SUMMARY
Pregnancy and its effects on breast cancer risk have been widely investigated; there is consensus among researchers that early pregnancy confers protection against breast cancer later in life, whereas nulliparity and late-age parity have been associated with increased risk of developing breast cancer. The answer to the question of how pregnancy reduces breast cancer risk has been elusive; however, pregnancy, like breast cancer, is a similar hormone-dependent entity under direct control of estrogen, progesterone and, of particular importance, human chorionic gonadotropin (hCG). In this report, we emphasize the main changes, previously described by our laboratory, in morphology and gene expression levels of the mammary gland of Sprague–Dawley rats exposed to known cancer-preventative conditions (pregnancy, hCG and progesterone + estrogen). In addition, we postulate a protective mechanism induced by hCG that could reduce the cell’s potential to be transformed by carcinogens.
It is well documented that pregnancy – single and multiple – offers women protection against breast cancer [1–10]. Many studies have investigated the effect of pregnancy on breast tissue development, morphology and gene expression [3,11–15]. The first major study that showed a correlation between parity and reduced breast cancer risk was by MacMahon and colleagues, in which they carried out an international case–control study analyzing patient epidemiological data, and concluded that early age of first full-term birth is protective against breast cancer [1]. Compared with nulliparous women, those whose first live birth occurred before 20 years of age had a 50% reduced risk of breast cancer, whereas those whose first full-term pregnancy occurred after 35 years of age had a 20% increased risk. Many other studies have analyzed patient data and revealed a correlation between early age at first birth, as well as multiparity, with reduced breast cancer risk [1–10,16].
There is a clear consensus that pregnancy, particularly at an early age, confers protection and reduces the risk of breast cancer later in life. Many studies have tried to understand the mechanisms underlying the protective effects of early pregnancy and multiparity in comparison with the breast cancer risk of nulliparous women or the progressive increase in risk when the first pregnancy occurs after 24 years of age, reaching a peak at first pregnancy after 35 years of age [1–10,16]. Many studies have identified sets of genes that are differentially regulated in nulliparous versus parous breast tissue [11,12,14,17,18]. The protection from breast cancer conferred by early pregnancy has more recently been found to be related to changes in the population of mammary epithelial stem cells [15,19]. A decrease in the number of these stem cells, through induction of differentiation into nonpluripotent offspring, has been correlated with decreased incidence of tumors in breast tissue [15,19]. The differentiation of these stem cells into the mature cells and tissues of the breast ductal tree in parous women has been found to be induced by the hormones of pregnancy, namely estrogen, progesterone and, of particular importance, human chorionic gonadotropin (hCG) [13–15,19–22]. Progesterone has been found to be essential for the maintenance of the ovarian corpus luteum after ovulation, which is stimulated by luteinizing hormone (LH) [22]. Thereafter, hCG promotes progesterone production for 3–4 weeks following pregnancy implantation and reaches a peak at 10 weeks of pregnancy, although it continues to be produced throughout pregnancy. hCG is a complex glycoprotein heterodimer molecule that fulfills numerous functions during pregnancy [22,23]. The hormone is composed of α- and β-subunits. The α-subunit is common to the pituitary hormones LH, follicle-stimulating hormone and thyroid-stimulating hormone, whereas the β-subunit confers specificity to the hormone, which binds to the G-protein-coupled receptor, the choriogonadotropin/LH receptor. Five variants of hCG have been identified: hCG, sulfated hCG, hyperglycosylated hCG, free β-hCG and hyperglycosylated free β-hCG. Hyperglycosylated hCG is the principal hCG form produced in early pregnancy, and its relative proportion rapidly declines after the fourth week of pregnancy. In early pregnancy, the high hyperglycosylated hCG is thought to be the driving signal of deep pregnancy implantation. Although hCG and hyperglycosylated hCG have identical amino acid sequences, they are produced by different cells, have independent functions and differ in their binding sites; hCG binds the choriogonadotropin/LH receptor, while hyperglycosylated hCG antagonizes a TGF-β receptor [22–24]. The autocrine and paracrine forms of these variants, such as hyperglycosylated hCG produced by gestational trophoblastic neoplasms, have been thoroughly reviewed by numerous authors [22–24], and are not within the scope of this article. Toniolo et al. observed a correlation between increased concentrations of hCG during a woman’s pregnancy and a corresponding decreased risk of breast cancer later in life [20]. In addition, studies by Russo et al. have demonstrated that hCG acts in a chemopreventative manner, that is, protecting normal cells from becoming cancerous [13–15]. hCG has also been shown to have anticancer effects when tested on breast cancer cell lines [21].
In this report, we summarize a previous study performed in our laboratory in which the gene-expression levels in the mammary glands of rats under different reproductive and hormonal conditions that have been shown to be protective against breast carcinogenesis (i.e., pregnancy, hCG and estrogen + progesterone [E+P]) [13,25–35] were compared with the gene-expression levels in the mammary glands of untreated rats [36]. Herein, the genes that we believe are essential for a common mechanism of protection among pregnancy, hCG and E+P, are emphasized, and we postulate a protective mechanism induced by hCG, which could represent an advantage of the use of this hormone as a prophylactic agent against breast cancer in high-risk women, due to its ability to induce additional differentiation of this organ.
Materials & methods
The following is a summary of the materials and methods used in the study previously described [36]. To analyze the gene-expression profile induced by pregnancy and the treatments that mimic pregnancy (hCG and an E+P pellet), we treated 50-day-old virgin female Sprague–Dawley rats. They were separated into four groups containing 20 animals each: control, which received only bacteriostatic water intraperitoneally (hCG vehicle) for 21 days; hCG, which received 100 IU/day of recombinant hCG (Ovidrel®) intraperitoneally for 21 days; pellet, which received a surgically implanted E+P pellet containing 0.72 mg 17-β estradiol and 200 mg progesterone with a 21-day release period; and pregnancy, in which the rats were mated, allowed to complete pregnancy (21 ± 2 days) and, after delivery, were separated from the offspring. Each group was exposed to pregnancy or hormone treatment for 21 consecutive days and, at the end of exposure, all groups had a resting period of 21 days to allow the involution of the mammary gland. At the end of the resting period, the animals were euthanized and their abdominal mammary glands were collected (Figure 1). Ten animals per group had their mammary glands collected for histological analysis through whole-mount preparations, and the other ten animals per group had their mammary glands snap frozen for later RNA extraction. Five samples per group were chosen based on the quality of the extracted RNA, and Agilent gene-expression microarrays, representing the whole genome of the rat, were performed to identify the changes induced by the different conditions. Relative gene expressions were identified between each of the treatment groups in comparison with the control group (hCG vs control, pellet vs control and pregnancy vs control). Initially, we applied different criteria (false-discovery rates, p-values and fold changes) to determine the genes that were differentially expressed in each comparison. The hCG treatment was shown to have the largest number of genes differentially expressed using different criteria, and to be the only treatment that induced significant transcriptomic changes using the most stringent criteria we have tested (false-discovery rate <5% and fold change of at least 2.0). Based on the exploratory nature of this project, we considered statistically differentially expressed genes with a fold change of at least 2.0 and p-values less than 0.01 for further analyses. The differentially expressed genes were individually analyzed using publications in PubMed, and classified according to their biological functions. In addition, they were submitted to Ingenuity Pathways Analysis software (Ingenuity® Systems [101]) version 14197757 from Ingenuity Systems, Inc., to investigate their pathways and the relationships among them.
Figure 1. Experimental design.
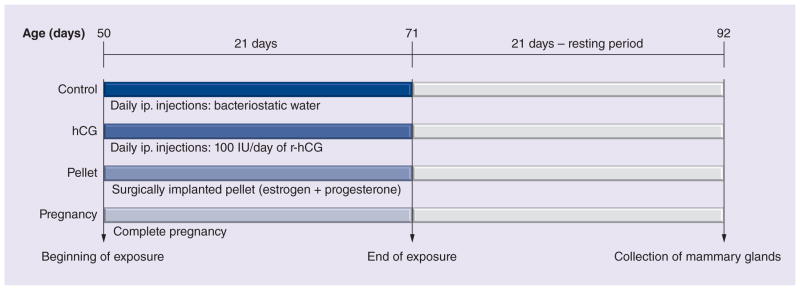
Fifty-day-old virgin female Sprague–Dawley rats were divided into four groups (20 animals each). The control group received ip. bacteriostatic water (hCG vehicle) daily for 21 days. The hCG group received 100 IU/day of r-hCG (Ovidrel®). The pellet group received a surgically implanted pellet containing estrogen and progesterone at 0.72 mg 17-β estradiol and 200 mg progesterone with a 21-day release period. The pregnancy group rats were mated, allowed to complete pregnancy (21 ± 2 days) and, after delivery, were separated from the offspring. Each group was exposed to pregnancy or hormone treatment for 21 consecutive days and, at the end of the exposure, they had a resting period of 21 days to allow the involution of the mammary gland. At the end of the resting period, the animals were euthanized and their abdominal mammary glands were collected. Ten animals per group had their mammary glands collected for histological analysis, and the other ten animals had their mammary glands collected for genomic analysis.
hCG: Human chorionic gonadotropin; ip.: Intraperitoneally; r-hCG: Recombinant human chorionic gonadotropin.
Adapted with permission from Springer Science+Business Media BV [36].
Discussion
Differentiation of the mammary glands
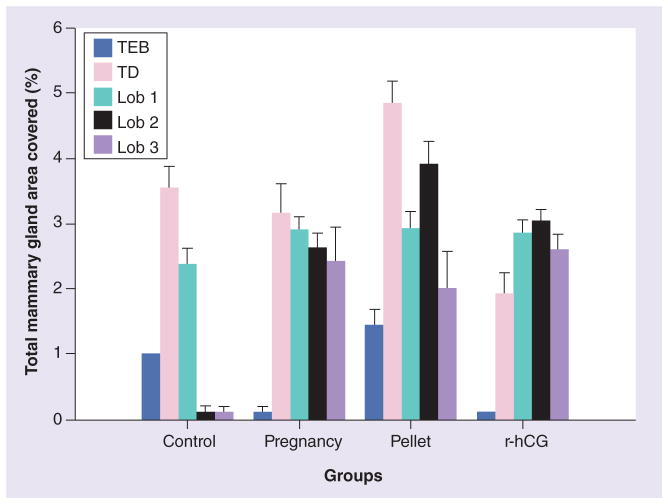
We analyzed the mammary gland whole mounts of the rats that had involuted after the 21-day resting period and observed the lasting effects of the three preventative conditions. The structures on the mammary glands were categorized as: terminal end buds (TEBs), terminal ducts (TDs) and lobules type 1 (lob 1), type 2 (lob 2) and type 3 (lob 3). TEBs are the least developed structures with the highest proliferative rates, characteristics that confer on them the highest susceptibility to carcinogenesis [26,27]. We counted the number of structures of the rat mammary gland and expressed them as a percentage of total mammary gland area (mm2; Figure 2) [36]. Mammary glands from the control group were devoid of lob 2 and lob 3, while pregnancy, E+P pellets and hCG stimulated gland differentiation to reach the stage of lob 3. After 21 days of mammary gland regression, the control group had TEBs, TDs and lob 1, whereas none of the rat mammary glands postpregnancy or post-hCG treatment contained TEBs. A slightly higher numbers of lobs 2 and 3 were found in the glands of hCG-treated animals when compared with the pregnancy group. The mammary glands of animals that had the E+P pellet implanted contained a larger percentage of TEBs than the controls, in addition to TDs and lobs 1, 2 and 3, indicating that the mammary gland had not been completely differentiated by this treatment (Figure 2). This indicates that, among the treatments that mimic pregnancy, hCG induces complete differentiation of the mammary gland more efficiently.
Figure 2. Percentage of structures in the total mammary gland area (mm2) in whole-mount preparations.
The structures on the mammary glands were categorized as TEBs, TDs and lobs 1, 2 and 3. The control rat mammary glands were devoid of lobs 2 and 3, while pregnancy, pellet and r-hCG stimulated the gland differentiation to reach lob 3. None of the rat mammary glands postpregnancy or post-hCG treatment contained TEBs, and they presented lobs 2 and 3, whereas r-hCG presented slightly higher numbers of lobs 2 and 3 compared with pregnancy. The animals treated with the pellet contained – in addition to TDs and lobs 1, 2 and 3 – a greater percentage of TEBs, compared with the control group, indicating that the mammary gland has not been completely differentiated by this treatment. Lob: Lobule type; r-hCG: Recombinant human chorionic gonadotropin; TD: Terminal duct; TEB: Terminal end bud.
Reproduced with permission from Springer Science+Business Media BV [36].
Transcriptome profile of the three preventative strategies
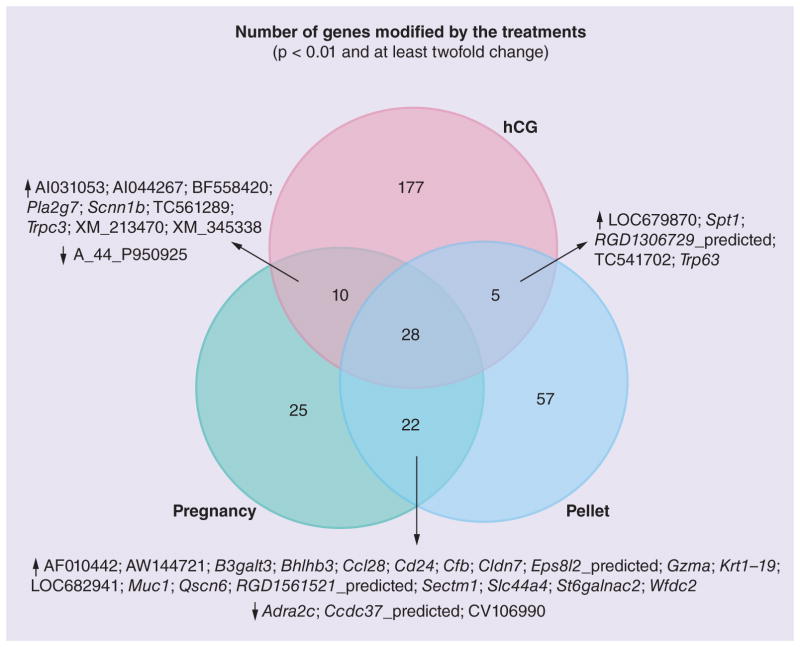
It is known that pregnancy, E+P and hCG confer protective effects against tumor formation in rats [13–15,25–35]. When we compared the gene expression of the mammary glands of the animals exposed to these conditions with the age-matched untreated group, we found 85, 112 and 220 genes modulated by pregnancy, pellet and hCG, respectively (Figure 3). Interestingly, we observed common patterns of expression in the mammary glands of the rats exposed to these three conditions (Figure 3). We found 27 genes that were upregulated in these three preventative modalities and one gene was downregulated (Table 1). In order to identify the possible mechanisms of prevention common to these three conditions, we further analyzed these genes (Table 1).
Figure 3. Venn diagram showing the number of genes differently expressed in the three preventative treatments (pregnancy, pellet and hCG).
↑ indicates the genes that were upregulated and ↓ indicates the genes that were downregulated by the treatments. We observed 28 genes being commonly regulated in the three preventative conditions.
hCG: Human chorionic gonadotropin.
Adapted with permission from Springer Science+Business Media BV [36].
Table 1.
Genes found to be differentially expressed in all three of the conditions (p < 0.01 and at least twofold change).
| Gene symbol | Systematic name | Gene name | hCG | Fold change E+P pellet | Preg |
|---|---|---|---|---|---|
| TC540900 | TC540900 | Unknown | 0.45 | 0.40 | 0.45 |
| AA874941 | AA874941 | AA874941 UI-R-E0-ci-d-06–0-UI.s1 UI-R-E0 Rattus norvegicus cDNA clone UI-R-E0-ci-d-06–0-UI 3′ similar to gi | 2.42 | 3.01 | 2.59 |
| Apod | NM_012777 | Rattus norvegicus apolipoprotein D (Apod), mRNA | 2.80 | 2.02 | 2.22 |
| Cfi | NM_024157 | Rattus norvegicus complement factor I (Cfi), mRNA | 7.57 | 6.72 | 6.05 |
| Cldn4 | NM_001012022 | Rattus norvegicus claudin 4 (Cldn4), mRNA | 3.57 | 3.70 | 3.84 |
| Crb3 | NM_001025661 | Rattus norvegicus crumbs homolog 3 (Drosophila) (Crb3), mRNA | 2.40 | 2.31 | 2.39 |
| Crhr2 | NM_022714 | Rattus norvegicus corticotropin releasing hormone receptor 2 (Crhr2), mRNA | 2.99 | 3.78 | 4.84 |
| Duox1 | NM_153739 | Rattus norvegicus dual oxidase 1 (Duox1), mRNA | 2.71 | 2.79 | 2.62 |
| Enc1 | NM_001003401 | Rattus norvegicus ectodermal-neural cortex 1 (Enc1), mRNA | 2.08 | 2.26 | 2.01 |
| Irx2 | NM_001039505 | Rattus norvegicus Iroquois-related homeobox 2 (Drosophila) (Irx2), mRNA | 3.08 | 3.50 | 3.07 |
| Kng1 | NM_012696 | Rattus norvegicus kininogen 1 (Kng1), mRNA | 4.42 | 3.14 | 3.69 |
| Lbp | NM_017208 | Rattus norvegicus lipopolysaccharide-binding protein (Lbp), mRNA | 3.53 | 4.84 | 6.57 |
| Lcn2 | NM_130741 | Rattus norvegicus lipocalin 2 (Lcn2), mRNA | 3.78 | 4.99 | 4.53 |
| LOC685608 | XM_001065355 | Predicted: Rattus norvegicus hypothetical protein LOC685608 (LOC685608), mRNA | 25.41 | 8.42 | 4.99 |
| Map3k6_predicted | XM_232732 | Predicted: Rattus norvegicus mitogen-activated protein kinase kinase kinase 6 (predicted) (Map3k6_predicted), mRNA | 2.04 | 2.09 | 2.00 |
| Kng1l1 | NM_001009628 | Rattus norvegicus kininogen 1-like 1 (Kng1l1), mRNA | 3.64 | 2.65 | 2.70 |
| Mmp12 | NM_053963 | Rattus norvegicus matrix metallopeptidase 12 (Mmp12), mRNA | 2.87 | 3.31 | 4.57 |
| Pdzk1ip1 | NM_130401 | Rattus norvegicus PDZK1-interacting protein 1 (Pdzk1ip1), mRNA | 2.27 | 2.97 | 2.87 |
| RGD1306601_predicted | XM_230527 | Predicted: Rattus norvegicus similar to cDNA sequence BC019755 (predicted) (RGD1306601_predicted), mRNA | 2.76 | 2.78 | 2.71 |
| Scnn1g | NM_017046 | Rattus norvegicus sodium channel, nonvoltage-gated 1 γ (Scnn1g), mRNA | 4.88 | 2.97 | 3.76 |
| Sectm1 | NM_199082 | Rattus norvegicus secreted and transmembrane 1 (Sectm1), mRNA | 3.52 | 4.44 | 4.77 |
| Spp1 | NM_012881 | Rattus norvegicus secreted phosphoprotein 1 (Spp1), mRNA | 2.46 | 2.85 | 3.98 |
| TC523154 | TC523154 | Q91842 (Q91842) Xenopus laevis U2 snRNA gene, partial (6%) | 2.16 | 2.33 | 2.19 |
| Tgfb3 | NM_013174 | Rattus norvegicus transforming growth factor β 3 (Tgfb3), mRNA | 2.54 | 2.85 | 3.07 |
| Thedc1 | NM_022705 | Rattus norvegicus thioesterase domain containing 1 (Thedc1), mRNA | 7.05 | 13.64 | 10.29 |
| Trim29_predicted | XM_236207 | Predicted: Rattus norvegicus tripartite motif protein 29 (predicted) (Trim29_predicted), mRNA | 3.05 | 2.64 | 2.54 |
| Wap | NM_053751 | Rattus norvegicus whey acidic protein (Wap), mRNA | 17.44 | 17.10 | 14.38 |
| XM_234581 | XM_234581 | Rattus norvegicus similar to immunoglobulin α heavy chain (LOC314487), mRNA | 3.32 | 4.05 | 10.42 |
E+P: Estrogen + progesterone; hCG: Human chorionic gonadotropin; Preg: Pregnancy. Reproduced with permission from Springer Science+Business Media BV [36].
These genes are related to processes such as cellular differentiation, maintenance of cellular polarity, tight junction formation and cellular communication.
An interesting gene with regards to cellular polarity found to be upregulated in all three treatment groups was Trim29. A study conducted by Liu et al. investigated the tumor-suppressor properties of the TRIM29 gene in nontumorigenic breast cells, as well as estrogen receptor-positive breast cancer; shRNA knockdown of TRIM29 in MCF10A cells resulted in an approximately twofold increase in growth at 72 h compared with parental or nonsilenced controls [37]. Parental MCF10A cells grown in 3D Matrigel™ formed acinus-like spheroids and had well-circumscribed staining of α-integrin, which demonstrates apicobasal polarization. When TRIM29 was knocked down in MCF10A cells, there was a lack of polarity, increased proliferation and reduced apoptosis compared with parental MCF10A cells. The expression of Trim29 helps to maintain the polarity of the cell and significantly reduces its susceptibility to transformation.
Another gene that was upregulated in all three treatment groups and that is related to both cell polarity and tight junction formation was Crb3. This gene encodes a small transmembrane protein mainly expressed in epithelial tissues and skeletal muscles [38]. Crb3 is localized to the apical membrane of epithelial cells and plays an important role in epithelial polarity and tight junction formation [38–41]. As shown in previous studies, fly embryos lacking Crb have been identified as having epithelial polarity defects and loss of epithelial tissue integrity [39]. A study by Fogg et al. investigated the expression of CRB3 in human mammary epithelial cell lines [41]. Nontumorigenic MCF10A cells that express low levels of CRB3 were not able to form tight junctions; however, when exogenous expression of CRB3 was induced, dramatic differences in tight junction formation were observed [41]. From this study and others, the importance of Crb3 to maintain epithelial cell polarity is shown [38–41].
The results found in our laboratory have identified the gene Cldn4 in all three treatment groups. A study conducted by Blanchard et al. reported the expression of Claudins 1, 3 and 4 in normal mammary gland development. The expression of Claudin 1 and 4 increased during pregnancy in CD1 mice and fell during lactation, but increased at day 1 of involution. This increase in tight junction protein activity is explained by the accumulation of milk in the alveoli after weaning, thereby increasing the mechanical stress placed on the alveolar junctions [42]. These results again showed the significance of Claudins in the formation and maintenance of tight junctions, as well as demon strating a possible role in mammary gland development.
Among the 28 genes evaluated, one gene was related to cell communication. Scnn1g, known to encode the epithelial sodium channel, ENaC, was upregulated in all three treatment groups. Epithelial sodium channels are located in the apical membrane of epithelial cells and consist of three homologous subunits (α, β and γ) [43]. ENaC is a sodium-selective ion channel that plays a role in the regulation of sodium homeostasis by controlling reabsorption of sodium across epithelia [43]. As mentioned in a study by Boyd and Naray-Fejes-Toth, few data have been obtained regarding ENaC expression in the mammary epithelium; their study observed the effects of glucocorticoids on ENaC expression, as well as steroid hormones. Both progesterone and glucocorticoids affect lactogenesis, and high concentrations of sodium in breast milk have been linked to impaired lactogenesis [44]. In both MCF10A and HC11 cells, γENaC mRNA had the largest and most rapid response to glucocorticoid treatment. Glucocorticoids are important for the induction and maintenance of lactation, as well as the formation of tight junctions in mouse mammary epithelial cells [44]. The response of ENaC to steroid hormones demonstrates its importance in the development and progression of the mammary gland.
The relevance of these genes to cell polarity emphasizes the importance of these preventative strategies in inducing differentiation in the mammary gland to reduce cancer effectiveness. By establishing tight junctions and polarity, the cell’s susceptibility to transformation, as well as the cell’s ability to metastasize, in the presence of a carcinogen, will be reduced.
Transcriptome profile induced by hCG
hCG treatment induced the largest changes in the transcriptomic profile of the mammary gland; 63 upregulated and 114 downregulated genes were solely differentially expressed by this treatment (Figure 3).
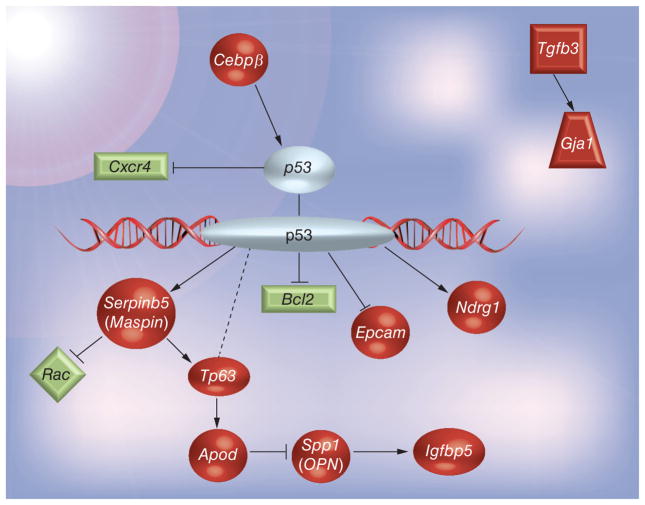
The roles and functions of some of these genes that are modified by hCG were clarified, and a possible pathway of action was constructed using the up- and down-regulated genes based on the literature. As previously postulated by our laboratory [45] and corroborated by others [34,46,47], these observed results and the pattern of gene expression induced by hCG have shown the involvement of the tumor-suppressor p53 gene. It is interesting to note that many of the genes upregulated in the current experiment correspond to literature findings of p53-dependent behavior. A possible pathway depicting the protective mechanisms of hCG treatment with regards to breast cancer was created based on the evaluation of the studies discussed below (Figure 4).
Figure 4. Postulated protective mechanism induced by human chorionic gonadotropin treatment.
Genes in red were upregulated by human chorionic gonadotropin, while genes in green were downregulated.
Data were analyzed with the use of Ingenuity Pathways Analysis (IPA®) software [101].
The first gene in this pathway is Cebpβ, which was upregulated by hCG treatment. A study by Boggs and Reisman, evaluated the relationship between Cebpβ and the expression of p53 in response to mitogen stimulation [48]. In vitro studies showed that one of the isoforms of Cebpβ had greater binding activity to the −972/−953 cis-acting regulatory element of the p53 promoter. Murine fibroblast cells (Swiss 3T3) were studied and the results indicated that endogenous Cebpβ found in these cells was able to bind to the −972/−953 site on the p53 promoter. Next, the cell lines that did not undergo normal p53 cell cycle activity (i.e., murine breast carcinoma cell lines NuMuMg, FSK-3, TM4O-A, TM-3, HC11 and 4T1) were tested for the level of p53 activity and Cebpβ DNA-binding activity. The results showed that cell lines NuMuMg, FSK-3, TM4O-A and TM-3 had elevated p53 levels in response to serum treatment. From these results, the authors concluded that Cebpβ is essential for the proper regulation of p53 transcription during the transition from growth arrest to entry into the cell cycle [48].
Another gene with increased expression was Ndrg1, whose expression has been noted to exert metastasis-suppressing qualities. The mechanisms by which Ndrg1 is able to suppress metastasis are obscure. However, a study by Stein et al. has shown that Ndgr1 is necessary for p53-mediated apoptosis and that it is a possible p53 target gene [49]. The inducible p53 human colon cancer cell line DLD-1 was screened using fluorescent differential display to identify early p53-responsive genes. After the screening, known p53 targets, such as p21, HDM2 and PIG3, were confirmed, and corresponding Ndrg1 cDNA fragments were amplified and showed upregulation following p53 induction. Northern and western blot analyses were performed to confirm the expression of Ndrg1. The induction of Ndrg1 expression by endogenous p53 expression due to DNA damage was also studied using the HCT116 p53+/+ cell line. p53 expression following treatment with doxorubicin, a DNA-damaging reagent, led to elevated levels of Ndrg1 expression. However, the data showed that p53-induced Ndrg1 expression occurs only in certain cells. The study also investigated potential binding sites for p53 upstream of the Ndrg1 gene and, using chromatin immunoprecipitation assays, the binding of p53 to these sites was demonstrated [49]. A study by Kovacevic et al. also focused on understanding the molecular mechanisms involved in Ndrg1 function. Three cell lines – human prostate cells PC3MM and DU145, and human lung cancer cells H1299 – were studied and transfected with NDRG1. Using western blot analysis, expression of the p21 protein was shown to be significantly increased by upregulation of NDRG1 in all three cell lines. The three cell lines have been characterized as being either p53-null or having mutated p53 that does not transactivate target genes [50]. Overall, the study showed that NDRG1 significantly increased the expression of the crucial CDK inhibitor, p21, which may be an underlying mechanism explaning its antitumor ability [50].
Another gene related to p53 expression is Maspin. A study by Zou et al. investigated whether the tumor suppressor p53 regulated the expression of maspin in prostate tumor cells, as well as in the breast tumor cell line MCF7 [51]. These cell lines were infected with an adeno-virus containing a wild-type p53 expression vector and were harvested at different time points to isolate RNA and to compare with the control. Only p53 stimulated the expression of maspin in these cell lines. DNA-damaging agents and cytotoxic drugs are inducers of p53 expression that lead to the induction of downstream target genes. In order to demonstrate whether maspin expression is inducible in response to DNA damage, cells were ultraviolet (UV) irradiated; MCF7 cells containing wild-type p53 showed an increased expression of maspin upon UV irradiation. These results suggest a role for wild-type p53 in the regulation of maspin function involved in cell invasion or metastasis [51]. A study by Kim et al. observed the expression of Maspin in various lung cancer cell lines in order to better understand the gene’s regulatory mechanisms; p63 and maspin expression were both high in squamous carcinoma tissues. Using immunohistochemical analysis, all six splice variants of p63 were tested to examine whether maspin was controlled by p63 in vitro; results showed that some splice variants of p63 transactivated maspin expression [52]. Next, gel-shift assays were performed to determine whether p63 directly interacts with the Maspin promoter; the p63 protein exhibited binding to the oligonucleotide with a p53 consensus sequence and the anti-p63 antibody caused a reduction in the intensity of the binding, thus indicating the presence of p63 in the binding complex [52]. In our study, both Maspin and Tp63 were overexpressed in the mammary gland of the hCG-treated group.
Igfbp5, which was also found to be upregulated by hCG, is one of the six members of the binding proteins in the IGF axis that bind with high affinity to IGFs and regulate their ability to interact with the type 1 IGF receptor [53]. Igfbp5 is the most conserved member of the Igfbp family and has been shown to regulate cell growth, determine cell fate and play a role in the metastatic process in cancer development [54]. Igfbp5 has also been shown to regulate extracellular matrix degradation due to its strong binding affinity [55]. A study by Nam et al. evaluated the ability of TSP-1 and OPN to bind to IGFBP5 [56]. IGFBP-5 was incubated with TSP-1 or OPN and coimmunoprecipitation was performed to determine the specificity of binding [56]. IGFBP5 was able to bind to both TSP-1 and OPN, but did not alter their affinities to IGF-1 [56]. Another study has shown that IGFBP5 causes apoptosis in mammary epithelial cells in vivo and in vitro at high concentrations [57]. IGFBP5 is also the only IGFBP that is highly expressed during involution of the mammary gland [57]. IGFBP5 has also been shown to prevent migration of MCF-7 cells, thereby indicating a possible role in the limitation of metastasis [57].
Tacstd1 or Epcam was also investigated for its relationship with p53. A study by Sankpal et al. evaluated the transcriptional repression of Epcam and its relationship with p53 expression in breast cancer cells [58]. Candidate p53 binding sites were located and chromatin immunoprecipitation assays confirmed p53 and p21 binding to Epcam. Gain-of-function experiments were conducted, and it was observed that the induction of p53 expression was associated with a dose-dependent decrease in EpCAM expression. EpCAM expression was increased in p53-deficient HCT116 cells, and ablated p53-expressing MCF-7 breast cancer cells treated with UV or camptothecin showed no change in Epcam expression, confirming that EpCAM is mediated by p53. Sankpal et al. also showed that p53 represses the transcription of EpCAM and that Epcam may have an intermediary role in breast and epithelial cancers [58].
In addition, the gene Bcl2, which is also related to p53, was repressed by hCG treatment in our study. Bcl2 has been shown to promote cell survival and to be antiapoptotic, being related to the development of tumors and considered to be an oncogene [59,60].
The genes Arhgap4 and A2m were also found to be downregulated. Arhgap4 is a complex protein that includes an N-terminal FCH domain and a C-terminal SH3 domain [61]. SH3 domains bind to many proteins, including actin-binding proteins. Although the function of the FCH domain is not well understood, current evidence proposes that proteins containing FCH domains are involved in the regulation of cytoskeletal rearrangements, vesicular transport and endocytosis [61]. Arhgap4 is believed to play an important role in the regulation of motility, especially since it has been shown that Arhgap4 has the ability to inhibit the function of Rac1 and Cdc42 [61], which are genes that belong to the Rho family and control several cellular processes, such as cell proliferation, differentiation, motility, adhesion, survival and secretion [62]. However, based on the literature, it seems that the function and role of Arhgap4 in breast cancer cells has yet to be studied. A2m is a major human blood glycoprotein that is known for its ability to inhibit a variety of proteases and to bind and clear endogenous and exogenous molecules [63]. A study by Misra et al. showed that A2m had the ability to activate PAK-2 in 1-LN prostate cancer cells and may be responsible for the metastasic potential of these cells [63]. In our study, we observed the repression of this gene by hCG treatment.
These changes in gene expression are induced by the direct effect of hCG on the mammary gland, but in addition, some of the changes observed could be also caused indirectly by hCG, through its effect on the ovaries. Previously, our laboratory demonstrated that hCG treatment induces an increase in the size of the ovaries, mainly due to the enlargement of the corpora lutea, and increases the serum levels of E+P. However, these effects on the ovaries and on the hormones they produce were transient and returned to normal after the cessation of the treatment, while the differentiation of the mammary gland persisted [64]. Independently of the ovary-dependent effects of hCG, our laboratory has also confirmed the preventative effects of hCG against mammary gland tumorigenesis in ovariectomized rats [65]; moreover, we have also observed that hCG was able to abrogate the transforming abilities of estradiol and demonstrated differentiating properties on epithelial mammary gland cells in vitro [66]. Other studies have suggested that the protective effects against breast cancer are due to AFP, a protein that is produced by the liver [16,67–71]. Jacobson et al. observed an increase of AFP in the serum of animals that were pregnant or exposed to pregnancy hormones and also observed cancer-inhibition properties of this protein in breast cancer cells [71]. In our studies, we have not found any gene directly or indirectly related to AFP to be modulated by any of the three preventative strategies.
Conclusion
Analysis of the genes expressed in all three preventative modalities shows that pregnancy, treatment with E+P pellet and hCG invoke cellular differentiation of the mammary gland, providing a protective barrier against breast cancer (Figure 3). With this is mind, the hCG preventative strategy expresses a larger group of genes that promotes additional cellular differentiation of the mammary gland and reduces the potential of mammary gland cells to be transformed by carcinogens (Figure 4). The expression of myoepithelial cell markers Tp63 and Serpinb5, also known as Maspin, in the hCG group supports the conclusion that hCG promotes the expression of genes that are responsible for the advanced state of differentiation in the mammary gland. In addition, the ability of hCG treatment to downregulate the expression of genes involved in cancer-promoting characteristics also highlights its ability to reduce the effect of carcinogens and provide a protective barrier for the mammary gland.
Future perspective
As the most dominant cancer in women, both in the developed and developing world [5], finding ways to prevent breast cancer is critical. In this study, we suggest some of the mechanisms that could be involved in the protective effects of pregnancy, hCG and the combination of progesterone and estrogen. Our studies describe the basis of why hCG could be used as a preventative strategy against breast cancer.
Practice Points.
Early-age parity is protective against breast cancer risk, and pregnancy hormone treatments can mimic this protection.
Sprague–Dawley rats were exposed to three preventative strategies (pregnancy, human chorionic gonadotropin [hCG] and progesterone + estrogen) and their mammary gland morphology and transcriptome were studied.
Pregnancy, progesterone + estrogen and hCG induced differentiation of the mammary gland.
Pregnancy, progesterone + estrogen and hCG induced changes in the transcriptome, with 28 genes commonly dysregulated by all three treatments.
hCG treatment induced the most complete differentiation and larger transcriptomic changes. Some of the upregulated genes were Cebpb, Ndrg1, Maspin and Epcam.
hCG had the strongest effect on genes related to differentiation and other genes that reduce the cell’s potential to be transformed by carcinogens.
hCG could potentially be used as a preventative strategy against breast cancer.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This work was supported by grant KG101080 from Susan G Komen for the Cure and NIH core grant CA06927 to the Fox Chase Cancer Center and an appropriation from the Commonwealth of Pennsylvania. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.MacMahon B, Cole P, Lin TM, et al. Age at first birth and breast cancer risk. Bull World Health Organ. 1970;43(2):209–221. [PMC free article] [PubMed] [Google Scholar]
- 2.Woods KL, Smith SR, Morrison JM. Parity and breast cancer: evidence of a dual effect. BMJ. 1980;281(6237):419–421. doi: 10.1136/bmj.281.6237.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ursin G, Bernstein L, Lord SJ, et al. Reproductive factors and subtypes of breast cancer defined by hormone receptor and histology. Br J Cancer. 2005;93(3):364–371. doi: 10.1038/sj.bjc.6602712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma H, Bernstein L, Pike MC, Ursin G. Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res. 2006;8(4):R43. doi: 10.1186/bcr1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lord SJ, Bernstein L, Johnson KA, et al. Breast cancer risk and hormone receptor status in older women by parity, age of first birth, and breastfeeding: a case–control study. Cancer Epidemiol Biomarkers Prev. 2008;17(7):1723–1730. doi: 10.1158/1055-9965.EPI-07-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang XR, Chang-Claude J, Goode EL, et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103(3):250–263. doi: 10.1093/jnci/djq526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SH, Akuete K, Fulton J, Chelmow D, Chung MA, Cady B. An increased risk of breast cancer after delayed first parity. Am J Surg. 2003;186(4):409–412. doi: 10.1016/s0002-9610(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 8.Li CI, Littman AJ, White E. Relationship between age maximum height is attained, age at menarche, and age at first full-term birth and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16(10):2144–2149. doi: 10.1158/1055-9965.EPI-07-0242. [DOI] [PubMed] [Google Scholar]
- 9.Newcomb PA, Trentham-Dietz A, Hampton JM, et al. Late age at first full term birth is strongly associated with lobular breast cancer. Cancer. 2011;117(9):1946–1956. doi: 10.1002/cncr.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamakoshi K, Yatsuya H, Wakai K, et al. Impact of menstrual and reproductive factors on breast cancer risk in Japan: results of the JACC study. Cancer Sci. 2005;96(1):57–62. doi: 10.1111/j.1349-7006.2005.00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asztalos S, Gann PH, Hayes MK, et al. Gene expression patterns in the human breast after pregnancy. Cancer Prev Res (Phila ) 2010;3(3):301–311. doi: 10.1158/1940-6207.CAPR-09-0069. [DOI] [PubMed] [Google Scholar]
- 12.Misra Y, Bentley PA, Bond JP, Tighe S, Hunter T, Zhao FQ. Mammary gland morphological and gene expression changes underlying pregnancy protection of breast cancer tumorigenesis. Physiol Genomics. 2012;44(1):76–88. doi: 10.1152/physiolgenomics.00056.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13■■.Russo IH, Koszalka M, Russo J. Comparative study of the influence of pregnancy and hormonal treatment on mammary carcinogenesis. Br J Cancer. 1991;64(3):481–484. doi: 10.1038/bjc.1991.335. Shows that human chorionic gonadotropin (hCG) is as efficient as pregnancy for significantly reducing mammary carcinogenesis, and that the protective effect of both pregnancy and hCG treatment is long lasting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russo J, Mailo D, Hu YF, Balogh G, Sheriff F, Russo IH. Breast differentiation and its implication in cancer prevention. Clin Cancer Res. 2005;11(2 Pt 2):931S–936S. [PubMed] [Google Scholar]
- 15.Russo IH, Russo J. Pregnancy-induced changes in breast cancer risk. J Mammary Gland Biol Neoplasia. 2011;16(3):221–233. doi: 10.1007/s10911-011-9228-y. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson HI, Thompson WD, Janerich DT. Multiple births and maternal risk of breast cancer. Am J Epidemiol. 1989;129(5):865–873. doi: 10.1093/oxfordjournals.aje.a115220. [DOI] [PubMed] [Google Scholar]
- 17.Belitskaya-Levy I, Zeleniuch-Jacquotte A, Russo J, et al. Characterization of a genomic signature of pregnancy identified in the breast. Cancer Prev Res. 2011;4(9):1457–1464. doi: 10.1158/1940-6207.CAPR-11-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18■.Peri S, de Cicco RL, Santucci-Pereira J, et al. Defining the genomic signature of the parous breast. BMC Med Genomics. 2012;5(1):46. doi: 10.1186/1755-8794-5-46. Demonstrated that the differentiation of the breast induced by pregnancy induces transcriptomic changes in postmenopausal women. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siwko SK, Dong J, Lewis MT, Liu H, Hilsenbeck SG, Li Y. Evidence that an early pregnancy causes a persistent decrease in the number of functional mammary epithelial stem cells – implications for pregnancy-induced protection against breast cancer. Stem Cells. 2008;26(12):3205–3209. doi: 10.1634/stemcells.2008-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toniolo P, Grankvist K, Wulff M, et al. Human chorionic gonadotropin in pregnancy and maternal risk of breast cancer. Cancer Res. 2010;70(17):6779–6786. doi: 10.1158/0008-5472.CAN-09-4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo S, Russo IH, Lareef MH, Russo J. Effect of human chorionic gonadotropin in the gene expression profile of MCF-7 cells. Int J Oncol. 2004;24(2):399–407. [PubMed] [Google Scholar]
- 22.Cole LA. hCG, the wonder of today’s science. Reprod Biol Endocrinol. 2012;10:24. doi: 10.1186/1477-7827-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cole LA. Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol. 2010;8:102. doi: 10.1186/1477-7827-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole LA. Hyperglycosylated hCG, a review. Placenta. 2010;31(8):653–664. doi: 10.1016/j.placenta.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Russo IH, Koszalka M, Russo J. Human chorionic gonadotropin and rat mammary cancer prevention. J Natl Cancer Inst. 1990;82(15):1286–1289. doi: 10.1093/jnci/82.15.1286. [DOI] [PubMed] [Google Scholar]
- 26■■.Russo IH, Koszalka M, Russo J. Effect of human chorionic gonadotropin on mammary gland differentiation and carcinogenesis. Carcinogenesis. 1990;11(10):1849–1855. doi: 10.1093/carcin/11.10.1849. Shows that hCG significantly reduces mammary carcinogenesis and induces the differentiation of the mammary gland. [DOI] [PubMed] [Google Scholar]
- 27.Russo J, Russo IH. Influence of differentiation and cell kinetics on the susceptibility of the rat mammary gland to carcinogenesis. Cancer Res. 1980;40(8 Pt 1):2677–2687. [PubMed] [Google Scholar]
- 28.Russo J, Russo IH. Susceptibility of the mammary gland to carcinogenesis. II. Pregnancy interruption as a risk factor in tumor incidence. Am J Pathol. 1980;100(2):497–512. [PMC free article] [PubMed] [Google Scholar]
- 29.Russo J, Tay LK, Russo IH. Differentiation of the mammary gland and susceptibility to carcinogenesis. Breast Cancer Res Treat. 1982;2(1):5–73. doi: 10.1007/BF01805718. [DOI] [PubMed] [Google Scholar]
- 30.Thordarson G, Jin E, Guzman RC, Swanson SM, Nandi S, Talamantes F. Refractoriness to mammary tumorigenesis in parous rats: is it caused by persistent changes in the hormonal environment or permanent biochemical alterations in the mammary epithelia? Carcinogenesis. 1995;16(11):2847–2853. doi: 10.1093/carcin/16.11.2847. [DOI] [PubMed] [Google Scholar]
- 31.Sivaraman L, Stephens LC, Markaverich BM, et al. Hormone-induced refractoriness to mammary carcinogenesis in Wistar–Furth rats. Carcinogenesis. 1998;19(9):1573–1581. doi: 10.1093/carcin/19.9.1573. [DOI] [PubMed] [Google Scholar]
- 32.Guzman RC, Yang J, Rajkumar L, Thordarson G, Chen X, Nandi S. Hormonal prevention of breast cancer: mimicking the protective effect of pregnancy. Proc Natl Acad Sci USA. 1999;96(5):2520–2525. doi: 10.1073/pnas.96.5.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajkumar L, Guzman RC, Yang J, Thordarson G, Talamantes F, Nandi S. Short-term exposure to pregnancy levels of estrogen prevents mammary carcinogenesis. Proc Natl Acad Sci USA. 2001;98(20):11755–11759. doi: 10.1073/pnas.201393798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34■.Medina D. Breast cancer: the protective effect of pregnancy. Clin Cancer Res. 2004;10(1 Pt 2):380S–384S. doi: 10.1158/1078-0432.ccr-031211. Demonstrates that p53 plays a pivotal role in hormone-induced protection. [DOI] [PubMed] [Google Scholar]
- 35.Rajkumar L, Guzman RC, Yang J, Thordarson G, Talamantes F, Nandi S. Prevention of mammary carcinogenesis by short-term estrogen and progestin treatments. Breast Cancer Res. 2004;6(1):R31–R37. doi: 10.1186/bcr734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russo J, Russo IH. Role of the Transcriptome in Breast Cancer Prevention. Springer; NY, USA: 2013. Comparative effects of the preventive effect of pregnancy, steroidal hormones, and hCG in the transcriptomic profile of the rat mammary gland; pp. 73–189. [Google Scholar]
- 37.Liu J, Welm B, Boucher KM, Ebbert MT, Bernard PS. TRIM29 functions as a tumor suppressor in nontumorigenic breast cells and invasive ER+ breast cancer. Am J Pathol. 2012;180(2):839–847. doi: 10.1016/j.ajpath.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lemmers C, Michel D, Lane-Guermonprez L, et al. CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol Biol Cell. 2004;15(3):1324–1333. doi: 10.1091/mbc.E03-04-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chartier FJ, Hardy EJ, Laprise P. Crumbs controls epithelial integrity by inhibiting Rac1 and PI3K. J Cell Sci. 2011;124(Pt 20):3393–3398. doi: 10.1242/jcs.092601. [DOI] [PubMed] [Google Scholar]
- 40.Fan S, Fogg V, Wang Q, Chen XW, Liu CJ, Margolis B. A novel Crumbs3 isoform regulates cell division and ciliogenesis via importin beta interactions. J Cell Biol. 2007;178(3):387–398. doi: 10.1083/jcb.200609096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fogg VC, Liu CJ, Margolis B. Multiple regions of Crumbs3 are required for tight junction formation in MCF10A cells. J Cell Sci. 2005;118(Pt 13):2859–2869. doi: 10.1242/jcs.02412. [DOI] [PubMed] [Google Scholar]
- 42.Blanchard AA, Watson PH, Shiu RP, et al. Differential expression of claudin 1, 3, and 4 during normal mammary gland development in the mouse. DNA Cell Biol. 2006;25(2):79–86. doi: 10.1089/dna.2006.25.79. [DOI] [PubMed] [Google Scholar]
- 43.Thibodeau PH, Butterworth MB. Proteases, cystic fibrosis and the epithelial sodium channel (ENaC) Cell Tissue Res. 2013;351(2):309–323. doi: 10.1007/s00441-012-1439-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boyd C, Naray-Fejes-Toth A. Steroid-mediated regulation of the epithelial sodium channel subunits in mammary epithelial cells. Endocrinology. 2007;148(8):3958–3967. doi: 10.1210/en.2006-1741. [DOI] [PubMed] [Google Scholar]
- 45■.Srivastava P, Russo J, Russo IH. Chorionic gonadotropin inhibits rat mammary carcinogenesis through activation of programmed cell death. Carcinogenesis. 1997;18(9):1799–1808. doi: 10.1093/carcin/18.9.1799. Demonstrates that hCG induces programmed cell death and postulates that this process is p53 dependent and is modulated by c-myc expression. [DOI] [PubMed] [Google Scholar]
- 46.Herr D, Keck C, Tempfer C, Pietrowski D. Chorionic gonadotropin regulates the transcript level of VHL, p53, and HIF-2alpha in human granulosa lutein cells. Mol Reprod Dev. 2004;69(4):397–401. doi: 10.1002/mrd.20137. [DOI] [PubMed] [Google Scholar]
- 47.Matsubara H, Ikuta K, Ozaki Y, et al. Gonadotropins and cytokines affect luteal function through control of apoptosis in human luteinized granulosa cells. J Clin Endocrinol Metab. 2000;85(4):1620–1626. doi: 10.1210/jcem.85.4.6509. [DOI] [PubMed] [Google Scholar]
- 48.Boggs K, Reisman D. C/EBPbeta participates in regulating transcription of the p53 gene in response to mitogen stimulation. J Biol Chem. 2007;282(11):7982–7990. doi: 10.1074/jbc.M611675200. [DOI] [PubMed] [Google Scholar]
- 49.Stein S, Thomas EK, Herzog B, et al. NDRG1 is necessary for p53-dependent apoptosis. J Biol Chem. 2004;279(47):48930–48940. doi: 10.1074/jbc.M400386200. [DOI] [PubMed] [Google Scholar]
- 50.Kovacevic Z, Sivagurunathan S, Mangs H, Chikhani S, Zhang D, Richardson DR. The metastasis suppressor, N-myc downstream regulated gene 1 (NDRG1), upregulates p21 via p53-independent mechanisms. Carcinogenesis. 2011;32(5):732–740. doi: 10.1093/carcin/bgr046. [DOI] [PubMed] [Google Scholar]
- 51.Zou Z, Gao C, Nagaich AK, et al. p53 regulates the expression of the tumor suppressor gene. Maspin J Biol Chem. 2000;275(9):6051–6054. doi: 10.1074/jbc.275.9.6051. [DOI] [PubMed] [Google Scholar]
- 52.Kim S, Han J, Kim J, Park C. Maspin expression is transactivated by p63 and is critical for the modulation of lung cancer progression. Cancer Res. 2004;64(19):6900–6905. doi: 10.1158/0008-5472.CAN-04-1657. [DOI] [PubMed] [Google Scholar]
- 53.Butt AJ, Dickson KA, McDougall F, Baxter RC. Insulin-like growth factor-binding protein-5 inhibits the growth of human breast cancer cells in vitro and in vivo. J Biol Chem. 2003;278(32):29676–29685. doi: 10.1074/jbc.M301965200. [DOI] [PubMed] [Google Scholar]
- 54.Gullu G, Karabulut S, Akkiprik M. Functional roles and clinical values of insulin-like growth factor-binding protein-5 in different types of cancers. Chin J Cancer. 2012;31(6):266–280. doi: 10.5732/cjc.011.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parker A, Clarke JB, Busby WH, Jr, Clemmons DR. Identification of the extracellular matrix binding sites for insulin-like growth factor-binding protein 5. J Biol Chem. 1996;271(23):13523–13529. doi: 10.1074/jbc.271.23.13523. [DOI] [PubMed] [Google Scholar]
- 56.Nam TJ, Busby WH, Jr, Rees C, Clemmons DR. Thrombospondin and osteopontin bind to insulin-like growth factor (IGF)-binding protein-5 leading to an alteration in IGF-I-stimulated cell growth. Endocrinology. 2000;141(3):1100–1106. doi: 10.1210/endo.141.3.7386. [DOI] [PubMed] [Google Scholar]
- 57.Sureshbabu A, Okajima H, Yamanaka D, et al. IGFBP5 induces cell adhesion, increases cell survival and inhibits cell migration in MCF-7 human breast cancer cells. J Cell Sci. 2012;125(Pt 7):1693–1705. doi: 10.1242/jcs.092882. [DOI] [PubMed] [Google Scholar]
- 58.Sankpal NV, Willman MW, Fleming TP, Mayfield JD, Gillanders WE. Transcriptional repression of epithelial cell adhesion molecule contributes to p53 control of breast cancer invasion. Cancer Res. 2009;69(3):753–757. doi: 10.1158/0008-5472.CAN-08-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang DC, O’Reilly LA, Strasser A, Cory S. The anti-apoptosis function of Bcl-2 can be genetically separated from its inhibitory effect on cell cycle entry. EMBO J. 1997;16(15):4628–4638. doi: 10.1093/emboj/16.15.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22(53):8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 61.Vogt DL, Gray CD, Young WS, 3rd, Orellana SA, Malouf AT. ARHGAP4 is a novel RhoGAP that mediates inhibition of cell motility and axon outgrowth. Mol Cell Neurosci. 2007;36(3):332–342. doi: 10.1016/j.mcn.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boureux A, Vignal E, Faure S, Fort P. Evolution of the Rho family of ras-like GTPases in eukaryotes. Mol Biol Evol. 2007;24(1):203–216. doi: 10.1093/molbev/msl145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Misra UK, Deedwania R, Pizzo SV. Binding of activated alpha2-macroglobulin to its cell surface receptor GRP78 in 1-LN prostate cancer cells regulates PAK-2-dependent activation of LIMK. J Biol Chem. 2005;280(28):26278–26286. doi: 10.1074/jbc.M414467200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Russo J, Russo IH. Molecular Basis of Breast Cancer: Prevention and Treatment. Springer-Verlag; Berlin, Germany: 2004. The new paradigm in breast cancer prevention; pp. 396–397. [Google Scholar]
- 65.Russo IH, Russo J. Physiological bases of breast cancer prevention. Eur J Cancer Prev. 1993;2(Suppl 3):101–111. [PubMed] [Google Scholar]
- 66.Kocdor H, Kocdor MA, Russo J, et al. Human chorionic gonadotropin (hCG) prevents the transformed phenotypes induced by 17 beta-estradiol in human breast epithelial cells. Cell Biol Int. 2009;33(11):1135–1143. doi: 10.1016/j.cellbi.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Janerich DT, Hoff MB. Evidence for a crossover in breast cancer risk factors. Am J Epidemiol. 1982;116(5):737–742. doi: 10.1093/oxfordjournals.aje.a113462. [DOI] [PubMed] [Google Scholar]
- 68.Bernstein L, Hanisch R, Sullivan-Halley J, Ross RK. Treatment with human chorionic gonadotropin and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 1995;4(5):437–440. [PubMed] [Google Scholar]
- 69.Bennett JA, Zhu S, Pagano-Mirarchi A, Kellom TA, Jacobson HI. Alpha-fetoprotein derived from a human hepatoma prevents growth of estrogen-dependent human breast cancer xenografts. Clin Cancer Res. 1998;4(11):2877–2884. [PubMed] [Google Scholar]
- 70.Richardson BE, Hulka BS, Peck JL, et al. Levels of maternal serum alpha-fetoprotein (AFP) in pregnant women and subsequent breast cancer risk. Am J Epidemiol. 1998;148(8):719–727. doi: 10.1093/oxfordjournals.aje.a009691. [DOI] [PubMed] [Google Scholar]
- 71.Jacobson HI, Lemanski N, Agarwal A, et al. A proposed unified mechanism for the reduction of human breast cancer risk by the hormones of pregnancy. Cancer Prev Res (Phila ) 2010;3(2):212–220. doi: 10.1158/1940-6207.CAPR-09-0050. [DOI] [PubMed] [Google Scholar]
Website
- 101.Ingenuity® Systems. www.ingenuity.com.