | 43.75 | |||
| Langdon et al. (2010) | 35 (23) SCZ | Age, gender, IQ | 35.9 | 0.52 |
| 34 (26) HC | 32.0 | |||
| Martino et al. (2007) | 21 (12.18) SCZ | Age, education, IQ | 32.66 | 1.30 |
| 15 (6) HC | 34.96 | |||
| Pijnenborg et al. (2009) | 46 (34) SCZ | No matched for age, gender, IQ | 27.4 | 0.68 |
| 53 (24) HC | 31.1 | |||
| Riveros et al. (2010) | 15 (12) SCZ | Age, gender, education | 37.57 | 0.92 |
| 18 (11) HC | 40.5 | |||
| Stanford et al. (2011) | 13 (6) SCZ | Age, sex, education | 33.6 | 1.03 |
| 14 (6) HC | 29.1 | |||
| Shur et al. (2008) | 26 (17) SCZ | Age, education | 32.58 | 0.90 |
| 35 (20) HC | 29.00 | |||
| Zhu et al. (2007) | 40 (18) SCZ | Age, education, sex | 30.2 | 1.27 |
| 31 (9) HC | 29.97 | |||
| Emotion Recognition From Eyes Task | ||||
| Study | Participants | Matched For | Age (M) | Hedges’ g |
| Bailey and Henry (2010) | 28 (14) SCZ | Age, education | 40.3 | 0.60 |
| 30 (15) HC | 36.4 | |||
| Bora et al. (2008) | 91 (60) SCZ | Education | 31.1 | 0.72 |
| 55 (34) HC | 35.6 | |||
| Couture et al. (2008) | 26 (22.88) SCZ | No matched for age and gender | 24.9 | 0.43 |
| 41 (38.13) HC | 23.0 | |||
| Couture et al. (2010)a | 41 (34) HC | Differ in age and IQ | 22.9 | 0.76 |
| 44 (39) SCZ | 27.5 | |||
| Craig et al. (2004)a | 16(11) SCZ | Age, IQ | 31.69 | 1.64 |
| 16 (11) HC | 29.44 | |||
| De Achával et al. (2010) | 20 (13) SCZ | Age, gender | 30.9 | 0.80 |
| 20 (13) HC | 28.2 | |||
| Hirao et al. (2008) | 20 (10) SCZ | Age, gender, and education | 36.7 | 1.89 |
| 20 (10) HC | 35.0 | |||
| Irani et al. (2006) | 10 (7) SCZ | Gender, ethnicity | 34.00 | 0.94 |
| 10 (5) HC | 38.00 | |||
| Kington et al. (2000) | 16 (13) SCZ | Age, gender, ethnicity | 34.07 | 0.55 |
| 16 (13) HC | 34.75 | |||
| Mcglade et al. (2008) | 73 (49) SCZ | Age, gender | 41.4 | 0.38 |
| 78 (45) HC | 38.3 | |||
| Riveros et al. (2010) | 15 (12) SCZ | Age, gender, education | 37.57 | 0.47 |
| 18 (11) HC | 40.5 | |||
| Russell et al. (2000) | 5 (5) SCZ | Age, gender, education | 36.00 | 1.37 |
| 7 (7) HC | 40.00 | |||
| Schimansky et al. (2010) | 40 (28) SCZ | Age | 38.0 | 0.71 |
| 40 (22) HC | 34.4 | |||
| Shur et al. (2008) | 26 (17) SCZ | Age, education | 32.58 | 0.53 |
| 35 (20) HC | 29.00 | |||
| Stanford et al. (2011) | 13 (6) SCZ | Age, gender, education | 33.6 | 0.79 |
| 14 (6) HC | 29.1 | |||
| Tso et al. (2010) | 33 (22) SCZ | Age, gender, education | 38.5 | 0.72 |
| 33 (23) HC | 38.2 | |||
Note: HC, healthy control; SCZ, schizophrenia.
aTwo studies included both individuals with schizophrenia and those with autism spectrum disorders.
Table 3.
Characteristics and Effect Sizes of Studies of Autism Spectrum Disorders Included in the Meta-Analysis
| Cognitive-Linguistic Mentalizing Tasks | ||||
|---|---|---|---|---|
| Study | Participants (Male) | Matched For | Age (M) | Hedges’ g |
| Adler et al. (2010) | 16 (15) HFA | Age, education, gender | 21.87 | 0.69 |
| 21 (20) HC | 22.90 | |||
| David et al. (2008) | 24 (14) HFA | Age, gender, IQ | 32.3 | 0.30 |
| 24 (13) HC | 30.6 | |||
| Happe et al. (1994) | 18 (13) ASD | Age | 20.6 | 2.88 |
| 10 (5) HC | 20.5 | |||
| Heavey et al. (2000) | 16 (15) HFA | Age | 34.7 | 1.56 |
| 15 (15) HC | 30.7 | |||
| Hill et al. (2004) | 15 (12) ASD | Age | 34.40 | 1.13 |
| 15 (7) HC | 34.00 | |||
| Jolliffe & Baron-Cohen (1999) | 17 (15) HFA | Age, gender, IQ | 30.71 | 1.73 |
| 17 (15) ASD | 27.77 | |||
| 17 (15) HC | 30.00 | |||
| Ponnet et al. (2004) | 19 (14) ASD | Age, IQ | 21.06 | 0.04 |
| 19 (14) HC | 21.93 | |||
| Roeyers et al. (2001) | 24 (22) ASD | Gender, age, IQ, education | 23.8 | 0.32 |
| 24 (22) HC | 23.1 | |||
| Spek et al. (2010) | 32 (27) HFA | Age, gender, IQ | 42.1 | 0.81 |
| 29 (25) ASD | 43.67 | |||
| 32 (24) HC | 38.68 | |||
| Zalla et al. (2009) | 15 (12) ASD | Age, gender, IQ, education | 28.00 | 1.72 |
| 15 (11) HC | 27.80 | |||
| Emotion Recognition From Eyes Task | ||||
| Study | Participants (Male) | Matched For | Age (M) | Hedges’ g |
| Adler et al. (2010) | 16 (15) HFA | Age, education, gender | 21.87 | 0.70 |
| 21 (20) HC | 22.90 | |||
| Baron-Cohen et al. (1997) | 16 (13) HFA | IQ, age | 28.6 | 1.74 |
| 16 (13) HC | 30.0 | |||
| Baron-Cohen et al. (1999) | 6 (4) ASD | IQ, age, education | 26.3 | 1.40 |
| 12 (6) HC | 25.5 | |||
| Baron-Cohen et al. (2001) | 15 (15) HFA/ASD | Age, IQ | 29.7 | 1.69 |
| 14 (14) HC | 28.0 | |||
| Couture et al. (2010)a | 36 (29) HFA | Differ in age and IQ | 20.9 | 0.61 |
| 41 (34) HC | 22.9 | |||
| Craig et al. (2004)a | 16 (11) HC | Age, IQ | 24.12 | 1.43 |
| 17 (15) ASD | 29.44 | |||
| David et al. (2008) | 24 (14) HFA | Age, gender, IQ | 32.3 | 1.23 |
| 24 (13) HC | 30.6 | |||
| Kleinman et al. (2001) | 30 (21) HFA | No matched for age, IQ, gender | 31.43 | 0.42 |
| 24 (10) HC | 22.33 | |||
| Ponnet et al. (2004) | 19 (14) ASD | Age, IQ, gender | 21.06 | 0.21 |
| 19 (14) HC | 21.93 | |||
| Roeyers et al. (2001) | 24 (22) ASD | Gender, education | 23.8 | 0.11 |
| 24 (22) HC | 23.1 | |||
| Spek et al. (2010) | 32 (27) HFA | Age, gender, IQ | 42.1 | 0.32 |
| 29 (25) ASD | 43.67 | |||
| 32 (24) HC | 38.68 | |||
Note: ASD, autism spectrum disorders; HC, healthy control; HFA, high-functioning autism; SCZ, schizophrenia.
aTwo studies included both individuals with schizophrenia and those with autism spectrum disorders.
Impairments on Cognitive-Linguistic Tasks in SCZ and ASD
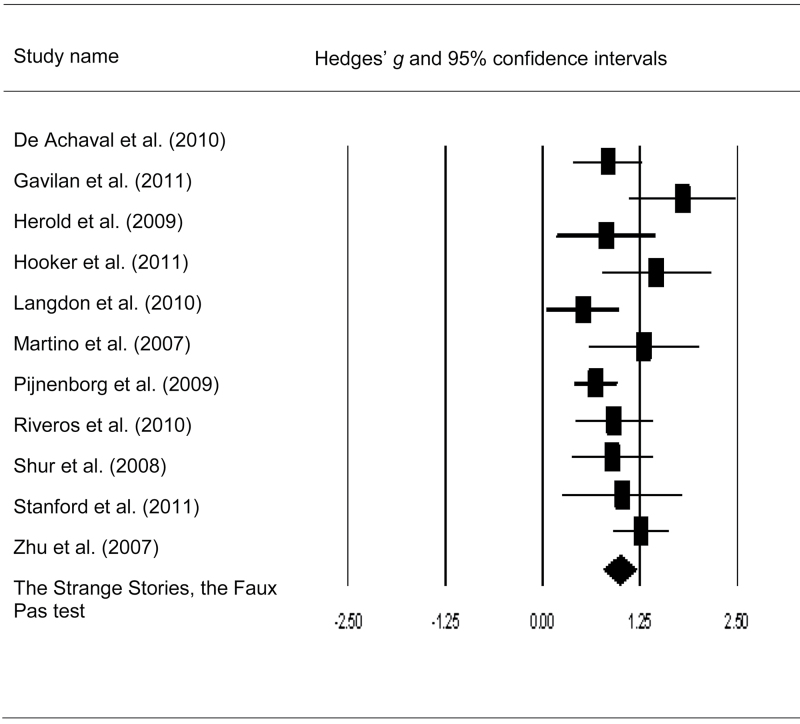
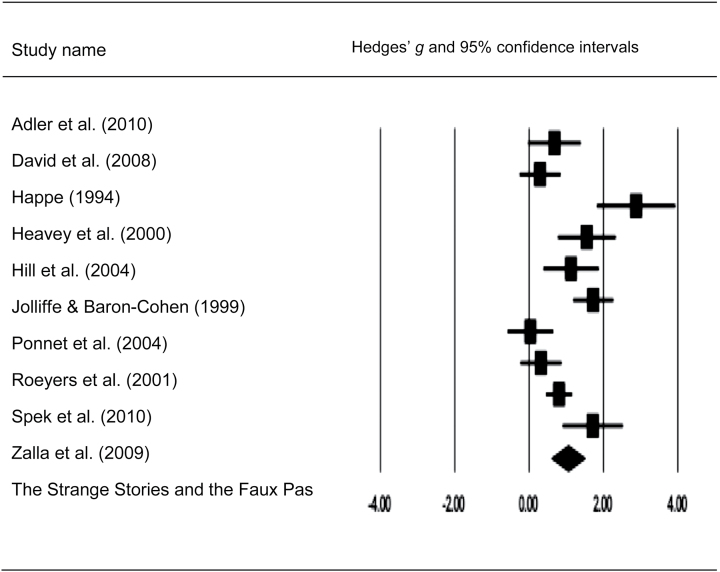
Meta-analysis of the cognitive-linguistic tests demonstrated highly significant mentalizing impairments in adults with SCZ and ASD compared with HC (table 4). Effect sizes of HC-SCZ and HC-ASD comparisons were quite large (overall Hedges’ g = 0.99, 1.05, respectively, both P < .001). Further, the magnitude of the deficits for HC-ASD and HC-SCZ were not significantly different according to the Q-test (Q bet = 0.00, df = 1, P = .96). The distribution of the effect sizes of cognitive-linguistic mentalizing impairments for HC-SCZ showed a trend for heterogeneity according to the Q-test (Q w = 18.59, P = .05, df = 10) but were significantly heterogeneous for the HC-ASD comparison (Q w = 45.38, P = .00, df = 9). Egger’s regression test showed no publication bias for the cognitive-linguistic tasks in either comparison (HC-ASD: b = 3.35, P = .19; HC-SCZ: b = 2.06, P = .12). The fail-safe number of missing studies required to make the group difference nonsignificant is also quite large (n = 239 and n = 449 for the comparison for HC-ASD and HC-SCZ, respectively) (see figures 2 and 3 for mean effect sizes and confidence intervals of SCZ and ASD samples).
Table 4.
Overall Effect Sizes for Group Differences on Cognitive-Linguistic and Eye-Gaze Mentalizing Tasks
| Task Type | Comparisons | Ka | Hedges’ g | 95% CI | z | P | Q-test P |
|---|---|---|---|---|---|---|---|
| Verbal | HC-SCZ | 11 | 0.99 | 0.78–1.20 | 9.15 | .00** | .05 |
| HC-ASD | 10 | 1.05 | 0.60–1.50 | 4.58 | .00** | .00** | |
| Visual | HC-SCZ | 16 | 0.73 | 0.56–0.90 | 8.36 | .00** | .06 |
| HC-ASD | 11 | 0.81 | 0.48–1.14 | 4.83 | .00** | .00** |
Note: ASD, autism spectrum disorders; HC, healthy control; SCZ, schizophrenia.aThe number of studies included.p < .01
Fig. 2.
Forest plot with mean effect size (g) and confidence intervals for cognitive linguistic–based mentalizing tasks in patients with schizophrenia.
Fig. 3.
Forest plot with mean effect size (g) and confidence intervals for cognitive linguistic–based mentalizing tasks in adults with autism spectrum disorders.
Impairments on the “Reading the Mind in the Eyes Task” in SCZ and ASD
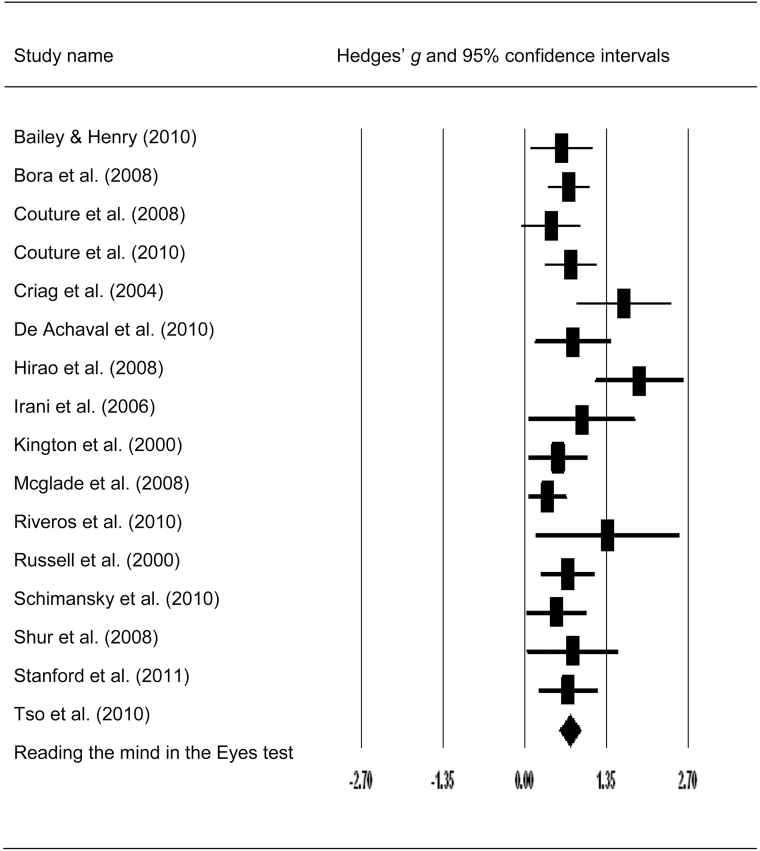
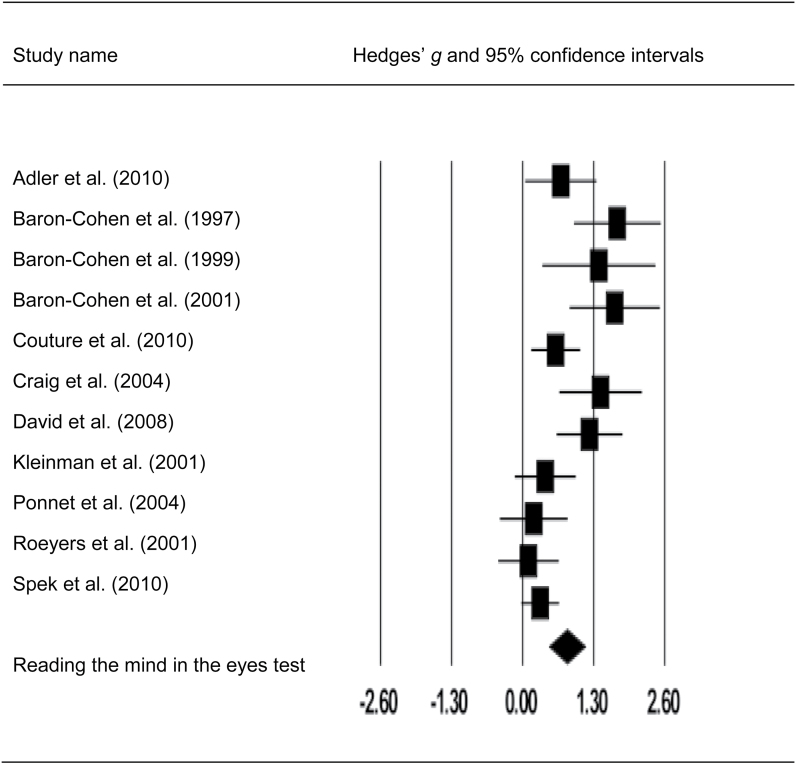
Meta-analysis of the Eyes test also demonstrated significant mentalizing impairments in both clinical groups. The overall effect sizes of HC-SCZ and HC-ASD comparisons were 0.73 and 0.81 (both P < .001). However, contrary to our predictions, the magnitude of effect sizes of HC-SCZ and HC-ASD comparisons was not significantly different according to the Q-test (Q bet = 0.27, P = .60, df = 1). Again, the distribution of the effect sizes of the Eyes test for HC-SCZ tended to be heterogeneous at a trend level (Q w = 23.73, P = .06, df = 15), whereas the distribution of the effect sizes of the Eyes test for HC-ASD comparisons was significantly heterogeneous according to the Q-test (Q w = 37.75, P < .001, df = 10) (table 4).
Egger’s regression test suggested that there might be some publication bias for performances on the Eyes test in adults with ASD (b = 4.66, df = 9, P = .01). However, the fail-safe number of missing studies needed to make the group difference nonsignificant was large (n = 217), considering the relatively small number of studies included in the analysis (k = 11 studies). Similarly, Egger’s regression test for SCZ also showed a possibility for publication bias (b = 2.38, P = .01). However, the fail-safe number of missing studies was again quite large (n = 473) (see figures 4 and 5 for mean effect sizes and confidence intervals of SCZ and ASD samples).
Fig. 4.
Forest plot with mean effect size (g) and confidence intervals for visual mentalizing tasks in patients with schizophrenia.
Fig. 5.
Forest plot with mean effect size (g) and confidence intervals for visual mentalizing tasks in adults with autism spectrum disorders.
Comparisons of Task Types Within Each Diagnostic Group
In SCZ, the magnitude of mentalizing impairments on the cognitive linguistic tasks was larger than that of mentalizing deficits on the Eyes test at a trend level (HC-SCZ: Q bet = 3.45, P = .06, df = 1). In the ASD group, the magnitude of mentalizing impairments on the Eyes test was not significantly different from the cognitive-linguistic mentalizing tasks (HC-ASD: Q bet = .08, P = .78, df = 1).
Meta-Regression Analyses
To further investigate the sources of the variance in effect sizes, we examined potential moderator effects on mentalizing impairments.
Cognitive-Linguistic Mentalizing Tasks
Schizophrenia.
No demographic characteristics or clinical symptoms had an impact on the magnitude of mentalizing impairments in SCZ (see table 5).
Autism Spectrum Disorder.
Due to the small number of studies reporting these variables, we included only 3 moderator factors: age, IQ, and the difference in the proportion of males in the ASD vs HC groups in meta-regression analysis (see table 6). Group differences in age (k = 9 studies) and full IQ scores (k = 7 studies) had no impact on the magnitude of mentalizing impairments in adults with ASD. However, differences in the proportion of males in the HC and ASD groups tended to moderate the magnitude of impairments on the cognitive-linguistic mentalizing tasks, such that a larger relative percentage of males in the ASD group was associated with greater impairments at a trend level (k = 10 studies, B = 1.67, SE = 0.96, Z = 1.73, P = .08).
Eyes Task
Schizophrenia and Autism Spectrum Disorder.
No moderator variables significantly moderated the variance in mentalizing impairments on the Eyes task in either group.
Discussion
The purpose of the current meta-analysis was to compare adults with SCZ and ASD on impairments in emotion recognition from eyes (visual) and intention/belief inference (cognitive-linguistic) tasks. The current meta-analysis showed that adults with SCZ and ASD exhibited large effect size deficits on both types of mentalizing tasks (SCZ: overall Hedges’ g = 0.56–1.20, ASD: overall Hedges’ g = 0.48–1.50). Impairments in cognitive-linguistic mentalizing tasks in SCZ were somewhat larger than on the Eyes test at a trend level but did not differ across tasks in ASD. Similarly, the magnitude of impairments on both types of tasks did not differ across groups.
The current meta-analysis findings indicating a similar pattern of mentalizing impairments in adults with SCZ and ASD are consistent with 2 studies that directly compared adults with SCZ and those with ASD regarding mentalizing performance. For example, Craig et al42 compared patients with paranoid delusions and those with Asperger syndrome. Their results showed that both clinical groups exhibited impaired performance on the Hinting task41 and the Eyes tests compared with the HC but that the scores for the 2 patient groups did not differ from each other. Consistent with our results, the direct comparison study between ASD and SCZ by Couture et al69 also found that both individuals with (High-Functioning Autism) HFA (n = 36) and SCZ (n = 44) showed impairments on the Eyes test compared with the HC. Importantly, there were no clinical group differences on the Eyes test47 after controlling for IQ.69
Contrary to our predictions, adults with ASD did not show greater impairments than adults with SCZ on the Eye’s test. The Eye’s test uses still photographs of the eyes. It is possible that subtle deficits on eye-gaze processing in HFA with above-average IQ would be more pronounced in natural settings that include less predictable and more complex online social interactions rather than in well-structured experimental settings.87,88 In addition, as previous reviews have pointed out,48,89 limited psychometric properties such as low discriminatory power might have also led to a failure to discriminate differentially impaired aspects of mentalizing performance in ASD vs SCZ.
It should be noted that the majority of the ASD studies we included had HFA and Asperger Syndrome participants with at least average intelligence (average IQ = 108.54). Therefore, generalizing current results showing similar pattern of mentalizing deficits between SCZ and ASD to full-blown autism with an intellectual disability (IQ < 70) may be limited, and we might have seen greater differences had we been able to include more studies with lower functioning ASD. Second, the average age for adults with SCZ (M = 46.55) was older compared with those with ASD (M = 29.65) and HC (M = 31.71). We cannot rule out the possibility that age may have influenced both verbally (Strange Stories) and visually measured (the Eyes test) mentalizing performance. However, the current meta-analysis found no age moderator effect on either type of mentalizing performance (tables 5 and 6). Further, consistent with our results, emerging evidence suggests there is no relationship between age and mentalizing abilities.90–92
To further investigate the source of heterogeneous effect sizes, we examined the effect of putative moderators. Consistent with a previous review of studies with SCZ samples,48 the current meta-regression results showed no moderating effects on the effect size of mentalizing impairments. In terms of the relationship between symptom severity and mentalizing impairments, converging evidence suggests the possibility that mentalizing impairments are a trait factor in SCZ rather than state-dependent factor, considering that remitted patients68 and first-degree relatives of patients with SCZ show mentalizing impairments.48,71,93
Regarding gender effects on mentalizing abilities, we found that a larger relative percentage of males in the ASD, but not in then SCZ group, tended to be associated with greater impairments in the cognitive-linguistic mentalizing tasks (k = 10 studies, B = 1.67, SE = 0.96, Z = 1.73, P = .08), consistent with “female superiority” in mentalizing, as argued by Baron-Cohen et al.94 However, except for early studies by Baron-Cohen et al26,47,49,94 later studies from different research groups have failed to find a significant gender difference on the Eyes test among adults with ASD,86 adults with SCZ,48 relatives of patients with SCZ,57,95 and other clinical groups.96 Thus, further research is needed to determine whether there are strong and consistent gender differences in mentalizing performance among individuals with ASD.
Despite limited information about the psychometric properties of mentalizing tasks, comparing cognitive-linguistic verbal mentalizing with visual mentalizing, especially for adults with ASD and SCZ, is a crucial attempt to tease apart multifaceted mentalizing abilities into specific aspects of impaired psychological or neural mechanisms in each disorder. The results of this meta-analysis suggest that both SCZ and ASD disorders share at least some common impairment in mentalizing. Emerging evidence from neuroimaging studies consistently indicates that several neural regions show altered activation in both disorders during mentalizing tasks. Such findings may suggest a common neural mechanism underlying the observed behavioral deficits in the 2 disorders. For example, a recent neuroimaging meta-analyses97 found that adults with SCZ and ASD both exhibited reduced engagement of medial PFC, thought with a greater degree of impairment in patients with ASD during facial emotional processing and the Eyes test47 compared with SCZ.
The current results may have been limited for the following reasons. The major limitation is that this study compared effects sizes across diagnostic groups from different studies, rather than from studies that directly compared SCZ and ASD, though our results are consistent with previous studies that directly compared between ASD and SCZ.42,69 Further, despite no significant impact of proportion of medicated patients on the magnitude of mentalizing impairments in SCZ, we cannot rule out the possibility that antipsychotic medication may have affected mentalizing abilities in SCZ, which led to similar degree of verbal-type mentalizing impairments between ASD and SCZ. However, there is little empirical evidence suggesting that antipsychotic medication improves mentalizing ability in SCZ. For example, Mizrahi et al98 examined drug-induced improvement in psychotic symptoms of SCZ and mentalizing ability measured by the Hinting task. They found positive scores and mentalizing ability in SCZ improved after the first 2 weeks of antipsychotic treatment. However, the changes observed in psychotic symptoms and the percentage improvements in mentalizing performance were not significantly associated, and they could not rule out the possibility that the improvements on the Hinting task reflected practice effects, though different versions were used across testing sessions. Further, the mentalizing tasks chosen in the current meta-analysis were quite selective. However, the comparison of cognitive-linguistic vs emotion recognition from eyes is consistent with a distinction made in the literature between mental state decoding (the Eyes test) and reasoning (cognitive-linguistic mentalizing tasks).99–102 To support this, a distinctive neural mechanism has been found to be involved in performance of the different sets of tasks. Whereas left ventromedial PFC regions appear to be involved in cognitive “reasoning” about mental states,103,104 the right inferior frontal and anterior temporal lobes may be relatively crucial for “decoding sensory processing” based mentalizing.100,104 Importantly, it should be noted that because most mentalizing tasks included in the current meta-analysis have been used in experimental settings, most of them have not been standardized, which led to investigators’ arbitrary choice of items and/or modifications for a given task. For example, in terms of the Strange Stories task, Gavilan et al58 reduced the number of passages considering patients’ cognitive difficulty and developed 3 types of story passages from the original version of the Strange Stories. Also, 2 versions of the Eyes test (eg, Baron-Cohen et al14,47) existed in the current analysis. However, given that each clinical group and HCs used the same mentalizing task in an individual study, observed mean difference of mentalizing impairments cannot simply reflect psychometric artifacts related to the use of slightly varying versions of the tasks instead of distinct aspects of mentalizing performance.
In summary, in the current meta-analysis, adults with SCZ and ASD exhibited similar levels of mentalizing impairments in the 2 types of mentalizing tasks. These results suggest at least in part, common cognitive behavioral deficits in mentalizing for these 2 clinical disorders. However, the current mentalizing tasks used in this field may not be sufficiently sensitive to distinguish visually (emotion recognition from eyes) vs verbally (cognitive-linguistic) measured mentalizing performance due to their uncertain psychometric properties. As mentioned in the Introduction, mentalizing impairments in ASD may evolve from impairments in deficits starting early in life, including alterations in eye contact and gaze orientation.39 On the other hand, mentalizing impairments in SCZ may arise later in life in patients with SCZ and may reflect greater contributions from cognitive deficits. As such, longitudinal studies that compare the evolution of mentalizing abilities across the 2 disorders may help identify and understand disorder-specific features of mentalizing. Furthermore, along with establishing psychometric properties of mentalizing tasks, future studies are required to develop novel tasks that tap into specific psychological constructs or neural circuitry, which potentially differentiate ASD and SCZ.
Supplementary Material
Supplementary material is available at http://schizophre niabulletin.oxfordjournals.org.
Acknowledgments
We thank the staff at each of the Cognitive Control and Psychopathology laboratory for their hard work and our participants for their time, energy, and cooperation. We also thank 3 anonymous reviewers for their helpful comments and suggestions, and Steven Tarlow for providing technical tips to run a Comprehensive Meta-Analysis Version 2.0. Financial Disclosures: Y.S.C. has no financial disclosures. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Penn DL, Sanna LJ, Roberts DL. Social cognition in schizophrenia: an overview. Schizophr Bull. 2008;34:408–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brüne M. “Theory of mind” in schizophrenia: a review of the literature. Schizophr Bull. 2005;31:21–42 [DOI] [PubMed] [Google Scholar]
- 3. Tager-Flusberg H. What neurodevelopmental disorders can reveal about cognitive architecture: the example of theory of mind. In: Carruthers P, Laurence S, Stich S, eds. The structure of the innate mind. Oxford: Oxford University Press; 2005:272–288 [Google Scholar]
- 4. Stone VE, Gerrans P. What’s domain-specific about theory of mind? Soc Neurosci. 2006;1:309–319 [DOI] [PubMed] [Google Scholar]
- 5. Wellman HM, Cross D, Watson J. Meta-analysis of theory-of-mind development: the truth about false belief. Child Dev. 2001;72:655–684 [DOI] [PubMed] [Google Scholar]
- 6. Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr Res. 2009;109:1–9 [DOI] [PubMed] [Google Scholar]
- 7. Scheeren AM, de Rosnay M, Koot HM, Begeer S. Rethinking theory of mind in high-functioning autism spectrum disorder. [published online ahead of print October 16, 2012]. J Child Psychol Psychiatry. doi: 10.1111/jcpp.12007 [DOI] [PubMed] [Google Scholar]
- 8. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed, revised. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 9. Mundy P, Sigman M, Ungerer J, Sherman T. Defining the social deficits of autism: the contribution of non-verbal communication measures. J Child Psychol Psychiatry. 1986;27:657–669 [DOI] [PubMed] [Google Scholar]
- 10. Baron-Cohen S, Campbell R, Karmiloff-Smith A, Grant J, Walker J. Are children with autism blind to the mentalistic significance of the eyes? Br J Dev Psychol. 1999;13:379–398 [Google Scholar]
- 11. Kanner L. Autistic disturbances of affective contact. Acta Paedopsychiatr. 1968;35:100–136 [PubMed] [Google Scholar]
- 12. Leekam SR, Hunnisett E, Moore C. Targets and cues: gaze-following in children with autism. J Child Psychol Psychiatry. 1998;39:951–962 [PubMed] [Google Scholar]
- 13. Ristic J, Mottron L, Friesen CK, Iarocci G, Burack JA, Kingstone A. Eyes are special but not for everyone: the case of autism. Brain Res Cogn Brain Res. 2005;24:715–718 [DOI] [PubMed] [Google Scholar]
- 14. Baron-Cohen S, Wheelwright S, Jolliffe T. Is there a “Language of the Eyes?” Evidence from Normal Adults, and Adults with Autism or Asperger Syndrome. Vis cogn. 1997;4:311–331 [Google Scholar]
- 15. Baron-Cohen S, Cambell R, Karmiloff-Smith A, Grant JJW. Are children with autism blind to the mentalistic significance of the eyes? Br J Develop Psychol. 1995;13:379–398 [Google Scholar]
- 16. Leekam S, Baron-Cohen S, Perrett DI, Milders M, Brown S. Eye-direction detection: a dissociation between geometric and joint attention skills in autism. Br J Develop Psychol. 1997;15:77–95 [Google Scholar]
- 17. Emery NJ. The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci Biobehav Rev. 2000;24:581–604 [DOI] [PubMed] [Google Scholar]
- 18. Hoehl S, Reid VM, Parise E, Handl A, Palumbo L, Striano T. Looking at eye gaze processing and its neural correlates in infancy-implications for social development and autism spectrum disorder. Child Dev. 2009;80:968–985 [DOI] [PubMed] [Google Scholar]
- 19. Padrao G, Mallorqui A, Cucurell D, Marco-Pallares J, Rodriguez-Fornells A. Neurophysiological differences in reward processing in anhedonics. Cogn Affect Behav Neurosci. 2012;13:102–115 [DOI] [PubMed] [Google Scholar]
- 20. Baron-Cohen S. How to build a baby that can read minds: Cognitive mechanisms in mindreading. Cahiers de Psychol Cog. 1994;13:513–552 [Google Scholar]
- 21. Charman T, Baron-Cohen S, Swettenham J, Baird G, Cox A, Drew A. Testing joint attention, imiation, and play as infancy precursors to language and theory of mind. Cognitive Dev. 2000;15:481–498 [Google Scholar]
- 22. Toth K, Munson J, Meltzoff AN, Dawson G. Early predictors of communication development in young children with autism spectrum disorder: joint attention, imitation, and toy play. J Autism Dev Disord. 2006;36:993–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harwood MD, Farrar MJ. Conflicting emotions: the connection between affective perspective taking and theory of mind. Brit Psychol Soc. 2006;24:401–418 [Google Scholar]
- 24. Hughes C, Dunn J. Understanding mind and emotion: longitudinal associations with mental-state talk between young friends. Dev Psychol. 1998;34:1026–1037 [DOI] [PubMed] [Google Scholar]
- 25. O’Brien M, Miner Weaver J, Nelson JA, Calkins SD, Leerkes EM, Marcovitch S. Longitudinal associations between children’s understanding of emotions and theory of mind. Cogn Emot. 2011;25:1074–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baron-Cohen S, O’Riordan M, Stone V, Jones R, Plaisted K. Recognition of faux pas by normally developing children and children with Asperger syndrome or high-functioning autism. J Autism Dev Disord. 1999;29:407–418 [DOI] [PubMed] [Google Scholar]
- 27. Tager-Flusberg H. Language and understanding minds: connections in autism. In: Baron-Cohen S, Tager-Flusberg H, Cohen DJ, eds. Understanding Other Minds: Perspectives from Developmental Cognitive Neuroscience. 2nd ed. Oxford: Oxford University Press; 2000:3–46 [Google Scholar]
- 28. Murray RM, O’Callaghan E, Castle DJ, Lewis SW. A neurodevelopmental approach to the classification of schizophrenia. Schizophr Bull. 1992;18:319–332 [DOI] [PubMed] [Google Scholar]
- 29. O’Carroll R. Cognitive impairment in schizophrenia. Adv Psychiatr Treat. 2000;6:161–168 [Google Scholar]
- 30. Bora E, Yücel M, Pantelis C. Theory of mind impairment: a distinct trait-marker for schizophrenia spectrum disorders and bipolar disorder? Acta Psychiatr Scand. 2009;120:253–264 [DOI] [PubMed] [Google Scholar]
- 31. Couture SM, Penn DL, Addington J, Woods SW, Perkins DO. Assessment of social judgments and complex mental states in the early phases of psychosis. Schizophr Res. 2008;100:237–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stanford AD, Messinger J, Malaspina D, Corcoran CM. Theory of Mind in patients at clinical high risk for psychosis. Schizophr Res. 2011;131:11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grossberg S, Mingolla E, Ross WD. Visual brain and visual perception: how does the cortex do perceptual grouping? Trends Neurosci. 1997;20:106–111 [DOI] [PubMed] [Google Scholar]
- 34. Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci (Regul Ed). 2012;16:27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McClure MM, Barch DM, Flory JD, Harvey PD, Siever LJ. Context processing in schizotypal personality disorder: evidence of specificity of impairment to the schizophrenia spectrum. J Abnorm Psychol. 2008;117:342–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Servan-Schreiber D, Cohen JD, Steingard S. Schizophrenic deficits in the processing of context. A test of a theoretical model. Arch Gen Psychiatry. 1996;53:1105–1112 [DOI] [PubMed] [Google Scholar]
- 37. Chung YS, Mathews JR, Barch DM. The effect of context processing on different aspects of social cognition in schizophrenia. Schizophr Bull. 2011;37:1048–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Green MJ, Waldron JH, Simpson I, Coltheart M. Visual processing of social context during mental state perception in schizophrenia. J Psychiatry Neurosci. 2008;33:34–42 [PMC free article] [PubMed] [Google Scholar]
- 39. Abu-Akel A, Bailey AL. The possibility of different forms of theory of mind impairment in psychiatric and developmental disorders. Psychol Med. 2000;30:735–738 [DOI] [PubMed] [Google Scholar]
- 40. Bölte S, Poustka F. The recognition of facial affect in autistic and schizophrenic subjects and their first-degree relatives. Psychol Med. 2003;33:907–915 [DOI] [PubMed] [Google Scholar]
- 41. Corcoran R, Mercer G, Frith CD. Schizophrenia, symptomatology and social inference: investigating “theory of mind” in people with schizophrenia. Schizophr Res. 1995;17:5–13 [DOI] [PubMed] [Google Scholar]
- 42. Craig JS, Hatton C, Craig FB, Bentall RP. Persecutory beliefs, attributions and theory of mind: comparison of patients with paranoid delusions, Asperger’s syndrome and healthy controls. Schizophr Res. 2004;69:29–33 [DOI] [PubMed] [Google Scholar]
- 43. Pilowsky T, Yirmiya N, Arbelle S, Mozes T. Theory of mind abilities of children with schizophrenia, children with autism, and normally developing children. Schizophr Res. 2000;42:145–155 [DOI] [PubMed] [Google Scholar]
- 44. Hala S, Chandler M, Fritz AS. Fledging theories of mind: Deception as a marker of three year olds' understanding of false belief. Child Devel. 1991;62:83–97 [Google Scholar]
- 45. Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21:37–46 [DOI] [PubMed] [Google Scholar]
- 46. Happé FG. An advanced test of theory of mind: understanding of story characters’ thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. J Autism Dev Disord. 1994;24:129–154 [DOI] [PubMed] [Google Scholar]
- 47. Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001;42:241–251 [PubMed] [Google Scholar]
- 48. Sprong M, Schothorst P, Vos E, Hox J, van Engeland H. Theory of mind in schizophrenia: meta-analysis. Br J Psychiatry. 2007;191:5–13 [DOI] [PubMed] [Google Scholar]
- 49. Baron-Cohen S, Knickmeyer RC, Belmonte MK. Sex differences in the brain: implications for explaining autism. Science. 2005;310:819–823 [DOI] [PubMed] [Google Scholar]
- 50. Brüne M, Bodenstein L. Proverb comprehension reconsidered–‘theory of mind’ and the pragmatic use of language in schizophrenia. Schizophr Res. 2005;75:233–239 [DOI] [PubMed] [Google Scholar]
- 51. Carlson SM, Moses LJ, Breton C. How specific is the relation between executive function and theory of mind? Contributions of inhibitory control and working memory. Infant Child Dev. 2002;11:73–92 [Google Scholar]
- 52. Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. J Cogn Neurosci. 1998;10:640–656 [DOI] [PubMed] [Google Scholar]
- 53. Baron-Cohen S, Jolliffe T, Mortimore C, Robertson M. Another advanced test of theory of mind: evidence from very high functioning adults with autism or asperger syndrome. J Child Psychol Psychiatry. 1997;38:813–822 [DOI] [PubMed] [Google Scholar]
- 54. Golan O, Baron-Cohen S, Hill JJ, Rutherford MD. The ‘Reading the Mind in the Voice’ test-revised: a study of complex emotion recognition in adults with and without autism spectrum conditions. J Autism Dev Disord. 2007;37:1096–1106 [DOI] [PubMed] [Google Scholar]
- 55. Borenstein M, Hedges L, Higgings J, Rothstein H, Englewood NJ. Comprehensive Meta-Analysis. A Computer Program for Meta-Analysis. [Computer software]. Englewood, NJ: Biostat Inc; 2007. [Google Scholar]
- 56. Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. J Edu Stat. 1981;6:107–128 [Google Scholar]
- 57. de Achával D, Costanzo EY, Villarreal M, et al. Emotion processing and theory of mind in schizophrenia patients and their unaffected first-degree relatives. Neuropsychologia. 2010;48:1209–1215 [DOI] [PubMed] [Google Scholar]
- 58. Gavilan JM, Garcia-Albea JE. Theory of mind and language comprehension in schizophrenia: poor mindreading affects figurative language comprehension beyond intelligence deficits. J Neurolinguist. 2011;24:54–69 [Google Scholar]
- 59. Herold R, Feldmann A, Simon M, et al. Regional gray matter reduction and theory of mind deficit in the early phase of schizophrenia: a voxel-based morphometric study. Acta Psychiatr Scand. 2009;119:199–208 [DOI] [PubMed] [Google Scholar]
- 60. Hooker CI, Bruce L, Lincoln SH, Fisher M, Vinogradov S. Theory of mind skills are related to gray matter volume in the ventromedial prefrontal cortex in schizophrenia. Biol Psychiatry. 2011;70:1169–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Langdon R, Ward PB, Coltheart M. Reasoning anomalies associated with delusions in schizophrenia. Schizophr Bull. 2010;36:321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Martino DJ, Bucay D, Butman JT, Allegri RF. Neuropsychological frontal impairments and negative symptoms in schizophrenia. Psychiatry Res. 2007;152:121–128 [DOI] [PubMed] [Google Scholar]
- 63. Pijnenborg GH, Withaar FK, Evans JJ, van den Bosch RJ, Timmerman ME, Brouwer WH. The predictive value of measures of social cognition for community functioning in schizophrenia: implications for neuropsychological assessment. J Int Neuropsychol Soc. 2009;15:239–247 [DOI] [PubMed] [Google Scholar]
- 64. Riveros R, Manes F, Hurtado E, et al. Context-sensitive social cognition is impaired in schizophrenic patients and their healthy relatives. Schizophr Res. 2010;116:297–298 [DOI] [PubMed] [Google Scholar]
- 65. Shur S, Shamay-Tsoory SG, Levkovitz Y. Integration of emotional and cognitive aspects of theory of mind in schizophrenia and its relation to prefrontal neurocognitive performance. Cogn Neuropsychiatry. 2008;13:472–490 [DOI] [PubMed] [Google Scholar]
- 66. Zhu CY, Lee TM, Li XS, Jing SC, Wang YG, Wang K. Impairments of social cues recognition and social functioning in Chinese people with schizophrenia. Psychiatry Clin Neurosci. 2007;61:149–158 [DOI] [PubMed] [Google Scholar]
- 67. Bailey PE, Henry JD. Separating component processes of theory of mind in schizophrenia. Br J Clin Psychol. 2010;49:43–52 [DOI] [PubMed] [Google Scholar]
- 68. Bora E, Gökçen S, Kayahan B, Veznedaroglu B. Deficits of social-cognitive and social-perceptual aspects of theory of mind in remitted patients with schizophrenia: effect of residual symptoms. J Nerv Ment Dis. 2008;196:95–99 [DOI] [PubMed] [Google Scholar]
- 69. Couture SM, Penn DL, Losh M, Adolphs R, Hurley R, Piven J. Comparison of social cognitive functioning in schizophrenia and high functioning autism: more convergence than divergence. Psychol Med. 2010;40:569–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hirao K, Miyata J, Fujiwara H, et al. Theory of mind and frontal lobe pathology in schizophrenia: a voxel-based morphometry study. Schizophr Res. 2008;105:165–174 [DOI] [PubMed] [Google Scholar]
- 71. Irani F, Platek SM, Panyavin IS, et al. Self-face recognition and theory of mind in patients with schizophrenia and first-degree relatives. Schizophr Res. 2006;88:151–160 [DOI] [PubMed] [Google Scholar]
- 72. Kington JM, Jones LA, Watt AA, Hopkin EJ, Williams J. Impaired eye expression recognition in schizophrenia. J Psychiatr Res. 2000;34:341–347 [DOI] [PubMed] [Google Scholar]
- 73. McGlade N, Behan C, Hayden J, et al. Mental state decoding v. mental state reasoning as a mediator between cognitive and social function in psychosis. Br J Psychiatry. 2008;193:77–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Russell TA, Rubia K, Bullmore ET, et al. Exploring the social brain in schizophrenia: left prefrontal underactivation during mental state attribution. Am J Psychiatry. 2000;157:2040–2042 [DOI] [PubMed] [Google Scholar]
- 75. Schimansky J, David N, Rössler W, Haker H. Sense of agency and mentalizing: dissociation of subdomains of social cognition in patients with schizophrenia. Psychiatry Res. 2010;178:39–45 [DOI] [PubMed] [Google Scholar]
- 76. Tso IF, Grove TB, Taylor SF. Emotional experience predicts social adjustment independent of neurocognition and social cognition in schizophrenia. Schizophr Res. 2010;122:156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Adler N, Nadler B, Eviatar Z, Shamay-Tsoory SG. The relationship between theory of mind and autobiographical memory in high-functioning autism and Asperger syndrome. Psychiatry Res. 2010;178:214–216 [DOI] [PubMed] [Google Scholar]
- 78. David N, Gawronski A, Santos NS, et al. Dissociation between key processes of social cognition in autism: impaired mentalizing but intact sense of agency. J Autism Dev Disord. 2008;38:593–605 [DOI] [PubMed] [Google Scholar]
- 79. Heavey L, Phillips W, Baron-Cohen S, Rutter M. The Awkward Moments Test: a naturalistic measure of social understanding in autism. J Autism Dev Disord. 2000;30:225–236 [DOI] [PubMed] [Google Scholar]
- 80. Hill EL, Sally D, Frith U. Does mentalising ability influence cooperative decision-making in a social dilemma? J Conscious Stud. 2004;11:144–161 [Google Scholar]
- 81. Jolliffe T, Baron-Cohen S. The Strange Stories Test: a replication with high-functioning adults with autism or Asperger syndrome. J Autism Dev Disord. 1999;29:395–406 [DOI] [PubMed] [Google Scholar]
- 82. Ponnet KS, Roeyers H, Buysse A, De Clercq A, Van der Heyden E. Advanced mind-reading in adults with Asperger syndrome. Autism. 2004;8:249–266 [DOI] [PubMed] [Google Scholar]
- 83. Roeyers H, Buysse A, Ponnet K, Pichal B. Advancing advanced mind-reading tests: empathic accuracy in adults with a pervasive developmental disorder. J Child Psychol Psychiatry. 2001;42:271–278 [PubMed] [Google Scholar]
- 84. Spek AA, Scholte EM, Van Berckelaer-Onnes IA. Theory of mind in adults with HFA and Asperger syndrome. J Autism Dev Disord. 2010;40:280–289 [DOI] [PubMed] [Google Scholar]
- 85. Zalla T, Sav AM, Stopin A, Ahade S, Leboyer M. Faux pas detection and intentional action in Asperger Syndrome. A replication on a French sample. J Autism Dev Disord. 2009;39:373–382 [DOI] [PubMed] [Google Scholar]
- 86. Kleinman J, Marciano PL, Ault RL. Advanced theory of mind in high-functioning adults with autism. J Autism Dev Disord. 2001;31:29–36 [DOI] [PubMed] [Google Scholar]
- 87. Castelli F, Frith C, Happé F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849 [DOI] [PubMed] [Google Scholar]
- 88. Ponnet K, Buysse A, Roeyers H, De Clercq A. Mind-reading in young adults with ASD: does structure matter? J Autism Dev Disord. 2008;38:905–918 [DOI] [PubMed] [Google Scholar]
- 89. Harrington L, Siegert RJ, McClure J. Theory of mind in schizophrenia: a critical review. Cogn Neuropsychiatry. 2005;10:249–286 [DOI] [PubMed] [Google Scholar]
- 90. Maylor EA, Moulson JM, Muncer AM, Taylor LA. Does performance on theory of mind tasks decline in old age? Br J Psychol. 2002;93:465–485 [DOI] [PubMed] [Google Scholar]
- 91. Sullivan S, Ruffman T. Social understanding: How does it fare with advancing years? Br J Psychol. 2004;95:1–18 [DOI] [PubMed] [Google Scholar]
- 92. Pardini M, Nichelli PF. Age-related decline in mentalizing skills across adult life span. Exp Aging Res. 2009;35:98–106 [DOI] [PubMed] [Google Scholar]
- 93. Janssen I, Krabbendam L, Jolles J, van Os J. Alterations in theory of mind in patients with schizophrenia and non- psychotic relatives. Acta Psychiatr Scand. 2003;108:110–117 [DOI] [PubMed] [Google Scholar]
- 94. Baron-Cohen S. The extreme male brain theory of autism. Trends Cogn Sci (Regul Ed). 2002;6:248–254 [DOI] [PubMed] [Google Scholar]
- 95. Kelemen O, Kéri S, Must A, Benedek G, Janka Z. No evidence for impaired ‘theory of mind’ in unaffected first-degree relatives of schizophrenia patients. Acta Psychiatr Scand. 2004;110:146–149 [DOI] [PubMed] [Google Scholar]
- 96. Bora E, Vahip S, Gonul AS, et al. Evidence for theory of mind deficits in euthymic patients with bipolar disorder. Acta Psychiatr Scand. 2005;112:110–116 [DOI] [PubMed] [Google Scholar]
- 97. Sugranyes G, Kyriakopoulos M, Corrigall R, Taylor E, Frangou S. Autism spectrum disorders and schizophrenia: meta-analysis of the neural correlates of social cognition. PLoS ONE. 2011;6:e25322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mizrahi R, Korostil M, Starkstein SE, Zipursky RB, Kapur S. The effect of antipsychotic treatment on Theory of Mind. Psychol Med. 2007;37:595–601 [DOI] [PubMed] [Google Scholar]
- 99. Pickup GJ. Relationship between Theory of Mind and executive function in schizophrenia: a systematic review. Psychopathology. 2008;41:206–213 [DOI] [PubMed] [Google Scholar]
- 100. Bora E, Eryavuz A, Kayahan B, Sungu G, Veznedaroglu B. Social functioning, theory of mind and neurocognition in outpatients with schizophrenia; mental state decoding may be a better predictor of social functioning than mental state reasoning. Psychiatry Res. 2006;145:95–103 [DOI] [PubMed] [Google Scholar]
- 101. Shamay-Tsoory SG, Shur S, Barcai-Goodman L, Medlovich S, Harari H, Levkovitz Y. Dissociation of cognitive from affective components of theory of mind in schizophrenia. Psychiatry Res. 2007;149:11–23 [DOI] [PubMed] [Google Scholar]
- 102. Donohoe G, Duignan A, Hargreaves A, et al. Social cognition in bipolar disorder versus schizophrenia: comparability in mental state decoding deficits. Bipolar Disord. 2012;14:743–748 [DOI] [PubMed] [Google Scholar]
- 103. Lee KH, Farrow TF, Spence SA, Woodruff PW. Social cognition, brain networks and schizophrenia. Psychol Med. 2004;34:391–400 [DOI] [PubMed] [Google Scholar]
- 104. Sabbagh MA. Understanding orbitofrontal contributions to theory-of-mind reasoning: implications for autism. Brain Cogn. 2004;55:209–219 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.