Abstract
Background
The aim of this study was to determine correlations between progression-free survival (PFS) and the objective response rate (ORR) with overall survival (OS) in glioblastoma and to evaluate their potential use as surrogates for OS.
Method
Published glioblastoma trials reporting OS and ORR and/or PFS with sufficient detail were included in correlative analyses using weighted linear regression.
Results
Of 274 published unique glioblastoma trials, 91 were included. PFS and OS hazard ratios were strongly correlated; R2 = 0.92 (95% confidence interval [CI], 0.71–0.99). Linear regression determined that a 10% PFS risk reduction would yield an 8.1% ± 0.8% OS risk reduction. R2 between median PFS and median OS was 0.70 (95% CI, 0.59–0.79), with a higher value in trials using Response Assessment in Neuro-Oncology (RANO; R2 = 0.96, n = 8) versus Macdonald criteria (R2 = 0.70; n = 83). No significant differences were demonstrated between temozolomide- and bevacizumab-containing regimens (P = .10) or between trials using RANO and Macdonald criteria (P = .49). The regression line slope between median PFS and OS was significantly higher in newly diagnosed versus recurrent disease (0.58 vs 0.35, P = .04). R2 for 6-month PFS with 1-year OS and median OS were 0.60 (95% CI, 0.37–0.77) and 0.64 (95% CI, 0.42–0.77), respectively. Objective response rate and OS were poorly correlated (R2 = 0.22).
Conclusion
In glioblastoma, PFS and OS are strongly correlated, indicating that PFS may be an appropriate surrogate for OS. Compared with OS, PFS offers earlier assessment and higher statistical power at the time of analysis.
Keywords: glioblastoma, meta-analysis, overall survival, progression-free survival, regression, response rate, surrogate endpoint
Traditionally, the success of new cancer treatments is gauged by their ability to improve overall survival (OS) in large, randomized, phase III trials. However, the use of OS as the primary endpoint is often limited by long trial times and confounding effects of postprotocol events, such as subsequent therapies. It is thus helpful to identify and validate surrogate endpoints to facilitate efficacy evaluation and drug approval. Proposed surrogates for OS include progression-free survival (PFS), time to progression, and objective response rate (ORR).1–6 Progression-free survival has many advantages over OS, including earlier assessment of efficacy, greater statistical power at the time of analysis, and lack of influence from postprogression therapies.
The relationship between PFS and OS has been studied in various tumors. Results vary greatly by tumor type, with some reinforcing PFS as a good surrogate endpoint for OS and others indicating weak PFS/OS correlation; it has been shown that PFS may be an appropriate surrogate for OS in colorectal cancer1,5 but may not be a good surrogate in breast cancer.7,8
Glioblastoma is a highly aggressive form of cancer that represents 15.8% of all brain and CNS tumors.9 Despite decades of research into its treatment, prognosis remains poor, with median OS of only 12–14 months.10 While the introduction of temozolomide (TMZ), an oral alkylating agent, into first-line standard of care11 achieved some survival improvement, nearly all patients relapse and treatment options are limited for recurrent patients, with no accepted standard of care.12 There is therefore an unmet need for effective, novel therapies for glioblastoma. However, with fewer than 20 000 new cases diagnosed in the United States each year,9 glioblastoma occurs much less frequently than other cancers, and consequently patient accrual is low in glioblastoma studies. Thus, the use of trial endpoints that require prolonged periods of follow-up or mix varying treatments into the primary endpoint are particularly undesirable in glioblastoma studies.
While PFS represents an attractive potential surrogate endpoint, the relationship between PFS and OS has not been extensively analyzed in glioblastoma. A recent pooled analysis13 focused on phase I and single-arm phase II trials, with specific treatments, and evaluated the PFS/OS correlation at the individual level in 5 glioblastoma trials. While good individual-level correlation was demonstrated, the small sample sizes precluded any conclusions regarding correlation at trial level. Our analysis evaluates the validity of PFS and ORR as surrogate endpoints for OS using a meta-analysis of completed published phase II and III glioblastoma trials and consideration of a greater range of variables than previously evaluated.
Materials and Methods
Literature Search and Data Extraction
Completed phase II, III, and IV trials in glioblastoma published between January 1, 1991 and June 4, 2012 were identified through a systematic search on MEDLINE/PubMed and Trialtrove (Citeline) using the following keywords: “oncology and CNS” OR “glioblastoma” OR “GBM” OR “glioblastoma multiforme” AND “survival” OR “PFS” OR “progression free survival” OR “progression-free survival” OR “overall survival” OR “OS” OR “progression”. Relevant sources identified in the bibliographies of reviewed papers were also included. Publications reporting PFS and OS data from unique glioblastoma trials utilizing standard tumor response criteria were included. Abstracts and other nonjournal publications were included if sufficient detail was provided. Duplicate publications of the same trial, pediatric studies, non–English language papers, sources lacking methodology detail, and review/summary papers were excluded. The analysis was performed using the original authors' and per protocol endpoint definitions. When available, hazard ratios (HRs) for PFS and OS were recorded. Treatment, patient, and clinical endpoint data from each study were included in the database. Endpoints of interest were OS, PFS, and ORR. Due to the small number of glioblastoma trials available, the analysis was not limited to randomized trials, and studies with mixed high-grade glioma (World Health Organization [WHO]14 grades III and IV) patient populations were included; these patients were denoted as “All” in the database, since they contained both glioblastoma and anaplastic glioma histologic subpopulations.
Statistical Methods and Analysis
Weighted linear regression analysis through the origin of the plot was used to evaluate correlation between the following pairs of endpoints: (i) HR in PFS and OS, (ii) median PFS (mPFS) and median OS (mOS), (iii) ORR and mOS, (iv) 6-month PFS and mOS, and (v) 6-month PFS and 1-year OS. Pearson's R2 coefficient was used as a correlation measure between these endpoints. An R2 value of greater than 0.9 is an indicator for a strong correlation, 0.89 to 0.6 for a good to moderate correlation, and below 0.6 for a weak correlation. Points were weighted by the number of patients in the intent-to-treat population. Linear scale or log scale was selected based on the data distribution. Confidence intervals (CIs) for R2 and the weighted fit were calculated using a bootstrapping method (resampling 1000 times) assuming a sample size of 60 patients in additional trials (mean sample size per arm in the included glioblastoma trials).
Differences in the PFS/OS correlations by response criteria that include tumor response and clinical symptomatology (Macdonald,15 Response Assessment in Neuro-Oncology [RANO]16), treatments (TMZ- or bevacizumab [BEV]-containing regimens), line settings (newly diagnosed, recurrent), and histology (glioblastoma only, mixed high-grade glioma populations) were evaluated using analysis of covariance for weighted linear regression. Glioblastoma-only trials were classified as those with ≥95% glioblastoma histology in actual accrual. Statistical analyses were performed using R 2.14.2.17 Lead-time that could be gained by using PFS instead of OS as the endpoint was calculated as mOS minus mPFS for each arm and compared with other cancer types. Digitization was performed using Plot Digitizer 2.6.2 (plotdigitizer.sourceforge.net).
Results
Database
A total of 274 references were identified in the initial search. After review, 183 were excluded: 139 due to insufficient information or publication type, 11 due to lack of survival data, 9 review/summary papers, 8 pediatric studies, 8 similar papers for the same studies, and 8 due to nonstandard criteria, unclear methodology, or non-English language. Ninety-one unique trials were eligible for the meta-analysis, including 7125 patients and 115 study arms (Table 1).18–108 Of these, 2 were published in nonjournal sources: 1 abstract60 and 1 trial summary.45 Eleven HR pairs for PFS and OS were available from 10 trials;18,22,33,58,63,73,78,84,86,94 historical data were used for the control arm to calculate HR in 3 single-arm trials,33,63,73 but in each case, the historical patient population had similar baseline and treatment characteristics compared with the study population. None of these 10 trials contained any agents targeting vascular endothelial growth factor (VEGF) or VEGF receptor (VEGFR).
Table 1.
Summary of data included in literature database
| Trial | Arm | Patients (ITT) | References | |
|---|---|---|---|---|
| Phase | ||||
| II | 84 | 99 | 5161 | 18–21,23,24,26–37,39–59,61–77,79–85,87–93,95–108 |
| III | 7 | 16 | 1964 | 22,25,38,60,78,86,94 |
| Maintenance | ||||
| Yes | 16a | 19 | 1357 | 32,33,38,49,61,63,64,66,73,78,80,81,87,99,104,107 |
| No | 76a | 96 | 5768 | 18–31,34–37,39–48,50–60,62,65,67–72,74–77,79,82–86,88–98, 100–103,105,106,108 |
| Treatment | ||||
| Temozolomide containing | 37 | 40 | 2555 | 18,21,23,27,30,31,33,34,37,43,44,48,49,52,55,62,63,69, 73–75,78–81,83,84,87,95,96,98,99,101,104,106,107 |
| Bevacizumab containing | 12 | 15 | 678 | 51,67,70,71,76,88,91,93,99,104,106,107 |
| Randomized | ||||
| Yes | 13 | 30 | 3128 | 18,22,25,38,58,60,67,78,79,84,86,94,96 |
| No (single arm) | 78 | 85 | 3997 | 19–21,23,24,26–37,39–57,59,61–66,68–77,80–83,85,87–93,95,97–108 |
| Treatment line setting | ||||
| Newly diagnosed | 30 | 35 | 2412 | 20,25,27,28,32,33,36,38,39,40,43,45,49,54,55,62,63,65,73,74,78,80,81,87,98, 99,103,104,107,108 |
| Recurrent | 57 | 75 | 4049 | 18,19,21,23,24,26,29,30,34,35,41,42,44,46–4,8,50–5,3,56–5,8,60,61,64, 66–72,75–7,7,79,82–86,88–97,100–10,2,105,106 |
| Adjuvant/neoadjuvant | 2 | 3 | 586 | 22/31 |
| Unknown | 2 | 2 | 78 | 37,59 |
| Histology | ||||
| Glioblastoma | 55 | 72 | 3722 | 22,24,26,27,29,30,32,33,35–39,45,46,48,49,51,53–56,59–61,63–69,71–74, 76,79,82,85,87–8,9,92–9,4,98,99,101–10,7 |
| Grade III + IV (mixed grouping) | 36 | 43 | 3403 | 18–21,23,25,28,31,34,40–4,4,47,50,52,57,58,62,70,75,77,78,80,81, 83,84,86,90,91,95–9,7,100,108 |
| OS data available (glioblastoma) | ||||
| 49 | 63 | 3494 | 22,26,27,29,32,33,35–3,9,49,51,53–5,6,59,61,63–6,9,71–7,4,76,79,82,85, 87–8,9,92–9,4,98,99,101–10,7 | |
| PFS data available (glioblastoma) | ||||
| Macdonald | 41 | 52 | 2352 | 22,24,26,27,30,33,35–3,7,39,45,46,48,51,53–5,6,59–61,63–6,5,68,69, 72–7,4,79,82,85,87–8,9,92,98,101–10,3,105 |
| RANO | 6 | 7 | 277 | 71,76,93,104,106,107 |
| Levin | 2 | 3 | 336 | 94,99 |
| RECIST | 2 | 2 | 107 | 29,66 |
| WHO | 1 | 2 | 167 | 67 |
| Unknown/unclear | 3 | 6 | 483 | 32,38,49 |
Abbreviation: ITT, intent-to-treat.
aOne trial78 had both a maintenance arm and a nonmaintenance arm.
Correlation Between HR for PFS and OS
R2 was 0.92 (95% CI, 0.71–0.99) for the weighted linear regression of HR in OS as a function of HR in PFS (Fig. 1), indicating strong correlation. Linear regression demonstrated that a 10% risk reduction for PFS would yield an 8.1% ± 0.8% risk reduction for OS.
Fig. 1.
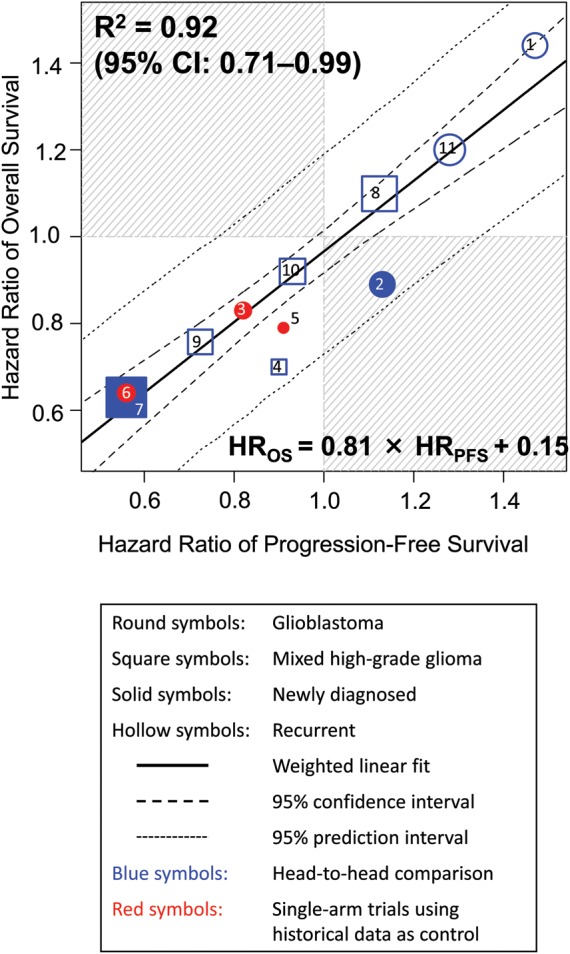
Correlation in treatment effects (HR) between PFS and OS. The linear regression shown in this and subsequent figures is all weighted by trial size. The sizes of the symbols are proportional to the number of patients included in the trial for this and subsequent figures. Treatments (year of publication) are: 1. procarbazine vs TMZ (2000)18; 2. carmustine plus radiotherapy (RT) with or without cisplatin (2003)22; 3. cis-retinoic acid vs thalidomide, both in combination with TMZ and RT (2005)33; 4. cilengitide 500 mg vs 2000 mg (2008)58; 5. TMZ plus RT with or without pegylated liposomal doxorubicin (2009)63; 6. erlotinib vs thalidomide/cis-retinoic acid, all in combination with TMZ and RT (2009)73; 7. RT with or without TMZ (2009)78; 8. procarbazine, lomustine, and vincristine vs TMZ (2010)84; 9. TMZ 200 mg/m2 for 5 days versus 100 mg/m2 for 21 days (2010)84; 10. hydroxyurea with or without imatinib (2010)86; 11. enzastaurin vs lomustine, modified Levin criterion (2010).94 All trials used Macdonald or RANO criteria except for number 11.
Correlation Between Median PFS and OS
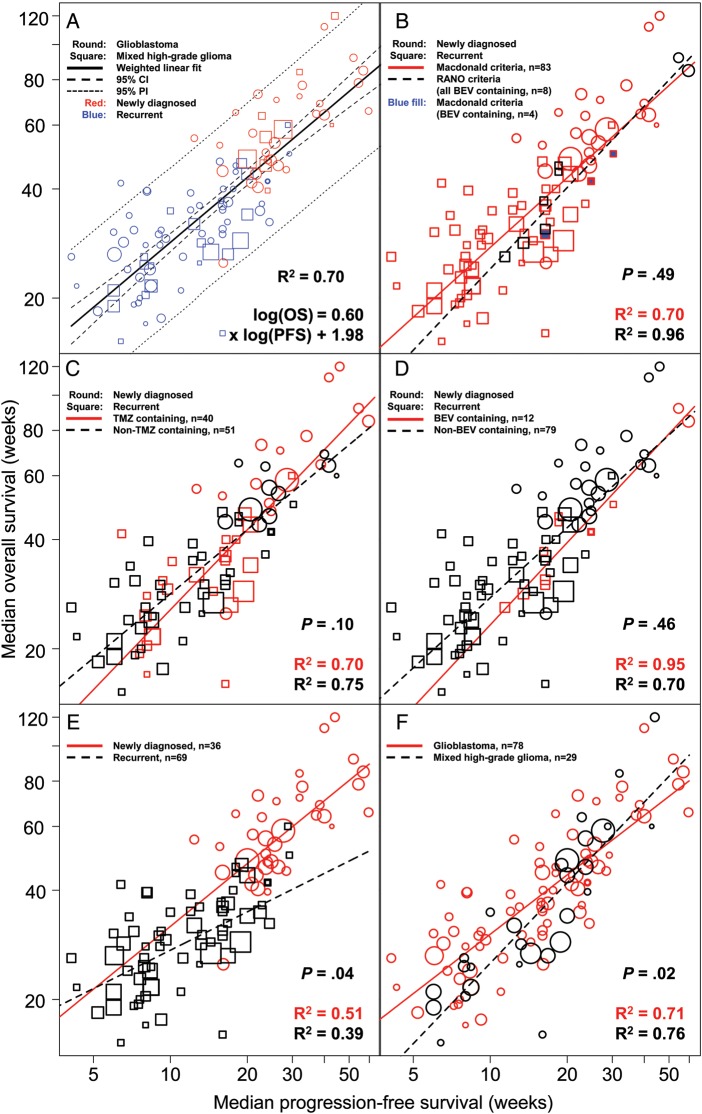
There was a good correlation between mPFS and mOS, with an R2 of 0.70 (95% CI, 0.59–0.79; Fig. 2A). There was no significant difference in the slope of the regression line between trials using Macdonald and RANO response criteria (P = .49), with good correlation between mPFS and mOS observed with both (Fig. 2B). Some trials (eg, Friedman et al.67) used criteria that were similar to RANO and thus were classified as RANO criteria. When the correlation between mPFS and mOS was evaluated by treatments, R2 was 0.70 (95% CI, 0.50–0.85) and 0.75 (95% CI, 0.60–0.86) for TMZ-containing and non-TMZ-containing regimens, respectively (Fig. 2C). No significant difference in the slope of the regression line was demonstrated between these 2 treatment types (P = .10). There was no significant difference in the slope of the regression line between mPFS and mOS for BEV-containing and non-BEV-containing treatments (P = .46), with good correlation between mPFS and mOS observed with both (R2 [95% CI]: 0.95 [0.65–0.99] vs 0.70 [0.56–0.80]; Fig. 2D). A significant difference in the slope of the regression line between mPFS and mOS was demonstrated between line settings (newly diagnosed vs recurrent, P = .04; Fig. 2E) and between histology types (glioblastoma only vs mixed histology, P = .02; Fig. 2F). When the correlation between mPFS and mOS was evaluated in trials conducted at different time periods (1991 to present), no significant difference in the slope of the regression line was demonstrated.
Fig. 2.
(A) Correlation between mPFS and mOS by study arm. (B) Correlation between mPFS and mOS in trials using Macdonald or RANO criteria for response evaluation. All RANO trials contain BEV test regimens, and the 3 BEV-containing trials using Macdonald criteria are indicated in blue. There are 83 arms using Macdonald criteria (red circle or square) and 8 arms (7 unique trials) using RANO criteria (black circle or square). (C) Correlation between mPFS and mOS separated by treatment (TMZ [red] vs non-TMZ [black]). Trials included used the Macdonald/RANO criteria for tumor assessment. (D) Correlation between mPFS and mOS separated by treatment (BEV [red] vs non-BEV [black]). Trials included used the Macdonald/RANO criteria for tumor assessment. (E) Correlation between mPFS and mOS separated by line settings (newly diagnosed vs recurrent). (F) Correlation between mPFS and mOS separated by histology (glioblastoma only vs mixed histology). Abbreviation: PI, prediction interval.
Correlation Between Other Endpoints and OS
Objective response rate was poorly correlated with mOS (R2 = 0.22; Fig. 3). For 6-month PFS versus 1-year OS (by study arm), R2 was 0.60 (95% CI, 0.37–0.77), indicating a moderate correlation between the 2 survival rates (Fig. 4A). The correlation between 6-month PFS and mOS yielded an R2 of 0.64 (95% CI, 0.42–0.77; Fig. 4B).
Fig. 3.
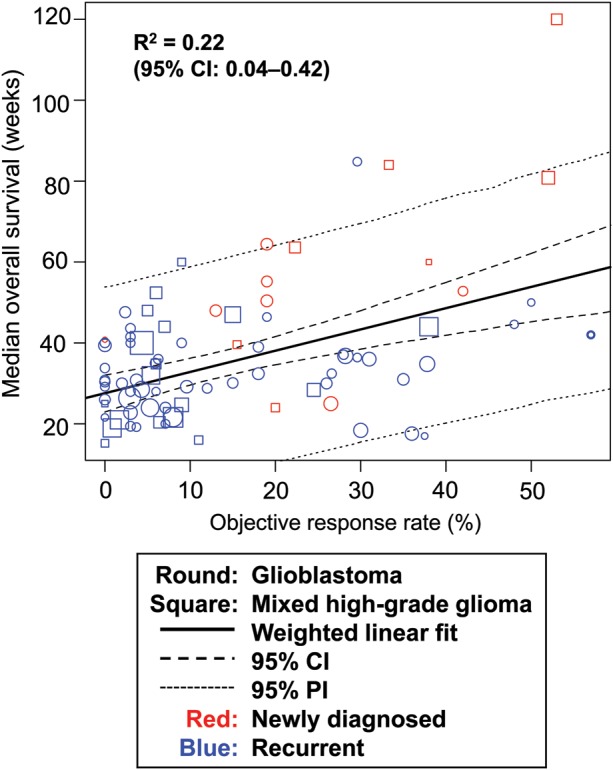
Correlation between ORR and median OS. Abbreviation: PI, prediction interval.
Fig. 4.
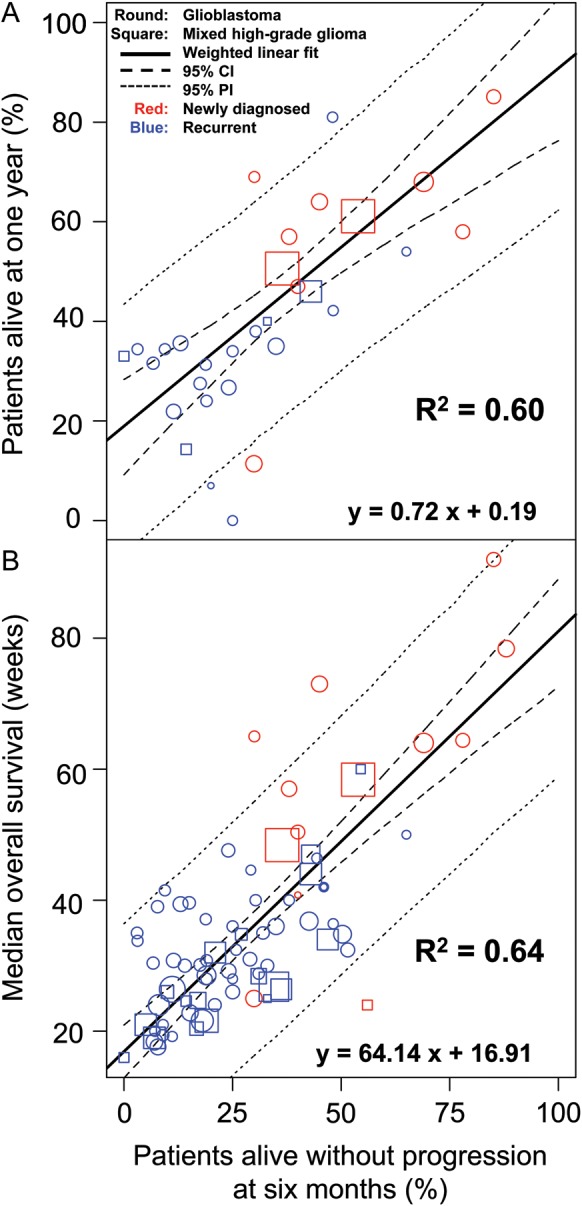
(A) Correlation between 6-month PFS and 1-year OS. (B) Correlation between 6-month PFS and median OS. Abbreviation: PI, prediction interval.
Lead-time Analysis
The lead-time that could be gained by using PFS instead of OS as the endpoint averaged 7.4 months (max 17.6 mo) and 4.2 months (max 8.1 mo) in newly diagnosed and recurrent cases, respectively (Fig. 5). The lead-time increased with increasing mOS: for newly diagnosed cases, it increased from 6–7 months for a mOS of 1 year to ∼9–10 months for a mOS of 1.5 years; for recurrent patients, it increased from 3–4 months for a mOS of half a year to ∼5–6 months for a mOS of 9 months.
Fig. 5.
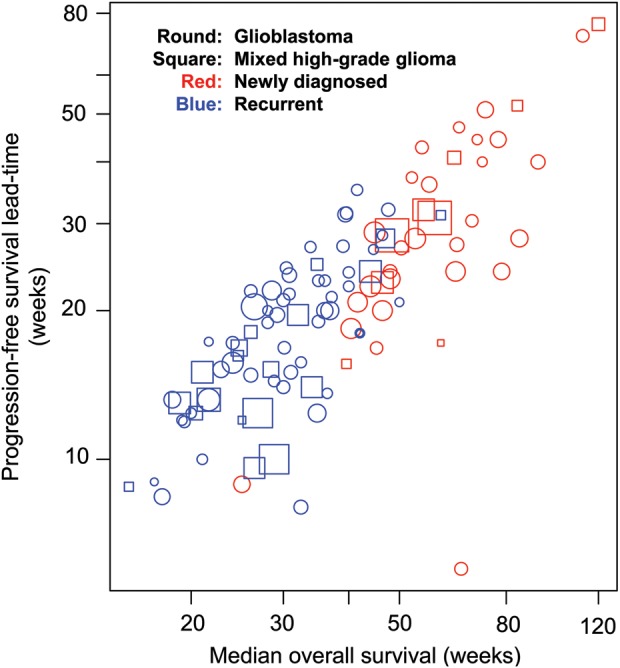
Lead-time gained by using PFS instead of OS as the endpoint plotted against mOS and mPFS. The lead-time was defined as mOS minus mPFS in each arm.
Discussion
This is a systematic evaluation of whether PFS is an appropriate surrogate endpoint for OS in glioblastoma clinical trials. We assembled the largest literature glioblastoma trial database to date, which included almost all published glioblastoma trials (phase II and beyond) since 1991, as well as the latest advances in treatment and clinical management of glioblastoma, such as trials evaluating targeted therapies and trials using RANO criteria. Our analysis demonstrated a good correlation between HR of PFS and OS and between median PFS and OS, with a 10% risk reduction for PFS yielding an 8.1% ± 0.8% risk reduction for OS. Objective response rate and OS were poorly correlated.
There are several advantages of using PFS as a surrogate endpoint for OS. Firstly, PFS offers the opportunity for early assessment. We demonstrated that a significant lead-time benefit is achieved using PFS instead of OS as an endpoint in glioblastoma. Notably, the lead-time in newly diagnosed glioblastoma is comparable to that in metastatic colorectal carcinoma (mean ∼8.5 mo, max ∼13.8 mo) based on digitized data.1 Secondly, delaying progression may represent a clinically significant benefit for glioblastoma patients. Glioblastoma is a highly infiltrative and destructive tumor that generates significant peritumoral edema and mass effect. Progressive underlying tumor is frequently associated with new or worsening neurologic deficits, which may in turn impact overall function and quality of life. Thirdly, PFS offers higher statistical power, since more PFS events usually have occurred by the time of analysis than OS events, especially given the significant PFS lead-time in glioblastoma. Notably, in our analysis, the observed percent risk reduction for PFS was higher than the percent risk reduction for OS, indicating that PFS is a more sensitive endpoint for treatment effect. Finally, PFS is independent of subsequent postprogression treatment.
However, the use of PFS is associated with several limitations that must also be considered. Firstly, it is important to standardize response criteria; a number of standard criteria are being used in glioblastoma trials and have been used in trials building the basis of our knowledge, such as Macdonald, Levin, Response Evaluation Criteria In Solid Tumors (RECIST), and RANO. Secondly, the discrepancy between the time of clinical event (progression or death) and radiologic assessment could be a confounding factor, and therefore the time interval between clinical and radiologic assessments should be minimized and consistent across studies. Thirdly, the association between radiologic progression, clinical benefit, and quality of life remains open for discussion.
The debate about the definition of a valid surrogate endpoint is ongoing, with many proposals under consideration.109 For example, it has been proposed that the conclusion of the statistical test based on the surrogate endpoint should be consistent with that based on the gold standard endpoint, and/or the treatment effect on the surrogate endpoint should predict the treatment effect on the gold standard endpoint.110–112 However, there is a general consensus that there should be good correlation between the surrogate and the gold standard endpoints and that the treatment effect on the gold standard endpoint should be captured by the surrogate endpoint.113
Our analysis demonstrated that the percentage risk reduction calculated from the HR of PFS is highly correlated with the percentage risk reduction calculated from the HR of OS in glioblastoma trials, indicating that the treatment effect on PFS can predict the treatment effect on OS in glioblastoma. A great portion (92%) of variability in OS difference can be explained by the PFS difference (R2 = 0.92). Notably, the 95% CI and prediction interval were relatively narrow. Median PFS and OS were also well correlated (R2 = 0.70). Taken together, these results lend substantial support to the use of PFS as a surrogate endpoint for OS in glioblastoma trials.
While the validity of PFS as a surrogate for OS can be demonstrated in some tumor types, the effect is not consistent.1,5,7,8 Broglio et al6 attributed differences to variations in survival postprogression (SPP), where SPP is the time difference between OS and PFS. Overall survival in cancers with long SPPs are more affected by the presence of confounding factors and therefore demonstrate weaker correlations with PFS. Glioblastoma patients have a median SPP of around 7 months,114 and therefore the PFS versus OS correlation should be fairly strong. Our analysis supports this premise.
Although sample sizes were small in our analysis, every attempt was made to standardize the data, with exclusion of trials including insufficient methodological detail and those not utilizing standardized response assessment and study endpoints.
The effects of TMZ and BEV on the correlation between mPFS and mOS were selected for study because these 2 treatments appeared most often in the literature, and too few trials report other specific treatments. Interestingly, despite differing mechanisms of action, these treatments demonstrated consistent correlations between mPFS and mOS. Although the small sample number in our analysis precludes any definitive conclusions with regard to treatment effect, the results warrant future studies.
Historically, it has been shown that patients with anaplastic glioma (WHO grade III) have a much better prognosis and survival115 than patients with glioblastoma. It would be logical to assume that a mixed grade III–IV group would have better survival because the anaplastic glioma patients' survival would increase the median values for the entire group. Our results confirmed this assumption: the slope of the regression line between mPFS and mOS is significantly higher in trials with mixed grade III–IV glioma compared with glioblastoma only. However, the difference was marginal. Possible explanations for this observation may include the fact that the lower left corner data points, which represent the mixed histology group, also represent recurrent trials (poor PFS and OS) predominantly, and most recurrent glioma patients have progressed from grade III to glioblastoma. Conversely, data in the upper right corner (high PFS, high OS) represent newly diagnosed cases predominantly and support a trend toward better OS for mixed patients (better prognosis) compared with glioblastoma-only patients.
The accrual period in the trials included in this analysis ranged from 1991 to the present. During this period, advances have been made in many aspects of glioblastoma clinical management, such as diagnostics, surgical and imaging technology, treatments, recurrence monitoring, and standard supportive care. However, the correlation between median PFS and OS seems to be consistent across different time periods, which supports the applicability of these results to future trials.
There was only a moderate correlation between 6-month PFS and mOS, which is consistent with the results of Ballman et al,116 who investigated the relationship between 6-month PFS and 1-year OS in phase II glioblastoma trials. However, it is impossible in our analysis to identify at which time point the PFS rate would be a good predictor for OS because most trials report only 6-month PFS and mPFS and individual patient data are not available to us. In addition, ORR and OS were poorly correlated (R2 = 0.22).
The applicability of our estimate of the linear relationship between the HR of OS and HR of PFS for trials evaluating anti-VEGF agents (ie, agents targeting VEGF or VEGFR) may require further validation because none of the trials in the HR correlation analysis contained an anti-VEGF agent, such as BEV (an anti-VEGF antibody) or cediranib (a VEGFR tyrosine kinase inhibitor). VEGF blockade decreases vascular permeability and normalizes vascular perfusion and the blood–brain barrier, often causing decreased contrast enhancement on MRI examinations without affecting the underlying tumor, a phenomenon called pseudoresponse.117 In contrast, radiochemotherapy could induce an inflammatory reaction (edema) and abnormal vessel permeability, causing new or increased contrast enhancement without affecting the underlying tumor, a phenomenon called pseudoprogression.118 Furthermore, radiochemotherapies preceding or following anti-VEGF therapies could further complicate MRI evaluation given the rapid onset of pseudoprogression and pseudoresponse. Therefore, models based purely on radiochemotherapies should not be extrapolated to anti-VEGF therapies without cautious validation.
There are some limitations in our analysis. Notably, we used literature instead of individual patient data, and HRs were reported in only a small number of studies due to lack of large, phase III, randomized glioblastoma trials. Furthermore, modifications to the standard response criteria were made in some trials, and details of treatments after progression were rarely reported, making it difficult to assess the potential confounding effects of subsequent treatments and crossover therapies on OS. The number of studies incorporating the RANO criteria was also small. Although these criteria have not been formally validated, they importantly address the phenomenon of pseudoprogression and pseudoresponse.16 Although we noted a consistent relationship between mPFS and mOS regardless of radiologic assessment methods, future analyses may further evaluate this relationship in more trials incorporating the RANO criteria.
In conclusion, our meta-analysis of 91 unique glioblastoma trials demonstrated a strong correlation between improvements in PFS and OS. There is also a good correlation between median PFS and OS in glioblastoma trials, regardless of response criteria, treatment, line settings, and histology. However, poor correlation was observed between ORR and OS, indicating that a high ORR may not translate into improved OS. Together these findings indicate that PFS may be an appropriate surrogate for OS in glioblastoma trials. Compared with OS, PFS offers the opportunity for earlier assessment of efficacy and higher statistical power, so establishment of these correlations may facilitate interpretation of interim analyses and future trial design.
Funding
This analysis was sponsored by Genentech Inc, South San Francisco, CA, USA.
Acknowledgments
We acknowledge Bert Lum for assistance. Support for editorial assistance for this manuscript was provided by F. Hoffmann–La Roche Ltd. These data were previously presented at the American Society of Clinical Pharmacology Therapeutics 2013 annual meeting.
Conflict of interest statement. Kelong Han is an employee of Genentech and holds stock in F. Hoffmann–La Roche. Melanie Ren was an employee of Genentech at the time of writing the manuscript. Wolfgang Wick has acted as a consultant for F. Hoffmann–La Roche, Eli Lilly (uncompensated), and MSD and received research support from Apogenix, Boehringer Ingelheim, Eli Lilly, and MSD. Lauren Abrey is an employee of F. Hoffmann–La Roche and holds stock in F. Hoffmann–La Roche. Asha Das is an employee of Genentech and holds stock in F. Hoffmann–La Roche. Jin Jin is an employee of Genentech and holds stock in F. Hoffmann–La Roche and Eli Lilly. David A. Reardon has received remuneration (other than research funding, honoraria, and consultancy fees) from F. Hoffmann–La Roche/Genentech and Merck & Co.
References
- 1.Tang PA, Bentzen SM, Chen EX, Siu LL. Surrogate end points for median overall survival in metastatic colorectal cancer: literature-based analysis from 39 randomized controlled trials of first-line chemotherapy. J Clin Oncol. 2007;25(29):4562–4568. doi: 10.1200/JCO.2006.08.1935. [DOI] [PubMed] [Google Scholar]
- 2.Saad ED, Katz A, Hoff PM, Buyse M. Progression-free survival as surrogate and as true end point: insights from the breast and colorectal cancer literature. Ann Oncol. 2010;21(1):7–12. doi: 10.1093/annonc/mdp523. [DOI] [PubMed] [Google Scholar]
- 3.Johnson KR, Ringland C, Stokes BJ, et al. Response rate or time to progression as predictors of survival in trials of metastatic colorectal cancer or non-small-cell lung cancer: a meta-analysis. Lancet Oncol. 2006;7(9):741–746. doi: 10.1016/S1470-2045(06)70800-2. [DOI] [PubMed] [Google Scholar]
- 4.Hotta K, Fujiwara Y, Matsuo K, et al. Time to progression as a surrogate marker for overall survival in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2009;4(3):311–317. doi: 10.1097/JTO.0b013e3181989bd2. [DOI] [PubMed] [Google Scholar]
- 5.Buyse M, Burzykowski T, Carroll K, et al. Progression-free survival is a surrogate for survival in advanced colorectal cancer. J Clin Oncol. 2007;25(33):5218–5224. doi: 10.1200/JCO.2007.11.8836. [DOI] [PubMed] [Google Scholar]
- 6.Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst. 2009;101(23):1642–1649. doi: 10.1093/jnci/djp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burzykowski T, Buyse M, Piccart-Gebhart MJ, et al. Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancer. J Clin Oncol. 2008;26(12):1987–1992. doi: 10.1200/JCO.2007.10.8407. [DOI] [PubMed] [Google Scholar]
- 8.Miksad RA, Zietemann V, Gothe R, et al. Progression-free survival as a surrogate endpoint in advanced breast cancer. Int J Technol Assess Health Care. 2008;24(4):371–383. doi: 10.1017/S0266462308080495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(suppl 5):v1–v49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koshy M, Villano JL, Dolecek TA, et al. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J Neurooncol. 2012;107(1):207–212. doi: 10.1007/s11060-011-0738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Central Nervous System Cancers V 1. 2013. Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed August 2, 2013.
- 13.Wu W, Galanis E, Buckner JC, Jaeckle KA, Sargent DJ. Relationship between overall survival and progression-free survival for recent NCCTG glioblastoma multiforme trials. J Clin Oncol. 2012;30(suppl) abstr 2004. [Google Scholar]
- 14.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 16.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology working group. J Clin Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 17.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. Available at http://www.R-project.org. Accessed August 2, 2013. [Google Scholar]
- 18.Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83(5):588–593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brada M, Hoang-Xuan K, Rampling R, et al. Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol. 2001;12(2):259–266. doi: 10.1023/a:1008382516636. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert MR, Friedman HS, Kuttesch JF, et al. A phase II study of temozolomide in patients with newly diagnosed supratentorial malignant glioma before radiation therapy. Neuro Oncol. 2002;4(4):261–267. doi: 10.1093/neuonc/4.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan RB, Raizer JJ, Malkin MG, Bazylewicz KA, Abrey LE. A phase II study of extended low-dose temozolomide in recurrent malignant gliomas. Neuro Oncol. 2002;4(1):39–43. doi: 10.1215/15228517-4-1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grossman SA, O'Neill A, Grunnet M, et al. Phase III study comparing three cycles of infusional carmustine and cisplatin followed by radiation therapy with radiation therapy and concurrent carmustine in patients with newly diagnosed supratentorial glioblastoma multiforme: Eastern Cooperative Oncology Group Trial 2394. J Clin Oncol. 2003;21(8):1485–1491. doi: 10.1200/JCO.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 23.Jaeckle KA, Hess KR, Yung WK, et al. Phase II evaluation of temozolomide and 13-cis-retinoic acid for the treatment of recurrent and progressive malignant glioma: a North American Brain Tumor Consortium study. J Clin Oncol. 2003;21(12):2305–2311. doi: 10.1200/JCO.2003.12.097. [DOI] [PubMed] [Google Scholar]
- 24.van den Bent MJ, Grisold W, Frappaz D, et al. European Organization for Research and Treatment of Cancer (EORTC) open label phase II study on glufosfamide administered as a 60-minute infusion every 3 weeks in recurrent glioblastoma multiforme. Ann Oncol. 2003;14(12):1732–1734. doi: 10.1093/annonc/mdg491. [DOI] [PubMed] [Google Scholar]
- 25.Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5(2):79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandes AA, Tosoni A, Amista P. How effective is BCNU in recurrent glioblastoma in the modern era? A phase II trial. Neurology. 2004;63(7):1281–1284. doi: 10.1212/01.wnl.0000140495.33615.ca. [DOI] [PubMed] [Google Scholar]
- 27.Chang SM, Lamborn KR, Malec M, et al. Phase II study of temozolomide and thalidomide with radiation therapy for newly diagnosed glioblastoma multiforme. Int J Rad Onc Biol Phys. 2004;60(2):353–357. doi: 10.1016/j.ijrobp.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 28.Lee S-J, Lee SH, Jung H-W, Kim C-Y, Heo DS, Kim IH. Phase II study of continuous ACNU (nimustine) and CDDP (cisplatin) infusion followed by conventional radiotherapy in patients with high grade astrocytomas. J Korean Neurosurg Soc. 2004;35(2):127–135. [Google Scholar]
- 29.Rich JN, Reardon DA, Peery T, et al. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol. 2004;22(1):133–142. doi: 10.1200/JCO.2004.08.110. [DOI] [PubMed] [Google Scholar]
- 30.Wick W, Steinbach JP, Küker WM, Dichgans J, Bamberg M, Weller M. One week on/one week off: a novel active regimen of temozolomide for recurrent glioblastoma. Neurology. 2004;62(11):2113–2115. doi: 10.1212/01.wnl.0000127617.89363.84. [DOI] [PubMed] [Google Scholar]
- 31.Brada M, Ashley S, Dowe A, et al. Neoadjuvant phase II multicentre study of new agents in patients with malignant glioma after minimal surgery: report of a cohort of 187 patients treated with temozolomide. Ann Oncol. 2005;16(6):942–949. doi: 10.1093/annonc/mdi183. [DOI] [PubMed] [Google Scholar]
- 32.Brem S, Grossman SA, Carson KA, et al. Phase 2 trial of copper depletion and penicillamine as antiangiogenesis therapy of glioblastoma. Neuro Oncol. 2005;7(3):246–253. doi: 10.1215/S1152851704000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butowski N, Prados MD, Lamborn KR, et al. A phase II study of concurrent temozolomide and cis-retinoic acid with radiation for adult patients with newly diagnosed supratentorial glioblastoma. Int J Radiat Oncol Biol Phys. 2005;61(5):1454–1459. doi: 10.1016/j.ijrobp.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 34.Chan DT, Poon WS, Chan YL, Ng HK. Temozolomide in the treatment of recurrent malignant glioma in Chinese patients. Hong Kong Med J. 2005;11(6):452–456. [PubMed] [Google Scholar]
- 35.Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23(23):5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 36.Aoki T, Takahashi JA, Ueba T, et al. Phase II study of nimustine, carboplatin, vincristine, and interferon-beta with radiotherapy for glioblastoma multiforme: experience of the Kyoto Neuro-Oncology Group. J Neurosurg. 2006;105(3):385–391. doi: 10.3171/jns.2006.105.3.385. [DOI] [PubMed] [Google Scholar]
- 37.Brandes AA, Tosoni A, Cavallo G, et al. Temozolomide 3 weeks on and 1 week off as first-line therapy for recurrent glioblastoma: phase II study from Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO) Br J Cancer. 2006;95(9):1155–1160. doi: 10.1038/sj.bjc.6603376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buckner JC, Ballman KV, Michalak JC, et al. Phase III trial of carmustine and cisplatin compared with carmustine alone and standard radiation therapy or accelerated radiation therapy in patients with glioblastoma multiforme: North Central Cancer Treatment Group 93-72-52 and Southwest Oncology Group 9503 Trials. J Clin Oncol. 2006;24(24):3871–3879. doi: 10.1200/JCO.2005.04.6979. [DOI] [PubMed] [Google Scholar]
- 39.Colman H, Berkey BA, Maor MH, et al. Phase II Radiation Therapy Oncology Group trial of conventional radiation therapy followed by treatment with recombinant interferon-beta for supratentorial glioblastoma: results of RTOG 9710. Int J Rad Onc Biol Phys. 2006;66(3):818–824. doi: 10.1016/j.ijrobp.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 40.Ogawa K, Yoshii Y, Toita T, et al. Hyperfractionated radiotherapy and multi-agent chemotherapy (procarbazine, ACNU and vincristine) for high-grade gliomas: a prospective study. Anticancer Res. 2006;26(3B):2457–2462. [PubMed] [Google Scholar]
- 41.Prados MD, Lamborn K, Yung WK, et al. A phase 2 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortium study. Neuro Oncol. 2006;8(2):189–193. doi: 10.1215/15228517-2005-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wen PY, Yung WK, Lamborn KR, et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99-08. Clin Cancer Res. 2006;12(16):4899–4907. doi: 10.1158/1078-0432.CCR-06-0773. [DOI] [PubMed] [Google Scholar]
- 43.Wong S, Rosenthal MA, Dowling A, et al. Phase II study of two-weekly temozolomide in patients with high-grade gliomas. J Clin Neurosci. 2006;13(1):18–22. doi: 10.1016/j.jocn.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 44.Yang SH, Kim MK, Lee TK, et al. Temozolomide chemotherapy in patients with recurrent malignant gliomas. J Korean Med Sci. 2006;21(4):739–744. doi: 10.3346/jkms.2006.21.4.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Astrazeneca Pharmaceuticals. A phase II exploratory, multicentre, open-label, non-comparative study of zd1839 (Iressa™) and radiotherapy in the treatment of patients with glioblastoma multiforme. 2007. Available at http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical-trials/resources/pdf/9255851. Accessed August 6, 2013.
- 46.Badruddoja MA, Penne K, Desjardins A, et al. Phase II study of cloretazine for the treatment of adults with recurrent glioblastoma multiforme. Neuro Oncol. 2007;9(1):70–74. doi: 10.1215/15228517-2006-022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franceschi E, Cavallo G, Lonardi S, et al. Gefitinib in patients with progressive high-grade gliomas: a multicentre phase II study by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO) Br J Cancer. 2007;96(7):1047–1051. doi: 10.1038/sj.bjc.6603669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Groves MD, Puduvalli VK, Chang SM, et al. A North American brain tumor consortium (NABTC 99-04) phase II trial of temozolomide plus thalidomide for recurrent glioblastoma multiforme. J Neurooncol. 2007;81(3):271–277. doi: 10.1007/s11060-006-9225-y. [DOI] [PubMed] [Google Scholar]
- 49.Jalali R, Basu A, Gupta T, et al. Encouraging experience of concomitant temozolomide with radiotherapy followed by adjuvant temozolomide in newly diagnosed glioblastoma multiforme: single institution experience. Br J Neurosurg. 2007;21(6):583–587. doi: 10.1080/02688690701604574. [DOI] [PubMed] [Google Scholar]
- 50.Kesari S, Schiff D, Doherty L, et al. Phase II study of metronomic chemotherapy for recurrent malignant gliomas in adults. Neuro Oncol. 2007;9:354–363. doi: 10.1215/15228517-2007-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 52.Balmaceda C, Peereboom D, Pannullo S, et al. Multi-institutional phase II study of temozolomide administered twice daily in the treatment of recurrent high-grade gliomas. Cancer. 2008;112(5):1139–1146. doi: 10.1002/cncr.23167. [DOI] [PubMed] [Google Scholar]
- 53.de Groot JF, Gilbert MR, Aldape K, et al. Phase II study of carboplatin and erlotinib (Tarceva, OSI-774) in patients with recurrent glioblastoma. J Neurooncol. 2008;90(1):89–97. doi: 10.1007/s11060-008-9637-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fadul CE, Kingman LS, Meyer LP, et al. A phase II study of thalidomide and irinotecan for treatment of glioblastoma multiforme. J Neurooncol. 2008;30(2):229–235. doi: 10.1007/s11060-008-9655-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kesari S, Schiff D, Henson JW, et al. Phase II study of temozolomide, thalidomide, and celecoxib for newly diagnosed glioblastoma in adults. Neuro Oncol. 2008;10(3):300–308. doi: 10.1215/15228517-2008-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Puduvalli VK, Giglio P, Groves MD, et al. Phase 2 trial of irinotecan and thalidomide in adults with recurrent anaplastic glioma. Neuro Oncol. 2008;10(2):216–222. doi: 10.1215/15228517-2007-060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raymond E, Brandes AA, Dittrich C, et al. Phase II study of imatinib in patients with recurrent gliomas of various histologies: a European Organisation for Research and Treatment of Cancer Brain Tumor Group study. J Clin Oncol. 2008;26(28):4659–4665. doi: 10.1200/JCO.2008.16.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reardon DA, Fink KL, Mikkelsen T, et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol. 2008;26(34):5610–5617. doi: 10.1200/JCO.2008.16.7510. [DOI] [PubMed] [Google Scholar]
- 59.Robins HI, O'Neill A, Gilbert M, et al. Effect of dalteparin and radiation on survival and thromboembolic events in glioblastoma multiforme. Cancer Chemother Pharmacol. 2008;62(2):227–233. doi: 10.1007/s00280-007-0596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schleyer E, Dresemann G, Christian H, Nikolova Z. Imatinib plus hydroxyurea versus hydroxyurea monotherapy in progressive glioblastoma (gbm) - an international multi-center, open label, randomised, phase III study (ambrosia-study) Ann Oncol. 2008;19(suppl 8):viii249. [Google Scholar]
- 61.Scoccianti S, Detti B, Sardaro A, et al. Second-line chemotherapy with fotemustine in temozolomide-pretreated patients with relapsing glioblastoma: a single institution experience. Anticancer Drugs. 2008;19(6):613–620. doi: 10.1097/CAD.0b013e3283005075. [DOI] [PubMed] [Google Scholar]
- 62.Beauchesne PD, Taillandier L, Bernier V, Carnin C. Concurrent radiotherapy: fotemustine combination for newly diagnosed malignant glioma patients, a phase II study. Cancer Chemother Pharmacol. 2009;64(1):171–175. doi: 10.1007/s00280-009-0993-x. [DOI] [PubMed] [Google Scholar]
- 63.Beier CP, Schmid C, Gorlia T, et al. RNOP-09: pegylated liposomal doxorubicine and prolonged temozolomide in addition to radiotherapy in newly diagnosed glioblastoma—a phase II study. BMC Cancer. 2009;9:308. doi: 10.1186/1471-2407-9-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brandes AA, Tosoni A, Franceschi E, et al. Fotemustine as second-line treatment for recurrent or progressive glioblastoma after concomitant and/or adjuvant temozolomide: a phase II trial of Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO) Cancer Chemother Pharmacol. 2009;64(4):769–775. doi: 10.1007/s00280-009-0926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Butowski N, Chang SM, Junck L, et al. A phase II clinical trial of poly-ICLC with radiation for adult patients with newly diagnosed supratentorial glioblastoma: a North American Brain Tumor Consortium (NABTC01-05) J Neurooncol. 2009;91(2):175–182. doi: 10.1007/s11060-008-9693-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fabrini MG, Silvano G, Lolli I, et al. A multi-institutional phase II study on second-line fotemustine chemotherapy in recurrent glioblastoma. J Neurooncol. 2009;92(1):79–86. doi: 10.1007/s11060-008-9739-6. [DOI] [PubMed] [Google Scholar]
- 67.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4743. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 68.Galanis E, Jaeckle KA, Maurer MJ, et al. Phase II trial of vorinostat in recurrent glioblastoma multiforme: a north central cancer treatment group study. J Clin Oncol. 2009;27(12):2052–2058. doi: 10.1200/JCO.2008.19.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Groves MD, Puduvalli VK, Gilbert MR, et al. Two phase II trials of temozolomide with interferon-alpha2b (pegylated and non-pegylated) in patients with recurrent glioblastoma multiforme. Br J Cancer. 2009;101(4):615–620. doi: 10.1038/sj.bjc.6605189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gutin PH, Iwamoto FM, Beal K, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Rad Onc Biol Phys. 2009;75(1):156–163. doi: 10.1016/j.ijrobp.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neyns B, Sadones J, Joosens E, et al. Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann Oncol. 2009;20(9):1596–1603. doi: 10.1093/annonc/mdp032. [DOI] [PubMed] [Google Scholar]
- 73.Prados MD, Chang SM, Butowski N, et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol. 2009;27(4):579–584. doi: 10.1200/JCO.2008.18.9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Quinn JA, Jiang SX, Reardon DA, et al. Phase II trial of temozolomide (TMZ) plus irinotecan (CPT-11) in adults with newly diagnosed glioblastoma multiforme before radiotherapy. J Neurooncol. 2009;95(3):393–400. doi: 10.1007/s11060-009-9937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Quinn JA, Jiang SX, Reardon DA, et al. Phase II trial of temozolomide plus o6-benzylguanine in adults with recurrent, temozolomide-resistant malignant glioma. J Clin Oncol. 2009;27(8):1262–1267. doi: 10.1200/JCO.2008.18.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reardon DA, Desjardins A, Vredenburgh JJ, et al. Metronomic chemotherapy with daily, oral etoposide plus bevacizumab for recurrent malignant glioma: a phase II study. Br J Cancer. 2009;101(12):1986–1994. doi: 10.1038/sj.bjc.6605412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Silvani A, Gaviani P, Fiumani A, et al. Systemic sagopilone (ZK-EPO) treatment of patients with recurrent malignant gliomas. J Neurooncol. 2009;95(1):61–64. doi: 10.1007/s11060-009-9890-8. [DOI] [PubMed] [Google Scholar]
- 78.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 79.van den Bent MJ, Brandes AA, Rampling R, et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol. 2009;27(8):1268–1274. doi: 10.1200/JCO.2008.17.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Balducci M, Apicella G, Manfrida S, et al. Single-arm phase II study of conformal radiation therapy and temozolomide plus fractionated stereotactic conformal boost in high-grade gliomas: final report. Strahlenther Onkol. 2010;186(10):558–564. doi: 10.1007/s00066-010-2101-x. [DOI] [PubMed] [Google Scholar]
- 81.Balducci M, D'Agostino GR, Manfrida S, et al. Radiotherapy and concomitant temozolomide during the first and last weeks in high grade gliomas: long-term analysis of a phase II study. J Neurooncol. 2010;97(1):95–100. doi: 10.1007/s11060-009-9997-y. [DOI] [PubMed] [Google Scholar]
- 82.Batchelor TT, Duda DG, di Tomaso E, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28(17):2817–2823. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berrocal A, Perez Segura P, Gil M, et al. Extended-schedule dose-dense temozolomide in refractory gliomas. J Neurooncol. 2010;96(3):417–422. doi: 10.1007/s11060-009-9980-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brada M, Stenning S, Gabe R, et al. Temozolomide versus procarbazine, lomustine, and vincristine in recurrent high-grade glioma. J Clin Oncol. 2010;28(30):4601–4608. doi: 10.1200/JCO.2009.27.1932. [DOI] [PubMed] [Google Scholar]
- 85.Carpentier A, Metellus P, Ursu R, et al. Intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma: a phase II study. Neuro Oncol. 2010;12(4):401–408. doi: 10.1093/neuonc/nop047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dresemann G, Weller M, Rosenthal MA, et al. Imatinib in combination with hydroxyurea versus hydroxyurea alone as oral therapy in patients with progressive pretreated glioblastoma resistant to standard dose temozolomide. J Neurooncol. 2010;96(3):393–402. doi: 10.1007/s11060-009-9976-3. [DOI] [PubMed] [Google Scholar]
- 87.Hainsworth JD, Ervin T, Friedman E, et al. Concurrent radiotherapy and temozolomide followed by temozolomide and sorafenib in the first-line treatment of patients with glioblastoma multiforme. Cancer. 2010;116(15):3663–3669. doi: 10.1002/cncr.25275. [DOI] [PubMed] [Google Scholar]
- 88.Hasselbalch B, Lassen U, Hansen S, et al. Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: a phase II trial. Neuro Oncol. 2010;12(5):508–516. doi: 10.1093/neuonc/nop063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iwamoto FM, Lamborn KR, Robins HI, et al. Phase II trial of pazopanib (GW786034), an oral multi-targeted angiogenesis inhibitor, for adults with recurrent glioblastoma (North American Brain Tumor Consortium Study 06-02) Neuro Oncol. 2010;12(8):855–861. doi: 10.1093/neuonc/noq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kreisl TN, Kotliarova S, Butman JA, et al. A phase I/II trial of enzastaurin in patients with recurrent high-grade gliomas. Neuro Oncol. 2010;12(2):181–189. doi: 10.1093/neuonc/nop042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Raizer JJ, Grimm S, Chamberlain MC, et al. A phase 2 trial of single-agent bevacizumab given in an every-3-week schedule for patients with recurrent high-grade gliomas. Cancer. 2010;116(22):5297–5305. doi: 10.1002/cncr.25462. [DOI] [PubMed] [Google Scholar]
- 92.Reardon DA, Desjardins A, Vredenburgh JJ, et al. Phase 2 trial of erlotinib plus sirolimus in adults with recurrent glioblastoma. J Neurooncol. 2010;96(2):219–230. doi: 10.1007/s11060-009-9950-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sathornsumetee S, Desjardins A, Vredenburgh JJ, et al. Phase II trial of bevacizumab and erlotinib in patients with recurrent malignant glioma. Neuro Oncol. 2010;12(12):1300–1310. doi: 10.1093/neuonc/noq099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wick W, Puduvalli VK, Chamberlain MC, et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28(7):1168–1174. doi: 10.1200/JCO.2009.23.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abacioglu U, Caglar HB, Yumuk PF, Akgun Z, Atasoy BM, Sengoz M. Efficacy of protracted dose-dense temozolomide in patients with recurrent high-grade glioma. J Neurooncol. 2011;103(3):585–593. doi: 10.1007/s11060-010-0423-2. [DOI] [PubMed] [Google Scholar]
- 96.Bogdahn U, Hau P, Stockhammer G, et al. Targeted therapy for high-grade glioma with the TGF-β2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol. 2011;13(1):132–142. doi: 10.1093/neuonc/noq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Groot JF, Lamborn KR, Chang SM, et al. Phase II study of aflibercept in recurrent malignant glioma: a North American Brain Tumor Consortium study. J Clin Oncol. 2011;29(19):2689–2695. doi: 10.1200/JCO.2010.34.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gállego Pérez-Larraya J, Ducray F, Chinot O, et al. Temozolomide in elderly patients with newly diagnosed glioblastoma and poor performance status: an ANOCEF phase II trial. J Clin Oncol. 2001;29(22):3050–3055. doi: 10.1200/JCO.2011.34.8086. [DOI] [PubMed] [Google Scholar]
- 99.Lai A, Tran A, Nghiemphu PL, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2011;29(2):142–148. doi: 10.1200/JCO.2010.30.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Neyns B, Sadones J, Chaskis C, et al. Phase II study of sunitinib malate in patients with recurrent high-grade glioma. J Neurooncol. 2011;103(3):491–501. doi: 10.1007/s11060-010-0402-7. [DOI] [PubMed] [Google Scholar]
- 101.Reardon DA, Vredenburgh JJ, Desjardins A, et al. Effect of CYP3A-inducing anti-epileptics on sorafenib exposure: results of a phase II study of sorafenib plus daily temozolomide in adults with recurrent glioblastoma. J Neurooncol. 2011;101(1):57–66. doi: 10.1007/s11060-010-0217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stupp R, Tosoni A, Bromberg JE, et al. Sagopilone (ZK-EPO, ZK 219477) for recurrent glioblastoma A phase II multicenter trial by the European Organisation for Research and Treatment of Cancer (EORTC) Brain Tumor Group. Ann Oncol. 2011;22(9):2144–2149. doi: 10.1093/annonc/mdq729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Uhm JH, Ballman KV, Wu W, et al. Phase II evaluation of gefitinib in patients with newly diagnosed Grade 4 astrocytoma: Mayo/North Central Cancer Treatment Group Study N0074. Int J Rad Onc Biol Phys. 2011;80(2):347–353. doi: 10.1016/j.ijrobp.2010.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vredenburgh JJ, Desjardins A, Reardon DA, et al. The addition of bevacizumab to standard radiation therapy and temozolomide followed by bevacizumab, temozolomide, and irinotecan for newly diagnosed glioblastoma. Clin Cancer Res. 2011;17(12):4119–4124. doi: 10.1158/1078-0432.CCR-11-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wen PY, Schiff D, Cloughesy TF, et al. A phase II study evaluating the efficacy and safety of AMG 102 (rilotumumab) in patients with recurrent glioblastoma. Neuro Oncol. 2011;13(4):437–446. doi: 10.1093/neuonc/noq198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Desjardins A, Reardon DA, Coan A, et al. Bevacizumab and daily temozolomide for recurrent glioblastoma. Cancer. 2012;118(5):1302–1312. doi: 10.1002/cncr.26381. [DOI] [PubMed] [Google Scholar]
- 107.Narayana A, Gruber D, Kunnakkat S, et al. A clinical trial of bevacizumab, temozolomide, and radiation for newly diagnosed glioblastoma. J Neurosurg. 2012;116(2):341–345. doi: 10.3171/2011.9.JNS11656. [DOI] [PubMed] [Google Scholar]
- 108.Ogawa K, Ishiuchi S, Inoue O, et al. Phase II trial of radiotherapy after hyperbaric oxygenation with multiagent chemotherapy (procar-bazine, nimustine, and vincristine) for high-grade gliomas: long-term results. Int J Radiat Oncol Biol Phys. 2012;82(2):732–738. doi: 10.1016/j.ijrobp.2010.12.070. [DOI] [PubMed] [Google Scholar]
- 109.Baker SG. Surrogate endpoints: wishful thinking or reality? J Natl Cancer Inst. 2006;98(8):502–503. doi: 10.1093/jnci/djj153. [DOI] [PubMed] [Google Scholar]
- 110.Rustin GJ, Nelstrop AE, Bentzen SM, Bond SJ, McClean P. Selection of active drugs for ovarian cancer based on CA-125 and standard response rates in phase II trials. J Clin Oncol. 2000;18(8):1733–1739. doi: 10.1200/JCO.2000.18.8.1733. [DOI] [PubMed] [Google Scholar]
- 111.Buyse M, Molenberghs G, Burzykowski T, Renard D, Geys H. The validation of surrogate endpoints in metaanalyses of randomized experiments. Biostatistics. 2000;1(1):49–67. doi: 10.1093/biostatistics/1.1.49. [DOI] [PubMed] [Google Scholar]
- 112.Begg CB, Leung DHY. On the use of surrogate endpoints in randomized trials. J R Stat Soc (Ser A) 2000;163(1):15–28. [Google Scholar]
- 113.Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8(4):431–440. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 114.Park JK, Hodges T, Arko L, et al. Scale to predict survival after surgery for recurrent glioblastoma multiforme. J Clin Oncol. 2010;28(24):3838–3843. doi: 10.1200/JCO.2010.30.0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17(8):2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 116.Ballman KV, Buckner JC, Brown PD, et al. The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro Oncol. 2007;9(1):29–38. doi: 10.1215/15228517-2006-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hygino da Cruz LC, Jr, Rodriguez I, Domingues RC, Gasparetto EL, Sorensen AG. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol. 2011;32(11):1978–1985. doi: 10.3174/ajnr.A2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brandes AA, Tosoni A, Spagnolli F, et al. Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: pitfalls in neurooncology. Neuro Oncol. 2008;10(3):361–367. doi: 10.1215/15228517-2008-008. [DOI] [PMC free article] [PubMed] [Google Scholar]