Abstract
Background
Prostaglandins play a major role in inflammation and pain. They are synthesised by the two cyclooxygenase (COX) isoforms: COX-1, which is expressed constitutively in many cell types and COX-2, which is induced at the site of inflammation. However, unlike peripheral tissues, COX-2 is expressed constitutively in the central nervous system and may play a role in nociceptive processes. The present study aimed to investigate the role of constitutive COX-2 in the spinal transmission of nociceptive signals in humans.
Methods
We used 12 healthy volunteers to compare the effects of the specific COX-2 inhibitor sodium parecoxib (1 mg/kg) or placebo, administered intravenously in a double-blind and cross-over fashion, on the electrophysiological recordings of the nociceptive flexion (RIII) reflex. The RIII reflex is an objective psychophysiological index of the spinal transmission of nociceptive signals and was recorded from the biceps femoris after electrical stimulation of the sural nerve. Two experiments, seven days apart, were carried out on each volunteer. On each experimental day, the effects of parecoxib or placebo were tested on: 1) the RIII reflex threshold, 2) the stimulus-response curves of the reflex up to the tolerance threshold (frequency of stimulation: 0.1 Hz); 3) the progressive increase of the reflex and pain sensations (i.e. “wind-up” phenomenon) induced by a series of 15 stimulations at a frequency of 1 Hz (intensity 20% above RIII threshold).
Results
Parecoxib, but not placebo, significantly reduced the slope of the stimulus-response curve, suggesting a reduction in the gain of the spinal transmision of nociceptive signals. By contrast, the “wind-up” phenomenon was not significantly altered after administration of parecoxib or placebo.
Conclusions
Our study shows that constitutive COX-2 modulates spinal nociceptive processes and that the anti-inflammatory and antinociceptive actions of COX-2 inhibitors are not necessarily related.
Keywords: Adult, Cross-Over Studies, Cyclooxygenase 2, physiology, Double-Blind Method, Female, Humans, Isoxazoles, pharmacology, Male, Pain, physiopathology, Pain Threshold, drug effects, Reflex, drug effects, Spinal Cord, physiology
Introduction
The analgesic and anti-inflammatory actions of nonsteroidal anti-inflammatory drugs (NSAIDs) have traditionally been attributed to their inhibition of peripheral prostaglandins (PG)[1,2]. PGs are synthesised from arachidonic acids by two cyclooxygenase isoforms (COX1 and COX2) and play a major role in sensitising nociceptors at the site of tissue injury[3]. COX-1 is constitutively expressed in many cell types whereas COX-2 is induced at the site of inflammation[4]. Most conventional NSAIDs non-preferentially inhibit both COX isozymes. Their analgesic effects are thought to be mostly due to their inhibiting the COX-2 isoform, and their adverse effects from inhibiting COX-1. Thus, the development of selective COX-2 inhibitors has contributed significantly to therapeutic progress as these molecules have similar anti-inflammatory and analgesic properties but are better tolerated clinically[5,6]. However, recent data on acute cardiovascular toxicity has lead to new recommendations for their use[7–9]. Thus, there is active research into the mechanisms of NSAIDs action with the aim of improving their clinical use.
A growing body of experimental evidence suggests that, in addition to their well established peripheral effects, NSAIDs may also exert their analgesic action directly within the central nervous system (CNS)[2,10]. Both COX isoforms are constitutively expressed in rat brain and spinal cord [11,12]. COX-2 is the predominant isoform in the spinal dorsal horn and could play a role, not only in pathological inflammatory pain, but also in normal physiological pain (i.e. without inflammation). There are few experimental data confirming the role of constitutive COX-2 in normal pain processing in animal models[2, 13] but no information in humans. More generally, there is no direct evidence for central antinociceptive effects of selective COX-2 inhibitors in humans. However, studies based on experimental models of inflammatory secondary hyperalgesia indirectly suggested a central action of parecoxib[14] and rofecoxib[15], but not valdecoxib[16].
In the present study we analysed the role of constitutive COX-2 in central nociceptive processes. We compared the effects on the nociceptive flexion RIII reflex of intravenous administration of parecoxib or placebo in healthy volunteers, administered according to a double-blind, cross-over design.
Parecoxib is the sulfonamide-based pro-drug of valdecoxib. It is a highly specific COX-2 inhibitor and is the only available parenterally-administered coxib[17]. The RIII reflex is elicited by electrical stimulation of a cutaneous sensory nerve and is recorded from a flexor muscle on the ipsilateral limb. This polysynaptic spinal reflex is considered to be a reliable index of spinal nociceptive signal transmission as its threshold and amplitude are closely related to those of painful cutaneous sensations evoked by electrical stimulation[18]. RIII reflex recordings have been used in numerous pharmacological studies related to analgesia in humans [19–20]. In particular, it was used to reveal the central action of conventional NSAIDs (i.e. mixed COX1-COX2 inhibitors) on nociceptive processes[21–23]. In the present study, we tested the effects of parecoxib on the RIII reflex stimulus-response curves. We also analysed the effects of parecoxib on the progressive increase of the reflex response and resulting sensation induced by repeated series of stimuli at relatively a high frequency (i.e. 1 Hz) of fixed intensity. This “wind-up” phenomenon, is due to the summation of nociceptive input over time (i.e. temporal summation) in the spinal cord and is considered to be an experimental elementary form of central sensitisation[24].
Thus, the goal of this electrophysiological study was to demonstrate in humans that COX-2 inhibitors have central effects and act on nociceptive processes independently of inflammation.
Methods
The experiments were approved by a local Ethics Committee and carried out on 12 paid healthy volunteers. The volunteers were carefully briefed about the experimental procedures and gave informed written consent for their participation in the study.
Electrophysiological recording of the RIII reflex
During the recordings, the subjects sat comfortably reclined to ensure a state complete muscular relaxation. (Fig 1) The nociceptive flexion reflex (RIII) was evoked and recorded with a computerised system (Notocord Systems, Croissy, France), using previously described techniques[25–26]. Briefly, the sural nerve was electrically stimulated at a rate of 0.1 Hz using a pair of surface electrodes placed 2 cm apart on the degreased skin overlying the nerve within its retromalleolar path. The electrical stimuli consisted of trains of six rectangular 1 ms pulses delivered over 12 ms from a constant current stimulator. Electromyographic responses were recorded from the ipsilateral biceps femoris muscle using a pair of surface electrodes placed 2 cm apart on the degreased skin over the muscle. The electromyographic responses were then amplified, digitised and full-wave rectified, and the RIII response from the resulting integrals. The reflex responses were identified as multiphasic signals and integrated in a time window from 90 to 180 ms after stimulus onset. This time-window restriction avoids any tactile (RII) reflex that can occur between 50 and 70 ms after stimulation or any artifacts produced by involuntary movements that can be observed as early as 250 to 300 ms after stimulation. Each individual experiment started with a control period during which the stimulus was applied at an intensity 20% higher than the threshold required for stable RIII reflex responses. This control period was considered a prerequisite before starting the pharmacological procedure. The stimuli elicited slightly painful sensations similar to pinprick and were described by the subjects as originating from the stimulating electrodes and projecting into the distal cutaneous receptive field of the sural nerve on the lateral side of the foot.
Figure 1.
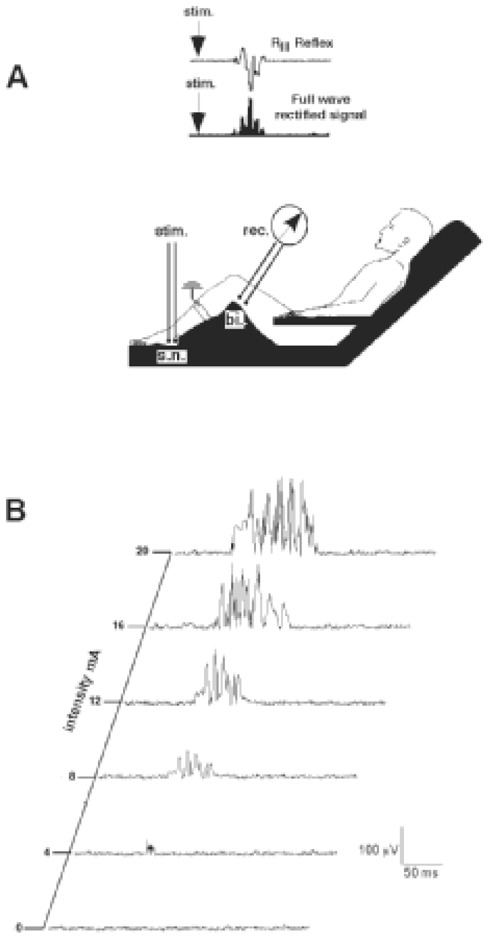
A) Experimental set-up for recording the RIII reflex. The sural nerve (sn) was stimulated behind the lateral malleolus, using a pair of surface electrodes. The electrical responses were recorded (rec) from the ipsilateral biceps femoris muscle (bi) using a pair of surface electrodes. An example of an RIII reflex response, and the corresponding full-wave rectified signal, are shown in the upper part of the figure. B) individual example of RIII reflex responses with increasing intensity of stimulation of the sural nerve at 0.1 Hz.
Experimental procedure
This pharmacological study was organised as a double-blind, cross-over trial. We randomly assigned a placebo (saline) or 1 mg/kg of parecoxib (Laboratoire Pfizer, Paris, France) in a 10 ml volume of 10 ml, which was injected intravenously over a period of 10 min, with a maximum parecoxib dose of 80 mg. This dose was chosen because it was in the range used in clinical trials[27–28]. The maximum dose corresponds to the daily maximum dose authorised for clinical use in France. The experiments were carried out in the volunteers twice, with each experiment separated by an interval of seven days. Six stimulation sequences were used on each experimental day: two before injection (i.e. control period), and then at 20, 40, 60 and 80 min after administration of parecoxib or placebo. Each sequence consisted of: (1) determining the RIII reflex threshold (defined as the average minimal current that elicited the reflex response) by four successive sequences of increasing and decreasing the stimulus intensity by steps of 0.5 mA; (2) building of the recruitment curve for the reflex as a function of stimulus intensity by increasing progressively the stimulus intensity by steps of 1 mA to the tolerance threshold at a frequency of 0.1 Hz (i.e. 6 stimuli/min); (3) applying a series of 15 stimuli at 1.2 times the threshold at a frequency of 1 Hz to analyse the “wind-up” phenomenon. After each “wind-up” sequence, the subjects were asked to use a 100 mm VAS, graduated from 0 (no pain) to 100 (worst possible pain), to rate both the sensation evoked by the first stimulus in each series and the maximum pain produced by any of the stimuli. Blood pressure, heart rate and SaO2 were monitored during the experimental sessions. Side effects such as nausea, vomiting, sedation, dysphoria and hallucinations were recorded when present. Sedation was scored using the following scale: 0: patient fully alert; 1: patient with intermittent sedation; 2: patient sedated but responsive to verbal stimuli; 3: patient unresponsive to verbal stimuli.
Data analysis
Data are expressed as means ± SEM. The reflex threshold was defined as the minimum intensity inducing an RIII response for at least 50% of the stimuli. The tolerance threshold was the maximum tolerable stimulation intensity defined by the volunteer during recording of the recruitment curve. Each reflex response was expressed as a percentage of the maximum response observed during recording of the control recruitment curve (i.e. before the injection) to allow analysis of the group data. Recruitment curves were normalised between 0 and 20 mA according to the last observation carried forward method, such that when the tolerance threshold was <20 mA, the final value obtained for the reflex was assigned to all the higher intensities in the series. The wind-up phenomenon was analysed by expressing each response during the sequence of 15 stimuli at 1 Hz as a percentage of the first response. We used Wilcoxon’s signed ranks test to compare paired data. The areas under the mean recruitment curves (AUC) and the mean “wind-up” curves were calculated and used to compare the effects of the placebo and the parecoxib. A repeated-measures analysis of variance was used to test treatment, subject, sequence and period effects. Results were considered significant at P < 0.05.
Results
Twelve volunteers (6 men, 6 women, aged 21–40 years), completed the two sessions of the study. The mean dose of parecoxib administered intravenously was: 57.0 ± 12.0 mg. No side effects were reported with either the placebo or parecoxib.
Effects of Parecoxib on the Recruitment Curve of the Nociceptive Flexion Reflex
The area under the RIII reflex recruitment curves (AUC) were similar before the injection of parecoxib or placebo (Fig 2). After administration of parecoxib, the AUC progressively and significantly decreased between 20 to 60 minutes, returning to baseline values at 80 minutes (Fig 2A). By contrast, we observed no significant change of AUC after administration of the placebo. The effects due to parecoxib were significantly different from those due to placebo (p< 0.05) at 40 and 60 minutes. The effects on the recruitment curve were not influenced by the sequence (parecoxib-placebo or placebo-parecoxib) or the period (first or second session).
Figure 2.
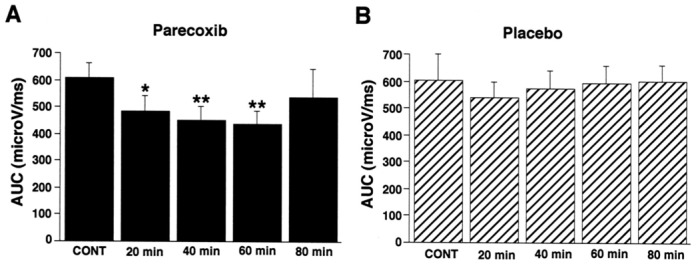
Comparisons of the area under the recruitment curves (AUC) from the control baseline period (CONT), and 20, 40, 60 and 80 minutes after the administration of (A) parecoxib (black columns), or (B) placebo (hatched columns). A significant reduction in the recruitment curve was observed from 20 to 60 minutes after the administration of parecoxib but not with the placebo. Data are means ± SEMs. * = P<0.05, **= P<0.01.
As illustrated in Fig 3, the decrease in AUC was due to the slope of the recruitment curve decreasing, with no significant change in the RIII reflex threshold at any time after the injection. Thus, the mean RIII threshold, which was not significantly different at baseline between the parecoxib (7.8 ± 1 mA) and placebo (7.0 ± 0.7 mA) groups, was not significantly altered 60 minutes after treatment (7.5 ± 1.0 mA after parecoxib and 6.2 ± 0.6 mA after placebo). By contrast, the mean tolerance threshold (i.e. the maximum stimulus intensity on the recruitment curve), which was not significantly different at baseline between the parecoxib (14.3±2.7 mA) and placebo (15.0± 3.6 mA) groups, was significantly higher (p < 0.05) 40 and 60 minutes after administration of parecoxib (i.e. 17.2 ± 2.4 mA and 17.3 ± 2.0 mA) than after the placebo (14.1 ± 1.1 mA and 14.0 ± 1.3 mA).
Figure 3.
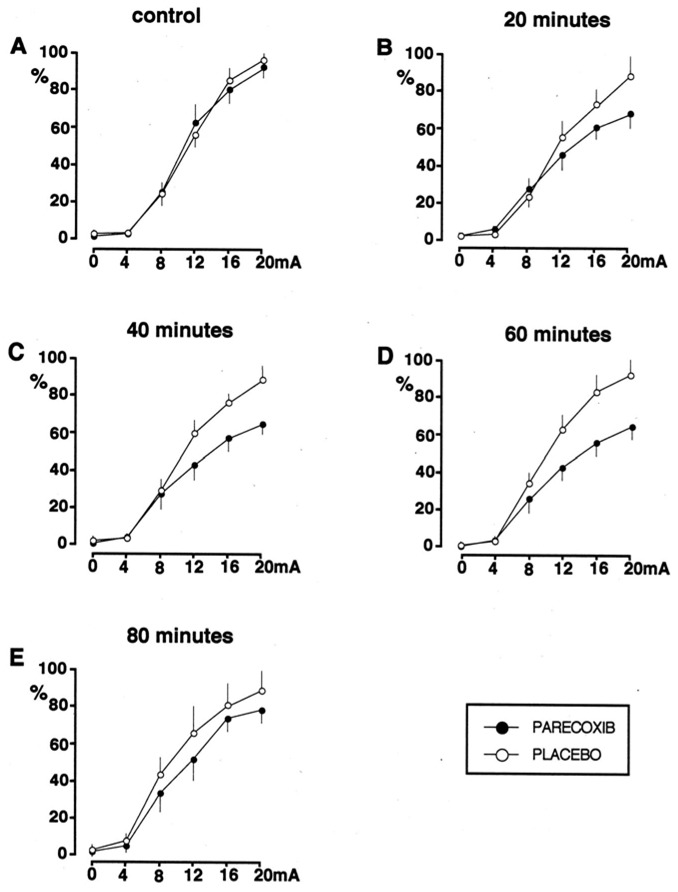
Effects of administration of placebo or parecoxib on the shape of the recruitment curves for the RIII reflex at baseline (A), and 20 (B), 40 (C) 60 (D) and 80 (E) minutes after the administration of placebo or parecoxib. Reflex responses are expressed as a percentage of the maximum response obtained when building the control curve. A progressive reduction of the slope of the recruitment curve was observed from 20 minutes after the administration of parecoxib.
Effects of parecoxib on the wind up phenomenon
As previously described[25–26], applying a series of 15 stimuli at 1.2 times the reflex threshold and at a frequency of 1 Hz, progressively increased the reflex responses (up to 250% of the first response). This wind-up of the RIII reflex, due to the temporal summation of nociceptive stimuli, was similar during the two control periods and did not significantly change after administration of the placebo or parecoxib (Fig 4). Consistent with these electrophysiological results, the progressive increase in pain sensation during the application of high frequency stimulation was similar in the two treatment groups at baseline (VAS score increased from 21.6±8 to 52 ± 12 in the parecoxib group and from 23.6±11 mm to 49± 15 in the placebo group) and was not significantly different after administration of parecoxib or placebo.
Figure 4.
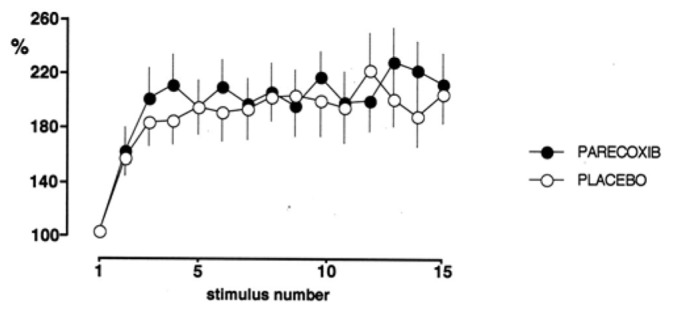
Effects of placebo and parecoxib on the wind-up of the RIII reflex. Each reflex response was expressed as a percentage of the first response in the series.
4. Discussion
The systemic administration of a clinically active dose of parecoxib significantly reduced the nociceptive flexion RIII reflex in healthy volunteers. This shows that COX-2 inhibitors act centrally and that the spinal transmission of nociceptive signals is affected by constitutively expressed COX-2 in the central nervous system. More generally, our results indicate that the anti-inflammatory and anti-nociceptive actions of COX-2 inhibitors are not necessarily related.
NSAIDs have long been suspected to affect the CNS[29] as numerous animals studies over the last fifteen years have suggested[2,13]. The finding that both COX isoenzymes are expressed in the CNS, that COX-2 is overexpressed in CNS neurons after peripheral inflammation, whereas COX-1 is overexpressed in the microglia[30], and that PG, which are released in the spinal cord during nociceptive stimuli, could facilitate central nociceptive transmission strongly support this idea[2,4,13,31]. These results challenged the classical notion that the antinociceptive action of NSAIDs was due to a reduction of nociceptor sensitisation. However, the peripheral and central modes of NSAIDs actions should not be considered as mutually exclusive but as complementary and possibly synergistic. In addition, the fact that COX-2 is also constitutively expressed in the CNS suggests that it could have a role in normal physiological pain (i.e. without inflammation).
These hypotheses were based mostly on animal data since few studies were performed in humans. Investigation of the central effects of COX-2 inhibitors in humans has relied on the analysis of primary and secondary hyperalgesia induced by cutaneous UV-B irradiation injury[15], electrical stimulation[14] or capsaicin application[16]. However, these models allow only an indirect approach to the central analgesics effects of treatment. In addition, the results seem to depend on the type of experimental pain model since negative results were reported with the capsaicin model[16]. Our data based on recordings of the nociceptive flexion RIII reflex which represents an objective and quantifiable electrophysiological correlate of the spinal transmission of nociceptive signals, show more directly the central action of COX-2 inhibitors. This methodology is particularly interesting as the cutaneous electrical stimulation of the sural nerve at the ankle bypasses the peripheral nociceptor. Thus, changes in the RIII reflex after administration of pharmacological agents, which do not act on the nerve conduction or muscular contraction, can be attributed to a central action of the drug. This methodology allows analysis of the pharmacological effects on the pain threshold and also over a wide range of supra-threshold stimulus intensities. The central effects of various mixed COX-1/COX-2 inhibitors (ketoprofen, ibuprofen, indomethacin) have been confirmed in healthy volunteers using this methodology[21–23]. Our finding that a more selective COX-2 inhibitor modulates the RIII reflex, complements these results and suggests that the central action of conventional NSAIDs involves the inhibition of central COX-2. This result, however, does not exclude that central COX-1 has also a role in analgesia, as suggested by experimental studies[30,32].
The preferential effects of parecoxib on the RIII reflex recruitment curve (i.e. intensity-response) may indicate that it more specifically acts on the “gain” of nociceptive signal transmission in the spinal cord. Consistent with this, COX-2 has been shown to be the predominant constitutive isoform expressed in the central nervous system and particularly in the spinal cord[12,13]. In accordance with electrophysiological and behavioral data in animals[13], our results indicate that constitutive COX-2 and probably prostaglandins are involved in normal nociceptive processing in humans. However, since in our study parecoxib was injected intravenously, we cannot exclude that it also acts supraspinally and the modulation of the RIII reflex involved descending controls. Interestingly, Willer et al.[33] showed that the effects of ketoprofen on the RIII reflex were reduced in paraplegic patients with a complete spinal cord transection, suggesting that mixed inhibitors could act supraspinally.
A series of animal studies have suggested that COX2 and prostaglandins, especially PGE2, are involved in central sensitisation (i.e. hyperexcitability of spinal nociceptive neurons) and hyperalgesia developing after peripheral inflammation and probably also nerve injury[2,30,32,34,35]. The fact that COX-2 inhibitors reduced secondary hyperalgesia also suggest an action on central sensitization[14,15]. In the present study, we investigated the effects of parecoxib on the progressive increase (i.e. “wind-up”) of the RIII reflex induced by the temporal summation of the nociceptive inputs, which may be related to central sensitisation[24,36]. Consistent with the results of animal studies, it has been shown previously, in humans, that the wind-up of the RIII reflex involves activation of N-methyl-D-aspartate (NMDA) receptors, since it is selectively reduced by low doses of ketamine[25]. In the present study, the wind-up phenomenon was not altered by parecoxib. This is consistent with the results of animal electrophysiological studies of the effects of NSAIDs on wind-up[37,38], although contradictory results have also been reported[39]. In any case, the opposite effects of parecoxib and ketamine on wind-up of the RIII reflex suggest that NMDA and COX-2 play different roles in the central nociceptive mechanisms.
In conclusion, our results provide the first electrophysiological experimental evidence showing that constitutive COX-2 can modulate central nociceptive processes in humans independent of peripheral inflammation. Thus, the development of centrally-acting COX-2 inhibitors may be of interest in the treatment of pain. The present study also shows that recordings of RIII reflex and analysis of its recruitment curve is a reliable experimental model for analysing the analgesics effects new COX-2 inhibitors.
Acknowledgments
Source of financial support : INSERM (Institut National de la Santé et de la Recherche Médicale)
This study was not founded by private companies. None of the authors has any conflict of interest with regard to this manuscript. The authors thank Valérie Gaudé-Joindreau (Study nurse, Ambroise Paré Hospital, Boulogne-Billancourt, France) and Valérie Casano (Study Nurse, Ambroise Paré Hospital, Boulogne-Billancourt, France) for technical assistance.
Abbreviations
- COX
Cyclooxygenase
- NSAID
nonsteroidal anti-inflammatory drug
- PG
prostaglandin
- CNS
central nervous system
- VAS
Visual Analog Scale
- NMDA
N-methyl-D-aspartate
References
- 1.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin- like drugs. Nat New Biol. 1971;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 2.Burian M, Geisslinger G. COX-dependent mechanisms involved in the antinociceptive action of NSAIDs at central and peripheral sites. Pharmacol Ther. 2005;107:139–154. doi: 10.1016/j.pharmthera.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Ito S, Okuda-Ashitaka E, Minami T. Central and peripheral roles of prostaglandins in pain and their interactions with novel neuropeptides nociceptin and nocistatin. Neurosci Res. 2001;41:299–332. doi: 10.1016/s0168-0102(01)00289-9. [DOI] [PubMed] [Google Scholar]
- 4.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 5.Stichtenoth DO, Frolich JC. The second generation of COX- 2 inhibitors: what advantages do the newest offer? Drugs. 2003;63:33–45. doi: 10.2165/00003495-200363010-00003. [DOI] [PubMed] [Google Scholar]
- 6.Warner TD, Mitchell JA. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J. 2004;18:790–804. doi: 10.1096/fj.03-0645rev. [DOI] [PubMed] [Google Scholar]
- 7.Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, Konstam MA, Baron JA Investigators tAPPoVAT. Cardiovascular Events Associated with Rofecoxib in a Colorectal Adenoma Chemoprevention Trial. N Engl J Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 8.Solomon SD, McMurray JJV, Pfeffer MA, Wittes J, Fowler R, Finn P, Anderson WF, Zauber A, Hawk E, Bertagnolli M The Adenoma Prevention with Celecoxib (APC) Study Investigators. Cardiovascular Risk Associated with Celecoxib in a Clinical Trial for Colorectal Adenoma Prevention. N Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 9.Nussmeier NA, Whelton AA, Brown MT, Langford RM, Hoeft A, Parlow JL, Boyce SW, Verburg KM. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005;352:1081–1091. doi: 10.1056/NEJMoa050330. [DOI] [PubMed] [Google Scholar]
- 10.McCormack K, Brune K. Dissociation between the antinociceptive and anti-inflammatory effects of the nonsteroidal anti-inflammatory drugs. A survey of their analgesic efficacy. Drugs. 1991;41:533–547. doi: 10.2165/00003495-199141040-00003. [DOI] [PubMed] [Google Scholar]
- 11.Breder CD, Dewitt D, RPK Characterization of inducible cyclooxygenase in rat brain. J Comp Neurol. 1995;355:296–315. doi: 10.1002/cne.903550208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beiche F, Scheuerer S, Brune K, Geisslinger G, Goppelt-Struebe M. Up-regulation of cyclooxygenase-2 mRNA in the rat spinal cord following peripheral inflammation. FEBS Lett. 1996;390:165–169. doi: 10.1016/0014-5793(96)00604-7. [DOI] [PubMed] [Google Scholar]
- 13.Vanegas H, Schaible HG. Prostaglandins and cyclooxygenases in the spinal cord. Prog Neurobiol. 2001;64:327–363. doi: 10.1016/s0301-0082(00)00063-0. [DOI] [PubMed] [Google Scholar]
- 14.Koppert W, Wehrfritz A, Korber N, Sittl R, Albrecht S, Schuttler J, MS The cyclooxygenase isozyme inhibitors parecoxib and paracetamol reduce central hyperalgesia in humans. Pain. 2004;108:148–153. doi: 10.1016/j.pain.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Sycha T, Anzenhofer S, Lehr S, Schmetterer L, Chizh B, Eichler HG, BG Rofecoxib attenuates both primary and secondary inflammatory hyperalgesia: a randomized, double blinded, placebo controlled crossover trial in the UV-B pain model. Pain. 2005;113:316–322. doi: 10.1016/j.pain.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Burns D, Hill L, Essandoh M, Jarzembowski TM, Schuler HG, Janicki PK. Effect of valdecoxib pretreatment on pain and secondary hyperalgesia: a randomized controlled trial in healthy volunteers. BMC Anesthesiol. 2006;10(6):3. doi: 10.1186/1471-2253-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barden J, Edwards JE, McQuay HJ, Moore RA. Oral valdecoxib and injected parecoxib for acute postoperative pain: a quantitative systematic review. BMC Anesthesiol. 2003;3:1–8. doi: 10.1186/1471-2253-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willer JC. Comparative study of perceived pain and nociceptive flexion reflex in man. Pain. 1977;3:69–80. doi: 10.1016/0304-3959(77)90036-7. [DOI] [PubMed] [Google Scholar]
- 19.Skljarevski V, Ramadan NM. The nociceptive flexion reflex in humans -- review article. Pain. 2002;96:3–8. doi: 10.1016/s0304-3959(02)00018-0. [DOI] [PubMed] [Google Scholar]
- 20.Sandrini G, Serrao M, Rossi P, Romaniello A, Cruccu G, Willer JC. The lower limb flexion reflex in humans. Prog Neurobiol. 2005;77:353–395. doi: 10.1016/j.pneurobio.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Willer JC, Harrewyn JM. Central inhibitory effect of intravenous ketoprofen on the nociceptive flexion reflex in man. Presse Med. 1987;16(2):63–67. [PubMed] [Google Scholar]
- 22.Sandrini G, Ruiz L, Capararo M, Garofoli F, Beretta A, GN Central analgesic activity of ibuprofen. A neurophysiological study in humans. Int J Clin Pharmacol Res. 1992;12(4):197–204. [PubMed] [Google Scholar]
- 23.Guieu R, Blin O, Pouget J, GS Analgesic effect of indomethacin shown using the nociceptive flexion reflex in humans. Ann Rheum Dis. 1992;51(3):391–393. doi: 10.1136/ard.51.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrero JF, Laird JM, JAL-G Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 25.Guirimand F, Dupont X, Brasseur L, Chauvin M, DB The effects of ketamine on the temporal summation “wind-up” of the nociceptive flexion (RIII) reflex and pain in humans. Anesth Analg. 2000;90:408–414. doi: 10.1097/00000539-200002000-00031. [DOI] [PubMed] [Google Scholar]
- 26.Bossard AE, Guirimand F, Fletcher D, Gaude-Joindreau V, Chauvin M, DB Interaction of a combination of morphine and ketamine on the nociceptive flexion reflex in human volunteers. Pain. 2002;98:47–57. doi: 10.1016/s0304-3959(01)00472-9. [DOI] [PubMed] [Google Scholar]
- 27.Karim A, Laurent A, Slater ME, Kuss ME, Qian J, Crosby-Sessoms SL, Hubbard RC. A pharmacokinetic study of intramuscular (i.m.) parecoxib sodium in normal subjects. J Clin Pharmacol. 2001;41:1111–1119. doi: 10.1177/00912700122012607. [DOI] [PubMed] [Google Scholar]
- 28.Mehlisch DR, Desjardins PJ, Daniels S, Hubbard RC. The analgesic efficacy of intramuscular parecoxib sodium in postoperative dental pain. J Am Dent Assoc. 2004;135:1578–1590. doi: 10.14219/jada.archive.2004.0085. [DOI] [PubMed] [Google Scholar]
- 29.Ferreira SH, Lorenzetti BB, Correa FM. Central and peripheral antialgesic action of aspirin-like drugs. Eur J Pharmacol. 1978;53:39–48. doi: 10.1016/0014-2999(78)90265-0. [DOI] [PubMed] [Google Scholar]
- 30.Zhu X, Conklin D, Eisenach JC. Cyclooxygenase-1 in the spinal cord plays an important role in postoperative pain. Pain. 2003;104:15–23. doi: 10.1016/s0304-3959(02)00465-7. [DOI] [PubMed] [Google Scholar]
- 31.Samad TA, Sapirstein A, Woolf CJ. Prostanoids and pain: unraveling mechanisms and revealing therapeutic targets. Trends Mol Med. 2002;8:390–396. doi: 10.1016/s1471-4914(02)02383-3. [DOI] [PubMed] [Google Scholar]
- 32.Martin TJ, Buechler NL, Eisenach JC. Intrathecal administration of a cylcooxygenase-1, but not a cyclooxygenase-2 inhibitor, reverses the effects of laparotomy on exploratory activity in rats. Anesth Analg. 2006;103:690–695. doi: 10.1213/01.ane.0000226093.46973.39. [DOI] [PubMed] [Google Scholar]
- 33.Willer JC, De Broucker T, Bussel B, Roby-Brami A, JMH Central analgesic effect of ketoprofen in humans: electrophysiological evidence for a supraspinal mechanism in a double-blind and cross-over study. Pain. 1989;38(1):1–7. doi: 10.1016/0304-3959(89)90065-1. [DOI] [PubMed] [Google Scholar]
- 34.Ma W, Du W, Eisenach JC. Role for both spinal cord COX-1 and COX-2 in maintenance of mechanical hypersensitivity following peripheral nerve injury. Brain Res. 2002;937:94–99. doi: 10.1016/s0006-8993(02)02593-3. [DOI] [PubMed] [Google Scholar]
- 35.Malmberg AB, Yaksh TL. Hyperalgesia mediated by spinal glutamate or substance P receptor blocked by spinal cyclooxygenase inhibition. Science. 1992;257:1276–1279. doi: 10.1126/science.1381521. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Simone DA, Larson AA. Wind-up leads to characteristics of central sensitization. Pain. 1999;79:75–82. doi: 10.1016/S0304-3959(98)00154-7. [DOI] [PubMed] [Google Scholar]
- 37.Chapman V, Dickenson AH. The spinal and peripheral roles of bradykinin and prostaglandins in nociceptive processing in the rats. Eur J Pharmacol. 1992;219:427–433. doi: 10.1016/0014-2999(92)90484-l. [DOI] [PubMed] [Google Scholar]
- 38.Laird JMA, Herrero JF, Garcia de la Rubia P. Analgesic avtivity of the novel COX-2 preferring NSAID, meloxicam in mono-arthritics rats: central and peripheral components. Inflamm Res. 1997;46:203–210. doi: 10.1007/s000110050174. [DOI] [PubMed] [Google Scholar]
- 39.Willingale HL, Gardiner NJ, McLymont N, Giblett S, Grubb BD. Prostanoids synthesized by cyclo-oxygenase isoforms in rat spinal cord and their contribution to the development of neuronal hyperexcitability. Br J Pharmacol. 1997;122:1593–1604. doi: 10.1038/sj.bjp.0701548. [DOI] [PMC free article] [PubMed] [Google Scholar]


