Abstract
Otitis media is an extremely common pediatric ailment caused by opportunists that reside within the nasopharynx. Inflammation within the upper airway can promote ascension of these opportunists into the middle ear chamber. Otitis media can be chronic/recurrent in nature, and a wealth of data indicates that in these cases the bacteria persist within biofilms. Epidemiological data demonstrates most cases of otitis media are polymicrobial, which may have significant impact on antibiotic resistance. In this study, we used in vitro biofilm assays and rodent infection models to examine the impact of polymicrobial infection with Moraxella catarrhalis and Streptococcus pneumoniae (pneumococcus) on biofilm resistance to antibiotic treatment and persistence in vivo. Consistent with prior work, M. catarrhalis conferred beta-lactamase dependent passive protection from beta-lactam killing to pneumococci within polymicrobial biofilms. Moreover, pneumococci increased resistance of M. catarrhalis to macrolide killing in polymicrobial biofilms. However, pneumococci increased colonization in vivo by M. catarrhalis in a quorum signal-dependent manner. We also found that co-infection with M. catarrhalis affects middle ear ascension of pneumococci in both mice and chinchillas. Therefore, we conclude that residence of M. catarrhalis and pneumococci within the same biofilm community significantly impacts resistance to antibiotic treatment and bacterial persistence in vivo.
Keywords: Otitis, persistence, biofilm, antibiotic
INTRODUCTION
Otitis media (OM) is a significant public health problem worldwide, affecting the majority of all children at least once by three years of age (Klein, 2000). OM is typically caused by colonization of the middle ear space by bacterial opportunists that normally reside within the nasopharyngeal microbiota. These infections can be chronic and/or recurrent in nature, and a wealth of data indicates that the bacterial populations persist within biofilm communities (Post, 2001, Ehrlich, et al., 2002, Hall-Stoodley, et al., 2006, Swords, 2012). Recent epidemiology data also clearly demonstrate that most cases of OM involve simultaneous infection with multiple agents (Chonmaitree, et al., 2008, Pettigrew, et al., 2008, Revai, et al., 2008, Holder, et al., 2012), and our recent work shows that otopathogens can coexist within biofilm communities (Armbruster, et al., 2010, Weimer, et al., 2010, Weimer, et al., 2011). Such polymicrobial infections can have a profound impact on the progression, severity, and response of infections to treatment. It is therefore of great importance to understand how different bacterial species interact during OM infections.
In particular, Moraxella catarrhalis has long been thought to be of importance in the context of polymicrobial infections due to the expression of beta-lactamase by virtually all clinical isolates (Bernhard, et al., 2012). It is for this reason that M. catarrhalis is frequently implicated as a cause of high treatment failures with beta-lactam antibiotics against pathogens that are otherwise susceptible. The general hypothesis is that the production of beta-lactamase affords passive protection (Budhani & Struthers, 1997, Budhani & Struthers, 1998).
In addition, many species of bacteria can produce and/or respond to small, diffusible molecules in a process termed quorum sensing. It has been hypothesized that production of interspecies quorum signal, auto-inducer 2 (AI-2), could have an effect on persistence and/or virulence of multiple species of bacteria residing within a polymicrobial community. AI-2 is produced as a bi-product of the activated methyl cycle where LuxS cleaves S-ribosylhomocysteine into homocysteine and 4,5-dihydroxy-2,3-pentanedione (DPD), which spontaneously cyclizes in solution into AI-2. First described in Vibrio species (Kuo, et al., 1994, Gilson, et al., 1995, Surette, et al., 1999), AI-2 production has been demonstrated in species of both gram-positive and gram-negative bacteria (Xavier & Bassler, 2003), including Streptococcus pneumoniae (pneumococcus). While M. catarrhalis cannot produce its own AI-2, our recent work highlights the importance of interspecies quorum signaling to the persistence of M. catarrhalis bacteria in vivo, with other otopathogens potentially augmenting biofilm formation and persistence by M. catarrhalis through production of AI-2 (Armbruster, et al., 2010). The objective of this study was to define interactions of M. catarrhalis and S. pneumoniae within polymicrobial biofilms, and their implications for resistance of bacteria within biofilm to antibiotic treatment or host clearance.
MATERIALS AND METHODS
Bacterial strains and growth conditions
A list of all bacterial strains, plasmids, and primers is provided in Table 1. S. pneumoniae EF3030 is a serotype 19F strain which typically establishes nasopharyngeal carriage or localized airway infection in murine models (Briles, et al., 1992, Palaniappan, et al., 2005). Pneumococci were grown on trypticase soy agar (BD) supplemented with 5% defibrinated sheep blood (Hemostat) and 4 μg ml−1 gentamicin. For freezer stocks, S. pneumoniae was grown in Todd Hewitt broth with 0.5% yeast extract (THY) additionally supplemented with 10% horse serum and ~2,500 U/ml of catalase to late logarithmic phase (OD600 0.850 – 1.000), then diluted 1:1 in 50% glycerol and frozen at −80°C.
Table 1.
Bacterial strains, plasmids, and primers
| Bacterial strains
| ||
|---|---|---|
| Species | Strain name | Reference |
| S. pneumoniae | EF3030 | Briles et al., 1992 |
| EF3030 luxS− | this study | |
| M. catarrhalis | O35E | Unhanand, et al., 1992 |
| O35E bro− | Balder, et al., 2013 | |
| Plasmids
| |
|---|---|
| Name | Reference |
| pCR2.1 | Invitrogen® |
| pSpR | Whitby et al., 1998 |
| pCRSpLuxSSp | this study |
| Primers
| ||
|---|---|---|
| Name | Sequence | Reference |
| Splux | this study | |
| Forward | 5′-CGTGTTCGTCGCAATCCATACTC-3′ | |
| Reverse | 5′-ACAAGAAATCATCCGCCGTTACTA-3′ | |
| Spluxver | this study | |
| Forward | 5′-GACGTTCAAAGGCATCATCTG-3′ | |
| Reverse | 5′-CCTACTGCCGGCCTTCACACTATC-3′ | |
| M13 | Invitrogen ® | |
| Forward (−20) | 5′-GTAAAACGACGGCCAGT-3′ | |
| Reverse | 5′-CAGGAAACAGCTATGAC-3′ | |
A DNA fragment containing the luxS open reading frame was amplified by PCR using S. pneumoniae genomic DNA using primers (SpluxF and SpluxR), and cloned using the TOPO-TA Cloning kit (Invitrogen). Presence of inserts within clones was verified via PCR with primers (SpLuxverF and SpLuxverR) and by DNA sequencing. A null allele of luxS was generated by ligation of a spectinomycin-resistance marker into an AleI restriction site within the coding sequence. The resulting plasmid (pLuxS::Sp) was used for natural transformation of S. pneumoniae EF3030 using established methods (Yother, et al., 1986); transformants were plated onto blood agar containing spectinomycin (100 μg ml−1).
Moraxella catarrhalis strain O35E is a well characterized laboratory strain (Unhanand, et al., 1992), and a beta-lactamase deficient mutant in this background (hereafter referred to as 035E bro−) has been recently described (Balder, et al., 2013). M. catarrhalis strains (O35E and O35E bro−) were grown on brain heart infusion (BHI) agar containing vancomycin (3 μg ml−1).
For in vitro biofilm assays, bacteria were grown in either THY broth supplemented with 10% horse serum and ~2,500 U ml−1 of catalase (hereby referred to as supplemented THY) or trypticase soy broth (TSB) supplemented with ~2,500 U ml−1 of catalase (hereby referred to as supplemented TSB). In each assay, M. catarrhalis was seeded 3 logs higher than pneumococcus in single species and polymicrobial biofilms for equivalent survival of both species at time of harvest in polymicrobial biofilms.
Antibiotic protection assays
Antibiotic protection assays were performed essentially as described previously (Armbruster, et al.,2010; Weimer, et al., 2011). S. pneumoniae EF3030 and/or M. catarrhalis O35E, or isogenic mutants as indicated in the text, were seeded into 24 well flat-bottom plates (Costar) using inocula of 105 and 108 colony-forming units (CFU) ml−1, respectively, in supplemented THY. After incubation (4 hours at 37°C), azithromycin (6 μg ml−1) or amoxicillin (1 μg ml−1) was added as indicated in the text, concentrations of both antibiotics were chosen based on minimal inhibitory concentrations for the strains used in this study; buffer was added to negative control wells. After incubation (16 hours at 37°C) the biofilms were scraped from the surface, resuspended in phospate-buffered saline (PBS; pH = 7.2) and serial dilutions were prepared and analyzed by plating on appropriate media to define viable counts of each species (blood agar plates supplemented with gentamicin to select for pneumococcus and BHI plates supplemented with vancomycin to select for M. catarrhalis).
Confocal scanning laser microscopy (CLSM)
CLSM was performed as previously described with some modifications (Armbruster, et al., 2010). S. pneumoniae EF3030 and/or M. catarrhalis O35E were seeded in 4 well Permanox chamber slides (Thermo Scientific) as previously stated and grown for 24 hours at 37°C in supplemented TSB. The biofilms were fixed (0.3% paraformaldehyde), frozen in embedding medium (Tissue-Tek), and cryosectioned laterally (~5 μm per section). Immunofluorescent staining was performed using rabbit polyclonal antiserum against pneumococcal surface protein A (PspA) and monoclonal antibody Mab 3F5-5E5 which recognizes a conserved M. catarrhalis surface epitope (Furano, et al., 2005) along with appropriate fluorescent secondary antibody conjugates (S. pneumoniae: Alexa Fluor 488 donkey anti-rabbit IgG, M. catarrhalis: Texas Red goat anti-mouse IgM) (Molecular Probes). Microscopy was performed using a Nikon Eclipse confocal laser scanning microscope. Images were analyzed using the COMSTAT program within MatLab 7.0.4 software.
Mouse infections
BALB/c mice (9 week old females, 5 per group) were serially infected for 3 days with 107 CFU of S. pneumoniae EF3030, its luxS− mutant, or M. catarrhalis O35E via intranasal inoculation either alone or both species of bacteria in a co-infection. At 3, 7, and 10 days post-infection (after the third inoculation), the bullae and nasopharynx were harvested from the mice, homogenized in PBS, serially diluted, and plated onto appropriate media to determine viability of either species of bacteria at each site. All mouse infection experiments were performed according to protocols approved by the Wake Forest Animal Care and Use Committee.
Chinchilla infections
Adult chinchillas (each weighing 400 – 600 g; 8 per group) were intranasally inoculated with either S. pneumoniae EF3030 (105 CFU), its luxS− mutant (105 CFU), M. catarrhalis O35E (108 CFU), or both species of bacteria simultaneously. Chinchillas were given a higher inoculum of M. catarrhalis to improve its survival in this particular animal model. Animals were monitored daily for clinical signs of infection and examined by otoscopy at 48 hour intervals. At 2 and 7 days post-infection, the bullae and nasopharyngeal epithelia were collected and homogenized in PBS. Samples were then serially diluted and plated onto appropriate media to obtain viable counts. All chinchilla infections were performed according to protocols approved by the Wake Forest Animal Care and Use Committee.
Statistics
Statistical analyses were performed using GraphPad Prism 5 software. In vitro data was analyzed using the Mann-Whitney U test for significance. A one-way ANOVA with Newman-Keuls post-test was used to determine statistical significance for in vivo bacterial counts. Incidence of OM was also assessed using a chi-square test; counts on or above the limit of detection (LOD) were considered infected while counts below the LOD were considered uninfected.
Results
Beta-lactamase mediates passive protection of pneumococci by M. catarrhalis
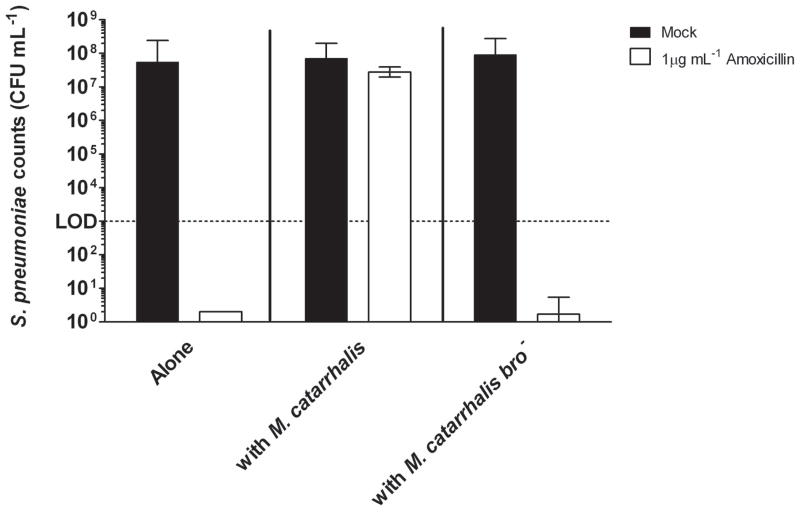
To determine whether the production of a beta-lactamase by M. catarrhalis provides protection of S. pneumoniae from beta-lactam antibiotic killing in polymicrobial biofilms, amoxicillin was added to static biofilms as previously described in the methods section. S. pneumoniae within monospecies biofilms was readily killed by amoxicillin (Fig. 1). However, growth of pneumococci with M. catarrhalis in polymicrobial biofilms completely abolished amoxicillin-mediated killing. Additional studies within the beta-lactamase deficient M. catarrhalis bro− mutant showed that this protective effect was dependent upon beta-lactamase (Fig. 1). Based on these data we conclude that beta-lactamase production by M. catarrhalis provides passive protection to S. pneumoniae in polymicrobial biofilms from beta-lactam antibiotic killing.
Figure 1. M. catarrhalis protects S. pneumoniae from beta-lactam killing.
S. pneumoniae EF3030 and M. catarrhalis O35E were seeded in a 24 well plate alone or together (at a ratio of 1:1,000) as described in the methods section. After 4 hours at 37°C, 1 μg/ml of amoxicillin or buffer was added to each well. Biofilms were resuspended, serially diluted, and plated at 16 hrs post-antibiotic treatment to determine viability. Results depict pneumococcal counts from 3 independent experiments.
S. pneumoniae passively protects M. catarrhalis from azithromycin
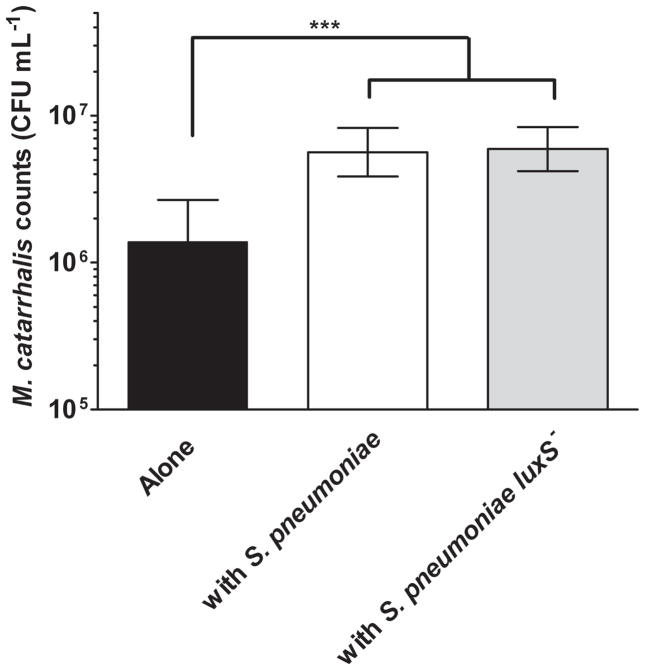
A luxS mutant was created in S. pneumoniae strain EF3030 as described in the methods, and the absence of detectable AI-2 quorum signal was confirmed (data not shown). Previous studies have shown that nontypeable Haemophilus influenzae stimulates formation of antibiotic-tolerant biofilms by M. catarrhalis, via interspecies quorum signaling (Armbruster, et al., 2010). To determine if S. pneumoniae promoted antibiotic resistance within M. catarrhalis biofilms in a similar fashion, biofilms containing S. pneumoniae and/or M. catarrhalis were tested for resistance to azithromycin. In the absence of a co-infecting species, M. catarrhalis within biofilm showed some resistance to azithromycin (Fig. 2). However, culture of M. catarrhalis within polymicrobial biofilm with S. pneumoniae enhanced resistance of M. catarrhalis to azithromycin. Surprisingly, polymicrobial biofilms with the luxS− mutant confered equivalent protection to M. catarrhalis as when it is in a polymicrobial biofilm with the parental strain (Fig. 2). This was in contrast to the previous work with nontypeable H. influenzae, this passive protection was unaltered by abolition of quorum signal production (Fig. 2). Based on these data we conclude that S. pneumoniae provides passive protection to M. catarrhalis from azithromycin killing by an AI-2 independent mechanism.
Figure 2. S. pneumoniae protects M. catarrhalis from macrolide killing.
S. pneumoniae EF3030 and M. catarrhalis O35E were seeded in a 24 well plate alone or together as described in the methods section. After 4 hours at 37°C, 6 μg/ml of azithromycin or buffer was added to each well. Biofilms were resuspended, serially diluted, and plated at 16 hrs post-antibiotic treatment to determine viability. Images depict M. catarrhalis counts from 5 independent experiments. *** denotes a P value < 0.001.
S. pneumoniae and M. catarrhalis form polymicrobial biofilms in vitro
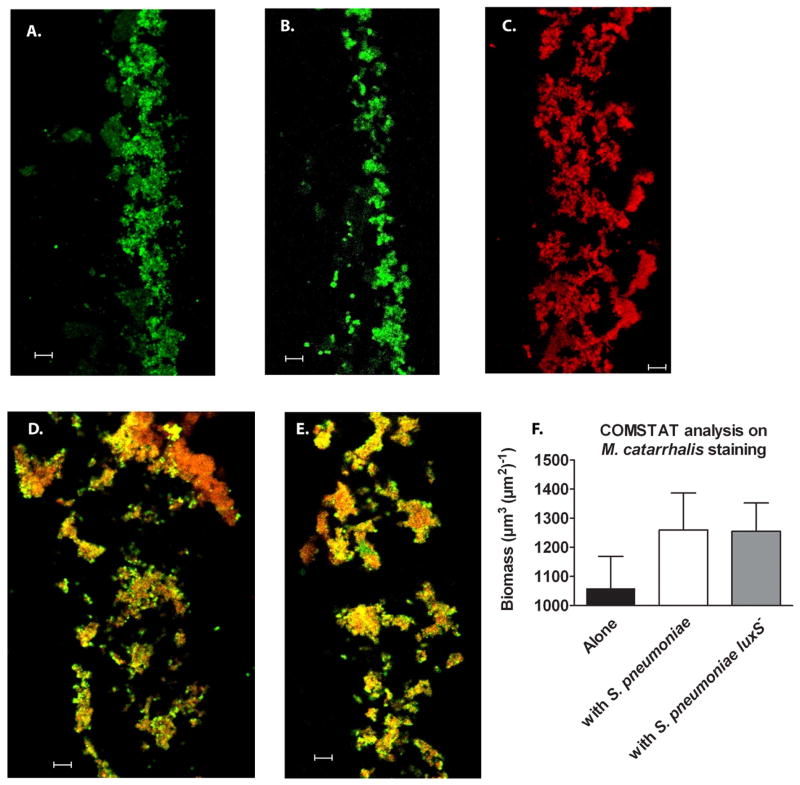
As the additional resistance was independent of quorum signal, we hypothesized that the polymicrobial biofilms may have increased density. This was addressed using confocal microscopy; polymicrobial biofilms grown in static conditions were cryosectioned and differentially stained to identify both species (Fig. 3 A–E). Cross-sections of the polymicrobial biofilms show that both species of bacteria were able to homogenously grow in dense clusters within the biofilm. Images of these cross-sections were then analyzed using the COMSTAT program and the biomass of M. catarrhalis was determined in each condition based on staining specifically identifying this species of bacteria (represented in red). Interestingly, while the biomass of M. catarrhalis increased in polymicrobial biofilms with either S. pneumoniae or its luxS− mutant (Fig. 3 F), viable counts were comparable between monospecies and polymicrobial biofilms (data not shown). Based on these data we conclude that the biomass of M. catarrhalis increases in polymicrobial biofilms with S. pneumoniae independent of AI-2 production.
Figure 3. M. catarrhalis forms polymicrobial biofilms with S. pneumoniae in vitro.
S. pneumoniae EF3030 (green) and M. catarrhalis O35E (red) were seeded in 4 well Permanox chamber slides alone or together at 1:1,000 (as described in the methods section) and grown for 24 hours at 37°C. Biofilms were then fixed, frozen within OCT medium and cryosectioned (~5 μm/slice) and placed on slides. Bacteria were visualized using antibodies specific for pneumococi (rabbit anti-PspA) and Moraxella (monoclonal antibody 4G5), along with relevant fluorescent secondary antibody conjugates (Molecular Probes). Representative images of S. pneumoniae (A.), its luxS− mutant (B.), M. catarrhalis (C.), polymicrobial biofilms with S. pneumoniae and M. catarrhalis (D.), and polymicrobial biofilms with S. pneumoniae luxS− and M. catarrhalis (E.) were taken using CLSM. Images (n=5 frames per group) were analyzed by COMSTAT to determine biomass of M. catarrhalis (F.) alone or in polymicrobial biofilms. Scale bar = 10 μm.
Quorum signal (AI-2) production promotes nasopharyngeal colonization and affects middle ear ascension in polymicrobial infections
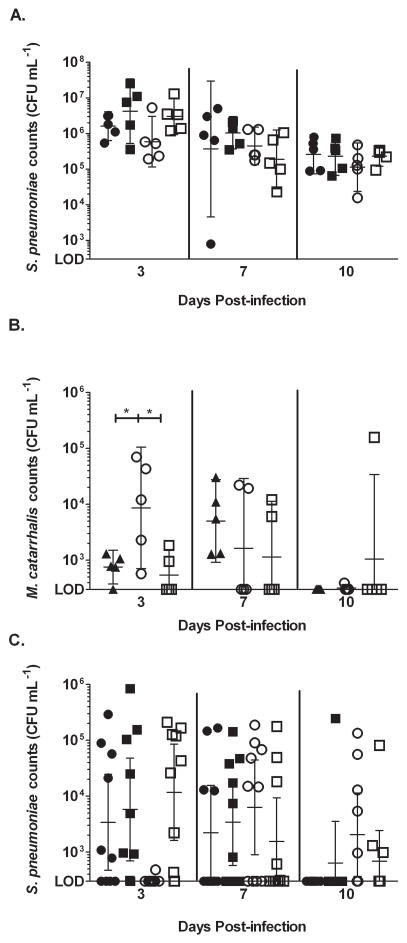
Previous studies have shown that production of the interspecies quorum signal, AI-2, by nontypeable H. influenzae improved M. catarrhalis survival and persistence in the middle ears of experimentally infected chinchillas (Armbruster, et al., 2010). To assess the role of AI-2 in co-infections with S. pneumoniae both a murine and chinchilla model was used. In mice, nasopharyngeal colonization was established after 3 serial inoculations of bacteria (Fig. 4 A). At all time points, S. pneumoniae was not affected by the presence or absence of M. catarrhalis (Fig. 4 A). However, at 3 days post-infection, the numbers of colonizing M. catarrhalis increased by a log when it was in a co-infection with S. pneumoniae (Fig. 4 B). Moreover, in co-infections with the luxS− mutant, the numbers of colonizing M. catarrhalis was equivalent to when it is alone. At later times post-infection, M. catarrhalis was quickly cleared from the nasopharynx. From these data, we conclude that during co-infection the production of AI-2 by S. pneumoniae increased colonization of M. catarrhalis in the nasopharynx.
Figure 4. Quorum sensing promotes nasopharyngeal colonization of M. catarrhalis and affects ascension of pneumococci during polymicrobial infection in mice.
9 week old female BALB/c mice were serially infected for 3 days with S. pneumoniae EF3030, its luxS− mutant, and M. catarrhalis O35E intranasally either alone or together as described in the methods section. At 3, 7, and 10 days post-infection, the superior middle-ear bullae and nasopharynx were harvested from the mice, homogenized, and serially diluted and plated to determine viability of S. pneumoniae in the nasopharynx (A.), M. catarrhalis in the nasopharynx (B.), and S. pneumoniae in the middle ear (C.). ● represents S. pneumoniae, ■ represents S. pneumoniae luxS− alone, ▲ represents M. catarrhalis alone, ○ represents polymicrobial infections with S. pneumoniae and M. catarrhalis, and □ represents polymicrobial infections with S. pneumoniae luxS− and M. catarrhalis. * denotes a P value between 0.01 and 0.05. n = 5 animals per group
Although M. catarrhalis was not recovered from any middle ear samples (data not shown), co-infection with M. catarrhalis did have an effect on the presence of S. pneumoniae in the middle ear. At day 3 post-infection, the CFU counts of S. pneumoniae were significantly reduced when it was in a co-infection with M. catarrhalis compared to when it was in a single infection (Fig. 4 C). Furthermore, this effect was not seen in co-infections with the luxS− mutant. However at later times post-infection (days 7 and 10), clearance of M. catarrhalis from the nasopharynx coincided with improved recovery and prolonged survival of S. pneumoniae in the middle ear (Fig. 4 B and C). Additionally, this effect was not seen in co-infections with the luxS− mutant, as the counts of pneumococcus in the middle ears of these animals peaked at day 3, similar to the single infections. All together, these data show that production of AI-2 by S. pneumoniae enhances colonization of M. catarrhalis in the nasopharynx. In turn, this alters the disease progression of AOM by delaying ascension of S. pneumoniae into the middle ear.
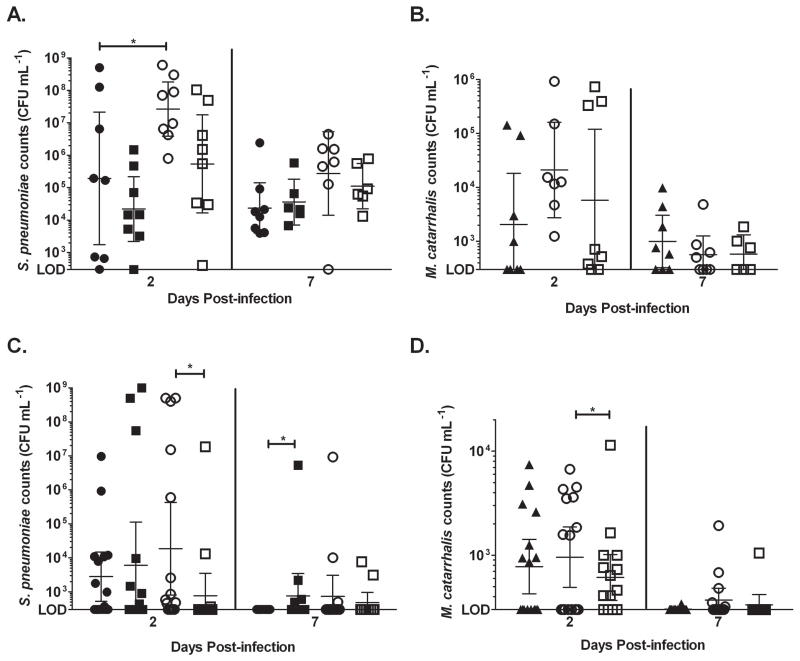
In addition, a chinchilla intranasal infection was performed as described in the methods. In the nasopharynx, S. pneumoniae colonization was increased during a co-infection with M. catarrhalis independent of quorum signal production(Fig. 5 A). There was a statistically significant increase in the bacterial load of pneumococcus when it was in a co-infection. Also, there was a log increase in the bacterial load of the luxS− mutant when it was in a co-infection. Although not statistically significant, there was an increase in both the number of colonized nasopharynxes (alone: 50%; co-infection: 100%) and bacterial load of M. catarrhalis in co-infected animals. Furthermore, during co-colonization with the luxS− mutant, the number of animals colonized with M. catarrhalis dropped to almost equivalent levels as the single-infected animals (~63%) (Fig. 5 B). At day 7 post-infection, M. catarrhalis and S. pneumoniae counts decreased in both single and co-infected animals, which may be due to host clearance of the bacteria from this site.
Figure 5. Quorum sensing promotes nasopharyngeal colonization and ascension of both S. pneumoniae and M. catarrhalis during polymicrobial infections in chinchillas.
Adult chinchillas were intranasally inoculated with S. pneumoniae EF3030, its luxS− mutant, and M. catarrhalis O35E either alone or together in a co-infection as described in the methods section. Nasopharyngeal epithelia and bullae were harvested at days 2 and 7 post-infection. Each sample was homogenized, serially diluted, and plated to determine the viability of S. pneumoniae in the nasopharynx (A.), M. catarrhalis in the nasopharynx (B.), S. pneumoniae in the middle ear (C.), and M. catarrhalis in the middle ear (D.). ● represents S. pneumoniae alone, ■ represents S. pneumoniae luxS− alone, ▲ represents M. catarrhalis alone, ○ represents polymicrobial infections with S. pneumoniae and M. catarrhalis, and □ represents polymicrobial infections with S. pneumoniae luxS− and M. catarrhalis. * denotes a P value between 0.01 and 0.05. n = 8 animals per group
Both S. pneumoniae and M. catarrhalis was able to ascend into the middle ear during single and co-infection (Fig. 5 C–D). While there was no difference in the bacterial load in the middle ears of single and co-infected animals, the incidence of OM increased during co-infection (percent of infected ears during single infection – 50% and co-infection – 78%). Also, during a co-infection, the bacterial load and number of colonized ears with S. pneumoniae was significantly higher during co-infections with the parental strain of pneumococcus as opposed to co-infection with the luxS− mutant at 2 days post-infection. Similarly, the bacterial load of M. catarrhalis in the middle ears of co-infected animals with the parental strain of pneumococcus was significantly higher at day 2 post-infection than in co-infections with the luxS− mutant. At day 7, both the number of infected ears and the bacterial load decreased, which may be due to host clearance of the bacteria. The results from the middle ear suggest that during co-infections, AI-2 production promotes ascension of both species of bacteria in the middle ear. Taken together, the results from both animal models suggest that quorum signaling plays an important role in nasopharyngeal colonization and middle ear ascension during co-infections with S. pneumoniae and M. catarrhalis.
Discussion
It has long been understood that the outcome, severity, and success of treatment of bacterial infection can be profoundly influenced by other microbes within the microbiota or in co-infection (Maddocks & May, 1969, Maddocks, 1980). Our previous work has clearly demonstrated that polymicrobial infection significantly influences persistence of otopathogens, at least in part, by affecting biofilm formation, with related impact on bacterial resistance to host clearance and antibiotics (Armbruster, et al., 2010, Weimer, et al., 2010, Weimer, et al., 2011). For pneumococcal infections, the incidence of antibiotic treatment failure dramatically exceeds the occurrence of antibiotic resistant pneumococcal strains (Harrison, et al., 2009). In the case of beta-lactam resistance, this has often led to speculation that co-infection with bacteria expressing beta-lactamase might confer passive protection (Kaieda, et al., 2005, Brook, 2009). In keeping with this hypothesis, experimental evidence has indicated that M. catarrhalis can confer such passive protection within biofilms (Budhani & Struthers, 1997, Budhani & Struthers, 1998, Armbruster, et al., 2010, Schaar, et al., 2011). The unique beta-lactamase produced by M. catarrhalis is encoded by the bro gene, which produces either one of two isoforms, BRO-1 or BRO-2 (Wallace, et al., 1989, Eliasson, et al., 1992, Bootsma, et al., 1996). The heavier isoform, BRO-1, is the most commonly found isoform among M. catarrhalis strains. It also differs from BRO-2 in the amounts that are produced and in vitro rate of substrate metabolism (Wallace, et al., 1989). The work presented in this study clearly demonstrates that M. catarrhalis can afford passive protection from beta-lactam killing upon pneumococci residing within the same biofilm. Importantly, our experiments conclusively point to beta-lactamase production as the sole determinant of this protection, as no passive resistance was observed with an isogenic M. catarrhalis bro− mutant lacking beta-lactamase activity.
Our previous work showed that M. catarrhalis uses quorum signal eavesdropping to enhance biofilm formation and, in turn, improve antibiotic resistance (Armbruster, et al., 2010). However in this study, we found that polymicrobial biofilms with S. pneumoniae enhanced antimicrobial resistance despite the production of a quorum signal. To further investigate this improved resistance we found that there was an AI-2-independent increase in the biomass of M. catarrhalis. In addition, there seemed to be a change in the overall structure of the polymicrobial biofilms. The bacteria formed dense clusters surrounded by an extensive amount of open space, which could be due to water channel formation or extracellular matrix material which was not accounted for in this study. The significance of these findings is important, especially given recent epidemiological evidence demonstrating the increased occurrence of M. catarrhalis in conjunction with other bacterial species as opposed to alone (Pettigrew, et al., 2008). These results demonstrate the resilient nature of polymicrobial biofilms and suggest other microbe-microbe interactions not characterized in this study may play a role in antimicrobial resistance.
Mice and chinchillas were infected via the intranasal route to assess how colonization of both M. catarrhalis and S. pneumoniae affect nasopharyngeal colonization and persistence, ascension of the Eustachian tube, and development of OM. Both murine and chinchilla models have been used to study colonization of the nasopharynx and middle ears by otopathogens (Krishnamurthy, et al., 2009, Hoopman, et al., 2012, Weimer, et al., 2010). Each model offers different advantages for studying OM (Chiavolini, et al., 2008), which provides a stronger argument for the trends observed herein. In both of these models, the results suggest a role for quorum sensing in nasopharyngeal colonization as well as middle ear ascension and colonization during co-infections. These results are quite convincing, especially considering the stark differences between these two models. Not only are these two different species of animals, but the infection schemes were different as well. Additionally, the increased bacterial load and incidence of OM in co-infected chinchillas was an interesting outcome, which seems to closely model what has been seen in young children (Ruohola, et al., 2013). This is tantalizing evidence that suggests communication between these two species via the interspecies quorum signal AI-2 could mediate the increased incidence of OM in children. luxS is widely expressed by a number of species of bacteria, including S. pneumoniae (Stroeher, et al., 2003). To date, there have not been any reports of S. pneumoniae strains that do not contain this gene. Therefore, it is possible that targeting AI-2 production could mitigate the incidence of OM in children that are colonized with these two species of bacteria.
In conclusion, these studies show that M. catarrhalis and S. pneumoniae can form polymicrobial communities which, under antibiotic and environmental pressure, can render either bacterium more resistant to clearance. It is important to understand the impact of polymicrobial communities on otopathogens and other nasopharyngeal normal flora to develop better strategies for preventing and treating OM.
Acknowledgments
The authors thank Anthony Campagnari and David Briles for providing antibodies and Eric R. Lafontaine for providing M. catarrhalis strain O35E bro−. This work was supported by grants from NIH/NIDCD (DC007444, DC10051, and DC12205) awarded to W.E.S. A.C.P. and K.A.M. were also supported by NIH training grant (T32AI007401).
References
- Armbruster CE, Hong W, Pang B, Weimer KE, Juneau RA, Turner J, Swords WE. Indirect pathogenicity of Haemophilus influenzae and Moraxella catarrhalis in polymicrobial otitis media occurs via interspecies quorum signaling. MBio. 2010;1:102–110. doi: 10.1128/mBio.00102-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balder R, Shaffer TL, Lafontaine ER. Moraxella catarrhalis uses a twin-arginine translocation system to secrete the beta-lactamase BRO-2. BMC Microbiol. 2013;13:140. doi: 10.1186/1471-2180-13-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard S, Spaniol V, Aebi C. Molecular pathogenesis of infections caused by Moraxella catarrhalis in children. Swiss Med Wkly. 2012;142:w13694. doi: 10.4414/smw.2012.13694. [DOI] [PubMed] [Google Scholar]
- Bootsma HJ, van Dijk H, Verhoef J, Fleer A, Mooi FR. Molecular characterization of the BRO beta-lactamase of Moraxella (Branhamella) catarrhalis. Antimicrob Agents Chemother. 1996;40:966–972. doi: 10.1128/aac.40.4.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook I. The role of beta-lactamase-producing-bacteria in mixed infections. BMC Infect Dis. 2009;9:202. doi: 10.1186/1471-2334-9-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles DE, Crain MJ, Gray BM, Forman C, Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992;60:111–116. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhani RK, Struthers JK. The use of Sorbarod biofilms to study the antimicrobial susceptibility of a strain of Streptococcus pneumoniae. J Antimicrob Chemother. 1997;40:601–602. doi: 10.1093/jac/40.4.601. [DOI] [PubMed] [Google Scholar]
- Budhani RK, Struthers JK. Interaction of Streptococcus pneumoniae and Moraxella catarrhalis: investigation of the indirect pathogenic role of beta-lactamase-producing Moraxellae by use of a continuous-culture biofilm system. Antimicrob Agents Chemother. 1998;42:2521–2526. doi: 10.1128/aac.42.10.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiavolini D, Pozzi G, Ricci S. Animal models of Streptococcus pneumoniae disease. Clin Microbiol Rev. 2008;21(4):666–85. doi: 10.1128/CMR.00012-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chonmaitree T, Revai K, Grady JJ, et al. Viral upper respiratory tract infection and otitis media complication in young children. Clin Infect Dis. 2008;46:815–823. doi: 10.1086/528685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich GD, Veeh R, Wang X, et al. Mucosal biofilm formation on middle-ear mucosa in the chinchilla model of otitis media. JAMA. 2002;287:1710–1715. doi: 10.1001/jama.287.13.1710. [DOI] [PubMed] [Google Scholar]
- Eliasson I, Kamme C, Vang M, Waley SG. Characterization of cell-bound papain-soluble beta-lactamases in BRO-1 and BRO-2 producing strains of Moraxella (Branhamella) catarrhalis and Moraxella nonliquefaciens. Eur J Clin Microbiol Infect Dis. 1992;11:313–321. doi: 10.1007/BF01962070. [DOI] [PubMed] [Google Scholar]
- Furano K, Luke NR, Howlett AJ, Campagnari AA. Identification of a conserved Moraxella catarrhalis haemoglobin-utilization protein, MhuA. Microbiology. 2005;151:1151–1158. doi: 10.1099/mic.0.27820-0. [DOI] [PubMed] [Google Scholar]
- Gilson L, Kuo A, Dunlap PV. AinS and a new family of autoinducer synthesis proteins. J Bacteriol. 1995;177:6946–6951. doi: 10.1128/jb.177.23.6946-6951.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L, Hu FZ, Gieseke A, et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296:202–211. doi: 10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison CJ, Woods C, Stout G, Martin B, Selvarangan R. Susceptibilities of Haemophilus influenzae, Streptococcus pneumoniae, including serotype 19A, and Moraxella catarrhalis paediatric isolates from 2005 to 2007 to commonly used antibiotics. J Antimicrob Chemother. 2009;63:511–9. doi: 10.1093/jac/dkn538. [DOI] [PubMed] [Google Scholar]
- Holder RC, Kirse DJ, Evans AK, Peters TR, Poehling KA, Swords WE, Reid SD. One third of middle ear effusions from children undergoing tympanostomy tube placement had multiple bacterial pathogens. BMC Pediatr. 2012;12:87. doi: 10.1186/1471-2431-12-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopman TC, Liu W, Joslin SN, et al. Use of the chinchilla model for nasopharyngeal colonization to study gene expression by Moraxella catarrhalis. Infect Immun. 2012;80 (3):982–995. doi: 10.1128/IAI.05918-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaieda S, Hisakazu Y, Okitsu N, Hosaka Y, Okamoto R, Inoue M, Takahashi H. In vitro investigation of the indirect pathogenicity of beta-lactamase-producing microorganisms in the nasopharyngeal microflora. Int J Pediatr Otorhinolaryngol. 2005;69:479–85. doi: 10.1016/j.ijporl.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Klein JO. The burden of otitis media. Vaccine. 2000;19:S2–8. doi: 10.1016/s0264-410x(00)00271-1. [DOI] [PubMed] [Google Scholar]
- Kuo A, Blough NV, Dunlap PV. Multiple N-acyl-L-homoserine lactone autoinducers of luminescence in the marine symbiotic bacterium Vibrio fischeri. J Bacteriol. 1994;176:7558–7565. doi: 10.1128/jb.176.24.7558-7565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy A, McGrath J, Cripps AW, Kyd JM. The incidence of Streptococcus pneumoniae otitis media is affected by the polymicrobial environment particularly Moraxella catarrhalis in a mouse nasal colonisation model. Microbes Infect. 2009;11:545–553. doi: 10.1016/j.micinf.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Maddocks JL. Indirect pathogenicity. J Antimicrob Chemother. 1980;6:307–309. doi: 10.1093/jac/6.3.307. [DOI] [PubMed] [Google Scholar]
- Maddocks JL, May JR. “Indirect pathogenicity” of penicillinase-producing enterobacteria in chronic bronchial infections. Lancet. 1969;1:793–795. doi: 10.1016/s0140-6736(69)92063-7. [DOI] [PubMed] [Google Scholar]
- Palaniappan R, Singh S, Singh UP, et al. Differential PsaA-, PspA-, PspC-, and PdB-specific immune responses in a mouse model of pneumococcal carriage. Infect Immun. 2005;73:1006–1013. doi: 10.1128/IAI.73.2.1006-1013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew MM, Gent JF, Revai K, Patel JA, Chonmaitree T. Microbial interactions during upper respiratory tract infections. Emerg Infect Dis. 2008;14:1584–1591. doi: 10.3201/eid1410.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post JC. Direct evidence of bacterial biofilms in otitis media. Laryngoscope. 2001;111:2083–2094. doi: 10.1097/00005537-200112000-00001. [DOI] [PubMed] [Google Scholar]
- Revai K, Mamidi D, Chonmaitree T. Association of nasopharyngeal bacterial colonization during upper respiratory tract infection and the development of acute otitis media. Clin Infect Dis. 2008;46:e34–37. doi: 10.1086/525856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruohola A, Pettigrew MM, Lindholm L, et al. Bacterial and viral interactions within the nasopharynx contribute to the risk of acute otitis media. J Infect. 2013;66:247–254. doi: 10.1016/j.jinf.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaar V, Nordstrom T, Morgelin M, Riesbeck K. Moraxella catarrhalis outer membrane vesicles carry beta-lactamase and promote survival of Streptococcus pneumoniae and Haemophilus influenzae by inactivating amoxicillin. Antimicrob Agents Chemother. 2011;55:3845–3853. doi: 10.1128/AAC.01772-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroeher UH, Paton AW, Ogunniyi AD, Paton JC. Mutation of luxS of Streptococcus pneumoniae affects virulence in a mouse model. Infect Immun. 2003;6:3206–12. doi: 10.1128/IAI.71.6.3206-3212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surette MG, Miller MB, Bassler BL. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc Natl Acad Sci USA. 1999;96:1639–1644. doi: 10.1073/pnas.96.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swords WE. Nontypeable Haemophilus influenzae biofilms: evidence and relevance. Frontiers in Cell Infect Microbiol. 2012 doi: 10.3389/fcimb.2012.00097. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unhanand M, Maciver I, Ramilo O, Arencibia-Mireles O, Argyle JC, McCracken GH, Jr, Hansen EJ. Pulmonary clearance of Moraxella catarrhalis in an animal model. J Infect Dis. 1992;165:644–650. doi: 10.1093/infdis/165.4.644. [DOI] [PubMed] [Google Scholar]
- Wallace RJ, Jr, Steingrube VA, Nash DR, et al. BRO beta-lactamases of Branhamella catarrhalis and Moraxella subgenus Moraxella, including evidence for chromosomal beta-lactamase transfer by conjugation in B. catarrhalis, M. nonliquefaciens, and M. lacunata. Antimicrob Agents Chemother. 1989;33:1845–1854. doi: 10.1128/aac.33.11.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer KE, Armbruster CE, Juneau RA, Hong W, Pang B, Swords WE. Coinfection with Haemophilus influenzae promotes pneumococcal biofilm formation during experimental otitis media and impedes the progression of pneumococcal disease. J Infect Dis. 2010;202(7):1068–1075. doi: 10.1086/656046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer KE, Juneau RA, Murrah KA, Pang B, Armbruster CE, Richardson SH, Swords WE. Divergent mechanisms for passive pneumococcal resistance to beta-lactam antibiotics in the presence of Haemophilus influenzae. J Infect Dis. 2011;203:549–555. doi: 10.1093/infdis/jiq087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitby PW, Morton DJ, Stull TL. Construction of antibiotic resistance cassettes with multiple paired restriction sites for insertional mutagenesis of Haemophilus influenzae. FEMS Microbiol Lett. 1998;158:57–60. doi: 10.1111/j.1574-6968.1998.tb12800.x. [DOI] [PubMed] [Google Scholar]
- Xavier KB, Bassler BL. LuxS quorum sensing: more than just a numbers game. Curr Opin Microbiol. 2003;6:191–197. doi: 10.1016/s1369-5274(03)00028-6. [DOI] [PubMed] [Google Scholar]
- Yother J, McDaniel LS, Briles DE. Transformation of encapsulated Streptococcus pneumoniae. J Bacteriol. 1986;168:1463–1465. doi: 10.1128/jb.168.3.1463-1465.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]