Abstract
Aims
Studies have shown association between common variants in the α6–β3 nicotinic receptor subunit gene cluster and nicotine dependence in European Ancestry populations. We investigate whether this generalizes to African Americans, whether the association is specific to nicotine dependence, and whether this region contains additional genetic contributors to nicotine dependence.
Design
We examined consistency of association across studies and race between the α6β3 nicotinic receptor subunit locus and nicotine, alcohol, marijuana, and cocaine dependence in three independent studies.
Setting
United States of America
Participants
European Americans and African Americans from three case control studies of substance dependence.
Measurements
Subjects were evaluated using the Semi-Structured Assessment for the Genetics of Alcoholism. Nicotine dependence was determined using the Fagerström Test for Nicotine Dependence.
Findings
rs13273442 was significantly associated to nicotine dependence across all three studies in both ancestry groups (OR=0.75, p=5.8 × 10−4 European Americans; OR=0.80, p=0.05 African Americans). No other substance dependence was consistently associated to this variant in either group. Another SNP in the region, rs4952, remains modestly associated with nicotine dependence in the combined data after conditioning on rs13273442.
Conclusions
The common variant rs13273442 in the CHRNB3-CHNRA6 region is significantly associated to nicotine dependence in European Americans and African Americans across studies recruited for nicotine, alcohol, and cocaine dependence. Although these data are modestly powered for other substances, our results provide no evidence that correlates of rs13273442 represent a general substance dependence liability. Additional variants likely account for some of the association of this region to nicotine dependence.
INTRODUCTION
Though the genetics of substance dependence are complex, recent studies have successfully identified several genes that contribute to the development of nicotine dependence. The region of chromosome 8p11 that includes the α6 –β3 nicotinic receptor subunit gene cluster has been associated with smoking behavior (1–7). A set of common, highly correlated variants (r2=1.0) tagged by rs13273442, rs6474412, and rs1451240 is associated with smoking behaviors at genome-wide significance in European ancestry populations: for the quantitative smoking phenotype “cigarettes per day” (p = 1.3 × 10−8 using rs6474412 (1)) and for dependence using a case-control phenotype based on the Fagerström Test for Nicotine Dependence (8) (p = 2.4 × 10−8 using rs1451240 (7)). When robust evidence is discovered that a genetic variant contributes to dependence on a particular substance, three questions logically arise: (1) Is the identified association robust across different ancestral populations? (2) Is the association specific to a single substance, or does it represent a general substance dependence risk? (3) Are there additional, statistically independent genetic associations in the region?
The goal of this study is to explore these three questions. To examine consistency of the genetic association across populations, we meta-analyzed three independent studies of substance dependence, which collectively include 5171 subjects of European American and African American descent. Comparing results in both populations is essential for determining whether a finding can be generalized and may help identify contributors to health disparities between African Americans and European Americans (9). For example, although African Americans smoke fewer cigarettes than European Americans, they have a higher incidence of lung cancer (76 vs. 70 per 100,000) (10). This health disparity underscores the need for studies to identify genetic factors contributing to nicotine dependence in African Americans. To test whether the genetic association is specific to nicotine dependence or whether rs13273442 tags a non-specific genetic liability to substance dependence, we utilized the fact that each study comprehensively assessed nicotine, alcohol, marijuana, and cocaine dependence. Whether or not the variation in this correlated cluster is specific to nicotine dependence is a key factor for improved understanding of the underlying biology of dependence. Finally, to examine the question of additional genetic associations to nicotine in this region, we performed analyses of rs4952 conditioned on the rs13273442 genotype. We targeted rs4952 as a potential second contributor to nicotine dependence in this region because it has been previously reported as associated to nicotine dependence (6), and it is only modestly correlated to rs13273442 (r2=0.103, D’=1.0 in European Americans, r2=0.005, D’=1.0 in African Americans). Knowledge of multiple signals in this region will help determine the focus of future genetic research.
METHODS
Data
Subjects were recruited by three independent studies of addiction: the Collaborative Genetic Study of Nicotine Dependence (COGEND), the Collaborative Study on the Genetics of Alcoholism (COGA), and the Family Study of Cocaine Dependence (FSCD). The Institutional Review Board at each contributing institution reviewed and approved the protocols for genetic studies under which all subjects were recruited. Subjects provided written informed consent. All subjects included in these analyses passed high quality control procedures for phenotypes and genotypes.
Collaborative Genetic Study of Nicotine Dependence (COGEND)
COGEND was designed as a community-based study of nicotine dependence. Subjects were recruited from Detroit, MI, and St. Louis, MO. More than 53,000 subjects were screened by telephone, more than 2,800 were personally interviewed, and over 2,700 donated blood samples for genetic studies (5, 6). To be recruited, subjects had to meet one of two ascertainment criteria. Nicotine dependent case subjects were current smokers who met criteria for nicotine dependence defined as having a current Fagerström Test for Nicotine Dependence (FTND) score of 4 or more (5). Non-dependent control subjects smoked at least 100 cigarettes in their lifetime with a lifetime maximum FTND score of 0 or 1. As part of the study design strategy, controls were over-sampled to have a lifetime maximum FTND score of 0, with 99.2% (910/917) of the European controls having a score of 0 and 82.1% (193/235) of the African American controls having a score of 0. There were no inclusionary or exclusionary criteria regarding alcohol or drug dependence. The present study includes 2597 unrelated, genotyped subjects from COGEND.
Collaborative Study on the Genetics of Alcoholism (COGA)
An alcohol dependent case and non-alcohol dependent control series of unrelated individuals was selected from more than 10,000 subjects who participated in the genetic arm of COGA. COGA systematically recruited families with multiple members affected with alcohol dependence and community-based comparison families from participating centers across the United States. From this larger sample, a subset of unrelated alcohol dependent individuals was selected for genotyping. (11). Control subjects, who used alcohol but never met criteria for alcohol or drug dependence, were selected for genotyping. Because this study focused on alcohol dependence, nicotine dependence was not an exclusionary criterion for the control subjects. The present study includes 1403 unrelated, genotyped subjects from COGA.
Family Study of Cocaine Dependence (FSCD)
FSCD was designed as a genetic and environmental study of drug dependence with a focus on cocaine dependence. Cocaine-dependent subjects were recruited systematically from chemical dependency treatment units in the greater St. Louis, MO, metropolitan area (12). Community-based control subjects were identified through the Missouri Family Registry and matched by age, race, gender, and residential zip code. The community-based control subjects had used alcohol, but never met criteria for any alcohol, nicotine, or other drug dependence. The present study includes 1171 unrelated genotyped subjects from FSCD.
Assessment
All three studies used an assessment of substance dependence based on the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) (13). This shared methodology of interview administration, question format, and queried domains allowed harmonization of phenotypic data across all studies. The Fagerström Test for Nicotine Dependence (FTND) was used to evaluate lifetime history of nicotine dependence, with a score of 4 or more defining “dependence” for this study. Lifetime history of dependence on alcohol, cocaine, and marijuana was determined according to DSM-IV criteria. Though opioid and sedative dependence were assessed, these diagnoses were not included in this analysis because of the small number of subjects dependent on these substances and reduced power to detect association.
Ancestry was assessed by self-report and confirmed for all participants through a principal component analysis of ancestry informative markers using EIGENSTRAT(14).
Demographics
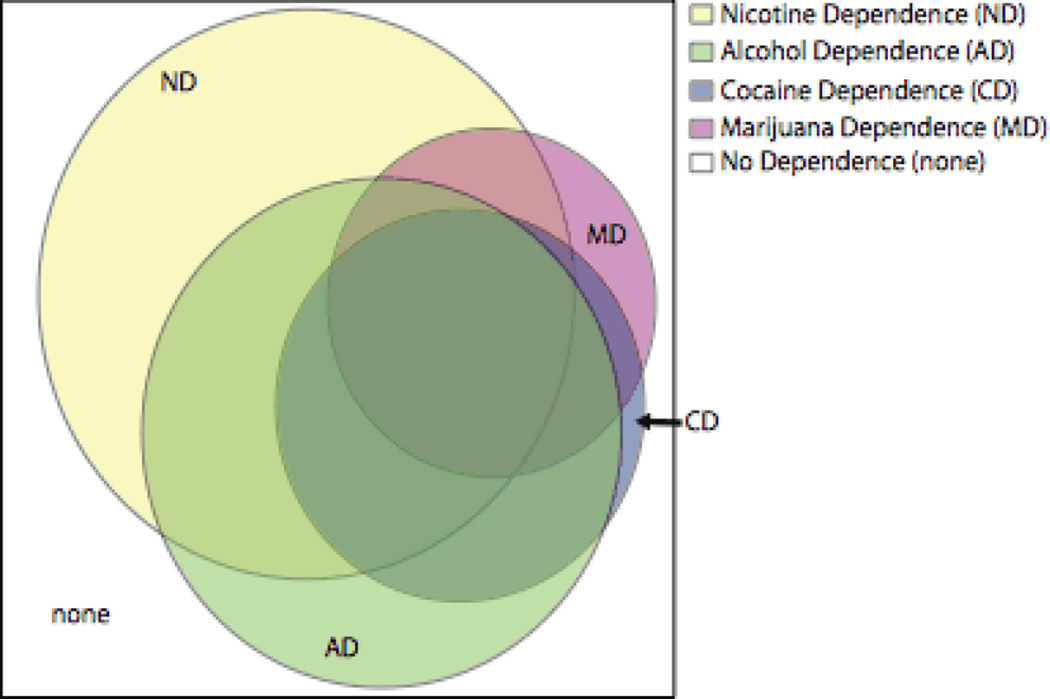
The characteristics of the study participants are listed in Table 1. The sample is 68% European American and 32% African-American. Comorbid substance dependence is common, with over half of the subjects dependent on any given substance being dependent on at least one other substance as well. The extent of comorbidity in these data is illustrated in Figure 1. Demographics broken down by ethnic subgroups can be found in Supplemental Table S1. Rates of substance use and rates of dependence among those who have used are shown in Supplemental Table S2. We define use of cigarettes as having smoked at least 100 cigarettes lifetime (a common definition). For the other substances, use is defined as having tried it at least once. Each study’s demographic characteristics are consistent with its recruitment design.
Table 1.
Characteristics of the sample
| Characteristic | COGEND | COGA | FSCD | Total |
|---|---|---|---|---|
| Sample size (N) | 2575 | 1347 | 1170 | 5092 |
| Sex, N (%) | ||||
| Males | 978 (38.0%) | 713 (52.9%) | 581 (49.7%) | 2319 (44.8%) |
| Females | 1597 (62.0%) | 634 (47.1%) | 589 (50.3%) | 2852 (55.2%) |
| Age, years | ||||
| Mean ± SD | 36.6 ± 5.5 | 42.9 ± 10.3 | 37.0 ± 8.8 | 38.4 ± 8.3 |
| Range | 23 – 47 | 18 – 77 | 18 – 60 | 18 – 77 |
| Ancestry, n (%) | ||||
| European-American | 1899 (73.7%) | 974 (72.3%) | 558 (47.7%) | 3498 (67.6%) |
| African-American | 676 (26.3%) | 373 (27.7%) | 612 (52.3%) | 1673 (32.4%) |
| Diagnoses, n (%) | ||||
| Nicotine dependence | 1423 (55.3%) | 634 (47.1%) | 448 (38.3%) | 2505 (49.2%) |
| Alcohol dependence | 574 (22.3%) | 855 (63.5%) | 548 (46.8%) | 1977 (38.8%) |
| Cocaine dependence | 209 (8.1%) | 384 (28.5%) | 555 (47.4%) | 1148 (22.6%) |
| Marijuana dependence | 295 (11.5%) | 289 (21.5%) | 300 (25.6%) | 884 (17.4%) |
COGEND: Collaborative Genetic Study of Nicotine Dependence
COGA: Collaborative Study on the Genetics of Alcoholism
FSCD: Family Study of Cocaine Dependence
Figure 1. Comorbidity in the combined COGEND, COGA, and FSCD data.
Areas of the circles are proportional to the fraction of the sample dependent on each substance. Alcohol Dependence (38.8% of the subjects), Cocaine Dependence (22.6%), and Marijuana Dependence (17.4%) were defined by DSM-IV criteria. Nicotine Dependence (49.2%) was defined as FTND score > 4. Overlap represents comorbidity. For instance, in these data, most of the subjects who were dependent on cocaine were also dependent on alcohol. In this sample, 34.4% of the individuals were not dependent on any of the 4 substances.
Genotyping and Data Cleaning
All genotyping was performed by the Center for Inherited Disease Research (CIDR). The COGA and FSCD samples were genotyped using Illumina Human1Mv1_C BeadChips. COGEND subjects were genotyped using two genome-wide SNP arrays: Illumina Human1Mv1_C and Illumina HumanOmni2.5M. Extensive cleaning was undertaken to ensure high-quality genotyping by examining batch effects, call rates, and other quality metrics (11).
Our analyses of consistency of association across populations and specificity of substance focused on correlates (r2=1.0 in the European population) of the common chromosome 8p11 variants rs6474412 and rs1451240 previously reported as having GWAS significant associations to nicotine dependence. The SNP Annotation and Proxy Search (SNAP) tool (15) using the 1000 Genomes CEU sample identified 18 additional SNPs that are highly correlated (r2=1.0) to these two SNPs in subjects of European ancestry. Only one SNP genotyped in our data, rs13273442, is highly correlated in both Europeans (r2=1.0) and in the African Yoruba (YRI) populations (r2≥0.89) to the two SNPs rs6474412 and rs1451240. The A allele displays a frequency of 0.25 in the European CEU reference sample and a frequency of 0.64 in the African YRI reference sample. Supplemental Table S3 provides information about the correlations in YRI for the 20 SNPs that are perfectly correlated in the CEU reference.
Our examination of whether variants in addition to rs13273442 may contribute to the association between the CHRNB3-CHRNA6 region and nicotine dependence focused on the nearby variant rs4952. The T allele of rs4952 is uncommon in both European Americans (3.0%) and African Americans (0.8%). The variant rs4952 is in linkage disequilibrium with rs13273442, but only modestly correlated to it (r2=0.103, D’=1.0 in European Americans, r2=0.005, D’=1.0 in African Americans).
Statistical Analyses
The primary analysis approach for all three arms of our investigation was a meta-analysis of odds ratios using a consistently coded reference allele across the three studies in our largest ethnic sample, the subjects of European ancestry. This strategy maximized power to detect robust association while recognizing the potential impact of differences in ascertainment protocols and environmental differences among the studies. The analysis of the African American samples explored the consistency of signals in a distinct ancestral group. Finally, we conducted meta-analyses of the 6 ancestry-by-study strata to evaluate evidence for or against a consistent association across ancestral groups.
All computations of odds ratios (OR) and standard errors for the sub-studies were performed using SAS (16). In each analysis, covariates representing sex and age [using quartiles, defined by 34 years and younger (reference), 35–39 years, 40–44 years, and 45 years and older] were included as categorical variables. Because use of a substance is required to develop dependence, binary covariates representing use of each of the 4 substances were also included. Meta-analyses were performed using the R package r-meta. None of the corresponding tests for heterogeneity based on Woolf’s Test were significant (range p=0.13 to p=0.95). For this reason, reported p-values for the meta-analyses are based on fixed effects models.
Primary Genetic Association Analyses
To account for high rates of comorbid substance dependence in these subjects, we chose a method that could simultaneously model these disorders and their association with genotype (17). We utilized a logistic regression method in which genotype (coded 0, 1, or 2 to represent the number of coded risk alleles) is expressed as the dependent outcome variable:
In this model P1 and P2 represent an individual’s probability of carrying one or two copies of the risk allele, respectively, and the Di represent diagnoses for dependence on the substances evaluated in this study: nicotine, alcohol, marijuana, and cocaine. This model makes a “proportional odds” assumption, which, in this case, is equivalent to assuming an additive genetic model. The demographic covariates used were sex and age quartiles. This model was then extended to include covariates for “use” of each substance as well as dependence. Inclusion of “use” covariates creates a more complex model, but has the advantage of distinguishing between individuals who have used a substance, but not become dependent from those who cannot be dependent because they have never used the substance. These primary models, which controlled for demographics and substance use covariates within each study-by-ancestry stratum, were then meta-analyzed. The stratified results along with the meta-analysis results were the primary tools we used to investigate the cross-population consistency of results and the question of whether rs13273442 represented a general liability to dependence on any substance, or if it appeared to be specific to nicotine dependence.
To investigate the question of whether rs4952 reflected additional association between this region and nicotine dependence, we used a traditional logistic regression model. Nicotine dependence was the dependent variable, and rs4952 was the predictor of interest. The variant rs13273442 was included as a conditioning covariate, and variables representing dependence on alcohol, cocaine, and marijuana were included to account for comorbidity, along with sex and age quartiles.
RESULTS
Association of rs13273442 in European and African Ancestry Samples
The variant rs13273442 is associated with nicotine dependence in the European Ancestry group (Table 2). The point estimates of the odds ratios display a consistent direction of effect for the A allele across all three studies, resulting in a highly significant association (p = 5.8 × 104) between the variant and nicotine dependence after adjusting for comorbid alcohol, marijuana, and cocaine dependence. The meta-analyzed European ancestry samples result in an estimated OR=0.75 (95% CI=(0.64–0.88)).
Table 2.
Association between rs13273442 genotype and dependence for multiple substances in each of the individual sub-samples together with meta-analysis within ancestry group and across all strata (Fixed effects meta-analysis model)
| Nicotine | Alcohol | Cocaine | Marijuana | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ancestry | Study | N | OR | (CI) | p | OR | (CI) | p | OR | (CI) | p | OR | (CI) | p |
| EA | COGEND | 1899 | 0.79 | (0.65–0.96) | 0.02 | 1.03 | (0.82–1.29) | 0.82 | 1.06 | (0.70–1.62) | 0.77 | 0.79 | (0.55–1.12) | 0.18 |
| EA | COGA | 974 | 0.56 | (0.39–0.80) | 1.4e-3 | 1.31 | (0.88–1.96) | 0.18 | 1.18 | (0.78–1.79) | 0.44 | 0.85 | (0.58–1.26) | 0.42 |
| EA | FSCD | 558 | 0.99 | (0.59–1.72) | 0.99 | 0.82 | (0.49–1.37) | 0.45 | 1.45 | (0.74–2.84) | 0.27 | 1.08 | (0.66–1.76) | 0.77 |
| EA | Meta-analysis | 3431 | 0.75 | (0.64–0.88) | 5.8e-4 | 1.05 | (0.87–1.27) | 0.59 | 1.17 | (0.89–1.53) | 0.26 | 0.87 | (0.69–1.09) | 0.22 |
| AA | COGEND | 676 | 0.97 | (0.71–1.32) | 0.84 | 1.19 | (0.80–1.76) | 0.39 | 0.93 | (0.51–1.68) | 0.80 | 1.31 | (0.86–1.97) | 0.20 |
| AA | COGA | 373 | 0.58 | (0.36–0.96) | 0.03 | 1.66 | (0.86–3.18) | 0.13 | 1.19 | (0.64–2.19) | 0.58 | 1.00 | (0.61–1.65) | 0.99 |
| AA | FSCD | 612 | 0.69 | (0.45–1.08) | 0.11 | 0.92 | (0.61–1.37) | 0.67 | 1.21 | (0.62–2.38) | 0.58 | 1.19 | (0.78–1.80) | 0.42 |
| AA | Meta-analysis | 1661 | 0.80 | (0.64–1.00) | 0.05 | 1.12 | (0.87–1.45) | 0.37 | 1.09 | (0.76–1.57) | 0.64 | 1.18 | (0.91–1.52) | 0.20 |
| EA+AA | Meta-analysis | 5092 | 0.77 | (0.67–0.88) | 8.6e-5 | 1.08 | (0.93–1.25) | 0.34 | 1.14 | (0.92–1.42) | 0.24 | 1.00 | (0.84–1.18) | 0.97 |
COGEND: Collaborative Genetic Study of Nicotine Dependence
COGA: Collaborative Study on the Genetics of Alcoholism
FSCD: Family Study of Cocaine Dependence
EA: European ancestry
AA: African American
Results for dependence variables from analyses predicting genotypes using all substance dependencies, sex, and age quartile as predictors
MODEL: genotype = sex + age quartile + nicotine dependence + alcohol dependence + marijuana dependence + cocaine dependence + smoking exposure (Y/N at least 100 cigs/lifetime) + ever drank alcohol (Y/N) + ever used cocaine (Y/N) + ever smoked marijuana (Y/N)
Minimum p-value range for tests of heterogeneity: (0.14 – 0.95).
In the African American sample (Table 2), the results for rs13273442 are consistent with those found in the European ancestry sample. After including all four substance dependence diagnoses in the model, the meta-analysis results for nicotine dependence indicate a protective effect for the A allele seen in the point estimates in each of the sub-studies. Meta-analyzing the 3 African American samples results in a nominally significant association (p=0.05) and an odds ratio estimate (OR=0.80, 95% CI (0.64–1.00)) very similar to the one estimated from the European samples.
Meta-analyzing the 6 race-by-study strata (Table 2) tightened the confidence interval for the estimated nicotine dependence odds ratio of 0.77, corresponding to an improvement in the p-value to 8.6 × 10−5.
Although no significant heterogeneity was detected for any of the meta-analyses, for completeness, we include the results of meta-analysis using random effects models in Supplemental Table S4. Results from traditional logistic regression (predicting dependence status using the variant as a predictor) were consistent with our primary analyses and are included in Supplemental Table S5.
Association of rs13273442 is specific for nicotine dependence
None of the other substance dependence covariates displayed statistically significant association with rs13273442. Alcohol dependence is common in the European American subjects (37.8%, N=1298), and hence we expect this meta-analysis to be well powered to detect association to this substance. The European ancestry meta-analysis estimated OR for alcohol, adjusted for all other substances, is a modest 1.05 (p=0.59), and the odds ratios point estimates in the different studies range from protective (0.82 for FSCD) to risk (1.31 for COGA). Similarly, there is no clear genetic contribution to cocaine or marijuana dependence (OR=1.17 and 0.87, respectively), but the power to detect an association for these substances is reduced due to the smaller number of dependence diagnoses (16.8% (N=578) and 15.8% (N=541), respectively).
Similar findings resulted from the African American analyses. Alcohol dependence does not demonstrate statistically significant independent association in these data (OR=1.12, p=0.37). Neither cocaine nor marijuana dependence are significantly associated with rs13273442, though the power to detect association is reduced due to the smaller number of individuals dependent on these substances (34.3%, (N=570) and 20.7%, (N=343), respectively). Furthermore, the direction of effect is not consistent across studies or populations.
By meta-analyzing the 6 race-by-study strata (Table 2), the differences between nicotine and the other substances became even more apparent. In contrast to the nicotine dependence meta-analysis p-value (8.6×10−5), the meta-analysis p-values for alcohol, cocaine, and marijuana are 0.34, 0.24, and 0.97, respectively, again demonstrating no statistical evidence for association between variation in rs13273442 and dependence on these substances independent of the association with nicotine dependence.
Results of testing for an additional genetic contributor beyond rs13273442
Because of the low allele frequency of rs4952, this association was modeled as homozygous for the common allele (CC) versus all other genotypes (CT and TT). Results from our analyses of rs4952, controlling for rs13273442 by including it as a covariate in the model, are listed in Table 3. The power is decreased for this analysis compared to our primary analysis of rs13273442 due to the low frequency of the T allele of rs4952. Nonetheless, in both ancestry groups the summary estimate of effect is strongly protective (OR=0.75 for European Americans, OR=0.59 for African Americans), though the confidence intervals are wide. Meta-analysis across the ancestral populations yields a statistically significant odds ratio between the common CC genotype and carriers of the T allele (OR=0.72, p=0.02).
Table 3.
Association between rs4952 and nicotine dependence, conditioned on rs13273442 (Fixed effects meta-analysis model)
| Nicotine (Fixed Effects) | Het p | |||||
|---|---|---|---|---|---|---|
| Ancestry | Study | N | OR | (CI) | p | |
| EA | COGEND | 1899 | 0.82 | (0.56–1.21) | 0.32 | |
| EA | COGA | 974 | 0.44 | (0.22–0.86) | 0.02 | |
| EA | FSCD | 558 | 1.39 | (0.47–4.12) | 0.55 | |
| EA | Meta-analysis | 3431 | 0.75 | (0.54–1.03) | 0.08 | 0.15 |
| AA | COGEND | 676 | 0.43 | (0.16–1.17) | 0.10 | |
| AA | COGA | 373 | 1.38 | (0.19–10.14) | 0.75 | |
| AA | FSCD | 612 | 1.81 | (0.19–17.19) | 0.61 | |
| AA | Meta-analysis | 1661 | 0.59 | (0.28–1.17) | 0.13 | 0.33 |
| EA+AA | Meta-analysis | 5092 | 0.72 | (0.53–0.96) | 0.02 | 0.26 |
Het p = p-value for a test of heterogeneity among the meta-analysis strata
Model: nicotine dependence = rs4952 + rs13273442 + sex + age quartile + alcohol dependence + cocaine dependence + marijuana dependence
DISCUSSION
When a genetic risk factor for the development of substance dependence is identified, several questions arise. These coordinated meta-analyses of data from three studies that focused on nicotine, alcohol, and cocaine dependence increase our understanding of the role that variants in the chromosome 8p11 region, which includes the α6-β3 nicotinic receptor subunit gene cluster, play in the development of substance dependence.
First, this study extends the finding of genetic association with nicotine dependence into an African American population. Thus far, the majority of genetic studies, including those of substance dependence, have been conducted using European ancestry samples (18). Our findings indicate that the A allele of rs13273442 is associated with protection from nicotine dependence in African American subjects as well as European American subjects. This allele, which is common in both populations, displays a consistent protective effect across all three of our African American samples and represents a similar strong decrease in risk for each copy of the A allele carried in both ancestral populations (OR=0.80 for African Americans; OR=0.75 for European Americans). Meta-analyzing the European Ancestry and African American datasets strengthened the statistical evidence for association, resulting in a p-value of 8.6 × 105 and further increasing confidence in the validity of this genetic finding. This conclusion is supported by a recent study of nicotine dependence that focused on this region (19) and reported significant association for rs6474412, a correlate of rs13273442 (r2 ≥ 0.89) in three populations: European-American, African-American, and Asian.
Next, these analyses demonstrate that the association between a common variant in the chromosome 8p11region is specific to nicotine dependence and does not represent a general substance dependence liability locus. These meta-analyses found that the nicotine dependence result is robust, even after adjusting for comorbidity with other substances. In contrast, none of the other three dependence diagnoses (alcohol, cocaine, marijuana) displayed a statistically significant association in the meta-analysis. The lack of association results must be interpreted with knowledge of the limitations of power. The sample sizes for the individual strata were modest. For the modest effect sizes expected for complex phenotypes such as alcohol and other drug dependence, large samples are needed to confidently rule out an association. Thus, while the evidence points to this locus having a specific risk effect associated with nicotine dependence, more modest genetic risks for alcohol, marijuana, or cocaine may be present, but beyond the limits of detection in this study.
We also examined this locus to see if the association signal was represented by one variant or if there were potentially multiple distinct variants associated with nicotine dependence. The variant rs4952 was the focus of this investigation, and we chose it because of its low correlation to rs13273442 in both ancestral populations (r2=0.10, in European Americans, r2<0.001 in African Americans) and a previous report of association to nicotine dependence (6). We recognized that the power of this analysis would be decreased due to the low frequency of the T allele for rs4952 (European Americans (3.0%) and African Americans (0.8%)). In spite of the modest power, tests of association between rs4952 and nicotine dependence conditioned on the common variant rs13273442 resulted in point estimates indicating a protective effect for the minor allele in both groups (OR=0.75 for European Americans, OR=0.59 for African Americans). Meta-analysis across the two ancestral groups tightened the confidence interval enough that the result is statistically significant (OR=0.72; p=0.02). We thus believe that multiple variants in this region contribute to the genetic risk for nicotine dependence.
In conclusion, substance dependence has a major impact on public health, and smoking is a leading modifiable contributor to death worldwide, killing more than 5 million people annually (20). Common variation in the chromosome 8p11 region, which contains the α6 and β3 nicotinic receptor subunit genes, contributes to heaviness of smoking in European Americans and is associated with nicotine dependence in African Americans. General liability and substance-specific genetic risk variants are predicted to contribute to the development of dependence. However, our data suggest that the genetic variants tagged by rs13273442 in this region most likely represent a risk factor that is specific to nicotine dependence. This variant does not appear to be associated with a general liability to alcohol or other drug dependence, though the power is reduced to confidently rule out a risk for other substance dependence. Finally, our findings suggest that multiple variants contribute to the association between this region and nicotine dependence. This work represents important next steps to improve our understanding of the genetic factors that underlie addiction, which we hope will provide insights into how to reduce nicotine dependence and improve smoking cessation.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by NIH grants R21DA033827 and R01DA026911 from the National Institute on Drug Abuse (NIDA).
The Collaborative Genetic Study of Nicotine Dependence (COGEND) is supported by NIH grant P01CA089392 from the National Cancer Institute (NCI).
The Collaborative Study on the Genetics of Alcoholism (COGA) is supported by NIH grant U10AA008401 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and NIDA.
The Family Study of Cocaine dependence was supported by NIH grants R01DA013423 and R01DA019963 from NIDA.
GWAS genotyping was performed at the Johns Hopkins University Center for Inherited Disease Research with support from NIAAA, NIDA, the NIH Gene Environment Initiative (U01HG004438, U01HG004422), NIH contract HHSN268200782096C, and NIH X01HG005274. Assistance with genotype cleaning was provided by the Gene Environment Association Studies Coordinating Center (U01HG004446).
Footnotes
Declarations of interest
Drs. Bierut and Goate are listed as inventors on Issued U.S. Patent 8,080,371,“Markers for Addiction” covering the use of certain SNPs in determining the diagnosis, prognosis, and treatment of addiction. Dr. NL Saccone is the spouse of Dr. SF Saccone, who is also listed as an inventor on the above patent. In 2013, Dr. Marc Schuckit gave a presentation on the genetics of alcohol to Anheuser-Busch-InBev executives at Yale University. He received the cost of his travel and an honorarium.
REFERENCES
- 1.Thorgeirsson TE, Gudbjartsson DF, Surakka I, et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet. 2010;42:448–453. doi: 10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoft NR, Corley RP, Mcqueen MB, et al. Genetic association of the CHRNA6 and CHRNB3 genes with tobacco dependence in a nationally representative sample. Neuropsychopharmacology. 2009;34:698–706. doi: 10.1038/npp.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeiger JS, Haberstick BC, Schlaepfer I, et al. The neuronal nicotinic receptor subunit genes (CHRNA6 and CHRNB3) are associated with subjective responses to tobacco. Hum Mol Genet. 2008;17:724–734. doi: 10.1093/hmg/ddm344. [DOI] [PubMed] [Google Scholar]
- 4.Ehringer MA, Mcqueen MB, Hoft NR, et al. Association of CHRN genes with "dizziness" to tobacco. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:600–609. doi: 10.1002/ajmg.b.31027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bierut LJ, Madden PA, Breslau N, et al. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet. 2007;16:24–35. doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saccone SF, Hinrichs AL, Saccone NL, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rice JP, Hartz SM, Agrawal A, et al. CHRNB3 is more strongly associated with Fagerstrom test for cigarette dependence-based nicotine dependence than cigarettes per day: phenotype definition changes genome-wide association studies results. Addiction. 2012;107:2019–2028. doi: 10.1111/j.1360-0443.2012.03922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. CDC Health Disparities and Inequalities Report — United States, 2011. Morbidity and Mortality Weekly Report. 2011 [Google Scholar]
- 10.Centers for Disease Control and Prevention. Racial/Ethnic Disparities and Geographic Differences in Lung Cancer Incidence --- 38 States and the District of Columbia, 1998–2006. Morbidity and Mortality Weekly Report. 2010:1434–1438. [PubMed] [Google Scholar]
- 11.Bierut LJ, Agrawal A, Bucholz KK, et al. A genome-wide association study of alcohol dependence. Proc Natl Acad Sci U S A. 2010;107:5082–5087. doi: 10.1073/pnas.0911109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bierut LJ, Strickland JR, Thompson JR, Afful SE, Cottler LB. Drug use and dependence in cocaine dependent subjects, community-based individuals, and their siblings. Drug Alcohol Depend. 2008;95:14–22. doi: 10.1016/j.drugalcdep.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bucholz KK, Cadoret R, Cloninger CR, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- 14.Price AL, Patterson NJ, Plenge RM, et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 15.Johnson AD, Handsaker RE, Pulit SL, et al. SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics. 2008;24:2938–2939. doi: 10.1093/bioinformatics/btn564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SAS/STAT. Cary, NC, USA: SAS Institute Inc; 2002–2003. [Google Scholar]
- 17.Grucza RA, Wang JC, Stitzel JA, et al. A risk allele for nicotine dependence in CHRNA5 is a protective allele for cocaine dependence. Biol Psychiatry. 2008;64:922–929. doi: 10.1016/j.biopsych.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartz SM, Johnson EO, Saccone NL, et al. Inclusion of African Americans in genetic studies: what is the barrier? Am J Epidemiol. 2011;174:336–344. doi: 10.1093/aje/kwr084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui WY, Wang S, Yang J, et al. Significant association of CHRNB3 variants with nicotine dependence in multiple ethnic populations. Mol Psychiatry. 2013 doi: 10.1038/mp.2012.190. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Tobacco fact sheet. 2011
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.