Abstract
Dyskeratosis congenita (DC) is an inherited bone marrow failure syndrome and telomere biology disorder characterized by dysplastic nails, reticular skin pigmentation and oral leucoplakia. Androgens are a standard therapeutic option for bone marrow failure in those patients with DC who are unable to undergo haematopoietic stem cell transplantation, but there are no systematic data on its use in those patients. We evaluated haematological response and side effects of androgen therapy in 16 patients with DC in our observational cohort study. Untreated DC patients served as controls. Seventy percent of treated DC patients had a haematological response with red blood cell and/or platelet transfusion independence. The expected age-related decline in telomere length was noted in androgen-treated patients. All treated DC patients had at least one significant lipid abnormality. Additional treatment-related findings included a significant decrease in thyroid binding globulin, accelerated growth in pre-pubertal children and splenic peliosis in two patients. Liver enzymes were elevated in both androgen-treated and untreated patients, suggesting underlying liver involvement in DC. This study suggests that androgen therapy can be effectively used to treat bone marrow failure in DC, but that side effects need to be closely monitored.
Keywords: Dyskeratosis Congenita, androgen, telomere
INTRODUCTION
Dyskeratosis congenita (DC), an inherited bone marrow failure (BMF) and cancer susceptibility syndrome, is characterized by very short germline telomeres and/or a mutation in a telomere biology gene (Dokal, 2011; Ballew & Savage, 2013). The diagnostic triad of DC consists of dysplastic nails, reticular skin pigmentation and oral leucoplakia. Patients with DC may also have pulmonary fibrosis, liver disease, neurological, gastrointestinal and ophthalmological problems. In addition, patients with DC are at a significantly increased risk of solid tumours, leukaemia and myelodysplastic syndrome (Alter et al., 2010). Telomere length less than the first percentile for age measured by leucocyte flow fluorescent in situ hybridization (FISH) is diagnostic of DC and differentiates DC from other inherited BMF syndromes (IBMFS), such as Fanconi Anaemia (FA) (Alter et al., 2007).
The cumulative incidence of BMF is at least 50% by age 50 years in patients with DC and is often life threatening (Alter et al., 2010; Vulliamy & Dokal, 2006). Patients with DC-related BMF do not respond to immunosuppressive therapy (Al-Rahawan et al, 2006). Haematopoietic stem cell transplant (HSCT) is the only current curative modality for DC-associated BMF. However, HSCT may not be feasible due to the presence of co-morbid conditions, lack of suitable donors or patient preferences; thus, Anabolic-androgenic steroid (androgen) treatment is often considered for DC-related BMF (Savage et al., 2009; Islam et al., 2013).
Androgens have been used to treat acquired and inherited BMF, both prior to the current era of HSCT and currently in patients who are not HSCT candidates (Shahidi, 2001; Allen et al, 1968; Alter et al, 2010; Diamond & Shahidi, 1967). Historically, BMF in FA patients has also been treated with androgens with an approximately 50% haematological response rate (Allen et al, 1968; Shimamura & Alter, 2010). With recent advances and improvements in HSCT for patients with FA, androgens are now less often used to treat FA-related BMF.
The specific mechanism(s) by which androgens stimulate haematopoiesis is (are) not well understood. It was postulated that androgens lead to an increase in erythropoietin, which stimulates erythropoietic stem cells and, to a lesser extent, myeloid progenitor cells in the bone marrow (Shahidi, 2001). Recent studies suggest that androgens such as testosterone do not increase erythropoietin levels, but rather work at the level of the erythropoietin receptor to elicit a haematological response (Maggio et al., 2013). Androgens also stimulate osteoblasts, bone matrix production, cytokine and growth factor synthesis (Shahidi, 2001).
The most common side effects of androgen therapy include virilization in females, priapism in males, acne, hepatotoxicity including hyperbilirubinaemia, transaminitis, hepatic and splenic peliosis, hepatic adenomas or hepatocellular carcinomas, lipid abnormalities and behavioural problems (Shahidi, 2001). Anecdotal data suggest that DC patients are particularly sensitive to the side effects of androgen therapy; thus, lower doses and more frequent monitoring have been recommended (Savage et al., 2009; Islam et al., 2013). However, androgen-related side effects have not been systematically studied in patients with DC. Here we describe the response to androgens and the side effects in DC patients enrolled in a natural history study of IBMFS.
METHODS
Patient Characteristics
This retrospective/prospective natural study included patients with DC enrolled in the National Cancer Institute’s (NCI) Institutional Review Board-approved IBMFS study (NCI 02-C-0052), NCT00027274, www.marrowfailure.cancer.gov) between March 2002 and December 2011 (Alter et al, 2010). Patients were classified as having DC if they had a germline mutation in one of the known DC genes, or if they had at least two features of the diagnostic triad and other clinical findings consistent with DC, such as haematological or neoplastic complications (Savage & Bertuch, 2010; Ballew et al, 2013).
We reviewed medical records from 16 DC patients who were treated with androgens, and compared their data with 28 DC patients never treated with androgens (Table I). All patients underwent evaluations at the National Institutes of Health (NIH) Clinical Center, and were examined by the same physicians, whether or not they were on androgens. The management of patients was at the discretion of their primary haematologist, with consultative input from the NCI IBMFS study physicians.
Table I.
Demographics of patients with dyskeratosis congenita
| Diagnosis | Dyskeratosis Congenita | ||
|---|---|---|---|
| Total | No Androgens | Treated with Androgens | |
| Number | 44 | 28 | 16 |
| Male:female | 17:5 | 11:3 | 3:1 |
| Median age, years (range) | 16 (1.5–69) | 18 (2.5–69) | 11 (1.5–47) |
Haematological response was defined as red blood cell (RBC) and/or platelet transfusion independence along with an increment in haemoglobin (Hb) of ≥20 g/l from the baseline, rise in the absolute neutrophil count (ANC) to ≥ 1.0 × 109/l without the use of granulocyte colony stimulating factor (G-CSF), and/or a platelet count increase to ≥ 40 × 109/l. We evaluated patients for androgen-related side effects by determining fasting serum lipid profile [total cholesterol, high density lipoprotein (HDL), low density lipoprotein (LDL) and triglycerides]; liver function tests (LFT) including serum bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and gamma-glutamyl transpeptidase (GGT); endocrine function [thyroid binding globulin (TBG), triiodothyronine (T3), thyroxine (T4) and thyroid-stimulating hormone (TSH) levels]. An abnormal lipid profile was defined as the presence of one or more of the following: total cholesterol more than 5.18 mmol/l, LDL more than 2.59 mmol/l, triglycerides more than 1.69 mmol/l, or HDL less than 1.03 mmol/l. LFT values above the normal range reported by the clinical laboratory were considered abnormal. Liver and spleen ultrasounds were performed in search of abnormalities in architecture, peliosis or tumours. Bone age and linear growth were evaluated in children and adolescents. Telomere length was determined on leucocyte subsets as previously described (Baerlocher et al., 2006; Alter et al., 2012). Clinical germline mutation testing was performed in Clinical Laboratory Improvement Amendments-certified laboratories.
Statistical analysis
All statistical analyses were two-sided. We used Fisher’s exact and Student’s T-test to compare pre- and post-treatment parameters. P-values less than 0.05 were considered significant. Analyses were done with Microsoft Excel (2007 release) and Stata Version 11 (StataCorp Release 11, College Station, TX, USA).
RESULTS
Patient Characteristics
The median age at study entry was 16 years (range 1.5–69 years) (Table I). Thirty-seven of the 44 patients (84%) had a germline mutation in one of the known DC-causing genes (11 DKC1, 12 TINF2, 5 TERT, 3 TERC, 2 WRAP53 [TCAB1], and 4 RTEL1) (Table 2). Thirty-four of the 44 patients (77%) had single or multi-lineage cytopenia. Ten (23%) had cytopenia with Hb >80 g/l but less than normal for age, ANC between 0.5–1.5 × 109/l and/or platelets between 30–150 ×109/l and did not require treatment. Twenty-four patients (54%) had severe single or multi-lineage cytopenia with Hb <80 g/l, ANC <0.5 × 109/l and/or platelets <30 ×109/l. Eight patients with severe cytopenias were not treated with androgens; four of these eight patients were transfusion dependent until receiving HSCT, one was treated with RBC and platelet transfusions as needed, two were treated with immunosuppressive agents (but did not respond) and one patient did not require treatment. Sixteen patients with severe cytopenias were treated with androgens (14 oxymetholone, one fluoxymesterone and one nandrolone decanoate). Of these 16 treated patients, germline mutations were known in 14 (88%) (3 DKC1, 6 TINF2, 1 TERC, 1 WRAP53 and 3 RTEL1) (Table II). The starting oxymetholone dose was 0.5–1 mg/kg, which was increased or decreased based on the patient’s haematological response. The maintenance dose was the minimum effective dose and ranged from 0.5 – 2.7 mg/kg/day (median 1.5 mg/kg/day). Of the 16 patients who received androgens, pre-treatment lipid panels were available for 10, LFTs and measures of endocrine function were available from 11, and complete blood counts (CBCs) were available for all patients. Twenty-eight DC patients who never received androgens served as controls. Germline mutations were known in 22 (79%) of the controls (8 DKC1, 6 TINF2, 5 TERT, 2 TERC and 1 WRAP53) (Table II).
Table II.
Genetic characteristics of dyskeratosis congenita patients by androgen treatment status and response.
| Treated with androgens | Not treated | Total | |||
|---|---|---|---|---|---|
| Responders | Non responders | % response | |||
| DKC1 | 2 | 1 | 67% | 8 | 11 |
| TINF2 | 4 | 2 | 67% | 6 | 12 |
| TERC | 1 | 0 | 100% | 2 | 3 |
| TERT | 0 | 0 | NA | 5 | 5 |
| WRAP53 | 1 | 0 | 100% | 1 | 2 |
| RTEL1 | 3 | 0 | 100% | 0 | 3 |
| Unknown | 0 | 2 | 0% | 6 | 8 |
| Total | 11 | 5 | 28 | 44 | |
Haematological response
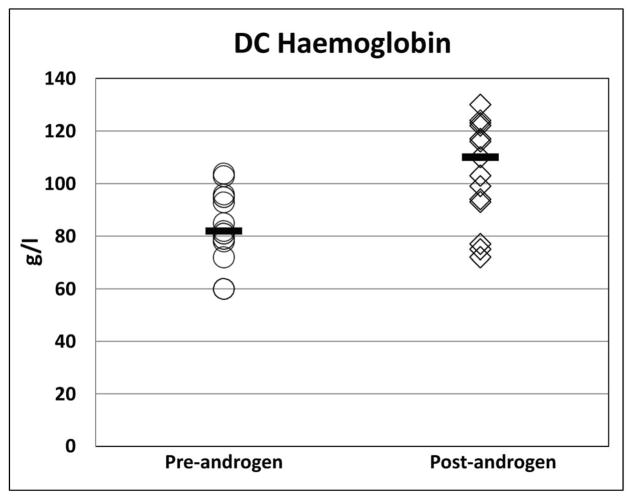
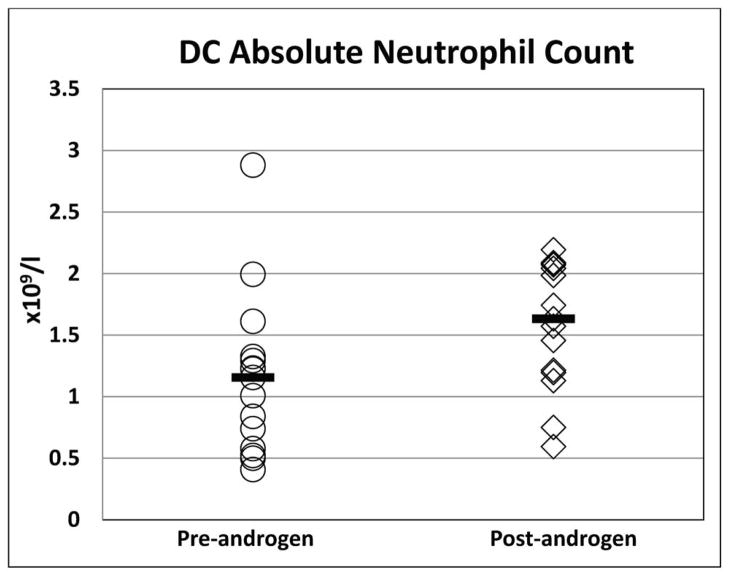
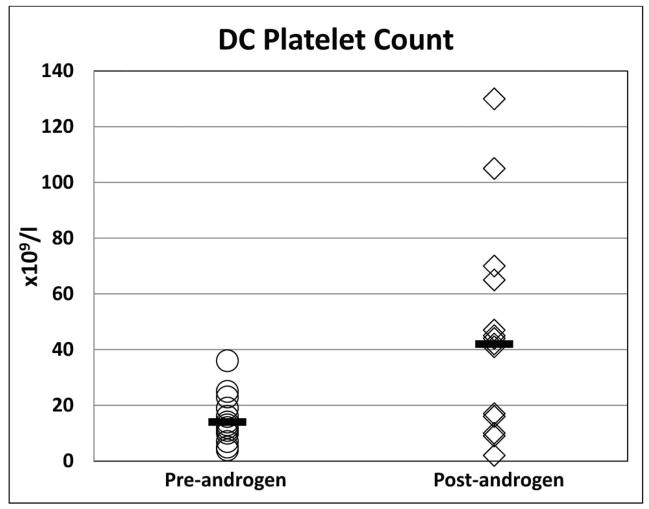
Eleven of the 16 patients (69%) treated with androgens showed a haematological response, 10 to oxymetholone and one to fluoxymesterone (Figure 1). All 11 patients who responded had a Hb increment of ≥20 g/l from baseline [median pre-treatment Hb 93 g/l (range 60 – 104 g/l) vs. post-treatment Hb 116 g/l (range 94 – 130 g/l); p<0.001]. The ANC improved in four out of the eight patients with an ANC below 1.0 × 109/l at treatment initiation [median pretreatment ANC 0.55 × 109/l (range 0.5 – 0.84 × 109/l); post treatment ANC 1.514 × 109/l (range 1.13–1.985 × 109/l); p<0.01]. Nine of 15 patients (60%) who had a platelet count <30 × 109/l prior to androgen therapy demonstrated an androgen response with increase from a median platelet count of 13.5 × 109/l (range 7–25 × 109/l) to 41.5 ×109/l (range 41–131 × 109/l); p<0.01. The median time to response was four months (range 2 – 9 months) for Hb, 1.6 months (range 1 – 2.6 months) for ANC, and 1.4 months (0.6–7.5 months) for platelets. A haematological response was observed in 2 of 3 (67%) patients with DKC1 mutations, 4 of 6 (67%) with TINF2, 1 of 1 (100%) with TERC, 1 of 1 (100%) with WRAP53, and 3 of 3 (100%) with RTEL1 mutations. Neither of the two treated patients with unknown genetic cause for DC responded to androgens.
Figure 1. Haematological response in all patients with DC treated with androgens.
The median is denoted by the solid horizontal line.
A) Haemoglobin (g/l)
B) Absolute Neutrophil Count (× 109/l)
C) Platelet count (× 109/l)
DC, dyskeratosis congenita
The median duration of androgen therapy was 2.2 years (range 0.4–10 years). Androgens were discontinued in 11 patients during the observation period. Four patients (one on nandrolone and three on oxymetholone) failed to respond after 4 to 5 months. Two patients on oxymetholone and G-CSF discontinued therapy after 1 and 2 years of treatment because of splenic peliosis (Giri et al., 2007). Three patients stopped responding to oxymetholone after 2.3, 3.9, and 5.5 years of therapy. Treatment was discontinued at 2.6 and 5.5 years for a hypoechoic liver lesion with a non-diagnostic biopsy and persistent transaminitis in one patient, and behavioural problems/noncompliance in another. Five haematological DC responders (one on fluoxymesterone, 4 on oxymetholone) were still responding to treatment at a median of 3.7 years (range 0.8 – 10 years) at the time these data were analysed.
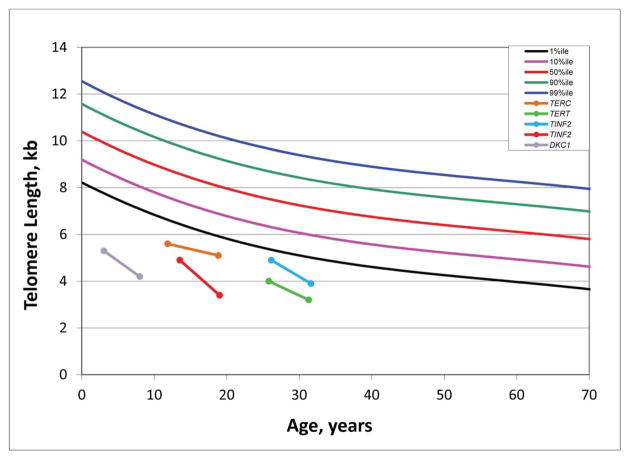
Telomere length in DC before and after androgens
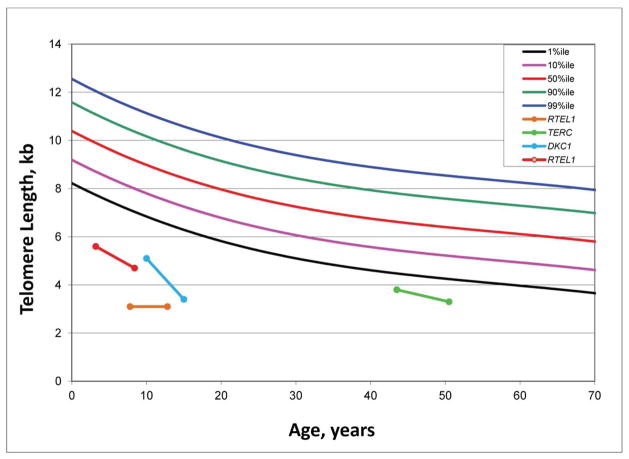
Four DC patients who responded to androgen (three to oxymetholone and one to fluoxymesterone) therapy had telomere length measurements prior to, and at a median of 2.6 years (range 0.8–7.8 years) after androgen treatment. Post-androgen telomere lengths were consistently the same or shorter than pre-androgen measurements in the total lymphocyte population from all four patients (Figure 2A) and in the four lymphocyte subsets measured (CD 20+ B cells, CD 45+ Naïve T cells, CD 45- Memory T cells, CD 57+ Natural Killer cells; data not shown). Telomere lengths declined over time (median 5.5 years, range 5–7 years) in five untreated patients, as previously shown in this population (Figure 2B) (Alter et al., 2012).
Figure 2. Longitudinal telomere lengths in patients with DC.
A) Pre- and post-androgen lymphocyte telomere lengths in 4 DC patients. The green line indicates the patient treated with fluoxymesterone. The other three patients received oxymetholone. Legend reflects the known germline mutation in each patient.
B) Lymphocyte telomere lengths in 5 DC patients not treated with androgens. Legend reflects the known germline mutation in each patient.
DC, dyskeratosis congenita
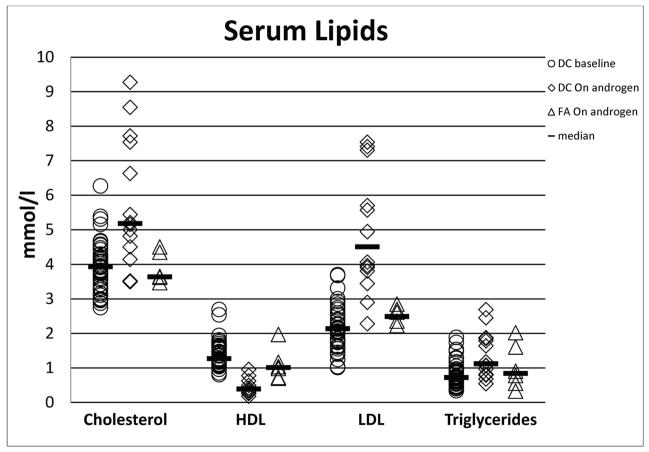
Lipid abnormalities
A fasting lipid panel was obtained in all 28 untreated patients and in 13 of the 16 patients while on androgen therapy; 10 of the latter also had pre-androgen lipid data. All 13 DC patients treated with androgens showed markedly abnormal lipid profiles compared with their own pre-androgen values. Total cholesterol was elevated (>5.18 mmol/l) in 54% after androgens vs. 8% prior to therapy (P=0.001). Similarly, all patients had significantly abnormally low HDL (100% on androgens vs. 5% prior to treatment, P<0.0001) and high LDL (100% on androgens vs. 16% prior to treatment, P<0.0001), and high triglycerides (38% on androgens vs. 5% prior to treatment, P=0.008). All P values for lipid levels on the treated patients compared with those never treated were statistically significant (P<0.0001) (Table III, Figure 3).
Table III.
Lipid profiles of untreated and treated patients with dyskeratosis congenita (DC) and treated Fanconi anaemia (FA) patients
| Parameter (normal range) | Cholesterol (< 5.18 mmol/l) | HDL (1.03–1.55 mmol/l) | LDL (<2.59 mmol/) | Triglyceride (<1.69 mmol/l) | |
|---|---|---|---|---|---|
| DC: not treated | Number abnormal/Total patients (%) | 3/38 (8%) | 2/38 (5%) | 6/37 (16%) | 2/38 (5%) |
| Median (range) | 3.94 (2.75–6.27) | 1.27 (0.8–2.69) | 2.14 (1.01–3.7) | 0.72 (0.34–1.89) | |
| DC: treated | Number abnormal/Total patients (%) | 7/13 (54%) | 13/13 (100%) | 13/13 (100%) | 5/13 (38%) |
| Median (range) | 5.18 (3.5–9.27) | 0.39 (0.18–0.96) | 4.51 (2.28–7.54) | 1.12 (0.54–2.69) | |
| P-value* | 0.001 | <0.0001 | <0.0001 | 0.008 | |
| FA: treated | Number abnormal/Total Patients (%) | 0/6 (0%) | 3/6 (50%) | 3/6 (50%) | 1/6 (17%) |
| Median (range) | 3.64 (3.47–4.51) | 1.01 (0.7–1.97) | 2.49 (2.23–2.85) | 0.84 (0.33–2.02) | |
| P-value** | 0.04 | 0.02 | 0.02 | NS | |
p value calculated by Fisher exact test. p ≤ 0.05 considered significant
: Comparison between untreated and treated DC patients
: Comparison between post-androgen DC and FA patients
HDL: high density lipoprotein, LDL: low density lipoprotein, NS: Not significant (p >0.05)
Figure 3. Summary of lipid changes in patients with DC and FA treated with androgens.
DC, dyskeratosis congenita; FA, Fanconi anaemia; HDL, high density lipoprotein; LDL, low density lipoprotein
Liver function
Twenty-one of the 28 (75%) untreated DC patients had elevated LFTs, suggesting possible underlying DC-related liver disease. Six of 11 (55%) treated patients had pre-treatment abnormalities in at least one liver parameter evaluated (Table IV). There was no statistically significant difference in liver function between treated versus never-treated DC patients. Though pre- and post-treatment LFT parameters did not differ statistically, liver enzyme elevation on androgens was noted in eight (50%) treated patients, one of whom had normal pretreatment liver function at the time of evaluation. Dose reduction was advised when patients on androgens developed an AST or ALT more than twice the upper limit of normal. One patient continued to have good haematological response and LFT normalization after dose reduction. Androgen therapy was discontinued in one of 16 (6%) patients due to persistent elevation of liver transaminases.
Table IV.
Liver function of untreated and treated dyskeratosis congenita (DC) patients
| Parameter (normal range) | AST (0–59 u/l) | ALT (5–30 u/l) | Total Bilirubin (0–17.1 μmol/l) | GGT (2–24 u/l) | |
|---|---|---|---|---|---|
| DC: not treated | Number abnormal/Total patients (%) | 4/40 (10%) | 15/40 (38%) | 10/40 (25%) | 6/36 (17%) |
| Median (range) | 25 (12–116) | 26 (7–171) | 11.97 (3.42–41) | 24 (0–114) | |
| DC: treated | Number abnormal/Total patients (%) | 2/13 (15%) | 6/14 (43%) | 3/14 (21%) | 4/10 (40%) |
| Median (range) | 31 (12–87) | 29 (15–553) | 11.97 (3.42–27.37) | 17 (9–247) | |
| P-value* | NS | NS | NS | NS | |
p value calculated by Fisher exact test. p ≤ 0.05 considered significant
: Comparison between untreated and treated DC patients
AST: aspartate aminotransferase, ALT: alanine aminotransferase, GGT: gamma-glutamyl transpeptidase; NS: Not significant (p >0.05).
Structural changes in the liver and spleen
Among the 37 DC patients who underwent liver and spleen ultrasound examinations, one treated patient (3%) developed a hypoechoic liver lesion that was non-diagnostic on biopsy but not suggestive of either an adenoma or liver fibrosis. Two patients who received G-CSF combined with androgens developed splenic peliosis leading to rupture requiring emergent surgical care for one patient (Giri et al, 2007). No other patients in our study reported concomitant use of growth factors and androgens.
Bone changes
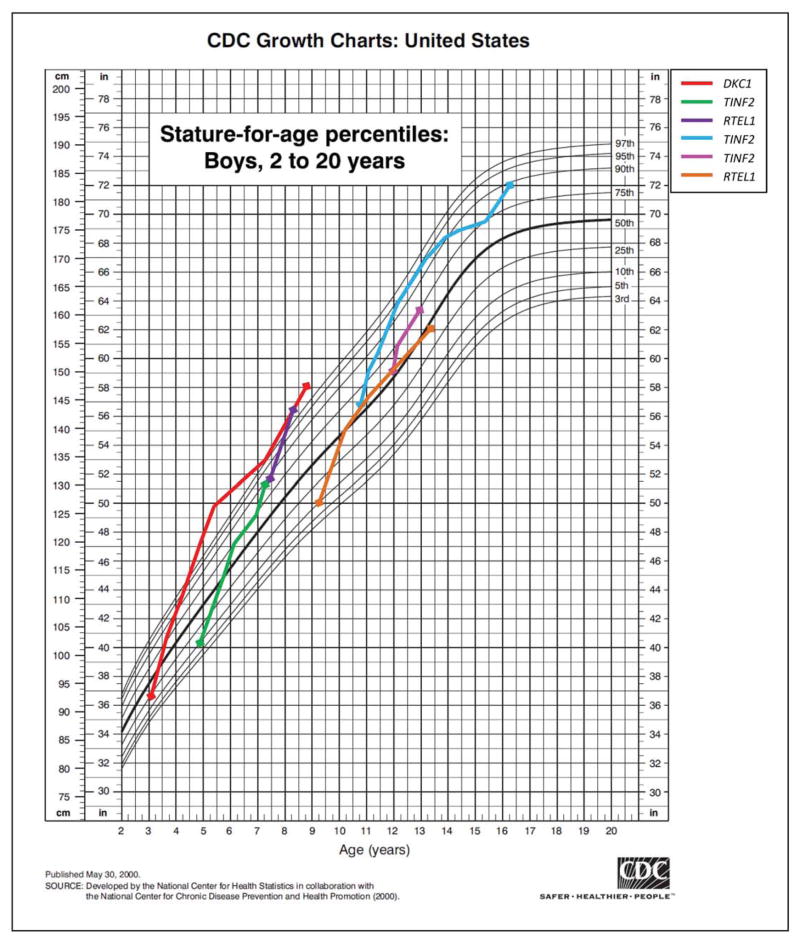
Six pre-pubertal male DC patients had accelerated growth that crossed at least two percentile curves on the growth chart during androgen treatment (Figure 4). One patient had marked growth acceleration from the 10th percentile to greater than the 97th percentile for height in a six-year period. Three patients on androgens developed bone fractures of the femur, tibia, humerus and clavicle after reporting minimal or no trauma (e.g., getting into a car). However, two patients in the untreated group also had bone fractures, one tibial fracture after minor trauma and one femur fracture after minor trauma in a post-HSCT patient.
Figure 4. Growth acceleration in six pre-pubertal male dyskeratosis congenita patients on androgen therapy.
Growth charts from CDC website, May 2000 (http://www.cdc.gov/growthcharts/clinical_charts.htm). Legend reflects the known germline mutation in each patient.
Endocrine function
All patients had normal thyroid function both prior to and during androgen therapy, although we observed a significant decrease in TBG in 10/11 (91%) patients after beginning androgen treatment (P=0.004). All treated patients reported masculinizing androgen effects; males reported priapism; and female patients reported hirsutism and voice hoarseness. We did not collect systematic information on behavioural complications.
Comparison with Fanconi anaemia
As an additional comparison group, we also reviewed the medical records for 11 patients with FA treated with androgens (three males and eight females) enrolled in the same NCI longitudinal IBMFS study. Median age at study entry for the FA group was 13 years (range 6–34 years). Two FA patients had single lineage and nine (82%) had multi-lineage cytopenia. Seven patients were treated with oxymetholone, two with danazol, one each with nandrolone decanoate and dehydroepiandrosterone (DHEA). Two FA patients, one DHEA- and one danazol-treated, had received oxymetholone in the past. Haematological data were available on eight treated FA patients, all of whom (100%) had a haematological response with an increase in Hb of at least 20 g/l higher than the pre-treatment value. Five of eight (63%) had a neutrophil response and seven patients (71%) had a platelet response. Time to response could not be determined due to lack of data.
Post-androgen lipid data were available from six of the 11 treated FA patients. Four (60%) had high LDL, low HDL or high triglycerides while on androgens (Table III, Figure 3). Lipid levels in FA patients could not be directly compared with the levels in DC patients because FA patients were on substantially lower doses of androgens at the time of evaluation (median oxymetholone dose of 0.2 mg/kg/day [range 0.02–0.6]). Liver function abnormalities in the FA patients were similar to those observed in DC patients. Five of six (83%) FA patients had abnormal liver parameters before treatment and eight of 10 (80%) androgen-treated patients developed at least one elevated serum transaminase. Review of records showed that like DC, FA patients had also required androgen dose-adjustments for high liver transaminases. None of the six FA patients who had TBG measurements while on androgens demonstrated a change in TBG.
DISCUSSION
Patients with DC have very high rates of BMF but may not always be HSCT candidates, whether due to personal choice, medical ineligibility or lack of suitable donors. Our study suggests that androgen therapy for DC-related BMF may be a reasonable therapeutic modality if carefully monitored and thoughtfully considered either in patients unable to undergo HSCT or as a bridge to HSCT further down the road.
It is very encouraging that 69% of our DC patients had a haematopoietic response, with notable improvements in erythroid and platelet lineages. Specifically, 14 of the 16 DC patients evaluated in our study were treated with oxymetholone, with two (14%) of them demonstrating a trilineage response; six (43%) had erythroid and platelet lineage responses (all of whom had normal pre-treatment ANC). This is similar to previous FA reports (Alter & Kupfer, 1993; Rose et al., 2014) and in our small FA cohort. Our patient treated with danazol did not have a haematological response, in contrast to a recent report of two patients with DC who did respond (Islam et al., 2013).
Patients with DC in our study had a surprisingly high prevalence of severe lipid profile abnormalities. This has neither been previously evaluated in DC nor reported in FA. Androgen-treated FA patients in our cohort did not have lipid abnormalities at the time of this evaluation, but their androgen doses were much lower than those used in DC. Abnormalities in lipid profile during androgen therapy for different indications, such as chronic obstructive pulmonary disease, constitutional growth retardation, muscle wasting or body-building, have been well documented in the past (Glazer, 1991; Shahidi, 2001). We cannot quantify the long-term risk of atherosclerosis from the sustained lipid profile abnormalities seen in our DC patients, but it is possible that annual monitoring of carotid intima-media thickness by Doppler ultrasound may prove helpful in the future, to identify early atherosclerotic changes so that timely discontinuation of androgen therapy or initiation of lipid-lowering therapy can be considered (Baldassarre et al., 2013).
Endocrine dysfunction is an important clinical concern for all patients treated with androgens. While we observed statistically significant decreased TBGs in treated DC patients, their thyroid function was normal. Previous studies (Tahboub & Arafah, 2009; Arafah, 1994) suggest that in patients with normal thyroid function, TBG levels markedly decrease after initiation of androgen therapy, but the precise mechanism is unknown.
Growth and development before, during and after androgen therapy in pre-pubertal and adolescent patients should be carefully monitored; patients and their parents need to understand the potential for endocrine-related side effects, including rapid bone growth, behaviour changes and masculinization. It is also important to note that patients with DC may have an increased prevalence of neuropsychiatric conditions (Rackley et al., 2012) that could be exacerbated by androgen therapy. Careful evaluation and management for behavioural complications of androgen therapy are warranted.
The occurrence of bone fractures in both treated and untreated DC patients makes it difficult to discern whether these events were treatment-related, an intrinsic component of the disease phenotype, a combination of both or a consequence of other unrecognized risk factors. The combination of androgen-related accelerated bone growth and increased muscle mass may have created a discrepancy between muscle strength and bone mineralization which resulted in increased skeletal fragility. It is also possible that telomere biology disorders, such as DC, have a bony phenotype that is not yet characterized. For example, a subset of patients with DC has mutations in CTC1, a telomere-capping gene. In addition, mutations in CTC1 also cause a disorder designated “Coats plus” (also known as cerebroretinal microangiopathy with calcifications and cysts), in which some patients have abnormal bone growth and poor bone healing, in addition to features of DC (Anderson et al., 2012; Polvi et al., 2012). The DC patients in our study did not have CTC1 mutations.
We found the expected age-related telomere attrition in patients with DC both before and after androgen therapy, observations which differ from recent case reports suggesting that telomere attrition may be mitigated by androgen therapy (Scheinberg, 2013; Chen et al., 2012; Ziegler et al., 2012). This difference could be due to the small size of our cohort, the sensitivity and specificity of the different telomere length measurement methods (e.g., quantitative polymerase chain reaction used by Scheinberg (2013), flow-FISH used by Chen et al (2012) and Alter et al 2012) and/or other clinical or methodological differences between studies.
A strength of this study is that, to our knowledge, it is the largest systematic study of therapeutic response to and side effects from androgen therapy for DC-related BMF. Our data suggest that a large proportion of DC patients with BMF can be given androgens safely, and can achieve a good haematological response. However, it is difficult to draw definitive conclusions from a small, observational non-randomized study. But, given the rarity of DC, a methodologically-rigorous study design may be difficult to implement.
In conclusion, androgen therapy for BMF in DC is a reasonable option in patients who are not HSCT candidates or as bridging therapy while awaiting HSCT. A longer, prospective study is needed to further characterize the observed adverse effects. Studies using danazol, an attenuated androgen, are underway in patients with FA, and various DC-related telomere biology disorders (NCT01001598 and NCT01441037). Until those results are available, we recommend that DC patients receiving androgens for BMF have frequent monitoring of their serum lipids, liver function, growth and development, as well as biannual liver/spleen ultrasounds.
Acknowledgments
We thank the patients, their families, and the referring clinicians for their valuable contributions to this study. Lisa Leathwood, RN, and Maureen Risch, RN, Westat, Inc. (NIH contracts N02-CP-11019, N02-CP-65504, and N02-CP-65501) provided outstanding study support. This work and the research of Drs. Khincha, Wentzensen, Giri, Alter and Savage were supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health. We thank Charleen Adams, NCI, for help with FA-related data abstraction and Dr. Mark H. Greene, NCI, for his helpful comments.
Footnotes
AUTHOR CONTRIBUTIONS
NG saw all patients, collected, analysed and interpreted the data, and wrote the manuscript. PK and IMW did the chart review, extracted and analysed the data, and wrote the manuscript. SAS designed the study, interpreted data and wrote the manuscript. BPA designed the parent IBMFS study, served as an advisor, saw the patients and edited the manuscript.
DISCLOSURE OF CONFLICTS OF INTEREST
The authors declare no conflict of interest.
References
- Allen DM, Fine MH, Necheles TF, Dameshek W. Oxymetholone therapy in aplastic anemia. Blood. 1968;32:83–89. [PubMed] [Google Scholar]
- Al-Rahawan MM, Giri N, Alter BP. Intensive immunosuppression therapy for aplastic anemia associated with dyskeratosis congenita. International Journal of Hematology. 2006;83:275–276. doi: 10.1532/IJH97.06030. [DOI] [PubMed] [Google Scholar]
- Alter BP, Kupfer G. Fanconi anemia. In: Pagon RA, Bird TD, Dolan CR, Stephens K, Adam MP, editors. GeneReviews. University of Washington, Seattle; Seattle (WA): 1993. [Google Scholar]
- Alter BP, Baerlocher GM, savage SA, Chanock SJ, Weksler BB, Willner JP, Peters JA, Giri N, Lansdorp PM. Very short telomere length by flow FISH identifies patients with Dyskeratosis Congenita. Blood. 2007;110:1439–1447. doi: 10.1182/blood-2007-02-075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter BP, Giri N, Savage SA, Peters JA, Loud JT, Leathwood L, Carr AG, Greene MH, Rosenberg PS. Malignancies and survival patterns in the national cancer institute inherited bone marrow failure syndromes cohort study. British Journal of Haematology. 2010;150:179–188. doi: 10.1111/j.1365-2141.2010.08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter BP, Rosenberg PS, Giri N, Baerlocher GM, Lansdorp PM, Savage SA. Telomere length is associated with disease severity and declines with age in dyskeratosis congenita. Haematologica. 2012;97:353–359. doi: 10.3324/haematol.2011.055269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BH, Kasher PR, Mayer J, Szynkiewicz M, Jenkinson EM, Bhaskar SS, Urquhart JE, Daly SB, Dickerson JE, O’Sullivan J, Leibundgut EO, Muter J, Abdel-Salem GM, Babul-Hirji R, Baxter P, Berger A, Bonafe L, Brunstom-Hernandez JE, Buckard JA, Chitayat D, Chong WK, Cordelli DM, Ferreira P, Fluss J, Forrest EH, Franzoni E, Garone C, Hammans SR, Houge G, Hughes I, Jacquemont S, Jeannet PY, Jefferson RJ, Kumar R, Kutschke G, Lundberg S, Lourenco CM, Mehta R, Naidu S, Nischal KK, Nunes L, Ounap K, Philippart M, Prabhakar P, Risen SR, Schiffmann R, Soh C, Stephenson JB, Stewart H, Stone J, Tolmie JL, van der Knaap MS, Vieira JP, Vilain CN, Wakeling EL, Wermenbol V, Whitney A, Lovell SC, Meyer S, Livingston JH, Baerlocher GM, Black GC, Rice GI, Crow YJ. Mutations in CTC1, encoding conserved telomere maintenance component 1, cause coats plus. Nature Genetics. 2012;44:338–342. doi: 10.1038/ng.1084. [DOI] [PubMed] [Google Scholar]
- Arafah BM. Decreased levothyroxine requirement in women with hypothyroidism during androgen therapy for breast cancer. Annals of Internal Medicine. 1994;121:247–251. doi: 10.7326/0003-4819-121-4-199408150-00002. [DOI] [PubMed] [Google Scholar]
- Baerlocher GM, Vulto I, de Jong G, Lansdorp PM. Flow cytometry and FISH to measure the average length of telomeres (flow FISH) Nature Protocols. 2006;1:2365–2376. doi: 10.1038/nprot.2006.263. [DOI] [PubMed] [Google Scholar]
- Baldassarre D, Veglia F, Hamsten A, Humphries SE, Rauramaa R, de Faire U, Smit AJ, Giral P, Kurl S, Mannarino E, Grossi E, Paoletti R, Tremoli E on behalf of the IMPROVE Study Group. Progression of Carotid Intima-Media Thickness as Predictor of Vascular Events: Results from the IMPROVE Study. Arteriosclerosis, Thrombosis and Vascular Biology. 2013;33:2273–2279. doi: 10.1161/ATVBAHA.113.301844. [DOI] [PubMed] [Google Scholar]
- Ballew BJ, Savage SA. Updates on the biology and management of Dyskeratosis Congenita and related telomere biology disorders. Expert Reviews in Hematology. 2013;6(3):327–27. doi: 10.1586/ehm.13.23. [DOI] [PubMed] [Google Scholar]
- Ballew BJ, Yeager M, Jacobs K, Giri N, Boland J, Burdett L, Alter BP, Savage SA. Germline mutations of regulator of telomere elongation helicase 1, RTEL1, in dyskeratosis congenita. Human Genetics. 2013;132:473–480. doi: 10.1007/s00439-013-1265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Desierto MJ, Dent J, Bachman MJ, Calado RT, Stratakis CA, Young NS. Androgen Treatment Mitigates Hematopoietic Cell Telomere Attrition In Vivo. Blood (ASH Annual Meeting Abstracts) 2012;120:516. [Google Scholar]
- Dokal I. Dyskeratosis Congenita. ASH Education Book 2011. 2011;2011:480–486. doi: 10.1182/asheducation-2011.1.480. [DOI] [PubMed] [Google Scholar]
- Diamond LK, Shahidi NT. Treatment of aplastic anemia in children. Seminars in Hematology. 1967;4:278–288. [PubMed] [Google Scholar]
- Glazer G. Atherogenic effects of anabolic steroids on serum lipid levels. A literature review. Archives of Internal Medicine. 1991;151:1925–1933. [PubMed] [Google Scholar]
- Giri N, Pitel PA, Green D, Alter BP. Splenic peliosis and rupture in patients with dyskeratosis congenita on androgens and granulocyte colony-stimulating factor. British Journal of Haematology. 2007;138:815–817. doi: 10.1111/j.1365-2141.2007.06718.x. [DOI] [PubMed] [Google Scholar]
- Islam A, Rafiq S, Kirwan M, Walne A, Cavenagh J, Vulliamy T, Dokal I. Haematological recovery in dyskeratosis congenita patients treated with danazol. British Journal of Haematology. 2013;162:854–856. doi: 10.1111/bjh.12432. [DOI] [PubMed] [Google Scholar]
- Maggio M, Snyder PJ, Ceda GP, Milaneschi Y, Luci M, Cattabiani C, Masoni S, Vignali A, Volpi R, Lauretani F, Peachey H, Valenti G, Cappola AR, Longo D, Ferrucci L. Is the haematopoietic effect of testosterone mediated by erythropoietin? the results of a clinical trial in older men. Andrology. 2013;1:24–28. doi: 10.1111/j.2047-2927.2012.00009.x. [DOI] [PubMed] [Google Scholar]
- Polvi A, Linnankivi T, Kivela T, Herva R, Keating JP, Makitie O, Pareyson D, Vainionpaa L, Lahtinen J, Hovatta I, Pihko H, Lehesjoki AE. Mutations in CTC1, encoding the CTS telomere maintenance complex component 1, cause cerebroretinal microangiopathy with calcifications and cysts. American Journal of Human Genetics. 2012;90:540–549. doi: 10.1016/j.ajhg.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackley S, Pao M, Seratti GF, Giri N, Rasimas JJ, Alter BP, Savage SA. Neuropsychiatric conditions among patients with dyskeratosis congenita: a link with telomere biology? Psychosomatics. 2012;53:230–235. doi: 10.1016/j.psym.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SR, Kim MO, Korbee L, Wilson KA, Douglas RM, Eyal O, Sherafat-Kazemzadeh R, Bollepalli S, Harris R, Jeng MR, Williams DA, Smith FO. Oxandrolone in the treatment of bone marrow failure in Fanconi Anemia. Pediatric Blood and Cancer. 2014;61:11–19. doi: 10.1002/pbc.24617. [DOI] [PubMed] [Google Scholar]
- Savage SA, Bertuch AA. The genetics and clinical manifestations of telomere biology disorders. Genetics in Medicine: Official Journal of the American College of Medical Genetics. 2010;12:753–764. doi: 10.1097/GIM.0b013e3181f415b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage SA, Dokal I, Armanios M, Aubert G, Cowen EW, Domingo DL, Giri N, Greene MH, Orchard PJ, Tolar J, Tsilou E, Van Waes C, Wong JM, Young NS, Alter BP. Dyskeratosis Congenita: The first NIH clinical research workshop. Pediatric Blood & Cancer. 2009;53 (3):520–3. doi: 10.1002/pbc.22061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinberg P. Prognostic value of telomere attrition in patients with aplastic anemia. International Journal of Hematology. 2013;97:553–557. doi: 10.1007/s12185-013-1332-x. [DOI] [PubMed] [Google Scholar]
- Shahidi NT. A review of the chemistry, biological action, and clinical applications of anabolic-androgenic steroids. Clinical Therapeutics. 2001;23:1355–1390. doi: 10.1016/s0149-2918(01)80114-4. [DOI] [PubMed] [Google Scholar]
- Shimamura A, Alter BP. Pathophysiology and management of inherited bone marrow failure syndromes. Blood Reviews. 2010;24:101–122. doi: 10.1016/j.blre.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahboub R, Arafah BM. Sex steroids and the thyroid. Best Practice & Research. Clinical Endocrinology & Metabolism. 2009;23:769–780. doi: 10.1016/j.beem.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Vulliamy T, Dokal I. Dyskeratosis congenita. Seminars in Hematology. 2006;43:157–166. doi: 10.1053/j.seminhematol.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Ziegler P, Schrezenmeier H, Akkad J, Brassat U, Vankann L, Panse J, Wilop S, Balabanov S, Schwarz K, Martens UM, Brummendorf TH. Telomere elongation and clinical response to androgen treatment in a patient with aplastic anemia and a heterozygous hTERT gene mutation. Annals of Hematology. 2012;91:1115–1120. doi: 10.1007/s00277-012-1454-x. [DOI] [PubMed] [Google Scholar]