Abstract
Moderate, yet relatively consistent, associations between cognitive performance and shyness have been reported throughout the child and adult literatures. The current study assessed longitudinal associations between cognition (i.e., executive functioning) and parent-report temperamental shyness from infancy to early childhood and used temporal order to explore directionality of the relations. Two hundred eleven children contributed data at multiple ages (5-months, 10-months, 2-years, 3-years, and 4-years). The results indicated a complex pattern of association between cognition and shyness in early development and provided tentative support for both cognitive ability and temperament as causal agents at different developmental time points.
Keywords: infancy, early childhood, temperament, shyness, executive function, cognition
Shy children and adults are wary during novel social events and/or exhibit self-conscious behavior in situations where there is a perception of being socially evaluated (Coplan & Rubin, 2010). Shyness has been identified with many labels, including behavioral inhibition, introversion, social inhibition, social reticence, social wariness, social anxiety, and social withdrawal. Notably, the term behavioral inhibition has been used to characterize a child who is wary in both social and nonsocial situations (Kagan, Snidman, Kahn, & Towsley, 2007). As such, shy behavior has been the topic of much theory and research (for reviews see Fox, Henderson, Marshall, Nichols, & Ghera, 2005, and Rubin & Coplan, 2010), and empirical reports show that shy children are at a disadvantage in social interactions with peers and adults and could be at risk for a variety of personal and academic adjustment problems (Fox et al., 2005; Hughes & Coplan, 2010; Rothbart, 2011; Rubin, Coplan, & Bowker, 2009). Because of our interest in individual differences in cognitive development, we focused on early associations between shyness and cognition.
Associations between Cognitive Performance and Shyness
There are reports of negative associations between cognitive performance and shyness in both the child (Asendorpf, 1994; Blankson, O’Brien, Leerkes, Marcovitch, & Calkins, 2011; Crozier & Hostettler, 2003; Hadwin, Brogan, & Stevenson, 2005; Hughes & Coplan, 2010; Ludwig & Lazarus, 1983; Ng & Lee, 2010; Tanwar & Malhotra, 1992) and adult research literatures (Derakshan & Eysenck, 1998; Elliman, Green, Rogers, & Finch, 1997; Eysenck & Calvo, 1992; Gray & Braver, 2002; Johnson & Gronlund, 2009; Lieberman, 2000; Lieberman & Rosenthal, 2001). In general, children who are categorized as temperamentally shy and adults who are comparably categorized as introverted or socially anxious are at a disadvantage on various measures of cognitive ability (e.g., executive function, working memory, inhibitory control, and attentional control) compared to individuals who are less shy and introverted.1
Although these studies are correlational and most capture the associations at a single point in time, it is the tendency of some authors to suggest that the cognitive performance differences are influenced by underlying differences in personal behavioral style; or perhaps that the cognitive deficits for introverts are the results of differences in arousal (Lieberman, 2000). A few researchers have entertained the possible causal role of cognition in the exhibition of shy behavior (e.g., Asendorpf, 1994; Blankson et al., 2011; Ludwig & Lazarus, 1983). However, no investigation has examined the potential developmental associations between cognition and shyness during infancy through early childhood, as previous studies reported cross-sectional analyses (e.g., Blankson et al., 2011; Ludwig & Lazarus, 1983) or used longitudinal designs without a consideration of the infant period (e.g., Asendorpf, 1994).
For example, Ludwig and Lazurus (1983) examined associations between cognition and shyness from an information processing standpoint, basing their work on the notion that cognitive style mediates the relation between stimulus and response. Citing Klein (1959), they argued that cognitive styles are organizational, selective in function, and become strongly associated with emotional, motivational, and affective meaning for the individual. Ludwig and Lazarus compared Stroop color-word performance for shy and non-shy children between the ages of 8 and 11 years. The authors found that shy children had significantly slower response times on the task but showed no differences on a standardized language assessment or general academic ability. The Stroop color-word task is an index of executive functioning and reflects focused attention, maintained memory for the rule, inhibition of a prepotent response, and processing efficiency. The authors interpreted these Stroop task performance differences as evidence of constricted cognitive control in the shy children and concluded that personality traits, such as shyness, are related to individual differences in how children process information.
However, Ludwig and Lazurus (1983) could not address the developmental associations between cognition and shyness because of their cross-sectional design. Given the dynamic and intricate relations between cognition and emotion (Bell & Calkins, 2012; Bell & Deater-Deckard, 2007; Bell & Wolfe, 2004; Blair & Dennis, 2010; Calkins & Bell, 2010; Rothbart, Ellis, Rueda, & Posner, 2003), a developmental account of developing interactions between cognition and temperamental shyness must include a longitudinal perspective that begins in early infancy.
Early Measures of Shyness and Cognition
Although shyness does not appear until about 1-year of age (Schmidt & Buss, 2010), temperamental fear (a potential early indicator of shyness and behavioral inhibition) has been measured behaviorally at 4-months of age (Kagan et al., 2007) and by parental report at 3-months (Gartstein & Rothbart, 2003). Individual differences in the early cognitive processes have been related to temperamental inhibition and shyness-related behaviors, such as fear. Ruff and Rothbart (1996) described a scenario in which infants who looked for longer periods of time at small toys they were holding, perhaps displaying a difficulty with attentional disengagement, were more fearful. This scenario was empirically demonstrated in a study of visual attention and temperament with infants 2, 4, and 6 months of age (McConnell & Bryson, 2005). These researchers found a concurrent link between the ability to disengage attention and fearfulness at 4-months of age; specifically, infants who shifted more slowly were rated as more fearful by parents. These associations extend beyond infancy. For example, infants with low sustained attention are more likely to demonstrate increases in behavioral inhibition over the course of early childhood (Pérez-Edgar et al., 2010). Further, for the children with low sustained attention, their initial behavioral inhibition score was positively associated with their social discomfort in adolescence but this was not the case for those children with high sustained attention (Pérez-Edgar et al., 2010).
Thus, the developmental literature provides some evidence for linking cognition with shyness, anxiety, and/or introversion. Much of this literature is based on a framework of personal behavioral style or temperament impacting cognitive performance, although most studies reported this correlational finding from a cross-sectional perspective and thus were inadequate for addressing questions of causality. Indeed, temperament is biologically-based, stabile, and hereditable (Kagan et al., 2007; Rothbart & Bates, 2006). However, it is important to consider the explanatory limitations of correlational research, to recognize that multiple and complex pathways of development exist, and likewise to consider the potential shortcomings of focusing on temperament as a single main effect predictor rather than as part of a developmental system (Wachs, 2006).
Current Study
We examined longitudinal associations between cognition and temperamental shyness. Rather than assuming that temperament influences cognition, we also considered that the development of shy behavior might be influenced by early individual differences in cognition (Asendorpf, 1994; Blankson et al., 2011; Ludwig & Lazarus, 1983). These cognitive differences likely influence the detection, evaluation, and response selection to social and nonsocial stimuli, and thus, would be related to the adaptability of the organism. From this perspective, early cognition would set the stage for all environmental interactions and potentially would influence a complex learning sequence and a resultant behavioral pattern or adaptive strategy for dealing with conditions that include novelty or complex stimuli (e.g., social situations).
Our study was focused on cognition-emotion integration across early development. Thus, multiple data points for the cognitive and temperament variables were available from early infancy and through early childhood. We assessed the executive functioning (EF) aspects of cognitive development. EF has been associated with shyness in the child literature (e.g., Blankson et al., 2011; Ludwig & Lazarus, 1983) and includes cognitive skills that have been related to fear and shyness in the infant literature (e.g., attentional engagement and disengagement; e.g., McConnell & Bryson, 2005). We measured EF with age-appropriate tasks that require focused attention, working memory, and inhibitory control.
We assessed temperamental shyness at each age using parent-report questionnaires. The use of parent-report temperament assessments increases the consistency of the repeated measures and allows for the consideration of shy behavior in a wide-range of situations and contexts. Caregiver-reports of temperament have been identified as reliable and valid, and they have been praised for providing valuable information on the general behavior patterns of children from an ecologically and ethically sound perspective (Rothbart & Bates, 2006).
Research Questions and Hypotheses
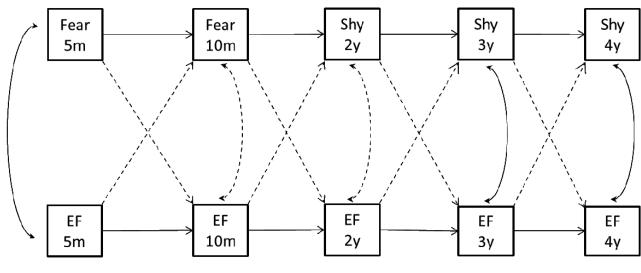
Our primary goal involved identifying directional relations between cognition and temperament from one time period to the next, beginning in early infancy and continuing through early childhood. Analyses were motivated by our research question: Can we identify predictive associations between cognition and temperamental shyness from infancy to early childhood that may lend support to a temporally-based causal argument? Testing of our model (see Figure 1) allowed us to examine the concurrent relations between cognition and temperament at each time period. Based on research demonstrating concurrent associations between temperament and cognition, we had the following hypotheses. First, at 5-months of age, a negative association was expected between fear and EF based on work by McConnel and Bryson (2005) establishing a link between slow attentional disengagement and fearfulness at 4-months. Likewise, at 3- and 4-years of age, a negative association was expected between shyness and EF based on work by Blankson et al. (2011) demonstrating a negative relation between EF and shyness at 3½-years. We are not aware of research measuring these specific associations late in the second half of the first year of life or during the toddler years, thus we had no hypotheses regarding the concurrent relations between cognition and temperament at 10-months and 2-years of age.
Figure 1.
Model tested and hypothesized associations.
Testing the predictive, cross-lagged associations also allowed us to measure the stability of temperamental fear and shyness as well as the stability of cognition across early development. The temperamental traits of fear and shyness were expected to demonstrate stability from infancy to early childhood based on such findings by Sanson, Pedlow, Cann, Prior, and Oberklaid (1996) with children between the ages of 4-months to 6-years. The EF measures, including memory and attention components, were expected to demonstrate positive associations from one time period to the next based on longitudinal work by Rose and colleagues (Rose & Feldman, 1995, 1997; Rose, Feldman, & Jankowski, 2012) with children between the ages of 7-months and 11-years of age.
Method
Participants
Participants were two cohorts of children in an on-going longitudinal study of cognition and emotion integration in early development. The research took place at a large rural university in the mid-Appalachian region. Approximately half of the children were in each cohort and the demographics for the two cohorts were similar. At 5 months of age, 211 infants and their mothers were recruited for participation (112 girls; 89 percent Caucasian). Mothers were approximately 30 years of age and fathers were approximately 33 years of age when the infants were born (SD = 5 and 6 years). For these cohorts, 72 percent of mothers and 65 percent of fathers had college degrees. All infants were full term and healthy at the time of testing and were seen within 3 weeks of their 5-month “birthday”; 187 of infants and mothers participated at 10-months; 153 at 2-years; 134 at 3-years; and 126 at 4-years. Attrition was primarily due to families moving out of the region. There were no differences in fear or shyness at any age measured between children who dropped-out of the study by age 4 and those who remained (all t’s < .64, all p’s > .52). Parents were paid for their children's participation in the study. Beginning at age 2, children were given a small gift for their participation.2 Study procedures were approved by the institutional review board.
Cognitive tasks
The cognitive tasks used in these analyses required age-appropriate EF skills of focused attention, working memory, and/or inhibitory control. These tasks are common in the developmental literature (e.g., Bell, 2012; Carlson, 2005). Descriptive statistics for all tasks can be found in Table 1. For all tasks, interrater reliability (Cronbach’s α ≥ .90) was established for at least 20% of our entire longitudinal sample.
Table 1.
Descriptive statistics for the executive function and temperament measures.
| Measure | n | M | SD | Min | Max | |
|---|---|---|---|---|---|---|
| 5-months | Fear (IBQ) | 196 | 2.18 | .79 | 1.00 | 4.25 |
| AB Scale Score | 201 | .87 | 1.07 | 0.00 | 7.00 | |
| AB Reversal | 40 | .28 | .39 | .00 | 1.00 | |
| EF Composite | 201 | .00 | 1.00 | −.87 | 5.27 | |
| 10-months | Fear (IBQ) | 182 | 2.86 | .95 | 1.07 | 5.50 |
| AB Scale Score | 187 | 3.84 | 1.58 | .00 | 8.00 | |
| AB Reversal | 173 | .48 | .37 | .00 | 1.00 | |
| EF Composite | 187 | .00 | 1.00 | −2.37 | 1.86 | |
| 2-years | Shyness (ECBQ) | 152 | 3.36 | .95 | 1.58 | 5.75 |
| DCCS Pre-switch | 137 | .69 | .25 | .00 | 1.00 | |
| Crayon Score | 150 | 3.10 | 2.12 | 1.00 | 6.00 | |
| Crayon Delay | 150 | 26.31 | 25.73 | .00 | 60.00 | |
| EF Composite | 152 | .00 | 1.00 | −1.75 | 1.68 | |
| 3-years | Shyness (CBQ) | 133 | 3.73 | 1.34 | 1.23 | 7.00 |
| DCCS Post-switch | 126 | .60 | .44 | .00 | 1.00 | |
| Tongue Task | 121 | .68 | .39 | .00 | 1.00 | |
| Simon Says | 114 | .33 | .43 | .00 | 1.00 | |
| EF Composite | 132 | .00 | 1.00 | −2.35 | 2.14 | |
| 4-years | Shyness (CBQ) | 124 | 3.46 | 1.33 | 1.00 | 6.83 |
| DCCS Post-switch | 117 | .77 | .38 | .00 | 1.00 | |
| Tongue Task | 120 | .90 | .24 | .00 | 1.00 | |
| Simon Says | 117 | .81 | .37 | .00 | 1.00 | |
| EF Composite | 120 | .00 | 1.00 | −4.13 | .76 |
Note. IBQ = Infant Behavior Questionnaire; AB = A-not-B; EF = Executive function; ECBQ = Early Childhood Behavior Questionnaire; DCCS = Dimensional Change Card Sort; CBQ = Children’s Behavior Questionnaire.
5-months and 10-months
At 5- and 10-months of age, a looking version of the A-not-B task was used in which the infant searched for a hidden toy by making eye movements to one of two possible hiding locations to assess rudiments of EF – including working memory and inhibitory control skills (Bell & Adams, 1999; Bell, 2001, 2012). A toy was hidden twice at the original location, and if the infant searched correctly both times, the hiding site was reversed and the toy was moved to the other hiding location. After the infant searched on the reversal trial, the process was repeated. Successful performance on this task was indicated by the infant correctly searching on the initial trials and the reversal trials. For the current analyses, the proportion of correct reversal trials and the highest earned A-not-B scale score (1 = Object partially covered with one cloth; 2 = Object completely covered with one cloth; 3 = Object hidden under one of two identical cloths; 4 = A-not-B at 0 delay; 5 = A-not-B at 2-sec delay; 6 = A-not-B at 4-sec delay; 7 = A-not-B with 6-sec delay; 8 = A-not-B at 8-sec delay; see Bell & Adams, 1999) were used as measures of infant EF.
2-years
For the Dimensional Change Card Sort task (Zelazo, Frye, & Rapus, 1996; Zelazo, Muller, Frye & Marcovitch, 2003), children were instructed to sort cards based on two dimensions (i.e., color, shape). Children first sorted six cards by one dimension (pre-switch; counterbalanced across participants) and then by the other dimension during the post-switch. Performance at age 2 was based on pre-switch proportion correct.
For the Crayon Task (Calkins, 1997), the experimenter placed a newly opened box of crayons on the table along with a blank half sheet of paper and asked the toddler to help her draw a picture with the new crayons. However, before the toddler could touch the crayons the experimenter told the toddler that she had to step out of the room to find something. She clearly instructed the toddler to not touch the crayons, box, or paper until she returned to the room. The experimenter then left the room for 60 seconds. Toddlers’ behavior during the delay was scored as latency (sec) to touch the either the paper or crayons. Behavior was also scored as to the level of inhibitory control (1 = colors with crayons; 2 = takes crayons out of box; 3 = picks up box; 4 = touches box; 5 = touches paper; 6 = does not touch; Morasch & Bell, 2011).
3 years and 4 years
For Dimensional Change Card Sort task (Zelazo, Frye, & Rapus, 1996), children were instructed to sort cards based on two dimensions (i.e., color, shape). Children first sorted six cards by one dimension (pre-switch; counterbalanced across participants) and then were instructed to switch and to sort the remaining six cards by the other dimension (post-switch). Performance was the proportion of correct post-switch responses.
The Tongue Task (Kochanska, Murray, & Harlan, 2000; Wolfe & Bell, 2007) required children to hold a goldfish cracker on their tongue without chewing it (three trials with delays of 10, 20, and 30 s). Performance was the proportion of successful trials.
The Simon-Says Task followed the Bear/Dragon procedure (Carlson, Moses, & Breton, 2002) and is detailed in Wolfe and Bell (2007). Children were instructed to do what the nice horse (48 months: pig) “tells us” and to not do what the mean cow (48 months: bull) “tells us.” Ten test trials (half for each type, alternating order) followed practice trials of each type, and children’s response on each trial was coded as either correct or incorrect (Carlson, 2005). Performance was the proportion of correct responses on inhibition (cow/bull) trials.
Cognitive composite measures
Composite scores of a latent construct are more reliable than individual indicators (Carlson, Mandell, & Williams, 2004; Rushton, Brainerd, & Pressley, 1983); thus, we created cognitive composites for each age. Using the tasks previously described, the first principal component explained 85% of the variance (λ = .81) at 5 months and 90% of the variance (λ = .92) and at 10 months. The first principal component explained 64% of the variance (λ = .42-.94) at 2 years, 43% (λ = .48-.74) at 3 years, and 45% (λ = .19-.82) at 4 years. To create the composite at each age, individual cognitive task scores were standardized, averaged, and standardized again to yield an EF composite z score. For missing data, composite scores included the remaining performance measures.
Temperament Assessments
We used Rothbart's parent-report temperament questionnaires across our longitudinal study for consistency in construct assessment. At 5- and 10-months of age, parents completed the Infant Behavioral Questionnaire – Revised (IBQ-R; Gartstein & Rothbart, 2003). We focused on the fear scale of the IBQ-R, which measures the infant’s startle or distress to sudden changes in stimulation and response to novel physical or social stimuli. As we noted in the introduction, temperamental fear is a potential early indicator of shyness. For our combined cohorts, the fear scale had an internal consistency of α = .88 at 5-months and α = .90 at 10-months.
At age 2, parents completed the Early Childhood Behavior Questionnaire (ECBQ; Putnam, Gartstein, & Rothbart, 2006). We focused on the shyness scale, an index of slow or inhibited approach and/or discomfort in social situations involving novelty or uncertainty. The shyness scale of the ECBQ had an internal consistency in our combined cohorts of α = .85.
At ages 3 and 4, the parents completed the Children’s Behavior Questionnaire (CBQ; Rothbart, Ahadi, Hershey, & Fisher, 2001) and we again focused on the shyness scale. For our first cohort of children (the first half of this total sample), parents completed the long form of the CBQ when children were ages 3 and 4. The internal consistencies for the shyness scale of the CBQ long-form were α = .93 at age 3 and α = .94 at age 4. For the second cohort of children, parents completed the short form of the CBQ when children were ages 3 and 4. The internal consistencies for the shyness scale of the CBQ short-form α = .86 at age 3 and α = .89 at age 4.
Results
Bivariate Correlations
Bivariate correlations were calculated between all EF and temperament measures (see Table 2). The correlation table shows concurrent associations between shyness and EF. Based on the literature, we predicted negative correlations at 5 months, as well as 3 and 4 years. Table 2 shows that these correlations were negative, with only the 4-year correlation being significant. In addition, the temperament measures showed strong intercorrelations across the ages, with the exception of 5-month fear being only marginally related to 3- and 4-year shyness. The intercorrelations for the composite EF scores were less robust (see the Discussion for an elaboration), yet the tendency existed for the EF composites to be associated across adjacent time periods (e.g., 5-month EF was associated with 10-month EF, and 3-year EF was associated with 4-year EF). The correlations for the EF composites and the fear/shyness measures did not yield any discernible patterns with the exception of the 4-year EF composite negatively relating to 10-month fear, 3-year shyness, and 4-year shyness.
Table 2.
Summary of bivariate correlations for the cognitive and temperament measures.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. 5mo fear | 1 | ||||||||||||||||||||||
| 2. l0mo fear | .49** | 1 | |||||||||||||||||||||
| 3. 2yr shyness | .19** | .42** | 1 | ||||||||||||||||||||
| 4. Syr shyness | .16† | .32** | .61** | 1 | |||||||||||||||||||
| 5. 4yr shyness | .16† | .29** | .39** | .77** | 1 | ||||||||||||||||||
| 6. 5mo EF Composite | −.12 | .06 | −.03 | −.05 | −.09 | 1 | |||||||||||||||||
| 7. 10mo EF Composite | .01 | .05 | −.06 | −.01 | −.002 | .16** | 1 | ||||||||||||||||
| 8. 2yr EF Composite | −.13 | −.13 | .07 | .01 | .05 | −.06 | .03 | 1 | |||||||||||||||
| 9. 3yr EF Composite | .13 | .02 | .01 | −.12 | −.04 | −.10 | .12 | .13 | 1 | ||||||||||||||
| 10. 4yr EF Composite | .01 | −.19** | −.08 | −.16† | −.32** | −.06 | −.04 | .14 | .19** | 1 | |||||||||||||
| 11. 5mo AB Scale | −.11 | .05 | −.05 | −.09 | −.13 | .95** | .13t | −.07 | −.09 | −.01 | 1 | ||||||||||||
| 12. 5mo AB Reversal | −.15 | .24 | −.01 | −.12 | −.01 | .93** | .3l† | .13 | −.03 | −.21 | .70** | 1 | |||||||||||
| I3. 10mo AB Scale | .01 | .06 | −.07 | −.04 | −.02 | .19** | .96** | .002 | .12 | −.004 | .16** | .41** | 1 | ||||||||||
| 14. 10mo AB Reversal | .04 | .01 | −.08 | .06 | .04 | .08 | .96** | .10 | .04 | −.10 | .05 | .17 | .81** | 1 | |||||||||
| 15. 2yr DCCS Pre-switch | −.24** | −.10 | .03 | −.06 | −.09 | −.04 | .09 | .62** | .07 | .15 | −.07 | .30 | .06 | .11 | 1 | ||||||||
| 16. 2yr Crayon Scale | −.06 | −.13 | .03 | .08 | .13 | −.07 | −.01 | .88** | .13 | .18† | −.07 | −.03 | −.03 | .07 | .22** | 1 | |||||||
| 17. 2yr Crayon Time | .01 | −.08 | .07 | .00 | .07 | −.03 | .001 | .88** | .15 | .08 | −.02 | .06 | −.02 | −.06 | .23** | .84** | 1 | ||||||
| 18. 3yr DCCS Post-switch | .12 | −.02 | .05 | −.01 | −.09 | −.10 | .18** | .12 | .70** | .13 | −.12 | −.12 | .24** | .03 | .01 | .12 | .14 | 1 | |||||
| 19. Syr Tongue Task | −.03 | −.10 | −.13 | −.19** | −.05 | −.08 | −.03 | .07 | .66** | .24** | −.05 | .02 | −.08 | .04 | .06 | .11 | .05 | .10 | 1 | ||||
| 20. Syr Simon Says | .18 | .10 | .06 | .09 | .09 | −.07 | .13 | .07 | .71** | .06 | −.06 | .12 | .10 | .13 | .01 | .08 | .16 | .25** | .12 | 1 | |||
| 21. 4yr DCCS Post-switch | −.17† | −.12 | −.03 | −.12 | −.19** | .11 | −.03 | .12 | .02 | .69** | .13 | .15 | .01 | −.06 | .23** | .09 | .02 | .02 | .09 | −.08 | 1 | ||
| 22. 4yr Tongue Task | .18 | −.05 | .05 | .03 | −.15 | −.29** | −.10 | .09 | .16t | .57** | −.27** | −.32† | −.14 | −.06 | .05 | .13 | .07 | .07 | .23** | .08 | .004 | 1 | |
| 23. 4yr Simon Says | .01 | −.21** | −.18t | −.18t | −.27** | .05 | .04 | .06 | .49** | .76** | .10 | −.14 | .10 | −.10 | .01 | .13 | .05 | .20** | .12 | .09 | .37** | .08 | 1 |
Note. AB = A-not-B; EF Comp = Executive function composite; DCCS = Dimensional Change Card Sort;
p <. 001,
p < .05,
p < .09 All tests are 2-tailed.
Cross-lagged Autoregressive Model
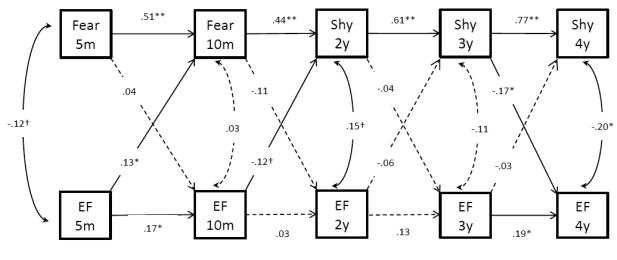
A cross-lagged autoregressive model was estimated in Mplus 7.0 to evaluate the cross-lagged, autoregressive, and concurrent pathways between cognition and temperament across five waves of data. Cross-lagged pathways estimated the reciprocal association between temperament and cognition over time. Autoregressive pathways evaluated the effects of previous temperament on subsequent temperament, as well as the effects of previous cognition on subsequent cognition. Concurrent pathways estimated the association between cognition and temperament at the same time. Full information maximum likelihood estimation (Arbuckle, 1996) was used to handle the missing data. Indexes of model fit included: root mean square error of approximation (RMSEA) with the cutoff value of .05, standardized root mean square residual (SRMR) with the cutoff value of .08, comparative fit index (CFI) with cutoff value of .95, and the Tucker-Lewis index (TLI) with cutoff value of .95 (Hu & Bentler, 1999). Our hypothesized model had a good fit with χ2(24) = 22.79, p > .05, RMSEA = .00, CFI = 1.00, and TLI = 1.00.
Cross-lagged pathways
Shyness at 3-years was a significant predictor of EF at 4-years (b = −.17, p =.05). EF at 5- and 10 - months predicted temperament at 10-months and 2-years, respectively, yet in different directions. EF at 5-months was positively associated with fear at 10-months (b = .13, p =.05), and EF at 10-months was marginally associated with shyness at 2-years in a negative direction (b = −.14, p =.06).
Autoregressive pathways
Consistent with our hypothesis, all coefficients for the autoregressive pathways of temperament were significant (p < .01), ranging from .44 to .77, indicating the stability of temperament from infancy to early childhood. Similarly, previous EF was a predictor for subsequent EF at 10-months (b = .17, p < .05) and 4-years (b =.19, p < .05), but not at 2-years or 3-years.
Residual correlations
Concurrent residual correlations between cognition and temperament were significant or marginally significant at 5-months (b = −.12, p = .09), 2-years (b = .15, p = .07), and 4-years (b = −.20, p < .05).
Discussion
The purpose of our study was to examine longitudinal associations between cognition and temperamental shyness and to use temporal order to explore the issue of directionality in their relation. The primary goal involved identifying predictive, cross-lagged relations between EF and temperamental shyness from infancy to early childhood. The results identified a complex pattern of associations between cognition and temperament across early development.
Cross-lagged Associations between Temperament and Cognition
The first cross-lagged pathway to reach significance was the association between 5-month EF and 10-month fear, and this association was positive. Thus, those infants with high EF performance at 5-months were more likely to be reported as more fearful by their parents at 10-months. Longitudinal work by Karrass and Braungart-Rieker (2004) found that distress to novelty at 4-months of age was positively associated with performance on an intelligence test at 36-months. They interpreted these findings by suggesting that infants who show distress to novelty at this early age might reflect a more precocious cognitive level than those who do not show this distress in that they might more readily recognize when a stimulus or event is discrepant with something earlier experienced. A similar explanation can be offered for the findings of the current study. Perhaps those 5-month-olds who were more cognitively advanced tended to approach the world in a more cautious and hesitant way than their counterparts and, thus, exhibited behaviors that were considered fearful in the subsequent months. Notably, the cross-lagged pathway between 5-month fear and 10-month EF revealed no association between these two measures.
The second cross-lagged pathway to reach significance was between 3-year shyness and 4-year EF, and this association was negative. Thus, those children who were rated highly by their parents on the shyness scale at age 3 tended to have lower scores on the EF assessment at age 4. Also, recall the results of the bivariate correlations that demonstrated relatively consistent negative associations between early temperament (i.e., 10-month fear and 3-year shyness) and 4-year EF. These findings are in line with the dominant perspective that one’s temperament impacts cognitive development and performance.
The cross-lagged pathway between 10-month EF and 2-year shyness demonstrated a marginally significant, negative association. Ten-month-old infants with lower scores on the EF task were rated as more shy by their parents at age 2. Importantly, the first measure of shyness per se (as opposed to infant fear) was taken at age 2. Thus, this finding, negatively linking infant cognition with toddler shyness, is particularly intriguing and lends support to the idea that cognitive processes affect later temperamental shyness. Indeed, within this time period, lower levels of EF in the second half of the first year of life may have set the stage for the development of shy behavior in the toddler years. Different from the positive 5-month EF to 10-month fear pathway linking high EF to high fear, more efficient cognitive processing at 10-months seemed to serve as a protective factor or buffer against exhibiting shy and wary behavior at age 2. This interpretation is consistent with existing longitudinal work that found negative temperament-related outcomes for those children with less advantageous cognitive skills (Asendorpf, 1994; Belsky, Friedman, & Hsieh, 2001; Pérez-Edgar et al20., 2010).
Thus, the earlier predictive relations were from EF to fear/shyness and the later predictive relations were from shyness to EF, with age 2 appearing to be a pivotal point in the developmental pattern. Whether this critical point is significant developmentally or whether it is artificially induced due to EF assessment is difficult to discern. The age 2 EF tasks were unique in our longitudinal study, with the 5 and 10 month tasks and the age 3 and age 4 tasks being identical. Age 24 months is a unique time for assessment of EF in the developmental literature, with fewer tasks available for administration (see Carlson, 2005) and compliance with assessment perhaps being more of an issue than at other ages (Morasch & Bell, 2011). More research is required to determine the nature of these predictive patterns.
Stability of Temperament and Cognition
The secondary hypotheses in this study included establishing the stability of fear and shyness as well as the stability of EF across early development. First, the temperamental traits of fear and shyness were expected to demonstrate stability from infancy to early childhood based on such findings by Sanson et al. (1996) with children between the ages of 4-months to 6-years. As anticipated, there was good stability of the temperament measures from one time period to the next. Even the association between 10-month fear and 2-year shyness held despite the assessment of two distinct, but related, constructs. This consistency also may be reflective of the facts that the temperament instruments used were all created by the same research group (e.g., Rothbart and colleagues), and they were all completed by the mothers.
The EF measures, including memory and attention components, also were expected to demonstrate positive associations from one time period to the next. In general, this hypothesis was supported showing positive, significant associations between 5-month and 10-month cognition and between 3-year and 4-year cognition. No association was found between 10-month and 2-year cognition, and the positive association between 2-year and 3-year cognition failed to reach significance.
Such results are not inconsistent with the literature tracking the longitudinal development of EF skills (Best & Miller, 2010). It is challenging to longitudinally assess the development of any cognitive construct. For one, tasks that are developmentally appropriate for one age are not necessarily appropriate for another. Also, although cognitive ability undergoes an increase in stability from early to middle childhood, there is great variability in child performance during early periods in development (Best & Miller, 2010; Carlson, 2005). For example, when comparing the performance of 2-to-6-year-olds on a battery of EF tasks, those tasks that required the skills of working memory and inhibitory control (the skills needed for successful performance on the tasks used in the current studies) were the most difficult for each age group and, thus, were most susceptible to large variability in performance (Carlson, 2005). Also, it has been suggested that some cognitive skills required by a task may develop more quickly than others (Best & Miller, 2010). With regard to the multicomponent tasks used in the current analyses, it is conceivable that any one of these components was more or less advanced than the other for some children from one testing time to the next, thereby, impacting the consistency measure of the construct.
Concurrent Associations between Cognition and Temperament
As shown in Table 2, the findings for concurrent associations are in line with what previous studies have reported. At 5-months of age, the two constructs are negatively related but the correlation is not statistically significant. This association disappears at 10-months and at 2-years, and then reemerges as a weak negative correlation at 3-years that is not statistically significant. At 4-years, there is significant, negative association between shy and EF. As expected, there was a trend of negative association between 5-month EF and 5-month fear, suggesting that young infants with high EF performance were less fearful than their lower EF counterparts. This result replicated the work by McConnell and Bryson (2005) with 4-month-olds in which lower cognitive skills (i.e., difficulty with attentional disengagement) were related to fearfulness at the same age. The finding extends their work by using a slightly older sample and a multidimensional cognitive construct.
Likewise, at 3- and 4-years of age, a negative association was expected between shyness and EF based on work by Blankson et al. (2011) demonstrating such a relation between EF and shyness at 3½-years. This hypothesis was partially supported. Although the negative association seen between EF and shyness failed to reach significance at age 3, the negative association between EF and shyness found at age 4 was significant. Thus, the work by Blankson and colleagues was replicated here with a different operationalization of the EF construct.
No specific hypotheses were made regarding the concurrent relations between EF and fear at 10-months of age and shyness at 2-years. In the bivariate correlations shown in Table 2, there was no association between shyness and EF at 10 month and at age 2. In our cross-lagged autoregressive model presented in Figure 2, the concurrent association at 5-month indicates the bivariate correlation between Shyness scores and EF scores, but concurrent associations in the following time points indicate bivariate correlation between residuals (i.e., unexplained variances). In general, the residual correlations are in line with the bivariate correlations of the corresponding variables. An exception, however, is a trend toward a positive association between the residual variances of EF and shyness. That is, after taking into account the positive autoregressive effect on shyness and the negative cross-lagged effects of the previous EF and shyness, there appeared to be something common about those with high shyness and those with high EF. This is an interesting finding, and in light of the other findings of this study, supports the complex cognitive and emotional context of toddler development. Given the positive links between EF skills and self-regulation (e.g., Rueda, Posner, & Rothbart, 2005), perhaps those 2-year-olds with high EF skills may be more behaviorally regulated (Morasch & Bell, 2011) and appear more cautious in novel and social situations. The explanation that was given for the earlier positive relation between fear and cognition in infancy (i.e., more precocious infants might approach the world in a more hesitant or cautious manner) might be applicable here in toddlerhood as well. No association was found between EF and fear at 10-months of age. Perhaps this absence is reflective of the transition from a negative EF-fear association at 5-months to the positive EF-shyness association at 2-years.
Figure 2.
Results of the cross-lagged autoregressive model analysis.
Note. **p ≤. 001, *p ≤ .05, †p ≤ .09
In sum, the patterns of negative associations between fear/shyness and EF at 5 months, 3 years, and 4 years were hypothesized based on previous literature. We had no hypotheses for the correlation patterns at 10 months and 2 years because of the lack of literature to guide us and found no evidence for significant associations. Our finding of the trend of positive association between shyness and EF may capture a temporary developmental characteristic shared by toddlers high in EF and those high in shyness at age 2. The nonsignificant association at 10 months, age 3 negative trend, and age 4 negative correlation found in the bivariate correlations were consistently reflected as residual correlations in the autoregressive model, giving us more confidence in those particular associations.
Strengths, Limitations, and Future Research
These analyses show a complex pattern of modest associations between cognition and temperamental fear and shyness from infancy to early childhood. Although assertions of causality were entertained, it is important to remember that these analyses are still correlational and do not account for third variables that may be linking temperament and cognition. It will be important for future studies to consider contributions from additional variables before confidence in making such a statement of bivariate causality can be increased.
In addition to being a longitudinal account, a strength of this research was the use of tasks that required the higher order cognitive skills of focused attention, working memory, and inhibitory control as measures of infant and young child cognition. A considerable number of research studies in the adult and child literatures focus on EF, especially the working memory system, when describing differences between shy or anxious individuals and those who are less so. Further, the cognitive tasks used in this study included an inhibitory control component and thus engaged the central executive processor and the controlled attention components of the working memory system (Baddeley, 2001; Engle, Kane, & Tuholski, 1999). Thus, this may begin to address the applicability of the processing efficiency theory (Eysenck & Calvo, 1992) and the more recent attentional control theory (Eysenck, Derakshan, Santos, & Calvo, 2007) to the infant and young child populations. These theories suggest that the deficits in cognitive performance for anxious individuals are related to the disruption of the working memory system due to the anxiety, and this deficit is especially pronounced when the task is complex and places high demands on the system.
Additionally, some of these tasks (e.g., A-not-B, day-night Stroop) have been empirically or hypothetically related to the functioning of the dorsolateral prefrontal cortex (Diamond, Prevor, Callender, & Druin, 1997), a cortical area that has been linked to processing differences between adults who are anxious or introverted and those who are less so (e.g., Gray & Braver, 2002; Lieberman, 2000). Further, fMRI work by Gray and Braver (2002) found that adults scoring higher on behavioral approach sensitivity (BAS; a measure comparable to extraversion) had better performance on a working memory task and displayed greater neural efficiency with lower task-related activation in the caudal anterior cingulate cortex – a brain area that has been associated with the executive control of behavior (Bush, Luu, & Posner, 2000). Although, the fMRI technique presents challenges for use with an infant and young child population, other neuroimaging procedures such as continuous electroencephalographic (EEG) and event related potential (ERP) measures are appropriate for use with these populations and could address potential group differences in cortical functioning.
A limitation of this study is that all assessments of temperamental shyness were based on maternal report of those behaviors. Although parent-reports of temperament are not seemingly characterized by control and objectivity as are laboratory assessments of temperament, they have been praised for their consideration of their child’s behavior in multiple contexts and across appreciable periods of time (Rothbart & Bates, 2006). This multi-contextual assessment of the child’s temperament allows for a valid description of the child’s general patterns of behavior and boasts high ecological validity.
Another potential limitation was the variation in tasks administered at each developmental time period (infancy, toddlerhood, early childhood). As noted, the 2-year EF tasks were different from the infant and preschool tasks. The infant tasks were designed for preverbal children and the preschool tasks had verbal instructions at an age when children typically have good command of language. The age 2 EF tasks also had verbal instructions, but there was a wide range of language skills in our sample at age 2 and thus the tasks were more simple than those administered at ages 3 and 4. The variation in EF tasks at each age, along with the greater levels of variability in task performance at younger ages (Best & Miller, 2010; Carlson, 2005), potentially combined to influence the pattern of results seen here.
In sum, knowledge about the nature of associations between cognition and shyness can inform and optimize the experience of those children demonstrating lower levels of cognitive performance, higher levels of temperamental shyness, or both. Coupled with previous longitudinal work on this topic, the findings in the current study suggest that early EF skills training may enhance the social experience for some children and may even serve as a protective factor in the development of shyness.
Acknowledgments
This research was supported by grants HD043057 and HD049878 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) awarded to Martha Ann Bell. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health. We are grateful to the families for their participation in our research and to our research team for their assistance with data collection and coding.
Footnotes
Footnotes
Personality rather than temperament (and thus introversion rather than shyness) is generally assessed in adult research. Notably, these constructs are related but are not equivalent. Temperament includes those biological- and constitutionally-based tendencies for reactivity, self-regulation, and attention processes. This construct is traditionally used in research with infants, children, and animals. The concept of personality encompasses temperament but also includes qualities such as thoughts, values, and social cognitions (Rothbart & Bates, 2006).
These participants comprise two cohorts from larger, on-going study. Research including these 5- and 10-month-old participants’ performance on an infant working memory task has been reported (Cuevas, Bell, Marcovitch, & Calkins, 2012; Cuevas, Swingler, Bell, Marcovitch, & Calkins, 2012). Research including some of these 2-year-olds participants performance on the crayon task has been reported (Morasch & Bell, 2011). Likewise, reports of the EF performance in some of these children at ages 3 and 4 have been published (Cuevas, Deater-Deckard, Watson, Kim-Spoon, Morasch, & Bell, in press; Cuevas & Bell, 2013; Cuevas, Hubble, & Bell, 2012; Kraybill & Bell, 2012; Watson & Bell, 2013).
References
- Arbuckle JL. Full information estimation in the presence of incomplete data. In: Marcoulides GA, Schumacker RE, editors. Advanced structural equation modeling: Issues and techniques. Erlbaum; Mahwah, NJ: 1996. pp. 243–277. [Google Scholar]
- Asendorpf JB. The malleability of behavioral inhibition: A study of developmental functions. Developmental Psychology. 1994;30:912–919. [Google Scholar]
- Baddeley AD. Is working memory still working? American Psychologist. 2001;56:851–864. doi: 10.1037/0003-066x.56.11.851. [DOI] [PubMed] [Google Scholar]
- Bell MA. Brain electrical activity associated with cognitive processing during a looking version of the A-not-B task. Infancy. 2001;2:311–330. doi: 10.1207/S15327078IN0203_2. [DOI] [PubMed] [Google Scholar]
- Bell MA. A psychobiological perspective on working memory performance at 8 months of age. Child Development. 2012;83:251–265. doi: 10.1111/j.1467-8624.2011.01684.x. [DOI] [PubMed] [Google Scholar]
- Bell MA, Adams SE. Comparable performance on looking and reaching versions of the A-not-B task at 8-months of age. Infant Behavior and Development. 1999;22:221–235. [Google Scholar]
- Bell MA, Calkins SD. Attentional control and emotion regulation in early development. In: Posner M, editor. Cognitive neuroscience of attention. 2nd Guilford Press; New York, NY: 2012. [Google Scholar]
- Bell MA, Deater-Deckard K. Biological systems and the development of self-regulation: Integrating behavior, genetics, and psychophysiology. Journal of Developmental and Behavioral Pediatrics. 2007;28:409–420. doi: 10.1097/DBP.0b013e3181131fc7. [DOI] [PubMed] [Google Scholar]
- Bell MA, Wolfe CD. Emotion and cognition: An intricately bound developmental process. Child Development. 2004;75:366–370. doi: 10.1111/j.1467-8624.2004.00679.x. [DOI] [PubMed] [Google Scholar]
- Belsky J, Friedman SL, Hsieh KH. Testing a core emotion-regulation prediction: Does early attentional persistence moderate the effect of infant negative emotionality on later development? Child Development. 2001;72:123–133. doi: 10.1111/1467-8624.00269. [DOI] [PubMed] [Google Scholar]
- Best JR, Miller PH. A developmental perspective on executive function. Child Development. 2010;81:1641–1660. doi: 10.1111/j.1467-8624.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Dennis T. An optimal balance: The integration of emotion and cognition in context. In: Calkins SD, Bell MA, editors. Child development at the intersection of emotion and cognition. American Psychological Association; Washington, DC: 2010. pp. 17–35. [Google Scholar]
- Blankson AN, O’Brien M, Leerkes EM, Marcovitch S, Calkins SD. Shyness and vocabulary: The roles of executive functioning and home environmental stimulation. Merrill-Palmer Quarterly. 2011;57:105–128. doi: 10.1353/mpq.2011.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Calkins SD. Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Developmental Psychobiology. 1997;31:125–135. doi: 10.1002/(sici)1098-2302(199709)31:2<125::aid-dev5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Bell MA. Child development at the intersection of emotion and cognition. American Psychological Association; Washington, DC: 2010. [Google Scholar]
- Carlson SM. Developmentally sensitive measures of executive function in preschool children. Developmental Neuropsychology. 2005;28:595–616. doi: 10.1207/s15326942dn2802_3. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Mandell DJ, Williams L. Executive function and theory of mind: Stability and prediction from ages 2 to 3. Developmental Psychology. 2004;40:1105–1122. doi: 10.1037/0012-1649.40.6.1105. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Moses LJ, Breton C. How specific is the relation between executive function and theory of mind? Infant and Child Development. 2002;11:73–92. [Google Scholar]
- Coplan RJ, Rubin KH. Social withdrawal and shyness in childhood: History, theories, definitions, and assessments. In: Coplan R, Rubin K, editors. The development of shyness and social withdrawal. Guilford Press; New York: 2010. pp. 3–20. [Google Scholar]
- Crozier WR, Hostettler K. The influence of shyness on children’s test performance. British Journal of Educational Psychology. 2003;73:317–328. doi: 10.1348/000709903322275858. [DOI] [PubMed] [Google Scholar]
- Cuevas K, Bell MA. Infant attention and early childhood executive function. Child Development. 2013 doi: 10.1111/cdev.12126. doi:10.1111/cdev.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas K, Bell MA, Marcovitch S, Calkins SD. Electroencephalogram and heart rate measures of working memory at 5 and 10 months. Developmental Psychology. 2012;48:907–917. doi: 10.1037/a0026448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas K, Deater-Deckard K, Watson AJ, Kim-Spoon J, Morasch KC, Bell MA. What’s mom got to do with it? Contributions of maternal executive function and caregiving to the development of executive function across early childhood. Developmental Science. doi: 10.1111/desc.12073. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas K, Hubble M, Bell MA. Early childhood predictors of post-kindergarten executive function: Behavior, parent-report, and psychophysiology. Early Education and Development. 2012;23:59–73. doi: 10.1080/10409289.2011.611441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas K, Swingler MM, Bell MA, Marcovitch S, Calkins SD. Measures of frontal functioning and the emergence of inhibitory control processes at 10 months of age. Developmental Cognitive Neuroscience. 2012;2:235–243. doi: 10.1016/j.dcn.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derakshan N, Eysenck MW. Working memory capacity in high trait-anxious and Repressor Groups. Cognition and Emotion. 1998;12:697–713. [Google Scholar]
- Diamond A, Prevor MB, Callender G, Druin DP. Prefrontal cortex deficits in children treated early and continuously for PKU. Monographs of the Society for Research in Child Development. 1997;62 4, Serial No. 252. [PubMed] [Google Scholar]
- Elliman NA, Green MW, Rogers PJ, Finch GM. Processing-efficiency theory and the working memory system: Impairments associated with sub-clinical anxiety. Personality and Individual Differences. 1997;23:31–35. [Google Scholar]
- Engle RW, Kane MJ, Tuholski SW. Individual differences in working memory capacity and what they tell us about controlled attention, general fluid intelligence, and functions of the prefrontal cortex. In: Miyake A, Shah P, editors. Models of working memory: Mechanisms of active maintenance and executive control. Cambridge University Press; New York: 1999. pp. 102–134. [Google Scholar]
- Eysenck MW, Calvo MG. Anxiety and performance: The processing efficiency theory. Cognition and Emotion. 1992;6:409–434. [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo M. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: Linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Gartstein MA, Rothbart MK. Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behavior & Development. 2003;26:64–86. [Google Scholar]
- Gray JR, Braver TL. Personality predicts working-memory-related activation in the caudal anterior cingulate cortex. Cognitive, Affective, and Behavioral Neuroscience. 2002;2:64–75. doi: 10.3758/cabn.2.1.64. [DOI] [PubMed] [Google Scholar]
- Hadwin JA, Brogan J, Stevenson J. State anxiety and working memory in children: A test of processing efficiency theory. Educational Psychology. 2005;25:379–393. [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Hughes K, Coplan RJ. Exploring processes linking shyness and academic achievement in childhood. School Psychology Quarterly. 2010;25:213–222. [Google Scholar]
- Johnson DR, Gronlund SD. Individuals lower in working memory capacity are particularly vulnerable to anxiety’s disruptive effect on performance. Anxiety, Stress, & Coping. 2009;22:201–213. doi: 10.1080/10615800802291277. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N, Kahn V, Towsley S. The preservation of two infant temperaments into adolescence. Monographs of the Society for Research in Child Development. 2007;72 doi: 10.1111/j.1540-5834.2007.00436.x. 2, Serial No. 287. [DOI] [PubMed] [Google Scholar]
- Karrass J, Braungart-Rieker JM. Infant negative emotionality and attachment: Implications for preschool intelligence. International Journal of Behavioral Development. 2004;28:221–229. [Google Scholar]
- Klein GS. Consciousness in psychoanalytic theory; Some implications for current research in perception. Journal of American Psychoanalytic Association, 1959. 1959;7:5–34. doi: 10.1177/000306515900700101. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Murray KT, Harlan ET. Effortful control in early childhood: Continuity and change, antecedents, and implications for social development. Developmental Psychology. 2000;36:220–232. [PubMed] [Google Scholar]
- Kraybill JH, Bell MA. Infancy predictors of preschool and post-kindergarten executive function. Developmental Psychobiology. 2013;55:530–538. doi: 10.1002/dev.21057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD. Introversion and working memory: Central executive differences. Personality and Individual Differences. 2000;28:479–486. [Google Scholar]
- Lieberman MD, Rosenthal R. Why introverts can’t always tell who likes them: Multitasking and nonverbal decoding. Journal of Personality and Social Psychology. 2001;80:294–310. doi: 10.1037/0022-3514.80.2.294. [DOI] [PubMed] [Google Scholar]
- Ludwig RP, Lazarus PJ. Relationship between shyness in children and constricted cognitive control as measured by the Stroop color-word test. Journal of Consulting and Clinical Psychology. 1983;51:386–389. doi: 10.1037//0022-006x.51.3.386. [DOI] [PubMed] [Google Scholar]
- McConnell BA, Bryson SE. Visual attention and temperament: Developmental data from the first 6 months of life. Infant Behavior and Development. 2005;28:537–544. [Google Scholar]
- Morasch KC, Bell MA. The role of inhibitory control in behavioral and physiological expressions of toddler executive function. Journal of Experimental Child Psychology. 2011;108:593–606. doi: 10.1016/j.jecp.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng E, Lee K. Children’s task performance under stress and non-stress conditions: A test of the processing efficiency theory. Cognition and Emotion. 2010;24:1229–1238. [Google Scholar]
- Pérez-Edgar K, Martin McDermott JN, Korelitz K, Degnan KA, Curby TW, Pine DS, Fox NA. Patterns of sustained attention in infancy shape the developmental trajectory of social behavior from toddlerhood through adolescence. Developmental Psychology. 2010;46:1723–1730. doi: 10.1037/a0021064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam SP, Gartstein MA, Rothbart MK. Measurement of fine-grained aspects of toddler temperament: The Early Childhood Behavior Questionnaire. Infant Behavior and Development. 2006;29:386–401. doi: 10.1016/j.infbeh.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SA, Feldman JF. Prediction of IQ and specific cognitive abilities at 11 years from infancy measures. Developmental Psychology. 1995;31:685–696. [Google Scholar]
- Rose SA, Feldman JF. Memory and speed: Their role in the relation of infant information processing to later IQ. Child Development. 1997;68:630–641. [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Implications of infant cognition for executive functions at age 11. Psychological Science. 2012;23:1345–1355. doi: 10.1177/0956797612444902. [DOI] [PubMed] [Google Scholar]
- Rothbart MK. Becoming who we are: Temperament and personality in development. Guilford Press; New York: 2011. [Google Scholar]
- Rothbart MK, Bates JE. In: Handbook of child psychology: Vol. 3. Social, emotional, and personality development. 6th. Damon W, Lerner RM, Eisenberg N, editors. Wiley; Hoboken, NJ: 2006. pp. 99–166. Series Eds. Vol. Ed. [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey K, Fisher P. Investigations of temperament at three to seven years: The children’s behavior questionnaire. Child Development. 2001;72:1394–1408. doi: 10.1111/1467-8624.00355. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ellis LK, Rueda MR, Posner MI. Developing mechanisms of temperamental effortful control. Journal of Personality. 2003;71:1113–1144. doi: 10.1111/1467-6494.7106009. [DOI] [PubMed] [Google Scholar]
- Rubin KH. In: The development of shyness and social withdrawal. Coplan RJ, editor. Guildford Press; New York: 2010. [Google Scholar]
- Rubin KH, Coplan RJ, Bowker J. Social withdrawal and shyness in childhood and adolescence. Annual Review of Psychology. 2009;60:141–171. doi: 10.1146/annurev.psych.60.110707.163642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda MR, Posner MI, Rothbart MK. Attentional control and self-regulation. In: Vohs K, Baumeister R, editors. Handbook of self-regulation: Research, theory, and applications. 2nd Guilford Press; New York, NY: 2005. pp. 284–299. [Google Scholar]
- Ruff HA, Rothbart MK. Attention in early development: Themes and variations. Oxford University Press; New York: 1996. [Google Scholar]
- Rushton JP, Brainerd CJ, Pressley M. Behavioral development and construct validity: The principle of aggregation. Psychological Bulletin. 1983;94:18–38. [Google Scholar]
- Sanson A, Pedlow R, Cann W, Prior M, Oberklaid F. Shyness ratings: Stability and correlates in early childhood. International Journal of Behavioral Development. 1996;19:705–724. [Google Scholar]
- Schmidt LA, Buss AH. Understanding shyness: Four questions and four decades of research. In: Coplan R, Rubin K, editors. The development of shyness and social withdrawal. Guilford Press; New York: 2010. pp. 3–20. [Google Scholar]
- Tanwar U, Malhotra D. Short-term memory as a function of personality and imagery. Personality and Individual Differences. 1992;13:175–180. [Google Scholar]
- Wachs TD. The nature, etiology, and consequences of individual differences in temperament. In: Balter L, Tamis-LeMonda C, editors. Child psychology: A handbook of contemporary issues. Psychology Press; New York: 2006. pp. 27–52. [Google Scholar]
- Watson AJ, Bell MA. Individual differences in inhibitory control skills at three years of age. Developmental Neuropsychology. 2013;38:1–21. doi: 10.1080/87565641.2012.718818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe CD, Bell MA. The integration of cognition and emotion during infancy and early childhood: Regulatory processes associated with the development of working memory. Brain and Cognition. 2007;65:3–13. doi: 10.1016/j.bandc.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Frye D, Rapus T. An age-related dissociation between knowing rules and using them. Cognitive Development. 1996;11:37–63. [Google Scholar]
- Zelazo PD, Müller U, Frye D, Marcovitch S. The development of executive function in early childhood. Monographs of the Society for Research in Child Development. 2003;68 doi: 10.1111/j.0037-976x.2003.00260.x. 3, Serial No. 274. [DOI] [PubMed] [Google Scholar]