Abstract
Objective
Determine the frequency of left ventricular (LV) amyloid in heart failure with preserved ejection fraction (HFpEF).
Background
LV amyloid deposition can cause diastolic dysfunction and HFpEF.
Methods
Autopsy LV specimens from patients with ante-mortem diagnosis of HFpEF without clinically apparent amyloid (n=109) and Control subjects (n=131) were screened with sulfated alcian blue and subsequent Congo red staining with micro-dissection for mass spectrometry-based proteomics to determine amyloid type. Fibrosis was assessed with quantitative whole-field digital microscopy.
Results
Presence of wtTTR amyloid was associated with age at death and male sex; but the age-and sex-adjusted prevalence of wtTTR amyloid was higher in HFpEF than Control (Odds ratio 3.8, 95% confidence interval 1.5–11.3; p=0.03). Among HFpEF patients, moderate or severe interstitial wtTTR deposition, consistent with senile systemic amyloidosis as the primary etiology of HFpEF, was present in five (5%) patients (80% male), with mild interstitial and/or variable severity of intramural coronary vascular deposition in 13 (12%). While wtTTR deposition was often mild, adjusting for age and presence of HFpEF, wtTTR amyloid was associated with more fibrosis (p=0.005) and lower age, sex, and body size-adjusted heart weight (p=0.04).
Conclusion
Given the age- and sex-independent association of HFpEF and wtTTR deposition and an emerging understanding of the pathophysiology of the amyloidoses, the current findings support further investigation of the role of wtTTR in the pathophysiology of HFpEF.
Keywords: Amyloid, Aging, Autopsy, Diastole, Heart failure with preserved ejection fraction, Mass spectrometry-based proteomics, Transthyretin
Background
Cardiac amyloid deposition can cause heart failure (HF) with preserved ejection fraction (HFpEF). While approximately 30 proteins have been linked to cardiac amyloidosis, monoclonal immunoglobin from clonal plasma cells (AL amyloid) and transthyretin (TTR amyloid) are the most common forms.
Transthyretin is a hepatic-derived, homotetrameric transporter protein which exists in equilibrium with TTR monomers. A number of genetic variations in the TTR gene cause hereditary amyloidosis affecting the nerves, heart or both. Cardiac amyloidosis (senile systemic amyloidosis; SSA) due to deposition of amyloid derived from wild type TTR (wtTTR) in the myocardial interstitium and intramural coronary vessels is associated with LV wall thickening, diastolic dysfunction and HFpEF (1–4).
While marked amyloid deposition is present in clinically diagnosed SSA, emerging evidence suggests that cell damage in TTR amyloid is due to deposition of lower molecular weight non-amyloid TTR species which precede detectable TTR amyloid fibril deposition (5–10). Variant TTR is structurally less stable and more prone to amyloid fibril formation (7), but the factors leading to wtTTR amyloid deposition have yet to be fully elucidated. In vitro studies suggest that oxidative modification of wtTTR increases its amyloidogenicity (7). As aging is associated with increased oxidative stress, oxidative modification of wtTTR may account for the association of the SSA with age.
HFpEF accounts for half of HF patients, and its prevalence increases dramatically with age and female sex (11). Clinical suspicion of amyloid in HFpEF patients may be low as they often have alternative explanations, such as chronic hypertension, for LV wall thickening and diastolic dysfunction.
Development of novel compounds that may attenuate TTR amyloid cardiac deposition has heightened interest in the significance of TTR amyloid as a cause of HF (12–14). While autopsy rates in elderly HF patients are low (15), autopsy specimens provide the optimal opportunity to assess the prevalence of unsuspected amyloid deposition in HFpEF, as endomyocardial biopsy is infrequent in elderly HFpEF patients (16) and fat aspirate has limited sensitivity for detection of wtTTR amyloid (17–19).
Accordingly, we sought to determine the frequency, extent and type of cardiac amyloid deposition in patients with an ante-mortem diagnosis of HFpEF (but not of amyloid) who subsequently underwent autopsy, as compared with age appropriate Control autopsy subjects.
Methods
The study was approved by Mayo Clinic Institutional Review Board and the Mayo Biospecimens Committee. Only autopsy specimens with consent for use of specimens for research purposes were utilized.
Identification of HFpEF cases with autopsy
HFpEF subjects were identified using cohorts previously assimilated from administrative data sets. Consecutive patients admitted to Mayo Clinic hospitals in Rochester, Minnesota, between January 1, 1986 and December 31, 2001 with a discharge diagnosis of HF confirmed by both the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 428 and the diagnosis related-group (DRG) code 127 for HF (n=6440), were identified as previously described (20,21).
This patient list was crossed to the Mayo Tissue Registry (MTR) (15,22) to identify patients who had undergone autopsy (n=441, 6.8%) (23). Characteristics, including EF distribution, of patients with and without autopsy were similar except for a slightly higher rate of hypertension and coronary disease in patients with autopsy (Supplemental Table 1). Many autopsies were restricted in extent (neurologic only). Thus, of the 441 patients with autopsy, 331 had EF measured at HF diagnosis and of these, 75 had EF >40% at HF diagnosis and autopsy.
To supplement this cohort, additional patients hospitalized with HF (ICD-9-CM code 428) at Mayo affiliated hospitals from 2003–2010 were identified and crossed with the MTR, yielding 25 additional cases with EF >40% at HF diagnosis and cardiac autopsy. Additionally, outpatients with HF (ICD-9-CM code 428) diagnosed between March, 1980 and July, 2009 who were residents of Olmsted County MN and had HF confirmed by medical record review (Framingham criteria) (24) were crossed with the MTR, yielding an additional 12 cases with EF >40% at HF diagnosis and autopsy, for a total of 112 cases with ante-mortem diagnosis of HFpEF and cardiac specimens from autopsy.
Medical records of these subjects were reviewed and any mention of definitive or probable cardiac amyloid in the clinical notes or reference to echocardiographic features of amyloid or cardiac biopsy findings suggestive of amyloid was considered pre-mortem suspicion. Three HFpEF cases had ante-mortem diagnosis of amyloidosis. Importantly, the amyloid type in all three patients was light chain type. Thus, exclusion of these patients does not affect estimates of the prevalence of wtTTR amyloid in HFpEF. As ante-mortem suspicion of cardiac amyloidosis could influence the decision to perform an autopsy, these three patients were excluded, leaving 109 patients with HFpEF and no clinical suspicion of cardiac amyloidosis prior to death and autopsy.
Identification of Control subjects
After review of the distribution of age at death and sex of the HFpEF autopsy cases, a minimum of 20 subjects per age at death decade, ≥40 years of age without ante-mortem HF diagnosis and who died of non-cardiovascular causes and had undergone autopsy between January 3, 1971 and October 12, 2010 were identified from the MTR database (n=131) to serve as age and sex appropriate Control subjects. While not formally age matched in a 1:1 ratio (where equal numbers of Control and HFpEF patients of same age would be studied, due to the small number of HFpEF patients in some age groups), appropriate numbers of Control subjects were selected to allow statistical assessment of age dependence of findings. Medical record review was performed to define clinical characteristics and exclude ante-mortem diagnosis of amyloid in Control patients.
Autopsy data and tissue processing
Finalized autopsy reports were reviewed and included assessment of absolute and % expected heart weight (percent of normative values based on age, sex and body size(22)), severity of coronary artery disease (semi-quantitative ordinal scale (0–4) reflecting no (0) to critical (4) atherosclerosis) and gross and microscopic hypertrophy, fibrosis or infarction. A sum coronary artery score was calculated by adding the individual artery scores (left main, left anterior descending, circumflex and right coronary arteries).
For this study, all histologic analysis was performed by a highly experienced cardiovascular pathologist (W.D.E.) who was blinded to clinical information. Hemotoxylin and eosin (H&E) stained samples from the LV inferior wall were reviewed to determine the quality of the specimen. If an infarct involved >50% of the inferior LV section, an adjacent non-infarcted section was chosen. Sections were then stained with sodium sulfate alcian blue (SAB) and counterstained with Van Gieson (25) to screen for the presence of amyloid. Amyloid positive slides were then stained with Congo red for confirmation and mass spectroscopy (MS)-based proteomics for determining the type of amyloid. Extent of interstitial amyloid deposition (green with SAB) was assessed semi-quantitatively as mild, moderate or severe, corresponding to <25%, 25–50% and >50% of myocardial surface area, with vascular involvement similarly graded and corresponding to <33%, 33–67 and >67% of vascular circumference (26).
Microdissection and MS-based proteomic analysis
Amyloid deposits were isolated with laser microdissection of 10 µm thick Congo red stained slides (performed by A.D.), subjected to trypsin digestion and analyzed by liquid chromatography electrospray ionization tandem mass spectrometry (27). For protein sequence identification, raw data were analyzed using 3 algorithms (SEQUEST, Mascot, and X!Tandem), which collectively provided coverage for all known TTR amyloid mutations. Subsequently, amyloid type and genotype were determined according to spectral matching as previously described (27). Mass spectroscopy (versus immunohistochemistry) was used as it allows screening for multiple proteins simultaneously (both known and unknown). We previously validated mass spectrometry in comparison to immunohistochemistry and the gold standard clinicopathologic criteria (27). When tissue quantity was adequate, mass spectrometry was superior to immunohistochemistry in identifying amyloid type with sensitivity and specificity over 98% (27). Of note, prior reports had shown lower sensitivity of mass spectrometry due to inadequate tissue specimen rather than misclassification (28).
Quantitative assessment of fibrosis
Because stains (Masson trichrome or picrosirus red) used to assess fibrosis in standard autopsy procedures can cause difficulty distinguishing between collagen and amyloid fibrils, SAB-stained sections (amyloid stains green and collagen stains red) were scanned using whole-field digital microscopy (WFDM) and the entire slide analyzed (blinded to group and patient characteristics) with an automated quantitative analysis software system (Definiens®) using a custom designed rule set to quantify fibrosis (red) as a percent of total tissue area (See Supplemental Methods).
Electrocardiograms
All available ECGs proximal to death were reviewed and voltages measured manually (S.F.M.). LV hypertrophy (LVH, Cornell and Sokolow criteria) and low voltage (all limb lead voltages <5 mm or all precordial voltages <10 mm (29)) were assessed. Ventricular paced QRS complexes were excluded from voltage analysis.
Statistical analysis
Data are presented as mean ± standard deviation or % frequency. Group comparisons were performed by unpaired Student’s T test for continuous variables, or Chi square test for categorical variables. Multivariable nominal logistic regression was used to elucidate the association of wtTTR (present or absent) with group (HFpEF vs Control) adjusting for age or sex. Multivariable standard least squares linear regression was used to elucidate the association between extent of fibrosis or heart weight and wtTTR presence adjusting for pertinent covariates (HF presence, age). All statistical analyses were 2-tailed and performed using JMP® software (version 9.0.1). A p-value <0.05 was considered statistically significant.
Results
Subject characteristics
Consistent with previous community-based reports, HFpEF subjects were elderly and predominantly women (Table 1). There was no difference in sex distribution between the HFpEF and Control groups. HFpEF subjects had more cardiovascular comorbidities. As Controls were not formally age-matched (equal numbers of patients of comparable age), as a group, Control patients were younger due to larger numbers of patients in their 40’s and 50’s (Figure 1). Compared to female HFpEF patients, male HFpEF patients were slightly but not significantly younger and had more coronary artery disease (Supplemental Table 2).
Table 1.
Patient Characteristics
| Control | HFpEF | p value | |
|---|---|---|---|
| N | 131 | 109 | |
| Male | 37% | 43% | 0.31 |
| Age at heart failure event, years | NA | 74±13 | |
| Age at death, years | 69 ± 16 | 76±13 | 0.002 |
| Hypertension | 27% | 78% | <0.001 |
| Diabetes mellitus | 10% | 42% | <0.001 |
| Clinical diagnosis of coronary disease | 0% | 62% | <0.001 |
| Ejection fraction at heart failure event, % | NA | 59±9 | |
| Autopsy Findings | |||
| Weight at autopsy, kg | 73±25 | 81±27 | 0.02 |
| Height at autopsy, cm | 165±10 | 166±10 | 0.31 |
| Body mass index at autopsy, kg/m2 | 26±8 | 29±9 | 0.02 |
| Body surface area at autopsy, m2 | 1.8±0.3 | 1.9±0.3 | 0.02 |
| Heart weight at autopsy, g | 343±77 | 541±163 | <0.001 |
| Percent expected heart weight, % | 116±21 | 176±50 | <0.001 |
| Gross left ventricular hypertrophy | 15% | 68% | <0.001 |
| Gross right ventricular hypertrophy | 9% | 47% | <0.001 |
| Gross infarct (old) | 2% | 43% | <0.001 |
| Gross fibrosis | 1% | 18% | <0.001 |
| Gross infarct (new) | 1% | 11% | <0.001 |
| Left ventricular dilatation | 3% | 39% | <0.001 |
| Right ventricular dilatation | 11% | 48% | <0.001 |
| Atrial dilatation | 7% | 55% | <0.001 |
| Microscopic fibrosis | 38% | 57% | 0.005 |
| Microscopic hypertrophy | 14% | 31% | 0.002 |
| Microscopic infarct | 1% | 17% | <0.001 |
| Coronary disease score | 6±3 | 11±4 | <0.001 |
| % Fibrosis (WFDM) | 7.6±3.4% | 12.6±8.9% | <0.001 |
Abbreviations: WFDM, whole-field digital microscopy
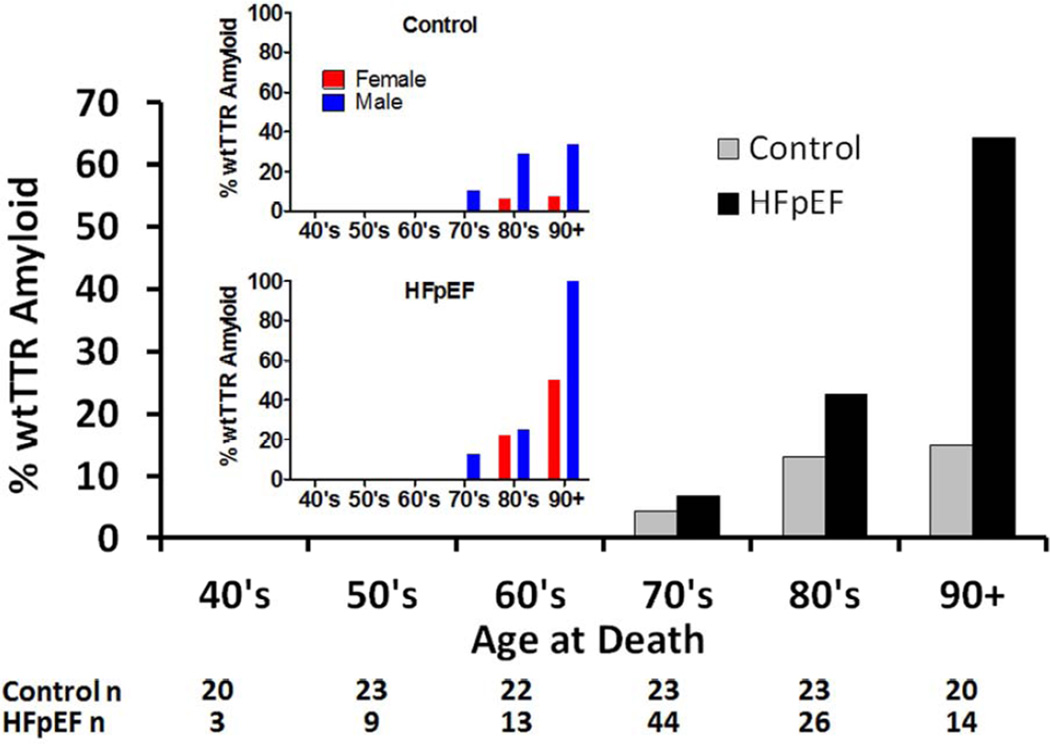
Figure 1. Prevalence of left ventricular wtTTR amyloid in Control (n=131) and HFpEF (n=109) patients by age.
Insert shows the prevalence of left ventricular wtTTR amyloid in males and females by age in Control (top insert panel) and HFpEF (bottom insert panel).
Autopsy findings
At autopsy, HFpEF patients had higher BMI compared with Control (Table 1). HFpEF subjects had more cardiac hypertrophy (by heart weight and microscopic analysis), chamber dilatation, coronary disease and evidence of old or new infarction. HFpEF patients had more gross and microscopic fibrosis as assessed by standard autopsy procedures and by quantitative analysis (WFDM) of SAB-stained sections. Compared to female HFpEF patients, male HFpEF patients were larger, had higher heart weight but similar % expected heart weight, a higher prevalence of old infarction on gross and microscopic inspection and more severe coronary artery disease (Supplemental Table 2).
Prevalence and type of left ventricular amyloid in Control and HFpEF
Overall, seven (5%) of the 131 Control and 21 (17%) of the 109 HFpEF patients without ante-mortem suspicion of amyloid had LV amyloid deposition at autopsy. Notably, Control subjects were seven years younger than HFpEF. Adjusting for age at death and sex, amyloid (any type) was more common in patients with HFpEF than Controls (Table 2).
Table 2. Prevalence of amyloid deposition is increased in HFpEF after adjustment for age and sex.
The odds ratio for amyloid (with 95% confidence intervals (CI)) and significance for each covariate adjusting for the other covariates in the model are shown.
| Odds Ratio | 95% CI | p | |
|---|---|---|---|
| Any Amyloid | |||
| Age at Death (per year) | 1.2 | 1.1–1.2 | <0.001 |
| Male sex (vs female) | 2.9 | 1.1–7.8 | 0.005 |
| HFpEF (vs Control) | 3.8 | 1.5–11.3 | 0.03 |
| wtTTR Amyloid | |||
| Age at Death (per year) | 1.18 | 1.11–1.28 | <0.001 |
| Male sex (vs female) | 4.75 | 1.69–14.7 | 0.004 |
| HFpEF (vs Control) | 3.29 | 1.18–10.27 | 0.02 |
Abbreviations: CI, confidence interval
All seven Control patients with amyloid had wtTTR amyloid. Two (2%) of the 83 females and five (10%) of the 48 males had wtTTR amyloid.
Among the HFpEF patients, two (2%) patients had unknown amyloid type due to inadequate specimens for microdissection, one (1%) had light chain amyloid, and 18 (17%) had wtTTR amyloid, involving nine (14%) of the 62 females and nine (19%) of the 47 males. The two patients with inadequate specimens for microdissection had mild interstitial (one) or mild focal non-obstructive vascular (one) deposition of amyloid.
Adjusting for age at death and sex, wtTTR amyloid was more common in patients with HFpEF than Controls (Figure 1 and Table 2).
wtTTR amyloid: deposition site and severity in Control and HFpEF
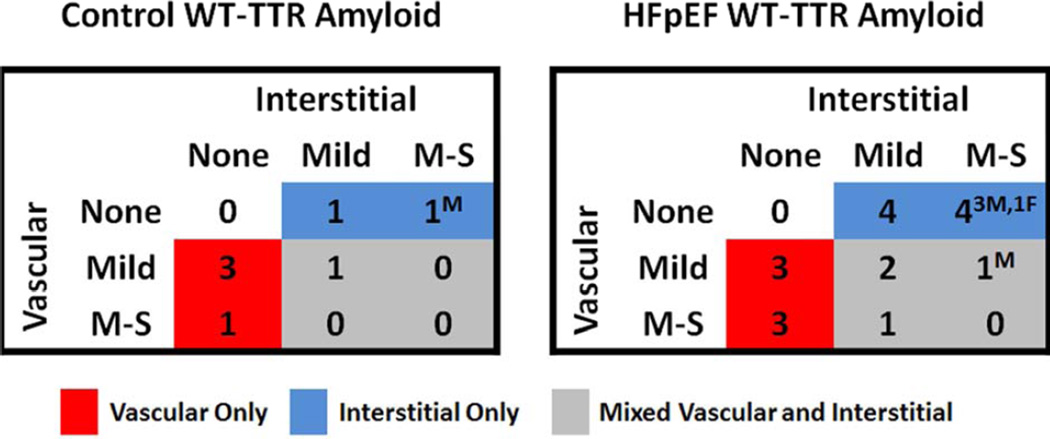
Representative examples of mild, moderate and severe interstitial or coronary vascular amyloid deposition are shown in Figure 2. Of the seven Control patients with wtTTR amyloid, deposition was vascular-only in four (57%), interstitial-only in 2 (29%) and both vascular and interstitial in one (14%) (Figure 3). The worst severity of deposition was mild in five (71%) patients and moderate-severe in two (29%) patients (one vascular and one interstitial). Only one Control patient (male) had more than mild interstitial amyloid deposition.
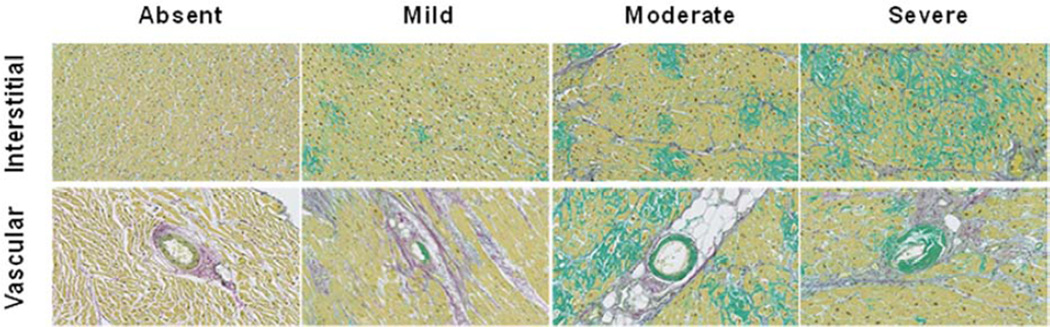
Figure 2. Semi-quantitative characterization of severity of amyloid deposition in sulfated alcian blue (SAB) stained left ventricular sections.
Representative examples demonstrate the extent of interstitial (top panels) and vascular (bottom panels) amyloid deposition (green), assessed semi-quantitatively as mild, moderate or severe.
Figure 3. Site and severity of amyloid deposition in Control and HFpEF patients with wtTTR amyloid.
The number of patients with no (none), mild, or moderate or severe (M–S) interstitial and vascular wtTTR amyloid is shown. Patients with only vascular deposition (red shading), only interstitial (blue shading) or mixed (grey shading) deposition are illustrated. Sex (M, male or F, female) of patients with M–S deposition is provided.
Of the 18 HFpEF patients with wtTTR amyloid, deposition was vascular-only in six (33%), interstitial-only in 8 (45%) and both vascular and interstitial in four (22%) (Figure 3). The worst severity of deposition was mild in nine (50%) patients and moderate-severe in nine (50%) patients (three vascular-only, four interstitial-only and two vascular and interstitial). Thus only five (5%; four males and one female) HFpEF patients had moderate or severe interstitial amyloid deposition consistent with HF due to SSA.
wtTTR amyloid: association with fibrosis and hypertrophy
Representative examples of SAB-stained LV sections from HFpEF patients with mild (<5%), moderate (5–10%) and severe (>15%) myocardial fibrosis along with the corresponding definition of fibrosis versus myocardial tissue by the Definiens® analysis program are shown in Figure 4. In the entire study population, adjusting for age and the presence of HFpEF, patients with wtTTR amyloid had more fibrosis as assessed by WFDM than those without amyloid. Adjusting for the presence of HFpEF, patients with wtTTR amyloid had lower age, sex and body size adjusted heart weight (% expected heart weight) than patients without wtTTR amyloid (Table 3).
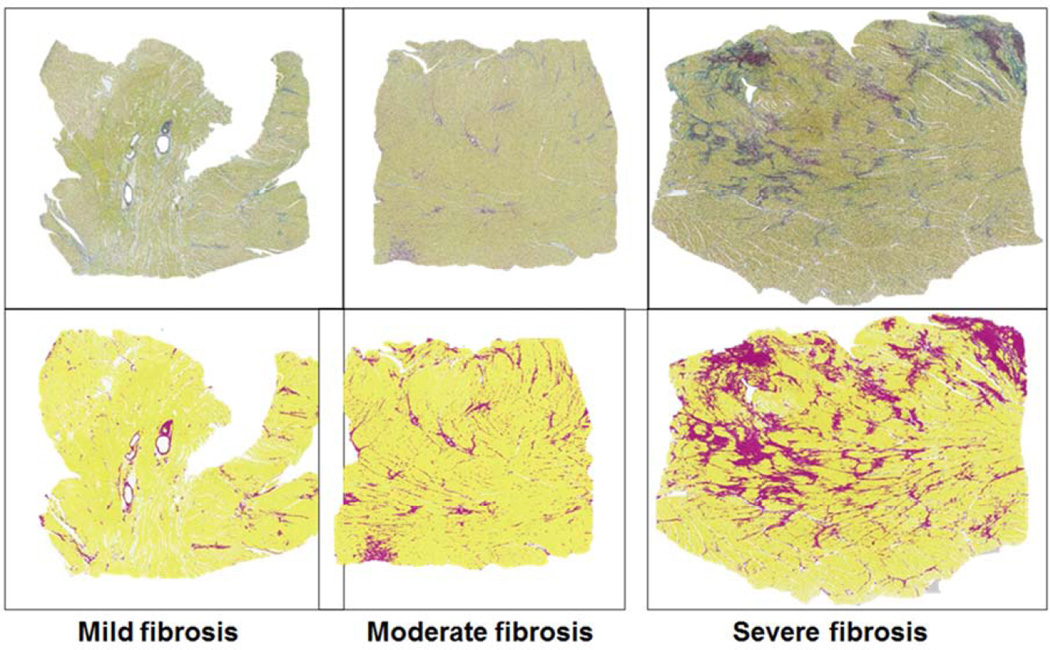
Figure 4. Assessment of LV fibrosis by whole-field digital microscopy and quantitative analysis in SAB-stained left ventricular (LV) sections.
Representative examples of SAB-stained LV sections from HFpEF patients with mild (<5%), moderate (5–10%) and severe (>15%) myocardial fibrosis (ratio of total fibrosis area (deep red) to total tissue area (yellow)) are shown (top panels), along with the corresponding definition of fibrosis (magenta) and myocardial tissue (yellow) by the Definiens® analysis program (bottom panels). Details of the custom analysis algorithm are provided in the online supplement.
Table 3. TTR amyloid deposition is associated with more LV fibrosis and lower heart weight after adjustment for age and presence of HFpEF.
The change in percent fibrosis or heart weight (parameter estimate and standard error (SE)) associated with each covariate and significance of the association adjusting for the other covariates in the model are shown.
| Regression coefficient | SE | p value | |
|---|---|---|---|
| Percent Fibrosis (WFDM) | |||
| Age at death (per 10 yrs) | 0.5% | 0.3% | 0.09 |
| HFpEF (vs Control) | 2.1% | 0.4% | <0.001 |
| wtTTR present (vs absent) | 2.0% | 0.7% | 0.005 |
| Percent Expected Heart Weight | |||
| HFpEF (vs Control) | 32.2% | 2.5% | <0.001 |
| wtTTR present (vs absent) | −8.2% | 4.0% | 0.04 |
Abbreviations: SE, standard error; WFDM, whole-field digital microscopy
In sensitivity analysis, when analysis was confined to the HFpEF groups only, wtTTR was associated with more fibrosis (5.6%; SE: 2.5%; p=0.02) and lower % expected heart weight (−31.9%; SE: 13.0%; p=0.02) compared with no TTR amyloid
wtTTR amyloid: impact of age at onset of HFpEF on prevalence of wtTTR amyloid
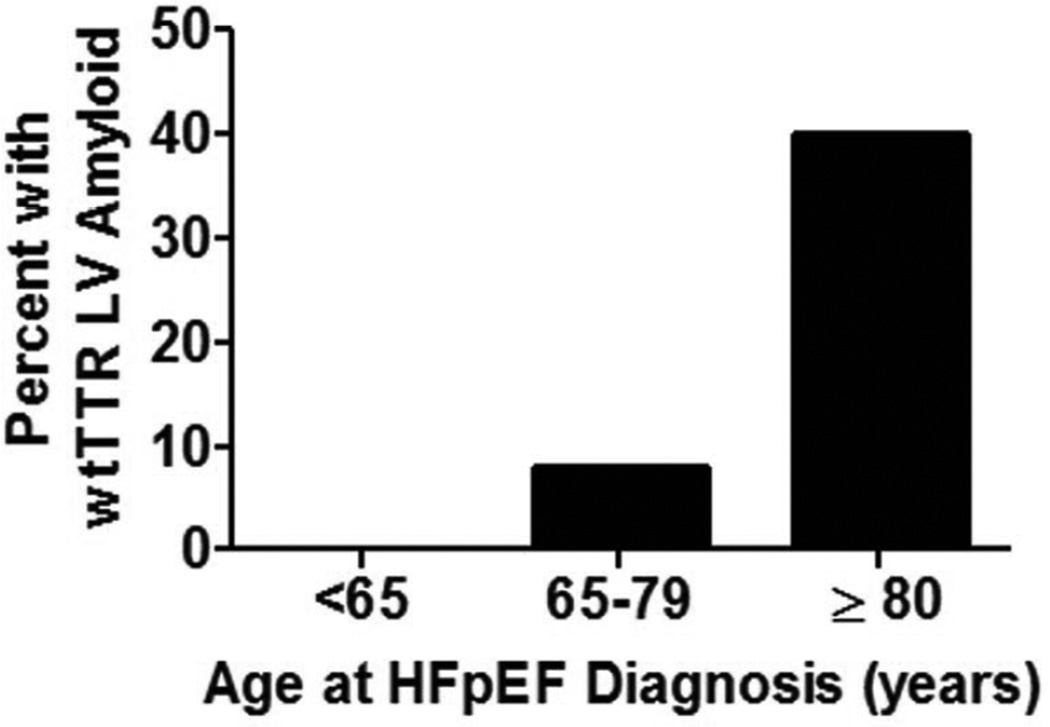
Left ventricular wtTTR amyloid deposition was not observed at autopsy in patients diagnosed with HFpEF before 65 years of age; but was very common at autopsy in those who were ≥80 years of age at the time of HFpEF diagnosis (Figure 5). Adjusting for sex, wtTTR amyloid was associated with age at HFpEF onset (p<0.001).
Figure 5. Prevalence of any or TTR amyloid at autopsy according to age at HFpEF diagnosis.
Frequency of wtTTR amyloid by in patients diagnosed with HFpEF at age <65 (n=21), age 65–79 (n=53) or age ≥ 80 (n=35).
HFpEF subjects with and without wtTTR cardiac amyloid
Compared to those without wtTTR, HFpEF subjects with wtTTR amyloid were older at HF diagnosis and death but had similar sex distribution and comorbidities (Table 4). HFpEF patients with wtTTR amyloid had smaller weight hearts, more fibrosis by WFDM and more microscopic infarcts than HFpEF patients without wtTTR amyloid. When available, the ECG proximal to death showed no difference in the prevalence of low voltage in HFpEF patients with or without wtTTR amyloid, with only 8% of wtTTR amyloid HFpEF patients showing low voltage.
Table 4.
Characteristics of HFpEF patients without or with wtTTR amyloid
| wtTTR Absent | wtTTR Present |
p value | |
|---|---|---|---|
| n | 88 | 18 | |
| Male | 43% | 43% | 0.98 |
| Age at heart failure event, years | 72±13 | 86±7 | <0.001 |
| Age at death, years | 74±12 | 89±8 | <0.001 |
| Hypertension | 78% | 78% | 0.95 |
| Diabetes mellitus | 44% | 33% | 0.39 |
| Coronary artery disease | 62% | 61% | 0.94 |
| Ejection fraction at heart failure event, % | 59±9 | 59±9 | 1.0 |
| Autopsy Findings | |||
| Heart weight at autopsy, g | 557±164 | 470±140 | 0.04 |
| Percent expected heart weight | 182±53 | 152±28 | 0.02 |
| Gross left ventricular hypertrophy | 68% | 67% | 0.90 |
| Gross right ventricular hypertrophy | 50% | 33% | 0.20 |
| Gross infarct (old) | 44% | 39% | 0.67 |
| Gross fibrosis | 19% | 11% | 0.41 |
| Gross infarct (new) | 11% | 11% | 0.98 |
| Left ventricular dilatation | 39% | 39% | 0.98 |
| Right ventricular dilatation | 49% | 44% | 0.73 |
| Atrial dilatation | 55% | 56% | 0.94 |
| Microscopic fibrosis | 53% | 72% | 0.14 |
| Microscopic hypertrophy | 27% | 44% | 0.15 |
| Microscopic infarct | 14% | 33% | 0.04 |
| Coronary disease score | 11±4 | 11±4 | 0.66 |
| % Fibrosis (WFDM) | 12±7 | 17±14 | 0.02 |
| ECG findings closest to Death | |||
| n | 66 | 12 | |
| Low QRS voltage (limb) | 5% | 8% | 0.58 |
| Low QRS voltage (precordial) | 3% | 8% | 0.38 |
Discussion
To our knowledge, this is the first study to examine the age- and sex-adjusted prevalence of LV amyloid deposition in HFpEF and was made possible by the unique resources afforded by Mayo Clinic HFpEF data bases and Tissue Registry. Rigorous methodology was used with blinded systematic review of Control and HFpEF LV specimens by a highly experienced cardiovascular pathologist, state-of-the-art pathology methodologies to determine amyloid type and fibrosis severity and statistical analysis of age and sex specific prevalence rates. Significant (moderate or severe) interstitial wtTTR amyloid deposition consistent with a diagnosis of SSA as the major cause of HFpEF was uncommon (5%) with the expected male (80%) predominance. However, the age and sex adjusted prevalence of wtTTR LV amyloid deposition was higher in HFpEF than Controls due to the identification of mild interstitial and/or variable severities of coronary vascular wtTTR deposition. Further, adjusting for age and the presence of HFpEF, wtTTR amyloid deposition, though often mild, was associated with more fibrosis and lower age, sex and body size adjusted heart weights.
The higher prevalence of wtTTR amyloid in HFpEF patients indicates a form of ascertainment bias whereby the HFpEF autopsy population is enriched for the presence of amyloid. This ascertainment bias could be due to bias towards obtaining autopsy owing to ante-mortem clinical suspicion of amyloid, amyloid deposition predisposing to HFpEF or HFpEF predisposing to amyloid deposition. Given the potent age association of both HFpEF and wtTTR amyloid, the age and sex independent association of HFpEF and wtTTR deposition and the emerging understanding of the pathophysiology of the amyloidoses (below), the current findings support the need for further investigation of the role of wtTTR in HFpEF.
Prevalence of amyloid in previous autopsy studies
Previous autopsy studies investigating the prevalence of cardiac amyloid have utilized varying patient selection criteria and often distinction of location, extent and type of amyloid have been lacking, with variable methods for determining the type of amyloid.
A Finnish population-based autopsy study of persons dying at age >84 (n=256) found LV TTR amyloid deposition (mostly mild) in 25% (33% of males and 23% of females) where TTR amyloid was confirmed by immunohistochemistry and genetic analysis for one TTR variant (2). TTR amyloid deposition was associated with age but not significantly with sex although males had more severe deposition. While clinically recognized SSA is well known to display a male predominance (17,30–32), in autopsy studies where detection of milder TTR deposition is possible, the association of TTR amyloid deposition with sex has varied.(2,4,33). We found that TTR amyloid was more common in males in both the Control and HFpEF populations after adjusting for age. However, both the male and female HFpEF patients had higher prevalence of TTR amyloid than Controls.
Hodkinson et al (4) screened for cardiac (atrial and LV) amyloid deposits at autopsy in an unselected series of 244 hospitalized geriatric patients who died of any cause and underwent autopsy. Amyloid deposition (screening with SAB and confirmation by Congo red staining) was characterized as atrial-only or LV in location. Any (atrial or LV) amyloid was more common in women (56%) than men (38%). Left ventricular amyloid was present in 14 (14.5%) of 96 men and was moderate or severe in 11(11.5%). Left ventricular amyloid was present in 24 of 148 (16.2%) women, with moderate or severe LV amyloid present in 10 (6.7%). The presence of any amyloid was associated with clinical HF or atrial fibrillation during the hospitalization but amyloid type and type of HF (HFpEF vs HF with reduced EF, HFrEF) were not specified.
Lie et al (33) examined consecutive cardiac autopsy specimens from persons dying from any cause (48% cardiovascular death) at age ≥90 years. Details on the severity of LV or coronary deposition, amyloid type or association with HF were not provided. Cardiac amyloid was detected in 66% of men and 65% of women but was restricted to the atria in two-thirds of patients, suggesting a prevalence of LV amyloid in approximately 22% of patients, which was lower than observed in HFpEF patients in their 90’s in the current study.
A study of 52,370 clinically reported autopsies showed a prevalence of presumed SSA (age >60, primarily cardiac involvement) of 0.42% in non-Hispanic whites and 1.6% in blacks (23,34). Immunohistochemistry for TTR with DNA analysis in a subset of SSA patients was performed and showed that the increased prevalence of SSA in blacks was actually related to late onset hereditary amyloidosis due to the V122I TTR variant, rather than wtTTR. The prevalence of milder amyloid deposition was not reported. The current study utilized MS characterization of the TTR amyloid to confirm wtTTR, and the patients were all Caucasian.
Potential for bias to obtain autopsy in amyloid positive HFpEF patients
The higher prevalence of wtTTR amyloid in HFpEF patients could be due to bias toward obtaining autopsy in patients in whom the presence of amyloid was suspected ante-mortem or due to more rigorous review for amyloid in HFpEF specimens. This is unlikely because review of medical records was specifically performed to determine if amyloid was suspected prior to death, the clinical characteristics of autopsied and non-autopsied HF patients were similar, the ECG changes were not suggestive of amyloid in HFpEF patients and the extent of amyloid was mild or limited to the intramural coronary vessels in a majority of patients, making it less likely to be suspected clinically on the basis of echocardiography. Screening of Control and HFpEF specimens for amyloid was performed blinded to clinical information and, as outlined above, the prevalence of amyloid in Control specimens was consistent with previous studies.
wtTTR amyloid deposition as a potential contributor to development of HFpEF
The pathophysiology of HFpEF is complex but hypertrophic and fibrotic LV remodeling, diastolic dysfunction, subtle systolic dysfunction, vascular dysfunction, adverse ventricular-vascular coupling and impaired cardiovascular reserve function are thought to contribute prominently to the pathophysiology of HFpEF (35). Structural and functional changes within aging myocytes, which negatively affect cellular plasticity, have yet to be explored in detail. Comorbidities (hypertension, diabetes, vascular disease and atrial fibrillation) are common in patients with HFpEF.
Senile systemic cardiovascular amyloidosis (SSA) is conventionally diagnosed when marked deposition producing wall thickening is present, and it has been assumed that such marked deposition is required to produce cardiac dysfunction (1,36). However, emerging evidence from in vitro and in vivo studies supports cytotoxic effects by lower molecular weight species that may be intermediates in the pathway of amyloid fibril deposition (5–10). These adverse effects occur before tissue deposition ensues and are associated with inflammation and cell death (6,10). These cytotoxic effects were observed with mutant and wild type TTR in human cardiomyocyte cell culture studies (6). Oxidized or carbonylated wtTTR has a higher propensity to form aggregates and fibrils and was cytotoxic in human cardiomyocytes (7). Further, in a murine model of human wtTTR overexpression, up-regulation of genes encoding for inflammatory and immune response pathways was observed in the heart prior to detectable amyloid deposition (10).
In asymptomatic carriers for familial amyloid polyneuropathy (PAN), aggregated, nonfibrillar, Congo Red birefringence negative TTR deposition was reported in association with early nerve damage as evidenced by inflammatory markers (8). In the current study, the presence of wtTTR amyloid was associated with more fibrosis and less hypertrophy, and these findings may support the presence of inflammation and cell death prior to marked amyloid deposition. Amyloid fibrils were also present in intramural coronary vessels. Coronary vascular dysfunction can contribute to diastolic dysfunction and/or ischemic injury and contribute to HFpEF pathophysiology (35).
HFpEF as a potential cause of wtTTR deposition
Alternatively, the increased prevalence of amyloid deposition in failing hearts may reflect a pro-oxidative state related to the neurohumoral activation and metabolic derangements seen in elderly HFpEF patients, as oxidative modification of wtTTR increases its cytotoxicity and alters its kinetic stability and amyloidogenicity (7). Both advanced age and the HF state are associated with increased oxidative stress, as are several of the comorbidities (chronic kidney disease, hypertension and vascular disease) commonly associated with HFpEF (37). Oxidative stress and associated redox changes has been implicated in the pathophysiology of HF (38).
Using a transgenic model of human wtTTR overexpression, Buxbaum et al (10) found that failure of hepatic proteostatic capacity enhanced age-related deposition of wtTTR in the heart. These data suggest that increased production and abnormal hepatic function may contribute to wtTTR amyloid deposition in the heart. Whether chronic hepatic congestion due to HF can alter hepatic proteostatic function is unclear.
Diagnostic and therapeutic implications
Currently, in elderly subjects presenting with HFpEF symptoms, the diagnosis of TTR amyloidosis is seldom entertained and HF is usually attributed to the underlying comorbidities. The variable yield of abdominal fat pad or rectal biopsies (17,18,19) limit the ability to diagnose wtTTR amyloid non-invasively, and the lack of effective therapy discourages use of endomyocardial biopsy. However, novel TTR proteostatic compounds and TTR gene silencing therapeutics offer the hope of specific preventative or disease-slowing therapy for TTR amyloidoses (12–14,39). As use of such therapeutics only in patients with advanced, clinically recognized SSA may have minimal impact, the current findings underscore the need for a better understanding of the natural history of wtTTR deposition and its impact on cardiac structure and function.
Limitations
Sample size was reduced by requirement for consent to use specimens for research. The autopsy rate observed among HF patients (Supplemental Table 1) is lower than reported nationally (~8%), but both advanced age and HF diagnosis are known to be associated with reduced autopsy rates (15). The present study is largely descriptive and cannot establish whether amyloid deposition is a contributor to or result of HFpEF pathophysiology. The clinical significance of amyloid deposits in elderly HFpEF subjects should be interpreted in the context of an autopsy study and may not be generalizable to younger HFpEF patients. Due to the limited statistical power, we were unable to adjust for comorbidities and other confounders that may influence amyloid deposition. The relationship between HFpEF and amyloid deposition could be explained by these confounders. We are unable to characterize the relative prevalence of wtTTR vs variant TTR amyloid in HFpEF patients of other ethnic groups.
Conclusions
While marked wtTTR amyloid deposition consistent with a diagnosis of SSA was an infrequent cause of HFpEF in this autopsy series, overall, the age- and sex-adjusted prevalence of wtTTR amyloid deposition in the LV and/or intramural coronary vessels was greater in HFpEF than in Controls and was associated with more fibrosis and lower cardiac mass, as adjusted for age, sex and body size. While this study cannot establish whether amyloid deposition is a contributor to or result of HFpEF pathophysiology, the findings and the emergence of disease modifying drugs for TTR amyloid suggest the need for further investigation of the natural history of wtTTR amyloid and its role in the pathophysiology of HFpEF.
Supplementary Material
Acknowledgement
This manuscript is dedicated to the memory of Dr. David C. Utz.
Grant Support: This study (HL72435 and HL 55502) and/or the investigators (MMR, U01HL 84907 and PO1HL 76611; SFM T32-HL0711 and TL1 TR000137) were supported by the National Institutes of Health and Mayo Clinic. Funds for technician support and mass spectrometry-based proteomics characterization were supplied by a grant from FoldRx®, a fully owned subsidiary of Pfizer®.
Abbreviations
- EF
ejection fraction
- H&E
hemotoxylin and eosin
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- ICD
International Classification of Diseases
- LV
left ventricle
- LVH
left ventricular hypertrophy
- MTR
Mayo Tissue Registry
- SAB
sodium sulfate alcian blue
- TTR
transthyretin
- WFDM
whole-field digital microscopy
- wtTTR
wild type transthyretin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cornwell GG, 3rd, Murdoch WL, Kyle RA, Westermark P, Pitkanen P. Frequency and distribution of senile cardiovascular amyloid. A clinicopathologic correlation. Am J Med. 1983;75:618–623. doi: 10.1016/0002-9343(83)90443-6. [DOI] [PubMed] [Google Scholar]
- 2.Tanskanen M, Peuralinna T, Polvikoski T, et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study. Ann Med. 2008;40:232–239. doi: 10.1080/07853890701842988. [DOI] [PubMed] [Google Scholar]
- 3.Buerger L, Braunstein H. Senile cardiac amyloidosis. Am J Med. 1960;28:357–367. doi: 10.1016/0002-9343(60)90167-4. [DOI] [PubMed] [Google Scholar]
- 4.Hodkinson HM, Pomerance A. The clinical significance of senile cardiac amyloidosis: a prospective clinico-pathological study. Q J Med. 1977;46:381–387. [PubMed] [Google Scholar]
- 5.Alhamadsheh MM, Connelly S, Cho A, et al. Potent kinetic stabilizers that prevent transthyretin-mediated cardiomyocyte proteotoxicity. Sci Transl Med. 2011;3:97ra81. doi: 10.1126/scitranslmed.3002473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourgault S, Choi S, Buxbaum JN, Kelly JW, Price JL, Reixach N. Mechanisms of transthyretin cardiomyocyte toxicity inhibition by resveratrol analogs. Biochem Biophys Res Commun. 2011;410:707–713. doi: 10.1016/j.bbrc.2011.04.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao L, Buxbaum JN, Reixach N. Age-related oxidative modifications of transthyretin modulate its amyloidogenicity. Biochemistry. 2013;52:1913–1926. doi: 10.1021/bi301313b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sousa MM, Cardoso I, Fernandes R, Guimaraes A, Saraiva MJ. Deposition of transthyretin in early stages of familial amyloidotic polyneuropathy: evidence for toxicity of nonfibrillar aggregates. Am J Pathol. 2001;159:1993–2000. doi: 10.1016/s0002-9440(10)63050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teng MH, Yin JY, Vidal R, et al. Amyloid and nonfibrillar deposits in mice transgenic for wild-type human transthyretin: a possible model for senile systemic amyloidosis. Lab Invest. 2001;81:385–396. doi: 10.1038/labinvest.3780246. [DOI] [PubMed] [Google Scholar]
- 10.Buxbaum JN, Tagoe C, Gallo G, Walker JR, Kurian S, Salomon DR. Why are some amyloidoses systemic? Does hepatic "chaperoning at a distance" prevent cardiac deposition in a transgenic model of human senile systemic (transthyretin) amyloidosis? FASEB J. 2012;26:2283–2293. doi: 10.1096/fj.11-189571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis. 2005;47:320–332. doi: 10.1016/j.pcad.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Said G, Grippon S, Kirkpatrick P. Tafamidis. Nat Rev Drug Discov. 2012;11:185–186. doi: 10.1038/nrd3675. [DOI] [PubMed] [Google Scholar]
- 13.Ackermann EJ, Guo S, Booten S, et al. Clinical development of an antisense therapy for the treatment of transthyretin-associated polyneuropathy. Amyloid. 2012;19(Suppl 1):43–44. doi: 10.3109/13506129.2012.673140. [DOI] [PubMed] [Google Scholar]
- 14.Vaishnaw AK, Gollob J, Gamba-Vitalo C, et al. A status report on RNAi therapeutics. Silence. 2010;1:14. doi: 10.1186/1758-907X-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Targonski P, Jacobsen SJ, Weston SA, et al. Referral to autopsy: effect of antemortem cardiovascular disease: a population-based study in Olmsted County, Minnesota. Ann Epidemiol. 2001;11:264–270. doi: 10.1016/s1047-2797(00)00220-9. [DOI] [PubMed] [Google Scholar]
- 16.Falk RH. Senile systemic amyloidosis: are regional differences real or do they reflect different diagnostic suspicion and use of techniques? Amyloid. 2012;19(Suppl 1):68–70. doi: 10.3109/13506129.2012.674074. [DOI] [PubMed] [Google Scholar]
- 17.Kyle RA, Spittell PC, Gertz MA, et al. The premortem recognition of systemic senile amyloidosis with cardiac involvement. Am J Med. 1996;101:395–400. doi: 10.1016/S0002-9343(96)00229-X. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda S, Sekijima Y, Tojo K, Koyama J. Diagnostic value of abdominal wall fat pad biopsy in senile systemic amyloidosis. Amyloid. 2011;18:211–215. doi: 10.3109/13506129.2011.623199. [DOI] [PubMed] [Google Scholar]
- 19.Fine NMOA, Zeldenrust S, Miller F, Bielinski S, Klarich K, Dispenzieri A, Scott C, Grogan M. Sensitivity of subcutaneous fat aspirate biopsy for detection of systemic amyloidosis in cardiac biopsy-proven transthyretin cardiomyopathy. J Am Coll Cardiol. 2013;61:E592. [Google Scholar]
- 20.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Secular trends in renal dysfunction and outcomes in hospitalized heart failure patients. J Card Fail. 2006;12:257–262. doi: 10.1016/j.cardfail.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 22.Kitzman DW, Scholz DG, Hagen PT, Ilstrup DM, Edwards WD. Age-related changes in normal human hearts during the first 10 decades of life. Part II (Maturity): A quantitative anatomic study of 765 specimens from subjects 20 to 99 years old. Mayo Clin Proc. 1988;63:137–146. doi: 10.1016/s0025-6196(12)64946-5. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson DR, Pastore RD, Yaghoubian R, et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med. 1997;336:466–473. doi: 10.1056/NEJM199702133360703. [DOI] [PubMed] [Google Scholar]
- 24.Dunlay SM, Weston SA, Redfield MM, Killian JM, Roger VL. Anemia and heart failure: a community study. Am J Med. 2008;121:726–732. doi: 10.1016/j.amjmed.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neben-Wittich MA, Wittich CM, Mueller PS, Larson DR, Gertz MA, Edwards WD. Obstructive intramural coronary amyloidosis and myocardial ischemia are common in primary amyloidosis. Am J Med. 2005;118:1287. doi: 10.1016/j.amjmed.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 26.Pellikka PA, Holmes DR, Jr, Edwards WD, Nishimura RA, Tajik AJ, Kyle RA. Endomyocardial biopsy in 30 patients with primary amyloidosis and suspected cardiac involvement. Arch Intern Med. 1988;148:662–666. [PubMed] [Google Scholar]
- 27.Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR, 3rd, Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood. 2009;114:4957–4959. doi: 10.1182/blood-2009-07-230722. [DOI] [PubMed] [Google Scholar]
- 28.Linke RP. On typing amyloidosis using immunohistochemistry. Detailled illustrations, review and a note on mass spectrometry. Prog Histochem Cytochem. 2012;47:61–132. doi: 10.1016/j.proghi.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Murtagh B, Hammill SC, Gertz MA, Kyle RA, Tajik AJ, Grogan M. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am J Cardiol. 2005;95:535–537. doi: 10.1016/j.amjcard.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 30.Pinney JH, Whelan CJ, Petrie A, et al. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc. 2013;2:e000098. doi: 10.1161/JAHA.113.000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dungu JN, Anderson LJ, Whelan CJ, Hawkins PN. Cardiac transthyretin amyloidosis. Heart. 2012;98:1546–1554. doi: 10.1136/heartjnl-2012-301924. [DOI] [PubMed] [Google Scholar]
- 32.Ng B, Connors LH, Davidoff R, Skinner M, Falk RH. Senile systemic amyloidosis presenting with heart failure: a comparison with light chain-associated amyloidosis. Arch Intern Med. 2005;165:1425–1429. doi: 10.1001/archinte.165.12.1425. [DOI] [PubMed] [Google Scholar]
- 33.Lie JT, Hammond PI. Pathology of the senescent heart: anatomic observations on 237 autopsy studies of patients 90 to 105 years old. Mayo Clin Proc. 1988;63:552–564. doi: 10.1016/s0025-6196(12)64885-x. [DOI] [PubMed] [Google Scholar]
- 34.Buck FS, Koss MN, Sherrod AE, Wu A, Takahashi M. Ethnic distribution of amyloidosis: an autopsy study. Mod Pathol. 1989;2:372–377. [PubMed] [Google Scholar]
- 35.Borlaug BA, Redfield MM. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation. 2011;123:2006–2014. doi: 10.1161/CIRCULATIONAHA.110.954388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright JR, Calkins E. Amyloid in the aged heart: frequency and clinical significance. J Am Geriatr Soc. 1975;23:97–103. doi: 10.1111/j.1532-5415.1975.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 37.van Heerebeek L, Franssen CP, Hamdani N, Verheugt FW, Somsen GA, Paulus WJ. Molecular and cellular basis for diastolic dysfunction. Curr Heart Fail Rep. 2012;9:293–302. doi: 10.1007/s11897-012-0109-5. [DOI] [PubMed] [Google Scholar]
- 38.Burgoyne JR, Mongue-Din H, Eaton P, Shah AM. Redox signaling in cardiac physiology and pathology. Circ Res. 2012;111:1091–1106. doi: 10.1161/CIRCRESAHA.111.255216. [DOI] [PubMed] [Google Scholar]
- 39.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Ann Rev Biochem. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.