Abstract
Recent results from 2 double-blind, placebo-controlled phase III trials (RTOG 0825) and (AVAglio) for first-line treatment of glioblastoma patients with the VEGF antibody bevacizumab, showed similar results, related to overall and progression-free survival. The RTOG 0825 trial indicated, opposed to the AVAglio trial, that patients treated with bevacizumab showed a decline in global neurocognitive function compared to untreated patients, -a decline that was most obvious after prolonged treatment. At present, there is a considerably controversy related to these observations. In the present work we point at the possibility that bevacizumab treatment of the normal brain can reduce synaptic plasticity in the hippocampus. We believe that such a phenomenon may partly explain the reduced cognitive function observed in patients in the RTOG 0825 trial. Since the same effects were not clearly defined in the AVAglio trial, further studies on putative neurocognitive effects after bevacizumab treatment are warranted.
Dear Editor,
The vascular endothelial growth factor (VEGF) inhibitor bevacizumab (Avastin) has since 2009 been extensively used in the clinic for the treatment of recurrent glioblastoma (GBM). Recently, results from two double-blind, placebo-controlled phase III trials, one publicly supported by the National Cancer Institute (NCI), conducted by the Radiation Therapy Oncology Group1 (RTOG 0825), and one industry sponsored trial2 (AVAglio) were released (The 2013 ASCO Annual meeting). The results from both trials show that bevacizumab treatment does not increase overall survival (OS) in the patient treatment group, whereas progression-free survival was improved in the bevacizumab arm, yet with some differences between the two trials. As a part of the results from the RTOG 0825 trial it was reported that patients in the bevacizumab arm showed less quality of life (QOL) and a worse symptom burden related to neurocognitive function1,2 an observation that was less recognized in the AVAglio trial. In the RTOG 0825 trial, 507 patients were evaluated at diagnosis and at intervals throughout bevacizumab treatment. Objective tests of cognitive function and subjective assessments of symptoms and quality of life were performed during periods were the tumors were not obviously progressing. Longitudinal analyses indicated that patients treated with bevacizumab showed a more pronounced decline in global neurocognitive function compared to untreated patients, a decline that was most obvious after prolonged treatment.
Based on these observations, there is an obvious need for further studies adding to the questions whether bevacizumab induces a putative cognitive impairment in the normal brain or whether such an impairment is a result of treatment effects on the tumor tissue.
It is well known that VEGF-A signaling modulates both vascular and neuronal behavior in the central nervous system (CNS). For instance it has been shown that VEGF-A can increase neurite number, length and size in the absence of glia cells, indicating an essential role in neuronal function.3–5 VEGF-A is also a potent survival factor for many neuronal populations5,6 and may provide neuroprotection from hypoxia7,8 and mechanical trauma9 which represent important environmental factors induced by progressive tumor growth. In the normal adult brain VEGF is expressed in a region specific manner that is incompatible with angiogenic growth10 indicating angiogenesis and perfusion independent functions of VEGF in the CNS. In particular, VEGF is expressed in the choroid plexus, in the olfactory bulb, in the cortex (pyramidal neurons), in the cerebellum (Purkinje cells) and in the hippocampus (by CA1 pyramidal neurons).10 The hippocampal regions known as CA1, CA3 and Dentate gyrus (DG) play an important role in spatial learning and short term memory function. At present there is considerable consensus that VEGF is not essential for maintaining basal neurogenesis but may be important in maintaining homeostatic functions in the CNS.10 In the hippocampus, it has been shown that VEGF overexpression can augment learning and memory whereas loss of function impairs this effect.11,12
Synaptic plasticity (SP) describes changes in synaptic strength that among others are important in learning and memory. SP is orchestrated by the amount of neurotransmitters available, the synaptic density and how effectively the cells respond to neurotransmitters. It is well known that a complex web of intracellular signaling pathways mediates SP. An informative study linking VEGF to synaptic plasticity was performed using long-term potentiation (LTP), which represents a method to determine dynamic plasticity changes in the hippocampus. This method measures the increase in synaptic response following potentiating pulses of electrical stimuli that are sustained at a level above the baseline response for hours or longer.13 In the context of VEGF, hippocampal slice cultures, as well as in vivo experiments have shown VEGF to increase LTP in CA1 pyramidal cells and that the absence of VEGF abrogates this effect.11,14 It is therefore highly likely that reduced VEGF levels in the CNS may affect neuronal populations. A key question in this context is the source of VEGF in the normal brain. In the brain parenchyma, co-expression studies have shown that both astrocytes and neurons can secrete VEGF, that is up-regulated by hypoxia inducible factor (HIF1) target genes.15–17
We have recently shown that vascular remodeling induced by bevacizumab treatment leads to a more hypoxic tumor microenvironment which favors metabolic changes in GBMs toward glycolysis.18 This study indicates that induced hypoxia, mediated by bevacizumab treatment, may also lead to an induction of VEGF production in neural cell populations to maintain homeostasis. It is therefore likely that the presence of the neutralizing antibody bevacizumab, during treatment abrogates the neuroprotective effects of VEGF.
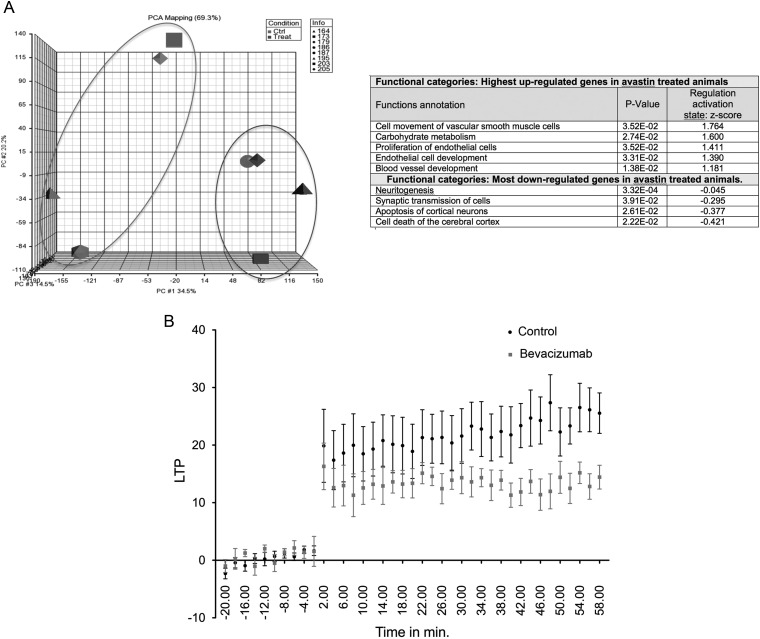
As a part of our bevacizumab treatment study18 we performed a number of control experiments where we treated normal non-tumor bearing rats with bevacizumab (10 mg/kg once a week during a 3-week period). We then performed gene expression analyses and observed significant changes in gene expression in the treatment group compared to the control group (3074 differentially expressed genes at a FDR of 5%) (Fig. 1A). Based on GO terms, not surprisingly, genes important for endothelial function and development were observed in the treatment group, but also a deregulation of genes involved in CNS function and development (Fig. 1A). More interesting, we then performed a highly standardized LTP experiment, comparing the bevacizumab treatment group with the control group. As seen in Fig. 1B, treatment caused a strong reduction in LTP compared to the controls animals, strongly implying a reduced neurocognitive function after bevacizumab treatment.
Fig. 1.
(A) Gene expression data from four rats in each of treated (bevacizumab) and control (vehicle) groups. Whole genome gene expression data was obtained using RNA extracted from rat brains of animals in the 2 groups. The Agilent 4 × 44k v3 rat Gene Expression Arrays were used. Principal Component Analysis (PCA) was carried out using Partek® and differentially expressed genes were obtained using Limma (Linear Models for Microarray Data in R) PCA shows that 2 main clusters are obtained (Ctrl and Treat). A functional analysis based on the list of differentially expressed genes was performed using Ingenuity Pathway analysis (IPA). The table shows a selection of gene ontology (GO) terms that are highly enriched in Avastin treated animals (right panel). (B) Hippocampal LTP recordings obtained from 6 rats (treatment group receiving 10 mg/kg bevacizumab twice a week during a 3-week period) and from 6 control rats (receiving sodium chloride) showing a significant LTP reduction in the bevacizumab treated group. Briefly, a bipolar concentric stimulating electrode was placed in the perforant path and a recording electrode was inserted into the dentate gyrus of the dorsal hippocampus according to stereotactic coordinates as described in.19 Evoked responses were amplified, filtered at 1 Hz to 1 kHz, and stored for later analysis. Input–output relations was examined by using three stimulus intensities (1.5, 3, and 4.5V). Paired-pulse responses were measured at three interstimulus intervals (15, 30, and 60 ms). LTP was induced by applying high frequency stimulation (HFS) of 5 trains of 8 0.4-ms, 400-Hz pulses of 1.5V. Ten measurements were taken every 5 min and LTP was computed as the change in the evoked responses measured during 60 min. after HFS in comparison with pre-HFS responses. 2-way Anova analysis using Matlab 8.2 (MathWorks Inc, Natick, MA, USA), using the treatment and time as factors of variation. The treatment significantly affected the LTP response (P value <10−5) throughout the whole duration of the LTP experiment. (ANOVA P < .02).
In conclusion, in light recent clinical trials and supporting the observations made in the RTOG 0825 trial,1 the relatively simple experiments performed here, strongly support the notion that bevacizumab can cause cognitive impairment. The fact that bevacizumab will reduce circulating as well as local VEGF levels in the brain may lead to a reduced accessibility of this growth factor to neurons that can lead to cognitive impairment. A central question, that warrants further studies, is if this impairment is reversible or not. In our mind this question should be addressed by in depth neuro-cognitive studies of cancer patients treated with bevacizumab for other malignancies (eg colorectal cancer).
Funding
This work was supported by the Norwegian Cancer Society, The Norwegian Research Council and Helse-Vest, Haukeland Hospital, Bergen, Norway.
Conflict of interest statement. None declared.
References
- 1.Gilbert MR, Dignam J, Won M, et al. RTOG 0825: Phase III double-blind placebo-controlled trial evaluating bevacizumab (Bev) in patients (Pts) with newly diagnosed glioblastoma (GBM). 2013 ASCO Annual Meeting; Chicago, Illinois, USA. 2013. J Clin Oncol 31, 2013 (suppl; abstr 1) [Google Scholar]
- 2.Henriksson R, Bottomley A, Mason W, et al. Progression-free survival (PFS) and health-related quality of life (HRQoL) in AVAglio, a phase III study of bevacizumab (Bv), temozolomide (T), and radiotherapy (RT) in newly diagnosed glioblastoma (GBM). 2013 ASCO Annual Meeting; Chicago, Illinois, USA. 2013. J Clin Oncol 31, 2013 (suppl; abstr 2005^) [Google Scholar]
- 3.Khaibullina AA, Rosenstein JM, Krum JM. Vascular endothelial growth factor promotes neurite maturation in primary CNS neuronal cultures. Brain Res Dev Brain Res. 2004;148(1):59–68. doi: 10.1016/j.devbrainres.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 4.Rosenstein JM, Krum JM. New roles for VEGF in nervous tissue-beyond blood vessels. Exp Neurol. 2004;187(2):246–253. doi: 10.1016/j.expneurol.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Rosenstein JM, Mani N, Khaibullina A, et al. Neurotrophic effects of vascular endothelial growth factor on organotypic cortical explants and primary cortical neurons. J Neurosci. 2003;23(35):11036–11044. doi: 10.1523/JNEUROSCI.23-35-11036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverman WF, Krum JM, Mani N, et al. Vascular, glial and neuronal effects of vascular endothelial growth factor in mesencephalic explant cultures. Neuroscience. 1999;90(4):1529–1541. doi: 10.1016/s0306-4522(98)00540-5. [DOI] [PubMed] [Google Scholar]
- 7.Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci USA. 2000;97(18):10242–7. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Svensson B, Peters M, Konig HG, et al. Vascular endothelial growth factor protects cultured rat hippocampal neurons against hypoxic injury via an antiexcitotoxic, caspase-independent mechanism. J Cereb Blood Flow Metab. 2002;22(10):1170–1175. doi: 10.1097/01.wcb.0000037988.07114.98. [DOI] [PubMed] [Google Scholar]
- 9.Ma Y, Liu W, Wang Y, et al. VEGF protects rat cortical neurons from mechanical trauma injury induced apoptosis via the MEK/ERK pathway. Brain Res Bull. 2011;86(5–6):441–446. doi: 10.1016/j.brainresbull.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Licht T, Keshet E. Delineating multiple functions of VEGF-A in the adult brain. Cell Mol Life Sci. 2013;70(10):1727–1737. doi: 10.1007/s00018-013-1280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Licht T, Goshen I, Avital A, et al. Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci U S A. 2011;108(12):5081–5086. doi: 10.1073/pnas.1007640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adamcio B, Sperling S, Hagemeyer N, et al. Hypoxia inducible factor stabilization leads to lasting improvement of hippocampal memory in healthy mice. Behav Brain Res. 2010;208(1):80–84. doi: 10.1016/j.bbr.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim BW, Choi M, Kim YS, et al. Vascular endothelial growth factor (VEGF) signaling regulates hippocampal neurons by elevation of intracellular calcium and activation of calcium/calmodulin protein kinase II and mammalian target of rapamycin. Cell Signal. 2008;20(4):714–725. doi: 10.1016/j.cellsig.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Licht T, Eavri R, Goshen I, et al. VEGF is required for dendritogenesis of newly born olfactory bulb interneurons. Development. 2010;137(2):261–271. doi: 10.1242/dev.039636. [DOI] [PubMed] [Google Scholar]
- 16.Weidemann A, Kerdiles YM, Knaup KX, et al. The glial cell response is an essential component of hypoxia-induced erythropoiesis in mice. J Clin Invest. 2009;119(11):3373–3383. doi: 10.1172/JCI39378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barouk S, Hintz T, Li P, et al. 17beta-estradiol increases astrocytic vascular endothelial growth factor (VEGF) in adult female rat hippocampus. Endocrinology. 2011;152(5):1745–1751. doi: 10.1210/en.2010-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keunen O, Johansson M, Oudin A, et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci U S A. 2011;108(9):3749–3754. doi: 10.1073/pnas.1014480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panja D, Dagyte G, Bidinosti M, et al. Novel translational control in Arc-dependent long term potentiation consolidation in vivo. J Biol Chem. 2009;284(46):31498–31511. doi: 10.1074/jbc.M109.056077. [DOI] [PMC free article] [PubMed] [Google Scholar]