Abstract
Olfactory sensory neurons that express transient receptor potential channel M5 (TrpM5) or neurotrophin-3 (NT-3) project to defined clusters of glomeruli situated ventrally in the main olfactory bulb. Using genetically labeled mice, we investigated whether expression of NT-3-driven βgal and TrpM5-driven GFP marked overlapping sets of glomeruli and whether expression of these markers was coordinated. Our results indicate that these markers largely characterize independent sets of olfactory sensory neuron axons and glomeruli. Further, in glomeruli in which both TrpM5-GFP and NT-3-βgal labeled axons occur, they are expressed independently. The nature of staining for these two markers also differs within glomeruli. Within each labeled TrpM5-positive glomerulus, the level of TrpM5-GFP expression was similar throughout the glomerular neuropil. In contrast, NT-3-driven βgal expression levels are heterogeneous even within heavily labeled glomeruli. In addition, a population of very small TrpM5-GFP positive glomeruli is apparent while no similar populations of NT-3-βgal glomeruli are evident. Taken together, these data suggest that TrpM5 and NT-3 characterize two largely independent receptor populations both conveying odorant information to the ventral olfactory bulb.
Introduction
The olfactory system in vertebrates comprises an array of olfactory sensory neurons (OSNs) scattered across the olfactory epithelia each extending a single axon into the olfactory bulb. Each OSN expresses one, or a very few, olfactory receptor molecules which dictate the chemical responsiveness of the particular OSN (Chess et al., 1994; Malnic et al., 1999; Rawson et al., 2000; Khan et al., 2011). OSNs expressing identical receptor proteins, although spaced widely in the epithelium, target one or two glomeruli within the olfactory bulb with the general area of termination being dictated by the particular receptor molecule being expressed by the OSN (Mombaerts et al., 1996, Feinstein and Mombaerts, 2004). While the final coalescence of axons into a glomerulus depends heavily on receptor expression, the initial targeting of the axon to the general bulbar region containing the destination glomerulus relies on many extracellular guidance factors, only some of which are known (Walz et al., 2002; McIntyre et al., 2010; Takahashi et al., 2010). For example, axons expressing the cell adhesion molecule OCAM target caudoventral glomeruli whereas axons lacking OCAM terminate dorsolaterally. Which factors determine the expression of these guidance molecules or growth factors remains unknown (Bozza et al., 2009). Since the position of glomeruli within the olfactory bulb relates to the types of odorants to which the OSNs respond (Johnson et al., 2009; Bozza et al., 2009), understanding the factors controlling axonal targeting is crucial to understanding how the olfactory system is wired functionally.
Previous investigations in our laboratories identified two proteins, transient receptor potential channel M5 (TrpM5) and neurotrophin-3 (NT-3), which are expressed by subpopulations of OSNs that preferentially project to ventrally positioned glomeruli in the MOB (Lin et al. 2007; Vigers et al. 2003). The transduction channel TrpM5 is expressed by a subset of OSNs (Lin et al., 2007) responsive to semiochemicals. The neurotrophin NT-3 is expressed in a population of OSNs (Feron et al., 1995; Liu et al., 2013) which target a small number of ventrally-situated glomeruli (Vigers et al., 2003). The apparent overlap in location of NT-3- and TrpM5-labeled glomeruli led us to question whether expression of these markers characterized identical or independent glomerular populations.
These glomerular targets lie within the ventral glomerular field of the MOB known to process odors of semiochemicals and urine (Schaefer et al., 2002; Xu et al., 2005; Johnson et al., 2009). Mitral cells that innervate ventrally-positioned glomeruli in the MOB project axons to the medial amygdala (Pro-Sistiaga et al., 2007; Kang et al., 2009; Thompson et al., 2012) and the hypothalamus (Bader et al., 2012), higher brain structures known to process semiochemical odorant information.
The present study investigates whether OSNs expressing TrpM5 and NT-3 project to overlapping or separate populations of MOB glomeruli, i.e. whether TrpM5 and NT-3 biochemically characterize distinct olfactory subsystems, or whether they exist in populations of OSNs that target specific glomeruli. Our results show that these two markers exist in largely distinct glomeruli although some overlap exists.
Materials and Methods
Experimental Animals
All procedures performed in the current study followed NIH guidelines and were approved by the University of Colorado Denver Animal Care and Use Committee. All animals used in the current study were bred in the animal facilities at the University of Colorado Denver and housed in ventilated cages under 14-hour light/10 h dark conditions and fed ad libitum on standard chow. Transgenic TrpM5-GFP (Clapp et al., 2006) and NT-3LacZ (Vigers et al., 2003; Vigers et al., 2000) mice were utilized in the experiments of the current study. TrpM5-GFP mice were developed by R. F. Margolskee (Monell Chem. Senses Ctr., Philadelphia) and contained 5′ to 3′: 11 kb of mouse TrpM5 5′ flanking sequence, TrpM5 Exon 1 (untranslated), Intron 1, and the untranslated part of Exon 2, and eGFP (Clapp et al., 2006). Immunohistochemical analysis shows nearly total overlap of TrpM5 immunoreactivity and of GFP transgene expression in the MOE (Lin et al., 2007; Oshimoto et al., 2013). The NT-3LacZ line, β-galactosidase (βgal) translation initiation start codon replaced the NT-3 start codon and the protein coding region of NT-3 is replaced by the LacZ gene (Farinas et al., 1994). Expression of βgal in this line faithfully reproduces expression of the NT-3 native gene in all cases examined to date.
Previous investigations in our laboratories utilized these two separate transgenic mouse lines to elucidate the populations of MOB glomeruli receiving efferent connections from TrpM5-GFP expressing and NT-3-βgal expressing OSNs. Both studies concluded that predominately ventrally positioned glomeruli received these projections from OSN subpopulations. The current investigation interbred the two mouse lines to form a new transgenic line having both reporter genes, TrpM5-GFP/NT-3LacZ. The olfactory bulbs of this new transgenic line were analyzed to determine if OSNs expressing TrpM5-GFP or NT-3-βgal project to glomerular fields resembling those described previously and the relative independence of each glomerular population to one another. All animals tested were two to eight months of age. Founder mice for our TrpM5-GFP line were provided by Dr. Robert Margolskee (Monell Chemical Senses Ctr., Philadelphia, PA). Founder mice for the NT-3LacZ line were originally supplied by K.R. Jones (Univ. Colorado Boulder) but are available from the Mutant Mouse Regional Resource Centers (MMRRC; Ntf3tm1Lfr/Mmucd; Stock Number: 000191-UCD). Polymerase chain reaction (PCR) was used to genotype experimental animals.
Tissue Preparation and Immunolabeling
Adult mice of both sexes were anesthetized with pentobarbital (20μg/g Nembutal), perfused transcardially with 0.9% NaCl with 0.5USP units/ml heparin followed by phosphate buffered saline (PBS) -fixative (4% paraformaldehyde; PFA). The whole brain was harvested and postfixed for 3–4 hours in PBS-fixative. The tissue was cryoprotected in 20% sucrose overnight. The olfactory bulbs were then sectioned at 18μm in the L-plane (i.e. perpendicular to the long axis of the olfactory bulb; Schaefer et al., 2001) and mounted on Superfrost Plus slides (VWR, West Chester, PA).
For immunolabeling of GFP and βgal, sections were incubated in blocking solution (BS) containing 0.1M PBS, 150mM NaCl, 0.03% Triton X-100, 1% bovine serum albumin and 1% normal goat serum for two hours. Sections were then incubated for 72 hrs in the BS containing primary antibodies against GFP and βgal (chicken anti-GFP, AB16901, 1:2,000, Chemicon [now Millipore], Temecula, CA); guinea pig anti-βgal, 1:1,000 prepared in-house and characterized in Yee et al., 2003. Specificity of the primary antisera was established first by lack of labeling of wildtype mouse tissues, and second, by unique labeling of different cell populations in the two transgenic lines. Further, specificity of both antisera was established by testing the pair of antisera on transgenic lines bearing only a single marker and finding reactivity for only the appropriate antiserum, (e.g. immunoreaction for anti-βgal but not anti-GFP in a LacZ line, and vice versa).
Sections were washed in 0.1M PBS then incubated in BS containing secondary antibodies (Alexa 488 goat anti-chicken, 1:400, Molecular Probes; and Alexa 568 goat anti-guinea pig, 1:400, Molecular Probes) for 2 hr at room temperature. Following incubation in secondary antibodies, sections were washed twice in 0.1M PBS, incubated in 0.1M PBS containing 1:30 DAPI for 30 min, and washed one additional time in 0.1M PB. Slides were then cover-slipped with Fluoromount-G (Fisher Biotech, Birmingham, AL). Control sections were treated in the same manner but without primary antibody, which resulted in no detectable labeling. A fluorescent microscope (Nikon Eclipse E600) was used to visualize the sections and to acquire composite images for mapping.
Mapping of Immunolabeled Glomeruli in the MOB
Immunofluorescence across the glomerular layer was detected and mapped using software and protocols developed in the Restrepo Laboratory and were described previously (Salcedo et al., 2005); GLOM-MAP mapping software written for MatLab (The MathWorks, Natick, MA). This customized software allows for the identification of individual glomeruli and defining characteristics (i.e. spatial location within the glomerular layer and mean immunofluorescence intensity within the glomerular neuropil). The coordinates for each glomerulus are given as the rostrocaudal distance along the L-plane and the radial angle around the section. After immunolabeling, every fourth section was imaged and mapped through the entire L-plane of the MOB, starting from the first section with a distinguishable external plexiform layer (EPL). The 0° – 180° axis was drawn from the dorsal most aspect of the granular cell layer to its most ventral aspect. Each glomerulus was tagged by tracing its boundary with juxtaglomerular cells labeled with DAPI counterstain. Arbitrary, glomerular-sized regions of interest (ROI) drawn within the EPL, served as controls by which to measure baseline fluorescence values in each section.
The fluorescence intensities emitted from the immunolabeled reporter gene products, GFP and βgal, were measured and mapped for 23,629 glomerular profiles in 8 MOBs from 4 adult TrpM5-GFP/NT-3LacZ mice. TrpM5-GFP positive and NT-3-βgal positive glomeruli were identified as having significantly higher fluorescence values than the fluorescence values measured in the ROIs drawn in the EPL. Intensity measurements for each glomerulus were calculated by averaging the mean pixel intensities contained within the circumscribed area of the glomerular cross-section. We then subtracted from this average glomerular intensity value, the mean intensity of pixels in the closest EPL ROI to obtain an absolute glomerular intensity value. The absolute intensity value was then normalized to the brightest glomerulus in the whole mapped bulb to obtain a relative brightness value for comparison across animals. For the intensity maps, these intensities were then summed in bins of 72μm and 10°, based on the location of the glomerular cross-sections.
High Resolution Confocal Imaging
For analysis of glomerular structure, confocal images were acquired by sequential acquisition on an Olympus Fluoview laser scanning confocal microscope equipped with UPlanApo 20x (nA 0.8) and PlanApo 60x (nA 1.4) objectives. Acquisition parameters were set so that no pixels in relevant structures were fully saturated. Most images were acquired using Kalman averaging of 2 or 3 frames. For preparation of figures in this publication, some z-stack confocal images were filtered with a median filter to reduce single pixel background noise.
Results
Axonal Projections of TrpM5-GFP and NT-3-βgal Expressing OSNs
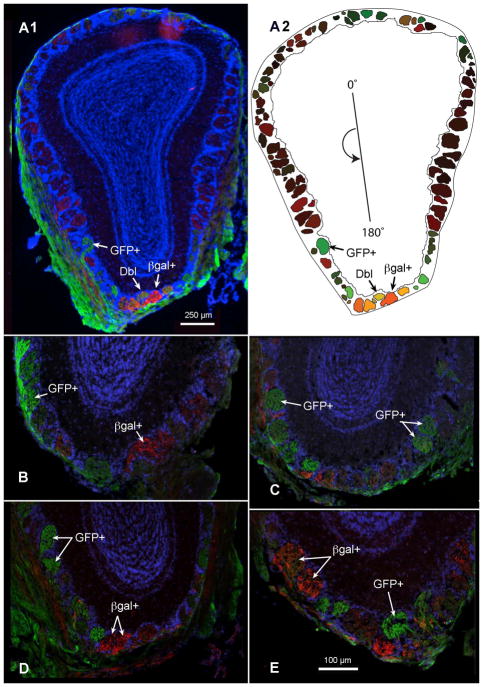
Previous investigations in our laboratories utilized two separate transgenic mouse lines to elucidate the populations of MOB glomeruli receiving input from TrpM5-GFP- and NT-3-βgal-expressing OSNs. Both studies concluded that ventrally positioned glomeruli predominately received projections from these OSN subpopulations. The current investigation combines both mouse lines to form a new transgenic line having both reporter genes, TrpM5-GFP/NT-3LacZ. The olfactory bulbs of this new transgenic line were analyzed to determine if OSNs expressing TrpM5-GFP or NT-3-βgal project to glomerular fields resembling those described previously and whether these markers are expressed independently of one another. Immunolabeled GFP and βgal positive glomeruli were easily discernable under low power magnification (4x) in MOB sections (Figure 1A). TrpM5-GFP positive and NT-3-βgal positive glomeruli were frequently observed adjacent to one another in ventral regions of the MOB (Figure 1B–E).
Figure 1.
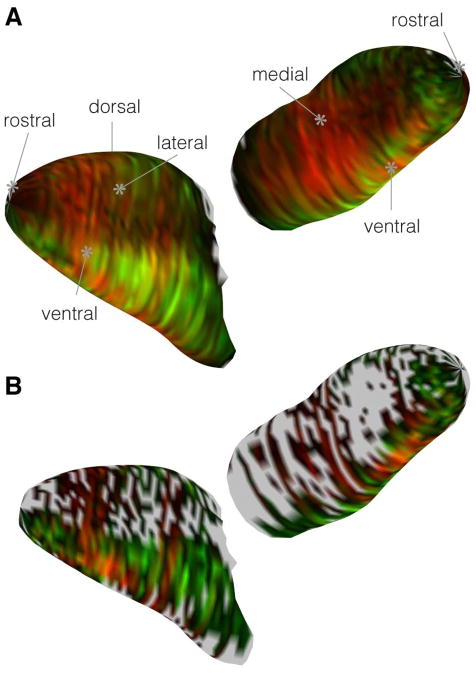
Cross-sections through the olfactory bulbs of double transgenic mice expressing TrpM5-driven GFP (GFP+, green) and NT-3-driven β-galactosidase (βgal+, red). (A1) Labeled axons for both reporter genes terminate in glomeruli throughout the glomerular layer of the MOB. Arrows indicate a few strongly labeled for the two reporters: GFP+ or βgal+; Dbl − indicates a double-labeled glomerulus. (A2) Plot of each mapped glomerulus from the bulb cross-section in (A1). The origin line in the center of the plot indicates the counter-clockwise cylindrical coordinates of each glomerulus, where 0, 90, 180, and 270° indicate dorsal, lateral, ventral, and medial, respectively. The color in each glomerulus is determined by an RGB triplet in which the red and green values correspond to the mean red and green intensities of the pixels contained within the glomerular profile, and the blue value is set to zero. (B and C) GFP+ and βgal+ glomeruli are shown in the ventral MOB of a single female mouse and (D and E) a single male mouse. Immunolabeling was performed with antibodies against GFP (green) and βgal (red), reporter proteins for TrpM5 and NT-3 expression, respectively; DAPI counterstain, blue A–E. Scale bar = 100μm.
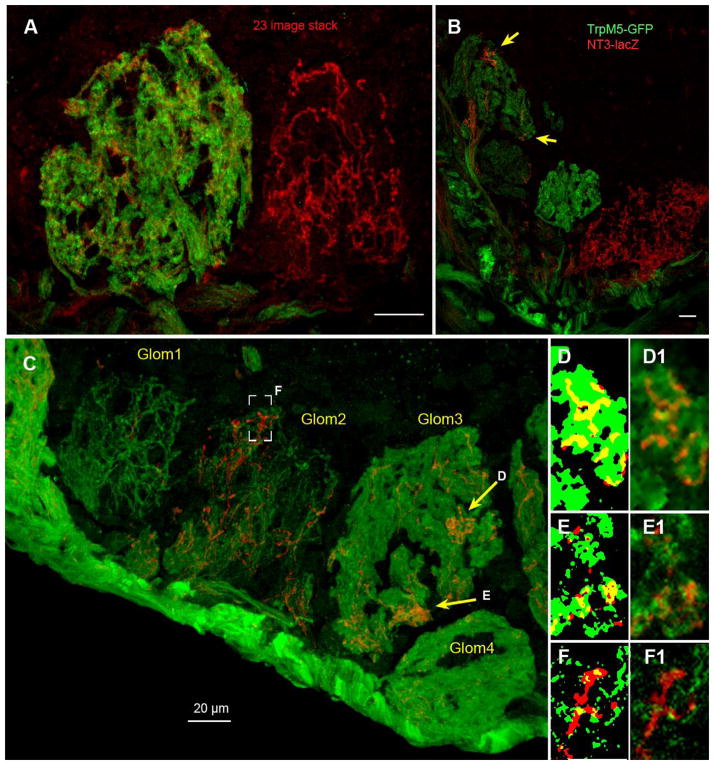
Detailed examination of the variously stained glomeruli reveals that the nature of GFP and βgal staining was qualitatively different in most glomeruli (Figure 2). In glomeruli heavily labeled for GFP (TrpM5), the stained fibers filled the glomerulus diffusely (Figure 2A,B and glomeruli 3 & 4 in Figure 2C) reminiscent of other markers of olfactory sensory cell axons, e.g. OMP. In contrast, even in glomeruli that were heavily labeled for NT-3, (Figure 2A,B), the glomerular neuropil was not completely filled by βgal-positive fibers. Rather, strongly positive fibers were distributed throughout the glomerulus, but leaving some glomerular neuropil regions void. That other olfactory axons filled these voids was evident in glomeruli labeled by both markers (e.g. left hand glomerulus of Figure 2A). In many glomeruli, the βgal-positive (NT-3) fibers filled glomerular subregions heavily while avoiding other territories in the same glomerulus (Figure 2B, yellow arrows; glom 3 in Figure 2C). Analysis of co-localization patterns in single z-plane images revealed that in heavily reactive neuropil, substantial regions of overlap occurred (Figure 2D, E) although many regions exhibited immunoreactivity for only one of the markers. Since individual olfactory axons and terminals are likely below the limits of Z-axis resolution of the confocal microscope, we cannot state definitively whether or not these markers were co-localized in individual olfactory axons – only that co-localization occurred in regions of glomerular neuropil. We do, however, see regions labeled by only one of the two markers in co-labeled glomerular neuropil, so we can conclude that even in such glomeruli, some axons express only one of the two markers.
Figure 2.
Confocal images of glomeruli in a double transgenic mouse: TrpM5-GFP/NT-3LacZ showing the variety and types of labeling observed. Scale bars = 20 μm. (A) Adjacent glomeruli in the ventral MOB can exhibit substantially different degrees of labeling. The left glomerulus shows the typical diffuse appearance of TrpM5-GFP staining (green) which is similar to that seen with OMP, i.e. when all incoming olfactory axons are labeled. In this same glomerulus, scattered NT-3-βgal fibers are evident throughout the glomerular neuropil. In contrast, the glomerulus on the right shows a moderately stained NT-3-positive glomerulus in which immunoreactive fibers appear as a bright network, suggesting that only some of the incoming axons exhibit immunoreactivity. Z-stack depth = 9.9 μm. (B) Three glomeruli in the ventromedial part of the MOB showing different degrees of labeling with the two markers. The glomerulus at upper left shows diffuse TrpM5-GFP label, but patchy distribution of fibers labeled for NT-3-βgal. Arrows indicate two areas of neuropil within this glomerulus with substantial NT-3-βgal innervation although the bulk of the glomerulus essentially lacks any NT-3-βgal+ fibers. Z-stack depth = 11.875 μm. (C) Four glomeruli along the ventral edge of the MOB showing different degrees and types of labeling for the two markers. Especially noteworthy is the patchy distribution (yellow arrows) of NT-3-βgal fibers (red) within Glom 3, which shows relatively homogeneous filling of the neuropil by TrpM5-GFP fibers (green). Glom1 is one of the relatively rare glomeruli, which exhibits sparse TrpM5-GFP label so that individual fibers can be discerned within the glomerular neuropil. Brackets indicate a subregion of Glom2 enlarged in panels F and F′. Z-stack depth = 8.7 μm. (D–F) Enlargements of single confocal planes from regions shown in Panel C to show degree of co-localization. The left hand image of each pair has been thresholded to yield a binary image in each color plane to enable comparison of co-localized regions (yellow) with single labeled regions of each color. (D and D1) Enlargement of area indicated by the top right arrow in panel C. Note that NT-3-βgal+ fibers (red) occupy areas of the glomerulus filled by TrpM5-positive fibers and show substantial co-localization within the limits of resolution of the microscope. (E1) Enlargement of area indicated by the bottom right arrow in panel C. Although many co-localized pixels are evident, some pixels display only NT-33-βgal label (red). (F & F1) Enlargement of a single plane image from area in brackets. Although this glomerulus is double-labeled, nearly all pixels show label for only one of the two markers indicating the independent origins of these fiber systems.
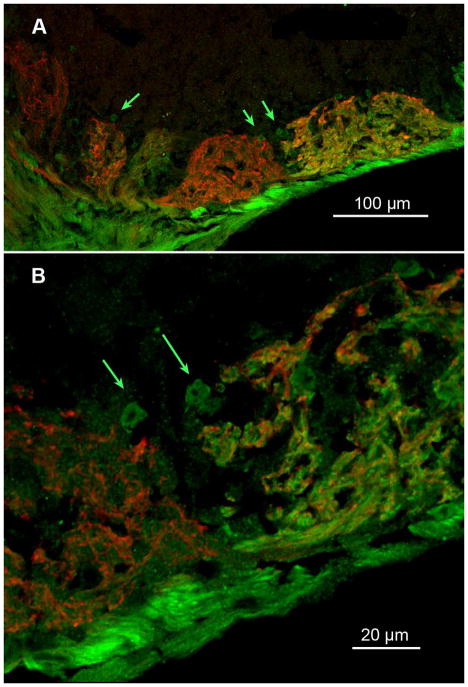
In many glomeruli in the ventral region of the MOB, we found small (5–10 μm) round areas of neuropil outside of the main body of a glomerulus that contained neuropil immunoreactive for TrpM5-GFP (green arrows, Figure 3). These small round areas abutted both TrpM5-positive and NT-3-positive glomeruli. These structures were reminiscent of the microglomeruli described by others (Lipscomb et al. 2002) but appeared somewhat larger than the microglomeruli described previously.
Figure 3.
(A) Confocal image (z-stack 6.0 μm deep) showing heterogeneous fields of glomerular neuropil in several glomeruli of the ventral MOB. The green arrows indicate small regions of neuropil that stain differently than the main body of the glomerulus. (B) Higher magnification of the region in panel A indicated by the paired arrows on the right side of (A) but shown in a z stack of 0.5 μm. Small round regions of TrpM5-GFP-positive neuropil are apparent at the margins of two neighboring glomeruli, one exhibiting dual label and the other being predominantly occupied by fibers exhibiting NT-3-βgal staining. A median digital filter was applied to reduce background pixel noise.
Mapping of NT-3-βgal and TrpM5-GFP Projection Patterns
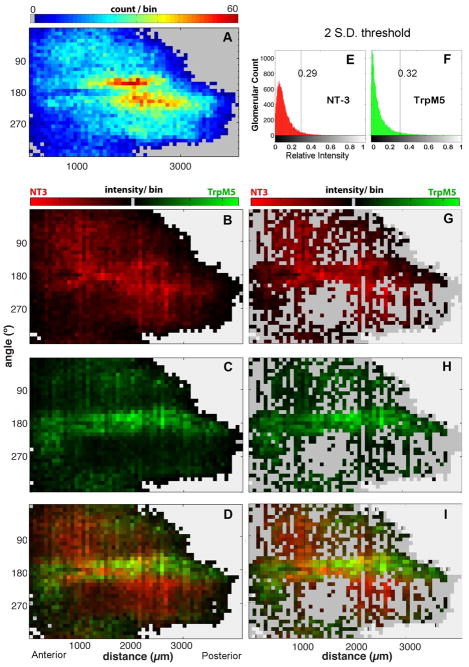
Glomerular maps show that the area of greatest density of TrpM5-positive and NT-3-βgal positive glomeruli was not simply a reflection of a higher number of total glomeruli in the ventral olfactory bulb. The density map in Figure 4A shows the location of all glomerular profiles. In this map, warmer colors represent more total glomeruli per bin. A region of increased glomerular density lies in the ventrocaudal region 1800 – 3000 μm in the AP axis (measured from the rostral tip of the MOB). Figures 4 B–D illustrate the distribution of immunoreactive glomeruli for the two markers, NT-3 in red and TrpM5 in green. Note that the location of the highest number of immunolabeled glomeruli of each type does not correspond to the region of highest overall glomerular density. The intensity map in Figure 4B reveals that NT-3-βgal+ glomeruli lie predominately along the ventral and lateral surfaces of the anterior half of the MOB (1000 – 2500 AP) (Figure 4C). In contrast, immunoreactivity for TrpM5-GFP (Fig. 4C) was located predominately along the ventral midline of the MOB with two smaller regions occurring in the anteromedial and the posterolateral MOB. GFP signal was remarkably low in a large portion of the posteromedial aspect of the bulb. Comparing these two distributions (Figure 4D), TrpM5-GFP positive and NT-3-βgal positive glomeruli appear to occupy largely segregated regions of the bulb, with the ventral midline in the anterior half of the MOB having the highest probability of containing glomeruli positive for both TrpM5-GFP and NT-3-βgal (Figure 4D). Even in this region, the number of double-labeled glomeruli is low. Furthermore, the immunoreactivity distributions shown in Figure 4B–D are not related to regional differences in total glomerular density (c.f. Fig. 4A & D), i.e. the region of potential co-localization is not simply an area containing more glomeruli.
Figure 4.
Standard GLOMMap 2D plots comparing staining patterns for glomeruli positive for TrpM5 or NT-3 with a plot of overall glomerular density in the MOB. Each square in the plots represents the collection of glomeruli in a bin of 72 μm and 10°. The X-axis plots the distance in microns from rostral tip of bulb (AP axis); the Y-axis gives the cylindrical coordinates around the perimeter of each bulb cross-section, where 0°, 90°, 180°, and 270° respectively indicate dorsal, lateral, ventral, and medial aspects. (A) A plot of the density of all glomeruli across location in the MOB (n = 8 bulbs). Warmer colors indicate more glomeruli per bin. Gray indicates outside of MOB. Two regions of increased glomerular density (red-yellow regions) occur on the ventrolateral and ventromedial surfaces of the mid-caudal bulb, between 160 and ~200° and between 1500 and 3000 μm AP. (B–D) 2D plots of the identical glomerular bins from A, but with color indicating average normalized staining intensity in each bin (instead of count) for the two markers: brighter greens indicate stronger TrpM5 staining intensity, while brighter reds indicate stronger NT-3 staining intensity. Black indicates a background subtracted binned intensity value of zero. B. 2D intensity plot for NT-3 positive glomeruli. C. 2D intensity plot for TrpM5 positive glomeruli. D. True color overlay of the green and red intensity plots shown individually in panels B and C so that a yellow hue indicates an equivalence in intensity values. The region of maximal co-localization lies along the ventral margin of the bulb (180°), but anterior (1000 – 1500 μm AP) to the region of highest overall glomerular density (yellow-red regions of panel A) (E & F) Normalized brightness histograms for all glomeruli for each channel after applying a 2 S.D. threshold cut-off. E: For NT-3, the normalized cut-off relative value is 0.29. F: For TrpM5, the normalized cut-off value is 0.32. G–I: GLOMMap 2D plots of normalized intensity of glomeruli above threshold similar to those of panels B–D, but showing only the brightest glomeruli (i.e. strongly positive for either marker) in which only those glomeruli with an intensity greater than two 2 S.D. above the mean in either color channel are plotted (leaving 9.3% of all glomeruli). Regions with no glomeruli above that cutoff are indicated by the darker gray. Few regions contain glomeruli that are heavily labeled by the two markers as indicated by yellow-orange hues.
In order to test whether strongly labeled glomeruli tended to be overlapping or distinct subpopulations, we generated a reduced glomerular dataset in which we excluded all glomeruli with intensities that fell below two standard deviations of the mean in both channels (i.e. below 0.29 and 0.32 for the red and green channels, respectively which left 2,214 glomerular cross-sections). Considering only these strongly labeled glomeruli, the distribution remains largely segregated (Fig. 4 G–I & Fig. 5). Few bins show a tendency toward containing glomeruli heavily labeled for both markers although a region of potential overlap does occur in the anteroventral region of the MOB, 1000 – 3000 AP (orange map elements, Figure 4H). Therefore, some glomeruli could potentially be strongly positive for both reporters, i.e. co-labeled. Examples of ventral MOB regions containing co-labeled glomeruli are shown in Figures 1A & E (Dbl arrow).
Figure 5.
3D visualization of the segregated intensity patterns. (A) The 2D histogram of the full glomerular dataset from 4D wrapped around a three-dimensional reconstruction of the MOB. (B) 3D plot of the brightest glomeruli reduced dataset from 4I.
Heavy TrpM5-GFP and NT-3-βgal Immunoreactivity Predominantly Occurs in Separate Populations of MOB Glomeruli
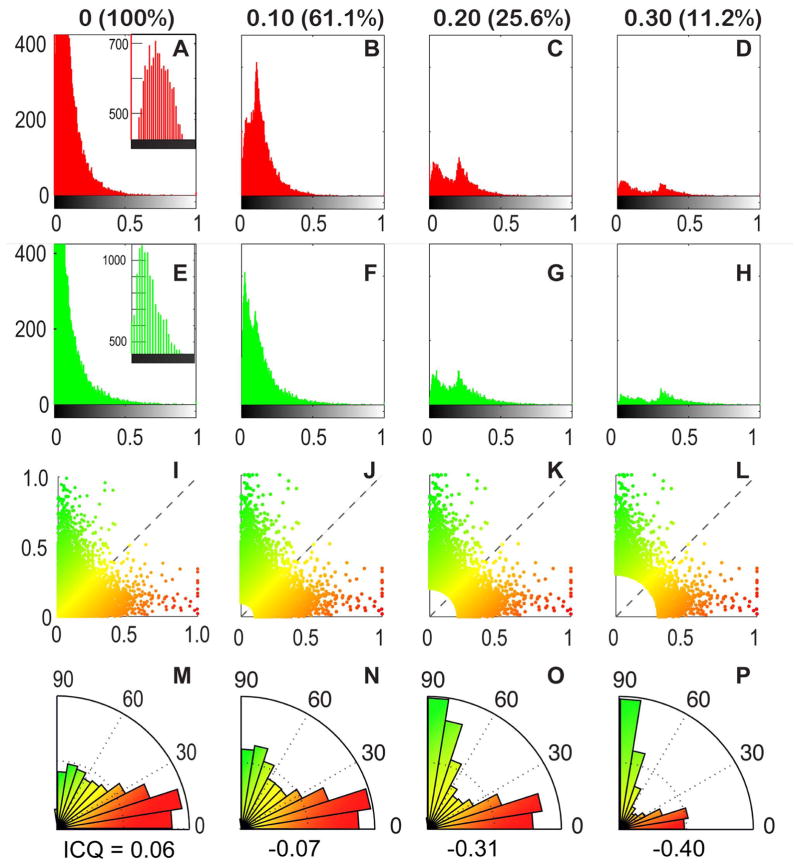
Since the brightest glomeruli tended to be non-overlapping, we wished to assess the degree of colocalization of fluorescence of the two labels by comparing the mean normalized immunofluorescence intensity in the red and green channels for each glomerular cross-section across the entire glomerular dataset. The histogram of intensities for each channel are given in Fig. 6A&E while Fig. 6I shows the 2-color scatterplot of green versus red intensities for each glomerular cross-section. While the shape of this 2D plot resembles histogram plots for immunolabels that have exclusive staining patterns (Bolte and Cordelieres, 2006), an intensity correlation analysis indicated a slightly colocalized staining pattern (intensity correlation quotient, ICQ = 0.0589, p = 3.3 x 10−73) (Li et al., 2004). Closer inspection of the 2D histogram in Figure 5I revealed that the majority of glomeruli with a colocalized signal pattern (i.e. along the 45° axis) appeared to be weakly stained by both immunolabels. Accordingly, we considered the pattern of co-localization after progressively removing the dimmest glomeruli from the dataset setting three different threshold values at 0.1, 0.2, and 0.3 for the normalized brightness signal (maximum = 1.0). Figs. 6B–D and F–G show the respective individual channel histograms while panels J–K show the corresponding 2-color scatterplots of these reduced datasets, Intensity correlation analysis of this reduced dataset indicated an exclusive staining pattern (ICQ = −0.07 for 0.1 cutoff; −0.31 for 0.2 cutoff and −0.40 with 0.3 cutoff) with exclusion of the dimmer glomeruli; thus, glomeruli exhibiting the highest intensity label for one marker were rarely labeled by the other. This tendency for segregation of label is obvious in a radial histogram plot (Fig. 6 M–P). The immunofluorescence distribution in these reduced datasets resembles the brightest aspects of the immunofluorescence distribution seen for the full glomerular dataset, indicating that glomeruli strongly positive for TrpM5-GFP most likely distinct from glomeruli strongly positive for NT-3-βgal although they may lie adjacent within the ventral portion of the MOB (Figure 4D& I; Fig. 5). Thus, expression of NT-3- and TrpM5-driven markers was statistically anti-correlated to segregated subpopulations of glomeruli, although both markers were preferentially localized to glomeruli along the ventral aspect of the MOB.
FIGURE 6.
Glomeruli positive for NT3 and TRPM5 show increasing anti-correlation at higher intensity. Each column in the figure (e.g. A–M, B–N, etc.) depicts glomeruli that exceed the intensity threshold criterion in 2 channel intensity space; the column headers indicate the intensity threshold (ranked from 0 to 1) and the percent of data remaining. In the first column (A–M), no threshold was applied and 100% of the data are represented. In the subsequent columns, incrementally higher intensity thresholds were used (B–N = 0.1; C–O = 0.2; D–P = 0.3) to reduce the dataset to smaller percentages representing brighter values. For each incremental subset, we increasingly eliminate glomeruli in which the normalized intensity of label fall below the indicated threshold in the 2 channels. For example, in the 0.1 column (B–N), all glomeruli with normalized intensities below 0.1 in the red and green channels are eliminated (61.1% of glomeruli remain). Correspondingly, in the 0.3 column, glomeruli with intensities below 0.3 in the 2 channels are eliminated (11.2% of glomeruli remain). Row 1 (A–D) Intensity histograms for the red channel across all datasets. In A, the inset shows the peak of the histogram. A large population of glomeruli with zero intensity can be seen here but are truncated in the full histogram. In B–D, the histograms have two peaks, indicating two populations of glomeruli: a more strongly labeled population and a weaker labeled one. The weakly labeled glomeruli are those that are above threshold due to brightness in the green channel although relatively dim in red. This bimodal distribution more clearly resolves into two distinct populations in C and D. Row 2 (E–H). Intensity histograms from the green channel displayed as in Row 1 and show a similar bimodal distribution. E. Inset shows peak of histogram truncated in the main panel. Row 3 (I–L) Color scatter plots in 2 color intensity space: red channel intensity (x-axis) plotted against green channel intensity (y-axis). Each dot represents one glomerulus. Glomeruli eliminated by the threshold cut-off occupy the lower left hand corner of plot as indicated by the increasingly larger white region in that corner (J–L). The statistical elimination of most glomeruli with even a low threshold indicates that most glomeruli are weakly or poorly stained by both TRPM5 and NT3. Furthermore, those glomeruli that are strongly labeled by one label tend to be weakly labeled by the other. Row 4 (M–P) Color-coded angle histograms showing the relative staining of suprathreshold glomeruli in red and green channels. For ease of comparison, the histograms are normalized to maximum bin count. In these histograms, a glomerulus with a normalized intensity of 1.0 in the red channel, and a normalized green intensity of 0 would fall in the 0–10° bin. By comparison, a glomerulus with green intensity of 1.0 and a red intensity of 0 would fall in the 80–90° bin. The low number of glomeruli near the 45 degree angle demonstrates the paucity of double label. The intensity correlation quotient (ICQ, Li et al., 2004) is listed below each histogram is listed the intensity correlation quotient. ICQ values range from −0.5 to 0.5; an ICQ value close to zero indicates a random (or mixed) staining pattern. Positive ICQ values indicated dependent staining patterns and negative ICQ values indicate a segregated staining pattern. These results further confirm that glomeruli strongly labeled by one marker tend to be weakly labeled by the other; few glomeruli are strongly labeled by both markers.
Discussion
The current study shows that although TrpM5 and NT-3 are expressed by populations of OSNs whose axons target similar glomerular territories along the ventral surface of the MOB in mice, these molecules are largely present in non-overlapping sets of glomeruli. Even within a single glomerulus, the expression of the two markers appears to occur in somewhat different populations of OSN axons. Since glomerular targeting is largely dependent on the particular odorant receptor expressed by an OSN (Mombaerts et al., 1996; Feinstein and Mombaerts, 2004), the presence of both markers in a single glomerulus suggests that expression of one or both of the markers is not linked tightly to expression of different odorant receptor molecules. Rather, expression of TrpM5 or NT-3 may be more related to the physiological or developmental status of the receptor cells. For example, TrpM5 expression increases following nares occlusion or genetic elimination of components of the canonical olfactory transduction cascade (Oshimoto et al., 2013) which suggests that TrpM5 expression may increase with reduced OSN activation. In the current work, we examined TrpM5 expression under basal conditions of conventional laboratory housing.
TrpM5 and NT-3 Characterize Separate Neural Subpathways in the Main Olfactory System
The findings of the present study support the current idea of a biochemical/functional organization of subpathways in the olfactory system. The current study provides evidence for two separate populations of OSNs, expressing either TrpM5 or NT-3, projecting axons to nearby glomerular fields in the ventral MOB. The fluorescence values measured from the immunolabeled reporter gene products (GFP for TrpM5; βgal for NT-3) were largely segregated to different glomerular fields in the MOB. TrpM5-GFP positive glomeruli were positioned along the ventral surface, with two smaller regions of the anteromedial and a posterolateral MOB. NT-3-βgal positive glomeruli were positioned along the ventral and lateral surfaces in the anterior half of the MOB. These finding are highly similar to the distributions reported previously (Vigers et al., 2003; Lin et al., 2007). However, it could not be discerned from these previous studies whether TrpM5 and NT-3 characterize the same or different olfactory subpathways. The findings of the current study resolve this question. TrpM5 and NT-3 expressing OSNs project to largely separate populations of glomeruli. TrpM5-GFP and NT-3-βgal immunolabeling were frequently observed in separate glomeruli within the ventral aspects of the MOB where these reporter gene products often labeled adjacent glomeruli. However, these labeled populations were not entirely mutually exclusive. A few glomeruli, positioned along the ventral surface of the anterior half of the MOB exhibited significant fluorescence values for both TrpM5-GFP and NT-3-βgal (co-labeled). The total number of these co-labeled glomeruli was small and co-localization analysis shows a significant tendency for mutual exclusion (Fig. 6), i.e. glomeruli heavily labeled for TrpM5 tended to exhibit relatively little NT3-driven label. Indeed, even within co-labeled glomeruli, regions of neuropil heavily labeled for TrpM5 often did not label for NT3 and vice-versa (Fig. 3). The current study did not examine labeling of the reporter gene products in the somas and dendrites of OSNs in the MOE. The possibility that a small number of OSNs express both proteins and project axons to the observed co-labeled glomeruli cannot be ruled out.
TrpM5-GFP and NT-3-βgal Positive Glomeruli Lie within a Region activated by semiochemicals
The main distribution of both TrpM5-GFP and NT-3-βgal positive glomeruli lies in the ventral and anterolateral portions of the mouse MOB – regions that process semiochemical odor information (Lin et al., 2007; Kang et al., 2011). A previous investigation suggested that TrpM5-GFP positive glomeruli were activated in response to semiochemicals (Lin et al. 2007); however, this study did not quantify the responsive proportion of the population. Similarly, the previous study that identified the glomerular region receiving inputs from NT-3-βgal expressing OSNs did not investigate the functionality of this glomerular population (Vigers et al., 2003). Both TrpM5-GFP and NT-3-βgal positive glomerular fields lie within the ventral portion of the MOB previously shown to be activated by odors of urine (Schaefer et al., 2001; Schaefer et al., 2002) and which project to the medial amygdala (Thompson et al., 2012), a region known to process semiochemical information (Fernandez-Fewell and Meredith, 1998; Meredith and Westberry, 2004; Samuelsen and Meredith, 2009; Kang et al., 2009; Kang et al., 2011). In light of the current study, the circuits characterized by TrpM5 and NT-3 are likely neural subpathways processing semiochemical information. Thompson et al. (Thompson et al., 2012) reported that some mitral cells projecting to the medial amygdala from the ventral MOB did not extend dendrites into TrpM5-GFP positive glomeruli. Given the location of NT-3+ glomeruli in the ventral OB, it is possible that those mitral cells that innervate NT-3-βgal positive glomeruli, also project to the medial amygdala and constitute a separate channel for semiochemical odor information.
Supplementary Material
Acknowledgments
This work was supported by NIH grants from the NIDCD: R01 DC006070 (D.R. & T.E.F.), P30 DC04657 (DR). and F32 DC 011690 (S.H.R.). The authors thank Kevin Jones of U. Colorado Boulder (Boulder, CO) for making available the NT3-lacZ animals and Robert Margolskee of Monell Chemical Senses Center (Philadelphia, PA) for the TrpM5-GFP line.
Footnotes
Author contributions: SHR helped design the study, carried out the histology and quantified the labeling, ES carried out most of the post-acquisition data analysis, DR helped design the study and analyze data, TEF helped design the study, acquire images and analyze data.
Conflict of Interest Statement: The authors have no known conflicts of interest in relation to this publication.
References Cited
- Bader A, Klein B, Breer H, Strotmann J. Connectivity from OR37 expressing olfactory sensory neurons to distinct cell types in the hypothalamus. Front Neural Circuits. 2012;6:84. doi: 10.3389/fncir.2012.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- Bozza T, Vassalli A, Fuss S, Zhang JJ, Weiland B, Pacifico R, Feinstein P, Mombaerts P. Mapping of class I and class II odorant receptors to glomerular domains by two distinct types of olfactory sensory neurons in the mouse. Neuron. 2009;61:220–233. doi: 10.1016/j.neuron.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess A, Simon I, Cedar H, Axel R. Allelic inactivation regulates olfactory receptor gene expression. Cell. 1994;78:823–834. doi: 10.1016/s0092-8674(94)90562-2. [DOI] [PubMed] [Google Scholar]
- Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 2006;4:7. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Backus C, Wang XY, Reichardt LF. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369:658–661. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- Feinstein P, Mombaerts P. A Contextual Model for Axonal Sorting into Glomeruli in the Mouse Olfactory System. Cell. 2004;117:817–831. doi: 10.1016/j.cell.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fewell GD, Meredith M. Olfactory contribution to Fos expression during mating in inexperienced male hamsters. Chem Senses. 1998;23:257–267. doi: 10.1093/chemse/23.3.257. [DOI] [PubMed] [Google Scholar]
- Feron F, Lizard G, Sicard G. Isolation of mature olfactory neurones using retrograde labelling and flow cytometry. Journal of neuroscience methods. 1995;57:9–14. doi: 10.1016/0165-0270(94)00105-p. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Xu Z, Ali SS, Leon M. Spatial representations of odorants in olfactory bulbs of rats and mice: similarities and differences in chemotopic organization. J Comp Neurol. 2009;514:658–673. doi: 10.1002/cne.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang N, Baum MJ, Cherry JA. A direct main olfactory bulb projection to the ‘vomeronasal’ amygdala in female mice selectively responds to volatile pheromones from males. Eur J Neurosci. 2009;29:624–634. doi: 10.1111/j.1460-9568.2009.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang N, McCarthy EA, Cherry JA, Baum MJ. A sex comparison of the anatomy and function of the main olfactory bulb-medial amygdala projection in mice. Neuroscience. 2011;172:196–204. doi: 10.1016/j.neuroscience.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Vaes E, Mombaerts P. Regulation of the probability of mouse odorant receptor gene choice. Cell. 2011;147:907–921. doi: 10.1016/j.cell.2011.09.049. [DOI] [PubMed] [Google Scholar]
- Li Q, Lau A, Morris TJ, Guo L, Fordyce CB, Stanley EF. A syntaxin 1, Galpha(o), and N-type calcium channel complex at a presynaptic nerve terminal: analysis by quantitative immunocolocalization. J Neurosci. 2004;24:4070–4081. doi: 10.1523/JNEUROSCI.0346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Margolskee R, Donnert G, Hell SW, Restrepo D. Olfactory neurons expressing transient receptor potential channel M5 (TRPM5) are involved in sensing semiochemicals. Proc Natl Acad Sci U S A. 2007;104:2471–2476. doi: 10.1073/pnas.0610201104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Lu M, Guthrie KM. Anterograde trafficking of neurotrophin-3 in the adult olfactory system in vivo. Exp Neurol. 2013;241:125–137. doi: 10.1016/j.expneurol.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- McIntyre JC, Titlow WB, McClintock TS. Axon Growth and Guidance Genes Identify Nascent, Immature, and Mature Olfactory Sensory Neurons. Journal of neuroscience research. 2010;88:3243–3256. doi: 10.1002/jnr.22497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith M, Westberry JM. Distinctive responses in the medial amygdala to same-species and different-species pheromones. J Neurosci. 2004;24:5719–5725. doi: 10.1523/JNEUROSCI.1139-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Oshimoto A, Wakabayashi Y, Garske A, Lopez R, Rolen S, Flowers M, Arevalo N, Restrepo D. Potential role of transient receptor potential channel M5 in sensing putative pheromones in mouse olfactory sensory neurons. PLoS One. 2013;8:e61990. doi: 10.1371/journal.pone.0061990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pro-Sistiaga P, Mohedano-Moriano A, Ubeda-Banon I, Del Mar Arroyo-Jimenez M, Marcos P, Artacho-Perula E, Crespo C, Insausti R, Martinez-Marcos A. Convergence of olfactory and vomeronasal projections in the rat basal telencephalon. J Comp Neurol. 2007;504:346–362. doi: 10.1002/cne.21455. [DOI] [PubMed] [Google Scholar]
- Rawson NE, Eberwine J, Dotson R, Jackson J, Ulrich P, Restrepo D. Expression of mRNAs encoding for two different olfactory receptors in a subset of olfactory receptor neurons. J Neurochem. 2000;75:185–195. doi: 10.1046/j.1471-4159.2000.0750185.x. [DOI] [PubMed] [Google Scholar]
- Salcedo E, Zhang C, Kronberg E, Restrepo D. Analysis of training-induced changes in ethyl acetate odor maps using a new computational tool to map the glomerular layer of the olfactory bulb. Chem Senses. 2005;30:615–626. doi: 10.1093/chemse/bji055. [DOI] [PubMed] [Google Scholar]
- Samuelsen CL, Meredith M. Categorization of biologically relevant chemical signals in the medial amygdala. Brain Res. 2009;1263:33–42. doi: 10.1016/j.brainres.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer ML, Finger TE, Restrepo D. Variability of position of the P2 glomerulus within a map of the mouse olfactory bulb. J Comp Neurol. 2001;436:351–362. [PubMed] [Google Scholar]
- Schaefer ML, Yamazaki K, Osada K, Restrepo D, Beauchamp GK. Olfactory fingerprints for major histocompatibility complex-determined body odors II: relationship among odor maps, genetics, odor composition, and behavior. J Neurosci. 2002;22:9513–9521. doi: 10.1523/JNEUROSCI.22-21-09513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Yoshihara S, Nishizumi H, Tsuboi A. Neuropilin-2 is required for the proper targeting of ventral glomeruli in the mouse olfactory bulb. Molecular and Cellular Neuroscience. 2010;44:233–245. doi: 10.1016/j.mcn.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Salcedo E, Restrepo D, Finger TE. Second-order input to the medial amygdala from olfactory sensory neurons expressing the transduction channel TRPM5. J Comp Neurol. 2012;520:1819–1830. doi: 10.1002/cne.23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigers AJ, Baquet ZC, Jones KR. Expression of neurotrophin-3 in the mouse forebrain: insights from a targeted LacZ reporter. J Comp Neurol. 2000;416:398–415. doi: 10.1002/(sici)1096-9861(20000117)416:3<398::aid-cne10>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Vigers AJ, Böttger B, Baquet ZC, Finger TE, Jones KR. Neurotrophin-3 is expressed in a discrete subset of olfactory receptor neurons in the mouse. J Comp Neurol. 2003;463:221–235. doi: 10.1002/cne.10752. [DOI] [PubMed] [Google Scholar]
- Walz A, Rodriguez I, Mombaerts P. Aberrant Sensory Innervation of the Olfactory Bulb in Neuropilin-2 Mutant Mice. 2002 doi: 10.1523/JNEUROSCI.22-10-04025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Schaefer M, Kida I, Schafer J, Liu N, Rothman DL, Hyder F, Restrepo D, Shepherd GM. Simultaneous activation of mouse main and accessory olfactory bulbs by odors or pheromones. J Comp Neurol. 2005;489:491–500. doi: 10.1002/cne.20652. [DOI] [PubMed] [Google Scholar]
- Yee CL, Jones KR, Finger TE. Brain-derived neurotrophic factor is present in adult mouse taste cells with synapses. J Comp Neurol. 2003;459:15–24. doi: 10.1002/cne.10589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.