Abstract
Background:
Altered transmission of gamma-aminobutyric acid (GABA), a major inhibitory neurotransmitter, may contribute to the development of schizophrenia. The purpose of the present study was to investigate the presence of GABA-A/benzodiazepine (BZ) receptor binding abnormalities in individuals at ultra-high risk (UHR) for psychosis in comparison with normal controls using [18F]-fluoroflumazenil (FFMZ) positron emission tomography (PET). In particular, we set regions of interest in the striatum (caudate, putamen, and nucleus accumbens) and medial temporal area (hippocampus and parahippocampal gyrus).
Methods:
Eleven BZ-naive people at UHR and 15 normal controls underwent PET scanning using [18F]-FFMZ to measure GABA-A/BZ receptor binding potential. The regional group differences between UHR individuals and normal controls were analyzed using Statistical Parametric Mapping 8 software. Participants were evaluated using the structured interview for prodromal syndromes and neurocognitive function tasks.
Results:
People at UHR demonstrated significantly reduced binding potential of GABA-A/BZ receptors in the right caudate.
Conclusions:
Altered GABAergic transmission and/or the imbalance of inhibitory and excitatory systems in the striatum may be present at the putative prodromal stage and play a pivotal role in the pathophysiology of psychosis.
Key words: GABA, schizophrenia, ultra-high, risk for psychosis, caudate, PET, fluoroflumazenil
Introduction
The dopamine theory of schizophrenia that proposes increased dopaminergic transmission in striatum is the oldest and most established hypothesis, having been in existence for more than 30 years.1,2 However, the pathophysiology of schizophrenia cannot be accounted for by dopamine alone.3,4 Intriguing evidence from clinical and basic science studies as well as from animal models have proposed the critical involvement of other neurotransmitter systems in the pathophysiology of schizophrenia, most notably of dysfunctions in the glutamate5,6 and gamma-aminobutyric acid (GABA)7,8 system. A striatal hyperdopaminergic state may be derived from the cascade of events involving glutamatergic and GABAergic neurotransmission.
In particular, alteration of the GABAergic system has been implicated in the pathophysiology of schizo phrenia.8,9 Converging evidence from postmortem studies in schizophrenia has shown altered GABA neurotransmission in the prefrontal cortex such as deficits of prefrontal GABA interneurons10 and decreased mRNA expression of glutamic acid decarboxylase (GAD), a rate limiting enzyme for GABA synthesis.7 Although primary emphasis has been placed on assessing the prefrontal cortex, these GABAergic dysfunctions have been observed in the striatum as well. The GABAergic alteration in the striatum has been supported by some postmortem evidence of GABA-A receptor alterations in the striatum11,12 and of epigenetic changes of decreased mRNA expression of reelin and GAD in basal ganglia GABA neurons from schizophrenic brain tissues.13 In vivo neuroimaging studies in schizophrenic patients have also provided evidence of GABAergic alteration in the striatum. A proton magnetic resonance spectroscopy (MRS) study showed that decreased GABA concentrations in the basal ganglia were present in early phase (duration of illness less than 6 months) schizophrenic patients.14 Furthermore, because the striatum is comprised almost entirely of GABAergic neurons15 and striatal neurons receive prominent inhibitory GABAergic inputs as well as massive dopaminergic and glutamatergic inputs,16 the striatal GABAergic system may play a key role in modulation of the cortico-striatal circuit through interactions among GABA, glutamate, and dopamine in schizophrenia. In addition to the striatum, the medial temporal area is another potential region of GABAergic alteration in schizophrenia. In 15 chronically medicated patients, a single photon emission computed tomography study using [123I]-iomazenil showed a significant negative relationship between binding in the left medial temporal region and positive psychopathology.17 In antipsychotic medication-naive (n = 6) or -free (n = 5) patients, though they were not young (mean age: about 33 years), a positron emission tomography (PET) study using a [11C]-Ro15-4513, a GABA-A/benzodiazepine (BZ) receptor partial inverse agonist, also showed a significant negative association between GABA-A/BZ receptor binding in the hippocampus and negative psychopathology.18 Although those previous reports showed no significant difference for GABA-A/BZ receptor bindings between schizophrenia patients and normal controls, the significant relationships of medial temporal (hippocampal) GABAergic binding with psychopathology suggest that inhibitory GABAergic transmission in this area may result in increased striatal glutamatergic afferents from the hippocampus leading to psychotic symptoms. Taken together, there may be altered GABA transmission in the striatum and the medial temporal area, which may play a pivotal role in the development of schizophrenia.
To examine the nature of GABAergic system alterations in the development of schizophrenia, changes to the GABAergic system should be evaluated in young patients at the prodromal phase, because prodromal young patients are relatively less affected by secondary processes including long-term effects of psychotropic medications and neurobiological compensatory changes, which are potential confounding factors of the above-mentioned postmortem and in vivo imaging studies. Recently, a novel strategy was developed to identify people at ultra-high risk (UHR) for developing psychosis, with a probability of 16%–35% within 2 years.19,20 A series of in vivo imaging studies were conducted by McGuire and his colleagues in UHR individuals,21–23 which revealed abnormal interactions between glutamate levels in the hippocampus and increased dopamine uptake in the dorsal striatum before the onset of psychosis.21 These findings suggested that altered signaling pathway between striatal dopamine neurons and hippocampal glutamate neurons in UHR individuals may be influenced by inhibitory GABAergic transmission in the hippocampus. However, as far as we know, there has been no in vivo research of GABAergic alteration in individuals at UHR, “putative” prodromal stage of psychosis.
In vivo measurement of central GABAergic alteration in UHR is crucial to examine the role of GABAergic system in the pathophysiology of psychosis development. Functional neuroimaging using radiolabeled flumazenil, a specific neutral competitive antagonist, at the BZ recognition site of GABA-A/BZ receptors containing α1, α2, α3, or α5 subunits has been widely used to measure central GABA-A/BZ receptor.24 In particular, [18F]-fluoroflumazenil (FFMZ) has a high affinity for the GABA-A/BZ receptor and a long half-life, enabling neuroimaging of GABA-A/BZ receptor distribution in the living human brain.25,26 In the present study, we investigated whether BZ-naive UHR individuals demonstrate GABA-A/BZ receptor binding potential (BP) abnormalities in comparison with normal controls by using [18F]-FFMZ PET. Based on previously established converging evidence, we performed not only exploratory whole-brain analysis but also confirmatory region of interest (ROI) analyses in the brain regions including the striatum (caudate, putamen, and nucleus accumbens) and the medial temporal area (hippocampus and parahippocampal gyrus). In addition, we examined the relationships between regional GABA-A/BZ receptor BP and findings of psychopathology and neurocognitive performance in UHR individuals.
Methods
Subjects
Eleven BZ-naive individuals at UHR and 15 normal controls participated in the present study. People at UHR were recruited from the Clinic FORYOU of the Green Program for Recognition And Prevention of Early Psychosis (GRAPE) project at Severance Hospital and Severance Mental Health Hospital of the Yonsei University Health System. The details of this GRAPE project have been described elsewhere.27 Axis I psychiatric disorders were assessed in all subjects by a trained psychiatrist (K.K.R.) using the Structured Clinical Interview for DSM-IV (SCID-IV).28 Subjects with past or current psychosis, any drug use disorders, neurological disorders, or mental retardation were excluded. In the control group, subjects with any past or current psychiatric or neurological illness were excluded. Subjects with prior history of radiation exposure for research purposes or in the workplace were excluded. In addition, subjects who have been exposed to BZs were excluded by history taking and chart reviews. All subjects were right handed.
The diagnosis of the UHR group was based on the SIPS,27,29 a specific assessment tool for people at UHR that assesses presence and severity of 4 prodromal symptom subdomains: positive, negative, disorganization, and general. Each subscale consists of 4–6 items (19 items total) rated on a 7-point severity scale (scored, 0–6). UHR subjects satisfied one or more of the 3 prodromal syndromes outlined in the SIPS: (1) brief intermittent psychotic syndrome (BIPS), which has emerging psychotic symptoms with spontaneous remission in <1 week; (2) attenuated positive prodromal syndrome (APS), which has attenuated subthreshold positive psychotic symptoms; or (3) genetic risk and deterioration syndrome (GRDS), which is a combination of genetic risk for schizophrenia and recent functional decline. All UHR individuals in the present sample met the APS criterion, and one and three subjects also met the GRDS and BIPS criteria, respectively. The UHR group members were also clinically rated with the Montgomery-Åsberg Depression Rating Scale (MADRS).30
In addition, neurocognitive functions were evaluated using the California Verbal Learning Test (CVLT),31 Continuous Performance Test (CPT),32 Stroop Test,33 Wisconsin Card Sorting Test (WCST),34 Controlled Oral Word Association Test (COWAT),35 and Figure Fluency Test36 for neurocognitive domains including verbal memory, continuous attention, and executive function that have previously been reported as impaired in both UHR37 and schizophrenia.38 All psychiatric evaluations were recorded within 1 week before or after scanning.
This study was performed under the guidelines established by the Institutional Review Board at Severance Hospital and Severance Mental Health Hospital of Yonsei University Health System. The study information, including the PET scanning procedures and specific information about the risks of the radiation exposure, was fully explained to the subjects. Written informed consent was obtained from all subjects before participating in the study and additionally from their parents if subjects were less than 18 years of age.
PET Protocol
All subjects underwent PET scanning using [18F]-FFMZ in a GE Discovery STE PET/CT scanner (GE) with 5.75-mm 3D transaxial resolution and 15.7-cm axial field of view. Each transmission scan was obtained using low-dose CT (140kV, 95 mA). Approximately 5.5 MBq (0.15 mCi)/kg [18F]-FFMZ was slowly injected intravenously. The estimated effective dose was around 5–8 mSv, which was based on the guidelines of the International Commission on Radiation Protection. A total of 150 frames of dynamic PET image acquisition data were obtained in 3D mode for 60min (60 × 10 s, 40 × 15 s, 20 × 30 s, and 30 × 60 s). PET images acquired from 20 to 40min after radiotracer injection were used for statistical analysis to avoid blood flow effects and nonspecific GABA-A/BZ receptor binding. Attenuation-corrected emission data were reconstructed in a 128×128×35 matrix with a voxel size of 1.95×1.95×4.25mm using Hanning and ramp filters. Regarding tracer pharmacokinetic characteristics of [18F]-FFMZ, please refer to our previous article.39
Data Analysis
Voxel-by-voxel BP was calculated from dynamic [18F]-FFMZ-PET images using a multilinear reference tissue method40 with reference tissue activity coming from mean activity within the manually delineated pons using PMOD software (PMOD Technologies Ltd). The BP in this study indicates the ratio of specifically bound radioligand to that of nondisplaceable radioligand in tissue at equilibrium, ie, BPND according to the consensus nomenclature.41 For this study, we assumed that there is no significant difference in nondisplaceable uptake at pons between groups. Several evaluations showed that the current reference tissue model with the pons activity as a reference is very reliable and has a very high correlation with the BP values estimated using arterial sampling method, which is difficult and invasive for patients.42,43
Spatial preprocessing and statistical analysis of BPND maps were performed using Statistical Parametric Mapping (SPM8, Institute of Neurology, University College of London). The [18F]-FFMZ-PET templates of both control and UHR groups were created by averaging all [18F]-FFMZ-PET images that were spatially normalized into the MNI (Montreal Neurological Institute, McGill University) standard [18F]-FDG-PET template with nonlinear transformation. All BPND maps were transformed to the group template using a nonlinear transform function. Spatially normalized BP maps were smoothed with an isotropic Gaussian kernel with 8mm full width at half maximum. To focus on the regional difference in BPND, the effects of global BPND difference were removed by scaling the BPND of each voxel to the mean BPND of the entire brain (proportional scaling in SPM).
In exploratory whole-brain SPM analyses, group differences in regional [18F]-FFMZ BPND between UHR and normal control groups were evaluated using 2-sample t statistics at every voxel. Regional [18F]-FFMZ BPND differences were considered statistically significant at a peak height threshold of uncorrected P < .005 with cluster size greater than 139 contiguous voxels, which corresponds to a threshold of P < .05 corrected by cluster level for multiple comparisons as estimated by 10 000 Monte Carlo simulations.44
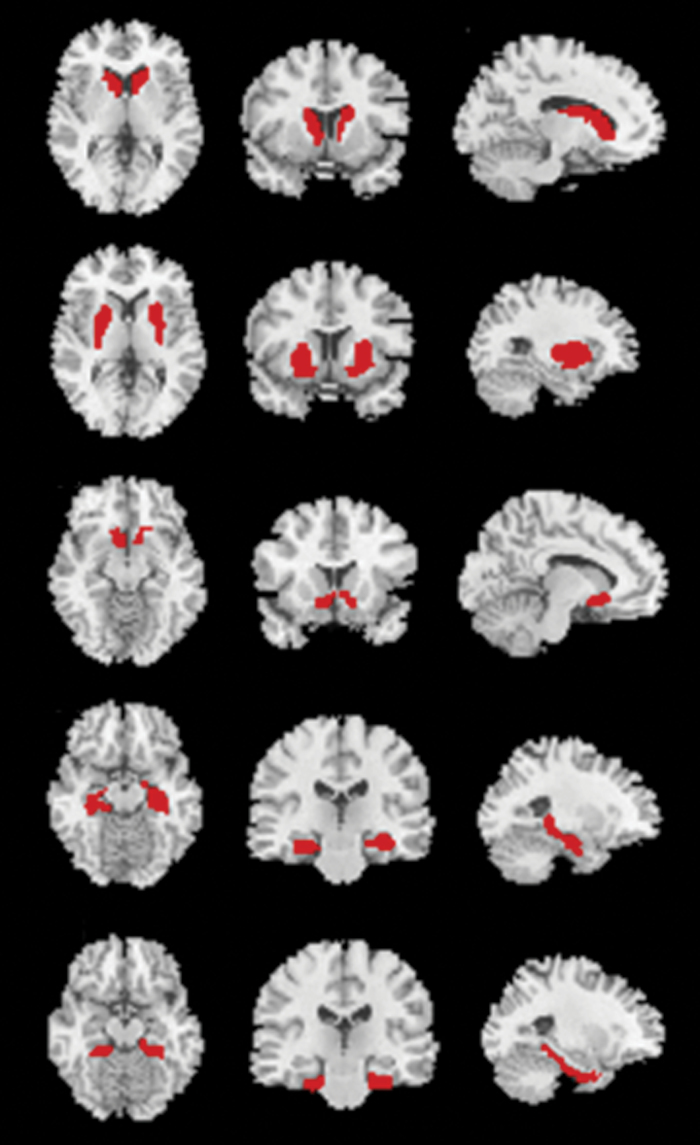
To confirm whole-brain SPM analysis results, ROI analyses were conducted. We calculated the mean BPND at the caudate, putamen, nucleus accumbens, hippocampus, and parahippocampal gyrus in both hemispheres (all 10 ROIs). These regions were defined using the Anatomical Automated Labeling Atlas,45 as shown in figure 1. FFMZ BPND in these ROIs was compared using MANOVA, which was performed using SPSS v.20 (SPSS). Bonferroni correction for multiple comparisons was applied by dividing the P value by 10 (all 10 ROIs).
Fig. 1.
Region of interest (ROI) placement for PET analysis, which was defined using the Anatomical Automated Labeling Atlas. From above, caudate, putamen, nucleus accumbens, hippocampus, and parahippocampal gyrus in both hemispheres.
To explore the relationships between the FFMZ BPND in regions identified to have significant between-group difference from the whole-brain SPM analyses and the degrees of psychopathology and neurocognitive function in UHR individuals, we conducted correlation analysis. Additionally, we examined the correlations between the regional FFMZ BPND in the 10 ROIs and clinical data. This analysis was performed using partial correlation analysis with age and years of education as covariates.
We used the Mann–Whitney U-test on continuous variables and Fisher’s exact tests on categorical variables to evaluate differences of demographic data between groups. Statistical analyses were performed using SPSS v.20. All tests were 2 tailed and the threshold of significance was set at P < .05.
Results
Sociodemographic and Clinical Characteristics of Subjects
Eleven people at UHR (mean age 19.04 years, SD 2.27; 6 men and 5 women) and 15 normal controls (mean age 20.98 years, SD 1.52; 9 men and 6 women) were included. Mean educational years were 12.27±1.74 for the UHR group and 13.47±0.92 for the normal controls. No significant differences were found between the UHR and control groups with respect to age, sex ratio, or years of education (P = .087, .78, and .097, respectively).
In the UHR group, the mean scores of positive, negative, disorganization, and general subscales of SIPS were 11.5±4.2, 13.0±7.5, 2.8±2.6, and 6.4±5.3, respectively. The depression score from MADRS was 15.0±9.9. In the neurocognitive task, scores of CVLT (total correct words) were 48.6±10.7 for CVLT learning trials (1–5), 12.0±3.1 for CVLT short-delay free recall, and 12.8±2.1 for CVLT long-delay free recall. In addition, the mean results of CPT (sensitivity; d′), Stroop Test (inference error), WCST (perseveration error), COWAT (total words), and Figure Fluency Test (total figures) were 4.8±0.7, 5.3±2.9, 14.0±10.7, 40.0±9.4, and 57.1 ± 15.4, respectively.
No control subjects were taking any medications. Two UHR individuals had been taking only atypical antipsychotics (aripiprazole 15mg [n = 1] or risperidone 4mg + quetiapine 25mg [n = 1]) for less than 4 weeks.
Between-Group Comparisons of GABA-A/BZ Receptor BPND
Whole-Brain SPM Analysis of Regional FFMZ BPND.
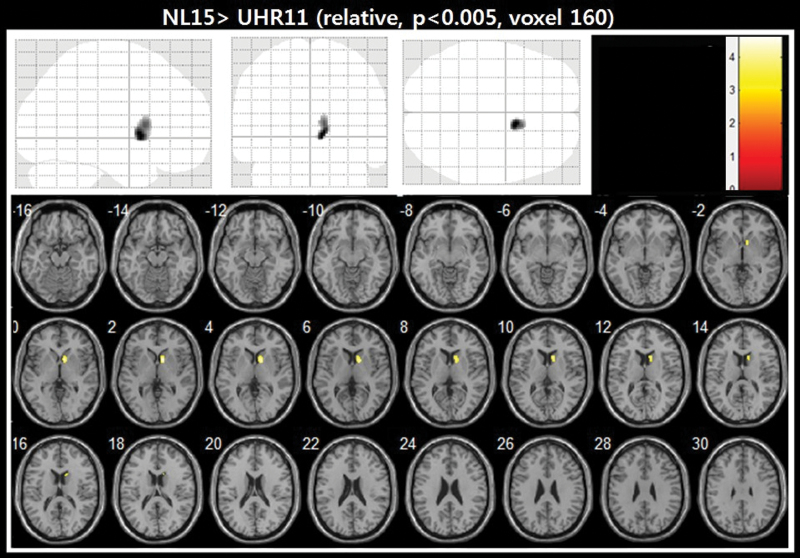
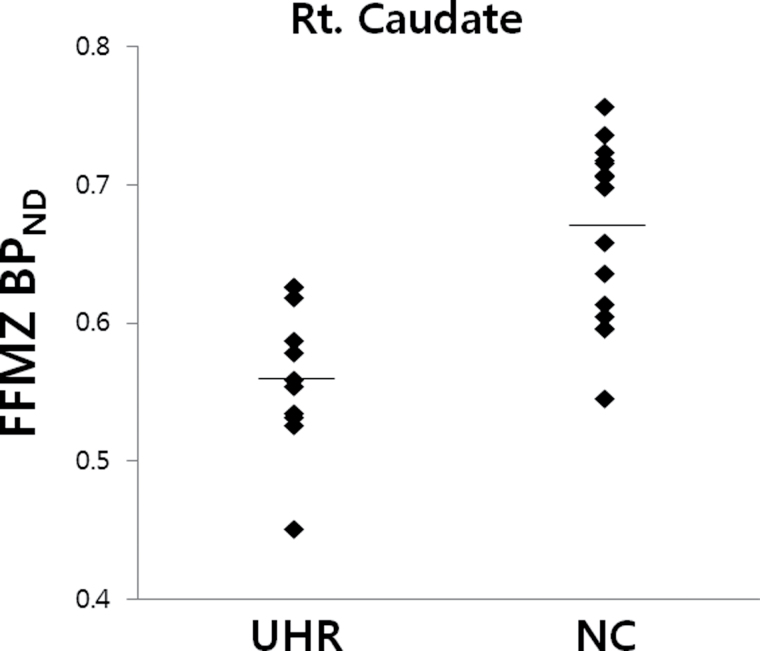
When the 11 BZ-naive individuals at UHR were compared with the 15 normal controls using the 2-sample t-test of SPM8, the UHR group demonstrated significantly decreased GABA-A/BZ receptor binding in the right caudate at the level of 160 contiguous voxels with the threshold of uncorrected P < .005, which corresponds to cluster level corrected P < .05. (figure 2 and table 1). The individual distribution of FFMZ BPND values in the caudate was presented by group of UHR and normal controls in figure 3.
Fig. 2.
Regions of decreased GABA-A/BZ receptor BPND in 11 individuals at UHR compared with 15 normal controls. The significance level of the image was set at uncorrected P < .005 with cluster size greater than 139 contiguous voxels. Decreased GABA-A/BZ receptor BPND in the right caudate was shown in the UHR subjects compared with normal controls by superimposing onto a series of axial brain slices extending from z-16 to z-30 in the Talairach coordinate space of the brain.
Table 1.
The Regions of Significantly Decreased [18F]-FFMZ BPND in 11 Individuals at UHR Compared With 15 Normal Controls
| Region | MNI (x, y, z [mm]) | Zmax | Cluster Size |
|---|---|---|---|
| NC > UHR Right caudate | 10, 10, 2 | 3.70 | 160 |
Note: UHR: ultra-high risk for psychosis; NC: normal controls. The significance level of a minimum of 139 contiguous voxels with the threshold of uncorrected P < .005.
Fig. 3.
Scatter plots showing distribution of [18F]-FFMZ BPND values in the right caudate identified to have significant between-group difference from the whole-brain SPM analyses by group of individuals at ultra-high risk (UHR) for psychosis and normal controls (NC). The presented BPND values indicate those to be scaled by global BPND for individuals.
Automated ROI Analysis of Regional FFMZ BPND.
In ROI-based approaches, to assess between-group differences of FFMZ BPND, MANOVA was applied to the FFMZ BPND in the preselected ROIs as a dependent variable and groups of UHR and normal controls as fixed factors. The MANOVA showed a significant main effect for the group of UHR and normal controls on the ROIs (Pillai’s trace = 0.66, F(10, 15) = 2.912, P = .030). Post hoc ANOVA in each ROI showed a significant difference in the right caudate (P = .007) among the 10 regions. The significance in the right caudate survived the Bonferroni correction for 10 multiple comparisons at trend level of P = .07. The mean and SD for regional FFMZ BPND values for all the ROIs and the individual values were presented in table 2.
Table 2.
Individual Values and the Mean and SD for Regional [18F]-FFMZ BPND for Each ROI in 15 Normal Controls and 11 Individuals at UHR
| Subjects | Left Caudate | Right Caudate | Left Putamen | Right Putamen | Left Nucleus Accumbens | Right Nucleus Accumbens | Left Hippocampus | Right Hippocampus | Left Para-hippocampus | Right Para-hippocampus |
|---|---|---|---|---|---|---|---|---|---|---|
| Normal control | ||||||||||
| NC_1, 21/M | 0.74 | 0.69 | 0.82 | 0.86 | 1.12 | 1.15 | 1.04 | 1.07 | 1.20 | 1.19 |
| NC_2, 21/M | 0.80 | 0.76 | 0.83 | 0.81 | 1.30 | 1.24 | 1.12 | 1.08 | 1.18 | 1.27 |
| NC_3, 21/F | 0.71 | 0.73 | 0.84 | 0.83 | 1.11 | 1.08 | 1.04 | 0.95 | 1.19 | 1.26 |
| NC_4, 21/M | 0.79 | 0.77 | 0.90 | 0.90 | 1.29 | 1.21 | 1.17 | 1.10 | 1.25 | 1.28 |
| NC_5, 22/M | 0.80 | 0.77 | 0.91 | 0.96 | 1.32 | 1.23 | 1.11 | 1.13 | 1.31 | 1.34 |
| NC_6, 20/F | 0.73 | 0.70 | 0.83 | 0.85 | 1.20 | 1.12 | 1.09 | 1.02 | 1.20 | 1.22 |
| NC_7, 22/M | 0.75 | 0.77 | 0.82 | 0.85 | 1.29 | 1.17 | 1.11 | 1.11 | 1.21 | 1.26 |
| NC_8, 20/M | 0.78 | 0.77 | 0.90 | 0.93 | 1.29 | 1.20 | 1.12 | 1.10 | 1.24 | 1.29 |
| NC_9, 25/F | 0.74 | 0.72 | 0.85 | 0.88 | 1.20 | 1.13 | 1.07 | 1.03 | 1.24 | 1.27 |
| NC_10, 20/F | 0.72 | 0.73 | 0.89 | 0.86 | 1.09 | 1.02 | 1.02 | 0.97 | 1.22 | 1.21 |
| NC_11, 21/M | 0.72 | 0.72 | 0.84 | 0.82 | 1.16 | 1.18 | 1.08 | 1.08 | 1.22 | 1.28 |
| NC_12, 18/F | 0.74 | 0.68 | 0.80 | 0.75 | 1.11 | 1.12 | 1.13 | 1.08 | 1.30 | 1.34 |
| NC_13, 23/F | 0.75 | 0.74 | 0.86 | 0.80 | 1.13 | 1.04 | 1.00 | 0.99 | 1.06 | 1.18 |
| NC_14, 20/M | 0.81 | 0.78 | 0.98 | 0.93 | 1.22 | 1.28 | 1.18 | 1.12 | 1.28 | 1.35 |
| NC_15, 20/M | 0.72 | 0.67 | 0.81 | 0.81 | 1.17 | 1.02 | 1.04 | 0.98 | 1.17 | 1.17 |
| Mean | 0.75 | 0.73 | 0.86 | 0.86 | 1.20 | 1.15 | 1.09 | 1.05 | 1.22 | 1.26 |
| SD | 0.03 | 0.04 | 0.05 | 0.06 | 0.08 | 0.08 | 0.05 | 0.06 | 0.06 | 0.06 |
| Ultra-high risk for psychosis | ||||||||||
| UHR_1, 20/M | 0.73 | 0.72 | 0.85 | 0.86 | 1.17 | 0.99 | 1.13 | 1.11 | 1.14 | 1.16 |
| UHR_2, 21/F | 0.72 | 0.69 | 0.84 | 0.88 | 1.10 | 1.08 | 1.06 | 1.08 | 1.17 | 1.22 |
| UHR_3, 20/M | 0.72 | 0.71 | 0.82 | 0.85 | 1.14 | 1.06 | 1.10 | 1.03 | 1.13 | 1.18 |
| UHR_4, 20/F | 0.75 | 0.68 | 0.87 | 0.85 | 1.21 | 1.22 | 1.09 | 1.02 | 1.24 | 1.27 |
| UHR_5, 22/M | 0.71 | 0.68 | 0.84 | 0.80 | 1.09 | 1.08 | 1.09 | 1.05 | 1.15 | 1.23 |
| UHR_6, 17/M | 0.77 | 0.75 | 0.96 | 0.92 | 1.18 | 1.16 | 1.11 | 1.08 | 1.28 | 1.28 |
| UHR_7, 17/F | 0.74 | 0.69 | 0.77 | 0.76 | 1.09 | 1.05 | 1.11 | 1.06 | 1.22 | 1.26 |
| UHR_8, 21/M | 0.78 | 0.68 | 0.91 | 0.87 | 1.22 | 1.11 | 1.11 | 1.09 | 1.17 | 1.24 |
| UHR_9, 19/F | 0.77 | 0.69 | 0.90 | 0.87 | 1.18 | 1.05 | 1.07 | 1.09 | 1.21 | 1.28 |
| UHR_10, 17/F | 0.69 | 0.66 | 0.81 | 0.80 | 1.09 | 1.12 | 1.02 | 1.01 | 1.13 | 1.23 |
| UHR_11, 16/M | 0.70 | 0.72 | 0.89 | 0.89 | 1.21 | 1.18 | 1.12 | 1.08 | 1.23 | 1.31 |
| Mean | 0.73 | 0.70 | 0.86 | 0.85 | 1.15 | 1.10 | 1.09 | 1.06 | 1.19 | 1.26 |
| SD | 0.03 | 0.03 | 0.05 | 0.05 | 0.05 | 0.07 | 0.03 | 0.03 | 0.05 | 0.04 |
Note: The presented BPND values indicate those to be scaled by global BPND for individuals.
Correlations Between Regional FFMZ BPND and Findings of Clinical Symptoms and Neurocognitive Performance in Individuals at UHR (n = 10)
For the right caudate identified to have significant between-group difference from the whole-brain SPM analyses, the individual mean values of FFMZ BPND showed some trend of correlations with SIPS positive and negative prodromal symptoms, although their relationship did not reach the level of significance (r = −.64, P = .09 and r = −.67, P = .067, respectively). For neurocognitive function, FFMZ BPND in the caudate did not demonstrate any significant correlations with neurocognitive tasks (P > .1). In the additional correlation analyses between the 10 ROIs and clinical characteristics, no significant relationships were observed.
Discussion
The present [18F]-FFMZ PET study aimed to investigate whether GABA-A/BZ receptor binding abnormalities have already emerged in people at UHR, “putative” prodromal phase of schizophrenia in comparison with normal controls, and their relationships with psychopathology and neurocognitive functions. To our knowledge, this study provides the first in vivo PET evidence of GABAergic system alterations in individuals at UHR for psychosis. UHR subjects showed a significant reduction of GABA-A/BZ receptor BPND in the right caudate compared with normal controls. The BPND reductions in the right caudate were negatively correlated with positive and negative prodromal symptoms at trend levels. The present findings suggest that altered GABAergic transmission may play a crucial role in the pathophysiology of developing psychosis.
Reduced GABA-A BPND in the Right Caudate in UHR Subjects
In the present [18F]-FFMZ PET study, the GABA-A/BZ receptor BPND in UHR subjects was significantly reduced in the right caudate for the whole-brain analysis. Moreover, in the confirmatory ROI analysis, the difference was shown only in the right caudate among the 10 ROIs. Although the significance in the caudate was at trend level of P = .07 after applying the Bonferroni correction, the finding would confirm the meaningful GABA-A/BZ receptor BPND reduction of the caudate in UHR, considering the small sample size. Consistent with this finding, a recent MRS study showed reduced GABA concentrations in the left basal ganglia, proposing a role of striatal GABAergic function in schizophrenia14 (see discussion below). These results were also compatible with previous postmortem findings showing reduced GABA uptake sites in the basal ganglia of schizophrenic patients,12 while a few postmortem findings of schizophrenic brains revealed no change46 or upregulation of GABA receptors in the caudate.11 These inconsistencies may be affected by differential down- or upregulation according to diverse subunit compositions of GABA-A receptors. It may also be contributed to by chronic exposure to antipsychotic medications and compensatory change. Indeed, a CSF study of GABA levels in recently ill medication-free schizophrenic patients showed that decreased GABA levels in the CSF may be found only in the early phase of the illness and that the GABA levels increase with time and with long-term medication.47 Because the present findings shown in UHR individuals are relatively preserved from the above-mentioned secondary changes, our findings provide crucial evidence that altered GABAergic transmission in the caudate may already be present in the “putative” prodromal stage of psychosis.
Furthermore, the caudate region shown in the present study likely corresponds to the associative subdivision of the striatum, which has been implicated in the UHR sample for higher dopamine uptake in a recent [18F]-DOPA PET study.21,22 It also corresponds to the associate caudate that showed higher glutamate level in the UHR and drug-naive first episode psychosis groups in another group’s MRS study.23 Because the striatum is a convergence region that is highly innervated by glutamatergic afferents from various regions and by dopaminergic inputs from the midbrain and that projects to a variety of brain networks,16 the striatal GABA system may be a key modulator of inhibitory and excitatory systems of the human brain. Therefore, the altered GABAergic transmission in the caudate in our UHR individuals may be implicated in the pathogenesis of psychosis through imbalance of brain inhibitory and excitatory systems.
No Regional Between-Group Difference in the Hippocampus or Parahippocampal Gyrus
On the other hand, the present data did not show any significant between-group difference in the hippocampus or parahippocampal gyrus. This finding is consistent with previous in vivo PET imaging studies that did not show binding reductions in the medial temporal lobe in schizophrenia.17,38,48 Meanwhile, the previous postmortem findings of schizophrenic brain showed selective loss of hippocampal pyramidal neurons with high densities of BZ binding site46 or GABA-A receptor upregulation in the hippocampal formation.49 However, these postmortem reports may be affected by compensatory upregulation of GABA-A receptors, as discussed above. The present findings did not support the possible role of hippocampal GABAergic interneuron proposed by the McGuire group.21 According to their postulation, deficits of GABAergic interneuron in the hippocampus may affect the relevance of dorsal striatal dopamine interaction with hippocampal glutamate in the development of psychosis. The present findings suggest that the striatal hyperdopaminergia in psychosis and UHR might be influenced by the GABAergic system in the striatum itself rather than that in the hippocampus. Future investigations are needed to provide greater understanding of GABAergic function and neurochemical interactions in the medial temporal lobe in the process of psychosis.
Possible Explanations for the Reduced BPND and Altered GABA Transmission
One possible explanation is that reduced GABA-A/BZ receptor binding in the caudate may be derived from striatal GABAergic neuronal loss and subsequent volume reduction, considering previous reports of reduced caudate volume in antipsychotic-naive schizophrenic patients.50,51 This issue may also be related to partial volume effect. However, a recent large-sized (n = 182), multicenter (5 different scanning sites) structural MRI study showed that there was no caudate volume reduction in UHR subjects.52 Therefore, our finding of reduced BPND in right caudate may not simply reflect the volume loss in UHR subjects.
A more plausible explanation is that it reflects primary or secondary pathophysiology of GABA-A/BZ receptor. Primary alteration of GABA-A receptor expression may lead to the finding of reduced GABA-A/BZ receptor binding. Because the subunit isoform expression patterns change during brain development,53 abnormal changes of GABA-A receptors during development may lead to altered GABA system in schizophrenia. A genetic association study showed an involvement of a GABRG2 SNP of GABA-A γ2 subunit sensitive to BZ binding in schizophrenia vulnerability.54 Another genetic study using knockout mice showed that a hyperdopaminergic state from midbrain dopamine neurons leading to deficits in sensorimotor information processing55 may be affected by disruption of the α3 GABA-A receptors. Alternatively, reduced [18F]-FFMZ BPND may be a secondary phenomenon of downregulation of postsynaptic GABA-A/BZ receptor to the preceding presynaptic GABAergic deficits. A BZ ligand PET study in healthy subjects reported that the BZ binding sites of the GABA-A/BZ receptor were increased through conformational changes (the so-called “GABA-shift”) in the presence of increased GABA levels in the synaptic cleft.56 Accordingly, decreased GABA levels in the caudate, which were found in early phase of schizophrenia patients in a previous MRS study,14 may lead to reduced affinity of the GABA-A/BZ receptor BZ site and subsequently reduced [18F]-FFMZ binding. The [18F]-FFMZ binding reduction in UHR subjects may be a plausible consequence of early insult to the striatal presynaptic GABAergic system.17 In any case, the decreased GABA-A/BZ receptor BPND in the striatum may reflect altered GABAergic transmission and/or inhibitory and excitatory system imbalance in UHR.
How Does Reduced GABA-A/BZ Receptor Binding Lead to the Subthreshold Psychotic Symptoms?
The present correlation analyses showed that the positive and negative symptoms in UHR individuals showed a negative trend with levels of GABA-A/BZ receptor BPND in the caudate. This finding indicates the possibility that altered GABAergic transmission in the caudate before the onset of frank psychosis might contribute to development of psychotic symptoms. Considering the interactions between GABA and dopamine systems in the striatum, although striatal dopamine transmission was not examined in the present study, an imbalance between inhibitory and excitatory systems through altered GABA-A receptors in the striatum may affect dysregulations of dopaminergic neuron firing and dopamine release, which may lead to acquisition of an aberrant sense of novelty and psychotic symptoms.57
Limitations
These findings should be interpreted with caution, given the limitations of the present study. First, as reported by almost all previous prodromal studies, a portion of UHR subjects represent false positives and do not progress to overt psychotic disorder. Such cases could contaminate the GABA-A/BZ receptor binding reduction of “true” prodromal cases. In the present study, the transition rate was 18.9% (2/11) during 12 months of follow-up. This conversion rate is consistent with those of previous reports showing a probability of 16%–35% within 2 years.19,20 Second, the sample size of the present study may be too small for generalization of our finding to UHR and schizophrenia individuals. Further investigation with a larger sample of UHR and schizophrenia subjects is needed to validate the present findings. Third, our UHR sample included 2 individuals taking antipsychotic agents. Considering the convergence and interactions of dopamine and GABA, antipsychotics may affect GABA-A/BZ receptor binding patterns. However, the regional differences in GABA-A/BZ receptor binding in the right caudate remained noticeable, even in the between-group analysis for 9 UHR individuals (significance level of a minimum of 139 contiguous voxels with the threshold of uncorrected P < .005). Fourth, it cannot be excluded that the reduced BPND may be related to altered endogenous ligand concentration, rather than changes to the GABA-A/BZ receptor itself or striatal volume loss. Finally, possible interactions between the GABA system and other neurotransmitters such as dopamine and glutamate were not examined. Further study combining neuroimaging techniques with other biological markers is needed to demonstrate these interactions in the pathophysiology of psychosis.
Conclusion
In summary, people at UHR demonstrated significantly reduced BPND of GABA-A/BZ receptors in the right caudate. The localized binding reduction in the right caudate was related to increased prodromal-like symptoms at the trend level. These findings suggest that altered GABAergic transmission and/or the imbalance of inhibitory and excitatory systems in the striatum, as reflected by reduced GABA-A/BZ receptor binding, may have already emerged at the UHR, “putative” prodromal stage and seem to play a pivotal role in the pathophysiology of frank psychosis development. This study is clinically relevant in that functional alterations to the regions identified in the UHR group may provide the opportunity for early detection during the prodromal phase of schizophrenia and targeted management including GABA substitution therapy (eg, MK077, a GABA-A receptor modulator), which might modify the early course of schizophrenia.
Funding
Korea Healthcare Technology R&D Project; Ministry for Health, Welfare & Family Affairs, Republic of Korea (A090096).
Acknowledgment
We thank Mr Maeng-Keun Oh for his assistance with PET data analysis. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35:549–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lyon GJ, Abi-Dargham A, Moore H, Lieberman JA, Javitch JA, Sulzer D. Presynaptic regulation of dopamine transmission in schizophrenia. Schizophr Bull. 2011;37:108–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benes FM. Neural circuitry models of schizophrenia: is it dopamine, GABA, glutamate, or something else? Biol Psychiatry. 2009;65:1003–1005 [DOI] [PubMed] [Google Scholar]
- 4. Rolls ET, Loh M, Deco G, Winterer G. Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nat Rev Neurosci. 2008;9:696–709 [DOI] [PubMed] [Google Scholar]
- 5. Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007 [DOI] [PubMed] [Google Scholar]
- 7. Volk D, Austin M, Pierri J, Sampson A, Lewis D. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons. Am J Psychiatry. 2001; 158: 256–265 [DOI] [PubMed] [Google Scholar]
- 8. Wassef A, Baker J, Kochan LD. GABA and schizophrenia: a review of basic science and clinical studies. J Clin Psychopharmacol. 2003;23:601–640 [DOI] [PubMed] [Google Scholar]
- 9. Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324 [DOI] [PubMed] [Google Scholar]
- 10. Beasley CL, Zhang ZJ, Patten I, Reynolds GP. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry. 2002; 52: 708–715 [DOI] [PubMed] [Google Scholar]
- 11. Hanada S, Mita T, Nishino N, Tanaka C. [3H]muscimol binding sites increased in autopsied brains of chronic schizophrenics. Life Sci. 1987; 40: 259–266 [DOI] [PubMed] [Google Scholar]
- 12. Simpson MD, Slater P, Royston MC, Deakin JF. Regionally selective deficits in uptake sites for glutamate and gamma-aminobutyric acid in the basal ganglia in schizophrenia. Psychiatry Res. 1992; 42: 273–282 [DOI] [PubMed] [Google Scholar]
- 13. Veldic M, Kadriu B, Maloku E, et al. Epigenetic mechanisms expressed in basal ganglia GABAergic neurons differentiate schizophrenia from bipolar disorder. Schizophr Res. 2007;91:51–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goto N, Yoshimura R, Moriya J, et al. Reduction of brain gamma-aminobutyric acid (GABA) concentrations in early-stage schizophrenia patients: 3T Proton MRS study. Schizophr Res. 2009;112:192–193 [DOI] [PubMed] [Google Scholar]
- 15. Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kreitzer AC. Physiology and pharmacology of striatal neurons. Annu Rev Neurosci. 2009;32:127–147 [DOI] [PubMed] [Google Scholar]
- 17. Busatto GF, Pilowsky LS, Costa DC, et al. Correlation between reduced in vivo benzodiazepine receptor binding and severity of psychotic symptoms in schizophrenia. Am J Psychiatry. 1997;154:56–63 [DOI] [PubMed] [Google Scholar]
- 18. Asai Y, Takano A, Ito H, et al. GABAA/benzodiazepine receptor binding in patients with schizophrenia using [11C]Ro15-4513, a radioligand with relatively high affinity for alpha5 subunit. Schizophr Res. 2008;99:333–340 [DOI] [PubMed] [Google Scholar]
- 19. Yung AR, Nelson B, Stanford C, et al. Validation of “prodromal” criteria to detect individuals at ultra high risk of psychosis: 2 year follow-up. Schizophr Res. 2008;105:10–17 [DOI] [PubMed] [Google Scholar]
- 20. Woods SW, Addington J, Cadenhead KS, et al. Validity of the prodromal risk syndrome for first psychosis: findings from the North American Prodrome Longitudinal Study. Schizophr Bull. 2009;35:894–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stone JM, Howes OD, Egerton A, et al. Altered relationship between hippocampal glutamate levels and striatal dopamine function in subjects at ultra high risk of psychosis. Biol Psychiatry. 2010; 68: 599–602 [DOI] [PubMed] [Google Scholar]
- 22. Howes OD, Montgomery AJ, Asselin MC, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20 [DOI] [PubMed] [Google Scholar]
- 23. de la Fuente-Sandoval C, Leon-Ortiz P, Favila R, Stephano S, Mamo D, Ramirez-Bermudez J, et al. Higher levels of glutamate in the associative-striatum of subjects with prodromal symptoms of schizophrenia and patients with first-episode psychosis. Neuropsychopharmacology. 2011; 36:1781–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salmi E, Aalto S, Hirvonen J, et al. Measurement of GABA(A) receptor binding in vivo with [C-11]flumazenil: a test-retest study in healthy subjects. Neuroimage. 2008; 41: 260–269 [DOI] [PubMed] [Google Scholar]
- 25. Mitterhauser M, Wadsak W, Wabnegger L, et al. Biological evaluation of 2′-[18F]fluoroflumazenil ([18F]FFMZ), a potential GABA receptor ligand for PET. Nucl Med Biol. 2004; 31: 291–295 [DOI] [PubMed] [Google Scholar]
- 26. Yoon YH, Jeong JM, Kim HW, et al. Novel one-pot one-step synthesis of 2′-[(18)F]fluoroflumazenil (FFMZ) for benzodiazepine receptor imaging. Nucl Med Biol. 2003; 30: 521–527 [DOI] [PubMed] [Google Scholar]
- 27. Jung MH, Jang JH, Kang DH, et al. The reliability and validity of the korean version of the structured interview for prodromal syndrome. Psychiatry Investig. 2010; 7: 257–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. First RLS, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders: Clinical Version (SCID-CV). Washington, DC: American Psychiatric Press; 1997 [Google Scholar]
- 29. McGlashan T, Miller T, Woods S, Rosen J, Hoffman R, Davidson L, eds. Structured Interview for Prodromal Syndromes (SIPS). Version 4.0. New Heaven: Yale University; 2003 [Google Scholar]
- 30. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389 [DOI] [PubMed] [Google Scholar]
- 31. Delis DC, Kramer JH, Kaplan E, Ober BA. The California Verbal Learning Test. San Antonio,TX: Psychological Corporation; 1987 [Google Scholar]
- 32. Nuechterlein KH, Edell WS, Norris M, Dawson ME. Attentional vulnerability indicators, thought disorder, and negative symptoms. Schizophr Bull. 1986;12:408–426 [DOI] [PubMed] [Google Scholar]
- 33. Golden CJ. Stroop Color and Word Test: Manual for Clinical and Experimental Uses. Chicago: IL: Stoelting; 1978 [Google Scholar]
- 34. Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test Manual: Revised and Expanded. Odessa,FL: Psychological Assessment Resources Inc; 1993 [Google Scholar]
- 35. Spreen O, Benton AL. Neurosensory Center Comprehensive Examination for Aphasia. Victoria, BC: University of Victoria Neuropsychology Laboratory; 1977 [Google Scholar]
- 36. Ruff RM. The ruff figural fluency test: a normative study with adults. Dev Neuropsychol. 1987(3):37–52 [Google Scholar]
- 37. Hawkins KA, Addington J, Keefe RS, et al. Neuropsychological status of subjects at high risk for a first episode of psychosis. Schizophr Res. 2004;67:115–122 [DOI] [PubMed] [Google Scholar]
- 38. Ball S, Busatto GF, David AS, et al. Cognitive functioning and GABAA/benzodiazepine receptor binding in schizophrenia: a 123I-iomazenil SPET study. Biol Psychiatry. 1998; 43: 107–117 [DOI] [PubMed] [Google Scholar]
- 39. Lee JD, Park HJ, Park ES, et al. Assessment of regional GABA(A) receptor binding using 18F-fluoroflumazenil positron emission tomography in spastic type cerebral palsy. Neuroimage. 2007; 34: 19–25 [DOI] [PubMed] [Google Scholar]
- 40. Ichise M, Ballinger JR, Golan H, et al. Noninvasive quantification of dopamine D2 receptors with iodine-123-IBF SPECT. J Nucl Med. 1996;37:513–520 [PubMed] [Google Scholar]
- 41. Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539 [DOI] [PubMed] [Google Scholar]
- 42. Klumpers UM, Veltman DJ, Boellaard R, et al. Comparison of plasma input and reference tissue models for analysing [(11)C]flumazenil studies. J Cereb Blood Flow Metab. 2008;28:579–587 [DOI] [PubMed] [Google Scholar]
- 43. Millet P, Graf C, Buck A, Walder B, Ibanez V. Evaluation of the reference tissue models for PET and SPECT benzodiazepine binding parameters. Neuroimage. 2002; 17: 928–942 [PubMed] [Google Scholar]
- 44. Ward D. 2000; Simultaneous Inference for FMRI Data. AFNI AlphaSim documentation. Biophysics Research Institute, Medical College of Wisconsin; 2000. [Google Scholar]
- 45. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289 [DOI] [PubMed] [Google Scholar]
- 46. Squires RF, Lajtha A, Saederup E, Palkovits M. Reduced [3H]flunitrazepam binding in cingulate cortex and hippocampus of postmortem schizophrenic brains: is selective loss of glutamatergic neurons associated with major psychoses? Neurochem Res. 1993; 18: 219–223 [DOI] [PubMed] [Google Scholar]
- 47. van Kammen DP, Sternberg DE, Hare TA, Waters RN, Bunney WE., Jr CSF levels of gamma-aminobutyric acid in schizophrenia. Low values in recently ill patients. Arch Gen Psychiatry. 1982;39:91–97 [DOI] [PubMed] [Google Scholar]
- 48. Abi-Dargham A, Laruelle M, Krystal J, et al. No evidence of altered in vivo benzodiazepine receptor binding in schizophrenia. Neuropsychopharmacology. 1999;20:650–661 [DOI] [PubMed] [Google Scholar]
- 49. Benes FM, Khan Y, Vincent SL, Wickramasinghe R. Differences in the subregional and cellular distribution of GABAA receptor binding in the hippocampal formation of schizophrenic brain. Synapse. 1996; 22: 338–349 [DOI] [PubMed] [Google Scholar]
- 50. Corson PW, Nopoulos P, Andreasen NC, Heckel D, Arndt S. Caudate size in first-episode neuroleptic-naive schizophrenic patients measured using an artificial neural network. Biol Psychiatry. 1999;46:712–720 [DOI] [PubMed] [Google Scholar]
- 51. Keshavan MS, Rosenberg D, Sweeney JA, Pettegrew JW. Decreased caudate volume in neuroleptic-naive psychotic patients. Am J Psychiatry. 1998; 155: 774–778 [DOI] [PubMed] [Google Scholar]
- 52. Mechelli A, Riecher-Rossler A, Meisenzahl EM, et al. Neuroanatomical abnormalities that predate the onset of psychosis: a multicenter study. Arch Gen Psychiatry. 2011;68:489–495 [DOI] [PubMed] [Google Scholar]
- 53. Lujan R, Shigemoto R, Lopez-Bendito G. Glutamate and GABA receptor signalling in the developing brain. Neuroscience. 2005; 130: 567–580 [DOI] [PubMed] [Google Scholar]
- 54. Zai CC, Tiwari AK, King N, et al. Association study of the gamma-aminobutyric acid type a receptor gamma2 subunit gene with schizophrenia. Schizophr Res. 2009;114:33–38 [DOI] [PubMed] [Google Scholar]
- 55. Yee BK, Keist R, von Boehmer L, et al. A schizophrenia-related sensorimotor deficit links alpha 3-containing GABAA receptors to a dopamine hyperfunction. Proc Natl Acad Sci USA. 2005;102:17154–17159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Frankle WG, Cho RY, Narendran R, et al. Tiagabine increases [11C]flumazenil binding in cortical brain regions in healthy control subjects. Neuropsychopharmacology. 2009;34: 624–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kapur S, Mizrahi R, Li M. From dopamine to salience to psychosis—linking biology, pharmacology and phenomenology of psychosis. Schizophr Res. 2005;79:59–68 [DOI] [PubMed] [Google Scholar]