Abstract
The epigenome is uniquely positioned as a point of convergence, integrating multiple intracellular signaling cascades into a cohesive gene expression profile necessary for long-term behavioral change. The last decade of neuroepigenetic research has primarily focused on learning-induced changes in DNA methylation and chromatin modifications. Numerous studies have independently demonstrated the importance of epigenetic modifications in memory formation and retention as well as Hebbian plasticity. However, how these mechanisms operate in the context of other forms of plasticity is largely unknown. In this review, we examine evidence for epigenetic regulation of Hebbian plasticity. We then discuss how non-Hebbian forms of plasticity, such as intrinsic plasticity and synaptic scaling, may also be involved in producing the cellular adaptations necessary for learning-related behavioral change. Furthermore, we consider the likely roles for transcriptional and epigenetic mechanisms in the regulation of these plasticities. In doing so, we aim to expand upon the idea that epigenetic mechanisms are critical regulators of both Hebbian and non-Hebbian forms of plasticity that ultimately drive learning and memory.
Keywords: Epigenetics, DNA methylation, Hebbian, histone modifications, homeostatic, intrinsic, metaplasticity, non-Hebbian, synaptic, synaptic scaling
1. Introduction
Long-term changes in neuronal function underlying learning and memory are driven by changes in gene expression with corresponding modifications in protein synthesis and neuronal connectivity (Barondes and Jarvik, 1964; Cohen and Barondes, 1966; Kim and Linden, 2007; Martin et al., 2000). Specifically, changes in the expression of growth factors, ion channels, ligand-gated receptors, and structural proteins are necessary to support long-lasting functional and structural changes within a neuronal circuit (Baker-Andresen et al., 2013a; McClung and Nestler, 2008). Recent evidence suggests epigenetic modifications that remodel chromatin, including DNA methylation and post-translational modifications (PTMs) of histones, likely serve as molecular mechanisms for bi-directional regulation of necessary gene expression (Chen et al., 2003a; 2003b; Levenson and Sweatt, 2005; Martinowich et al., 2003; Nelson and Turrigiano, 2008). This is supported by experimental evidence demonstrating the pathways upstream and downstream of chromatin remodeling are necessary components in synaptic plasticity and long-term behavioral memory (Day and Sweatt, 2011; Levenson et al., 2004a; 2006; Lipsky, 2013; Roberson and Sweatt, 1999; Roberson et al., 1999; Selcher et al., 2002; Sweatt, 2010).
At present, there are several broad questions that remain unanswered. What is the complete transcriptional profile necessary for acquisition and consolidation of long-term memory? How is the epigenome dynamically regulated to subserve these changes in gene expression? More importantly, how do the resulting gene products interact concordantly to produce neuronal plasticity and long-term behavioral adaptation? Historically, the field has focused on how epigenetic mechanisms modulate Hebbian plasticity. However, it is becoming increasingly evident that memory is also reliant on non-Hebbian forms of plasticity, such as intrinsic plasticity and synaptic scaling (Figure 1) (Baker-Andresen et al., 2013b; Nelson and Turrigiano, 2008). We propose that a thorough examination of how epigenetic mechanisms drive Hebbian and non-Hebbian forms of plasticity will allow for a more comprehensive understanding of the global transcriptional and epigenetic changes necessary for long-term behavioral memory. This review will examine a role for epigenetic regulation first in Hebbian plasticity, and later, in two forms of non-Hebbian plasticity – intrinsic plasticity and synaptic scaling. Additionally, we discuss each form of plasticity in the process of memory formation and explore how each is driven by transcriptional and epigenetic mechanisms.
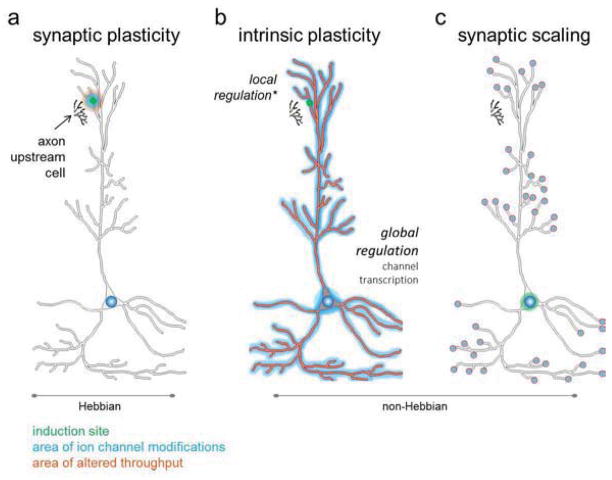
Figure 1. Induction and Expression Sites for Hebbian and non-Hebbian Forms of Plasticity.
Hebbian plasticity involves the modulation of synaptic efficacy due to precise coordination of pre- and post-synaptic activity. In contrast, non-Hebbian forms of plasticity are not dependent on coincident activity. Hebbian and non-Hebbian plasticities are induced and expressed differently, suggesting each possesses specific functions in the memory process. (a) The modulation of synaptic efficacy in Hebbian plasticity is synapse-specific; as a result, the sites of induction (green) and expression (blue) are co-localized. Classically expression is thought to occur post-synaptically via trafficking of ligand-gated ionotropic receptors (e.g., AMPARs), although there is evidence for the involvement of presynaptic modifications. The throughput (red), or the ability of synaptic activity to elicit an action potential, is altered only at those synapses expressing changes in synaptic efficacy. (b) Intrinsic plasticity is a form of non-Hebbian plasticity where modulation of voltage- and calcium-gated ion channels regulates synaptic integration and action potential generation. There is evidence that changes in intrinsic plasticity can be induced by local synaptic activity (as shown) as well as global changes in action potential firing. Similarly, intrinsic plasticity can be expressed locally (restricted to a subset of distal dendrites) or globally (as shown; involving broader changes along the dendritic tree and/or the axo-somatic membrane). In the setting of global changes, there is potential for throughput of all synapses to be altered. (c) Synaptic scaling is a form of non-Hebbian plasticity involving the multiplicative modification of postsynaptic ligand-gated ionotropic receptor (e.g., AMPAR) density across all synapses. Such changes occur in response to a given neuron sensing chronic alterations in its own firing rate through variations in Ca2+ influx at the soma. Since modifications occur at all synapses the throughput of all synapses are changed. However, relative weights of preexisting synaptic changes are maintained since scaling occurs in a multiplicative fashion. Adapted from Zhang and Linden (2003).
2. Hebbian Plasticity
2.1. Relevance to Learning and Memory
Hebbian plasticity is defined as synapse-specific changes in strength driven by the coordination of pre-synaptic input and post-synaptic depolarization (see Figure 1A). Long-term potentiation (LTP) is a form of Hebbian plasticity characterized by long-lasting enhancement in synapse-specific neurotransmission in response to repetitive, high frequency stimulation. LTP is a widely accepted cellular mechanism underlying long-term memory formation (Bauer et al., 2001; Blair et al., 2001; Bliss and Collingridge, 1993; Lynch et al., 1988; 2008; 2007; 2013; Malenka and Bear, 2004; Maren, 2005). Potentiation of excitatory synaptic transmission can be induced in various regions of the mammalian brain, including the hippocampus, amygdala, striatum, and cortex (Fourcaudot et al., 2009; Huang and Kandel, 1998; Huang et al., 2000; Iriki et al., 1989; Lee and Kirkwood, 2011; Maren, 1999; Rex et al., 2010; Weisskopf et al., 1999). Enhancements and deficits in memory are often correlated with increases or decreases in LTP, respectively, across many behavioral tasks and corresponding brain regions mediating the behaviors (Izquierdo and Medina, 1995; Lynch, 2002; Martin et al., 2000; Rodrigues et al., 2004; Staubli et al., 1994). In addition, LTP induction mechanisms are similar to those necessary for long-term memory formation (Klann et al., 2004; Pittenger and Kandel, 2003). Classical LTP of hippocampal cornu ammonis (CA)1 excitatory synapses is driven by N-Methyl-D-Aspartate (NMDA)-dependent Ca2+ influx, which subsequently activates, directly or indirectly, signaling cascades that modify the strength of targeted synapses (Malenka and Nicoll, 1993). The blockade of these receptors, or their downstream effectors, inhibits both LTP in vitro and memory in vivo. Furthermore, pharmacological and genetic manipulations of epigenetic targets affect the induction of LTP and memory formation (Levenson and Sweatt, 2006).
It should be noted that for subsequent discussions we have chosen to group together the two topics of transcriptional and epigenetic regulation as we believe that both processes are required to achieve a coordinated orchestration of gene expression and nuclear output that in turn effects cellular physiology and animal behavior. However, we readily acknowledge that although intimately coupled, each process likey possesses specific functions and limitations. We define transcriptional regulation as those mechanisms that are directly involved in the synthesis of RNA (either coding or non-coding) like transcription factor activation/binding and RNA polymerase association/activity. As such, their functionality is dependent on their ability to act as singaling relays between cystolic and nuclear mechanisms in order to set in motion precise gene expression profiles that are specific to a particular transcription factor and its associated upstream signaling cascades. In contrast, we find epigenetic mechanisms to act as powerful modulators of the aformentioned transcriptional machinery with their strength inherent in their capacity to serve as molecular tags of present and past neuronal activity and behavioral experience. The capability of epigenetic mechanisms to produce long-lasting cellular change provides a platform with extensive computational power that integrates stimuli across time to more appropriately fine-tune the transcriptional potential of the genome.
2.2. Transcriptional and Epigenetic Regulation
Eukaryotic DNA is tightly packaged into a DNA-protein complex known as chromatin. Positively-charged histones serve as a core around which negatively-charged DNA is tightly coiled. Conventionally, transcription is repressed by spatial restrictions caused by interactions of DNA with histones, which occludes RNA polymerase II/DNA interaction. Initiation of transcription requires the disruption of chromatin’s tightly compacted structure through the PTMs of histones (Roth and Sweatt, 2009; Varga-Weisz and Becker, 1998). At present, the most frequently characterized PTMs of histones are acetylation, methylation, ubiquitination, and phosphorylation; each modification serves as a distinct functional epigenetic tag (Rea et al., 2000; Strahl and Allis, 2000). The most extensively studied histone modification in the context of learning and memory is the acetylation of lysine residues on histone tails through the activity of histone acetyltransferases (HATs)(Lau et al., 2000; Tanner et al., 2000a; 2000b; 1999), an effect reversed by histone deacetylase (HDAC) activity (Fischle et al., 2003; Saha and Pahan, 2006; Varga-Weisz et al., 1999).
Recent reports demonstrate that histone-modifying enzymes and histone acetylation are necessary for mammalian associative learning and Hebbian plasticity (for a review of these mechanism in invertebrates please see Rahn et al., 2013) (Alarcon et al., 2004; Chen et al., 2003a; Chwang et al., 2007; Guan et al., 2009; Gupta et al., 2010; Koshibu et al., 2009; Levenson et al., 2004b; Vecsey et al., 2007). For example, mice with genetic mutations in the HAT cyclic adenosine monophosphate (cAMP)/Ca2+-response element binding protein (CREB) binding protein (CBP), have decreased histone acetylation and deficits in transcription-dependent LTP (Alarcon et al., 2004). Interestingly, those deficits were ameliorated by administration of the HDAC inhibitor (HDACi) suberoylanilide hydroxamic acid. In contrast, mice with deletion of HDAC2, displayed enhanced hippocampal LTP, whereas overexpression in the hippocampus blunted LTP (Guan et al., 2009). Moreover, LTP induction resulted in increased histone H3 and H4 acetylation and the enhancement of histone acetylation and LTP induction were both facilitated by HDACi application (Levenson et al., 2004b; Miller et al., 2008; Sui et al., 2012; Vecsey et al., 2007; Yeh et al., 2004; Zeng et al., 2011). Furthermore, LTP specifically increased changes in histone acetylation at the promoter regions of Bdnf and Reln, genes involved in synaptic transmission (Sui et al., 2012). Collectively, these studies argue for an intimate relationship between levels of histone acetylation and LTP.
In addition to histone modifications, DNA methylation is a canonical regulator of gene transcription. Methylation is the most common covalent modification occurring in eukaryotic DNA and has been studied extensively in development as a static process following cell differentiation (Rakyan et al., 2001). Recent reports have challenged the established dogma by demonstrating that DNA methylation is dynamically regulated in the adult nervous system and that this cellular mechanism is a crucial step in memory formation (Day et al., 2013; Feng et al., 2010; Lubin et al., 2008; Miller and Sweatt, 2007; Miller et al., 2010). Importantly, both DNA methylation and DNA methyl-binding proteins have been implicated in the induction of long-term synaptic plasticity (Cortés-Mendoza et al., 2013).
DNA methylation is a reaction catalyzed by DNA methyltransferase (DNMT) enzymes, during which a methyl group is added to the carbon at the 5′ position of the pyrimidine ring (Chen et al., 1991). Methylation was thought to only occur at cytosine bases followed by a guanine base, however this notion has recently been challenged (Lister et al., 2013; Varley et al., 2013; Xie et al., 2012). This dinucleotide sequence (designated CpG, with p corresponding to a phosphate group)is highly underrepresented in the genome and often found in both high-density clusters called CpG islands and low-density regions near CpG islands called CpG shores (Bird, 1978; Deaton and Bird, 2011; Guo et al., 2011b). Activity-induced changes in methylation occur predominantly in low-density regions in both inter- and intra-genic locations (Guo et al., 2011b). Although much attention has been paid to changes in promoter methylation, recent findings highlight changes in intragenic methylation observed with memory formation (Day et al., 2013).
There are two classes of DNMTs: maintenance and de novo DNMTs. The de novo DNMTs (DNMT3a and DNMT3b) methylate sites lacking methyl-cytosine on either DNA strand, while the maintenance DNMT isoform, DNMT1, methylates hemi-methylated DNA (Goll and Bestor, 2005). It should be noted that DNMT1 can also regulate de novo methylation under certain circumstances (Fatemi et al., 2002; Hsieh, 2005). Maintenance DNMTs perpetuate methylation after cell division by regenerating the methyl-cytosine marks on the newly synthesized complementary DNA strand that arises with DNA replication (Feng and Fan, 2009; Feng et al., 2010; Okano et al., 1999a; 1999b). Although there are examples of DNA methylation associated with increased gene transcription (Chahrour et al., 2008; Day et al., 2013; Uchida et al., 2011), it is commonly accepted that methylation of DNA suppresses gene transcription, and in specific circumstances extensive DNA methylation triggers complete silencing of the associated gene (Sweatt et al., 2012). Methylation can repress gene expression by directly interfering with binding of transcription factors to regulatory elements or by actively recruiting methyl-CpG-binding proteins. These methyl-CpG binding proteins repress transcription by recruiting other chromatin-remodeling enzymes such as HDACs, repressor element 1 (RE1) silencing transcription factor/ neuron-restrictive silencing factor (REST/NRSF), and CoREST (an associated HDAC), among others (Ballas and Mandel, 2005; Ballas et al., 2005; Klose et al., 2005; Levenson and Sweatt, 2005).
The link between DNA methylation and Hebbian plasticity represents a molecular mechanism of memory storage, and investigating this relationship has been approached via pharmacological and genetic methods. Blocking DNA methylation prior to LTP induction with the DNMT inhibitors zebularine or 5-aza-2-deoxycytidine disrupted hippocampal LTP and resulted in significant demethylation of Reln and Bdnf promoters (Levenson et al., 2006). Both genes are associated with synaptic plasticity and undergo comparable changes in methylation following fear conditioning (Lubin et al., 2008; Miller and Sweatt, 2007). A subsequent study demonstrated that the LTP deficit produced by DNMT inhibition could be reversed by pretreatment with the HDACi trichostatin A (TSA), suggesting cross-talk between histone acetylation and DNA methylation during plasticity (Miller et al., 2008). In support of these pharmacological studies, Feng and colleagues (2010) recently reported that mice with a double knockout of DNMT1 and DNMT3a in forebrain post-mitotic neurons have impaired hippocampal LTP and enhanced LTD. The effects on synaptic function were further correlated with a reduction in global DNA methylation and deregulation of specific genes. Furthermore, neurons lacking only one DNMT isoform had normal hippocampal plasticity, indicating that DNMT1 and DNMT3a may have overlapping roles in adult neurons, and that at least one form is required to maintain normal hippocampal LTP (Feng et al., 2010).
Though passive DNA demethylation is a largely accepted mechanism in dividing cells, the presence of active DNA demethylation (i.e., demethylation that occurs in the absence of DNA replication) in neurons has been controversial (Ooi and Bestor, 2008; Wu and Zhang, 2010). However, mounting evidence suggests that active demethylation does occur in the adult nervous system and regulates synaptic plasticity (Guo et al., 2011a; 2011b; Li et al., 2013; Sultan et al., 2012). One recent report provides evidence for activity-dependent DNA demethylation of Reln and Bdnf genes following induction of LTP in the medial pre-frontal cortex (Sui et al., 2012). Additionally, a landmark study by Ma and colleagues (2009) demonstrated that GADD45B (a member of the growth arrest and DNA damage inducible 45 family and an activity-induced immediate early gene) is necessary for the demethylation and transcriptional activation of both Bdnf promoter IX and Fgf1 promoter B following electroconvulsive stimulation of the dentate gyrus (Ma et al., 2009). A subsequent study by Sultan and colleagues (2012) reported that GADD45B regulated hippocampal LTP and memory formation. Extracellular recordings from hippocampal slices showed genetic deletion of Gadd45b resulted in a selective enhancement of late-phase LTP despite using a near-threshold stimulus. Additionally, mutant mice exhibited enhanced memory in tasks including motor performance, aversive conditioning, and spatial navigation (Sultan et al., 2012). Further studies are needed to elucidate detailed demethylation mechanisms, but these data highlight the importance of GADD45B and other modulators of DNA demethylation in plasticity and memory.
The studies presented here clearly implicate PTMs of histones and DNA methylation as necessary epigenetic mechanisms subserving Hebbian plasticity and long-term memory. Owing to space restrictions, we are unable to explore the topic of synaptogenesis in the context of synaptic plasticity and long-term behavioral memory. However, there is growing evidence for a role of epigenetic mechanisms in the regulation of spine and synapse formation both in the context of activity-dependent processes as well as aging and disease states with deficits in learning and memory (for reviews on the topics please see Kavalali et al., 2011; McEwen et al., 2012; Na et al., 2013). It should be noted that epigenetic regulation of structural plasticity at large warrants further consideration and integration with the topics covered in this review. In the subsequent sections we will examine how transcriptional and epigenetic mechanisms may subserve modulation of non-Hebbian forms of plasticity. We will focus our discussion on intrinsic plasticity and a form of homeostatic plasticity known as synaptic scaling.
3. Intrinsic Plasticity
The involvement of enduring, synapse-specific, Hebbian modifications in memory formation and storage is readily evident and well-documented. However, emerging evidence suggests that activity-dependent alterations in intrinsic neuronal excitability, termed intrinsic plasticity, may also be a necessary component of the cellular processes underlying learning and memory (Daoudal, 2003; Frick and Johnston, 2005; Sehgal et al., 2013; Zhang and Linden, 2003), in addition to regulating network function and informational processing at large (Nelson and Turrigiano, 2008; Remme and Wadman, 2012). Intrinsic plasticity involves the attunement of passive and/or active membrane properties as to modulate the input/output relationships that govern action potential (AP) firing rates. Modification of a neuron’s intrinsic properties can be mediated by regulating the expression or the biophysical properties of voltage- and calcium-gated ion channels (see Figure 2). In addition, the specific location on the neuron where these adaptations take place also determines how intrinsic plasticity manifests itself (see Figure 1B). For example, alterations in active and passive membrane properties can occur locally, targeting specific dendrites and influencing synaptic throughput of a small set of synapses. Some of the relevant currents involved in this process include the afterhyperpolarization (AHP) current (generated by Ca2+-activated K+ channels) (Storm, 1990), the IA current (subserved by rapidly inactivating A-type K+ channels) (Storm, 1990), and the Ih current (produced by hyperpolarization-activated cyclic nucleotide-gated (HCN) channels) (Biel et al., 2009); together these currents modify the degree of summation and propagation of synaptic input to the soma, as well as the amplitude and duration of back propagating APs. Intrinsic alterations can also occur globally, impacting the axo-somatic membrane as well as larger portions of proximal dendrites as to modify throughput for all synapses. Global alterations largely involve modulation of Na+ and K+ currents to regulate AP initiation (which depends on parameters like AP threshold and resting membrane potential), spike frequency adaptation or accommodation, and AP properties (e.g., amplitude and duration). Together, these local and global alterations ultimately dictate how information flows within and between neurons (for a more in depth examination of these topics see Daoudal, 2003; Frick and Johnston, 2005; Sehgal et al., 2013; Zhang and Linden, 2003).
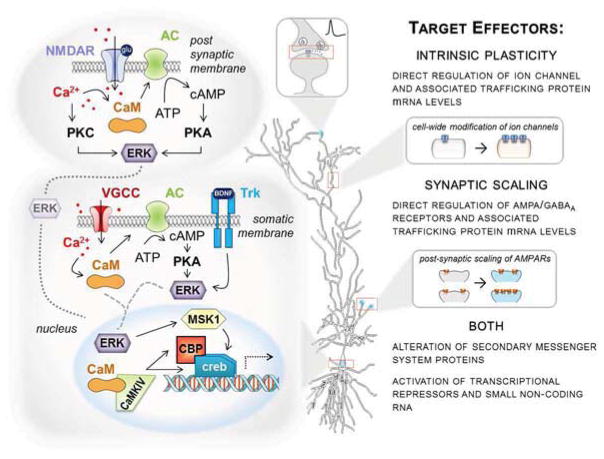
Figure 2. Potential Shared Molecular Mechanisms of Transcriptional Regulation between Intrinsic Plasticity and Synaptic Scaling.
Although clear distinctions exist in the induction and expression of intrinsic plasticity (IP) and synaptic scaling (SS), accumulating evidence suggests both plasticities rely on conserved molecular mechanisms also known to be involved in the long-term changes in gene expression necessary for Hebbian plasticity. Here we present a simplified model intended to demonstrate likely points of molecular convergence between IP and SS that require further experimental confirmation and elucidation. Despite differences in induction site (synaptic as in IP and somatic as in SS), there is a clear role for transcriptional regulation via Ca2+-mediated signaling. Ca2+ entry either through synaptic NMDA receptors or somatic voltage-gated calcium channels (VGGCs) directly and/or indirectly activates protein kinases like protein kinase C (PKC) and cAMP-dependent protein kinase (PKA) which converge on extracellular receptor kinase (ERK) and lead to its nuclear translocation. In SS, brain-derived neurotrophic factor (BDNF) binding of TrkB receptors likely serves as a level of higher-order control in the regulation of ERK nuclear translocation. Nuclear ERK may engage cAMP-response element (CREB)-mediated gene transcription through activation of downstream kinases such as mitogen- and stress-activated protein-kinase 1 (MSK1). Additionally, nuclear translocation of Ca2+/calmodulin (CaM) regulates Ca2+/calmodulin-dependent kinase IV (CaMKIV) activity, a key mediator of both CREB and CREB-binding protein (CBP) phosphorylation and activation. Furthermore, it is likely both IP and SS engage transcriptional repressors and small non-coding RNAs along with transcriptional activators like CREB. Coordinated expression of specific ion channels/receptors, associated trafficking proteins, and secondary messenger proteins will dictate how each form of plasticity manifests at the level of the cell membrane.
3.1. Relevance to Learning and Memory
Experimental evidence for learning-induced changes in intrinsic plasticity stems from a variety of model systems and behavioral paradigms. We will focus our discussion on studies performed on mammals (for additional information on invertebrates see Mozzachiodi and Byrne, 2010). One of the first reported studies examining learning-induced changes in intrinsic plasticity involved a feline associative conditioning task where a cat associated an auditory click (conditioned stimulus) with a tap between the eyebrows (unconditioned stimulus), such that future clicks elicited both an eyeblink and a nose twitch (conditioned responses) (Brons and Woody, 1980). Intracellular recordings from the pericruciate sensorimotor cortex of conditioned animals revealed an increase in neuronal excitability evidenced by a reduction in the threshold current needed for spike initiation. Similarly, whole-cell electrophysiological recordings from rabbit hippocampal slices following acquisition of trace eyeblink conditioning (EBC) revealed increased excitability in approximately 50% of CA1 and CA3 pyramidal neurons (Coulter et al., 1989; Disterhoft et al., 1986; 1988; Thompson et al., 1996). This hyperexcitability was characterized by an increased number of spikes elicited by a sustained depolarizing current injection, also termed reduced spike-frequency adaptation or accommodation, and a marked reduction in the AHP amplitude evoked by a spike burst.
Learning-related changes in intrinsic plasticity have also been observed in other species and additional behavioral paradigms such as Morris water maze (MWM) (Oh et al., 2003; Ohno et al., 2006), odor fear conditioning (Motanis et al., 2012; Rosenkranz and Grace, 2002), rule learning on odor discrimination tasks (Motanis et al., 2012; Saar et al., 1998; Zelcer et al., 2006), auditory fear conditioning (both delay and trace versions) (Motanis et al., 2012; Santini et al., 2008), and contextual fear conditioning (Kaczorowski and Disterhoft, 2009; McKay et al., 2009). In most cases, learning is associated with increased intrinsic excitability, although exceptions to this rule have been found in the infralimbic prefrontal cortex with tone fear conditioning (Santini et al., 2008) and the basolateral amygdala (BLA) with odor fear conditioning (Motanis et al., 2012). Overall, reductions in spike threshold, spike accommodation, and amplitude of burst-evoked AHPs are the electrophysiological changes most often observed following learning.
It is clear from the previously outlined studies that changes in intrinsic excitability appear to be evolutionarily conserved across species and evidenced in a variety of behavioral tasks. However, many questions remain regarding the exact mechanisms underlying the induction, expression, and maintenance of intrinsic plasticity in the context of animal behavior. Moreover, determining the functional role of intrinsic plasticity is an active area of ongoing research. As previously suggested (Sehgal et al., 2013; Zhang and Linden, 2003), growing evidence indicates that intrinsic plasticity may in fact serve three distinct, yet overlapping, functions: as part of the memory engram itself, as a modulator of behavioral memory and Hebbian plasticity, and as a component in the overall repertoire of homeostatic adaptations (for a review on this last topic see Nelson and Turrigiano, 2008).
Although intrinsic plasticity is associated with learning, a mnemonic function for intrinsic plasticity seems unlikely given that changes in excitability are short-lived in comparison to memory of the behavioral task (Motanis et al., 2012; Moyer et al., 1996; Saar et al., 1998; Thompson et al., 1996; Zelcer et al., 2006). For example, changes in intrinsic excitability of rabbit CA1 and CA3 pyramidal neurons with EBC are present for 1–3 days after training before returning to baseline values by 5–7 days, even though the behavioral memory is present for at least 6 months (Moyer et al., 1996; Thompson et al., 1996). Such data suggests that intrinsic plasticity might not comprise the actual memory engram itself, but may instead serve an early role during the acquisition and consolidation processes. However, the transient nature of hippocampal excitability changes may also reflect the time-limited involvement of the hippocampus in remote memory storage (Frankland and Bontempi, 2005; Kim et al., 1995; Wiltgen et al., 2004). Additionally, there are reports of persistent changes in intrinsic plasticity with learning that can last as long as one month (Brons and Woody, 1980; Schreurs et al., 1998) suggesting there may be instances in which intrinsic plasticity indeed possesses a mnemonic function.
Instead, the majority of existing data make a stronger case for intrinsic plasticity as a modulator of behavioral memory and Hebbian plasticity (a prime example of metaplasticity, which will be covered in more depth in section 4). At the behavioral level, learning-induced increases in excitability are associated with enhanced learning of the same or different behavioral tasks (Saar et al., 1998; Zelcer et al., 2006). For example, training rats in the MWM soon after olfactory learning takes place (during the time point where hyperexcitability is observed in CA1 pyramidal neurons) results in enhanced acquisition of the spatial task (Zelcer et al., 2006). Furthermore, the enhanced spatial learning capability of olfactory-trained rats is no longer observed once excitability levels revert to baseline. These observations suggest that by rendering neurons more excitable, the circuit may be primed to readily acquire future information. Indeed, this interpretation is supported by pharmacological and genetic interventions that increase neuronal excitability which in turn increase learning rate and/or capability (Disterhoft and Oh, 2006; Han et al., 2007; Zhou et al., 2009). At the electrophysiological level, manipulations of intrinsic excitability can also influence Hebbian plasticity, often facilitating the induction of LTP (Chen et al., 2006; Cohen and Abraham, 1996; Cohen et al., 1999; Kramár et al., 2004; Sah and Bekkers, 1996).
3.2. Transcriptional and Epigenetic Regulation
Given the involvement of intrinsic plasticity in learning, memory, and Hebbian plasticity, investigations have shifted towards understanding the molecular substrates that underlie this process. Mechanistic investigations have revealed intrinsic plasticity requires many of the same molecular mechanisms implicated in Hebbian plasticity; roles for Ca2+ signaling and intracellular signaling cascades like protein kinases C (PKC), cAMP-dependent protein kinase (PKA), cyclic guanosine monophosphate (cGMP)-dependent protein kinase (PKG), and Ca2+/calmodulin-dependent protein kinase II (CaMKII) are well documented (Daoudal, 2003; Zhang and Linden, 2003). Similar to Hebbian plasticity, the long-term maintenance of intrinsic plasticity is also protein-synthesis dependent (Cohen-Matsliah et al., 2010; Xu et al., 2005). However, only recently has transcriptional and epigenetic regulation of intrinsic plasticity begun to be examined in more depth.
Transcriptional involvement in intrinsic plasticity is strongly evidenced by a series of studies in which manipulations of the level or activity of CREB bidirectionally modulated neuronal intrinsic excitability (for review see Benito and Barco, 2010). Dong and colleagues were the first to demonstrate a positive correlation between intrinsic excitability and levels of CREB (Dong et al., 2006). Overexpression of constitutively active CREB increased the firing rate of medium spiny neurons (MSN) in the nucleus accumbens (NA) as well as decreased the threshold to elicit an AP and the minimal current needed to fire a spike. In contrast, overexpression of dominant-negative CREB revealed the opposite effect: decreased firing rate and increased threshold for eliciting an AP.
Several laboratories have recapitulated similar findings in the locus coeruleus (Han et al., 2006), CA1 region of the hippocampus (Lopez de Armentia et al., 2007), and the BLA (Viosca et al., 2009; Zhou et al., 2009) using recombinant neurotropic viral vectors, transgenic mice, and gene-targeting techniques to manipulate CREB levels and activity (for a thorough review on these methodologies see Barco and Marie, 2011). Overall, gain-of-function manipulations of CREB were associated with increased neuronal excitability, whereas loss-of-function interventions decreased excitability. Electrophysiological recordings revealed CREB modulated AP threshold, firing rate, AHP amplitude, input resistance, and resting membrane potential, although differences were observed depending on cell-type examined and methodology used to manipulate CREB. It is worth mentioning many of these studies found concurrent changes in Hebbian plasticity, which is not surprising considering CREB’s well-documented role in regulating long-term memory processes as well as underlying changes in Hebbian plasticity (Alberini, 2009; Josselyn and Nguyen, 2005; Sakamoto et al., 2011; Silva et al., 1998). Together these data demonstrate CREB-mediated gene transcription is capable of modulating intrinsic plasticity in addition to Hebbian plasticity.
However, the complete transcriptional profile underlying these observed changes in excitability is only beginning to be characterized. As previously mentioned, modulation of intrinsic neuronal properties occurs by regulating expression level or biophysical properties of voltage- and calcium-gated ion channels. As suggested by others (Benito and Barco, 2010; Won and Silva, 2008), CREB-mediated changes in intrinsic excitability likely involve direct and indirect modulation of positive and negative regulators of intrinsic excitability. Specifically, modifications of positive regulators could involve direct regulation of ion channel mRNA levels (including specific splice variants) as well as alteration of secondary messenger systems (kinases and phosphatases) that modulate ion channel function. Given that CREB is a transcriptional activator, inhibition of negative regulators would likely require activation of transcriptional repressors and small non-coding RNAs, like microRNAs and piwi-interacting RNAs, whose activation would inhibit downstream effectors of intrinsic plasticity (see Figure 2). CREB-mediated transcription is known to rely on the recruitment of co-activator complexes, like p300 (another HAT) and CBP, which subsequently restructure chromatin to influence gene expression (Alarcon et al., 2004; Barrett et al., 2011; Bourtchouladze et al., 2003; Chen et al., 2010; Korzus et al., 2004; Maurice et al., 2008; Oike et al., 1999; Oliveira et al., 2011; Valor et al., 2011; Wood et al., 2005). Therefore, one would expect to detect changes in epigenetic modifications, including histone acetylation, at CREB target genes that are positive and negative regulators of intrinsic excitability. This is in fact the case for genes implicated in Hebbian plasticity, like Bdnf, Egr1, and Pp1, which are not only known CREB targets but also genes that undergo epigenetic regulation during the acquisition and consolidation of long-term memories (Day et al., 2013; Lubin et al., 2008; Miller and Sweatt, 2007).
Efforts to characterize the transcriptional profile underlying changes in excitability have primarily focused on the level or function of voltage-gated Na+ and K+ channels, as well as potentiation of the adenylyl cyclase (AC)/cAMP/PKA pathway. Microarray analysis of the NA from inducible transgenic animals overexpressing CREB revealed upregulation of the voltage-dependent Na+ channel 1β subunit (Scn1b) and downregulation of the voltage-dependent K+ channel KV1.4 subunit (Kcna4) (McClung and Nestler, 2003). Likewise, current-clamp recordings from MSN neurons overexpressing CREB showed a potentiation of Na+ conductances and an inhibition of K+ conductances, effects reversed by expression of a dominant-negative CREB (Dong et al., 2006).
Converging evidence suggests CREB may regulate intrinsic plasticity via positive feedback onto the AC/cAMP/PKA pathway. Interestingly, viral-mediated overexpression of wild-type CREB in the locus coeruleus enhanced the excitatory effect of forskolin (an activator of AC) on these neurons (Han et al., 2006). The increased basal firing rate observed with forskolin could occur via CREB-mediated induction of AC8. Adcy8, which encodes AC8, is a direct target for CREB (Lane-Ladd et al., 1997), and its expression is regulated by CREB in vitro and in vivo (Chao et al., 2002). Furthermore, activation of the cAMP pathway has been shown to increase neuronal excitability in noradrenergic neurons of the locus coeruleus (Alreja and Aghajanian, 1995; Ivanov and Aston-Jones, 2001; Wang and Aghajanian, 1987). More specifically, the AC/cAMP/PKA pathway regulates many of the previously mentioned currents involved in modulating intrinsic plasticity, including the AHP current (Haug and Storm, 2000; Oh et al., 2009; Pedarzani and Storm, 1995; 1993), the Ia current (Hoffman and Johnston, 1998), and the Ih current (Pape, 1996). Together these observations support the hypothesis that CREB-mediated changes in intrinsic plasticity are mediated via potentiation of the AC/cAMP/PKA pathway. It is evident more research is necessary to completely understand the transcriptional changes underlying CREB-mediated increases in excitability; and to differentiate these changes from those underlying Hebbian plasticity.
It is worth noting that although CREB-mediated modulation of intrinsic plasticity is well supported, there is a shortage of direct evidence confirming epigenetic regulation of intrinsic plasticity in the context of learning and memory. However, recent evidence from the pain and epilepsy fields demonstrate epigenetic mechanisms underlie the characteristic neuronal hyperexcitability in these disease states (Beck and Yaari, 2008). As with long-term memory formation and synaptic plasticity, there is growing evidence that epigenetic mechanisms also play an important in role in the development and maintenance of different pain states and epileptogenesis (for pain-relevant reviews see: Denk and McMahon, 2012; Géranton, 2012; Rahn et al., 2013; for epileptogenesis-relevant reviews see: Lubin, 2012; Qureshi and Mehler, 2010; Roopra et al., 2012).
In animal models of neuropathic pain, sustained downregulation of genes encoding for sodium channel Nav1.8, the μ-opiod receptor, and potassium channel Kv4.3, occurred in the dorsal root ganglion (DRG) following nerve injury (Uchida et al., 2010a; 2010b). These decreased transcript levels were associated with enhanced binding of NRSF and hypoacetylation of histone H3 and H4 at neuron-restrictive silencer elements (NRSEs, also known as RE1s) in the promoter regions of these genes. NRSF is an activity-regulated transcription factor that targets genes containing NRSE sites and silences their expression by actively recruiting chromatin modifying and remodeling complexes that can include proteins like methyl CpG binding protein 2 (MeCP2), Co-REST, Sin3a, HDACs, histone methyltransferases, and histone demethylases (Roopra et al., 2001; 2012). Interestingly, nerve injury resulted in a chronic elevation of NRSF transcript and protein levels that were further correlated with increased acetyl H4 enrichment at the NSRF promoter II (Uchida et al., 2010a; 2010b).
NRSF-mediated repression of ion channels is also implicated in epileptogenesis. Seizure activity resulted in NRSF binding to the Hcn1 promoter in the hippocampus of kainite-treated animals (McClelland et al., 2011); decreased Hcn1 transcript levels as well as attenuation of Ih were observed. As mentioned previously, HCN channels dampen dendritic excitability in hippocampal cortical neurons and modify overall synaptic integration and somatic-dendritic coupling (Magee, 1999; Poolos et al., 2002; Santoro et al., 2000). Pharmacological blockade of HCN channels causes neuronal and network hyperexcitability (Albertson et al., 2013; 2011). Furthermore, animals lacking Hcn1 have more excitable neurons, are more prone to seizures, and have higher seizure-induced mortality (Huang et al., 2009; Santoro et al., 2010). Administration of oligonucleotides targeting the Hcn1-NRSE blocked REST binding of Hcn1, thereby restoring HCN1 protein levels and Ih current amplitudes as well as producing fewer spontaneous seizures (McClelland et al., 2011).
In addition to ion channels, there is evidence of epigenetic regulation of secondary messenger systems that modulate ion channel function. Following neuronal injury, p300 and cyclooxygenase 2 (COX-2) were upregulated in the lumbar spinal cord of rats (Zhu et al., 2012). More importantly, the degree of p300 binding to the COX-2 promoter dictated subsequent COX-2 transcript and protein level. COX-2 regulates the production of several prostaglandins that contribute to the development and maintenance of spinal cord hyperexcitability (Latremoliere and Woolf, 2009; Willingale et al., 1997). Similar gene-specific regulation was also observed in a model of inflammatory pain where peripheral infusion of an inflammatory agent resulted in demethylation of the cystathionine-β-synthase (Cbs) gene promoter with subsequent upregulation of Cbs mRNA and protein in the DRG (Qi et al., 2013). Like COX-2, CBS synthesizes an endogenous molecule, in this case hydrogen sulfide (H2S), whose activity was necessary and sufficient to elicit enhanced excitability of DRG neurons (Qi et al., 2013; Xu et al., 2009). More specifically, in vitro addition of NaHS (an H2S donor) significantly depolarized the resting membrane of DRG neurons, reduced rheobase and AP threshold, and increased firing frequency. This effect was mediated, in part, via potentiation of tetrodotoxin-resistant sodium channel currents, an effect dependent on the PKA pathway. These data are consistent with previous in vitro studies showing H2S modulates the AC/cAMP/PKA pathway (Muzaffar et al., 2009; Shao et al., 2011; Smith, 2009). That the AC/cAMP/PKA pathway mediates changes in excitability in both pain and memory circuits suggests shared homologous cellular and molecular mechanisms and underscores the fundamental importance of this pathway in regulating intrinsic neuronal properties (Rahn et al., 2013). Hence, it is conceivable that, in learning and memory, epigenetic mechanisms may impinge on intrinsic excitability directly via modulation of signaling proteins in the AC/cAMP/PKA pathway or indirectly via intermediary effectors as is seen in the pain system.
These studies collectively demonstrate the existence of epigenetic regulation of ion channels and associated signaling pathways involved in intrinsic plasticity. More specifically, REST appears to play an essential function in this regulation, which is not surprising given that many genes involved in neuronal excitability contain NRSE consensus sequences (Roopra et al., 2001). These and other mechanisms should be examined more closely in the context of learning and memory.
Are there learning-associated changes in epigenetic regulators or modifications at genes implicated in intrinsic plasticity? Besides CREB, are there additional transcription factors that may also mediate the necessary changes in gene expression contributing to intrinsic plasticity? Based on the pain and epilepsy literature, both of these scenarios seem likely. REST appears to be an excellent candidate transcription factor that may orchestrate specialized epigenetic machinery to relevant genes of interest. Other potential candidates include nuclear factor-κB (NF-κB) as it too is known to associate with epigenome-modifying complexes (Chen et al., 2011; Lanzillotta et al., 2010). In fact, hippocampal neurons from mice with mutations in the IκBα promoter, the primary inhibitor of NF-κB, exhibit spontaneous burst firing and hyperexcitability (Shim et al., 2011) that could be explained via modulation of voltage-dependent calcium channels and ionotropic glutamate receptor channels (Furukawa and Mattson, 2002). Finally, do genetic or pharmacological manipulations of epigenetic enzymes modulate neuronal intrinsic excitability? To our knowledge this last question remains completely unanswered, as it has not been directly investigated in the learning and memory, pain, or epilepsy fields.
3. Synaptic Scaling
Homeostatic plasticity refers to the cellular changes, both synaptic (Turrigiano and Nelson, 2004) and intrinsic (Zhang and Linden, 2003), that allow neurons to maintain relatively stable firing rates; thus mediating one of the most salient and paradoxical characteristics of neuronal networks: robust stability in the face of remarkable plasticity (Nelson and Turrigiano, 2008). While functionally distinct, synaptic and intrinsic homeostatic mechanisms are not completely independent and can influence one another in a manner directed from membrane-to-synapse (Ibata et al., 2008) or synapse-to-membrane (Ishikawa et al., 2009). A well-characterized form of homeostatic synaptic plasticity, synaptic scaling, involves bidirectional compensatory changes in post-synaptic receptor density in response to chronically elevated or depressed activity levels (Kilman et al., 2002; Rannals and Kapur, 2011; Shin et al., 2012; Turrigiano et al., 1998; Wierenga et al., 2005). Importantly, although homeostatic synaptic plasticity has been shown to operate at local synaptic inputs (Hou et al., 2011; Lee et al., 2013; 2010; Pozo and Goda, 2010) and even at the individual synapse level (Hou et al., 2011; Lee et al., 2010), synaptic scaling occurs via a highly coordinated, cell-wide program that multiplicatively adjusts post-synaptic weights across all synapses (see Figure 1C) (Turrigiano et al., 1998; Turrigiano, 2008). This program is initiated in a cell-autonomous manner, as neurons respond robustly to fluctuations in their own spiking rates by sensing concomitant changes in intracellular Ca2+ (Blackman et al., 2012; Goold and Nicoll, 2010; Ibata et al., 2008; Peng et al., 2013). Additionally, soluble factors such as brain-derived neurotrophic factor (BDNF) provide higher order control to scaling processes, through the coordination of transcription-dependent and independent processes (see section 3.2).
3.1 Relevance to Learning and Memory
Theoretical arguments detailing the role of synaptic scaling in learning and memory have been discussed extensively (Nelson and Turrigiano, 2008; Pozo and Goda, 2010; Queenan et al., 2012). The significance of global, multiplicative adjustments in post-synaptic strength lies in the fact that this mechanism has been posited to maintain relative synaptic weights in the context of a highly active and plastic neural network. It is hypothesized that scaling allows for preservation of information acquired through experience-dependent, Hebbian plasticity. As synapse-specific changes are thought to underlie memory storage, synaptic scaling allows for relative preservation of these changes and thus preservation of the memory trace itself. Furthermore, feedforward processes such as LTP and LTD have the potential to create positive feedback loops, driving gain to infinity or zero, respectively. Synaptic scaling is thought to act as negative feedback to these processes, therefore providing a cohesive solution to this theoretical problem. Supporting evidence comes from computational models that suggest within networks utilizing Hebbian plasticity, synaptic scaling, in cooperation with other homeostatic mechanisms such as the dynamic regulation of intrinsic excitability (Remme and Wadman, 2012), robustly increases network stability and information storage capacity (Tetzlaff et al., 2011; 2012).
While theoretical and computational models aid our understanding of how synaptic scaling may operate within in vivo circuitry, there is currently a need for evidence demonstrating a direct biological role for synaptic scaling in learning and memory. However, indirect evidence comes from studies in hippocampal slice cultures. These studies demonstrate that scaling, alongside other homeostatic adaptations, occurs within the intact hippocampal circuitry, setting it within proper anatomical context to participate in memory formation, consolidation, and storage. For example, in response to chronic inactivity, throughput circuits such as dentate gyrus (DG)-CA3 and CA3-CA1 scale up post-synaptic excitatory strength, while in the recurrent CA3-CA3 circuit, excitatory strength scales down (Kim and Tsien, 2008). Even within the CA3 region itself, homeostatic adaptions to inactivity modulate connectivity and synaptic strength in a complex manner, whereby certain contacts between pairs of pyramidal neurons are strengthened while others are silenced (Mitra et al., 2012). An interesting hypothesis put forward by Kim and Tsien (2008) is that homeostatic adaptions within the hippocampal circuit, including synaptic scaling, are necessary for maintaining proper directionality of information flow while concomitantly keeping potentially destabilizing reverberations in check.
3.2. Transcriptional and Epigenetic Regulation
Even as the studies discussed in the previous section move us closer to understanding the biological roles synaptic scaling may play within the context of learning and memory, the precise molecular mechanisms underlying its induction, maintenance, and expression are only beginning to be understood. For example, scaling up and down are not simply regulated in an inverse manner, but are mediated by non-overlapping molecular pathways (Pozo and Goda, 2010; Seeburg et al., 2008; Siddoway et al., 2013; Sun and Turrigiano, 2011). However, with rare exceptions (Aoto et al., 2008; Soden and Chen, 2010; Wang et al., 2011), the coordinated, cell-wide expression of synaptic scaling suggests an equally coordinated and integral role for transcriptional regulation. Indeed, the induction of bidirectional scaling across a range of modalities employed to manipulate activity can be inhibited by the transcription inhibitor, actinomycin-D (Goold and Nicoll, 2010; Han and Stevens, 2009; Ibata et al., 2008; Seeburg et al., 2008). In this section, we will examine salient studies that are beginning to dissect the transcriptional mechanisms at play, with a focused discussion of their place within the context of transcriptional regulation of synaptic plasticity in general. Furthermore, as epigenetic mechanisms are now being recognized as key regulators of gene expression and neuronal function in general, we will discuss how synaptic scaling may be driven through epigenetic modifications and suggest areas for further investigation.
As opposed to Hebbian plasticity, where coordinating pre-synaptic input with post-synaptic depolarization drives changes in synaptic strength, synaptic scaling occurs in a cell-autonomous manner. This is important as it suggests the initial wave of transcriptional changes are mediated not via cell-to-cell signaling, but that a given neuron adjusts its own synaptic weights by responding to fluctuations in Ca2+ entry through voltage-gated channels. Of particular interest here are those studies demonstrating a role for CaMKIV in regulating synaptic scaling in response to both increased (Goold and Nicoll, 2010) and decreased (Ibata et al., 2008) activity. CaMKIV activity itself is regulated via changes in Ca2+ flux through L-type voltage-gated Ca2+ channels (LTCCs) (see Figure 2) (Deisseroth et al., 1998); increased Ca2+ entry activated CaMKIV and led to downscaling, whereas decreased Ca2+ entry decreased CaMKIV activity and caused upscaling (Goold and Nicoll, 2010; Ibata et al., 2008). CaMKIV-regulated gene expression has been well-described in the context of Hebbian plasticity (Kang et al., 2001); however, besides a requirement for its activity, very little else is known about its role in synaptic scaling or how it interacts with other signaling events to regulate transcription.
How do prolonged changes in CaMKIV activity lead to scaling of excitatory synaptic weights? When activity is increased acutely, a rise in intracellular Ca2+ through LTCCs leads to nuclear translocation of Ca2+/calmodulin and increased CaMKIV acitivity (Deisseroth et al., 1998). Active CaMKIV may phosphorylate CREB and CBP, increasing CREB/CBP-dependent gene transcription and leading to histone acetylation via CBP’s HAT activity (Deisseroth et al., 1998; Vo and Goodman, 2001). There is a clear role for CREB/CBP-dependent transcription in promoting memory (Korzus et al., 2004; Silva et al., 1998; Tully et al., 2003). CBP+/- mice exhibit disrupted chromatin acetylation, impaired memory, and decreased Hebbian plasticity (Alarcon et al., 2004). The memory promoting effects of the broad-spectrum HDACi, TSA, have been attributed to specifically promoting CREB/CBP-dependent transcription (Levenson et al., 2004b; Vecsey et al., 2007). However, there is no established role for these players in synaptic scaling. Experiments to determine if neuronal cultures from CBP+/- mice have deficits in synaptic scaling and if these deficits may be rescued with HDAC inhibitors may provide an interesting initial approach. Furthermore, in light of the memory- and LTP-promoting effects of activated CaMKIV, it is interesting to note that the scaling down of excitatory synaptic weights is driven by a chronic increase in CaMKIV activity (Goold and Nicoll, 2010). Clearly, time course effects are playing an important role and need to be worked out in future experiments. The findings suggest CaMKIV signaling within an initial time window promotes the potentiation of synaptic strength, as in LTP, but if activity remains continually elevated, CaMKIV signaling promotes scaling down. As synaptic scaling is proposed to provide negative feedback to positive feedback processes such as LTP, this order of events fits the role of scaling processes within the current learning and memory model.
In addition to elucidating the mechanisms downstream of CaMKIV activity, investigations into the effect of mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK)-mediated signaling and its interactions with CaMKIV will likely provide further insight into the transcriptional and epigenetic regulation of synaptic scaling. The prolonged time course to induce scaling fits with a time frame involving an influential role for MAPK/ERK (Wu et al., 2001). Although there is overwhelming evidence demonstrating a clear role for MAPK/ERK-signaling in mediating the long-term changes in gene expression and epigenetic modifications necessary for Hebbian plasticity and learning and memory (Chwang et al., 2006; Impey et al., 1998; Levenson et al., 2004b; Roberson et al., 1999), evidence for this pathway in synaptic scaling is scant. Ca2+/calmodulin-stimulated AC activity and the subsequent activation of PKA may provide an exciting focal point for initial studies, especially as this pathway has been shown to be critical for the nuclear translocation of ERK, the induction of CREB-dependent transcription, and the induction of histone PTMs (Chwang et al., 2006; Ferguson and Storm, 2004; Impey et al., 1998; Levenson et al., 2004b; Wang and Zhang, 2012). Indeed, the activation of AC1 via Ca2+ entry through LTCCs has been implicated in synaptic scaling (Gong et al., 2007). Furthermore, a recent study using a kinase-dead knock-in mutation of mitogen- and stress-activated protein kinase-1 (MSK1), a component of the MAPK/ERK pathway critical for mediating histone H3 and CREB Ser133 phosphorylation (Arthur et al., 2004; Soloaga et al., 2003), showed its kinase activity to be necessary for the scaling up of excitatory strength in response to activity deprivation (Corrêa et al., 2012). However, one caveat is that although MSK1 can be activated through the activity of Ca2+ -stimulated ACs (Sindreu et al., 2007), the authors of this study focused on its activation via BDNF signaling (see below for further discussion).
Although somatic Ca2+ fluctuations are currently thought to serve as the initial induction locus, leading to the first wave of signaling pathways impinging on the nucleus to regulate the transcriptional and epigenetic programs necessary for the expression of synaptic scaling, the release of the neurotrophin BDNF has been implicated as well (see Figure 2). As one of the most widely studied and influential regulators of synaptic transmission (Elmariah et al., 2004; Marty et al., 2000; Nelson et al., 2008), plasticity (Bramham and Messaoudi, 2005; Figurov et al., 1996), and behavior (Lubin et al., 2008; Mizuno et al., 2012; Rattiner et al., 2005), it is no surprise that BDNF regulates synaptic scaling. However, BDNF operates in an incredibly complex manner, and its precise role in the context of synaptic scaling is unclear. In networks homeostatically adapting to inactivity, BDNF signaling can produce state-dependent, pre-synaptic effects (Jakawich et al., 2010; Lindskog et al., 2010), and its effects on synaptic scaling are cell-type specific (pyramidal vs. interneuron; (Rutherford et al., 1998; Wenner, 2011)) and receptor-specific (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPA) vs. γ-Aminobutyric acid ligand gated receptors (GABAA)); (Bolton et al., 2000; Peng et al., 2010; Swanwick et al., 2006)). Further complicating the story, BDNF may act through transcriptionally-dependent (Calfa et al., 2012; Corrêa et al., 2012) or independent (Fortin et al., 2012; Jakawich et al., 2010) mechanisms.
From a transcriptional and epigenetic standpoint, two broad questions remain regarding BDNF and synaptic scaling. First, how do the chronic, cell-autonomous changes in somatic Ca2+ entry required to induce synaptic scaling affect the transcription of the Bdnf gene, and are these changes mediated via epigenetic modifications? Indeed, studies have found that prolonged elevations in AP firing lead to increased Bdnf expression mediated via decreased promoter methylation (Nelson et al., 2008). Chronic inhibition of DNMT activity seemed to mimic this decrease in methylation (Nelson et al., 2008), a finding especially relevant in light of evidence showing DNMT inhibition in vivo also leads to promoter demethylation and altered expression of the Bdnf gene (Lubin et al., 2008). In both cases, it was argued these changes were regulated through NMDA receptor-mediated signaling, leaving the role of prolonged changes in somatic Ca2+ undefined. Yet, there is likely a capacity for chronic changes in somatic Ca2+ to mediate epigenetic changes at the Bdnf gene as acute, strongly depolarizing stimuli can lead to changes in Bdnf promoter methylation, and these changes are partly mediated through the Ca2+-dependent phosphorylation and unbinding of a repressive MeCP2 complex (Chen et al., 2003a; Martinowich et al., 2003). Although there is an abundance of evidence for the transcriptional regulation of Bdnf by MeCP2 (for review see Li and Pozzo-Miller, 2013), their relationship in the context of synaptic scaling is unclear. Interestingly, MeCP2 itself has been shown to be necessary to scale up (Blackman et al., 2012) and scale down (Qiu et al., 2012) post-synaptic strength, and it does so in a cell-autonomous manner (Blackman et al., 2012). Furthermore, as the Bdnf gene is known to contain multiple promoter regions (Aid et al., 2007; Liu et al., 2006; Timmusk et al., 1993), and the methylation status of these regions may be specifically regulated by Hebbian plasticity (Sui et al., 2012) and learning (Lubin et al., 2008; Mizuno et al., 2012), it may be helpful to determine the promoter-specific methylation changes induced during synaptic scaling.
The second broad question: how does BDNF signaling interact with the Ca2+-mediated pathways discussed above to further modify the epigenetic landscape during synaptic scaling? Clearly, this is a complex question, especially given the heterogeneous data regarding BDNF’s effects on scaling processes. However, there are certainly targets to be investigated. For instance, we should continue to dissect the interaction between BDNF-mediated ERK signaling and Ca2+/calmodulin-mediated signaling. As both BDNF signaling and Ca2+/calmodulin-stimulated AC are known to activate MSK1 (Alonso et al., 2004; Arthur et al., 2004; Corrêa et al., 2012; Sindreu et al., 2007; Soloaga et al., 2003), it is particularly interesting to consider how these pathways converge on this kinase and cooperate with CaMKIV to control CREB/CBP-dependent transcription and PTMs of histones. Moreover, how might these pathways antagonize each other? For example, how is synaptic scaling influenced by the competition of HAT/HDAC activity? BDNF has been shown to affect quantal neurotransmission via the activity of HDACs (Calfa et al., 2012), and HDACs themselves have been shown to affect neurotransmission in a class specific manner (Akhtar et al., 2009; Hanson et al., 2013; Kim et al., 2012). As synaptic scaling is a modulation of baseline neurotransmission in response to chronic changes in activity, there is likely a role for these mechanisms in its induction, maintenance, or expression. Therefore, it may be beneficial to determine the time course of global histone modifications during synaptic scaling and how these changes are influenced via BDNF/TrkB antagonization or HDAC inhibition.
4. Concluding Remarks
In the last decade, the field of neuroepigenetics has made tremendous progress in recognizing the importance of epigenetic mechanisms in the memory process. It is now evident that, in order to generate a lasting effect on behavior, neuronal circuits must modify their function in a persistent yet flexible manner. Currently the field has focused on examining how individual genes and epigenetic modifications drive these necessary long-lasting changes in neuronal function. However, technological advancements offered by the “-omics” revolution are making it increasingly possible to understand how entire programs of genes are epigenetically regulated to impact overall neuronal function and behavior. Advances in next-generation sequencing will allow investigators not only to fully characterize genome-wide changes in gene expression associated with a particular learning experience but also to define the accompanying epigenetic modifications regulating these changes. For example, bisulfite sequencing (BS-seq) in combination with oxidative bisulfite sequencing (oxBS-seq) or Tet-assisted bisulfite sequencing (TAB-seq) will allow for single-nucleotide resolution of cytosine methylation and 5-hydroxy-methylation (Booth et al., 2012; Yu et al., 2012). Similarly, chromatin immunoprecipitation followed by sequencing (ChIP-Seq) will allow for large-scale mapping of transcription factor binding and histone PTMs as has recently been done following fear memory acquisition (Park et al., 2013). Characterizing the memory transcriptome and epigenome will undoubtedly further our understanding of the molecular underpinnings of long-term memory, identifying new gene products that can be further targeted and explored.
Additional technological innovations are also making it increasingly possible to determine the precise functional role of individual DNA or histone modifications. Although genetic and pharmacological manipulations of epigenetic machinery have revealed the necessity of epigenetic mechanisms in learning and memory, dissecting the causal role of individual modifications has been challenging due to the correlative nature of existing chromatin-based approaches. Utilization of customizable zinc-finger arrays and transcription activator–like effector (TALE) proteins will allow investigators to activate or repress specific genes (Joung and Sander, 2013), to catalyze locus-specific DNA demethylation (Maeder et al., 2013) and to direct the addition or removal of specific histone modifications (Konermann et al., 2013; Mendenhall et al., 2013). These systems are further amenable to optogenetic modulation allowing for sophisticated manipulation of the epigenome in an inducible and reversible manner (Konermann et al., 2013).
However, as with all new technological developments, the newly acquired information will be hard to make sense of if we are unable to extract functional relevance from the data as it relates to neuronal function and plasticity. To obtain a better understanding of what these genome-wide expression and epigenetic changes mean for neuronal plasticity and behavior overall, two goals should be undertaken: (1) understanding how different forms of plasticity (Hebbian vs. non-Hebbian) are epigenetically regulated on an individual level, and (2) understanding how these forms of plasticity interact and modulate each other at level of the epigenome.
In this review, we hope to have broadened the functional relevance of epigenetic mechanisms to include regulation of both Hebbian and non-Hebbian forms of plasticity. In doing so, we provide a series of experimental starting points that will hopefully spur further exploration of these topics. Interestingly, our examination revealed these distinct plasticities rely on overlapping induction mechanisms like intracellular Ca2+ signaling and subsequent activation of several second-messenger pathways (see Figure 2). It is possible that once the upstream initiating signal is propagated to the nucleus, the epigenome acts as a point of convergence and divergence integrating upstream signals into a particular epigenetic and transcriptional signature that is plasticity-specific (e.g., LTP or synaptic scaling). Understanding how these different forms of plasticity are epigenetically regulated on an individual level will help us compartmentalize network topologies when analyzing whole-genome studies. Therefore, when examining learning-induced genome-wide changes in expression and epigenetic modifications, there will be a subset of changes that are inherently mnemonic in function. However, as previously mentioned there will be concurrent changes underlying other forms of plasticity (e.g., intrinsic plasticity and synaptic scaling) that may not constitute part of the molecular memory engram but are nonetheless critical to the overall memory process.
This latter point is becoming increasingly salient considering these different forms of plasticity are able to interact with one another in a metaplastic manner. Both intrinsic plasticity and synaptic scaling are able to modulate LTP (Arendt et al., 2013; Chen et al., 2006; Cohen and Abraham, 1996; Cohen et al., 1999; Kramár et al., 2004; Roth-Alpermann et al., 2006; Sah and Bekkers, 1996; Thiagarajan et al., 2007). These plasticities also interact at the level of the behaving animal (Lambo and Turrigiano, 2013; Maffei and Turrigiano, 2008; Nataraj et al., 2010; Saar et al., 1998; Zelcer et al., 2006). These observations are interesting given that HDAC inhibitors are able to facilitate both LTP induction and learning (Fass et al., 2013; Levenson et al., 2004b; Miller et al., 2008; Stafford et al., 2012; Sui et al., 2012; Vecsey et al., 2007; Yeh et al., 2004; Zeng et al., 2011), suggesting the underlying mechanism of action for these drugs may involve alterations in intrinsic and homeostastic processes as well as synapse-specific changes. Furthermore, intrinsic plasticity and homeostatic plasticity are intimately tied as chronic changes in neuronal activity can dynamically regulate intrinsic excitability thereby modulating the whole neuron’s response to incoming stimuli (Desai et al., 1999). CREB-mediated changes in intrinsic excitability have also been shown to influence which neurons get recruited to a given memory trace (Han et al., 2007; Kim et al., 2013; Zhou et al., 2009), a process termed memory allocation (Silva et al., 2009; Won and Silva, 2008). Although it is evident these different forms of plasticity interact at the level of the cell membrane to influence behavior, it is conceivable they also interact at the level of epigenome where a given neuron’s epigenetic “state” may ultimately dictate a neuron’s cellular “state” via the simultaneous regulation of Hebbian and non-Hebbian plasticity. In such a case, the epigenome would be a prime candidate for metaplasticity at large, which is an idea recently put forth in the literature (Baker-Andresen et al., 2013b).
As the field of neuroepigenetics expands into exciting and new territories, these topics amongst others will need to be addressed to obtain a comprehensive understanding of the global transcriptional and epigenetic changes necessary for long-term behavioral memory. Using the previously outlined ideas as foundational points, we propose the following simplified operational model to better understand and integrate such changes. A given learning experience drives nuclear change by impinging on a variety of well-conserved signaling cascades. Once at the nucleus, these signaling cascades engage a parallel set of chromatin remodeling processes that produce several overlapping, yet independent, gene expression profiles that ultimately regulate the levels/activity of ion channels, receptors, trafficking proteins, and signaling molecules. These cell-wide changes in transcriptional output ultimately modulate long-term behavioral memory by regulating synapse-specific Hebbian plasticity either directly or indirectly. Direct regulation may be accomplished by targeted transport of necessary gene products to tagged synapses, whereas indirect regulation may occur through non-Hebbian cell-wide functional changes. As a result, the combined output of transcriptional and epigenetic mechanisms may serve multifaceted functions including encoding of mnemonic information, adaptation to plasticity-inducing experiences, and subsequent modulation of future plasticity.
Research Highlights.
Hebbian and non-Hebbian forms of plasticity are critical to the memory process.
We review the data supporting epigenetic mechanisms in Hebbian plasticity (LTP).
Non-hebbian plasticity includes intrinsic plasticity and synaptic scaling.
Transcriptional and epigenetic regulation of non-Hebbian plasticity is examined.
Acknowledgments
We thank Jeremy Day, Dinesh Kumar, Elizabeth Rahn, Laura Qadri, and Iva Zovkic for helpful commentary on the manuscript and members of the Sweatt lab for stimulating discussion. This work was supported by MH57014, NIMH091122, Civitan International, and Evelyn F. McKnight Brain Research Foundation, the Pitt-Hopkins Syndrome (PTHS) Foundation (JDS). MCGK and JPM are supported by NINDS 3T32NS061788 and the UAB Medical Scientist Training Program, T32 GM008361. CFG is supported by the PTHS Foundation.
Abbreviations
- AC
adenylyl cyclase
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AP
action potential
- BDNF
brain-derived neurotrophic factor
- BLA
basolateral amygdala
- CA
cornus ammonis
- CaMKII/IV
Ca2+/calmodulin-dependent protein kinase II/IV
- CBP
CREB binding protein
- CREB
cAMP/Ca2+-response element binding protein
- CNS
central nervous system
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- DG
dentate gyrus
- ERK
extracellular-signal regulated kinase
- GABAA
γ-Aminobutyric acid ligand gated receptors
- H2S
hydrogen sulfide
- HATs
histone acetyltransferases
- HCN
hyperpolarization-activated cyclic nucleotide-gated
- HDAC
histone deacetylase
- HDACi
HDAC inhibitor
- DRG
dorsal root ganglion
- LA
lateral amygdala
- LTCCs
L-type voltage-gated Ca2+ channels
- MAPK
mitogen-activated protein kinase
- MeCP2
methyl CpG binding protein 2
- MSK1
mitogen- and stress-activated protein kinase-1
- MSN
medium spiny neurons
- NMDA
N-Methyl-D-Aspartate
- NRSE
neuron-restrictive silencer elements
- NRSF
neuron-restrictive silencing factor
- NA
nucleus accumbens
- PKA
cAMP-dependent protein kinase
- PKC
protein kinase C
- PKG
cGMP-dependent protein kinase
- PTMs
post-translational modifications
- RE1
repressor element 1
- REST
RE1 silencing transcription factor
- TSA
trichostatin A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aid TT, Kazantseva AA, Piirsoo MM, Palm KK, Timmusk TT. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar MW, Raingo J, Nelson ED, Montgomery RL, Olson EN, Kavalali ET, Monteggia LM. Histone deacetylases 1 and 2 form a developmental switch that controls excitatory synapse maturation and function. J Neurosci. 2009;29:8288–8297. doi: 10.1523/JNEUROSCI.0097-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon JMJ, Malleret GG, Touzani KK, Vronskaya SS, Ishii SS, Kandel ERE, Barco AA. Chromatin Acetylation, Memory, and LTP Are Impaired in CBP^+^/^- Mice - A Model for the Cognitive Deficit in Rubinstein-Taybi Syndrome and Its Amelioration. Neuron. 2004;42:13–13. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertson AJ, Williams SB, Hablitz JJ. Regulation of epileptiform discharges in rat neocortex by HCN channels. J Neurophysiol. 2013 doi: 10.1152/jn.00955.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertson AJ, Yang J, Hablitz JJ. Decreased hyperpolarization-activated currents in layer 5 pyramidal neurons enhances excitability in focal cortical dysplasia. J Neurophysiol. 2011;106:2189–2200. doi: 10.1152/jn.00164.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso MM, Medina JHJ, Pozzo-Miller LL. ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learn Mem. 2004;11:172–178. doi: 10.1101/lm.67804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alreja M, Aghajanian GK. Use of the whole-cell patch-clamp method in studies on the role of cAMP in regulating the spontaneous firing of locus coeruleus neurons. J Neurosci Methods. 1995;59:67–75. doi: 10.1016/0165-0270(94)00195-m. [DOI] [PubMed] [Google Scholar]
- Aoto J, Nam CI, Poon MM, Ting P, Chen L. Synaptic Signaling by All-Trans Retinoic Acid in Homeostatic Synaptic Plasticity. Neuron. 2008;60:13–13. doi: 10.1016/j.neuron.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt KL, Sarti F, Chen L. Chronic inactivation of a neural circuit enhances LTP by inducing silent synapse formation. J Neurosci. 2013;33:2087–2096. doi: 10.1523/JNEUROSCI.3880-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur JSC, Fong AL, Dwyer JM, Davare M, Reese E, Obrietan K, Impey S. Mitogen- and stress-activated protein kinase 1 mediates cAMP response element-binding protein phosphorylation and activation by neurotrophins. J Neurosci. 2004;24:4324–4332. doi: 10.1523/JNEUROSCI.5227-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Andresen D, Flavell CR, Li X, Bredy TW. Activation of BDNF signaling prevents the return of fear in female mice. Learning & Memory. 2013a;20:237–240. doi: 10.1101/lm.029520.112. [DOI] [PubMed] [Google Scholar]
- Baker-Andresen D, Ratnu VS, Bredy TW. Dynamic DNA methylation: a prime candidate for genomic metaplasticity and behavioral adaptation. Trends Neurosci. 2013b;36:3–13. doi: 10.1016/j.tins.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Ballas N, Mandel G. The many faces of REST oversee epigenetic programming of neuronal genes. Curr Opin Neurobiol. 2005;15:500–506. doi: 10.1016/j.conb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Ballas N, Grunseich C, Lu DD, Speh JC, Mandel G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell. 2005;121:645–657. doi: 10.1016/j.cell.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Barco A, Marie H. Genetic approaches to investigate the role of CREB in neuronal plasticity and memory. Mol Neurobiol. 2011;44:330–349. doi: 10.1007/s12035-011-8209-x. [DOI] [PubMed] [Google Scholar]
- Barondes SH, Jarvik ME. The influence of actinomycin-D on brain RNA synthesis and on memory. J Neurochem. 1964;11:187–195. doi: 10.1111/j.1471-4159.1964.tb06128.x. [DOI] [PubMed] [Google Scholar]
- Barrett R, Malvaez M, Kramar E. Hippocampal focal knockout of CBP affects specific histone modifications, long-term potentiation, and long-term memory. 2011;36:1545–1556. doi: 10.1038/npp.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer EP, LeDoux JE, Nader K. Fear conditioning and LTP in the lateral amygdala are sensitive to the same stimulus contingencies. Nat Neurosci. 2001;4:687–688. doi: 10.1038/89465. [DOI] [PubMed] [Google Scholar]
- Beck H, Yaari Y. Plasticity of intrinsic neuronal properties in CNS disorders. Nat Rev Neurosci. 2008;9:357–369. doi: 10.1038/nrn2371. [DOI] [PubMed] [Google Scholar]
- Benito E, Barco A. CREB’s control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci. 2010;33:230–240. doi: 10.1016/j.tins.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Biel M, Wahl-Schott C, Michalakis S, Zong X. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev. 2009;89:847–885. doi: 10.1152/physrev.00029.2008. [DOI] [PubMed] [Google Scholar]
- Bird AP. The occurrence and transmission of a pattern of DNA methylation in Xenopus laevis ribosomal DNA. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 1978;283:325–327. doi: 10.1098/rstb.1978.0032. [DOI] [PubMed] [Google Scholar]
- Blackman MP, Djukic B, Nelson SB, Turrigiano GG. A critical and cell-autonomous role for MeCP2 in synaptic scaling up. J Neurosci. 2012;32:13529–13536. doi: 10.1523/JNEUROSCI.3077-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn Mem. 2001;8:229–242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bolton MM, Pittman AJ, Lo DC. Brain-derived neurotrophic factor differentially regulates excitatory and inhibitory synaptic transmission in hippocampal cultures. J Neurosci. 2000;20:3221–3232. doi: 10.1523/JNEUROSCI.20-09-03221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth MJ, Branco MR, Ficz G, Oxley D, Krueger F, Reik W, Balasubramanian S. Quantitative Sequencing of 5-Methylcytosine and 5-Hydroxymethylcytosine at Single-Base Resolution. Science. 2012;336:934–937. doi: 10.1126/science.1220671. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Lidge R, Catapano R, Stanley J, Gossweiler S, Romashko D, Scott R, Tully T. A mouse model of Rubinstein-Taybi syndrome: defective long-term memory is ameliorated by inhibitors of phosphodiesterase 4. Proc Natl Acad Sci USa. 2003;100:10518–10522. doi: 10.1073/pnas.1834280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Brons JF, Woody CD. Long-term changes in excitability of cortical neurons after Pavlovian conditioning and extinction. J Neurophysiol. 1980;44:605–615. doi: 10.1152/jn.1980.44.3.605. [DOI] [PubMed] [Google Scholar]
- Calfa G, Chapleau CA, Campbell S, Inoue T, Morse SJ, Lubin FD, Pozzo-Miller L. HDAC activity is required for BDNF to increase quantal neurotransmitter release and dendritic spine density in CA1 pyramidal neurons. Hippocampus. 2012;22:1493–1500. doi: 10.1002/hipo.20990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong STC, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao JR, Ni YG, Bolaños CA, Rahman Z, DiLeone RJ, Nestler EJ. Characterization of the mouse adenylyl cyclase type VIII gene promoter: regulation by cAMP and CREB. Eur J Neurosci. 2002;16:1284–1294. doi: 10.1046/j.1460-9568.2002.02186.x. [DOI] [PubMed] [Google Scholar]
- Chen G, Zou X, Watanabe H, van Deursen JM, Shen J. CREB binding protein is required for both short-term and long-term memory formation. J Neurosci. 2010;30:13066–13077. doi: 10.1523/JNEUROSCI.2378-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, MacMillan AM, Chang W, Ezaz-Nikpay K, Lane WS, Verdine GL. Direct identification of the active-site nucleophile in a DNA (cytosine-5)-methyltransferase. Biochemistry. 1991;30:11018–11025. doi: 10.1021/bi00110a002. [DOI] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003a;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- Chen WG, West AE, Tao X, Corfas G, Szentirmay MN, Sawadogo M, Vinson C, Greenberg ME. Upstream stimulatory factors are mediators of Ca2+-responsive transcription in neurons. J Neurosci. 2003b;23:2572–2581. doi: 10.1523/JNEUROSCI.23-07-02572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yuan LL, Zhao C, Birnbaum SG, Frick A, Jung WE, Schwarz TL, Sweatt JD, Johnston D. Deletion of Kv4.2 Gene Eliminates Dendritic A-Type K+ Current and Enhances Induction of Long-Term Potentiation in Hippocampal CA1 Pyramidal Neurons. J Neurosci. 2006;26:12143–12151. doi: 10.1523/JNEUROSCI.2667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YY, Wang HH, Yoon SOS, Xu XX, Hottiger MOM, Svaren JJ, Nave KAK, Kim HAH, Olson ENE, Lu QRQ. HDAC-mediated deacetylation of NF-κB is critical for Schwann cell myelination. Nat Neurosci. 2011;14:437–441. doi: 10.1038/nn.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwang WB, Arthur JS, Schumacher A, Sweatt JD. The nuclear kinase mitogen- and stress-activated protein kinase 1 regulates hippocampal chromatin remodeling in memory formation. J Neurosci. 2007;27:12732–12742. doi: 10.1523/JNEUROSCI.2522-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwang WB, O’Riordan KJ, Levenson JM, Sweatt JD. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem. 2006;13:322–328. doi: 10.1101/lm.152906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Abraham WC. Facilitation of long-term potentiation by prior activation of metabotropic glutamate receptors. J Neurophysiol. 1996 doi: 10.1152/jn.1996.76.2.953. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Coussens CM, Raymond CR, Abraham WC. Long-Lasting Increase in Cellular Excitability Associated With the Priming of LTP Induction in Rat Hippocampus. … Of Neurophysiology. 1999 doi: 10.1152/jn.1999.82.6.3139. [DOI] [PubMed] [Google Scholar]
- Cohen HD, Barondes SH. Further studies of learning and memory after intracerebral actinomycin-D. J Neurochem. 1966;13:207–211. doi: 10.1111/j.1471-4159.1966.tb06793.x. [DOI] [PubMed] [Google Scholar]
- Cohen-Matsliah SI, Motanis H, Rosenblum K, Barkai E. A Novel Role for Protein Synthesis in Long-Term Neuronal Plasticity: Maintaining Reduced Postburst Afterhyperpolarization. The Journal of …. 2010 doi: 10.1523/JNEUROSCI.5005-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrêa SAL, Hunter CJ, Palygin O, Wauters SC, Martin KJ, McKenzie C, McKelvey K, Morris RGM, Pankratov Y, Arthur JSC, et al. MSK1 Regulates Homeostatic and Experience-Dependent Synaptic Plasticity. The Journal of …. 2012 doi: 10.1523/JNEUROSCI.0930-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortés-Mendoza J, Díaz de León-Guerrero S, Pedraza-Alva G, Pérez-Martínez L. Shaping synaptic plasticity: the role of activity-mediated epigenetic regulation on gene transcription. International Journal of Developmental Neuroscience: the Official Journal of the International Society for Developmental Neuroscience. 2013;31:359–369. doi: 10.1016/j.ijdevneu.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Coulter DA, Turco, Lo JJ, Kubota M, Disterhoft JF, Moore JW, Alkon DL. Classical conditioning reduces amplitude and duration of calcium-dependent afterhyperpolarization in rabbit hippocampal pyramidal cells. J Neurophysiol. 1989;61:971–981. doi: 10.1152/jn.1989.61.5.971. [DOI] [PubMed] [Google Scholar]
- Daoudal G. Long-Term Plasticity of Intrinsic Excitability: Learning Rules and Mechanisms. Learn Mem. 2003;10:456–465. doi: 10.1101/lm.64103. [DOI] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. Epigenetic mechanisms in cognition. Neuron. 2011;70:813–829. doi: 10.1016/j.neuron.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Childs D, Guzman-Karlsson MC, Kibe M, Moulden J, Song E, Tahir A, Sweatt JD. DNA methylation regulates associative reward learning. Nat Neurosci. 2013;16:1445–1452. doi: 10.1038/nn.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaton AM, Bird A. CpG islands and the regulation of transcription. Genes Dev. 2011;25:1010–1022. doi: 10.1101/gad.2037511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Heist EK, Tsien RW. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature. 1998;392:198–202. doi: 10.1038/32448. [DOI] [PubMed] [Google Scholar]
- Denk F, McMahon SB. Chronic Pain: Emerging Evidence for the Involvement of Epigenetics. Neuron. 2012;73:435–444. doi: 10.1016/j.neuron.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci. 1999;2:515–520. doi: 10.1038/9165. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Coulter DA, Alkon DL. Conditioning-specific membrane changes of rabbit hippocampal neurons measured in vitro. Proc Natl Acad Sci USa. 1986;83:2733–2737. doi: 10.1073/pnas.83.8.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disterhoft JFJ, Oh MMM. Learning, aging and intrinsic neuronal plasticity. Trends Neurosci. 2006;29:13–13. doi: 10.1016/j.tins.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Golden DT, Read HL, Coulter DA, Alkon DL. AHP reductions in rabbit hippocampal neurons during conditioning correlate with acquisition of the learned response. Brain Res. 1988;462:118–125. doi: 10.1016/0006-8993(88)90593-8. [DOI] [PubMed] [Google Scholar]
- Dong Y, Green T, Saal D, Marie H, Neve R, Nestler EJ, Malenka RC. CREB modulates excitability of nucleus accumbens neurons. Nat Neurosci. 2006;9:475–477. doi: 10.1038/nn1661. [DOI] [PubMed] [Google Scholar]
- Elmariah SB, Crumling MA, Parsons TD, Balice-Gordon RJ. Postsynaptic TrkB-mediated signaling modulates excitatory and inhibitory neurotransmitter receptor clustering at hippocampal synapses. J Neurosci. 2004;24:2380–2393. doi: 10.1523/JNEUROSCI.4112-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass DM, Reis SA, Ghosh B, Hennig KM, Joseph NF, Zhao WN, Nieland TJF, Guan JS, Kuhnle CEG, Tang W, et al. Crebinostat: a novel cognitive enhancer that inhibits histone deacetylase activity and modulates chromatin-mediated neuroplasticity. Neuropharmacology. 2013;64:81–96. doi: 10.1016/j.neuropharm.2012.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi MM, Hermann AA, Gowher HH, Jeltsch AA. Dnmt3a and Dnmt1 functionally cooperate during de novo methylation of DNA. FEBS Journal. 2002;269:4981–4984. doi: 10.1046/j.1432-1033.2002.03198.x. [DOI] [PubMed] [Google Scholar]
- Feng J, Fan G. The role of DNA methylation in the central nervous system and neuropsychiatric disorders. International Review of Neurobiology. 2009;89:67–84. doi: 10.1016/S0074-7742(09)89004-1. [DOI] [PubMed] [Google Scholar]
- Feng J, Zhou Y, Campbell SL, Le T, Li E, Sweatt JD, Silva AJ, Fan G. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat Neurosci. 2010;13:423–430. doi: 10.1038/nn.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson GD, Storm DR. Why calcium-stimulated adenylyl cyclases? Physiology (Bethesda) 2004;19:271–276. doi: 10.1152/physiol.00010.2004. [DOI] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Fischle W, Wang Y, Allis CD. Histone and chromatin cross-talk. Curr Opin Cell Biol. 2003;15:172–183. doi: 10.1016/s0955-0674(03)00013-9. [DOI] [PubMed] [Google Scholar]
- Fortin DA, Srivastava T, Dwarakanath D, Pierre P, Nygaard S, Derkach VA, Soderling TR. Brain-derived neurotrophic factor activation of CaM-kinase kinase via transient receptor potential canonical channels induces the translation and synaptic incorporation of GluA1-containing calcium-permeable AMPA receptors. J Neurosci. 2012;32:8127–8137. doi: 10.1523/JNEUROSCI.6034-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourcaudot E, Gambino F, Casassus G, Poulain B, Humeau Y, Lüthi A. L-type voltage-dependent Ca(2+) channels mediate expression of presynaptic LTP in amygdala. Nat Neurosci. 2009;12:1093–1095. doi: 10.1038/nn.2378. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Frick A, Johnston D. Plasticity of dendritic excitability. J Neurobiol. 2005;64:100–115. doi: 10.1002/neu.20148. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Mattson MP. The Transcription Factor NF-κB Mediates Increases in Calcium Currents and Decreases in NMDA- and AMPA/Kainate-Induced Currents Induced by Tumor Necrosis Factor-α in Hippocampal Neurons. J Neurochem. 2002;70:1876–1886. doi: 10.1046/j.1471-4159.1998.70051876.x. [DOI] [PubMed] [Google Scholar]
- Géranton SM. Targeting epigenetic mechanisms for pain relief. Curr Opin Pharmacol. 2012;12:35–41. doi: 10.1016/j.coph.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Goll MG, Bestor TH. Eukaryotic cytosine methyltransferases. Annual Review of Biochemistry. 2005;74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721. [DOI] [PubMed] [Google Scholar]
- Gong B, Wang H, Gu S, Heximer SP, Zhuo M. Genetic evidence for the requirement of adenylyl cyclase 1 in synaptic scaling of forebrain cortical neurons. Eur J Neurosci. 2007;26:275–288. doi: 10.1111/j.1460-9568.2007.05669.x. [DOI] [PubMed] [Google Scholar]
- Goold CP, Nicoll RA. Single-cell optogenetic excitation drives homeostatic synaptic depression. Neuron. 2010;68:512–528. doi: 10.1016/j.neuron.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJF, Zhou Y, Wang X, Mazitschek R, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JU, Su Y, Zhong C, Ming GL, Song H. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell. 2011a;145:423–434. doi: 10.1016/j.cell.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JU, Ma DK, Mo H, Ball MP, Jang M-H, Bonaguidi MA, Balazer JA, Eaves HL, Xie B, Ford E, et al. Neuronal activity modifies the DNA methylation landscape in the adult brain. Nat Neurosci. 2011b doi: 10.1038/nn.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Kim SY, Artis S, Molfese DL, Schumacher A, Sweatt JD, Paylor RE, Lubin FD. Histone methylation regulates memory formation. J Neurosci. 2010;30:3589–3599. doi: 10.1523/JNEUROSCI.3732-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han EB, Stevens CF. Development regulates a switch between post- and presynaptic strengthening in response to activity deprivation. Proc Natl Acad Sci USa. 2009;106:10817–10822. doi: 10.1073/pnas.0903603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ, Josselyn SA. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- Han MH, Bolaños CA, Green TA, Olson VG, Neve RL, Liu RJ, Aghajanian GK, Nestler EJ. Role of cAMP response element-binding protein in the rat locus ceruleus: regulation of neuronal activity and opiate withdrawal behaviors. J Neurosci. 2006;26:4624–4629. doi: 10.1523/JNEUROSCI.4701-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JE, Deng L, Hackos DH, Lo SC, Lauffer BE, Steiner P, Zhou Q. Histone deacetylase 2 cell autonomously suppresses excitatory and enhances inhibitory synaptic function in CA1 pyramidal neurons. J Neurosci. 2013;33:5924–5929. doi: 10.1523/JNEUROSCI.3162-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug T, Storm JF. Protein kinase A mediates the modulation of the slow Ca(2+)-dependent K(+) current, I(sAHP), by the neuropeptides CRF, VIP, and CGRP in hippocampal pyramidal neurons. J Neurophysiol. 2000;83:2071–2079. doi: 10.1152/jn.2000.83.4.2071. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Johnston D. Downregulation of Transient K+ Channels in Dendrites of Hippocampal CA1 Pyramidal Neurons by Activation of PKA and PKC. The Journal of Neuroscience. 1998 doi: 10.1523/JNEUROSCI.18-10-03521.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q, Gilbert J, Man HY. Homeostatic regulation of AMPA receptor trafficking and degradation by light-controlled single-synaptic activation. Neuron. 2011;72:806–818. doi: 10.1016/j.neuron.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CL. The de novo methylation activity of Dnmt3a is distinctly different than that of Dnmt1. BMC Biochem. 2005;6:6–6. doi: 10.1186/1471-2091-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Postsynaptic induction and PKA-dependent expression of LTP in the lateral amygdala. Neuron. 1998;21:169–178. doi: 10.1016/s0896-6273(00)80524-3. [DOI] [PubMed] [Google Scholar]
- Huang YY, Martin KC, Kandel ER. Both protein kinase A and mitogen-activated protein kinase are required in the amygdala for the macromolecular synthesis-dependent late phase of long-term potentiation. J Neurosci. 2000;20:6317–6325. doi: 10.1523/JNEUROSCI.20-17-06317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Walker MC, Shah MM. Loss of dendritic HCN1 subunits enhances cortical excitability and epileptogenesis. J Neurosci. 2009;29:10979–10988. doi: 10.1523/JNEUROSCI.1531-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibata KK, Sun QQ, Turrigiano GGG. Rapid Synaptic Scaling Induced by Changes in Postsynaptic Firing. Neuron. 2008;57:8–8. doi: 10.1016/j.neuron.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Iriki A, Pavlides C, Keller A, Asanuma H. Long-term potentiation in the motor cortex. Science. 1989;245:1385–1387. doi: 10.1126/science.2551038. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Mu P, Moyer JT, Wolf JA, Quock RM, Davies NM, Hu XT, Schlüter OM, Dong Y. Homeostatic synapse-driven membrane plasticity in nucleus accumbens neurons. J Neurosci. 2009;29:5820–5831. doi: 10.1523/JNEUROSCI.5703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A, Aston-Jones G. Local Opiate Withdrawal in Locus Coeruleus Neurons In Vitro. J Neurophysiol. 2001 doi: 10.1152/jn.2001.85.6.2388. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Medina JH. Correlation between the pharmacology of long-term potentiation and the pharmacology of memory. Neurobiol Learn Mem. 1995;63:19–32. doi: 10.1006/nlme.1995.1002. [DOI] [PubMed] [Google Scholar]
- Jakawich SK, Nasser HB, Strong MJ, McCartney AJ, Perez AS, Rakesh N, Carruthers CJL, Sutton MA. Local presynaptic activity gates homeostatic changes in presynaptic function driven by dendritic BDNF synthesis. Neuron. 2010;68:1143–1158. doi: 10.1016/j.neuron.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA, Nguyen PV. CREB, synapses and memory disorders: past progress and future challenges. Curr Drug Targets CNS Neurol Disord. 2005;4:481–497. doi: 10.2174/156800705774322058. [DOI] [PubMed] [Google Scholar]
- Joung JK, Sander JD. TALENs: a widely applicable technology for targeted genome editing. Nat Rev Mol Cell Biol. 2013;14:49–55. doi: 10.1038/nrm3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski CC, Disterhoft JF. Memory deficits are associated with impaired ability to modulate neuronal excitability in middle-aged mice. Learn Mem. 2009;16:362–366. doi: 10.1101/lm.1365609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Sun LD, Atkins CM, Soderling TR, Wilson MA, Tonegawa S. An important role of neural activity-dependent CaMKIV signaling in the consolidation of long-term memory. Cell. 2001;106:771–783. doi: 10.1016/s0092-8674(01)00497-4. [DOI] [PubMed] [Google Scholar]
- Kavalali ETE, Nelson EDE, Monteggia LML. Role of MeCP2, DNA methylation, and HDACs in regulating synapse function. J Neurodev Disord. 2011;3:250–256. doi: 10.1007/s11689-011-9078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilman V, van Rossum MCW, Turrigiano GG. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J Neurosci. 2002;22:1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav Neurosci. 1995;109:195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- Kim J, Kwon J-T, Kim H-S, Josselyn SA, Han J-H. Memory recall and modifications by activating neurons with elevated CREB. Nat Neurosci. 2013 doi: 10.1038/nn.3592. [DOI] [PubMed] [Google Scholar]
- Kim J, Tsien RW. Synapse-specific adaptations to inactivity in hippocampal circuits achieve homeostatic gain control while dampening network reverberation. Neuron. 2008;58:925–937. doi: 10.1016/j.neuron.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Akhtar MW, Adachi M, Mahgoub M, Bassel-Duby R, Kavalali ET, Olson EN, Monteggia LM. An essential role for histone deacetylase 4 in synaptic plasticity and memory formation. J Neurosci. 2012;32:10879–10886. doi: 10.1523/JNEUROSCI.2089-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Linden DJ. Ubiquitous Plasticity and Memory Storage. Neuron. 2007;56:582–592. doi: 10.1016/j.neuron.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Klann E, Antion MD, Banko JL, Hou L. Synaptic plasticity and translation initiation. Learn Mem. 2004;11:365–372. doi: 10.1101/lm.79004. [DOI] [PubMed] [Google Scholar]
- Klose RJ, Sarraf SA, Schmiedeberg L, McDermott SM, Stancheva I, Bird AP. DNA binding selectivity of MeCP2 due to a requirement for A/T sequences adjacent to methyl-CpG. Molecular Cell. 2005;19:667–678. doi: 10.1016/j.molcel.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Konermann S, Brigham MD, Trevino AE, Hsu PD, Heidenreich M, Cong L, Platt RJ, Scott DA, Church GM, Zhang F. Optical control of mammalian endogenous transcription and epigenetic states. Nature. 2013;500:472–476. doi: 10.1038/nature12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshibu K, Graff J, Beullens M, Heitz FD, Berchtold D, Russig H, Farinelli M, Bollen M, Mansuy IM. Protein Phosphatase 1 Regulates the Histone Code for Long-Term Memory. J Neurosci. 2009;29:13079–13089. doi: 10.1523/JNEUROSCI.3610-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramár EA, Lin Bin, Lin C-Y, Arai AC, Gall CM, Lynch G. A Novel Mechanism for the Facilitation of Theta-Induced Long-Term Potentiation by Brain-Derived Neurotrophic Factor. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambo ME, Turrigiano GG. Synaptic and intrinsic homeostatic mechanisms cooperate to increase L2/3 pyramidal neuron excitability during a late phase of critical period plasticity. J Neurosci. 2013;33:8810–8819. doi: 10.1523/JNEUROSCI.4502-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane-Ladd SB, Pineda J, Boundy VA, Pfeuffer T, Krupinski J, Aghajanian GK, Nestler EJ. CREB (cAMP response element-binding protein) in the locus coeruleus: biochemical, physiological, and behavioral evidence for a role in opiate dependence. J Neurosci. 1997;17:7890–7901. doi: 10.1523/JNEUROSCI.17-20-07890.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzillotta A, Sarnico I, Ingrassia R, Boroni F, Branca C, Benarese M, Faraco G, Blasi F, Chiarugi A, Spano P, et al. The acetylation of RelA in Lys310 dictates the NF-κB-dependent response in post-ischemic injury. Cell Death Dis. 2010;1:e96. doi: 10.1038/cddis.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau OD, Courtney AD, Vassilev A, Marzilli LA, Cotter RJ, Nakatani Y, Cole PA. p300/CBP-associated factor histone acetyltransferase processing of a peptide substrate. Kinetic analysis of the catalytic mechanism. J Biol Chem. 2000;275:21953–21959. doi: 10.1074/jbc.M003219200. [DOI] [PubMed] [Google Scholar]
- Lee HK, Kirkwood A. AMPA receptor regulation during synaptic plasticity in hippocampus and neocortex. Seminars in Cell & Developmental Biology. 2011;22:514–520. doi: 10.1016/j.semcdb.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KFH, Soares C, Béïque J-C. Tuning into diversity of homeostatic synaptic plasticity. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.03.016. [DOI] [PubMed] [Google Scholar]
- Lee MCM, Yasuda RR, Ehlers MDM. Metaplasticity at single glutamatergic synapses. Neuron. 2010;66:859–870. doi: 10.1016/j.neuron.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, Sweatt JD. Epigenetic mechanisms: a common theme in vertebrate and invertebrate memory formation. Cell Mol Life Sci. 2006;63:1009–1016. doi: 10.1007/s00018-006-6026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, Choi S, Lee SY, Cao YA, Ahn HJ, Worley KC, Pizzi M, Liou HC, Sweatt JD. A bioinformatics analysis of memory consolidation reveals involvement of the transcription factor c-rel. J Neurosci. 2004a;24:3933–3943. doi: 10.1523/JNEUROSCI.5646-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004b;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- Levenson JM, Roth TL, Lubin FD, Miller CA, Huang IC, Desai P, Malone LM, Sweatt JD. Evidence That DNA (Cytosine-5) Methyltransferase Regulates Synaptic Plasticity in the Hippocampus. Journal of Biological Chemistry. 2006;281:15763–15773. doi: 10.1074/jbc.M511767200. [DOI] [PubMed] [Google Scholar]
- Levenson JM, Sweatt JD. Epigenetic mechanisms in memory formation. Nat Rev Neurosci. 2005;6:108–118. doi: 10.1038/nrn1604. [DOI] [PubMed] [Google Scholar]
- Li W, Pozzo-Miller L. BDNF deregulation in Rett syndrome. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wei W, Ratnu VS, Bredy TW. On the potential role of active DNA demethylation in establishing epigenetic states associated with neural plasticity and memory. Neurobiol Learn Mem. 2013 doi: 10.1016/j.nlm.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Lindskog M, Li L, Groth RD, Poburko D, Thiagarajan TC, Han X, Tsien RW. Postsynaptic GluA1 enables acute retrograde enhancement of presynaptic function to coordinate adaptation to synaptic inactivity. Proceedings of the National Academy of Sciences. 2010;107:21806–21811. doi: 10.1073/pnas.1016399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky RH. Epigenetic mechanisms regulating learning and long-term memory. International Journal of Developmental Neuroscience: the Official Journal of the International Society for Developmental Neuroscience. 2013;31:353–358. doi: 10.1016/j.ijdevneu.2012.10.110. [DOI] [PubMed] [Google Scholar]
- Lister R, Mukamel EA, Nery JR, Urich M, Puddifoot CA, Johnson ND, Lucero J, Huang Y, Dwork AJ, Schultz MD, et al. Global epigenomic reconfiguration during mammalian brain development. Science. 2013;341:1237905. doi: 10.1126/science.1237905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QR, Lu L, Zhu XG, Gong JP, Shaham Y, Uhl GR. Rodent BDNF genes, novel promoters, novel splice variants, and regulation by cocaine. Brain Res. 2006;1067:1–12. doi: 10.1016/j.brainres.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Lopez de Armentia M, Jancic D, Olivares R, Alarcon JM, Kandel ER, Barco A. cAMP response element-binding protein-mediated gene expression increases the intrinsic excitability of CA1 pyramidal neurons. J Neurosci. 2007;27:13909–13918. doi: 10.1523/JNEUROSCI.3850-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin FD. Epileptogenesis: can the science of epigenetics give us answers? Epilepsy Curr. 2012;12:105–110. doi: 10.5698/1535-7511-12.3.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G. Memory enhancement: the search for mechanism-based drugs. Nat Neurosci. 2002;5(Suppl):1035–1038. doi: 10.1038/nn935. [DOI] [PubMed] [Google Scholar]
- Lynch G, Muller D, Seubert P, Larson J. Long-term potentiation: persisting problems and recent results. Brain Res Bull. 1988;21:363–372. doi: 10.1016/0361-9230(88)90148-7. [DOI] [PubMed] [Google Scholar]
- Lynch G, Rex CS, Chen LY, Gall CM. The substrates of memory: defects, treatments, and enhancement. European Journal of Pharmacology. 2008;585:2–13. doi: 10.1016/j.ejphar.2007.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch GG, Rex CSC, Gall CMC. LTP consolidation: substrates, explanatory power, and functional significance. Neuropharmacology. 2007;52:12–23. doi: 10.1016/j.neuropharm.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Lynch G, Kramár EA, Babayan AH, Rumbaugh G, Gall CM. Differences between synaptic plasticity thresholds result in new timing rules for maximizing long-term potentiation. Neuropharmacology. 2013;64:27–36. doi: 10.1016/j.neuropharm.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeder ML, Angstman JF, Richardson ME, Linder SJ, Cascio VM, Tsai SQ, Ho QH, Sander JD, Reyon D, Bernstein BE, et al. Targeted DNA demethylation and activation of endogenous genes using programmable TALE-TET1 fusion proteins. Nat Biotechnol. 2013;31:1137–1142. doi: 10.1038/nbt.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Turrigiano GG. Multiple modes of network homeostasis in visual cortical layer 2/3. J Neurosci. 2008;28:4377–4384. doi: 10.1523/JNEUROSCI.5298-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic lh normalizes temporal summation in hippocampal CA1 neurons. Nat Neurosci. 1999;2:508–514. doi: 10.1038/9158. [DOI] [PubMed] [Google Scholar]
- Malenka RCR, Nicoll RAR. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends Neurosci. 1993;16:521–527. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Maren S. Long-term potentiation in the amygdala: a mechanism for emotional learning and memory. Trends Neurosci. 1999;22:561–567. doi: 10.1016/s0166-2236(99)01465-4. [DOI] [PubMed] [Google Scholar]
- Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- Marty S, Wehrlé R, Sotelo C. Neuronal activity and brain-derived neurotrophic factor regulate the density of inhibitory synapses in organotypic slice cultures of postnatal hippocampus. J Neurosci. 2000;20:8087–8095. doi: 10.1523/JNEUROSCI.20-21-08087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T, Duclot F, Meunier J, Naert G, Givalois L, Meffre J, Célérier A, Jacquet C, Copois V, Mechti N, et al. Altered Memory Capacities and Response to Stress in p300/CBP-Associated Factor (PCAF) Histone Acetylase Knockout Mice. Neuropsychopharmacology. 2008;33:1584–1602. doi: 10.1038/sj.npp.1301551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland S, Flynn C, Dubé C, Richichi C, Zha Q, Ghestem A, Esclapez M, Bernard C, Baram TZ. Neuron-restrictive silencer factor-mediated hyperpolarization-activated cyclic nucleotide gated channelopathy in experimental temporal lobe epilepsy. Ann Neurol. 2011;70:454–465. doi: 10.1002/ana.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Neuroplasticity Mediated by Altered Gene Expression. Neuropsychopharmacology. 2008;33:3–17. doi: 10.1038/sj.npp.1301544. [DOI] [PubMed] [Google Scholar]
- McClung CAC, Nestler EJE. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat Neurosci. 2003;6:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62:3–12. doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BM, Matthews EA, Oliveira FA, Disterhoft JF. Intrinsic neuronal excitability is reversibly altered by a single experience in fear conditioning. J Neurophysiol. 2009;102:2763–2770. doi: 10.1152/jn.00347.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendenhall EM, Williamson KE, Reyon D, Zou JY, Ram O, Joung JK, Bernstein BE. Locus-specific editing of histone modifications at endogenous enhancers. Nat Biotechnol. 2013;31:1133–1136. doi: 10.1038/nbt.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Miller CA, Campbell SL, Sweatt JD. DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity. Neurobiol Learn Mem. 2008;89:599–603. doi: 10.1016/j.nlm.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Gavin CF, White JA, Parrish RR, Honasoge A, Yancey CR, Rivera IM, Rubio MD, Rumbaugh G, Sweatt JD. Cortical DNA methylation maintains remote memory. Nat Neurosci. 2010;13:664–666. doi: 10.1038/nn.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A, Mitra SS, Tsien RW. Heterogeneous reallocation of presynaptic efficacy in recurrent excitatory circuits adapting to inactivity. Nat Neurosci. 2012;15:250–257. doi: 10.1038/nn.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K, Dempster E, Mill J, Giese KP. Long-lasting regulation of hippocampal Bdnf gene transcription after contextual fear conditioning. Genes Brain Behav. 2012;11:651–659. doi: 10.1111/j.1601-183X.2012.00805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motanis H, Maroun M, Barkai E. Learning-Induced Bidirectional Plasticity of Intrinsic Neuronal Excitability Reflects the Valence of the Outcome. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs394. [DOI] [PubMed] [Google Scholar]
- Moyer JRJ, Thompson LTL, Disterhoft JFJ. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J Neurosci. 1996;16:5536–5546. doi: 10.1523/JNEUROSCI.16-17-05536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozzachiodi R, Byrne JH. More than synaptic plasticity: role of nonsynaptic plasticity in learning and memory. Trends Neurosci. 2010;33:17–26. doi: 10.1016/j.tins.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzaffar S, Jeremy JY, Sparatore A, Del Soldato P, Angelini GD, Shukla N. H2S-donating sildenafil (ACS6) inhibits superoxide formation and gp91phox expression in arterial endothelial cells: role of protein kinases A and G. British Journal of Pharmacology. 2009;155:984–994. doi: 10.1038/bjp.2008.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na ES, Nelson ED, Kavalali ET, Monteggia LM. The Impact of MeCP2 Loss- or Gain-of-Function on Synaptic Plasticity. Neuropsychopharmacology. 2013;38:212–219. doi: 10.1038/npp.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataraj K, Le Roux N, Nahmani M, Lefort S, Turrigiano G. Visual deprivation suppresses L5 pyramidal neuron excitability by preventing the induction of intrinsic plasticity. Neuron. 2010;68:750–762. doi: 10.1016/j.neuron.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ED, Kavalali ET, Monteggia LM. Activity-Dependent Suppression of Miniature Neurotransmission through the Regulation of DNA Methylation. J Neurosci. 2008;28:395–406. doi: 10.1523/JNEUROSCI.3796-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB, Turrigiano GG. Strength through diversity. Neuron. 2008;60:477–482. doi: 10.1016/j.neuron.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MM, Kuo AG, Wu WW, Sametsky EA, Disterhoft JF. Watermaze learning enhances excitability of CA1 pyramidal neurons. J Neurophysiol. 2003;90:2171–2179. doi: 10.1152/jn.01177.2002. [DOI] [PubMed] [Google Scholar]
- Oh MM, McKay BM, Power JM, Disterhoft JF. Learning-related postburst afterhyperpolarization reduction in CA1 pyramidal neurons is mediated by protein kinase A. Proceedings of the National Academy of Sciences. 2009;106:1620–1625. doi: 10.1073/pnas.0807708106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Sametsky EA, Silva AJ, Disterhoft JF. Differential effects of αCaMKII mutation on hippocampal learning and changes in intrinsic neuronal excitability. European Journal of Neuroscience. 2006;23:2235–2240. doi: 10.1111/j.1460-9568.2006.04746.x. [DOI] [PubMed] [Google Scholar]
- Oike Y, Hata A, Mamiya T, Kaname T, Noda Y, Suzuki M, Yasue H, Nabeshima T, Araki K, Yamamura K. Truncated CBP protein leads to classical Rubinstein-Taybi syndrome phenotypes in mice: implications for a dominant-negative mechanism. Hum Mol Genet. 1999;8:387–396. doi: 10.1093/hmg/8.3.387. [DOI] [PubMed] [Google Scholar]
- Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999a;99:247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- Okano M, Takebayashi S, Okumura K, Li E. Assignment of cytosine-5 DNA methyltransferases Dnmt3a and Dnmt3b to mouse chromosome bands 12A2-A3 and 2H1 by in situ hybridization. Cytogenet Cell Genet. 1999b;86:333–334. doi: 10.1159/000015331. [DOI] [PubMed] [Google Scholar]
- Oliveira AMM, Estévez MA, Hawk JD, Grimes S, Brindle PK, Abel T. Subregion-specific p300 conditional knock-out mice exhibit long-term memory impairments. Learning & Memory. 2011;18:161–169. doi: 10.1101/lm.1939811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi SKT, Bestor TH. The colorful history of active DNA demethylation. Cell. 2008;133:1145–1148. doi: 10.1016/j.cell.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- Park CS, Rehrauer H, Mansuy IM. Genome-wide analysis of H4K5 acetylation associated with fear memory in mice. BMC Genomics. 2013;14:539. doi: 10.1186/1471-2164-14-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedarzani P, Storm JF. Dopamine modulates the slow Ca(2+)-activated K+ current IAHP via cyclic AMP-dependent protein kinase in hippocampal neurons. J Neurophysiol. 1995;74:2749–2753. doi: 10.1152/jn.1995.74.6.2749. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Storm JF. Pka mediates the effects of monoamine transmitters on the K+ current underlying the slow spike frequency adaptation in hippocampal neurons. Neuron. 1993;11:1023–1035. doi: 10.1016/0896-6273(93)90216-e. [DOI] [PubMed] [Google Scholar]
- Peng YR, Hou ZH, Yu X. The kinase activity of EphA4 mediates homeostatic scaling-down of synaptic strength via activation of Cdk5. Neuropharmacology. 2013;65:232–243. doi: 10.1016/j.neuropharm.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Peng YR, Zeng SY, Song HL, Li MY, Yamada MK, Yu X. Postsynaptic spiking homeostatically induces cell-autonomous regulation of inhibitory inputs via retrograde signaling. J Neurosci. 2010;30:16220–16231. doi: 10.1523/JNEUROSCI.3085-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Kandel ER. In search of general mechanisms for long-lasting plasticity: Aplysia and the hippocampus. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2003;358:757–763. doi: 10.1098/rstb.2002.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolos NP, Migliore M, Johnston D. Pharmacological upregulation of h-channels reduces the excitability of pyramidal neuron dendrites. Nat Neurosci. 2002;5:767–774. doi: 10.1038/nn891. [DOI] [PubMed] [Google Scholar]
- Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66:337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi F, Zhou Y, Xiao Y, Tao J, Gu J, Jiang X, Xu GY. Promoter demethylation of cystathionine-β-synthetase gene contributes to inflammatory pain in rats. Pain. 2013;154:34–45. doi: 10.1016/j.pain.2012.07.031. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Sylwestrak EL, Lieberman DN, Zhang Y, Liu XY, Ghosh A. The Rett syndrome protein MeCP2 regulates synaptic scaling. J Neurosci. 2012;32:989–994. doi: 10.1523/JNEUROSCI.0175-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queenan BN, Lee KJ, Pak DTS. Wherefore art thou, homeo(stasis)? Functional diversity in homeostatic synaptic plasticity. Neural Plasticity. 2012;2012:718203. doi: 10.1155/2012/718203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi IA, Mehler MF. Epigenetic mechanisms underlying human epileptic disorders and the process of epileptogenesis. Neurobiol Dis. 2010;39:53–60. doi: 10.1016/j.nbd.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn EJ, Guzman-Karlsson MC, David Sweatt J. Cellular, molecular, and epigenetic mechanisms in non-associative conditioning: Implications for pain and memory. Neurobiol Learn Mem. 2013;105:133–150. doi: 10.1016/j.nlm.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakyan VK, Preis J, Morgan HD, Whitelaw E. The marks, mechanisms and memory of epigenetic states in mammals. Biochem J. 2001;356:1–10. doi: 10.1042/0264-6021:3560001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannals MD, Kapur J. Homeostatic strengthening of inhibitory synapses is mediated by the accumulation of GABA(A) receptors. J Neurosci. 2011;31:17701–17712. doi: 10.1523/JNEUROSCI.4476-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, Ressler KJ. Brain-derived neurotrophic factor in amygdala-dependent learning. Neuroscientist. 2005;11:323–333. doi: 10.1177/1073858404272255. [DOI] [PubMed] [Google Scholar]
- Rea S, Eisenhaber F, O’Carroll D, Strahl BD, Sun ZW, Schmid M, Opravil S, Mechtler K, Ponting CP, Allis CD, et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406:593–599. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- Remme MWH, Wadman WJ. Homeostatic scaling of excitability in recurrent neural networks. PLoS Comp Biol. 2012;8:e1002494. doi: 10.1371/journal.pcbi.1002494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Gavin CF, Rubio MD, Kramár EA, Chen LY, Jia Y, Huganir RL, Muzyczka N, Gall CM, Miller CA, et al. Myosin IIb regulates actin dynamics during synaptic plasticity and memory formation. Neuron. 2010;67:603–617. doi: 10.1016/j.neuron.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Sweatt JD. A biochemical blueprint for long-term memory. Learn Mem. 1999;6:381–388. [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, English JD, Adams JP, Selcher JC, Kondratick C, Sweatt JD. The mitogen-activated protein kinase cascade couples PKA and PKC to cAMP response element binding protein phosphorylation in area CA1 of hippocampus. J Neurosci. 1999;19:4337–4348. doi: 10.1523/JNEUROSCI.19-11-04337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Roopra A, Huang Y, Dingledine R. Neurological disease: listening to gene silencers. Mol Interv. 2001;1:219–228. [PubMed] [Google Scholar]
- Roopra A, Dingledine R, Hsieh J. Epigenetics and epilepsy. Epilepsia. 2012;53(Suppl 9):2–10. doi: 10.1111/epi.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature. 2002;417:282–287. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sweatt JD. Regulation of chromatin structure in memory formation. Curr Opin Neurobiol. 2009;19:336–342. doi: 10.1016/j.conb.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth-Alpermann C, Morris RGM, Korte M, Bonhoeffer T. Homeostatic shutdown of long-term potentiation in the adult hippocampus. Proc Natl Acad Sci USa. 2006;103:11039–11044. doi: 10.1073/pnas.0600894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford LC, Nelson SB, Turrigiano GG. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron. 1998;21:521–530. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- Saar D, Grossman Y, Barkai E. Reduced after-hyperpolarization in rat piriform cortex pyramidal neurons is associated with increased learning capability during operant conditioning. European Journal of Neuroscience. 1998;10:1518–1523. doi: 10.1046/j.1460-9568.1998.00149.x. [DOI] [PubMed] [Google Scholar]
- Sah P, Bekkers JM. Apical dendritic location of slow afterhyperpolarization current in hippocampal pyramidal neurons: implications for the integration of long-term potentiation. J Neurosci. 1996;16:4537–4542. doi: 10.1523/JNEUROSCI.16-15-04537.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha RN, Pahan K. HATs and HDACs in neurodegeneration: a tale of disconcerted acetylation homeostasis. Cell Death Differ. 2006;13:539–550. doi: 10.1038/sj.cdd.4401769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Karelina K, Obrietan K. CREB: a multifaceted regulator of neuronal plasticity and protection. J Neurochem. 2011;116:1–9. doi: 10.1111/j.1471-4159.2010.07080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Quirk GJ, Porter JT. Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J Neurosci. 2008;28:4028–4036. doi: 10.1523/JNEUROSCI.2623-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Chen S, Lüthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci. 2000;20:5264–5275. doi: 10.1523/JNEUROSCI.20-14-05264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Lee JY, Englot DJ, Gildersleeve S, Piskorowski RA, Siegelbaum SA, Winawer MR, Blumenfeld H. Increased seizure severity and seizure-related death in mice lacking HCN1 channels. Epilepsia. 2010;51:1624–1627. doi: 10.1111/j.1528-1167.2010.02554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG, Gusev PA, Tomsic D, Alkon DL, Shi T. Intracellular correlates of acquisition and long-term memory of classical conditioning in Purkinje cell dendrites in slices of rabbit cerebellar lobule HVI. J Neurosci. 1998;18:5498–5507. doi: 10.1523/JNEUROSCI.18-14-05498.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg DP, Feliu-Mojer M, Gaiottino J, Pak DTS, Sheng M. Critical role of CDK5 and Polo-like kinase 2 in homeostatic synaptic plasticity during elevated activity. Neuron. 2008;58:571–583. doi: 10.1016/j.neuron.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal M, Song C, Ehlers VL, Moyer JR. Learning to learn - Intrinsic plasticity as a metaplasticity mechanism for memory formation. Neurobiol Learn Mem. 2013;105:186–199. doi: 10.1016/j.nlm.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selcher JC, Weeber EJ, Varga AW, Sweatt JD, Swank M. Protein kinase signal transduction cascades in mammalian associative conditioning. Neuroscientist. 2002;8:122–131. doi: 10.1177/107385840200800208. [DOI] [PubMed] [Google Scholar]
- Shao JL, Wan XH, Chen Y, Bi C, Chen HM, Zhong Y, Heng XH, Qian JQ. H2S protects hippocampal neurons from anoxia-reoxygenation through cAMP-mediated PI3K/Akt/p70S6K cell-survival signaling pathways. J Mol Neurosci. 2011;43:453–460. doi: 10.1007/s12031-010-9464-4. [DOI] [PubMed] [Google Scholar]
- Shim DJ, Yang L, Reed JG, Noebels JL, Chiao PJ, Zheng H. Disruption of the NF-κB/IκBα Autoinhibitory Loop Improves Cognitive Performance and Promotes Hyperexcitability of Hippocampal Neurons. Mol Neurodegener. 2011;6:42. doi: 10.1186/1750-1326-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SM, Zhang N, Hansen J, Gerges NZ, Pak DTS, Sheng M, Lee SH. GKAP orchestrates activity-dependent postsynaptic protein remodeling and homeostatic scaling. Nat Neurosci. 2012;15:1655–1666. doi: 10.1038/nn.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddoway B, Hou H, Xia H. Neuropharmacology. Neuropharmacology. 2013:1–7. [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Zhou Y, Rogerson T, Shobe J, Balaji J. Molecular and cellular approaches to memory allocation in neural circuits. Science. 2009;326:391–395. doi: 10.1126/science.1174519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindreu CB, Scheiner ZS, Storm DR. Ca2+-stimulated adenylyl cyclases regulate ERK-dependent activation of MSK1 during fear conditioning. Neuron. 2007;53:79–89. doi: 10.1016/j.neuron.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HS. Hydrogen sulfide’s involvement in modulating nociception. Pain Physician. 2009;12:901–910. [PubMed] [Google Scholar]
- Soden ME, Chen L. Fragile X protein FMRP is required for homeostatic plasticity and regulation of synaptic strength by retinoic acid. J Neurosci. 2010;30:16910–16921. doi: 10.1523/JNEUROSCI.3660-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloaga A, Thomson S, Wiggin GR, Rampersaud N, Dyson MH, Hazzalin CA, Mahadevan LC, Arthur JSC. MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14. Embo J. 2003;22:2788–2797. doi: 10.1093/emboj/cdg273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford JM, Raybuck JD, Ryabinin AE, Lattal KM. Increasing histone acetylation in the hippocampus-infralimbic network enhances fear extinction. Biol Psychiatry. 2012;72:25–33. doi: 10.1016/j.biopsych.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli U, Rogers G, Lynch G. Facilitation of glutamate receptors enhances memory. Proc Natl Acad Sci USa. 1994;91:777–781. doi: 10.1073/pnas.91.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. Progress in Brain Research. Elsevier; 1990. Chapter 12 Potassium currents in hippocampal pyramidal cells; pp. 161–187. [DOI] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Sui L, Wang Y, Ju LH, Chen M. Epigenetic regulation of reelin and brain-derived neurotrophic factor genes in long-term potentiation in rat medial prefrontal cortex. Neurobiol Learn Mem. 2012;97:425–440. doi: 10.1016/j.nlm.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Sultan FA, Wang J, Tront J, Liebermann DA, Sweatt JD. Genetic deletion of Gadd45b, a regulator of active DNA demethylation, enhances long-term memory and synaptic plasticity. J Neurosci. 2012;32:17059–17066. doi: 10.1523/JNEUROSCI.1747-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Turrigiano GG. PSD-95 and PSD-93 play critical but distinct roles in synaptic scaling up and down. J Neurosci. 2011;31:6800–6808. doi: 10.1523/JNEUROSCI.5616-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanwick CC, Murthy NR, Kapur J. Activity-dependent scaling of GABAergic synapse strength is regulated by brain-derived neurotrophic factor. Mol Cell Neurosci. 2006;31:481–492. doi: 10.1016/j.mcn.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Neuroscience. Epigenetics and cognitive aging. Science. 2010;328:701–702. doi: 10.1126/science.1189968. [DOI] [PubMed] [Google Scholar]
- Sweatt JD, Meaney MJ, Nestler EJ, Akbarian S. Epigenetic Regulation in the Nervous System: Basic Mechanisms and Clinical Impact. 2012. [Google Scholar]
- Tanner KG, Langer MR, Denu JM. Kinetic mechanism of human histone acetyltransferase P/CAF. Biochemistry. 2000a;39:11961–11969. doi: 10.1021/bi001272h. [DOI] [PubMed] [Google Scholar]
- Tanner KG, Langer MR, Kim Y, Denu JM. Kinetic mechanism of the histone acetyltransferase GCN5 from yeast. J Biol Chem. 2000b;275:22048–22055. doi: 10.1074/jbc.M002893200. [DOI] [PubMed] [Google Scholar]
- Tanner KG, Trievel RC, Kuo MH, Howard RM, Berger SL, Allis CD, Marmorstein R, Denu JM. Catalytic mechanism and function of invariant glutamic acid 173 from the histone acetyltransferase GCN5 transcriptional coactivator. J Biol Chem. 1999;274:18157–18160. doi: 10.1074/jbc.274.26.18157. [DOI] [PubMed] [Google Scholar]
- Tetzlaff C, Kolodziejski C, Timme M, Wörgötter F. Synaptic scaling in combination with many generic plasticity mechanisms stabilizes circuit connectivity. Front Comput Neurosci. 2011;5:47. doi: 10.3389/fncom.2011.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzlaff C, Kolodziejski C, Timme M, Wörgötter F. Analysis of synaptic scaling in combination with hebbian plasticity in several simple networks. Front Comput Neurosci. 2012;6:36. doi: 10.3389/fncom.2012.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Malgaroli A, Tsien RW. LTP and adaptation to inactivity: overlapping mechanisms and implications for metaplasticity. Neuropharmacology. 2007;52:156–175. doi: 10.1016/j.neuropharm.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Moyer JR, Disterhoft JF. Transient changes in excitability of rabbit CA3 neurons with a time course appropriate to support memory consolidation. J Neurophysiol. 1996;76:1836–1849. doi: 10.1152/jn.1996.76.3.1836. [DOI] [PubMed] [Google Scholar]
- Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, Persson H. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10:475–489. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- Tully T, Bourtchouladze R, Scott R, Tallman J. Targeting the CREB pathway for memory enhancers. Nat Rev Drug Discov. 2003;2:267–277. doi: 10.1038/nrd1061. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Uchida H, Sasaki K, Ma L, Ueda H. Neuron-restrictive silencer factor causes epigenetic silencing of Kv4.3 gene after peripheral nerve injury. Neuroscience. 2010a;166:1–4. doi: 10.1016/j.neuroscience.2009.12.021. [DOI] [PubMed] [Google Scholar]
- Uchida H, Ma L, Ueda H. Epigenetic gene silencing underlies C-fiber dysfunctions in neuropathic pain. J Neurosci. 2010b;30:4806–4814. doi: 10.1523/JNEUROSCI.5541-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S, Hara K, Kobayashi A, Otsuki K, Yamagata H, Hobara T, Suzuki T, Miyata N, Watanabe Y. Epigenetic status of Gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events. Neuron. 2011;69:359–372. doi: 10.1016/j.neuron.2010.12.023. [DOI] [PubMed] [Google Scholar]
- Valor LM, Pulopulos MM, Jimenez-Minchan M, Olivares R, Lutz B, Barco A. Ablation of CBP in forebrain principal neurons causes modest memory and transcriptional defects and a dramatic reduction of histone acetylation but does not affect cell viability. J Neurosci. 2011;31:1652–1663. doi: 10.1523/JNEUROSCI.4737-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga-Weisz PD, Becker PB. Chromatin-remodeling factors: machines that regulate? Curr Opin Cell Biol. 1998;10:346–353. doi: 10.1016/s0955-0674(98)80010-0. [DOI] [PubMed] [Google Scholar]
- Varga-Weisz PD, Bonte EJ, Becker PB. Analysis of modulators of chromatin structure in Drosophila. Methods in Enzymology. 1999;304:742–757. doi: 10.1016/s0076-6879(99)04045-8. [DOI] [PubMed] [Google Scholar]
- Varley KE, Gertz J, Bowling KM, Parker SL, Reddy TE, Pauli-Behn F, Cross MK, Williams BA, Stamatoyannopoulos JA, Crawford GE, et al. Dynamic DNA methylation across diverse human cell lines and tissues. Genome Res. 2013;23:555–567. doi: 10.1101/gr.147942.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, Cabrera SM, McDonough CB, Brindle PK, Abel T, et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci. 2007;27:6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viosca J, Lopez de Armentia M, Jancic D, Barco A. Enhanced CREB-dependent gene expression increases the excitability of neurons in the basal amygdala and primes the consolidation of contextual and cued fear memory. Learning & Memory. 2009;16:193–197. doi: 10.1101/lm.1254209. [DOI] [PubMed] [Google Scholar]
- Vo N, Goodman RH. CREB-binding protein and p300 in transcriptional regulation. J Biol Chem. 2001;276:13505–13508. doi: 10.1074/jbc.R000025200. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang M. The role of Ca2-stimulated adenylyl cyclases in bidirectional synaptic plasticity and brain function. Rev Neurosci. 2012;23:67–78. doi: 10.1515/revneuro-2011-0063. [DOI] [PubMed] [Google Scholar]
- Wang HL, Zhang Z, Hintze M, Chen L. Decrease in calcium concentration triggers neuronal retinoic acid synthesis during homeostatic synaptic plasticity. J Neurosci. 2011;31:17764–17771. doi: 10.1523/JNEUROSCI.3964-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, Aghajanian GK. Excitation of locus coeruleus neurons by an adenosine 3″,5-″ cyclic monophosphate-activated inward current: extracellular and intracellular studies in rat brain slices. Synapse. 1987;1:481–487. doi: 10.1002/syn.890010512. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Bauer EP, LeDoux JE. L-type voltage-gated calcium channels mediate NMDA-independent associative long-term potentiation at thalamic input synapses to the amygdala. J Neurosci. 1999;19:10512–10519. doi: 10.1523/JNEUROSCI.19-23-10512.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenner P. Mechanisms of GABAergic homeostatic plasticity. Neural Plasticity. 2011;2011:489470. doi: 10.1155/2011/489470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CJ, Ibata K, Turrigiano GG. Postsynaptic expression of homeostatic plasticity at neocortical synapses. J Neurosci. 2005;25:2895–2905. doi: 10.1523/JNEUROSCI.5217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingale HL, Gardiner NJ, McLymont N, Giblett S, Grubb BD. Prostanoids synthesized by cyclo-oxygenase isoforms in rat spinal cord and their contribution to the development of neuronal hyperexcitability. British Journal of Pharmacology. 1997;122:1593–1604. doi: 10.1038/sj.bjp.0701548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Brown RAM, Talton LE, Silva AJ. New circuits for old memories: the role of the neocortex in consolidation. Neuron. 2004;44:101–108. doi: 10.1016/j.neuron.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Won J, Silva AJ. Molecular and cellular mechanisms of memory allocation in neuronetworks. Neurobiol Learn Mem. 2008;89:285–292. doi: 10.1016/j.nlm.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MA, Kaplan MP, Park A, Blanchard EJ, Oliveira AMM, Lombardi TL, Abel T. Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage. Learn Mem. 2005;12:111–119. doi: 10.1101/lm.86605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GY, Deisseroth K, Tsien RW. Activity-dependent CREB phosphorylation: convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. Proc Natl Acad Sci USa. 2001;98:2808–2813. doi: 10.1073/pnas.051634198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SC, Zhang Y. Active DNA demethylation: many roads lead to Rome. Nat Rev Mol Cell Biol. 2010;11:607–620. doi: 10.1038/nrm2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Barr CL, Kim A, Yue F, Lee AY, Eubanks J, Dempster EL, Ren B. Base-resolution analyses of sequence and parent-of-origin dependent DNA methylation in the mouse genome. Cell. 2012;148:816–831. doi: 10.1016/j.cell.2011.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu GY, Winston JH, Shenoy M, Zhou S, Chen JDZ, Pasricha PJ. The endogenous hydrogen sulfide producing enzyme cystathionine-beta synthase contributes to visceral hypersensitivity in a rat model of irritable bowel syndrome. Mol Pain. 2009;5:44. doi: 10.1186/1744-8069-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kang N, Jiang L, Nedergaard M, Kang J. Activity-dependent long-term potentiation of intrinsic excitability in hippocampal CA1 pyramidal neurons. J Neurosci. 2005;25:1750–1760. doi: 10.1523/JNEUROSCI.4217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh SH, Lin CH, Gean PW. Acetylation of nuclear factor-kappaB in rat amygdala improves long-term but not short-term retention of fear memory. Mol Pharmacol. 2004;65:1286–1292. doi: 10.1124/mol.65.5.1286. [DOI] [PubMed] [Google Scholar]
- Yu M, Hon GC, Szulwach KE, Song CX, Zhang L, Kim A, Li X, Dai Q, Shen Y, Park B, et al. Base-resolution analysis of 5-hydroxymethylcytosine in the mammalian genome. Cell. 2012;149:1368–1380. doi: 10.1016/j.cell.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelcer I, Cohen H, Richter-Levin G, Lebiosn T, Grossberger T, Barkai E. A cellular correlate of learning-induced metaplasticity in the hippocampus. Cereb Cortex. 2006;16:460–468. doi: 10.1093/cercor/bhi125. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Tan M, Kohyama J, Sneddon M, Watson JB, Sun YE, Xie CW. Epigenetic enhancement of BDNF signaling rescues synaptic plasticity in aging. J Neurosci. 2011;31:17800–17810. doi: 10.1523/JNEUROSCI.3878-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J, Neve R, Poirazi P, Silva AJ. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat Neurosci. 2009;12:1438–1443. doi: 10.1038/nn.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XY, Huang CS, Li Q, Chang RM, Song ZB, Zou WY, Guo QL. p300 exerts an epigenetic role in chronic neuropathic pain through its acetyltransferase activity in rats following chronic constriction injury (CCI) Mol Pain. 2012;8:84. doi: 10.1186/1744-8069-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]