Abstract
Cognitive remediation improves cognition in patients with schizophrenia, but its effect on other relevant factors such as negative symptoms and functional outcome has not been extensively studied. In this hospital-based study, 84 inpatients with chronic schizophrenia were recruited from Alava Hospital (Spain). All of the subjects underwent a baseline and a 3-month assessment that examined neurocognition, clinical symptoms, insight, and functional outcome according to the Global Assessment of Functioning (GAF) scale and Disability Assessment Schedule from World Health Organization (DAS-WHO). In addition to receiving standard treatment, patients were randomly assigned either to receive neuropsychological rehabilitation (REHACOP) or to a control group. REHACOP is an integrative program that taps all basic cognitive functions. The program included experts’ latest suggestions about positive feedback and activities of daily living in the patients’ environment. The REHACOP group showed significantly greater improvements at 3 months in the areas of neurocognition, negative symptoms, disorganization, and emotional distress compared with the control group (Cohen’s effect size for these changes ranged from d = 0.47 for emotional distress to d = 0.58 for disorganization symptoms). The REHACOP group also improved significantly in both the GAF (d = 0.61) and DAS-WHO total scores (d = 0.57). Specifically, the patients showed significant improvement in vocational outcomes (d = 0.47), family contact (d = 0.50), and social competence (d = 0.56). In conclusion, neuropsychological rehabilitation may be useful for the reduction of negative symptoms and functional disability in schizophrenia. These findings support the integration of neuropsychological rehabilitation into standard treatment programs for patients with schizophrenia.
Key words: schizophrenia, negative symptoms, functional outcome, cognitive rehabilitation, REHACOP
Introduction
Negative symptoms and cognitive impairment are 2 of the most common dimensions in schizophrenia. More than 50% of patients with schizophrenia suffer from negative and/or cognitive symptoms1 in the prodromal phase,2 during psychosis3 and even after the remission of positive symptoms.4 In addition, negative and cognitive symptoms are strong predictors of the transition to psychosis in ultrahigh-risk samples5 and of poorer prognosis and functional outcome.6 More specifically, evidence suggests that the early presence of negative symptoms is associated with a worse course, more psychotic episodes, and greater impairments in adaptive life skills.7
The NIMH-MATRICS consensus statement on negative symptoms6 suggested that persistent and clinically significant negative symptoms are a distinct and important therapeutic target. Unfortunately, although they are recognized as an important factor in schizophrenia, negative symptoms have received less attention in the literature than positive symptoms.1 Moreover, first- and second-generation antipsychotic drugs have demonstrated limited efficacy on the improvement of negative symptoms8 although promising new glutamatergic agents are being explored to target them.9 Therefore, new treatment efforts should be focused on negative symptoms.6
A recent meta-analysis performed by Wykes et al10 reported data on the effectiveness of cognitive remediation on clinical symptoms in general but not on negative symptoms in particular. A closer inspection of the studies included in this meta-analysis and of studies published after the meta-analysis reveals inconsistent results. Despite using different modalities of cognitive remediation, some researchers found positive significant effects on negative symptoms.11–13 However, other studies have not identified evidence supporting the efficacy of cognitive remediation on negative symptoms.14–20 Most of the programs used in these clinical trials were not specifically designed to target improvements in negative symptoms but were focused on the amelioration of cognitive deficits and daily living.
However, most authors agree that the ultimate goal of any treatment is to improve the functional capacity and quality of life of patients.10 According to the meta-analysis described above,10 cognitive remediation is effective for improving functioning although the effect sizes obtained were small to medium. As with negative symptoms, functioning was included as a single construct in this meta-analysis. Therefore, we cannot conclude whether cognitive remediation is equally effective for different functional outcome domains, such as social functioning, vocational outcome, or self-care management. A closer review of the literature indicates that the most frequently studied functional domains were vocational and occupational outcome. The data reveal generally positive improvements regarding occupational outcome.12,17,19,21,22
Social functioning has also been analyzed, but studies have produced more inconsistent results. Some studies report that cognitive remediation improved social functioning,12,13,22,23 whereas other studies report conflicting results.14,15 Less attention has been paid to other outcome domains, such as self-care management or family contact. To the best of our knowledge, only 1 study has directly addressed self-care management, and that study revealed nonsignificant improvements18
According to the most recent meta-analyses,10,13,24 the following factors predict treatment benefits: the number of hours of training, drill and practice plus strategy coaching; a combination of cognitive remediation with adjunctive psychiatric rehabilitation; and transfer techniques. A recent study22 partially supported these findings in schizophrenia outpatients. In this study, patients who received cognitive rehabilitation with adjunctive treatment (ie, functional adaptation skills training) exhibited improved functional outcome compared with those who did not receive combined treatment. The Spanish REHACOP is a new generation multidimensional remediation program that includes the above-mentioned treatment approaches with a specific emphasis on the implementation of learned skills in activities of daily living in their real environment.
Factors that have not been related to treatment benefits include age, clinical symptoms, chlorpromazine equivalence, computer use, and inpatient/outpatient settings. However, there are a few hospital-based cognitive remediation studies with chronic schizophrenia inpatients in the literature.17,22,25–28 Of these, only 217,18,25,26,29,30 simultaneously considered the impact of cognitive remediation on the clinical symptoms and specific domains of functional outcome, including work functioning17,25,29,30 and social competence.30
Therefore, in this study, we aimed to evaluate the efficacy of cognitive remediation with REHACOP among inpatients with chronic schizophrenia. The primary targets of this study were negative symptoms and functional outcome, including specific domains such as self-care management, vocational outcome, family contact and social competence.
Methods
Participants
Eighty-four inpatients with schizophrenia were recruited from the Alava Psychiatric Hospital. To be included in the study, patients had to meet the diagnostic criteria for schizophrenia according to the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR).31 Exclusion criteria included the following:
evidence of alcohol or drug abuse in the last 30 days;
previous history of a significant lack of consciousness;
mental retardation; and
relevant neurological or medical conditions.
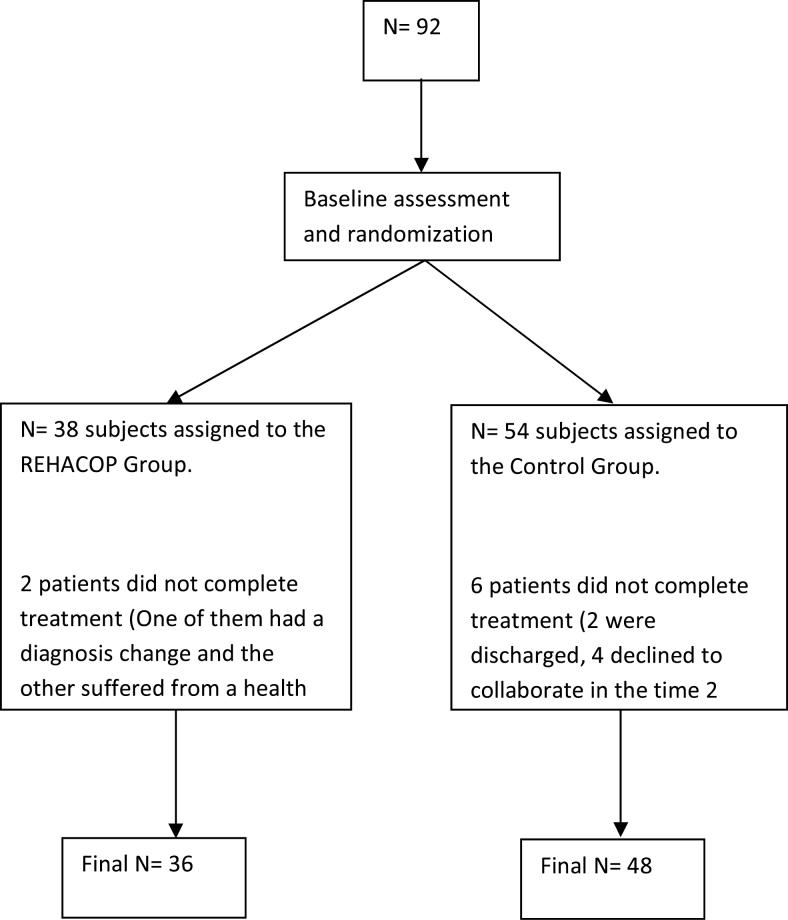
Ten days after admission, clinical, cognitive, and functional evaluations were performed. Then patients were randomly assigned to either the REHACOP group or the control group (see figure 1). Assignment to the program was performed using a computer-generated randomization list. Both groups received standard treatment, which included individual case management and medical reviews. All patients participated voluntarily and provided written informed consent to participate in the study. The study protocol was approved by the Ethics Committee at the Health Department of the Basque Mental Health System in Spain.
Fig. 1.
Flow card of recruitment procedure and study profile of participants.
Measures
Clinical symptoms, neurocognitive performance, and functional outcome were assessed before and after treatment. Baseline assessments were performed at the hospital when patients were stable with respect to psychopathology and medication after intake. Post-treatment assessment was performed within the first week after the intervention was completed. All raters were blind to the treatment condition and had no other role in the project that would undermine the blinding.
Clinical Assessment
After admission to the hospital, patients completed a psychiatric interview and evaluation with the Spanish version32 of the Positive and Negative Syndrome Scale (PANSS),33 which was scored using a 5-factor model. The 5 components were as follows: positive, negative, disorganization, excitement, and emotional distress (see Van der Gaag et al 200634 for details). The Premorbid Adjustment Scale was administered to obtain a measure of clinical premorbid adjustment. Insight was evaluated with the Schedule for the Assessment of Insight.35 After the training period, interrater reliability coefficients were obtained for the clinical scales (ICC ranged from 0.83 for clinical global impression [CGI] scale to 0.91 for PANSS).
Cognitive Evaluation
The evaluation of cognitive functioning included tests to assess processing speed, working memory, verbal learning and memory, verbal fluency, and executive functioning. All cognitive measures were converted into Z-scores, and the sign of some measures was adjusted so that higher scores indicated better performance. All composite cognitive domains obtained an acceptable internal consistency. Processing speed (Cronbach’s α = .76) was obtained from the Stroop-color test,36 Trail Making Test-A,37 and Symbol Digit (Wechsler General Intelligence Scale [WAIS-III]).38 For learning and verbal memory, authors included learning and long-term recall from the Hopkins Verbal Learning Test39 (Cronbach’s α = .86). Working memory (Cronbach’s α = .73) was assessed using Digit Forward and Digit Backwards from WAIS-III.38 Executive functioning (Cronbach’s α = .75) was measured with the Stroop test36 (word-color and interference scores), and verbal fluency (Cronbach’s α = .78) was measured with the Semantic and Phonological Fluency Subtests from the Barcelona Test.40 The Accentuation Reading Test, Test de Acentuación de Palabras (TAP),41 which is the Spanish version of the National Adult Reading Test, was also administered to obtain an estimation of each patient’s premorbid abilities.
Functional Outcome
Functional disability was assessed with the Global Assessment of Functioning (GAF) scale,42 the CGI scale,43 and the Disability Assessment Schedule scale from World Health Organization (DAS-WHO).44 The 4 functional disability characteristic indicators offered by the DAS-WHO were analyzed. These indicators are self-care management, social competence, vocational outcomes, and family contact.
Intervention
Developed by Ojeda and Peña in 2007,45 the REHACOP program is the first Spanish cognitive remediation program specifically designed for patients with psychosis and schizophrenia. REHACOP is a structured program based on paper-pencil tasks and uses the principles of restoration, compensation, and optimization. Training procedures gradually increase the level of cognitive effort and demand. REHACOP trains patients in traditionally impaired cognitive domains such as attention, memory, processing speed, language, and executive functioning. Additionally, the program includes 3 units related to functional outcome treatment: social skills training, activities of daily living, and psychoeducation. Patients’ relatives also take part in psychoeducation groups that provide family members with a better understanding of the illness, ways to cope with symptoms, the ability to identify early signs of relapse, and information about available clinical and social resources.
The REHACOP includes tasks hierarchically organized into 3 levels of difficulty and subtypes of abilities. Once a basic cognitive strategy has been trained and well acquired, the therapist moves on to the next level. Feedback is provided in each session after the tasks are completed. Each task includes fixed instructions for the therapist to be read to the patients, verbal and visual materials, and the patient’s response sheet for the answers. The program also provides the therapist with a solution sheet for tasks with nonopen responses to facilitate quick correction and interpretation. In addition, patients are required to put the obtained benefits into practice with homework activities. The format of the program allows for both individual and group administration (5–8 patients per group).
In this study, the REHACOP group attended 90-min sessions at least 3 days per week. Over 3 months, patients are supposed to integrate the learning experience, practice in a real-life context, and bring to the sessions feedback about possible new difficulties that must be faced. In this study, REHACOP groups were led by a trained neuropsychologist although the program’s highly structured design, instructions, and materials could allow its administration by other trained professionals.
The control group received standard treatment and participated in group activities including drawing, reading the daily news, and constructing objects using different materials (such as paper or wood). These activities were accomplished in a group format and with the same frequency as the implementation of REHACOP.
Data Analysis
The normality of the data was tested with the Kolmogorov-Smirnov Test. All variables resembled the normal distribution, with the exception of CGI, which was log-transformed for further analysis. Raw scores are presented for all variables. The χ2 test was used to analyze any differences in gender between the 2 groups, and ANOVA tests were used to analyze differences in sociodemographic or clinical variables at baseline.
Repeated measures of multiple analysis of variance (MANOVA) was performed for clinical, cognitive, and functional variables with group (REHACOP vs control group) as the between-subject factor and time (pretreatment and posttreatment) as the within-group factor. The main effects of time (longitudinal dimension), group (cross-sectional dimension), and time × group (interaction effect) were examined. Regression analyses were performed to analyze the predictors of functional changes. The significance level was set at 0.05. All tests were 2-tailed.
Results
Sociodemographic Characteristics of the Groups
The sociodemographic characteristics of the REHACOP group and the control group are shown in table 1. The general sample was a group of patients with chronic schizophrenia with severe clinical symptomatology and a high number of previous hospitalizations. The differences between the groups were analyzed to confirm the success of the randomization. There were no significant differences between the groups in any of the sociodemographic characteristics studied. Both groups were equivalent in terms of age, gender distribution, marital status, occupation, education, premorbid adjustment, and premorbid intelligence quotient. There were no significant differences in other clinical characteristics, such as age at onset, number of previous hospitalizations, schizophrenia diagnosis or alcohol, and tobacco consumption. The dose of antipsychotic medication (mg/day of chlorpromazine equivalents) was also equivalent at both baseline (F = 0.54, P = .61) and follow-up (F = 3.83, P = .06). Moreover, baseline medication and symptom severity did not correlate with the degree to which patients improved with REHACOP (Pearson’s r ranged from −.01 to .09, P values from .91 to .39).
Table 1.
Sociodemographic and Diagnostic Differences of Participants by Treatment Group (REHACOP; Control Group)
| REHACOP | Control Group | Group Differences | P | |
|---|---|---|---|---|
| Age (y) | 33.60 (9.4) | 36.92 (10.5) | F = 2.50 | .118 |
| Years of education (y) | 9.23 (2.7) | 10.24 (2.8) | F = 2.17 | .146 |
| Gender: n (%) | ||||
| Males | 27 (75.0) | 37 (77.1) | χ2 = 0.05 | .824 |
| Females | 9 (25.0) | 11 (22.9) | ||
| Age at onset | 22.28 (6.1) | 22.04 (6.1) | F = 0.03 | .863 |
| Number of previous hospitalizations | 7.11 (6.6) | 8.98 (10.3) | F = 0.89 | .349 |
| Accentuation reading test (TAP) | 18.50 (4.5) | 19.56 (6.3) | F = 0.67 | .414 |
| Premorbid adjustment (Cannon-Spor) | 49.64 (25.5) | 46.75 (22.2) | F = 0.30 | .582 |
| Dose of antipsychotic medication (converted to mg/day chlorpromazine) | 678.64 (349.2) | 747.97 (463.8) | F = 0.54 | .461 |
| DSM-IV-TR: n (%) | ||||
| Paranoid | 29 (80.6) | 40 (77.9) | χ2 = 0.73 | .866 |
| Disorganized | 4 (11.1) | 3 (6.3) | ||
| Residual | 2 (5.6) | 3 (6.3) | ||
| Nonspecified | 1 (2.8) | 2 (4.2) | ||
| Occupation: n (%) | ||||
| Employed | 3 (8.3) | 2 (4.2) | χ2 = 2.35 | .308 |
| Unemployed | 9 (25.0) | 7 (14.6) | ||
| Disabled | 24 (66.7) | 39 (81.3) | ||
| Marital status: n (%) | ||||
| Single | 33 (91.7) | 45 (93.8) | χ2 = 0.13 | .935 |
| Married | 1 (2.1) | 1 (2.8) | ||
| Separated or divorced | 2 (5.6) | 2 (4.2) | ||
| Alcohol consumption (g/day) | 0.92 (1.5) | 0.96 (1.5) | F = 0.06 | .809 |
| Tobacco smoking (cigarettes/day) | 20.56 (11.2) | 18.04 (12.4) | F = 0.90 | .345 |
Note: DSM-IV-TR, The Diagnostic and Statistical Manual of Mental Disorders 4th edition, Text Revised; TAP, Test de Acentuación de Palabras.
Patients received REHACOP treatment for a mean of 48.7 h (SD = 3.3; range 40.5–54). The length of REHACOP treatment did not correlate with improvements in cognitive, clinical, or functional measures (Pearson’s r values ranged from −.02 to .22, and P values ranged from .91 to .33).
Cognitive Changes in the REHACOP and Control Groups After the Intervention
The pattern of cognitive severity at baseline was similar for both groups (see table 2). However, the REHACOP group improved significantly compared with the control group, according to the significant group × time interactions for processing speed, working memory, verbal learning and memory, verbal fluency, and executive functioning. The effect size of these changes fell mostly in the medium range, with the exception of verbal learning and memory and working memory, which exhibited a large effect (d = 0.88).
Table 2.
Changes in Cognitive Functioning From Baseline to Posttreatment by Treatment Group (REHACOP; Control Group)
| Mean (SD) | Main Effect Group | Main Effect Time | Group × Time Interaction | Effect Size | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| REHACOP | Control Group | F | P | F | P | F | P | d | ||
| Processing speed | Pre | 0.08 (0.1) | 0.26 (0.1) | 0.01 | .979 | 3.55 | .064 | 5.91 | .018 | 0.63 |
| Post | 0.12 (0.1) | −0.05 (0.1) | ||||||||
| Verbal memory | Pre | −0.21 (0.3) | 0.16 (0.2) | 0.02 | .886 | 0.33 | .589 | 5.35 | .029 | 0.88 |
| Post | 0.32 (0.3) | −0.15 (0.2) | ||||||||
| Verbal fluency | Pre | 0.01 (0.1) | 0.07 (0.1) | 0.37 | .546 | 0.82 | .369 | 4.75 | .033 | 0.51 |
| Post | 0.11 (0.1) | −0.17 (0.1) | ||||||||
| Working memory | Pre | −0.08 (0.1) | 0.30 (0.1) | 0.03 | .857 | 1.21 | .274 | 14.21 | <.001 | 0.88 |
| Post | 0.16 (0.2) | −0.14 (0.1) | ||||||||
| Executive functioning | Pre | −0.07 (0.2) | 0.25 (0.2) | 0.45 | .505 | 0.15 | .703 | 9.88 | .003 | 0.51 |
| Post | 0.31 (0.2) | −0.23 (0.2) | ||||||||
Clinical Changes in the REHACOP and Control Groups After the Intervention
As observed in table 3, both groups exhibited a similar pattern of clinical symptom severity at baseline and a general improvement in clinical measures. However, the REHACOP group improved significantly more than the control group according to the significant group × time interactions for negative symptoms, disorganization symptoms, emotional distress, and the PANSS total score. The effect size of these changes fell mostly in the medium range (negative symptoms d = 0.48, disorganization d = 0.58, emotional distress d = 0.47, and PANSS total score d = 0.50). However, group × time interactions for CGI scores were not significant. Finally, although it was not significant, there was a trend toward significance in insight.
Table 3.
Changes in Clinical Symptoms From Baseline to Posttreatment by Treatment Group (REHACOP; Control Group)
| Mean (SD) | Main Effect Group | Main Effect Time | Group × Time Interaction | Effect Size | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| REHACOP | Control Group | F | P | F | P | F | P | d | ||
| Positive symptoms | Pre | 18.47 (7.4) | 16.47 (7.4) | 0.54 | .464 | 46.15 | <.001 | 2.10 | .151 | 0.32 |
| Post | 12.72 (5.1) | 12.75 (7.0) | ||||||||
| Negative symptoms | Pre | 27.23 (11.6) | 24.85 (9.7) | 0.10 | .749 | 23.54 | <.001 | 4.89 | .030 | 0.48 |
| Post | 21.91 (9.4) | 22.84 (10.1) | ||||||||
| Disorganization | Pre | 17.03 (7.2) | 14.13 (5.4) | 1.58 | .212 | 32.27 | <.001 | 7.32 | .008 | 0.58 |
| Post | 12.91 (5.6) | 12.67 (6.1) | ||||||||
| Excitement | Pre | 12.61 (5.2) | 9.56 (5.0) | 7.45 | .008 | 27.72 | <.001 | 1.64 | .204 | 0.27 |
| Post | 9.36 (4.2) | 7.58 (3.7) | ||||||||
| Emotional distress | Pre | 10.97 (6.2) | 7.95 (4.7) | 5.32 | .024 | 38.01 | <.001 | 4.42 | .039 | 0.47 |
| Post | 7.66 (3.9) | 6.33 (3.5) | ||||||||
| Insight | Pre | 6.09 (3.7) | 6.31 (4.3) | 0.15 | .694 | 41.81 | <.001 | 2.98 | .088 | 0.37 |
| Post | 8.64 (3.1) | 7.79 (3.7) | ||||||||
| PANSS total | Pre | 99.39 (34.8) | 84.56 (25.1) | 2.77 | .100 | 48.13 | <.001 | 4.71 | .033 | 0.50 |
| Post | 74.83 (23.5) | 71.70 (25.6) | ||||||||
Note: PANSS, Positive and Negative Syndrome Scale.
As previously mentioned, we reported data for negative symptoms according to the meta-analysis of Van der Gaag et al.34 However, to make our results easier to compare with that of other studies that used the conventional PANSS scores (positive, negative, and general psychopathology) and to make possible future meta-analyses, we decided to provide results according to the negative symptom scale (items N1 to N7). The REHACOP group exhibited reduced negative symptoms from 26.08 (SD = 12.37) at baseline to 20.94 (SD = 10.56) after treatment, whereas the control group exhibited a reduction in negative symptoms from 23.74 (SD = 9.57) to 21.63 (SD = 10.24). The interaction effect was significant (F = 5.30, P < .05), with Cohen’s effect size falling in the medium range (d = 0.51).
Functional Outcome Changes in the REHACOP and Control Groups After the Intervention
Both groups exhibited a similar pattern of functional outcomes at baseline, with a general pattern of improvement over the treatment period (see table 4). The repeated measures of MANOVA revealed significant group × time interactions in GAF and DAS-WHO, suggesting that the REHACOP group exhibited a significantly larger improvement after treatment than did the control group. For example, the REHACOP group’s improvement in GAF was twice the improvement obtained by the control group. The effect size for GAF was d = 0.61, and the total score for DAS-WHO was d = 0.57.
Table 4.
Functional Outcome From Baseline to Posttreatment by Treatment Group (REHACOP; Control Group)
| Mean (SD) | Main Effect Group | Main Effect Time | Group × Time Interaction | Effect Size | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| REHACOP | Control Group | F | P | F | P | F | P | d | ||
| GAF | Pre | 38.88 (13.5) | 43.33 (16.3) | 0.00 | .983 | 64.42 | <.001 | 5.64 | .020 | 0.61 |
| Post | 58.06 (17.3) | 53.75 (20.4) | ||||||||
| DAS-WHO | Pre | 14.50 (4.1) | 13.70 (3.8) | 0.03 | .855 | 63.08 | <.001 | 6.26 | .014 | 0.57 |
| Post | 10.50 (5.1) | 11.63 (4.7) | ||||||||
| CGI | Pre | 5.08 (1.4) | 4.64 (1.3) | 0.48 | .490 | 66.04 | <.001 | 2.74 | .102 | 0.36 |
| Post | 3.66 (1.4) | 3.71 (1.5) | ||||||||
| Self-care | Pre | 2.70 (1.4) | 2.40 (1.1) | 0.04 | .851 | 16.44 | <.001 | 1.22 | .272 | 0.23 |
| Post | 2.11 (1.3) | 2.07 (1.2) | ||||||||
| Social competence | Pre | 3.78 (1.1) | 3.71 (1.2) | 1.99 | .164 | 54.57 | <.001 | 5.90 | .017 | 0.56 |
| Post | 2.72 (1.3) | 3.18 (1.3) | ||||||||
| Vocational outcome | Pre | 4.00 (1.0) | 3.97 (0.9) | 2.41 | .127 | 48.01 | <.001 | 4.28 | .042 | 0.47 |
| Post | 2.88 (1.3) | 3.38 (1.2) | ||||||||
| Family contact | Pre | 3.72 (0.9) | 3.46 (1.1) | 0.11 | .746 | 64.64 | <.001 | 4.88 | .030 | 0.50 |
| Post | 2.47 (1.2) | 2.76 (1.2) | ||||||||
Note:GAF, Global Assessment of Fucntioning; DAS-WHO, Disability Assessment Schedule from WHO; CGI, Clinical General Impression Scale.
A closer inspection of the DAS-WHO specific domains revealed significant group × time interactions in vocational outcomes (d = 0.47), family contact (d = 0.50), and social competence (d = 0.56). However, the results for self-care management did not reach statistical significance.
Predictors of Change in Functional Outcome
The baseline level of medication and the symptom severity did not correlate with the degree to which patients improved with REHACOP. Changes in negative symptoms (B = −0.58, P < .001) and the total cognitive score (B = −0.36, P < .05) predicted changes in GAF scores in the REHACOP group, whereas different predictors emerged in the control group. Only changes in negative symptoms (B = −0.45, P < .001) predicted changes in GAF scores among patients in the control group. Changes in negative symptoms (B = 0.62, P < .001) predicted changes in CGI among patients in the control group, whereas changes in negative symptoms (B = 0.67, P < .001) and cognition (B = 0.39, P < .01) predicted changes in CGI scores in the REHACOP group.
Finally, negative symptoms (B = −0.60, P < .001) and cognition (B = −0.36, P < .01) predicted changes in DAS-WHO scores in the REHACOP group. Only changes in negative symptoms (B = −0.45, P < .001) predicted changes in DAS-WHO scores among patients in the control group.
Discussion
In this hospital-based study, patients with chronic schizophrenia undergoing cognitive remediation with REHACOP experienced improvements in neurocognition, clinical symptoms, and functional outcomes. Moreover, improvements in cognition and negative symptoms predicted the changes in functional outcome in the REHACOP group but not in the control group. This general improvement cannot be explained by increased professional attention, differences in medication, or other sample characteristics such as chronicity or clinical profile. The improvements cannot be attributed to the number of hours of exposure to treatment, because both groups received equivalent treatment time, or to group vs individual interventions, because both formats were alike.
The improvement in cognition is consistent with the findings of previous studies.10,24 One major finding is that REHACOP significantly reduced patients’ negative symptoms. These results support some previous studies that indicated improvements in negative symptoms11–13,28,46 but contradict others.14–20 It is difficult to identify a clear reason for these inconsistent results due to the enormous methodological heterogeneity. McGurk et al24 indicated that only cognitive remediation programs that provide positive learning experiences to bolster self-esteem and perceived self-efficacy have beneficial effects on symptom improvement. Grant and Beck47 supported this idea by demonstrating that cognitive impairment imposes discouraging life experiences on patients. These experiences may lead them to perceive negative expectancies and develop defeatist beliefs, especially among patients with schizophrenia. REHACOP exhibits several characteristics that can provide patients with positive learning experiences, which at least partially explains its benefits in altering negative symptoms. These characteristics include hierarchically organized exercises, positive feedback, and the use of homework to support the transfer of learned strategies to daily life activities. Patients may feel themselves to be more capable of overcoming daily problems caused by cognitive impairments. As a result, patients may be more encouraged to take part in activities that they tended to avoid previously, improving negative symptomatology.
Unexpectedly, the REHACOP intervention was an effective tool for improving other clinical symptoms, namely emotional distress and disorganization symptoms. Very few prior studies have identified a significant improvement in disorganization symptoms.17,19,28 In addition, contrary to previous studies that reported data on emotional distress,17,18 we observed significant improvements in emotional distress symptoms. Group support, positive feedback, the involvement of family members in the patients’ treatment, and the improvements in other areas may account for these positive results in emotional distress. However, REHACOP did not produce significant improvements in positive symptoms, excitement, or insight. These results are consistent with that of many similar studies.14,16,17,19,20,25
Consistent with previous articles,10 the improvement in functional outcome in the experimental group was significantly greater than in the control group. Likewise, the intervention with the REHACOP program enabled patients to obtain unexpectedly higher values on some assessment scales. In fact, unlike other studies,23 participants in this trial improved twice as much as the control group in general functioning (GAF). The benefit of the cognitive remediation intervention in this study could be reinforced by the inclusion of strategy learning and transfer techniques and through combination with treatment as usual. These results are similar to the benefits obtained by Bowie et al22 in a recent study that combined cognitive remediation with functional skills.
Because functional outcome consists of many different domains, it is useful to discriminate in greater detail the obtained information on functional disability. Observed improvements in vocational outcomes in our study are consistent with most of the previous studies published in this field.12,17,19,21 Contrary to the methodology of our study, however, most other groups have combined cognitive remediation with various types of vocational rehabilitation in the experimental group. Despite the absence of vocational rehabilitation, REHACOP was effective. Only one of the mentioned studies12 did not combine cognitive remediation with vocational rehabilitation but exhibited positive results. Similar to our study, Eack et al12 trained their patients in various cognitive domains and social skills. In both studies, learned strategies were generalized to patients’ daily living using homework, and therapists provided patients with positive feedback. Therefore, we may hypothesize that the mentioned characteristics play an important role in the improvement of vocational outcome.
However, the efficacy of cognitive remediation in improving social competence has been inconsistent. REHACOP improved patients’ social competence as previous studies have reported.12,22,23 Studies that have not identified positive changes in social competence14,15,18,20 have some features in common; most were administered individually, and none included social skills or daily life activities. Therefore, the inclusion of social skill training and working with other patients simultaneously with feedback from both the therapist and colleagues is a key factor for improving social competence.
Consistent with previous findings,18 the REHACOP group did not significantly improve in self-care management compared with the control group. However, the degree of improvement was reduced, and the baseline status of the groups in self-care (good enough) could have introduced a ceiling effect. Unfortunately, none of the reviewed studies have reported data on family contact, so very little is known about this functional domain. However, the family involvement in the rehabilitation process benefits the patients’ recovery, and it seems reasonable to think that good management of the family intervention can contribute to the patients’ general status.
Despite these promising findings, this study has several limitations. First, we did not assess social cognition. At the beginning of the study, REHACOP did not yet include a social cognition-training unit. A social cognition unit has just been recently added to REHACOP, and we hope to present our results in future studies. Second, the study lacks measures of subjective quality of life, and it is important to know whether REHACOP exhibits a significant influence on patients’ self-esteem. Unfortunately, we did not include any of these variables in the first study protocol. Future studies should note the effect of rehabilitation on these factors. The clinical profile of the sample must also be considered. The severity of the clinical profile does not seem to be a relevant factor for improvement during cognitive rehabilitation.10,13,24 However, more research is needed to shed light on this important issue. Finally, a longitudinal follow-up must be performed to understand the long-term effects.
Funding
Health Department of the Basque Government (2010111136, 2011111102); Educational and Science Department of the Basque Government (BFI09.123).
Acknowledgment
The authors declare that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Klingberg S, Wölwer W, Engel C, et al. Negative symptoms of schizophrenia as primary target of cognitive behavioral therapy: results of the randomized clinical TONES study. Schizophr Bull. 2011; 37(suppl 2):S98–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seidman LJ, Giuliano AJ, Meyer EC, et al. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Arch Gen Psychiatry. 2010;67:578–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schretlen DJ, Cascella NG, Meyer SM, et al. Neuropsychological functioning in bipolar disorder and schizophrenia. Biol Psychiatry. 2007; 62 179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Demjaha A, Valmaggia L, Stahl D, Byrne M, McGuire P. Disorganization/cognitive and negative symptom dimensions in the at-risk mental state predict subsequent transition to psychosis. Schizophr Bull. 2012; 38: 351–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koutsouleris N, Davatzikos C, Bottlender R, et al. Early recognition and disease prediction in the at-risk mental states for psychosis using neurocognitive pattern classification. Schizophr Bull. 2012; 38: 1200–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kirkpatrick B, Fenton WS, Carpenter WT, Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006; 32: 214–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Corcoran CM, Kimhy D, Parrilla-Escobar MA, et al. The relationship of social function to depressive and negative symptoms in individuals at clinical high risk for psychosis. Psychol Med. 2011;41:251–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goldberg TE, Goldman RS, Burdick KE, et al. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch Gen Psychiatry. 2007; 64: 1115–1122 [DOI] [PubMed] [Google Scholar]
- 9. Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006; 26: 365–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011; 168: 472–485 [DOI] [PubMed] [Google Scholar]
- 11. Bellucci DM, Glaberman K, Haslam N. Computer-assisted cognitive rehabilitation reduces negative symptoms in the severely mentally ill. Schizophr Res. 2003; 59: 225–232 [DOI] [PubMed] [Google Scholar]
- 12. Eack SM, Greenwald DP, Hogarty SS, et al. Cognitive enhancement therapy for early-course schizophrenia: effects of a two-year randomized controlled trial. Psychiatr Serv. 2009;60:1468–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roder V, Mueller DR, Schmidt SJ. Effectiveness of integrated psychological therapy (IPT) for schizophrenia patients: a research update. Schizophr Bull. 2011; 37 Suppl 2: S71–S79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. d’Amato T, Bation R, Cochet A, et al. A randomized, controlled trial of computer-assisted cognitive remediation for schizophrenia. Schizophr Res. 2011; 125: 284–290 [DOI] [PubMed] [Google Scholar]
- 15. Dickinson D, Tenhula W, Morris S, et al. A randomized, controlled trial of computer-assisted cognitive remediation for schizophrenia. Am J Psychiatry. 2010; 167: 170–180 [DOI] [PubMed] [Google Scholar]
- 16. Hodge MA, Siciliano D, Withey P, et al. A randomized controlled trial of cognitive remediation in schizophrenia. Schizophr Bull. 2010; 36: 419–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lindenmayer JP, McGurk SR, Mueser KT, et al. A randomized controlled trial of cognitive remediation among inpatients with persistent mental illness. Psychiatr Serv. 2008;59:241–247 [DOI] [PubMed] [Google Scholar]
- 18. Penadés R, Catalán R, Salamero M, et al. Cognitive remediation therapy for outpatients with chronic schizophrenia: a controlled and randomized study. Schizophr Res. 2006; 87: 323–331 [DOI] [PubMed] [Google Scholar]
- 19. Vauth R, Corrigan PW, Clauss M, et al. Cognitive strategies versus self-management skills as adjunct to vocational rehabilitation. Schizophr Bull. 2005; 31: 55–66 [DOI] [PubMed] [Google Scholar]
- 20. Wykes T, Reeder C, Landau S, et al. Cognitive remediation therapy in schizophrenia: randomised controlled trial. Br J Psychiatry. 2007; 190: 421–427 [DOI] [PubMed] [Google Scholar]
- 21. Bell MD, Zito W, Greig T, Wexler BE. Neurocognitive enhancement therapy with vocational services: work outcomes at two-year follow-up. Schizophr Res. 2008; 105: 18–29 [DOI] [PubMed] [Google Scholar]
- 22. Bowie CR, McGurk SR, Mausbach B, Patterson TL, Harvey PD. Combined cognitive remediation and functional skills training for schizophrenia: effects on cognition, functional competence, and real-world behavior. Am J Psychiatry. 2012; 169: 710–718 [DOI] [PubMed] [Google Scholar]
- 23. Hogarty GE, Flesher S, Ulrich R, et al. Cognitive enhancement therapy for schizophrenia: effects of a 2-year randomized trial on cognition and behavior. Arch Gen Psychiatry. 2004; 61: 866–876 [DOI] [PubMed] [Google Scholar]
- 24. McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007; 164: 1791–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bark N, Revheim N, Huq F, Khalderov V, Ganz ZW, Medalia A. The impact of cognitive remediation on psychiatric symptoms of schizophrenia. Schizophr Res. 2003; 63: 229–235 [DOI] [PubMed] [Google Scholar]
- 26. McGurk SR, Mueser KT, Pascaris A. Cognitive training and supported employment for persons with severe mental illness: one-year results from a randomized controlled trial. Schizophr Bull. 2005;31:898–909 [DOI] [PubMed] [Google Scholar]
- 27. Sartory G, Zorn C, Groetzinger G, Windgassen K. Computerized cognitive remediation improves verbal learning and processing speed in schizophrenia. Schizophr Res. 2005; 75: 219–223 [DOI] [PubMed] [Google Scholar]
- 28. Silverstein SM, Hatashita-Wong M, Solak BA, et al. Effectiveness of a two-phase cognitive rehabilitation intervention for severely impaired schizophrenia patients. Psychol Med. 2005; 35: 829–837 [DOI] [PubMed] [Google Scholar]
- 29. Silverstein SM, Schenkel LS, Valone C, Nuernberger SW. Cognitive deficits and psychiatric rehabilitation outcomes in schizophrenia. Psychiatr Q. 1998; 69: 169–191 [DOI] [PubMed] [Google Scholar]
- 30. Spaulding WD, Reed D, Sullivan M, Richardson C, Weiler M. Effects of cognitive treatment in psychiatric rehabilitation. Schizophr Bull. 1999; 25: 657–676 [DOI] [PubMed] [Google Scholar]
- 31. American Psychiatric Association Diagnostic and Statistical Manual Of Mental Disorders DSM-IV. 4thed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 32. Peralta V, Cuesta MJ. Validación de la escala de los síndromes positivo negativo (PANSS) en una muestra de esquizofrénicos españoles Actas Luso-Españolas de Neurología Psiquiatría. 1994; 4: 44–50 [PubMed] [Google Scholar]
- 33. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987; 13: 261–276 [DOI] [PubMed] [Google Scholar]
- 34. van der Gaag M, Cuijpers A, Hoffman T, et al. The five-factor model of the Positive and Negative Syndrome Scale I: confirmatory factor analysis fails to confirm 25 published five-factor solutions. Schizophr Res. 2006; 85: 273–279 [DOI] [PubMed] [Google Scholar]
- 35. David AS. Insight and psychosis. Br J Psychiatry. 1990; 156: 798–808 [DOI] [PubMed] [Google Scholar]
- 36. Golden CJ. STROOP: Test de colores y palabras. Madrid: TEA Ediciones; 2001. [Google Scholar]
- 37. Reitan R, Wolfson D. The Haldstead-Reitan Neuropsy- chological Test Battery. Tucson: Neuropsychology Press; 1985. [Google Scholar]
- 38. Wechsler D. WAIS-III Manual: Wechsler Adult Intelligence Scale-III. New York: Psychological Corporation; 1997. [Google Scholar]
- 39. Brandt J, Benedict RHB. Hopkins Verbal Learning Test-Revised. Lutz, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- 40. Peña-Casanova J. Programa integrado de exploración neuropsicológica. Manual. Barcelona: Masson; 1990. [Google Scholar]
- 41. González Montalvo JI. Creación y validación de un test de lectura para el diagnóstico del deterioro mental en el anciano. Madrid: Universidad Complutense de Madrid; 1991. [Google Scholar]
- 42. Frances A, Pincus HA, First MB. The Global Assessment of Functioning Scale (GAF). Diagnostic and Statistical Manual of Mental Disorders. 4thed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 43. Guy W. CGI: Clinical Global Impressions. Manual for the ECDEU Assessment Battery. Chevy Chase: National Institute of Mental Health; 1970. [Google Scholar]
- 44. World Health Organisation Psychiatric Disability Assessment Schedule. Ginebra: World Health Organisation; 1988. [Google Scholar]
- 45. Ojeda N, Peña J, Sánchez P, Bengoetxea E. La rehabilitación neuropsicológica en psicosis II: El programa rehacop. In: Ezcurra J, Gutiérrez M, Gonzalez-Pinto A, eds. Esquizofrenia: Sociogénesis, psicogénesis y condicionamiento biológico. Madrid: Aula Médica; 2010; 471–495 [Google Scholar]
- 46. Vita A, De Peri L, Barlati S, et al. Effectiveness of different modalities of cognitive remediation on symptomatological, neuropsychological, and functional outcome domains in schizophrenia: a prospective study in a real-world setting. Schizophr Res. 2011; 133: 223–231 [DOI] [PubMed] [Google Scholar]
- 47. Grant PM, Beck AT. Defeatist beliefs as a mediator of cognitive impairment, negative symptoms, and functioning in schizophrenia. Schizophr Bull. 2009; 35: 798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]