Abstract
Background: Neurological soft signs (NSS) are associated with schizophrenia and related psychotic disorders. NSS have been conventionally considered as clinical neurological signs without localized brain regions. However, recent brain imaging studies suggest that NSS are partly localizable and may be associated with deficits in specific brain areas. Method: We conducted an activation likelihood estimation meta-analysis to quantitatively review structural and functional imaging studies that evaluated the brain correlates of NSS in patients with schizophrenia and other psychotic disorders. Six structural magnetic resonance imaging (sMRI) and 15 functional magnetic resonance imaging (fMRI) studies were included. Results: The results from meta-analysis of the sMRI studies indicated that NSS were associated with atrophy of the precentral gyrus, the cerebellum, the inferior frontal gyrus, and the thalamus. The results from meta-analysis of the fMRI studies demonstrated that the NSS-related task was significantly associated with altered brain activation in the inferior frontal gyrus, bilateral putamen, the cerebellum, and the superior temporal gyrus. Conclusions: Our findings from both sMRI and fMRI meta-analyses further support the conceptualization of NSS as a manifestation of the “cerebello-thalamo-prefrontal” brain network model of schizophrenia and related psychotic disorders.
Key words: neurological, soft, signs, brain, imaging, activation, likelihood, estimation, meta-analysis, schizophrenia, psychosis
Introduction
Neurological soft signs (NSS) have received continuing interest in psychosis research. One of the main reasons is that these signs have been considered the main target features of neurological abnormalities for psychosis and related disorders,1,2 as well as one of the promising endophenotypes linking genotypes and clinical phenotypes for psychotic disorders.3–5 However, the reason of the high prevalence rate of NSS in psychosis is still unsolved. Traditionally, NSS are defined as minor neurological abnormalities without definite localizable brain regions responsible for the corresponding clinical manifestations, eg, simple motor coordination, complex motor sequencing, sensory integration, and disinhibition signs.6–10 The distinction and classification of NSS have also been criticized as artifactual, possibly reflecting an inability to define a clear brain-behavior relationship underlying their presence.6,9 Nevertheless, NSS have been frequently reported in schizophrenia spectrum disorders8,11 and sometimes also in related psychotic disorders such as bipolar disorder.11,12 However, whether the NSS are also associated with other mood disorders such as major depression is still unclear.13,14 Furthermore, studies have consistently shown that the high prevalence of NSS found in these disorders cannot be explained as a side effect of antipsychotic or other psychotropic medications.13,15
With the advent of neuroimaging technologies, an increasing amount of evidence suggests that NSS can be localized, at least partially, to specific cortical and subcortical structures that may be responsible for these behavioral and clinical manifestations.9,11,16–18 For example, reduced volumes of the inferior frontal lobe19 and bilateral precentral gyrus (BA6)16 have been reported in patients with psychotic disorders exhibiting NSS.20 Bilateral reduction of the size of the cerebellum, especially in the right hemisphere, has also been reported to correlate with total score of NSS in patients with psychotic disorders.9,16,19,21,22 A recent cortex morphology study found the bilateral global cortical sulci to be significantly reduced in size in psychosis patients with high levels of NSS.23 This kind of brain morphology findings suggest that NSS may reflect a series of early neurodevelopment deviation in psychosis.23 Moreover, the neural substrates for NSS have been suggested not to be limited to cortical regions but included subcortical regions such as the basal ganglia11,19,24 and the thalamus.16
Apart from the investigation of brain morphological correlates of NSS, several functional magnetic resonance imaging (fMRI) paradigms have also been used to examine the neural bases of NSS. Most of the paradigms were limited to the evaluation of motor coordination signs such as the finger-tapping task25–27 or of disinhibition signs such as the go/no-go task.28–30 Furthermore, most studies used region-of-interest approaches and did not provide information on whole-brain patterns of activation.
Data from whole-brain evaluation studies of the go/no-go task, one of the common disinhibition NSS items, showed that when performing the inhibition response, several brain regions, including the frontal gyrus, the temporal gyrus, the parietal lobule, the precuneus, and the posterior cingulate gyrus, were identified across both patients with psychosis and healthy controls.28–30 Patients with psychotic disorders had abnormal activation in several cortical regions such as the inferior frontal gyrus,31–34 the anterior cingulate,28,34,35 and the middle frontal gyrus35,36 and subcortical regions such as the hippocampus,34 the midbrain,37 and the amygdala.29
Because there is emerging evidence that supports the presence of a substrate of morphofunctional alterations that could explain the presence of neurological abnormalities in psychosis, it is important to review and estimate from the existing literature quantitatively the level of NSS. Meta-analysis is a method to combine numerical data from former studies.38 Meta-analysis also provides an overall estimate of the effect size of any behavioral impairment observed in clinical groups. The recent advance of linking behavioral data to neuroimaging data using activation likelihood estimation (ALE) meta-analysis39 further allows us to examine the specific brain activations associated with behavioral tasks such as NSS. The GingerALE2.1 software39–41 was utilized in this study to give a systematic assessment of the brain regions associated with NSS. The ALE also has the additional advantage of accommodating large amounts of data generated across multiple neuroimaging studies and mapping the involvement of sublobar components of the brain with good spatial resolution. The output identifies brain areas most consistently replicated thereby reducing the chances of false positive findings.42 The purpose of this study was to conduct a meta-analysis to quantitatively review the neural bases of NSS, based on both structural and functional studies, in patients with schizophrenia and related psychotic disorders.
Methods
Literature Searching
Articles were searched from 4 online databases: PubMed, Web of Knowledge, Elsevier, and EBSCO (PsyINFO, PsycARTICLES, PsycBOOKS, PsycCRITIQUES, and PsycEXTRA). The published period was selected from the earliest date of each database to August 31, 2012.
Twenty-five key words classified into 3 categories were used for the search:
1. key words of neuroimaging techniques: MRI, imaging, and positron emission tomography (PET);
2. key words of clinical diagnoses: psychosis, mental disorder, schizo*, depression, bipolar, and psychotic; and
3. key words of NSS and related test items: neurological AND soft AND signs, “Heidelberg Scale,” finger AND movement, “finger opposition,” “motor coordination,” pronation AND supination, “23 items from Krebs,” “Cambridge Neurological Inventory (CNI),” NSS, “Neurological Evaluation Scale,” complex AND motor AND sequencing, Fist-edge-palm (FEP), finger AND thumb AND opposition, fist AND edge AND palm, finger AND tapping, and go AND no-go.
Article Selection
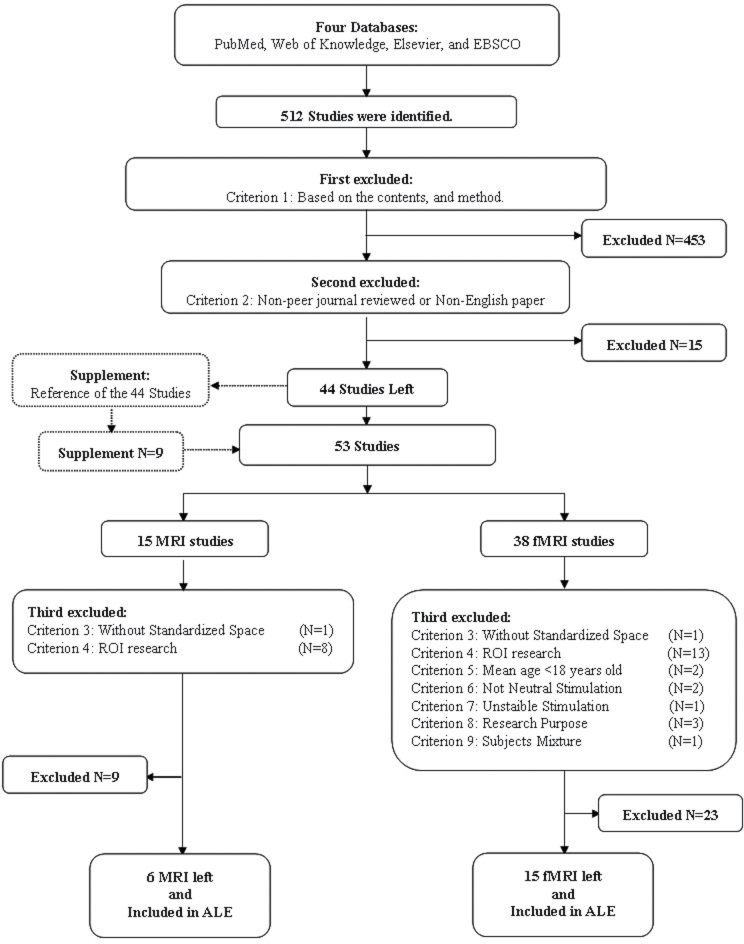
A total of 512 articles were identified. All articles were reviewed for inclusion in the ALE analysis by 2 independent raters (Q.Z. and Z.L.) according to a specified set of criteria. The process is illustrated in figure 1. After applying the first 2 exclusion criteria, 468 unrelated articles were excluded and 44 related articles were left. Besides, 9 additional related articles were also identified from the reference lists of the 44 articles. All of the 9 additional articles satisfied the first 2 criteria. Therefore, a total of 53 articles were considered (15 structural MRI [sMRI] and 38 fMRI). These articles were then subject to further consideration on the basis of exclusion Criteria 3–9:
Fig. 1.
The flow chart of articles selecting for activation likelihood estimation-structural magnetic resonance imaging (ALE-sMRI) and activation likelihood estimation-functional magnetic resonance imaging (ALE-fMRI) analyses.
1. Unrelated studies or studies using neuroimaging techniques other than sMRI, fMRI, or PET (excluded 453 studies).
2. Studies not published in peer-review journals or not published in English (excluded 15 studies).
3. The foci coordinates were not given in standardized space of the Montreal Neurological Institute (MNI)43 or Talairach space44 (excluded 2 studies).
4. The studies were not related to whole-brain investigation (excluded 21 studies).
5. The age of the participants was less than 18 years (excluded 2 studies).
6. The stimulation used in the go/no-go studies was not neutral or not a mixture of neutral, positive, and negative (excluded 2 studies).
7. The stimulation appearance rate used in the go/no-go task was not stable (excluded 1 study).
8. The purpose of the experimental task was not to test the categories of NSS, including motor coordination, disinhibition, or sensory integration (excluded 3 studies).
9. The activation foci were not reported separately for patients and healthy controls (excluded 1 study).
Articles Included in the ALE Analysis
A total of 21 articles were selected for the final ALE analysis using the search strategy given above. They were 6 sMRI studies and 15 fMRI studies (table 1 and Appendix 1).
Table 1.
Studies Included in ALE-sMRI and ALE-fMRI Analyses
| Author | Year | Test/Tasks | Design | Coordination | Transform | Healthy | Patients | Diagnosis | Episode | Medication | Analyses | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies included in ALE-sMRI | Gray matter | White matter | — | — | |||||||||||
| 1 | P. Dazzan | 2004 | NES | Volume | Talairach | — | — | 77 | PS | First | — | √ | — | — | — |
| 2 | G. Venkatasubramanian | 2008 | NES | Volume | Talairach | Brett transform | 27 | 30 | SZ | First | — | √ | — | — | — |
| 3 | P. A. Thomann | 2009 | Heidelberg | Density | MNI | MNI to Talairach | 22 | 42 | SZ | First | Atypical | √ | √ | — | — |
| 4 | S. Mouchet-Mages | 2011 | 23 items of Kreb | Volume | Talairach | — | — | 42 | PS | First | Mix | √ | √ | — | — |
| 5 | M. Heuser | 2011 | Heidelberg | Density | Talairach | — | — | 102 | PS | First | Atypical | √ | √ | — | — |
| 6 | K. Li | 2012 | Heidelberg | Volume | Talairach | — | 10 | 10 | SZ | Longitudinal | Atypical | √ | — | — | — |
| Studies included in ALE-fMRI (Go/no-go) | Healthy | Patients | HC > PA | PA > HC | |||||||||||
| 7 | K. Rubia | 2001 | Simple task | Block | Talairach | — | 7 | 6 | SZ | Chronic | Atypical | √ | √ | √ | — |
| 8 | K. R. Laurens | 2003 | Simple task | Event | Talairach | — | 16 | 10 | SZ | Chronic | Atypical | — | — | — | √ |
| 9 | R. Elliott | 2004 | Emotional task | Block | Talairach | — | 11 | 8 | BD | Mix | — | — | — | √ | √ |
| 10 | L. L. Altshuler | 2005 | Simple task | Block | MNI | MNI to Talairach | 13 | 11 | BD | Mix | Mix | √ | √ | √ | — |
| 11 | E. Arce | 2006 | Simple task | Block | Talairach | — | 17 | 17 | SZ | Chronic | Atypical | — | — | √ | — |
| 12 | D. Silbersweig | 2007 | Emotional task | Block | MNI | MNI to Talairach | 14 | 16 | BPD | Mix | — | — | — | √ | √ |
| 13 | C. C. Joyal | 2007 | Simple task | Block | Talairach | — | 12 | 12 | SZ | Chronic | — | — | — | √ | √ |
| 14 | A. Kaladjian | 2007 | Simple task | Event | Talairach | Brett transform | 21 | 21 | SZ | Chronic | Mix | √ | √ | √ | — |
| 15 | R. M. Roth | 2007 | Simple task | Event | Talairach | — | 14 | 12 | OCD | Mix | — | √ | √ | √ | √ |
| 16 | A. Kaladjian | 2009 | Simple task | Event | Talairach | — | 10 | 10 | BD | Chronic | Mix | √ | √ | √ | — |
| 17 | A. Kaladjian | 2009 | Simple task | Event | Talairach | Brett transform | 20 | 20 | BD | Chronic | Mix | √ | √ | √ | — |
| 18 | A. S. Welander-Vatn | 2009 | Simple task | Block | MNI | MNI to Talairach | 28 | 27 | BD | Chronic | Mix | √ | √ | — | — |
| 19 | P. Mazzola-Pomietto | 2009 | Simple task | Event | Talairach | Brett transform | 16 | 16 | BD | Chronic | Mix | √ | √ | √ | — |
| 20 | D. E. Fleck | 2011 | Simple task | Event | MNI | MNI to Talairach | 10 | 18 | BD | Chronic | — | — | — | — | √ |
| 21 | J. D. Townsend | 2012 | Simple task | Block | Talairach | — | 32 | 30 | BD | Chronic | Atypical | √ | √ | √ | — |
Note: NES, Neurological Evaluation Scale; MNI, Montreal Neurological Institute; BD, bipolar disorder; BPD, borderline personality disorder; OCD, obsessive compulsive disorder; PS, psychosis; SZ, schizophrenia;
HC > PA, attenuated brain activation of patients; PA > HC, hyperactivation of brain in patients; ALE, activation likelihood estimation; fMRI, functional magnetic resonance imaging; sMRI, structural magnetic resonance imaging; —, information unavailable; and √, information available.
The 6 sMRI studies included 5 cross-sectional studies and 1 longitudinal study.20 Both the cross-sectional and the longitudinal studies recruited patients with first-episode psychosis, including schizophrenia, schizophrenia spectrum disorder, and related psychosis. Three of these studies used the Heidelberg scale, 2 used the Neurological Evaluation Scale, and 1 used the 23 items of the Krebs’ scale to assess NSS (cf Appendix 2 for brief description of the scales). All of the 5 cross-sectional studies provided NSS scores negatively correlated brain regions. For the longitudinal design, the atrophic brain regions related to higher NSS scores were treated as negatively correlated.
Very few studies reported the positive correlation of brain regions with NSS. For white matter, 1 cross- sectional study reported a positive correlation of the left internal capsule volume with motor coordination signs and sensory integration signs.11 For gray matter, 1 cross-sectional study reported a positive correlation of bilateral thalamic volumes with motor coordination signs.16
Given the limited number of studies reporting the positive correlation of NSS with gray and white matters, we only included the negatively correlated foci in the sMRI-ALE analysis because the positively correlated foci were insufficient to allow for meaningful ALE analysis.
Among the 15 fMRI articles, there were 13 simple go/no-go studies and 2 emotional go/no-go studies.31,36 Five of the studies investigated patients with schizophrenia, 8 examined patients with bipolar disorder, 1 studied borderline personality disorder, and 1 investigated obsessive-compulsive disorder. All of the go/no-go studies provided inhibitory response activation to the neural stimulation or a mixture of positive and negative stimulation. Two inhibition-related contrasts, go/no-go condition minus go condition and go/no-go condition minus rest condition, were included in the final ALE-fMRI analysis (tasks introduced in Appendix 3).
Activation Likelihood Estimation
The software GingerALE2.139–41 was used for these ALE meta-analyses. The GingerALE is a widely used meta-analysis software for systematic review of neuroimaging data.39 The principle of ALE meta-analysis can be summarized into 3 steps: (1) each given focus was treated as a coordinate center of a probability Gaussian distribution, (2) the combined probability distribution map of the whole brain was calculated, and (3) the peak foci of the distribution value over the threshold were reported and anatomically labeled.39–41
For this ALE analysis, the standardized coordinates were set in Talairach space.44 All of the other foci reported in MNI space43 were converted into Talairach space with the Convert Foci tool in GingerALE2.1.39–41 Some of the foci transformed through Brett’s formation45 were converted in the following 2 steps by the tool Convert Foci: (1) convert to MNI space by Brett: Talairach to MNI and (2) convert back to Talairach space by MNI (SPM [Statistical Parametric Mapping software], http://www.fil.ion.ucl.ac.uk/spm/software/spm8/ ) to Talairach. The conversion information of each study is listed in table 1. The default parameters were chosen as follows: for the ALE-sMRI analysis, the false discovery rate (FDR) was set at “0.05” and for the ALE-fMRI analysis, the FDR was set at “0.01.” The results of these ALE analyses were viewed with the Mango Version 2.4 software (http://ric.uthscsa.edu/mango//download.html). The template was “Colin 1.1.nii” (http://www.brainmap.org/ale/).
Process of ALE Analyses
After standardization of coordinates, the ALE-sMRI and the ALE-fMRI data were meta-analyzed separately according to the following steps:
ALE-sMRI Analysis.
1. Gray or white matter discrimination: the original foci were separated into gray or white matter according to the original articles. If the location label was not provided in the original study, the foci’s labels were generated by the Talairach Client 2.4.2 software’s single point tool.46,47
2. Grouping: the gray matter and white matter foci were separately analyzed by 2 ALE analyses: ALE-sMRI (gray matter) and ALE-sMRI (white matter).
ALE-fMRI Analyses.
The ALE-fMRI analyses were conducted in 4 groups separately. First, the ALE-fMRI (healthy controls only) analyzed the activated foci of healthy controls; second, the ALE-fMRI (patients only) analyzed the activated foci of patients with psychotic disorders; third, the ALE-fMRI (healthy controls > patients) analyzed the attenuated brain foci of patients with psychotic disorders; finally, the ALE-fMRI (patients > healthy controls) analyzed the hyperactivated brain foci of patients with psychotic disorders.
Results
ALE of the sMRI Studies
The ALE-sMRI results are summarized in table 2 and illustrated in figure 2.
Table 2.
ALE-sMRI and ALE-fMRI Analyses Results (FDR of MRI < 0.05, FDR of fMRI < 0.01)
| Methods | Contrast | Contribution Information | ALE Results | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experiment | Foci | Participants | Cluster (No.) | ALE | Vol (mm3) | Talairach | Laterality | Broadman Area | Label | ||||
| X | Y | Z | |||||||||||
| MRI | Gray matter | 6 | 70 | 257 | 1 | 0.023 | 424 | −56 | −6 | 40 | Left | BA6 | Frontal lobe: precentral gyrus |
| 2 | 0.018 | 308 | 2 | −12 | 14 | Right | Thalamus | Sublobar: thalamus | |||||
| 3 | 0.018 | 296 | −46 | −8 | 50 | Left | BA4 | Frontal lobe: precentral gyrus | |||||
| 4 | 0.016 | 280 | −54 | 20 | 20 | Left | BA45 | Frontal lobe: inferior frontal gyrus | |||||
| 5 | 0.020 | 280 | 56 | −4 | 40 | Right | BA6 | Frontal lobe: precentral gyrus | |||||
| 6 | 0.017 | 224 | −48 | −26 | 52 | Left | BA2 | Parietal lobe: postcentral gyrus | |||||
| 7 | 0.014 | 184 | −50 | −40 | 44 | Left | BA40 | Parietal lobe: inferior parietal lobule | |||||
| White matter | 5 | 21 | 186 | 1 | 0.018 | 288 | 44 | −68 | 22 | Right | — | Temporal lobe: middle temporal gyrus | |
| 2 | 0.016 | 272 | 0 | −56 | −16 | Right | — | Anterior lobe: cerebellum culmen | |||||
| 3 | 0.015 | 184 | −36 | 34 | −6 | Left | — | Frontal lobe: inferior frontal gyrus | |||||
| fMRI | Healthy controls | 9 | 117 | 159 | 1 | 0.017 | 552 | 40 | 28 | 0 | Right | BA47 | Frontal lobe: inferior frontal gyrus |
| 2 | 0.018 | 320 | 44 | −58 | 22 | Right | BA39 | Temporal lobe: middle temporal gyrus | |||||
| 3 | 0.017 | 280 | −38 | −64 | −8 | Left | BA19 | Occipital lobe: fusiform gyrus | |||||
| 4 | 0.016 | 184 | 8 | −94 | 2 | Right | BA17 | Occipital lobe: lingual gyrus | |||||
| 5 | 0.014 | 120 | −26 | −8 | −12 | Left | Amygdala | Limbic lobe: parahippocampal gyrus | |||||
| 6 | 0.014 | 120 | −40 | 12 | 44 | Left | BA6 | Frontal lobe: middle frontal gyrus | |||||
| Patients | 9 | 91 | 155 | 1 | 0.017 | 680 | −32 | 22 | −2 | Left | BA13 | Sublobar: insula | |
| 2 | 0.016 | 464 | 50 | −54 | 18 | Right | BA22 | Temporal lobe: superior temporal gyrus | |||||
| 3 | 0.014 | 432 | −40 | −60 | 26 | Left | BA39 | Temporal lobe: middle temporal gyrus | |||||
| 4 | 0.015 | 312 | 18 | 0 | 4 | Right | Lateral globus pallidus | Sublobar: lentiform nucleus | |||||
| 5 | 0.014 | 192 | 36 | 16 | 6 | Right | BA13 | Sublobar: insula | |||||
| 6 | 0.012 | 120 | 24 | −70 | 42 | Right | BA7 | Parietal lobe: precuneus | |||||
| Healthy > patients | 13 | 52 | 189 | 1 | 0.013 | 416 | −24 | 10 | −4 | Left | Putamen | Sublobar: lentiform nucleus | |
| 2 | 0.012 | 336 | 20 | 4 | −4 | Right | Putamen | Sublobar: lentiform nucleus | |||||
| 3 | 0.011 | 320 | −22 | −6 | 12 | Left | Putamen | Sublobar: lentiform nucleus | |||||
| 4 | 0.012 | 216 | 40 | 22 | 4 | Right | BA45 | Frontal lobe: inferior frontal gyrus | |||||
| 5 | 0.012 | 160 | −2 | −30 | −10 | Left | Midbrain | Brainsteam | |||||
| Patients > healthy | 7 | 32 | 78 | 1 | 0.008 | 160 | −46 | 0 | −10 | Left | BA38 | Temporal lobe: superior temporal gyrus | |
Note: —, information unavailable.
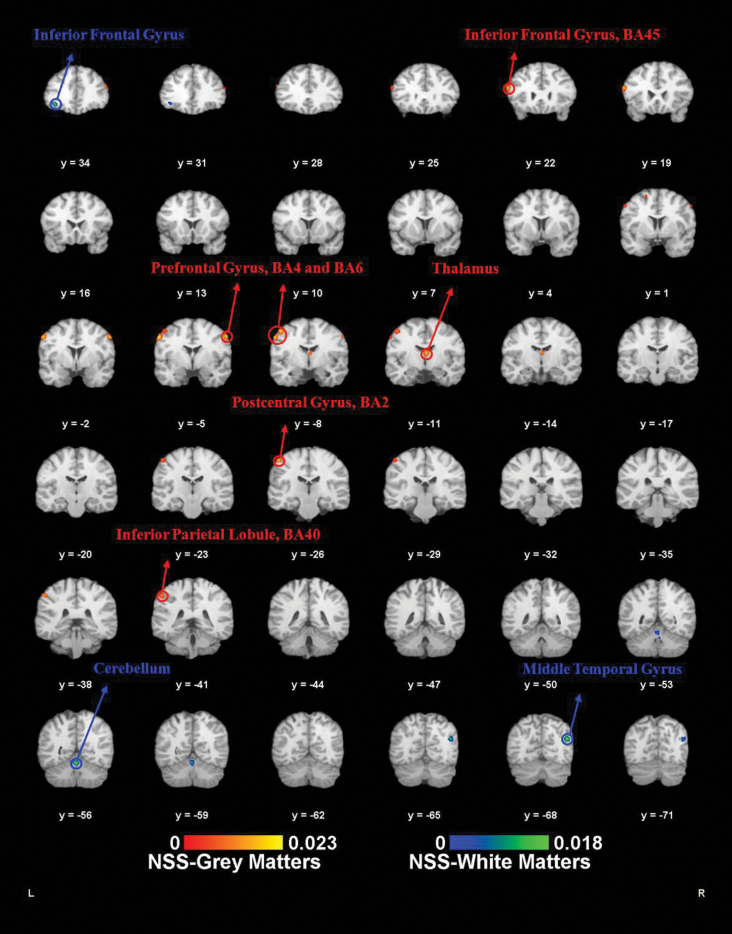
Fig. 2.
Activation likelihood estimation-structural magnetic resonance imaging (ALE-sMRI) results. ALE meta-analysis results of the sMRI. Red labels (neurological soft signs [NSS] gray matters) are the ALE meta-analysis results of the NSS scores negative correlated gray matter foci. Blue labels (NSS white matters) are the ALE meta-analysis results of the NSS scores negative correlated white matter foci (P < .05, false discovery rate [FDR] corrected).
Gray Matter Correlates of NSS.
The 6 MRI articles reported a total of 70 gray matter foci that were negatively correlated with NSS scores and included 257 patients. At the 0.05 FDR level, 7 foci were reported: the precentral gyrus foci (BA6), the right thalamus, the left precentral gyrus (BA4), the left inferior frontal gyrus (BA45), the right precentral gyrus (BA6), the left postcentral gyrus (BA2), and the left inferior parietal lobule (BA40).
White Matter Correlates of NSS.
Three sMRI studies16,19,48 reported 23 white matter foci that were negatively correlated with NSS and included 196 patients. At the 0.05 FDR level, 3 foci were reported: the right temporal lobe, the right culmen of the cerebellum, and the left inferior frontal gyrus.
ALE of the fMRI Studies
The fMRI ALE results are summarized in table 2 and illustrated in figures 3 and 4.
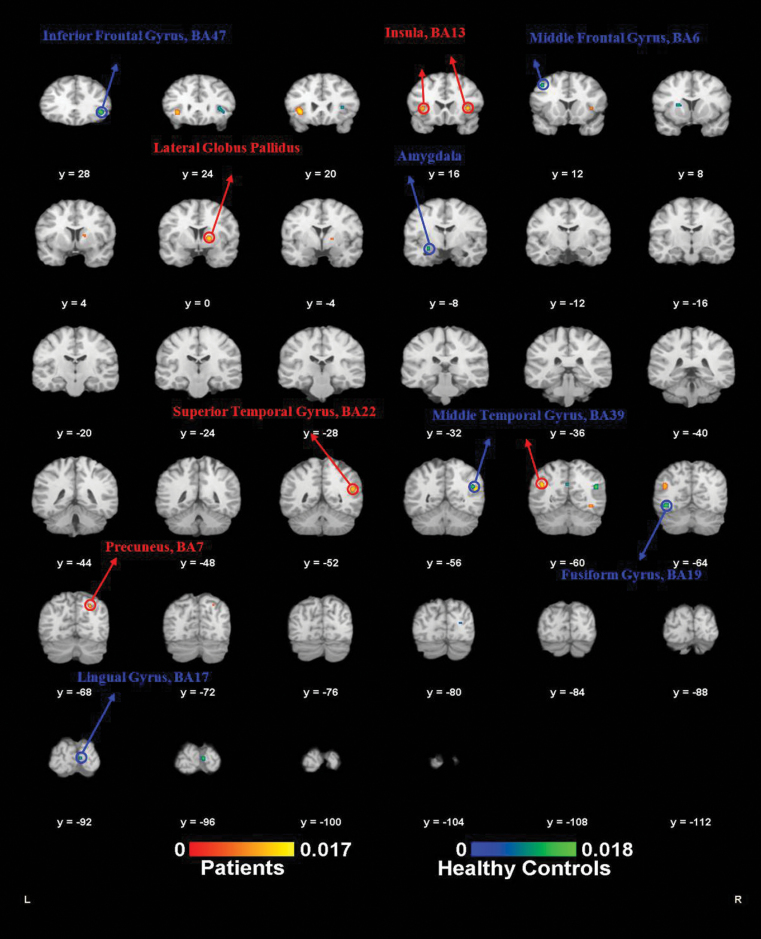
Fig. 3.
Activation likelihood estimation-functional magnetic resonance imaging (ALE-fMRI) results of individual groups. ALE meta-analysis results of the fMRI (go/no-go task) of mental disorders and healthy controls separately. Red labels (patients) are the ALE meta-analysis results of the brain activation foci of the mental disorder patients when doing the inhibition response. Blue labels (healthy controls) are the ALE meta-analysis results of the brain activation foci of the healthy controls when doing the inhibition response (P < .01, false discovery rate [FDR] corrected).
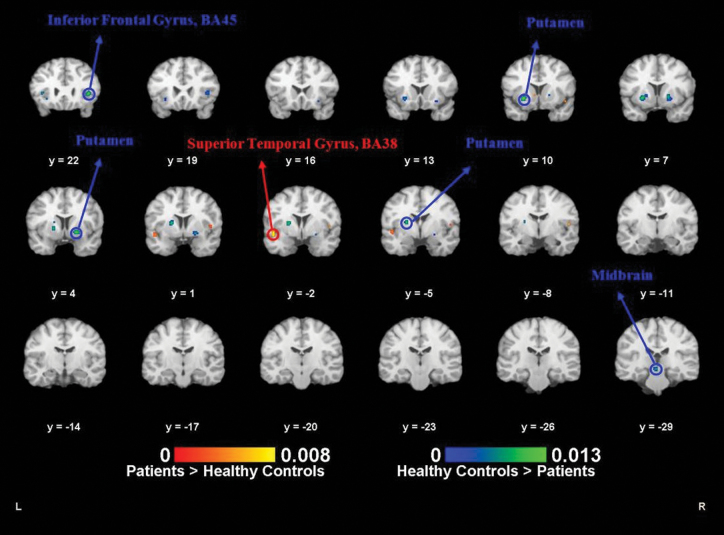
Fig. 4.
Activation likelihood estimation-functional magnetic resonance imaging (ALE-fMRI) results of groups difference. ALE meta-analysis results of the fMRI (go/no-go task) for the group difference between mental disorders and healthy controls. Red labels (patients > healthy controls) are the ALE meta-analysis results of the hyperactivated brain foci of the mental disorder patients when doing the inhibition response. Blue labels (healthy controls > patients) are the ALE meta-analysis results of the attenuated activation brain foci of the mental disorders when doing the inhibition response (P < .01, false discovery rate [FDR] corrected).
Healthy Controls Alone.
Nine of the 16 fMRI (go/no-go task) studies reported activated foci in healthy controls alone. The analysis included a total of 117 foci and 159 healthy controls. At an FDR level of 0.01, 6 foci were identified. They were the right inferior frontal gyrus (BA47) extending to the right insula (BA13), the right middle temporal gyrus (BA39), the left fusiform gyrus (BA19), the right lingual gyrus (BA17), the left amygdala, and the left middle frontal gyrus (BA6).
Psychosis Patients Alone.
Nine studies showed activated foci in patients with psychotic disorders when performing the go/no-go task. These included a total of 71 foci and 155 patients with psychotic disorders. At an FDR level of 0.01, the left insula (BA13), the right superior temporal gyrus (BA22), the left middle temporal (BA39), the right lateral globus pallidus, the right insula (BA13), and the right precuneus (BA7) were identified in the ALE analysis.
Healthy Controls > Psychosis Patients.
Twelve go/no- go studies provided information on attenuated brain activation of psychosis patients when performing the inhibition response, in comparison with healthy controls. The study of Joyal et al37 provided 2 comparisons between healthy controls and 2 different groups of patients with psychosis, and these were treated as 2 experiments. The ALE included 13 experiments, 52 foci, and 189 participants. At an FDR level of 0.01, the right and left putamen, the left lateral globus pallidus, the right inferior frontal gyrus (IFG; BA45), and the left culmen of cerebellum were identified as areas in which the patients showed reduced activation.
Psychosis Patients > Healthy Controls.
Six studies reported brain foci with higher activation in patients with psychosis. Once again the Joyal’s study37 was treated as reporting on 2 separate experiments. As a result, the ALE included 7 experiments, 32 foci, and 78 participants. At an FDR level of 0.01, the left superior temporal gyrus (BA38) was identified by the ALE as more activated in the patient population.
Discussion
This is the first meta-analysis that has estimated the extent of the morphological and neural functional correlates of NSS in patients with schizophrenia and other psychoses. The results from the sMRI analysis showed that NSS were associated with reduced gray matter at the precentral gyrus, the cerebellum, the inferior frontal gyrus, and the thalamus and associated with reduced white matter at the temporal lobe, the cerebellum, and the inferior frontal gyrus. The results from fMRI analysis showed that one of the inhibition NSS items such as the go/no-go task was significantly correlated with reduced brain activation at the inferior frontal gyrus, bilateral putamen, and the cerebellum and increased activation in the superior temporal gyrus.
ALE of the sMRI Studies
In the ALE-sMRI analyses, a smaller volume of foci located in the precentral gyrus (Brodman area 6, BA6) was found to be correlated with higher NSS scores. The BA6 area includes the presupplementary motor area (preSMA), the SMA, and the premotor cortex. This is an area that is crucial for motor coordination.48 The BA6 area has multiple roles in both integrating information from cognitive association areas (the IFG and the dorsolateral prefrontal cortex), and selecting or suppressing motor response to the basal ganglia, which further connects with the thalamus in an inhibitory pathway.49 Therefore, smaller volume of the BA6 area may influence both information relay and proper motor response selection. In single studies, smaller volume of the BA6 area has been associated with high total NSS score,19,48 high motor NSS subscore,48 and high sensory integration NSS subscore.11 In addition, volume reduction of this area has been frequently reported in brain structural imaging studies in individuals with schizophrenia,50 with bipolar disorders,51 and at high risk of developing psychosis.52
Apart from the precentral gyrus, several other brain areas were also found to be significantly associated with higher NSS scores, including the cerebellum, the IFG, and the thalamus. The cerebellum is closely involved in voluntary movement and motor coordination.22,53 Studies have found that, in comparison with healthy controls, patients with schizophrenia have smaller cerebellum.9,21 In several individual studies, a smaller volume of the cerebellum has been found to be significantly correlated with high total NSS scores,9,19,21 the motor subscore of NSS,16,19,21,22,53 finger-tapping task, and right-left extinction task deficient performance.53 The frontal gyrus is associated with action monitoring and executive functioning, and an intact IFG is fundamental to action monitoring,54 go/no-go tasks,55 and attentional set shifting.56 The IFG is considered a stimulus-response association information maintainer49 and functions as an unrelated response filter.32 Deficiencies in the IFG have been frequently reported in previous studies to be correlated with NSS deficits, such as motor coordination48 and sensory integration.11 A recent cortex morphology study also found that patients with first-episode psychosis with high scores in total NSS, motor coordination, and sensory integration showed a significant sulcation reduction in the bilateral dorsolateral prefrontal cortex and left medial frontal cortex.23 Finally, the thalamus is known as a relay station to select and relay information between peripheral, cortical, and subcortical regions.57,58 In the NSS cerebello-thalamo-prefrontal model, the thalamus is also an important key node.16 Smaller volume of the thalamus has been reported to correlate with both the total and motor subscores of NSS.19 In comparison with healthy controls, first-episode psychosis patients have been reported to have smaller thalami, independently from neurological performance.50 It is possible that changes in the thalamus may cause an inefficient communication between widespread brain regions and then cause the abnormal behavioral expression of NSS.
ALE of the fMRI Studies
Inhibition response in the go/no-go task requires cooperation between several brain regions: the frontal lobe, the temporal lobe, the parietal lobe, the occipital lobe, and subcortical regions.59 The connection can be conceptualized as a fronto-basal-ganglia circuitry divided into 3 parts: input, subcortical, and output processes.60 The input process is an activation process from the cortex to the striatum; the subcortical process is an inhibitory process from the striatum, through the globus pallidus, to the thalamus; and the output process is an activation process from the thalamus to the cortex.60
Our ALE-fMRI analysis identified several brain regions related to these processes, especially in the input and the subcortical ones. In the input process, a key area is the right inferior frontal lobe (rIFG), including BA45, BA46, and BA47, which is crucial in blocking the “go” response in the go/no-go task.59 The rIFG is described as an unrelated response filter,32 and a stimulus-response association information maintainer.49 Most importantly, the right IFG is responsible for both left and right hand-movement inhibitions.49 During the inhibition task, the greater the activation of the rIFG, the better the inhibition.61 The activation of the IFG has frequently been reported to be attenuated in patients with psychosis when performing the go/no-go task.32–34 From our results of ALE-fMRI analyses, the rIFG was only reported in the analysis of healthy controls but not in the analysis of patients. Furthermore, the ALE-fMRI (healthy controls > patients) also identified the rIFG (BA45), showing more directly that a lower activation of this area in patients with psychosis than healthy controls when performing the inhibition response. These ALE results suggest that the processes of inhibition in patients with psychotic disorders were already dampened at the information input stage.
In the subcortical process, when the striatum (putamen and caudate) is activated by signals from the frontal lobe, the globus pallidus is activated, which may further inhibit the thalamus. The decreased activation of the basal ganglia will cause an attenuation of the inhibition of the thalamus and therefore the whole inhibition response will be unsuccessful.59 Interestingly, patients with lesions in the basal ganglia (putamen, caudate, and globus pallidus) show an increased false alarm rate than healthy controls when performing the go/no-go task.62 Furthermore, activation of the basal ganglia seems attenuated in patients with psychotic disorders compared with healthy controls.59 Our ALE-fMRI (healthy > patients) also found the regions of the putamen and the globus pallidus to be hypoactivated in psychosis patients. Unfortunately, from the ALE analyses it is impossible to establish whether the attenuated activation of the putamen and the globus pallidus were simply caused by reduced signals from the rRFG or whether the hypoactivated basal ganglia would further attenuate the inhibition signal to the thalamus.
In addition to the brain areas mentioned above, abnormalities were also identified in the preSMA and the cerebellum. Our ALE-fMRI (healthy controls only) showed significant activation of the preSMA. The preSMA is critical for preparing a correct motor response and suppressing an incorrect motor prepotent in the go/no-go task.49 Similar to the rIFG, the greater the activation at the preSMA, the better is the inhibition response.61 Of note, a previous study found that patients with lesion at the BA6 (the premotor areas and the SMAs) had a higher false alarm rate.63 However, activations in the BA6 were not observed in our ALE-fMRI (patients only). Apart from the preSMA, abnormalities in the culmen of the cerebellum were also observed in our ALE-fMRI analysis (healthy controls > patients). The cerebellum is a brain region involved in motor processes. However, the cerebellum is also involved in higher cognitive function, such as selective attention and working memory.64 The cerebellum communicates with multiple cortical areas through the thalamus and also interacts with the basal ganglia at a subcortical level.65 Impaired cerebellar function may cause false alarms and disturb normal inhibition response.64
The only brain region in which patients showed higher activation in comparison to healthy controls in the ALE-fMRI analysis was the left superior temporal lobe. It has been frequently reported that patients with psychotic disorders showed accentuated activation of the superior temporal lobe compared with healthy controls on carrying out inhibition responses.31,66 Previous studies have suggested that the temporal region may be related to impulsivity and error commission.67 In performing the go/no-go task, the temporal region is related to awareness and negative emotional reaction associated with error commission.68 The hyperactivation of the superior temporal lobe may reflect an exaggerated negative emotional state in patients with psychotic disorders who commit errors.68
From our ALE-fMRI analysis, we found that impairment in the inhibition of the go/no-go task of NSS involved the cortical regions such as the IFG and several subcortical regions including the putamen, the globus pallidus, the thalamus, and the cerebellum. These brain areas are also overlapped with the cerebello-thalamo- prefrontal NSS-related circuit.16
Revisiting the Neuroanatomical and Functional Connectivity of NSS
In 1998, Andreasen et al69 proposed a theory of “cognitive dysmetria” in schizophrenic patients. These authors suggested that a scattered disturbance in the cortico-cerebellar- thalamic-cortical circuit in patients with schizophrenia could explain the diversity of schizophrenic symptoms. In 2011, Mouchet-Mages found that this cerebello-thalamo-prefrontal circuit may also be related to NSS abnormalities in first-episode psychosis.16 Both ALE-sMRI and ALE-fMRI analyses support Mouchet-Mages’ postulation. Moreover, our ALE results also imply that the temporal lobe, the basal ganglia, and the premotor area, all of which are connected with the cerebellum and the thalamus directly or indirectly, may also be potential brain locations of NSS.
Limitations
This study had a number of limitations. First, the number of studies included was relatively small, especially for the sMRI analysis. Similarly, the second limitation was about the inclusion of other psychotic disorders in addition to schizophrenia. The different clinical groups such as bipolar disorder and obsessive-compulsive disorder might have influenced the final results because whether they are also associated with high levels of NSS is still unclear. It has been shown that patients with early onset of obsessive-compulsive disorder did not have significant NSS compared with healthy controls.70 However, some other behavioral studies and meta-analysis have suggested that both bipolar and obsessive-compulsive disorders (particularly those experiencing psychotic symptoms) are strongly associated with NSS.71–73 Given the main aim of this study was to examine the neural basis of NSS both structurally and functionally, the findings generated from our meta-analysis provide valuable information contributing to understanding the neural basis of NSS in a more general clinical group of psychotic disorders rather than a specific group with schizophrenia. Having said that, further studies focusing on patients with schizophrenia are needed to examine the specific neural mechanism of NSS in this clinical group. The third limitation is that in this ALE analysis, all the NSS-related scores were considered as a whole. In future investigations, ALE analysis based on subscales of NSS is recommended because the heterogeneity of NSS items constitution may influence the outcome of analysis.
Finally, analyses of the fMRI data were mainly limited to the go/no-go paradigm, which is only one of the NSS disinhibition signs. The neural correlation or mechanisms for the other categories of NSS such as sensory integration has not been investigated thoroughly. Furthermore, in the go/no-go fMRI studies, researchers have used different analysis contrasts. For example, some studies have used the “go/no-go condition vs go condition,” while others have used the “go/no-go condition vs rest condition.” Compared with the first contrast, the second contrast may be confounded because a motor-related brain activation component is also involved. Fortunately, in this ALE meta-analysis, only 2 of the 15 studies used the second contrast, thus minimizing the potential confounding effect. However, in future ALE meta-analysis, the issue of inclusion and exclusion of the distinct contrasts should be addressed.
Conclusion
The results of this ALE meta-analysis further support that NSS may not be completely “soft” in nature. The structural abnormalities or functional deficiencies in the frontal lobe, the temporal lobe, the basal ganglia, and the thalamus, may be correlated with the expression of NSS, which further supports the conceptualization of NSS as a manifestation of the cerebello-thalamo-prefrontal brain network alteration in schizophrenia and related psychotic disorders. More rigorous and well-controlled experiments should be conducted to test this hypothesis in the near future.
Funding
National Science Fund China Outstanding Investigator Award (81088001 to R.C.K.C.); National Key Technologies R&D Programme (2012BAI36B01 to R.C.K.C.); Key Laboratory of Mental Health; Knowledge Innovation Project of the Chinese Academy of Sciences (KSCX2-EW-J-8 to R.C.K.C.); National Alliance for Research on Schizophrenia and Depression (to P.D.); National Health and Medical Research Council Senior Principal Research Fellowship (628386 to C.P.); National Alliance for Research on Schizophrenia and Depression Distinguished Investigator Award (to C.P.); National Health and Medical Research Council Program Grant (566529 to C.P.).
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Appendix 1. Studies Included in the Meta-analysis
MRI studies
1. Dazzan P, Morgan KD, Orr KG, et al. The structural brain correlates of neurological soft signs in AESOP first-episode psychoses study. Brain. 2004;127:143–153.
2. Venkatasubramanian G, Jayakumar PN, Gangadhar BN, Keshavan MS. Neuroanatomical correlates of neurological soft signs in antipsychotic-naive schizophrenia. Psychiatry Res. 2008;164:215–222.
3. Thomann PA, Wüstenberg T, Santos VD, Bachmann S, Essig M, Schröder J. Neurological soft signs and brain morphology in first-episode schizophrenia. Psychol Med. 2009;39:371–379.
4. Mouchet-Mages S, Rodrigo S, Cachia A, et al. Correlations of cerebello-thalamo-prefrontal structure and neurological soft signs in patients with first-episode psychosis. Acta Psychiatr Scand. 2011;123:451–458.
5. Heuser M, Thomann PA, Essig M, Bachmann S, Schröder J. Neurological signs and morphological cerebral changes in schizophrenia: an analysis of NSS subscales in patients with first episode psychosis. Psychiatry Res. 2011;192:69–76.
6. Kong L, Bachmann S, Thomann PA, Essig M, Schröder J. Neurological soft signs and gray matter changes: a longitudinal analysis in first-episode schizophrenia. Schizophr Res. 2012;134:27–32.
fMRI studies
7. Rubia K, Russell T, Bullmore ET, et al. An fMRI study of reduced left prefrontal activation in schizophrenia during normal inhibitory function. Schizophr Res. 2001;52:47–55.
8. Laurens KR, Ngan ET, Bates AT, Kiehl KA, Liddle PF. Rostral anterior cingulate cortex dysfunction during error processing in schizophrenia. Brain. 2003;126:610–622.
9. Elliott R, Ogilvie A, Rubinsztein JS, Calderon G, Dolan RJ, Sahakian BJ. Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biol Psychiatry. 2004;55:1163–1170.
10. Altshuler LL, Bookheimer SY, Townsend J, et al. Blunted activation in orbitofrontal cortex during mania: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:763–769.
11. Arce E, Leland DS, Miller DA, Simmons AN, Winternheimer KC, Paulus MP. Individuals with schizophrenia present hypo- and hyperactivation during implicit cueing in an inhibitory task. Neuroimage. 2006;32:704–713.
12. Silbersweig D, Clarkin JF, Goldstein M, et al. Failure of frontolimbic inhibitory function in the context of negative emotion in borderline personality disorder. Am J Psychiatry. 2007;164:1832–1841.
13. Joyal CC, Putkonen A, Mancini-Marïe A, et al. Violent persons with schizophrenia and comorbid disorders: a functional magnetic resonance imaging study. Schizophr Res. 2007;91:97–102.
14. Kaladjian A, Jeanningros R, Azorin JM, Grimault S, Anton JL, Mazzola-Pomietto P. Blunted activation in right ventrolateral prefrontal cortex during motor response inhibition in schizophrenia. Schizophr Res. 2007;97:184–193.
15. Roth RM, Saykin AJ, Flashman LA, Pixley HS, West JD, Mamourian AC. Event-related functional magnetic resonance imaging of response inhibition in obsessive-compulsive disorder. Biol Psychiatry. 2007;62:901–909.
16. Kaladjian A, Jeanningros R, Azorin JM, et al. Remission from mania is associated with a decrease in amygdala activation during motor response inhibition. Bipolar Disord. 2009;11:530–538.
17. Kaladjian A, Jeanningros R, Azorin JM, Nazarian B, Roth M, Mazzola-Pomietto P. Reduced brain activation in euthymic bipolar patients during response inhibition: an event-related fMRI study. Psychiatry Res. 2009;173:45–51.
18. Welander-Vatn AS, Jensen J, Lycke C, et al. No altered dorsal anterior cingulate activation in bipolar II disorder patients during a Go/No-go task: an fMRI study. Bipolar Disord. 2009;11:270–279.
19. Mazzola-Pomietto P, Kaladjian A, Azorin JM, Anton JL, Jeanningros R. Bilateral decrease in ventrolateral prefrontal cortex activation during motor response inhibition in mania. J Psychiatr Res. 2009;43:432–441.
20. Fleck DE, Kotwal R, Eliassen JC, et al. Preliminary evidence for increased frontosubcortical activation on a motor impulsivity task in mixed episode bipolar disorder. J Affect Disord. 2011;133:333–339.
21. Townsend JD, Bookheimer SY, Foland-Ross LC, et al. Deficits in inferior frontal cortex activation in euthymic bipolar disorder patients during a response inhibition task. Bipolar Disord. 2012;14:442–450.
Appendix 2. Description of Different Neurological Soft Signs (NSS) Scales
| NSS Scales | Subscales | Tests |
|---|---|---|
| Heidelberg Scales | Motor coordination | Ozeretzki’s test |
| Diadochokinesia | ||
| Pronation/supination | ||
| Finger-to-thumb oppostion | ||
| Speech articulation | ||
| Complex motor tasks | Finger-to-nose test | |
| Fist-edge-palm test | ||
| Integration function | Station and gait | |
| Tandem walking | ||
| Two-point discrimination | ||
| Right/left orientation | Right/left orientation | |
| Graphesthesia | ||
| Face-hand sensory test | ||
| Stereognosis | ||
| Neurological Evaluation Scale | Motor coordination signs | Tandem walk |
| Rapid alternating movements | ||
| Finger-thumb opposition | ||
| Finger-nose test | ||
| Motor sequencing signs | Fist-ring test | |
| Fist-edge-palm test | ||
| Ozeretski test | ||
| Sensory integration | Audiovisual integration | |
| Stereognosis | ||
| Graphaesthesia | ||
| Extinction | ||
| Right/left confusion | ||
| 23 items of the Krebs’ | Motor coordination | Rapid alternative of foot |
| rapid alternative of hand | ||
| Finger opposition | ||
| Foot and hand dysrhythmia | ||
| Fist-edge-palm test | ||
| Motor integration | Balance | |
| Romberg | ||
| Finger to nose | ||
| Gait | ||
| Sensory integration | Hand-face test | |
| Graphesthesia | ||
| Constructive apraxia | ||
| Stereognosia | ||
| Right/left recognition |
Appendix 3. Paradigm of Go/No-Go Task
| Study | Author | Year | Designs | Go/No-Go Taska | Handedness | Performing Hand | Contrast Included in ALE | Rate of Go/No-Go |
|---|---|---|---|---|---|---|---|---|
| 7 | K. Rubia | 2001 | Block | Simple task | Right | Right | Go/no-go block—go block | 70%/30% |
| 8 | K. R. Laurens | 2003 | Event | Simple task | Right | Right | Correct no-go trail—correct go trail | 84%/16% |
| 9 | R. Elliott | 2004 | Block | Emotional task | Right | Right | Mix-stimuli Go/no-go block—rest block | 50%/50% |
| 10 | L. L. Altshuler | 2005 | Block | Simple task | Right | Right | Go/no-go block—go block | 50%/50% |
| 11 | E. Arce | 2006 | Block | Simple task | 82% Right | NA | Go/no-go block—go block | 68%/31% |
| 12 | D. Silbersweig | 2007 | Block | Emotional task | 90% Right | Right | Netural go/no-go block—go block | 63%/37% |
| 13 | C. C. Joyal | 2007 | Block | Simple task | Right | NA | Go/no-go block—go block | NA |
| 14 | A. Kaladjian | 2007 | Event | Simple task | Right | NA | Correct no-go trail—correct go trail | 50%/50% |
| 15 | R. M. Roth | 2007 | Event | Simple task | Right | Right | No-go trail—go trail | Gradient |
| 16 | A. Kaladjian | 2009 | Event | Simple task | Right | NA | Correct no-go trail—correct go trail | 50%/50% |
| 17 | A. Kaladjian | 2009 | Event | Simple task | Right | NA | Correct no-go trail—correct go trail | 50%/50% |
| 18 | A. S. Welander-Vatn | 2009 | Block | Simple task | Right | NA | Go/no-go block—rest block | 75%/25% |
| 19 | P. Mazzola-Pomietto | 2009 | Event | Simple task | Right | Right | Correct no-go trail—correct go trail | 50%/505 |
| 20 | D. E. Fleck | 2011 | Event | Simple task | Right | Right | No-go trail—go trail | 83%/17% |
| 21 | J. D. Townsend | 2012 | Block | Simple task | Right | NA | Go/no-go block—go block | 50%/50% |
Note: ALE, activation likelihood estimation; NA, not applicable.
aAll of the go/no-go task instructed the participants to respond to the go signal as soon as possible and suppress response to no-go signal.
References
- 1. Tsuang MT, Gilbertson MW, Faraone SV. The genetics of schizophrenia. Current knowledge and future directions. Schizophr Res. 1991;4:157–171 [DOI] [PubMed] [Google Scholar]
- 2. Tsuang MT, Faraone SV, Lyons MJ. Identification of the phenotype in psychiatric genetics. Eur Arch Psychiatry Clin Neurosci. 1993;243:131–142 [DOI] [PubMed] [Google Scholar]
- 3. Chan RC, Xu T, Heinrichs RW, Yu Y, Wang Y. Neurological soft signs in schizophrenia: a meta-analysis. Schizophr Bull. 2010;36:1089–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan RC, Gottesman II. Neurological soft signs as candidate endophenotypes for schizophrenia: a shooting star or a Northern star? Neurosci Biobehav Rev. 2008;32:957–971 [DOI] [PubMed] [Google Scholar]
- 5. Smith RC, Hussain MI, Chowdhury SA, Stearns A. Stability of neurological soft signs in chronically hospitalized schizophrenic patients. J Neuropsychiatry Clin Neurosci. 1999;11:91–96 [DOI] [PubMed] [Google Scholar]
- 6. Heinrichs DW, Buchanan RW. Significance and meaning of neurological signs in schizophrenia. Am J Psychiatry. 1988;145:11–18 [DOI] [PubMed] [Google Scholar]
- 7. Chen EYH, Hui CLM, Chan RCK, et al. A 3-year prospective study of neurological soft signs in first-episode schizophrenia. Schizophr. Res. 2005;75:45–54 [DOI] [PubMed] [Google Scholar]
- 8. Buchanan RW, Heinrichs DW. The Neurological Evaluation Scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Res. 1989;27:335–350 [DOI] [PubMed] [Google Scholar]
- 9. Bottmer C, Bachmann S, Pantel J, et al. Reduced cerebellar volume and neurological soft signs in first-episode schizophrenia. Psychiatry Res. 2005;140:239–250 [DOI] [PubMed] [Google Scholar]
- 10. Bombin I, Arango C, Buchanan RW. Assessment tools for soft signs-a review of the major scales used in research on neurological soft signs in schizophrenia, with recommendations for future directions of soft sign assessment. Psychiatric Annals. 2003;33:170–180 [Google Scholar]
- 11. Dazzan P, Morgan KD, Orr KG, et al. The structural brain correlates of neurological soft signs in AESOP first-episode psychoses study. Brain. 2004;127:143–153 [DOI] [PubMed] [Google Scholar]
- 12. Negash A, Kebede D, Alem A, et al. Neurological soft signs in bipolar I disorder patients. J Affect Disord. 2004;80:221–230 [DOI] [PubMed] [Google Scholar]
- 13. Boks MP, Liddle PF, Burgerhof JG, Knegtering R, van den Bosch RJ. Neurological soft signs discriminating mood disorders from first episode schizophrenia. Acta Psychiatr Scand. 2004;110:29–35 [DOI] [PubMed] [Google Scholar]
- 14. Whitty P, Clarke M, McTigue O, et al. Diagnostic specificity and predictors of neurological soft signs in schizophrenia, bipolar disorder and other psychoses over the first 4 years of illness. Schizophr Res. 2006;86:110–117 [DOI] [PubMed] [Google Scholar]
- 15. Prikryl R, Ceskova E, Kasparek T, Kucerova H. Neurological soft signs and their relationship to 1-year outcome in first-episode schizophrenia. Eur Psychiatry. 2007;22:499–504 [DOI] [PubMed] [Google Scholar]
- 16. Mouchet-Mages S, Rodrigo S, Cachia A, et al. Correlations of cerebello-thalamo-prefrontal structure and neurological soft signs in patients with first-episode psychosis. Acta Psychiatr Scand. 2011;123:451–458 [DOI] [PubMed] [Google Scholar]
- 17. Keshavan MS, Sanders RD, Sweeney JA, et al. Diagnostic specificity and neuroanatomical validity of neurological abnormalities in first-episode psychoses. Am J Psychiatry. 2003;160:1298–1304 [DOI] [PubMed] [Google Scholar]
- 18. Schröder J, Essig M, Baudendistel K, et al. Motor dysfunction and sensorimotor cortex activation changes in schizophrenia: A study with functional magnetic resonance imaging. Neuroimage. 1999;9:81–87 [DOI] [PubMed] [Google Scholar]
- 19. Thomann PA, Wüstenberg T, Santos VD, Bachmann S, Essig M, Schröder J. Neurological soft signs and brain morphology in first-episode schizophrenia. Psychol Med. 2009;39:371–379 [DOI] [PubMed] [Google Scholar]
- 20. Kong L, Bachmann S, Thomann PA, Essig M, Schröder J. Neurological soft signs and gray matter changes: a longitudinal analysis in first-episode schizophrenia. Schizophr Res. 2012;134:27–32 [DOI] [PubMed] [Google Scholar]
- 21. Thomann PA, Roebel M, Dos Santos V, Bachmann S, Essig M, Schröder J. Cerebellar substructures and neurological soft signs in first-episode schizophrenia. Psychiatry Res. 2009;173:83–87 [DOI] [PubMed] [Google Scholar]
- 22. Ho BC, Mola C, Andreasen NC. Cerebellar dysfunction in neuroleptic naive schizophrenia patients: clinical, cognitive, and neuroanatomic correlates of cerebellar neurologic signs. Biol Psychiatry. 2004;55:1146–1153 [DOI] [PubMed] [Google Scholar]
- 23. Gay O, Plaze M, Oppenheim C, et al. Cortex morphology in first-episode psychosis patients with neurological soft signs. Schizophr. Bull. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hirjak D, Wolf RC, Stieltjes B, Seidl U, Schröder J, Thomann PA. Neurological soft signs and subcortical brain morphology in recent onset schizophrenia. J Psychiatr Res. 2012;46:533–539 [DOI] [PubMed] [Google Scholar]
- 25. Rogowska J, Gruber SA, Yurgelun-Todd DA. Functional magnetic resonance imaging in schizophrenia: cortical response to motor stimulation. Psychiatry Res. 2004;130:227–243 [DOI] [PubMed] [Google Scholar]
- 26. Müller JL, Röder CH, Schuierer G, Klein H. Motor-induced brain activation in cortical, subcortical and cerebellar regions in schizophrenic inpatients. A whole brain fMRI fingertapping study. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:421–426 [DOI] [PubMed] [Google Scholar]
- 27. Kodama S, Fukuzako H, Fukuzako T, et al. Aberrant brain activation following motor skill learning in schizophrenic patients as shown by functional magnetic resonance imaging. Psychol Med. 2001;31:1079–1088 [DOI] [PubMed] [Google Scholar]
- 28. Rubia K, Russell T, Bullmore ET, et al. An fMRI study of reduced left prefrontal activation in schizophrenia during normal inhibitory function. Schizophr Res. 2001;52:47–55 [DOI] [PubMed] [Google Scholar]
- 29. Kaladjian A, Jeanningros R, Azorin JM, et al. Remission from mania is associated with a decrease in amygdala activation during motor response inhibition. Bipolar Disord. 2009;11:530–538 [DOI] [PubMed] [Google Scholar]
- 30. Kaladjian A, Jeanningros R, Azorin JM, Nazarian B, Roth M, Mazzola-Pomietto P. Reduced brain activation in euthymic bipolar patients during response inhibition: an event-related fMRI study. Psychiatry Res. 2009;173:45–51 [DOI] [PubMed] [Google Scholar]
- 31. Silbersweig D, Clarkin JF, Goldstein M, et al. Failure of frontolimbic inhibitory function in the context of negative emotion in borderline personality disorder. Am J Psychiatry. 2007;164:1832–1841 [DOI] [PubMed] [Google Scholar]
- 32. Mazzola-Pomietto P, Kaladjian A, Azorin JM, Anton JL, Jeanningros R. Bilateral decrease in ventrolateral prefrontal cortex activation during motor response inhibition in mania. J Psychiatr Res. 2009;43:432–441 [DOI] [PubMed] [Google Scholar]
- 33. Kaladjian A, Jeanningros R, Azorin JM, Grimault S, Anton JL, Mazzola-Pomietto P. Blunted activation in right ventrolateral prefrontal cortex during motor response inhibition in schizophrenia. Schizophr Res. 2007;97:184–193 [DOI] [PubMed] [Google Scholar]
- 34. Altshuler LL, Bookheimer SY, Townsend J, et al. Blunted activation in orbitofrontal cortex during mania: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:763–769 [DOI] [PubMed] [Google Scholar]
- 35. Arce E, Leland DS, Miller DA, Simmons AN, Winternheimer KC, Paulus MP. Individuals with schizophrenia present hypo- and hyperactivation during implicit cueing in an inhibitory task. Neuroimage. 2006;32:704–713 [DOI] [PubMed] [Google Scholar]
- 36. Elliott R, Ogilvie A, Rubinsztein JS, Calderon G, Dolan RJ, Sahakian BJ. Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biol Psychiatry. 2004;55:1163–1170 [DOI] [PubMed] [Google Scholar]
- 37. Joyal CC, Putkonen A, Mancini-Marïe A, et al. Violent persons with schizophrenia and comorbid disorders: a functional magnetic resonance imaging study. Schizophr Res. 2007;91:97–102 [DOI] [PubMed] [Google Scholar]
- 38. Rosenthal R, DiMatteo MR. Meta-analysis: recent developments in quantitative methods for literature reviews. Annu Rev Psychol. 2001;52:59–82 [DOI] [PubMed] [Google Scholar]
- 39. Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780 [DOI] [PubMed] [Google Scholar]
- 40. Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Laird AR, Fox PM, Price CJ, et al. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lancaster JL, Laird AR, Fox PM, Glahn DE, Fox PT. Automated analysis of meta-analysis networks. Hum Brain Mapp. 2005;25:174–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205 [PubMed] [Google Scholar]
- 44. Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. New York, NY: Thieme; 1988. [Google Scholar]
- 45. Brett M. The MNI Brain and the Talairach Atlas. Cambridge, MA: MRC Cognition and Brain Sciences Unit; 1999. [Google Scholar]
- 46. Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lancaster JL, Rainey LH, Summerlin JL, et al. Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp. 1997;5:238–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Heuser M, Thomann PA, Essig M, Bachmann S, Schröder J. Neurological signs and morphological cerebral changes in schizophrenia: an analysis of NSS subscales in patients with first episode psychosis. Psychiatry Res. 2011;192:69–76 [DOI] [PubMed] [Google Scholar]
- 49. Mostofsky SH, Simmonds DJ. Response inhibition and response selection: two sides of the same coin. J Cogn Neurosci. 2008;20:751–761 [DOI] [PubMed] [Google Scholar]
- 50. Zhou SY, Suzuki M, Hagino H, et al. Volumetric analysis of sulci/gyri-defined in vivo frontal lobe regions in schizophrenia: precentral gyrus, cingulate gyrus, and prefrontal region. Psychiatry Res. 2005;139:127–139 [DOI] [PubMed] [Google Scholar]
- 51. Lyoo IK, Kim MJ, Stoll AL, et al. Frontal lobe gray matter density decreases in bipolar I disorder. Biol Psychiatry. 2004;55:648–651 [DOI] [PubMed] [Google Scholar]
- 52. Meisenzahl EM, Koutsouleris N, Gaser C, et al. Structural brain alterations in subjects at high-risk of psychosis: a voxel-based morphometric study. Schizophr Res. 2008;102:150–162 [DOI] [PubMed] [Google Scholar]
- 53. Giuseppe B, Marco P, Adele Q, et al. Neurological soft signs and cerebral measurements investigated by means of MRI in schizophrenic patients. Neurosci. Lett. 2007;413:82–87 [DOI] [PubMed] [Google Scholar]
- 54. Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nat Neurosci. 2000;3:516–520 [DOI] [PubMed] [Google Scholar]
- 55. Pantelis C, Brewer W. Neuropsychological and olfactory dysfunction in schizophrenia: relationship of frontal syndromes to syndromes of schizophrenia. Schizophr Res. 1995;17:35–45 [DOI] [PubMed] [Google Scholar]
- 56. Pantelis C, Wood SJ, Proffitt TM, et al. Attentional set-shifting ability in first-episode and established schizophrenia: relationship to working memory. Schizophr Res. 2009;112:104–113 [DOI] [PubMed] [Google Scholar]
- 57. Janssen J, Diaz-Caneja A, Reig S, et al. Brain morphology and neurological soft signs in adolescents with first-episode psychosis. Br J Psychiatry. 2009;195:227–233 [DOI] [PubMed] [Google Scholar]
- 58. Maltby N, Tolin DF, Worhunsky P, O’Keefe TM, Kiehl KA. Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: an event-related fMRI study. Neuroimage. 2005;24:495–503 [DOI] [PubMed] [Google Scholar]
- 59. Townsend JD, Bookheimer SY, Foland-Ross LC, et al. Deficits in inferior frontal cortex activation in euthymic bipolar disorder patients during a response inhibition task. Bipolar Disord. 2012;14:442–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Marchand WR, Lee JN, Suchy Y, Johnson S, Thatcher J, Gale P. Aberrant functional connectivity of cortico-basal ganglia circuits in major depression. Neurosci Lett. 2012;514:86–90 [DOI] [PubMed] [Google Scholar]
- 61. Hughes ME, Fulham WR, Johnston PJ, Michie PT. Stop-signal response inhibition in schizophrenia: behavioural, event-related potential and functional neuroimaging data. Biol Psychol. 2012;89:220–231 [DOI] [PubMed] [Google Scholar]
- 62. Thoma P, Koch B, Heyder K, Schwarz M, Daum I. Subcortical contributions to multitasking and response inhibition. Behav Brain Res. 2008;194:214–222 [DOI] [PubMed] [Google Scholar]
- 63. Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, Gillingham S. Effects of focal frontal lesions on response inhibition. Cereb Cortex. 2007;17:826–838 [DOI] [PubMed] [Google Scholar]
- 64. Gottwald B, Mihajlovic Z, Wilde B, Mehdorn HM. Does the cerebellum contribute to specific aspects of attention? Neuropsychologia. 2003;41:1452–1460 [DOI] [PubMed] [Google Scholar]
- 65. Bostan AC, Strick PL. The cerebellum and basal ganglia are interconnected. Neuropsychol Rev. 2010;20:261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Roth RM, Saykin AJ, Flashman LA, Pixley HS, West JD, Mamourian AC. Event-related functional magnetic resonance imaging of response inhibition in obsessive-compulsive disorder. Biol Psychiatry. 2007;62:901–909 [DOI] [PubMed] [Google Scholar]
- 67. Horn NR, Dolan M, Elliott R, Deakin JF, Woodruff PW. Response inhibition and impulsivity: an fMRI study. Neuropsychologia. 2003;41:1959–1966 [DOI] [PubMed] [Google Scholar]
- 68. Goldberg MC, Spinelli S, Joel S, Pekar JJ, Denckla MB, Mostofsky SH. Children with high functioning autism show increased prefrontal and temporal cortex activity during error monitoring. Dev Cogn Neurosci. 2011;1:47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218 [DOI] [PubMed] [Google Scholar]
- 70. Jaafari N, Baup N, Bourdel MC, et al. Neurological soft signs in OCD patients with early age at onset, versus patients with schizophrenia and healthy subjects. J Neuropsychiatry Clin Neurosci. 2011;23:409–416 [DOI] [PubMed] [Google Scholar]
- 71. Jaafari N, de la Cruz LF, Grau M, et al. Neurological soft signs in obsessive-compulsive disorder: two empirical studies and meta-analysis. Psychol Med. 2013;43:1069–1079 [DOI] [PubMed] [Google Scholar]
- 72. Peng ZW, Xu T, Miao GD, et al. Neurological soft signs in obsessive-compulsive disorder: the effect of co-morbid psychosis and evidence for familiality. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:200–205 [DOI] [PubMed] [Google Scholar]
- 73. Zhao Q, Ma YT, Lui SS, et al. Neurological soft signs discriminate schizophrenia from major depression but not bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:72–78 [DOI] [PubMed] [Google Scholar]