Abstract
OBJECTIVES
We previously demonstrated that sphingosine 1-phosphate (S1P) bimodally regulates epithelial ovarian cancer (EOC) cell invasiveness: low-concentration S1P stimulates invasion similar to lysophophatidic acid (LPA), while high-concentration S1P inhibits invasion. In this study, we investigated the mechanisms through which S1P affects EOC cell proteolysis, invasion, and adhesion in two cultured epithelial ovarian cancer cell lines.
METHODS
G-protein Gi was inhibited by pertussis toxin (PTX) and GTP binding protein Rac by NSC23766. S1P conditioned media of DOV13 and OVCA429 cells were evaluated via gel zymography, fluorometric gelatinase assay, urokinase plasminogen activator (uPA) activity assay, and Western Blot for MT1-MMP. Cell invasion was analyzed in Matrigel chambers. Membrane-N-cadherin was localized via fluorescence microscopy.
RESULTS
Zymography revealed pro-MMP2 in conditioned media of EOC cells regardless of treatment. Gelatinase activity was increased by low-concentration S1P. In DOV13 cells this effect was Gi and Rac dependent. In all OVCA429 and control DOV13 cells, PTX enhanced gelatinolysis, suggesting an MMP2-inhibitory pathway via Gi. MT1-MMP was decreased Gi-dependently by high-concentration S1P. Rac inhibition significantly counteracted low-S1P enhancement and high-S1P reduction of DOV13 invasiveness; and uPA activity in conditioned media of invading cells correlated significantly. Immunohistochemistry revealed Gi-dependent clustering of membrane-N-cadherin in DOV13 cells treated with 0.5µM S1P or 10µM LPA.
CONCLUSIONS
S1P influences EOC invasion by regulating ECM-proteolysis and cell-cell attachment via MMP2, uPA, and membrane-N-cadherin. Furthermore, this study illustrates that the net effect of S1P on each of these processes reflects a complex interplay of multiple GPCR pathways involving Gi and downstream Rac.
Keywords: S1P, LPA, invasion, MMP-2, MT1-MMP, uPA, N-cadherin, ovarian cancer
Introduction
Ovarian cancer remains a significant cause of morbidity and mortality as most women are diagnosed with advanced stage disease and have a 20–30% five year survival. However, when the tumor is limited to the ovary, the surgical morbidity is greatly reduced, adjuvant chemotherapy may not be required, and approximately 90% are alive at five years [1, 2]. In order to improve patient outcome it is essential that we gain better understanding of epithelial ovarian cancer (EOC) metastasis [3].
Two mediators of the complex ovarian cancer metastatic cascade are the bioactive phospholipids sphingosine 1-phosphate (S1P) and lysophophatidic acid (LPA). Both LPA and S1P are found in high levels in malignant ovarian ascites, have similar enhancing effects on proliferation, and repression of apoptosis in vitro, and affect invasion [4–9]. LPA and S1P have potential beyond their mechanistic role in EOC progression as diagnostic and prognostic biomarkers [4, 10, 11]. While LPA has been extensively studied, less is known about the mechanisms and behaviors of S1P. Recently, we reported that S1P induces EOC invasiveness similar to LPA at low concentrations yet inhibits invasion at high concentrations [12]. The pathways leading to the concentration dependant effect are unknown. In this study, we investigated the mechanisms through which S1P affects EOC cell proteolysis, invasion, and adhesion.
S1P, like LPA, acts at the cell surface via G-protein coupled receptors (GPCRs). There are five known SP1 receptors encoded by members of the endothelial differentiation gene (Edg) family, S1P1–5 [9]. We have recently shown that S1P regulates transcription and surface presentation of its own receptors [12]. The G proteins that mediate the biologic effects of S1P are associated with particular Edg’s, and pathways have been identified to all known S1P receptors through coupled G-Proteins, including the Gi subgroup [13]. Further downstream is Rac, a small GTP binding protein of the Rho family. Rac is a downstream affector in GPCR pathways that has been shown to be regulated by S1P [14] and LPA [15] and is known to induce cell motility via urokinase plasminogen activator (uPA) receptor induced actin cytoskeletal changes [16] and the loss of stress fibers as result of Rho inactivation [17].
uPA is a serine protease that converts plasminogen into active plasmin and initiates a cascade leading to extracellular matrix (ECM) degradation. Plasmin directly degrades various ECM components as well as activating matrix metalloproteinases (MMP’s), a family of zinc-dependent endopeptidases capable of degrading virtually all ECM components and correlated with cellular invasiveness [7, 18–20]. uPA activity is upregulated by LPA in DOV 13 cells [21]; however, the effect of S1P on uPA activity is unknown. Rac is also a mediator in MMP-activating pathways [22]. Rac enhancement of invasion through collagen requires MMP-2 activation, suggesting that Rac activity has the potential to induce invasion not only via cytoskeletal regulation but also through enhanced proteolysis [23]. In a complex chain of events, pro-MMP-2 is activated by MT1-MMP, a transmembrane MMP itself implicated in a broad spectrum of proteolysis of ECM components [24].
Recent data implicates Rac in a number of additional cellular processes essential to invasion, including cadherin-mediated cell adhesion. While E-cadherin suppresses invasion and is downregulated in most cancers, N-cadherin encourages invasion and is usually upregulated in malignant cells [25]. N-cadherin is present in the membranes of DOV 13 cells, while OVCA 429 membranes contain E-cadherin. We recently found that DOV 13 membrane-N-cadherin was increased by high concentration S1P (20µM) and reduced by LPA [26]. The inverse correlation observed between SIP and LPA’s effects on membrane-N-cadherin and invasiveness suggests that membrane presentation of N-cadherin and its related cell-cell attachment may constitute another mechanism by which these phospholipids regulate EOC invasion and migration.
Based on these findings, we hypothesized that upstream Gi and downstream Rac are instrumental mediators in several of the pathways by which S1P regulates EOC invasiveness. Therefore, we evaluated the impact of sequentially inhibiting Gi and Rac on invasion, gelatinase and uPA activities, as well as N-cadheren surface distribution in S1P treated ovarian cancer cells.
Methods
Cell lines and stock solutions
The DOV 13 and OVCA 429 ovarian carcinoma cell lines, kind gifts from Dr. Robert Bast, Jr. (M. D. Anderson Cancer Center, Houston, TX), were cultured as previously described [27]. All cells were grown in humidified atmosphere incubators at 37°C and 4.5% CO2. Sphingosine 1-phosphate (S1P) and 18:1 lysophosphatidic acid (LPA) were obtained from Avanti Polar Lipids (Alabaster, AL). LPA stock solution (2mM) was prepared in phosphate buffered saline (PBS) containing 0.1% fat free BSA. S1P was dissolved in DMSO at 20 mM and diluted to 1 mM with 5mM HEPES pH 7.5 containing 3% fat free BSA. The vehicle used for control cells in experiments involving S1P consisted of 3% BSA in 5mM HEPES pH 7.5 containing 5% DMSO. Control vehicle for LPA was PBS containing 0.1% fat free BSA.
Inhibitors
Signaling pathways coupled through Gi were specifically inhibited by pertussis toxin (PTX), and signaling through Rac was inhibited by NSC23766, both purchased from Calbiochem (San Diego, CA).
Invasion and viability assays
Invasion assays were performed using Matrigel coated invasion chambers (Bedford, MA) as previously described [20] with several modifications. Cells were pretreated with vehicle or S1P with or without 25µM Rac inhibitor for the 6 hours in most experiments, detached, and counted. 500,000 cells per half ml were applied to triplicate chambers, which were soaked in medium containing 40µM LPA chemoattractant at the bottom wells. After 21 hours incubation at 37°C 4.5% CO2, non-invading cells were wiped and invading cells were stained and four fields per membrane were counted at 25× magnification. Quadruplicate samples containing 100 µl (50000 cells) of the cells prepared for the invasion assay were assayed for viability in 96 wells plate using the Aqueous Cell Proliferation assay (Promega, Madison, WI) as per the manufacturer’s instructions. Conditioned media from the inserts were collected for gelatinase and uPA assays.
Gelatinase Activity Assay
To begin, we looked for MMPs and measured gelatinase activity in the conditioned media of DOV 13 cells after 6 hours’ treatment with various concentrations of S1P to determine whether ECM proteolysis correlates with previously observed S1P induced changes in EOC cell invasiveness. We then investigated the significance of Gi and Rac in MMP secretion and activity by treating control and S1P-treated cells with 50ng/mL PTX or 25µM Rac inhibitor. Conditioned media (CM) was collected and centrifuged. Supernatant from each treatment (100 µl) was then incubated with quenched fluorogenic DQ-gelatin in reaction buffer for 6–48 hours depending on experiment and the concentration of DQ-gelatin used. Fluorescence was excited by 495nm and measured at 515nm using the fluorescence microplate reader Spectra Max Gemini XS (Molecular Devices, Sunnyvale, CA).
Gel Zymography
The above CM samples, were evaluated for functional activity of MMPs by gelatin zymography as previously described [8]. CM samples (32µl) were electrophoresed at 26mA on 9% SDS–-polyacrylamide gels containing 0.1% porcine skin gelatin (Sigma, St Louis, MO). After electrophoresis, the gels were rinsed twice in 2.5% Triton X-100 and incubated at 37°C for 36 h in 0.15 M NaC1, 10 mM CaCl2, and 50 mM Tris HC1 buffer (pH 7.5) containing 0.05% NaN3. The gels were stained with 0.05% Coomassie Blue and destained in 15% methanol and 10% acetic acid in water. Gelatinolytic enzymes were detected as transparent bands on the blue background of the Coomassie Blue-stained slab gel.
uPA Activity Assays
We evaluated conditioned media from both attached (after 6 hours’ treatment) and invading cells (pretreated for 6 hours, then applied to invasion assay for 21 hours) via colorimetric assay for uPA activity. CM from S1P-treated DOV 13 cells with or without PTX or Rac inhibitor were applied to a coupled plasminogen activation assay to detect uPA activity, as described previously [28]: CM media was incubated at 37°C in the presence of 0.3µM plasminogen and 0.3 mM of the plasmin substrate D-Val-Leu-Lys 4-nitroanilide dihyrochloride (Sigma, St Louis, MO). Rate of plasmin hydrolysis of D-Val-Leu-Lys 4-nitroanilide dihyrochloride was measured by monitoring absorbance at 405nm in a OptiMax microplate reader (Molecular Devices, Sunnyvale, CA). This assay detects both uPA and tissue-type plasminogen activator. uPA stop (American Diagnostica, Stamford, CT) was added to each condition to determine specificity of activity to uPA.
Western analysis
OVCA 429 and DOV 13 cells were cultured as above and subjected to conditions as indicated. For whole cell lysates, cells were washed with ice-cold PBS and then extracted with lysis buffer (Radioimmunoprecipitation assay, RIPA, buffer containing 2 mM PMSF, 2 mM Na3VO4, 2 mM NaF, and aprotinin, leupeptin and pepstatin of 2 µg/ml each). Cell surface proteins of treated cells were biotin labeled and isolated as described [12] using the Pierce Biotinylation kit (Rockford, IL). Briefly, cells in plates were labeled with Sulfo-NHS-SS-Biotin, quenched, scraped off plates, sonicated in lysis buffer, and affinity purified by Immobilized NeutrAvidin. Protein content of eluted (biotinylated) and non-bound fractions and whole cell lysates was measured by DC Protein Assay Kit (Bio-Rad, Hercules, CA), and total protein content was calculated. Equal volumes (3% of total volume) of the biotinylated fractions per mg of total protein were separated on 12.5% SDS PAGE gels. 0.6 µg/µl protein concentration was used for whole cell lysates, and samples were separated on gradient 4–12% SDS PAGE gels. Proteins were transferred to Immobilon-P (Millipore, Billerica, MA) PVDF membrane and detected by western analysis using Rabbit anti MMP14 (MT-MMP hidge region) monoclonal antibody and peroxidase-conjugated goat anti mouse IgG and were visualized by chemiluminescence (Perkin Elmer, Boston, MA).
Immunohistochemistry
We analyzed S1P effects on cell surface localization of N-cadherin in DOV 13 cells by immunohistochemical staining and to begin to identify pathways involved by challenging with PTX. DOV 13 cells were plated sparcely at 125,000 cells per 10.5 cm2 well, cultured and starved as above and treated for 6hrs in indicated conditions, then fixed by 4% paraformaldehyde in PBS, blocked with 1% BSA in PBS, treated with anti-N-cadherin antibody (1:100 in 1% BSA), followed by FITC-anti-mouse IgG (1:200 in 1%BSA), then photographed via fluorescence microscopy at 20× magnification (Jackson Immuno Research, West Grove, PA). Chambers and slides for immunohistochemistry were purchased from Nalgene Nunc International (Rochester, NY).
Statistical analysis
Student’s t-test performed with Microsoft Excel was used for statistical significance analysis.
Results
S1P Regulation of DOV 13 invasiveness correlates with MMP-2 activity and MT1-MMP expression
Cell migration and invasion are coordinated active processes involving ECM degradation by proteases such as MMPs [19, 20]. At baseline we studied the effects of S1P on MMP expression and activity. Conditioned media (CM) from S1P treated DOV 13 cells were first tested for proteases and proteolytic activity. Low-concentration S1P increased gelatinase activity relative to vehicle alone, with 0.5µM S1P approximately twice that of control (p=0.028). Higher concentrations of S1P diminished gelatinase activity. Though this decrease was not statistically significant, it was highly reproducible (figure 1A). As we previously published, LPA increased gelatinase activity in a dose dependent manner (data not shown).
Fig. 1. S1P affects expression, secretion and activation of gelatinases.
DOV13 cells were plated and serum starved, as described in methods, and treated with the indicated S1P concentrations in SFM for 6 hours before CM was collected and (A) applied to the DQ gelatinase activity assay; and (B) separated on 15% SDS-PAGE zymography gels and stained. (C) Cell were treated as above for 4 or 24hrs, then cell surface proteins were biotin labeled and isolated from cell extracts through affinity streptavidin binding and separated on gradient 12.5% SDS PAGE gels, and analyzed by western analysis. Bars represent average of 2–3 assays, and gelatinolytic activity is represented relative to control (vehicle for S1P). Error bars=SEM. * Indicates p<0.05; ** = p<0.005.
To further characterize the MMPs responsible for the gelatinase activity, we analyzed media from the same conditions using gelatin zymography, which revealed the 72-kDa pro-MMP2 in all conditions and demonstrated increased and decreased intensity relative to control in the bands produced by conditioned media of cells treated with 0.5 and 5µM S1P, respectively (figure 1B). This correlated with both decreased invasiveness of these cells and with the gelatinase activity of their conditioned media. However, the active 67-kDa form of MMP2 could not be detected using 8–9% or 15% SDS-PAGE gels; additionally, MMP9 was not detected.
As MMP2 appeared to be a major contributor to the activity in the gelatinase assay, we next compared the expression of MT1-MMP -- the activating protease of MMP2 -- in cell surface biotin-labeled fractions of cells treated with 20µM S1P to control cells via western blot (figure 1C). Both the inactive ~63 kDa zymogen and the cleaved active 57kDa form of MT1-MMP were present in all samples. However, the active form, in which the catalytic domain faces the extracellular space and activates pro-MMP2, was significantly depleted from the membranes of the 20µM S1P treated cells at 4 and 24 hours treatment and the pro-form at 24 hours. This finding indicates diminished MT1-MMP activation of pro-MMP2 is a mediator of high-concentration S1P’s downregulation of EOC invasion.
S1P effects on gelatinase activity in DOV 13 and OVCA 429 cells are Gi dependent
Since Edg receptors affect cell motility via the Gi pathway [13, 14], we next tested the dependence of gelatinase activity on Gi using its specific inhibitor, PTX. We chose also to evaluate OVCA 429, another EOC cell line that does not invade well through matrigel, as a means of comparison.
In Dov 13 cells, PTX insignificantly (p=0.067) decreased the gelatinase activity induced by 0.5µM S1P and had minimal effect on high concentration S1P (figure 2A). In OVCA 429 cells, gelatinase activity was significantly increased by PTX in all treatments relative to S1P alone (p=0.028). The interval increase induced by PTX was similar regardless of treatment. The activity of control Dov 13 cells was similarly increased by PTX to a level similar to that of the 5µM S1P. This result indicates that baseline MMP-2 activity in OVCA 429 and Dov 13 cells is suppressed in a Gi-dependent manner. However, S1P modulation of MMP-2 activity in OVCA 429 cells is via a separate pathway that is additive to baseline gelatinolytic activity. Although the effect of PTX on control cells was consistent in both cell lines, the addition of PTX to S1P treated OVCA 429 cells altered gelatinase activity differently than in DOV 13 cells. In conclusion, Gi seems to have a robust inhibitory effect on gelatinase activity of EOC cells at baseline.
Figure 2. Pertussis toxin (PTX) increases gelatinase activity in OVCA429 cells but reverses S1P effects on proteolytic activity in DOV13 cells.
Dov13 and OVCA429 cells were treated as above for 6hrs with S1P +/− 50 ng/ml PTX, before CM was collected and analyzed through (A) DQ gelatinase activity assay; and (B) gel zymography. 72kDa pro-MMP2 and 67kDa active MMP2 are seen in PTX treated control OVCA429 cells. (C) MT1-MMP in whole cell lysates of DOV13 cells treated with LPA +/− 50ng/ml PTX and OVCA429 cells treated with S1P +/− PTX were analyzed by western blot. Bars represent average of 2–4 assays Error bars denote SEM. Significance is indicated: *=p<0.05, **=p<0.005 for S1P treated vs control cells, ◆ = p<0.05, ◆ ◆= p<0.005 for S1P and PTX vs same S1P treatment alone.
Gelatin zymography identified pro-MMP2 in the conditioned media of both cell lines regardless of treatment, with no other MMPs detected. However, the proteolytic band of the 67kDa active form of MMP-2 was noted in OVCA 429 cells treated with vehicle and PTX (figure 2B). A smaller band of 67kDa was also seen in OVCA 429 cells treated with LPA and PTX (not shown). These results are consistent with the hypothesis that a Gi-mediated process inhibits MMP-2 activation.
MT1-MMP was detected in whole cell lysates of both cell lines treated with S1P and LPA in combination with PTX. Different forms of the enzyme were seen than those found in cell surface fractions (figure 2C). Both DOV 13 and OVCA 429 cells revealed the 44kDa degradation form of the enzyme as well as the 50kDa form, previously reported as a shed ectodomain. The 50kDa soluble form in whole cell lysates was either excised after cell lysis or represents enzyme bound to the cell surface. The 44kDa degradation form is inactive, lacking the catalytic domain; however, it is associated with and results from the increased activation of pro-MMP-2 [24].
In DOV 13 cells, MT1-MMP was not present in S1P-treated cell-lysates (data not shown). In contrast, MT1-MMP was isolated from OVCA 429 cell lysates treated with S1P (figure 2C). The 50kDa soluble form was present in vehicle treated cells and was diminished by 5µM S1P and PTX, contrary to PTX’s enhancing effect on baseline gelatinase activity and to the strong MMP-2 active band induced by PTX in OVCA 429 cells. This finding could be explained by more of the 55kDa membrane bound MT1-MMP being present in the PTX treated cells. It is possible that this form was not visible in whole cell lysates because of degradation by cytoplasmic proteases, to which the membrane bound fractions were not exposed. The 44kDa form was seen in 5µM S1P treated OVCA 429 cells with a marked increase in band intensity after addition of PTX corresponding to the increased gelatinase activity observed.
S1P effects on DOV 13 cell invasiveness are Rac dependent
Activated Rac is a known inducer of cell motility and invasion. We hypothesized that S1P’s regulation of DOV 13 cell invasion may involve Rac downstream of Gi. We further analyzed Rac’s role on invasiveness of Dov13 cells through matrigel-coated chambers in medium containing LPA as a chemoattractant. We used the NSC23766 selective blocker of Rac GTPase activity. We found a similar effect on invasion by S1P as in our previous studies, with low concentration S1P (0.5µM) enhancing invasion and high concentration (5µM) decreasing it relative to control (figure 3A). Surprisingly, addition of 25µM Rac inhibitor significantly increased invasion relative to control (p=0.013) and relative to high-concentration S1P (p=0.00025). Contrastingly, Rac inhibition decreased invasion when added to low-concentration S1P (p=0.0001). We also assayed cell proliferation/viability after 6–8 hours treatment and found no significant difference across treatments, thus demonstrating that differences in the number of cells having traversed the Matrigel were not due to toxic effects of the treatments themselves (data not shown). OVCA 429 cells invade poorly through Matrigel (not shown), and therefore were not used in this set of experiments.
Fig. 3. Rac1 Inhibition counteracts S1P effects on invasion and gelatinase activity.
DOV13 cells were treated with the indicated S1P concentrations +/− 25µM Rac inhibitor (Rac-I) A- Invasion assay performed as described in methods. Rac inhibition is here seen to counteract S1P’s effects on Dov13 cell invasion. B- Gelatinase activity in CM from invasion chambers, performed as above, correlates with invasion results of 0.5µM S1P treated cells but shows negative correlation to invasion of control and 20µM S1P treated cells. Bars represent average activity of 2–3 assays relative control without S1P and inhibitors, with error bars=SEM. ***, ◆ ◆ ◆=p<0.0005; **, ◆ ◆=p<0.005; *, ◆=p<0.05. Stars (*)-S1P treatment relative to control, diamonds (◆) - Rac-I treated cells relative to respective cells without inhibitor.
We found that inhibition of Rac significantly decreased gelatinase activity when added to all conditions assayed (figure 3B). The decrease in activity was most significant relative to low concentration (0.5µM) S1P (p=0.00004), which corresponded to the reduced cell invasion through Matrigel. However, Rac inhibition affected gelatinase activity in control and high concentration S1P-treated cells oppositely to its effects on invasion, decreasing both. These findings suggests that Rac stimulates MMP-2 activation at baseline and cells treated with 20µM S1P in contrast to its effect on invasion..
uPA activity in invading cells correlates with S1P’s Rac-dependent effects on DOV 13 Invasiveness
Since the effects of Rac and Gi inhibitors on gelatinase activity did not correlate in all cases with their effect on invasion, we looked at the effects of these inhibitors on uPA, a key initiator of ECM degradation. Addition of uPA synthetic inhibitor to all assays significantly inhibited activity (not shown), demonstrating that the plasmin hydrolysis seen in our experiments was due to uPA. We found that although uPA activity was generally reduced by Rac inhibition and PTX in attached cells, the effects of the inhibitors were not significant and did not demonstrate dependence of uPA activity on Gi or Rac (figure 4A). We did find, however, a significant decrease in uPA activity induced by 20µM S1P relative to control (p=0.016) in correlation with inhibition of invasion as previously seen with this concentration (figure 3A).
Figure 4. S1P, PTX, and Rac inhibitor alone or in combination have higher impact on uPA activity of invading than attached cells.
(A) uPA activity secreted by DOV13 Cells in conditioned media (CM) after 6 hours’ treatment with S1P +/− PTX or Rac inhibitor. (B) uPA activity of CM taken from inserts containing invading DOV13 cells after 6hrs pretreatment and 21hrs in invasion chambers. PA- plasminogen activation. Bars represent average activity of 2–3 assays relative control without S1P and inhibitors, with error bars=SEM. ***, ◆ ◆ ◆=p<0.0005; **, ◆ ◆=p<0.005; *, ◆=p<0.05. Stars (*)- S1P treatment relative to control, diamonds (◆)- inhibitor treatment relative to respective conditioned media without inhibitor.
The differences among treatments and the absolute levels of uPA activity in the conditioned media of invading cells were more pronounced, with uPA activity in invading cells up to six times that seen in conditioned media of attached cells (not shown). Changes in uPA activity were induced by both S1P alone and with inhibitors (figure 4B). Interestingly, very little uPA activity was observed in the media of invading cells not treated with S1P, suggesting that similar to LPA, S1P induces uPA activity in EOC cells, particularly during ECM invasion. Both 0.5µM and 20µM S1P increased activity 14-fold relative to control (p=0.0001, 0.007). PTX and Rac inhibition both significantly inhibited the 0.5µM S1P induced uPA activity (p=0.002, 0.0001). PTX also significantly inhibited the 20µM S1P induced uPA activity (p=0.009), while Rac-inhibition slightly increased uPA activity, in concordance to its effects on invasion (Fig 4B). However, this effect was not statistically significant.
Low S1P concentrations and LPA induce clustering of membrane-N-Cadherin into microdomains
We previously found that the effects of S1P and LPA on invasion of Dov 13 cells inversely correlated with their effects on the expression of N-cadherin in the cell membrane [26]. We further evaluated effects of S1P and LPA on the distribution of N-cadherin on the cell surface using immunofluorescence, as both previously demonstrated induction of invasion and suppression of surface N-cad in Dov-13 cells [26]. Membrane-N-cadherin (m-N-cad) was visible in all cells regardless of treatment (figure 5). However, a difference in the distribution of m-N-cad was noted in 0.5µM S1P and 10 µM LPA-treated cells which showed a marked clustering into microdomains within the cell membrane. This appearance was in contrast to the uniform pattern of membrane-N-cadherin staining seen in control cells as well as in cells treated with 0.5µM S1P or 10 µM LPA together with PTX, indicating that the S1P and LPA effects on m-N-cad distribution was Gi-mediated (figure 5). Furthermore, the treatments inducing this effect were associated in previous studies with depletion of m-N-cad from cell membranes, decreased cell-cell adhesion, and increased invasion. This indicates that this pattern could be associated with decreased cellular adhesion and propensity to migrate and invade. E-cadherin was also assessed in OVCA 429 cells under the same conditions; however, no differences in the quantity or quality of membrane E-cad were observed (data not shown).
Figure 5. Characteristic Gi dependent cell surface N-cadherin clustering correlates with the LPA and S1P concentrations that enhance invasion.
DOV13 Cells cultured as above and treated for 6 hrs with 0.5 µM S1P and 10 µM LPA +/− 50 ng/mL PTX were coated first with antibody to membrane-N-cadherin and then with anti-mouse-IgG conjugated to FITC. Note clustering of N-cad in S1P and LPA treated cells, which correlates with increased invasion. Note also the blunting of this effect by the addition of PTX, which suggests a Gi-mediated process.
Discussion
This study identifies new mechanisms by which S1P regulates EOC invasion (figure 6). We found that MMP2, uPA activity and membrane cadherin distribution are regulated by S1P and LPA in correlation with invasion. The effect of S1P on these enzymes and adhesion molecules are significantly altered by the inhibition of Gi and Rac, giving us new information about the Edg-coupled pathways that influence EOC invasion. Our previous finding that invading cells respond differently from attached cells [26] was reinforced by this study: gelatinase and uPA activities were significantly increased in the conditioned media of invading versus attached cells, indicating the important role of proteolysis in invasion. In aggregate, these results appear to demonstrate that uPA activity in invading cell conditioned media correlates with Rac-dependent S1P regulation of DOV 13 cell invasion.
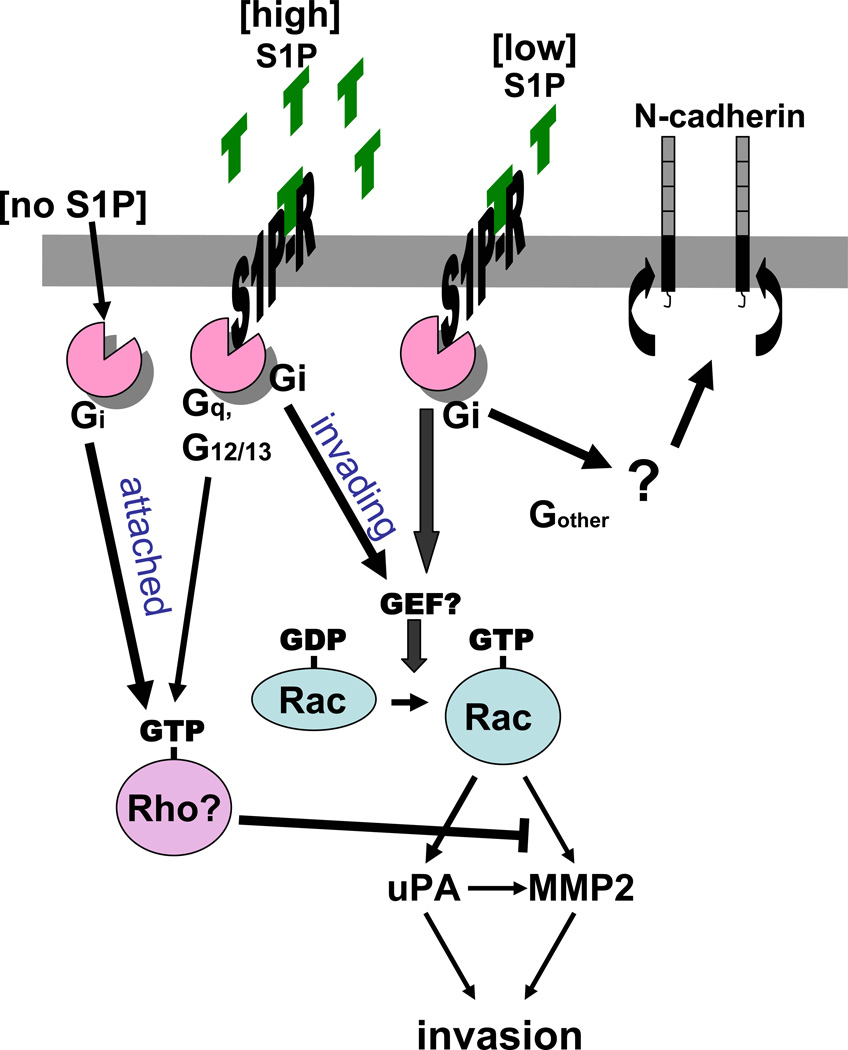
Figure 6. S1P mediated invasion pathways.
Low concentration S1P enhances invasion through activation of uPA and MMP2 via Gi and Rac. Low concentration S1P also leads to Gi dependent clustering of N-cadherin. Baseline invasion of cells in the absence of S1P is inhibited through a Gi pathway. High concentration S1P enhances this inhibition.
We have previously demonstrated that the five known S1P specific receptors are differently expressed in the EOC cell lines DOV 13 and OVCA 429 [12]. This may explain the different response of OVCA 429 and Dov13 cells to the PTX inhibition of the low-dose-S1P induced increase of gelatinase activity. We also reported that treatment with S1P alters the surface expression of S1P1, 2, and 3 in a concentration-dependent fashion [12]. As discussed, multiple S1P-receptor-coupled pathways converge at Rac, some that are Gi dependent and some that act through G12/13 or Gq [14]. Therefore, we might expect the impact of Rac inhibition to outweigh that of PTX, which could explain our observation that the effects of Rac inhibition on both gelatinase and uPA activity in the current study are more significant than the effects of PTX. A similar concept holds true for our observation that S1P effect on invasion correlate with its action on uPA activity, as uPA plays both direct and indirect (via MMP2 activation) roles in ECM degradation. Studies demonstrate that S1P regulates Rho, and that Rac and Rho have an antagonistic counter-modulatory relationship with Rho activation inhibiting Rac and Rac activation being dependent on Gi [14].
Recent studies in various non-EOC cell lines have reported that S1P differentially up- or down-regulated migration depending on the predominant GPCR: S1P1and S1P3 enhance migration, while S1P2 inhibits [29, 30]. Using mouse melanoma cells S1P1 mediated increased migration particularly at low concentrations [31]. Similar to our findings, Takuwa et. al [30] found in chinese hamster ovarian cells and B16-F10 mouse melanoma cells that Rac activation is regulated in the same direction as migration by S1P; and, that while Rac activation via S1P1 and S1P3 is Gi dependent, Rac inhibition via S1P2 is not [31].
In contrast, Lepler et al. demonstrated in glioblastoma cells that Rho activity was upregulated through S1P2 and likely accounted for decreased migration [29]. The consistency of increased gelatinase activity by the addition of PTX to controls in the present study suggests either an inhibitory Gi mediated pathway at baseline leading to decreased MMP-2 expression and/or activation in EOC cells (figure 6), or a Gi mediated proteosomal degradation. Jordan et al. recently described a Gi coupled cannabionoid receptor pathway in Neuro-2A cells that target its interactor protein Rap1GAPII to proteasomal degradation, which was inversed by PTX [32]. One possible explanation of increased MMP2 activity in conditioned media of PTX-treated EOC cells is a similar Gi mediated targeting of the molecule for proteosomal degradation. This explanation would also account for the observed active MMP2 band presented only by the PTX treated OVCA 429 cells.
The minimal effect of PTX on gelatinolytic activity and elevation of uPA activity by high concentration S1P in Dov13 cells suggests that higher concentration S1P decreases activity relative to low concentration by increasing Rho activity via S1P2 or S1P3. Both may inhibit Rac, Rac derived gelatinase and uPA by coupling with Gq or G12/13 [14]. In OVCA 429 cells, there is a baseline suppression of MMP-2 activity through Gi while changes in activity induced by S1P occur through a PTX-insensitive pathway.
Our previous data also demonstrated that attached and invading cells represent two very different functional states, with contrasting presentation of adhesion molecules [26]. The current study is consistent in that gelatinase and uPA activities secreted by invading cells are many fold higher than activities secreted by attached cells. This finding correlates with the effects of S1P and Rac-inhibition on invasion.
In other cancers, N-cadherin is associated with epithelial-mesenchymal transformation and aggressive tumor behavior, while E-cadherin suppresses tumor progression [33]. Studies on ovarian cancer, however, have been historically anomalous with regards to cadherins. Pathologic examination of ovarian cancer biopsies have found E-cadherin to be more commonly upregulated than N-cadherin [34]. One small series showed that differential expression of E- and N-cadherin expression in ovarian carcinomas have little prognostic significance [35]. However, a recent study of ovarian cancer cell lines, including OVCA 429, challenges the current belief that cadherins behave differently in EOC and indicates that N- and E-cadherin may have similar regulatory functions to those seen in other malignancies [36].
DOV 13 cells express N-cadherin, which may explain their higher rate of invasion through matrigel as compared to OVCA 429 cells. In our previous study, invasion was inversely correlated with expression of membrane-N-cadherin in invading cells [26]. In the present study, clustering of m-N-cadherin into microdomains correlated with conditions that promote EOC invasion and was Gi-dependent. It is possible that this clustering effect conditions N-cadherin to internalization or shedding thus decreasing its membrane expression. This clustering was not seen with E-cadherin in OVCA 429 cells, which may explain the differences in invasiveness of the two cell lines.
Collectively our results find S1P as a highly active biological molecule within the tumor microenvironment which regulates EOC cell invasion through a variety of mechanisms, including modulation of extracellular protease activity and N-cadherin. Furthermore the upstream mediators Gi and Rac affect each of these pathways. Clearly, the net result of the tumor cellular response to S1P is concentration dependent and represents the aggregate effect of multiple receptors and their various downstream mediators. Further elucidation of mechanisms by which S1P regulates local invasion of EOC cells and processing of proteinases may lead to new insights in ovarian metastasis and the design of novel therapeutic targets.
Acknowledgments
Grant Support for the study: NCI UO1CA85133, NCI P50 CA83639, NIH R01 CA89503, NIH-RO1CA82562, NIH RO1 CA01015, R21CA125227, Kaleidoscope of Hope Foundation, 100 Women in Hedge Fund National Ovarian Cancer Early Detection Program and the Sailfish Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statment
The authors declare that there is no conflict of interest.
References
- 1.Menon U, Skates SJ, Lewis S, Rosenthal AN, Rufford B, Sibley K, et al. Prospective study using the risk of ovarian cancer algorithm to screen for ovarian cancer. Journal of Clinical Oncology. 2005;23(31):7919–7926. doi: 10.1200/JCO.2005.01.6642. [DOI] [PubMed] [Google Scholar]
- 2.Society A. Cancer facts and figures. 2003;2003 [Google Scholar]
- 3.Tan DS, Agarwal R, Kaye SB. Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet Oncology. 2006;7(11):925–934. doi: 10.1016/S1470-2045(06)70939-1. [Review] [85 refs]. [DOI] [PubMed] [Google Scholar]
- 4.Westermann AM, Havik E, Postma FR, Beijnen JH, Dalesio O, Moolenaar WH, et al. Malignant effusions contain lysophosphatidic acid (LPA)-like activity. Annals of Oncology. 1998;9(4):437–442. doi: 10.1023/a:1008217129273. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y, Gaudette DC, Boynton JD, Frankel A, Fang XJ, Sharma A, et al. Characterization of an ovarian cancer activating factor in ascites from ovarian cancer patients. Clinical Cancer Research. 1995;1(10):1223–1232. [PubMed] [Google Scholar]
- 6.Hong G, Baudhuin LM, Xu Y. Sphingosine-1-phosphate modulates growth and adhesion of ovarian cancer cells. FEBS Letters. 1999;460(3):513–518. doi: 10.1016/s0014-5793(99)01400-3. [DOI] [PubMed] [Google Scholar]
- 7.Fishman DA, Liu Y, Ellerbroek SM, Stack MS. Lysophosphatidic acid promotes matrix metalloproteinase (MMP) activation and MMP-dependent invasion in ovarian cancer cells. Cancer Research. 2001;61(7):3194–3199. [PubMed] [Google Scholar]
- 8.So J, Navari J, Wang FQ, Fishman DA. Lysophosphatidic acid enhances epithelial ovarian carcinoma invasion through the increased expression of interleukin-8. Gynecologic Oncology. 2004;95(2):314–322. doi: 10.1016/j.ygyno.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signaling and biology. Annual Review of Biochemistry. 2004;73:321–354. doi: 10.1146/annurev.biochem.73.011303.073731. [Review] [210 refs]. [DOI] [PubMed] [Google Scholar]
- 10.Sutphen R, Xu Y, Wilbanks GD, Fiorica J, Grendys EC, Jr, LaPolla JP, et al. Lysophospholipids are potential biomarkers of ovarian cancer. Cancer Epidemiology, Biomarkers & Prevention. 2004;13(7):1185–1191. [PubMed] [Google Scholar]
- 11.Xu Y, Shen Z, Wiper DW, Wu M, Morton RE, Elson P, et al. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. Jama. 1998;280(8):719–723. doi: 10.1001/jama.280.8.719. [see comment]. [DOI] [PubMed] [Google Scholar]
- 12.Smicun Y, Reierstad S, Wang FQ, Lee C, Fishman DA. S1P regulation of ovarian carcinoma invasiveness. Gynecol Oncol. 2006;103:952–959. doi: 10.1016/j.ygyno.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 13.Radeff-Huang J, Seasholtz TM, Matteo RG, Brown JH. G protein mediated signaling pathways in lysophospholipid induced cell proliferation and survival. Journal of Cellular Biochemistry. 2004;92(5):949–966. doi: 10.1002/jcb.20094. [Review] [124 refs]. [DOI] [PubMed] [Google Scholar]
- 14.Taha TA, Argraves KM, Obeid LM. Sphingosine-1-phosphate receptors: receptor specificity versus functional redundancy. Biochimica et Biophysica Acta. 2004;1 doi: 10.1016/j.bbalip.2004.01.006. [Review] [59 refs]. [DOI] [PubMed] [Google Scholar]
- 15.Moolenaar WH, van Meeteren LA, Giepmans BN. The ins and outs of lysophosphatidic acid signaling. Bioessays. 2004;26(8):870–881. doi: 10.1002/bies.20081. [Review] [92 refs]. [DOI] [PubMed] [Google Scholar]
- 16.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279(5350):509–514. doi: 10.1126/science.279.5350.509. [Review] [50 refs]. [DOI] [PubMed] [Google Scholar]
- 17.Do TV, Symowicz JC, Berman DM, Liotta LA, Petricoin EF, 3rd, Stack MS, et al. Lysophosphatidic acid down-regulates stress fibers and up-regulates pro-matrix metalloproteinase-2 activation in ovarian cancer cells. Molecular Cancer Research: MCR. 2007;5(2):121–131. doi: 10.1158/1541-7786.MCR-06-0319. [DOI] [PubMed] [Google Scholar]
- 18.Vihinen P, Kahari VM. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. International Journal of Cancer. 2002;99(2):157–166. doi: 10.1002/ijc.10329. [Review] [165 refs]. [DOI] [PubMed] [Google Scholar]
- 19.Yoon SO, Park SJ, Yun CH, Chung AS. Roles of matrix metalloproteinases in tumor metastasis and angiogenesis. Journal of Biochemistry & Molecular Biology. 2003;36(1):128–137. doi: 10.5483/bmbrep.2003.36.1.128. [Review] [85 refs]. [DOI] [PubMed] [Google Scholar]
- 20.Wang FQ, So J, Reierstad S, Fishman DA. Matrilysin (MMP-7) promotes invasion of ovarian cancer cells by activation of progelatinase. International Journal of Cancer. 2005;114(1):19–31. doi: 10.1002/ijc.20697. [DOI] [PubMed] [Google Scholar]
- 21.Pustilnik TB, Estrella V, Wiener JR, Mao M, Eder A, Watt MA, et al. Lysophosphatidic acid induces urokinase secretion by ovarian cancer cells. Clinical Cancer Research. 1999;5(11):3704–3710. [PubMed] [Google Scholar]
- 22.Keely P, Parise L, Juliano R. Integrins and GTPases in tumour cell growth, motility and invasion. Trends in Cell Biology. 1998;8(3):101–106. doi: 10.1016/s0962-8924(97)01219-1. [Review] [70 refs]. [DOI] [PubMed] [Google Scholar]
- 23.Zhuge Y, Xu J. Rac1 mediates type I collagen-dependent MMP-2 activation. role in cell invasion across collagen barrier. Journal of Biological Chemistry. 2001;276(19):16248–16256. doi: 10.1074/jbc.m010190200. [DOI] [PubMed] [Google Scholar]
- 24.Osenkowski P, Toth M, Fridman R. Processing, shedding, and endocytosis of membrane type 1-matrix metalloproteinase (MT1-MMP) Journal of Cellular Physiology. 2004;200(1):2–10. doi: 10.1002/jcp.20064. [Review] [77 refs]. [DOI] [PubMed] [Google Scholar]
- 25.Derycke LD, Bracke ME. N-cadherin in the spotlight of cell-cell adhesion, differentiation, embryogenesis, invasion and signalling. International Journal of Developmental Biology. 2004;48(5–6):463–476. doi: 10.1387/ijdb.041793ld. [Review] [140 refs]. [DOI] [PubMed] [Google Scholar]
- 26.Smicun Y, Gil O, Devine K, Fishman DA. S1P and LPA have an attachment dependent regulatory effect on invasion of epithelial ovarian cancer cells. Gynecol Oncol. 2007;107:298–309. doi: 10.1016/j.ygyno.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 27.Moser TL, Young TN, Rodriguez GC, Pizzo SV, Bast RC, Jr, Stack MS. Secretion of extracellular matrix-degrading proteinases is increased in epithelial ovarian carcinoma. International Journal of Cancer. 1994;56(4):552–559. doi: 10.1002/ijc.2910560415. [DOI] [PubMed] [Google Scholar]
- 28.Moser TL, Enghild JJ, Pizzo SV, Stack MS. Specific binding of urinary-type plasminogen activator (u-PA) to vitronectin and its role in mediating u-PA-dependent adhesion of U937 cells. Biochemical Journal. 1995;307(Pt 3):867–873. doi: 10.1042/bj3070867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lepley D, Paik JH, Hla T, Ferrer F. The G protein-coupled receptor S1P2 regulates Rho/Rho kinase pathway to inhibit tumor cell migration. Cancer Research. 2005;65(9):3788–3795. doi: 10.1158/0008-5472.CAN-04-2311. [DOI] [PubMed] [Google Scholar]
- 30.Takuwa Y, Takuwa N, Sugimoto N. The Edg family G protein-coupled receptors for lysophospholipids: their signaling properties and biological activities. Journal of Biochemistry. 2002;131(6):767–771. doi: 10.1093/oxfordjournals.jbchem.a003163. [Review] [39 refs]. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi H, Kitayama J, Takuwa N, Arikawa K, Inoki I, Takehara K, et al. Sphingosine-1-phosphate receptor subtype-specific positive and negative regulation of Rac and haematogenous metastasis of melanoma cells. Biochemical Journal. 2003;374(Pt 3):715–722. doi: 10.1042/BJ20030381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jordan JD, He JC, Eungdamrong NJ, Gomes I, Ali W, Nguyen T, et al. Cannabinoid receptor-induced neurite outgrowth is mediated by Rap1 activation through G(alpha)o/i-triggered proteasomal degradation of Rap1GAPII. Journal of Biological Chemistry. 2005;280(12):11413–11421. doi: 10.1074/jbc.M411521200. [DOI] [PubMed] [Google Scholar]
- 33.Jaffer ZM, Chernoff J. The cross-Rho'ds of cell-cell adhesion. Journal of Biological Chemistry. 2004;279(34):35123–35126. doi: 10.1074/jbc.R400010200. [Review] [50 refs]. [DOI] [PubMed] [Google Scholar]
- 34.Sundfeldt K. Cell-cell adhesion in the normal ovary and ovarian tumors of epithelial origin; an exception to the rule. Molecular & Cellular Endocrinology. 2003;202(1–2):89–96. doi: 10.1016/s0303-7207(03)00068-6. [Review] [88 refs]. [DOI] [PubMed] [Google Scholar]
- 35.Darai E, Scoazec JY, Walker-Combrouze F, Mlika-Cabanne N, Feldmann G, Madelenat P, et al. Expression of cadherins in benign, borderline, and malignant ovarian epithelial tumors: a clinicopathologic study of 60 cases. Human Pathology. 1997;28(8):922–928. doi: 10.1016/s0046-8177(97)90007-1. [DOI] [PubMed] [Google Scholar]
- 36.Zeineldin R, Rosenberg M, Ortega D, Buhr C, Chavez MG, Stack MS, et al. Mesenchymal transformation in epithelial ovarian tumor cells expressing epidermal growth factor receptor variant III. Molecular Carcinogenesis. 2006;45(11):851–860. doi: 10.1002/mc.20237. [DOI] [PubMed] [Google Scholar]