Abstract
Background
The aim of this study was to assess the objective response rate (ORR) of children and young adults with recurrent medulloblastoma/primitive neuroectodermal tumor (MB/PNET) treated with temozolomide (TMZ). The secondary purpose was to analyze the toxicity profile of TMZ when administered orally for 5 days in 3 divided daily doses every 28 days.
Methods
Forty-two patients with recurrent MB/PNET, aged 21 years and younger, were recruited. Patients were treated with oral TMZ. Starting doses ranged from 120 to 200 mg/m2/day based on previous treatments. A craniospinal MRI was performed prior to the first cycle of TMZ and following every 2 cycles of treatment.
Results
Median age was 10 years (range, 2–21 years). Forty of 42 patients were assessed for response and toxicity. The objective response rate was 42.5%: 6 patients achieved a complete response, 11 had a partial response, and 10 had stable disease. Progression-free survival rates for all patients at 6 and 12 months were 30% and 7.5%, respectively. Their median overall survival rates at 6 and 12 months were 42.5% and 17.5%, respectively. No major extrahematological effects or life-threatening events were reported. The most common grade 3/4 toxicity included thrombocytopenia (17.5%), neutropenia (7.5%), and anemia (2.5%).
Conclusions
TMZ proved to be an effective agent in children and young adults with MB/PNET, heavily pre-treated, with a tolerable toxicity profile.
Keywords: medulloblastoma, phase II trial, temozolomide
Medulloblastoma (MB) is the most common malignant brain tumor of the posterior fossa in children. This tumor has the tendency to spread within the brain and along the neuroaxis. Approximately one-third of children with MB have positive cerebrospinal fluid and cranial or spinal metastases at diagnosis.
Current treatment modalities include neurosurgery, craniospinal irradiation (CSI), and chemotherapy. Radiotherapy is an essential component of treatment for CNS tumors and is normally utilized in children older than aged 3 years to avoid the high incidence of long-term neurocognitive and neuroendocrine deficits reported in younger children.1–4
Despite aggressive treatment protocols, the prognosis for relapsed patients remains poor, with a median overall survival of <1 year.5 In a report from the Children's Hospital of Philadelphia, 23 patients with recurrent MB had a median overall survival of 5 months, with the longest survivor living for 38 months after recurrence.6 A recent paper reported a median progression-free survival (PFS) and overall survival (OS) after a first relapse of 7 months.7 No long-term survivor was reported from other large studies in children with relapsed MB.8
A number of salvage treatments, including high-dose chemotherapy followed by peripheral blood stem cell rescue (PBSCR), have been attempted. However, results are poor, with few short-lasting responses.9 Moreover, salvage treatments may result in a higher incidence of toxicity due to CSI and/or intensive chemotherapy already received by patients in the early stages before the relapse occurs.10
Temozolomide (TMZ) is currently part of standard treatment for high-grade gliomas in adults. TMZ is spontaneously activated into its active metabolite by hydrolysis at physiological pH. It has almost 100% oral bioavailability when taken in the fasting state and is able to cross the blood-brain barrier because of its small size and lipophilic properties.11–13
Following initial studies conducted in children to confirm the activity observed in adult high-grade glioma patients, we planned 2 national multi-institutional trials to evaluate the response rate in childhood high-grade glioma already reported14 and in recurrent MB. We adopted the 5-day schedule every 28 days, derived by the 2 phase I studies reported in 1998.15,16 However, we made a slight modification to the daily schedule of administration, changing from a single daily dose to 3 divided daily doses. This modification was introduced because of the relatively short half-life of the active metabolite and the marked schedule-dependency demonstrated in preclinical studies.17 Schedule dependency was confirmed in clinical trials in which activity was shown with repeated multiple oral doses but not with single intravenous administration.18
Materials and Methods
Eligibility
Patients with histologically proven MB/PNET and recurrent disease were recruited between January 1, 2002, and May 31, 2005. All patients were required to be <21 years old, have a measurable tumor mass on a gadolinium-enhanced MRI scan and have a life expectancy of at least 8 weeks.
Eligible patients had not received radiotherapy for at least 6 weeks nor chemotherapy for at least 4 weeks prior to entering the study (6 weeks after nitrosoureas). Other eligibility criteria included a WHO performance status of ≤2 or a Lansky Play Scale ≥50%, except for motor impairments related to the disease. Adequate bone marrow function was required, including absolute neutrophil count ≥1 × 109/L, platelet count ≥100 × 109/L, and hemoglobin level ≥8 g/dL. Other required laboratory values included creatinine clearance corrected for body surface of 70 mL/min or more, AST/ALT ratio, and total bilirubin ≤2.5 times the normal value.
The study was approved by the local ethics committee, and all patients provided informed consent. Informed consent was obtained from either patients or their legal guardians, and evidence of assent was obtained from the children.
Patients were also required to be on a stable corticosteroid therapy for at least 2 weeks before baseline MRI evaluation.
Treatment was discontinued if progressive disease or unacceptable toxicity occurred.
Study Design
The main purpose was to assess the objective response rate (ORR) in children and young adults with recurrent MB/PNET treated with oral TMZ. The secondary purpose was to analyze the toxicity profile of oral TMZ given for 5 days in 3 divided doses.
In accordance with the minimax 2-stage phase II study design by Simon, the treatment program was designed to refuse response rate of 10% (P0) and to provide a significance level of 0.05 with a statistical power of 80% in assessing the activity of the regimen as a 25% response rate (P1). The upper limit for first-stage drug rejection was 2 responses in the 22 assessable patients; the upper limit of the second-stage rejection was 7 responses within the cohort of 40 assessable patients.19
Patients were treated with oral TMZ, divided into 3 daily doses for 5 consecutive days every 28 days, in the fasting state. Patients who had previously received heavy treatment with high dose chemotherapy (HD-CT) and PBSCR received a starting dose of 120 mg/m2/day, which was escalated to 150 mg/m2/day if no adverse effects occurred. Patients who had received CSI without HD-CT started treatment at a dose of 180 mg/m2/day. Patients not previously treated, either with CSI or HD-CT, received 200 mg/m2/day of TMZ as a starting dose. The drug formulation was available commercially as either a 20 mg or 100 mg hard gelatin capsule; therefore, the calculated dose of TMZ was rounded up to the nearest 20 mg to accommodate capsule strength.
Doses of TMZ were reduced by 20% if neutropenia (nadir count <0.5 × 109/L) or severe or protracted thrombocytopenia (defined as a platelet count nadir of <50 × 109/L) occurred and persisted for at least 7 days.
Evaluation of Response
Baseline evaluation included a complete medical history, general and neurological examination, and hematological and biochemical assessments. Complete blood counts and serum biochemistry tests were performed weekly or more often as needed. Medical and neurological examinations were performed prior to each cycle. Toxicities were graded according to National Cancer Institute-Common Toxicity Criteria (version 3.0).
A craniospinal MRI scan was performed within 21 days before the first cycle of TMZ and following every 2 cycles of treatment. Moreover, if clinical signs or symptoms of disease progression were noted, patients underwent an additional MRI scan.
A complete response (CR) was defined as the disappearance of all detectable lesions for at least 4 weeks; partial response (PR) was defined as a decrease of at least 50% of the sum of the products of the largest perpendicular diameters over the same time span without appearance of new tumor lesions; and stable disease was defined as a reduction in tumor size of <50% or a small increase of <25%. Progression of disease was defined as a ≥25% increase in tumor size or appearance of new tumor lesions or evidence of cerebrospinal fluid dissemination.
Patients experiencing stable disease or any response to the treatment continued to receive TMZ chemotherapy. If disease progression occurred, they were withdrawn from the study. PFS and OS were measured from the first day of TMZ administration until disease progression or death occurred, respectively.
Results
Patient Characteristics
Forty-two patients with a median age of 10 years (range, 2–21 years) were enrolled in this multicenter, phase II study. All patients had a histological diagnosis of MB (39 patients) or PNET (3 patients). M-stage (Chang stage) at the time of the enrollment was M2 in 9 patients (22.5%) and M3 in 31 patients (77.5%) (Table 1). Among the enrolled patients, 2 patients were withdrawn from the study. One patient was withdrawn because of a prior encephalopathy following the first course of therapy, and the other patient underwent total surgical removal of the tumor after only one course of TMZ. Therefore, 40 patients were evaluable for the study.
Table 1.
Characteristics of enrolled patients
| Patient | Age at Dx (years) | Sex | Dx | Rx Prestudy | Time From Dx to Study (months) | M-stage Prestudy | Time From Last Rx to Study (months) | Relapses (n) | TMZ Cycles (n) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 | M | MB | S,RT,CT,PBSCR | 34 | M3 | 9 | 4 | 10 |
| 2 | 7 | M | MB | S,CT,RT | 18 | M3 | 3 | 3 | 6 |
| 3 | 10 | M | MB | S,CT,RT,PBSCR | 42 | M3 | 2 | 4 | 17 |
| 4 | 18 | M | MB | S,CT,RT,PBSCR | 14 | M3 | 3 | 2 | 2 |
| 5 | 10 | M | MB | S,CT,RT,PBSCR | 19 | M3 | 7 | 2 | 6 |
| 6 | 5 | F | MB | S,CT,RT,PBSCR | 20 | M3 | 6 | 2 | 2 |
| 7 | 10 | M | MB | S,CT,RT,PBSCR | 19 | M3 | 9 | 2 | 6 |
| 8 | 21 | M | MB | S,CT,RT,PBSCR | 33 | M3 | 21 | 2 | 10 |
| 9 | 4 | M | MB | S,CT,RT,PBSCR | 27 | M3 | 15 | 2 | 2 |
| 10 | 10 | M | MB | S,CT,RT,PBSCR | 35 | M3 | 22 | 2 | 15 |
| 11 | 18 | F | PNET | S,CT,RT | 60 | M2 | 55 | 2 | 10 |
| 12 | 13 | M | MB | S,RT,CT | 43 | M3 | 30 | 1 | 11 |
| 13 | 7 | M | MB | S,CT,RT | 17 | M3 | 5 | 2 | 5 |
| 14 | 4 | F | MB | S,CT,RT | 10 | M2 | 2 | 1 | 2 |
| 15 | 20 | F | MB | S,RT,CT | 26 | M3 | 18 | 2 | 4 |
| 16 | 7 | M | MB | S,RT,CT | 12 | M3 | 2 | 2 | 11 |
| 17 | 6 | M | MB | S,RT,CT | 45 | M3 | 33 | 1 | 22 |
| 18 | 6 | M | PNET | S,RT,CT,PBSCR | 25 | M3 | 2 | 3 | 2 |
| 19 | 10 | M | PNET | S,CT,RT | 18 | M2 | 5 | 2 | 2 |
| 20 | 13 | M | MB | S,RT,CT | 36 | M3 | 17 | 3 | 6 |
| 21 | 2 | M | MB | S,CT,PBSCR | 5 | M2 | 2 | 2 | 4 |
| 22 | 16 | M | MB | S,RT,CT | 6 | M3 | 1 | 1 | 4 |
| 23 | 11 | M | MB | S,RT,CT,PBSCR | 48 | M3 | 6 | 2 | 3 |
| 24 | 11 | M | MB | S,RT,CT | 15 | M2 | 8 | 2 | 10 |
| 25 | 2 | M | MB | S,CT,PBSCR | 10 | M3 | 4 | 2 | 9 |
| 26 | 3 | F | MB | S,CT | 6 | M3 | 3 | 2 | 2 |
| 27 | 18 | M | MB | S,RT,CT,PBSCR | 10 | M2 | 2 | 2 | 4 |
| 28 | 3 | M | MB | S,CT | 5 | M3 | 2 | 2 | 4 |
| 29 | 6 | F | MB | S,RT,CT | 12 | M2 | 4 | 2 | 12 |
| 30 | 12 | F | MB | S,RT,CT | 12 | M3 | 4 | 2 | 8 |
| 31 | 17 | M | MB | S,RT,CT | 15 | M3 | 8 | 2 | 9 |
| 32 | 9 | M | MB | S,RT,CT,PBSCR | 27 | M3 | 8 | 2 | 6 |
| 33 | 15 | F | MB | S,RT,CT,PBSCR | 29 | M2 | 4 | 2 | 2 |
| 34 | 15 | M | MB | S,RT,CT | 6 | M3 | 2 | 2 | 7 |
| 35 | 18 | F | MB | S,RT,CT,PBSCR | 17 | M3 | 17 | 3 | 13 |
| 36 | 9 | M | MB | S,RT,CT,PBSCR | 16 | M3 | 9 | 2 | 7 |
| 37 | 17 | M | MB | S,RT,CT | 9 | M3 | 7 | 2 | 4 |
| 38 | 8 | M | MB | S,RT,CT | 20 | M3 | 24 | 2 | 11 |
| 39 | 9 | M | MB | S,RT,CT | 11 | M2 | 14 | 2 | 6 |
| 40 | 9 | M | MB | S,RT,CT | 13 | M3 | 2 | 1 | 2 |
Abbreviations: Dx, diagnosis; PBSCR, peripheral blood stem cell rescue; RT, craniospinal irradiation plus boost to the posterior fossa; Rx, treatment; S, surgery; CT, chemotherapy.
All patients included in the study had undergone surgical resection at the time of initial diagnosis. Thirty-six patients received previous CSI plus a boost to the posterior fossa, and 4 children had received tumor bed irradiation. Five patients had received 1 line of chemotherapy, and 35 had received 2 or more lines. Eighteen patients had been treated with high-dose chemotherapy (including thiotepa) and PBSCR at first relapse. Initial chemotherapy regimens included nitrosourea compounds, vincristine, methotrexate, etoposide, cyclophosphamide, or platinum derivative (carboplatin or cisplatin).
Toxicity
Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria (version 3.0).
A total of 278 courses were included for toxicity. No major extrahematological effects or life-threatening events occurred during treatment with oral TMZ. No Pneumocystis carinii pneumonia complicated the clinical course of our patients.
Myelosuppression occurred on days 14 to 26.
The most common grade 3 and 4 adverse events included thrombocytopenia (17.5%), neutropenia (7.5%), and anemia (2.5%) (Table 2). Other nonhematological adverse effects, such as nausea and vomiting, were mild and were promptly controlled with standard antiemetics. A 25% dose reduction of TMZ occurred in 4 (10%) patients, primarily for myelosuppression.
Table 2.
Summary of grade 3/4 hematological toxicities
| Courses (n = 278) | Patients (n = 40) | |
|---|---|---|
| Thrombocytopenia | 28 (10%) | 7 (17.5%) |
| Neutropenia | 3 (1%) | 3 (7.5%) |
| Anemia | 1 (0.3%) | 1 (2.5%) |
No patients were withdrawn from the study due to adverse events, and there was no evidence of chronic toxicity with multiple courses.
Treatment Response
A total of 278 cycles were administered to 40 patients. The number of cycles per patient ranged between 2 and 22 (median, 6 cycles). All patients were followed up until progressive disease or death occurred. The response rate was 42.5%: 6 patients achieved a CR and 11 a PR (2 of them had supratentorial PNET) (Figs 1 and 2). Ten children had stable disease, and 13 experienced progressive disease. In 14 out of 17 responders, initial tumor shrinkage was observed after the second course of TMZ at the time of the first MRI assessment. Interestingly, a CR was registered in a child treated at the dose of 120 mg/m2/day for 5 days.
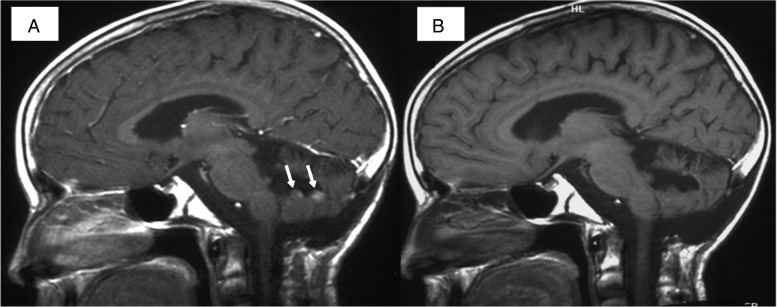
Fig. 1.
(A) Pretreatment sagittal contrast-enhanced T1-WI. Recurrence is seen as 2 contrast-enhancing nodules (arrows) along the walls of the surgical cavity. (B) Posttreatment sagittal contrast-enhanced T1-WI. Contrast enhancing nodules are no longer visible.
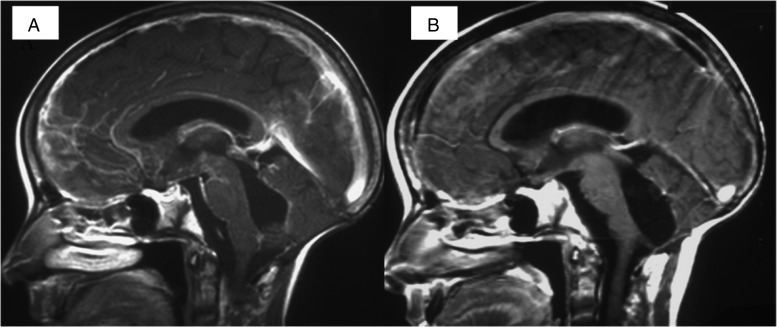
Fig. 2.
(A) Pretreatment sagittal contrast-enhanced T1-WI. Diffuse leptomeningeal seeding is seen, with “sugar coating” appearance along the brainstem, fourth ventricle, and anterior third ventricle. (B) Posttreatment sagittal contrast-enhanced T1-WI. The diffuse leptomeningeal enhancement has completely disappeared.
The PFS rate for all patients at 6 and 12 months was 30% and 7.5%, respectively. Their median OS rate at 6 and 12 months was 42.5% and 17.5%, respectively. Among patients with an objective response rate, PFS at 6 and 12 months was 70.6% and 17.5%, respectively. The median OS rate at 6 and 12 months was 94% and 41.2%, respectively.
Discussion
Treatment options for children with relapsed MB/PNET remain limited, and chances of an effective therapy are slim. Very few children can be cured following recurrence,20 and myeloablative chemotherapy followed by autologous stem cell rescue may be successful for some patients, especially when high-dose thiotepa-based chemotherapy is adopted.21–23 However, this approach appears to be rarely effective for children with bulky metastatic disease or with recurrence after definitive radiotherapy; the salvage rates do not exceed 10%.9 Besides, the prognosis is really poor in patients with subsequent recurrences, particularly when relapses occur after craniospinal radiotherapy or high-dose chemotherapy.7–9
The results of our study appear very interesting, both in terms of response rate and toxicity profile. It is noteworthy that this treatment was carried out in patients who had been heavily pretreated already. In addition, objective responses were observed at relatively low doses of TMZ, such as 120 mg/m2/day for 5 days. This observation may be relevant to the design of new multiagent treatment protocols that include TMZ.
Moreover, TMZ was administered at home without requiring hospitalization, and only a few outpatient appointments were required. Oral TMZ was well tolerated and accepted in our patient group. The toxicity profile was quite tolerable. Grade 3/4 hematological toxicity was noted in 10% of courses, although 18 of 40 patients had HD-CT with PBSCR as part of their previous treatment.
Objective responses to TMZ have been previously reported in the literature. Nicholson et al.15 enrolled 12 children with MB/PNET in a phase 1 study conducted in the United States. Two objective responses were reported: one CR in a patient with supratentorial PNET lasted more than 24 months, and a PR in a MB patient after 2 courses of TMZ at 180 mg/m2. Baruchel et al.24 reported their experience in 28 patients with recurrent brain tumors treated with continuous low-dose TMZ. Seven children had a MB/PNET, and 6 had been heavily pretreated. The authors reported 2 CRs. However, De Sio et al.25 treated 10 patients (8 MB, 2 PNET) and reported no objective response, such as CR or PR. Two patients with MB experienced a decrease in size between 25% and 50%, defined as minor responses. Nicholson et al. (2007),26 in their phase II study of TMZ, enrolled 104 children and adolescents with recurrent CNS tumors including 25 MB/PNET patients. The overall response rate was 16% (1 CR, 5 PRs). Interestingly, 4 out of the 6 responses occurred in children with MB/PNET. In addition, a few case reports have been published concerning impressive responses in relapsed MB/PNET in children and adults.27–29 In summary, the literature supports the activity of TMZ in MB/PNET with a number of CRs and PRs observed in some patients. However, the response rate reported in other clinical trials appears less impressive compared with our study. This could potentially be related to the intensity of the pre-recurrence treatment received, particularly exposure to nitrosoureas.
What made our study unique was the different schedule of administration. Our patients received their daily dose of TMZ in 3 divided doses for 5 consecutive days to obtain a longer block of O6-methylguanine DNA methyltransferase (MGMT). We had already adopted this approach in 2 different trials in children with high-grade glioma and proved its safety.14,30 In view of the schedule-dependency of TMZ in preclinical studies and the peculiar mechanism of cytotoxicity of TMZ through the production of O6-methylguanidine-DNA adducts, administering a single dose once a day could allow restoration of the cytoprotective DNA repair protein MGMT. Dosing TMZ more frequently than once a day for 5 days may result in a prolonged inhibition of the MGMT, hence improving the treatment efficacy.
Unfortunately, MGMT expression and methylation of gene promoter were not tested in our patients, so we cannot correlate patients’ responses with expression or methylation of the enzyme. Some studies seem to indicate that the MGMT expression alone is insufficient to predict the response rate to TMZ, as mechanisms other than MGMT have been demonstrated to mediate response or tolerance of O6-methylguanine lesions.31,32
In conclusion, TMZ appears to be an active agent against recurrent MB/PNET, both in children and in young adults who are heavily pretreated. Our data suggest that TMZ may warrant further study in a larger cohort of patients with MB/PNET, in combination with other agents such as oral etoposide, irinotecan, or new agents interfering with MB molecular targets.33–37 Taking into account the activity and the tolerability of TMZ, we think this compound should be included in prospective phase III trials for the treatment of high-risk subset patients such as very young children or those with unresectable/disseminated MB/PNET at diagnosis.
Infants and young children with MB or PNET responsive to TMZ, might obtain a meaningful delay of CSI thus decreasing the risk of neuroendocrine and neuropsychological morbidity associated with craniospinal RT. Moreover, responding children might be candidate to a high-dose chemotherapy regimen if not previously performed. Other potential studies could investigate the role of TMZ with concomitant CSI or as maintenance therapy after high-dose chemotherapy or radiotherapy in children with MB/PNET.
Funding
None declared.
Acknowledgments
Fondazione per l'Oncologia Pediatrica (Rome) and Fondazione Bianca Garavaglia (Milan).
Conflict of interest statement. None declared.
References
- 1.Taylor RE, Bailey CC, Robinson K, et al. Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: The International Society of Paediatric Oncology/United Kingdom Children's Cancer Study Group PNET-3 Study. J Clin Oncol. 2003;21:1581–1591. doi: 10.1200/JCO.2003.05.116. [DOI] [PubMed] [Google Scholar]
- 2.Rutkowski S, Bode U, Deinlein F, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;35:2978–2986. doi: 10.1056/NEJMoa042176. [DOI] [PubMed] [Google Scholar]
- 3.Evans AE, Jenkin RD, Sposto R, et al. The treatment of medulloblastoma. Results of a prospective randomized trial of radiation therapy with and without CCNU, vincristine, and prednisone. J Neurosurg. 1990;72:572–582. doi: 10.3171/jns.1990.72.4.0572. [DOI] [PubMed] [Google Scholar]
- 4.Bailey CC, Gnekow A, Wellek S, et al. Prospective randomised trial of chemotherapy given before radiotherapy in childhood medulloblastoma. International Society of Paediatric Oncology (SIOP) and the (German) Society of Paediatric Oncology (GPO): SIOP II. Med Pediatr Oncol. 1995;25:166–178. doi: 10.1002/mpo.2950250303. [DOI] [PubMed] [Google Scholar]
- 5.Gatta G, Capocaccia R, Coleman MP, et al. Childhood cancer survival in Europe and the United States. Cancer. 2002;95(8):1767–1772. doi: 10.1002/cncr.10833. [DOI] [PubMed] [Google Scholar]
- 6.Dunkel IJ, Boyett JM, Yates A, et al. High-dose carboplatin, thiotepa, and etoposide with autologous stem-cell rescue for patients with recurrent medulloblastoma. Children's Cancer Group. J Clin Oncol. 1998;16(1):222–228. doi: 10.1200/JCO.1998.16.1.222. [DOI] [PubMed] [Google Scholar]
- 7.Massimino M, Casanova M, Polastri D, et al. Relapse in medulloblastoma: what can be done after abandoning high-dose chemotherapy? A mono-institutional experience. Childs Nerv Syst. 2013;29:1107–1112. doi: 10.1007/s00381-013-2104-x. [DOI] [PubMed] [Google Scholar]
- 8.Bouffet E, Doz F, Demaille MC, et al. Improving survival in recurrent medulloblastoma: earlier detection, better treatment or still an impasse? Br J Cancer. 1998;77:1321–1326. doi: 10.1038/bjc.1998.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gajjar A, Pizer B. Role of high-dose chemotherapy for recurrent medulloblastoma and other CNS primitive neuroectodermal tumors. Pediatr Blood Cancer. 2010;54(4):649–651. doi: 10.1002/pbc.22378. [DOI] [PubMed] [Google Scholar]
- 10.Walter AW, Mulhern RK, Gajjar A, et al. Survival and neurodevelopmental outcome of young children with medulloblastoma at St Jude Children's Research Hospital. J Clin Oncol. 1999;17:3720–3728. doi: 10.1200/JCO.1999.17.12.3720. [DOI] [PubMed] [Google Scholar]
- 11.Ostermann S, Csajka C, Buclin T, et al. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res. 2004;10:3728–3736. doi: 10.1158/1078-0432.CCR-03-0807. [DOI] [PubMed] [Google Scholar]
- 12.Panetta JC, Kirstein MN, Gajjar A, et al. Population pharmacokinetics of temozolomide and metabolites in infants and children with primary central nervous system tumors. Cancer Chemother Pharmacol. 2003;52:435–441. doi: 10.1007/s00280-003-0670-4. [DOI] [PubMed] [Google Scholar]
- 13.Patel M, McCully C, Godwin K, et al. Plasma and cerebrospinal fluid pharmacokinetics of intravenous temozolomide in non-human primates. J Neurooncol. 2003;61:203–207. doi: 10.1023/a:1022592913323. [DOI] [PubMed] [Google Scholar]
- 14.Ruggiero A, Cefalo G, Garré ML, et al. Phase II trial of temozolomide in children with recurrent high-grade glioma. J Neurooncol. 2006;77:89–94. doi: 10.1007/s11060-005-9011-2. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson HS, Krailo M, Ames MM, et al. Phase I study of temozolomide in children and adolescents with recurrent solid tumors: a report from the Children's Cancer Group. J Clin Oncol. 1998;16:3037–3043. doi: 10.1200/JCO.1998.16.9.3037. [DOI] [PubMed] [Google Scholar]
- 16.Estlin EJ, Lashford L, Ablett S, et al. Phase I study of temozolomide in paediatric patients with advanced cancer. United Kingdom Children's Cancer Study Group. Br J Cancer. 1998;78(5):652–661. doi: 10.1038/bjc.1998.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens MF, Hickman JA, Langdon SP, et al. Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-methyl-imidazo[5.I-d]-l.2.3.5-tetrazin-4(3H)-one (CCRG 81045;M&B39831). A novel drug with potential as an alternative to dacarbazine. Cancer Res. 1987;47:5846–5852. [PubMed] [Google Scholar]
- 18.Newlands ES, Stevens MF, Wedge SR, et al. Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat Rev. 1997;23(1):35–61. doi: 10.1016/s0305-7372(97)90019-0. [DOI] [PubMed] [Google Scholar]
- 19.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 20.Palmer SL, Goloubeva O, Reddick WE, et al. Patterns of intellectual development among survivors of pediatric medulloblastoma: a longitudinal analysis. J Clin Oncol. 2001;19:2302–2308. doi: 10.1200/JCO.2001.19.8.2302. [DOI] [PubMed] [Google Scholar]
- 21.Dunkel IJ, Gardner SL, Garvin JH, Jr, et al. High-dose carboplatin, thiotepa, and etoposide with autologous stem cell rescue for patients with previously irradiated recurrent medulloblastoma. Neuro Oncol. 2010;12(3):297–303. doi: 10.1093/neuonc/nop031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grill J, Dufour C, Kalifa C. High-dose chemotherapy in children with newly-diagnosed medulloblastoma. Lancet Oncol. 2006;7:787–789. doi: 10.1016/S1470-2045(06)70872-5. [DOI] [PubMed] [Google Scholar]
- 23.Finlay JL, Goldman S, Wong MC, et al. Pilot study of high-dose thiotepa and etoposide with autologous bone marrow rescue in children and young adults with recurrent CNS tumors. The Children's Cancer Group. J Clin Oncol. 1996;14:2495–2503. doi: 10.1200/JCO.1996.14.9.2495. [DOI] [PubMed] [Google Scholar]
- 24.Baruchel S, Diezi M, Hargrave D, et al. Safety and pharmacokinetics of temozolomide using a dose-escalation, metronomic schedule in recurrent paediatric brain tumours. Eur J Cancer. 2006;42:2335–2342. doi: 10.1016/j.ejca.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 25.De Sio L, Milano GM, Castellano A, et al. Temozolomide in resistant or relapsed pediatric solid tumors. Pediatr Blood Cancer. 2006;47:30–36. doi: 10.1002/pbc.20516. [DOI] [PubMed] [Google Scholar]
- 26.Nicholson HS, Kretschmar CS, Krailo M, et al. Phase 2 study of temozolomide in children and adolescents with recurrent central nervous system tumors: a report from the Children's Oncology Group. Cancer. 2007;110:1542–1545. doi: 10.1002/cncr.22961. [DOI] [PubMed] [Google Scholar]
- 27.Hongeng S, Visudtibhan A, Dhanachai M, et al. Treatment of leptomeningeal relapse of medulloblastoma with temozolomide. J Pediatr Hematol Oncol. 2002;24:591–593. doi: 10.1097/00043426-200210000-00024. [DOI] [PubMed] [Google Scholar]
- 28.Poelen J, Bernsen HJ, Prick MJ. Metastatic medulloblastoma in an adult; treatment with temozolomide. Acta Neurol Belg. 2007;107(2):51–54. [PubMed] [Google Scholar]
- 29.Durando X, Thivat E, Gilliot O, et al. Temozolomide treatment of an adult with a relapsing medulloblastoma. Cancer Invest. 2007;25(6):470–475. doi: 10.1080/07357900701518164. [DOI] [PubMed] [Google Scholar]
- 30.Riccardi A, Mazzarella G, Cefalo G, et al. Pharmacokinetics of temozolomide given three times a day in pediatric and adult patients. Cancer Chemother Pharmacol. 2003;52:459–464. doi: 10.1007/s00280-003-0677-x. [DOI] [PubMed] [Google Scholar]
- 31.Sardi I, Cetica V, Massimino M, et al. Promoter methylation and expression analysis of MGMT in advanced pediatric brain tumors. Oncol Rep. 2009;22(4):773–779. doi: 10.3892/or_00000499. [DOI] [PubMed] [Google Scholar]
- 32.Bobola MS, Tseng SH, Blank A, et al. Role of O6-methylguanine-DNA methyltransferase in resistance of human brain tumor cell lines to the clinically relevant methylating agents temozolomide and streptozotocin. Clin Cancer Res. 1996;2(4):735–741. [PubMed] [Google Scholar]
- 33.Ruggiero A, Rizzo D, Attinà G, et al. Phase I study of temozolomide combined with oral etoposide in children with recurrent or progressive medulloblastoma. Eur J Cancer. 2010;46(16):2943–2949. doi: 10.1016/j.ejca.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 34.Quinn JA, Jiang SX, Reardon DA, et al. Phase II trial of temozolomide (TMZ) plus irinotecan (CPT-11) in adults with newly diagnosed glioblastoma multiforme before radiotherapy. J Neurooncol. 2009;95(3):393–400. doi: 10.1007/s11060-009-9937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sasine JP, Savaraj N, Feun LG. Topoisomerase I inhibitors in the treatment of primary CNS malignancies: an update on recent trends. Anticancer Agents Med Chem. 2010;10(9):683–696. doi: 10.2174/187152010794479825. [DOI] [PubMed] [Google Scholar]
- 36.Lo HW, Cao X, Zhu H, et al. Constitutively activated STAT3 frequently coexpresses with epidermal growth factor receptor in high-grade gliomas and targeting STAT3 sensitizes them to Iressa and alkylators. Clin Cancer Res. 2008;14(19):6042–6054. doi: 10.1158/1078-0432.CCR-07-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldhoff P, Warrington NM, Limbrick DD, Jr, et al. Targeted inhibition of cyclic AMP phosphodiesterase-4 promotes brain tumor regression. Clin Cancer Res. 2008;14(23):7717–7725. doi: 10.1158/1078-0432.CCR-08-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]