Abstract
Natural killer (NK) cells recognize deranged cells that display stress receptors or loss of major histocompatibility complex (MHC) class I. During development, NK cells become “licensed” only after they encounter cognate human leucocyte antigen (HLA) class I, leading to the acquisition of effector function. NK cells can be exploited for cancer therapy in several ways. These include targeting within monoclonal antibodies alone or combined with ex vivo and in vivo NK cell activation to facilitate adoptive immunotherapy using donor-derived NK cell products to induce graft-vs-tumor effects. In the adoptive transfer setting, persistence and in vivo expansion requires lymphodepleting chemotherapy to prevent rejection and provide homeostatic cytokines (such as IL-15) that activate NK cells. IL-15 has the advantage of avoiding regulatory T-cell expansion. Clinical applications are currently being tested. To enhance in vivo expansion, IL-2 has been used at low doses. However, low dose administration also leads to the stimulation of regulatory T cells. Monoclonal antibodies and bispecific killer engagers (BiKEs) may enhance specificity by targeting CD16 on NK cells to tumor antigens. Inhibition of CD16 shedding may also promote enhanced cytotoxicity. Future strategies include exploiting favorable donor immunogenetics or ex vivo expansion of NK cells from blood, progenitors, or pluripotent cells. Comparative clinical trials are needed to test these approaches.
Keywords: NK cells, adoptive cell therapy, immunotherapy, immunomodulation, acute myeloid leukemia
1.0 Introduction
The use of cellular immunotherapy has increased significantly over the past two decades. Discoveries in basic NK cell biology, together with growing clinical experience in the setting of hematopoietic cell transplantation (HCT), have placed NK cells along a trajectory of translational development. A deeper understanding of the interactions between active and suppressive immune cells in response to infected or malignantly transformed cells has been critical in the development of NK cell therapies for cancer. Major clinical advances include the use of cytokines to activate NK cells in vivo [1–3], use of monoclonal antibodies to redirect or enhance NK cell function [4–6], adoptive transfer of T cells or natural killer (NK) cells with anti-tumor activity [7, 8] and engineering adoptively transferred cells with artificial receptors specific for particular cell surface tumor antigens [9–11].
This review is focused upon the clinical utility of NK cells in patients with hematologic malignancies. We will review NK cell biology, the role of NK cells in hematopoietic cell transplantation, the rationale for using NK cells in cancer therapy, strategies to activate and expand NK cells and advances in adoptive transfer of autologous or allogeneic NK cells to treat cancer. Methods to optimize NK cell expansion and alloreactivity and limitations of current approaches are also considered, as are novel therapies intended to exploit unique properties of NK cells to advance cancer treatment. The ultimate goal of NK cell research is to transition from ex vivo isolation, expansion, and activation to optimizing therapeutic efficacy in appropriately selected patients.
2.0 NK cell biology
Overview of NK cell phenotype, development, and receptors
Natural killer cells are innate lymphocytes with the capacity to recognize cells that have undergone viral infection or malignant transformation. They are so named for their “natural” ability to kill cancer cells without prior sensitization [12]. As demonstrated in patients lacking functional NK cells, they are important for defense against a variety of cancers and viral infections [13–15]. NK cells develop in the bone marrow [16] and then home to secondary lymphoid tissues where they undergo distinct developmental intermediates [17–19]. In humans, they are defined by the expression of the surface marker CD56 and the lack of the T cell marker CD3. NK cells account for 10–15% of peripheral blood lymphocytes. The CD56 subset can be further divided into “bright” and “dim” populations. CD56bright cells are primarily located in secondary lymphoid tissues and are thought to support adaptive immunity through secretion of cytokines [20]. CD56dim NK cells are the cytotoxic population and comprise approximately 90% of the NK cells circulating in the peripheral blood. In addition to their cytolytic capacity, CD56dim NK cells can also rapidly release cytokines upon receptor-mediated crosslinking [21]. They are dependent on the cytokine IL-15 for development, as demonstrated by their absence in mice lacking this cytokine, receptor or signaling pathway [22–25].
2.1 NK cell receptors
Unlike T or B lymphocytes containing somatically rearranged receptors specific for antigens, NK cells contain a germ line-encoded set of receptors governing both their development and function. These receptors transmit either activating or inhibitory signals. Integration of all of these downstream signaling pathways dictates how an NK cell will respond when interacting with its target. The most notable inhibitory receptors are the killer-immunoglobulin like (KIR) receptors that recognize human leukocyte antigen (HLA) class I proteins (HLA-A, -B, and –C). Individuals may have up to 15 KIR genes. These genes differ in both their extracellular and intracytoplasmic domains [26]. Discovery of antibodies recognizing the extracellular portion of inhibitory and activating KIR were pivotal to further studies investigating the mechanisms of NK cell mediated killing [27–29]. The extracellular domain of a KIR receptor determines which HLA class I molecules an NK cell can recognize. The intracytoplasmic domains can transmit either an activating or inhibitory signal. Inhibitory KIR contain immunoreceptor tyrosine-based inhibitory motifs (ITIMs) which, upon phosphorylation, recruit tyrosine phosphatases that inhibit cellular activation. In contrast to inhibitory KIR, activating KIR have a shorter intracellular domain that non-covalently associates with the signaling adapter DAP12 which contains an immunoreceptor tyrosine-based activation motif (ITAM). The ligands for the activating receptors are incompletely understood.
The 15 KIR genes reside in a single complex on chromosome 19. These genes are stochastically expressed throughout development [30, 31] and are dependent on DNA methylation status. Treatment with DNA methyltransferase inhibitors is known to increase KIR transcript levels [32]. The KIR locus is highly polymorphic. Individuals carrying an A haplotype have mainly inhibitory KIR with a single activating KIR. In contrast, KIR B haplotype individuals have more activating KIR [33, 34]. The heterodimer CD94/NKG2A is another inhibitory receptor expressed by NK cells. Its activating counterpart is NKG2C and both recognize conserved HLA class I leader sequences presented in the context of HLA-E [35]. Both KIR and NKG2A are important for NK cell development and function. Although the absolute deficiency of KIR has not been described in humans, mice lacking the homologous family of receptors (Ly49 family) have NK cells with diminished function [35, 36]. Conversely, mice lacking the homolog of NKG2A/CD94 have normal NK cells and are functionally competent [37].
Activating receptors recognize “stress ligands” on virally or malignantly transformed cells. NKG2D is constitutively expressed on all NK cells and recognizes two groups of proteins: 1) the major histocompatibility complex class I chain-related genes A and B (MICA and MICB) and 2) UL16 binding protein family members 1–6 (ULBP1–6) [38, 39]. Natural cytotoxicity receptors (NCR) NKp30 [40], NKp44 [41, 42], and NKp46 [43, 44] bind to either viral hemaglutinins, other viral ligands (CMV pp65), heat shock- associated proteins, or tumor antigens (B7-H6, BAT-3, MLL5 and proliferating cell nuclear antigen (PCNA)) [39, 41, 45–48]. Additional ligands likely exist [35]. Other activating molecules include DNAM-1 and those from the 2B4 family [49, 50].
One of the most potent mechanisms of NK cell activation is through the Fc receptor (FcγRIIIA, CD16) via a process called antibody-dependent cellular cytotoxicty (ADCC). Antibodies crosslink CD16 on the surface triggering a rise in intracellular calcium, activation of NFAT, production of cytokines (IFNγ, GM-CSF) and degranulation. Understanding the receptors dictating NK cell function is fundamental to advancing NK cell cancer therapeutics.
2.2 Missing-self hypothesis and licensing
NK cells in peripheral blood are thought to have a short half-life of less than 10 days. However, they are continually replenished through differentiation from hematopoietic stem cells [51]. During this interval, immature NK cells must undergo an education process leading to a functional NK cell maintaining tolerance to “self” but also with the ability to recognize “non-self.” NK cells express inhibitory KIR receptors specific for MHC class I antigens in a stochastic and variegated manner. They become functionally competent only after they encounter self-HLA molecules (e.g. HLA-C1, -C2, -Bw4) during a process termed “licensing”[52–54], or NK cell education. About 10–20% of NK cells remain “unlicensed”. These cells lack receptors that recognize self MHC and are consequently functionally hyporesponsive [55, 56]. However, such cells can become activated by endogenous cytokines or cytokine administration and therefore have the capacity to become allo-reactive upon adoptive transfer and homoeostatic proliferation in a recipient environment. This occurs after donor transplantation or adoptive transfer in both matched and mismatched HLA recipients. HCT recipients who receive grafts mismatched between inhibitory KIR and cognate HLA class I ligands have a higher proportion of allo-reactive NK cells.
As NK cells undergo this developmental process they can be separated into different stages based on expression of several surface markers [20]. After they have obtained functional competence, they then exit the bone marrow or secondary lymphoid tissue to survey secondary sites. The mechanisms of education remain under investigation.
2.3 NK cell function
NK cell “licensing” depends on having inhibitory KIR recognizing self-HLA ligands. The level of KIR expression on NK cells directly correlates with function against malignant targets [57, 58]. There are four major NK cell functions: 1) secretion of cytokines/chemokines (interferon-γ, TNF-α, RANTES, MIP1-α and –β), 2) direct cytotoxicity by release of perforins and granzyme, 3) target killing through apoptotic pathways using FasL and TRAIL receptors and 4) target killing through antibody-dependent cellular cytotoxicity. Blocking any of these pathways often results in a decrease in NK cell-mediated killing. Several cancers have evolved mechanisms to subvert these pathways and NK cell mediated recognition [59–61]. Interferon-γ is one of the most important cytokines secreted by NK cells and serves as an crucial bridge in forming adaptive immune responses [62]. It serves to upregulate death receptors and stress ligands on target cells. Cytokines such as IL-2, IL-15, IL-12, IL-18, and IL-21 activate NK cells in vitro and enhance NK cytotoxicity against malignant cells [3, 63, 64], even if these NK cells are “unlicensed”. The use of cytokines, while promising, can be limited by in vivo competition with other lymphocyte populations. [8]. This challenge can be partially overcome by implementing lymphodepleting chemotherapy to “create space” or with higher doses of cytokines, but both approaches increase risk of systemic toxicity. Because cytokines are essential for the in vivo survival and expansion of adoptively transferred cells, methods that improve patient safety are prerequisite.
3.0 The role of NK cells in hematopoietic stem cell transplantation
3.1 NK cell alloreactivity in allogeneic HSCT
Natural killer cells were initially recognized for their ability to lyse leukemic cells in mice [12, 65]. In 2002, Ruggeri et. al. demonstrated superior disease-free survival in acute myologenous leukemia (AML) patients receiving bone marrow grafts from HLA-haploidentical donors who expressed KIR receptors which bound to MHC class I molecules absent in the host (i.e., KIR ligand mismatch in the graft versus host (GVH) direction) [66]. They also had a decreased incidence of graft versus host disease (GVHD). The probability of survival in KIR-ligand mismatched patients was 65% at 5 years versus only 5% for those without a predicted alloreactive NK repertoire.
In the missing self-model, donor NK cells express inhibitory KIR receptors for which HLA class I molecules are missing in the recipient. When transferred (or developing from HSCs in vivo) these NK cells become alloreactive as they are not restrained by MHC class I. Interestingly, the effect is mostly limited to patients with AML. In a large cohort of over 2000 T cell-replete, HLA-matched unrelated hematopoietic cell transplant (HCT) recipients, we found that lack of at least 1 KIR ligand in the recipient led to less relapse from AML and chronic myeloid leukemia (CML). In another large study of 1770 patients, Hsu and colleagues used HLA-C group 1, group 2, and Bw6 as markers of missing recipient ligands, finding a beneficial effect on post-transplantation relapse from leukemia. Although not all studies agree, this effect is likely due to differences in donor source, T lymphocyte content and post-transplant immunosuppression [67–70]
With better understanding of KIR immunogenetics, we and others began to investigate KIR genotype and outcomes in HCT. KIR genes can be divided into two main haplotypes, A or B [34]. The A haplotype consists of predominately inhibitory KIR and a single activating KIR, KIR2DS4. The B haplotype contains both inhibitory and several activating KIRs that can be further subdivided into two separate regions, centromeric (Cen) or telomeric (Tel). Activating KIR, such as KIR2DS1, are likely involved in the anti-leukemia effects of NK cells. It has been shown that signaling through this receptor can overcome inhibitory signals mediated by NKG2A [71, 72].
McQueen and Parham were the first to report that transplants from KIR A/A donors into B/x recipients led to decreased survival in HLA-matched, T cell-replete sibling transplants [73]. When using T cell-depleted grafts, the KIR B haplotype (genes for KIR2DS1, KIR3DS1, and KIR2DL5A) reduced the rate of relapse and improve overall survival [74]. This effect was specific for AML. In larger patient cohorts, it has been shown that those who received transplants from donors homozygous for the centromeric B haplotype (Cen-B/B, containing more activating KIR) have improved outcomes. In 1409 patients with AML undergoing transplantation, Cooley et.al. found that donors with KIR-B haplotypes had lower rates of relapse with improved overall survival [33]. These data are currently being evaluated in a prospective trial to select the “best” donors using KIR immunogenotype in patients with AML for whom unrelated donor HCT is planned (see ClinicalTrials.gov and search NCT01288222).
In HCT, manipulating NK cell function during immune reconstitution could further enhance the graft-vs-leukemia (GVL) effects. While NK cells are the first lymphocyte subset to recover (prior to T cells), the earliest cells to reconstitute resemble CD56bright NK cells and are functionally immature, expressing high levels of NKG2A/CD94 and lower levels of inhibitory KIR [75, 76]. Foley et. al. found that regardless of stem cell source (unmanipulated, CD34+ selected, or umbilical cord blood UCB), NK cell function is suppressed early on during recovery. In each of the groups the ability to produce IFN-γ in response to malignant target cells was suppressed. NK cell function typically recovers 6 months post-transplantation except in the UCB donor group [57]. This difference is consistent with observations that NFAT expression (which drives IFN-γ production) is lower in UCB-derived lymphocytes [77]. In these and other studies, KIR expression correlated with improved function and supports the model of high affinity KIR-HLA allotype interactions leading to enhanced functional capacity. Interestingly, it has also been demonstrated that T cells in the graft may play a role in NK cell development and KIR expression [57, 78, 79]. Whether this process is solely dependent on T cell help and IL-2 production remains to be established [80].
3.2 CMV has a profound effect on NK cell reconstitution after HCT
A more recently appreciated variable in transplant responses is the expansion and enhancement of donor NK cell function following reactivation of cytomegalovirus (CMV) [81]. Although this potentially fatal pathogen is a concern in immunosuppressed patients, its presence may be beneficial in patients with AML. CMV typically lies latent in endothelial and myeloid cells and can reactivate to cause life-threatening illness in immunocompromised individuals. Patients remain at risk until they seroconvert and establish suppressive adaptive immunity through development of antigen-specific T cells and the production of CMV-specific antibodies. This fails to occur in the immunosuppressed state and therefore such patients require rapid identification and anti-viral therapy. A number of groups have recently reported that CMV reactivation early on following allogeneic transplant leads to reduced relapse in AML [82–84]. In one report, CMV reactivation did not appear to be detrimental to overall survival. Patients with CMV reactivation showed a decrease in the rate of leukemic relapse at 10 years from 42% to only 9%. In cases where all of the donors are CMV naïve, such as in umbilical cord blood (UCB) transplantation, CMV reactivation leads to enhanced reconstitution of functional NK cells [85, 86].
Several groups have reported induction of long-lived, or “memory-like”, NK cells in response to CMV infection [86, 87]. NK cells are traditionally thought to be short-lived cells [51] without antigen specificity. However, in response to mouse CMV (mCMV) infection, murine NK cells expressing the Ly49H receptor undergo a proliferative phase that is dependent on the mCMV antigen m157. The expanded cells then undergo a contraction phase to establish a pool of long-lived memory-like cells that persist for at least 70 days. This pool of memory cells also shows enhanced expansion and effector function following a secondary challenge with virus.
In humans, a clonal population of NK cells expanding in response to CMV infection expresses the activating receptor NKG2C, self-inhibitory KIR and CD57. Unlike the mouse, a CMV-specific ligand for NKG2C has not been identified. These human NK cells also show enhanced function, including cytokine production, direct cytotoxicity, and ADCC [81, 85]. It remains to be shown whether NKG2C+ cells induced by CMV reactivation play a direct role in the graft-vs-leukemia (GLV) effect and protection from relapse. If NKG2C+ NK cells were shown to be the optimal population for anti-leukemic activity, it would be worthwhile to explore this differentiation in the adoptive transfer setting as well.
CMV has a powerful effect in shaping the immune response. Such knowledge suggests the possibility that CMV vaccine strategies may mimic this effect. This approach is currently under study.
4.0 NK cells for cancer therapy
4.1 NCI trials provide lymphodepleting platform for adoptive immunotherapy
In pioneering studies at the NCI, Rosenberg and colleagues infused melanoma and renal cell carcinoma patients with autologous peripheral blood cells treated ex vivo with IL-2. The product was enriched with NK cells and named “lymphocyte activated killer” (LAK) cells. High dose IL-2 was administered to patients after LAK infusions to promote their in vivo persistence and activity. In a subsequent trial, the NCI group adoptively transferred in vitro-expanded autologous tumor-infiltrating lymphocytes (TILs) to 20 patients with metastatic melanoma. Objective responses were observed in 11 patients. Given the limited persistence of the transferred tumor-specific T-cells in vivo, a second course of TIL cells was infused following lympho-depleting chemotherapy combining high dose cyclophosphamide with fludarabine [88]. Objective responses were observed with TILs in patients with melanoma. These and other studies have contributed important new knowledge: 1) high-dose IL-2 used in vivo with the goal of activating NK cells has significant but manageable toxicity owing to severe capillary leak syndrome, whereas low-dose subcutaneous IL-2 was well tolerated, 2) lymphodepleting chemotherapy using high-dose cyclophosphamide and fludarabine facilitated in vivo expansion of autologous adoptively transferred cytotoxic T lymphocytes and lead to enhanced efficacy,3) chemotherapy induces lymphopenia, changes the competitive balance between transferred lymphocytes and endogenous lymphocytes, changes the cytokine milieu and depletes inhibitory cell populations (T regulatory cells [Tregs]) [8, 89–91].
4.2 Autologous NK cells with IL-2
At the University of Minnesota, we first tested the use of low dose IL-2 daily to expand NK cells after autologous HCT in patients with non-Hodgkin lymphoma and breast cancer. Later, we activated autologous NK cells ex vivo with IL-2 for 24 hours, infused them into patients and administered daily subcutaneous IL-2 [92]. Although analysis of these phase II autologous NK cell studies showed limited efficacy, they did yield important findings: 1) IL-2 can be administered safely at daily or 3 times weekly intervals, 2) IL-2 can induce an increase in circulating cytotoxic lymphocytes with a disproportionate increase in NK cells and, 3) recipient lymphocytes can compete for cytokines and “space”. Concurrently, the role of inhibitory KIR receptors expressed on the surface of NK cells was becoming clearer. Autologous NK cells were unlikely to kill tumors because of inhibitory signals delivered by self-MHC, leading the field to test the use of allogenic NK cell therapies.
Adoptive transfer of allogeneic NK cells
4.3 NK cells to treat acute myeloid leukemia
In the initial studies testing allogeneic NK cells in a non-transplantation setting, we compared two preparative regimens for optimal in vivo expansion (71). Family-related HLA haploidentical donors were used. Apheresis products were CD3-depleted and activated with 1000 IU IL-2 ex vivo for 24 hours. Patients received either low dose fludarabine for five days (25 mg/m2/day) or high dose cyclophosphamide for two days (60 mg/kg/day), followed by the same dose of fludarabine (Hi-Cy/Flu) prior to NK cell infusion. This regimen was chosen on the basis of an NCI platform in which lack of a preparative regimen depleting host hematopoietic cells (lymphoid and myeloid) limited survival of adoptively transferred cells. IL-2 was administered daily (1.75 million units/m2) for 14 days (subsequently modified to 6 higher doses [10 million units without m2 correction] over 2 weeks). Only patients receiving Hi-Cy/Flu had in vivo detectable donor-derived NK cells which persisted in peripheral blood by day 14. A marked increase in IL-15 concentration (up to 100 pg/dl) was detected in patients receiving Hi-Cy/Flu but not with fludarabine alone [89]. In addition, we reported the inverse correlation between the absolute lymphocyte count and the IL-15 concentration (r=0.62, p-value < .0001). These data suggest that decreasing numbers of mature lymphocytes, which utilize IL-15, can result in elevated plasma IL-15 concentrations. This approach allowed for optimal homeostatic proliferation of infused NK cells through depletion of host lymphocytes functioning as cytokine “sinks” [8, 88]. Aside from the expected toxicities associated with IL-2 administration, infusion of allogeneic NK cells appears safe without an increased risk of GVHD. Notably, 5 out of 19 patients with poor prognosis AML went into complete remission. Leukemia clearance correlated with the persistence and in vivo expansion of donor NK cells after adoptive transfer, which was assessed using a PCR-based chimerism assay for informative donor HLA alleles not present in the background of the recipient. Following enrollment of 23 additional patients with advanced AML, 10% of the subjects met criteria for successful NK cell expansion. Our group has prospectively defined successful expansion as measurement of >100 donor NK cells/µL of peripheral blood at day +14 after NK cell infusion [(absolute lymphocyte count/µL × % of lymphocyte gate that are CD56+/CD3− NK cells) × (% donor chimerism using standard short tandem repeat testing)]. This proof-of-principle clinical study laid the groundwork for future trials using haploidentical NK cells in the treatment of cancer (71). Long-term disease-free survivors included only those patients that went on to receive allogeneic transplantation. Patients not meeting transplant eligibility criteria relapsed within a year after NK cell therapy. These outcomes have guided protocol improvements discussed in section 4.4. Among additional studies of NK cell infusions following chemotherapy Rubnitz et.al. studied a small cohort of children (n=10) with intermediate risk AML who had been treated with front line chemotherapy for 5 cycles [93]. Patients then received lymphodepeleting chemotherapy with cyclophosphamide and fludarabine and NK cell infusions from a haploidentical family member followed by IL-2 for six doses. NK cells could be found in all patients after infusion, with 3 children having cells detected up to 1 month after infusion. The NK infusions were well tolerated. One patient had prolonged lymphocytopenia but no other toxicity was noted. Significantly, while these patients were expected to have a disease free survival rate of 60%, the actual rate was 100% at a median of 2 years follow-up. Because this was a pediatric study, the cell dose was relatively high. Outcomes suggest that perhaps higher doses of NK cells may be more efficacious. The fact that the NK cells were infused shortly after intensive AML therapy might also account for their persistence and possible efficacy. In another study, Curti et.al. tested allogeneic KIR ligand mismatched NK cell infusions in older AML patients (median age, 62 years; range 52–73) who had received the Cy/Flu regimen described above [94]. A transient response occurred in only one patient with active disease. Three of 6 patients in complete remission (CR) remained disease-free at 34,32, and 18 months.
4.4 NK cells and regulatory T cells
IL-2 administered to patients to expand NK cells in vivo has been observed to also expand suppressive T lymphocytes, called Tregs. Tregs express the high affinity IL-2 receptor (CD25, IL2Rα chain) and can both directly and indirectly suppress NK cell activity through either receptor-ligand interactions or by competition for IL-2 [95, 96]. Tregs also secrete high levels of TGF-β which can directly inhibit NK cell cytokine secretion and cytotoxicity [59]. Tumor derived TGF-β can also directly support the differentiation of Tregs within the tumor microenvironment. Our recent collective experience using donor-derived NK cells with IL-2 in AML and solid tumor patients suggests that future trials should incorporate strategies to interfere with suppressive elements such as Tregs and myeloid-derived suppressor cells.
In two subsequent clinical trials, we treated additional refractory AML patients with Hi-Cy/Flu lymphodepleting chemotherapy and allogeneic NK cells. Fifteen patients also received IL-2 diphtheria toxin (IL2DT), a recombinant cytotoxic fusion protein composed of the amino acid sequences for diphtheria toxin and truncated amino acid sequences for IL-2. We designed the study to test whether IL2DT depletes Tregs and improves donor NK cell persistence/expansion. All patients tolerated this regimen well. Donor NK cells were detectable at day 7 in 10 patients (median 68% donor DNA). At day 14, 27% had successfully expanded NK cells in vivo, with median absolute donor-derived NK cell counts of 1000 cells/µL blood. These results improved upon our previous rate of 10% observed with the same regimen but without Treg depletion. In vivo NK cell expansion at day 14 correlated with the absence of a bona fide Treg population at either day 7 or day 14. Fifty-three percent of patients receiving Treg depletion with IL2DT attained complete hematologic remission. Outcomes are significantly better compared to strategies without IL2DT, suggesting that the NK cells themselves played a role in the anti-leukemia response over and above the activity of the high-dose chemotherapy preparative regimen. Results further suggest that lower donor NK cell levels or donor chimerism for shorter time intervals (e.g., day 7 but not day 14) may be sufficient for clinical response.
4.5 NK cells for other cancers
Other trials using allogeneic NK cells have targeted breast, ovarian, non-Hodgkin lymphoma and non-small cell lung cancer [97–100]. Sixteen patients with high risk lung cancer were treated with haploidentical NK cells that were activated with IL-15 and hydrocortisone [98]. Patients could receive multiple infusions. Two patients demonstrated partial responses and six showed stable disease. Geller et. al. tested allogeneic NK cells in a phase II trial in 20 patients (14 ovarian cancer, 6 breast cancer). Following a non-myeloblative preparative regimen, investigators were able to detect donor NK cells 1 week after NK cell infusion in 15/19 patients. However, the absolute number of NK cells in peripheral blood was below 100 cells/µL. After a 2-week course of IL-2, mostly recipient T cells were detected. Those cells were predominantly of a Treg phenotype. Given additional data from the NCI on the role of total body irradiation (TBI) in enhancing the lymphodepleting regimen, 200 cGy TBI was added to the study protocol. Unfortunately, this did not result in better NK cell expansion because it lengthened the time to hematopoietic recovery and was judged too toxic based on excess myelosuppression. Other modifications, including addition of cyclosporine, gave no indication of improved NK cell expansion and were poorly tolerated by a few ovarian cancer patients.
In a small pilot study, we also evaluated infusion of haploidentical donor NK cells with rituximab and IL-2 for antitumor efficacy in patients with advanced chemotherapy-refractory lymphoma (including rituximab) [101]. At two months, four of six patients showed a demonstrable clinical response. We observed evidence of donor DNA in nodal sites, suggesting that donor-derived NK cells could home back to secondary lymphoid tissues. All patients showed substantial increases in host Treg after haploidentical NK cells and IL-2 therapy (180 ± 80 cells/ml at day 14 vs. baseline: 58 ± 24 cells/l, p = 0.04). This data suggests that inadequate immunodepletion and Treg persistence may contribute to a hostile milieu for NK cell survival and expansion. Although these studies proved safety and feasibility of allogeneic NK cells, lack of consistent NK cell expansion and interference of a tumor-induced suppressive environment remains a major barrier to clinical application.
4.6 NK cell production under Good Manufacturing Practice (GMP) conditions
Our NK product has changed over the years. Given the safety of apheresis methods for the donor, we have replaced a 3-hour apheresis product with a 5-hour product depleted of T cells and B cells using CD3 and CD19 beads. GMP cell processing resulted in a significant reduction of T cells in all products, decreasing to < 1% following CD3-depletion, yielding a final T cell dose of <3 × 105 cells/kg. There was an average of 40-fold less T cells than NK cells. Monocytes (sometimes >50%) comprised the other major component of the final product. While monocytes express IL-15 receptor alpha important for trans-presentation of IL-15, we do not yet understand their contribution to successful adoptive transfer. Although 5-hour apheresis allows for enhanced NK cell doses up to 20 × 106 cells/kg, definitive studies need to be done to determine if differences in dose have an effect. In using ex vivo expanded products, up to 1 × 108 cells/kg have been infused without major toxicities [102]. Depletion of CD3 cells below 0.1% prevents transfer of T cells leading to GVHD. Depletion of CD19+ B cells prevents passenger lymphocyte syndrome and autoimmune phenomena. We observed passenger lymphocyte syndrome in 2 patients prior to B-depletion [103]. We also recognized that transfer of EBV-transformed B cells leading to donor-derived post-transplant lymphoproliferative disorder could be prevented.
5.0 Strategies for NK cell expansion
5.1 Ex vivo expansion methods
Because NK cells comprise only 10–15% of PB lymphocytes and their isolation requires a costly selection process, several groups have developed methods to expand NK cells in vitro [100]. Initially, this approach used cytokines which proved successful but predisposed the NK cells to activation-induced cell death when in contact with the vascular endothelium [104]. IL-15, however, does not exert this effect on expanded NK cells. Instead, it promotes their survival and expansion [2]. Over the years, alternative methods of expansion have been developed using human-derived feeder cells. Pioneering groups including Campana, June, Lee, and Cooper have explored the use of artificial antigen-presenting cells (aAPCs) to markedly activate and expand the NK cells ex vivo [105–107]. The use of more standardized feeder lines provides a clinically amenable and genetically modifiable system. Impressively, these cell lines can expand NK cells from PB of patients 500-to 1000-fold in a matter of weeks. The aAPCs have been further modified with costimulatory molecules including CD137 ligand and membrane-bound cytokines such as IL-15 or IL-21. The expanded cells have an activated phenotype maintaining high-level surface expression of KIR, activating receptors, and CD16. They produce large amounts of cytokines and are potent mediators of cytotoxicity [106–108]. However, problems have emerged. At the end of the expansion period, the NK cells appeared to become “exhausted”. They also demonstrated replicative senescence and a shortening of telomere length [109]. Lee et. al. were able to overcome this by modifying the aAPCs with a membrane-bound form of IL-21 that out-performed the membrane-bound IL-15 based APC while also maintaining telomere length [107]. An additional practical problem is that NK cell therapy is most feasible with patient specific or off-the-shelf frozen products. With either strategy, frozen products demonstrate diminished function, decreased expression of surface receptors and lower survival after thawing. It remains unclear how the activated and expanded cells will behave in vivo, especially in relation to persistence and homing. These cells change shape in culture and demonstrate alterations in adhesion receptor profiles important for in vivo targeting to tissue sites. Although adhesion receptors can be re-engineered through genetic modification [108], the procedure requires increased effort and costs. Moreover, such products may have a shorter half-life in vivo. To evaluate potential differences in survival and homing, we recently compared freshly isolated versus ex vivo-expanded NK cells using aAPCs expressing membrane-bound IL-15. By adoptively transferring 1 × 106 NK cells in NOD/SCID/γc−/− mice, followed by post-infusional IL-15, we found the survival of ex vivo-expanded NK cells decreased by 90% 1 week after cytokine withdrawal in contrast to a 45% decrease when using freshly activated NK cells (IL-2 activated overnight). It is possible that long-term culture with aAPCs leads to a state of cytokine “addiction”. Several clinical trials in progress are utilizing these different expanded products [100]. Optimization of cytokines in vivo using IL-15 to enhance NK cell survival may improve product efficacy [1].
Other groups have taken different approaches to generate NK cells for clinical use. Some Epstein-Barr virus (EBV)-infected cell lines have been shown to dramatically expand and activate NK cells, yielding up to 500-fold expansion over 21 days [110]. An alternative approach for NK cell expansion utilizes co-culture with an acute lymphoid leukemia cell line that is resistant to lysis [111, 112]. These investigators found a key aspect of this mode of NK activation and expansion involves the ligation of CD2 on NK cells by CD15 on the target cells [112]. Interestingly, even frozen lysate from this cell line can activate and expand NK cells. (see ClinicalTrials.gov and search NCT00720785 and NCT01520558) [113].
Research groups have also attempted generation of “memory-like” NK cells through brief pre-activation with a cytokine combination including IL-12, IL-15, and IL-18 [64, 114]. Sun et. al. demonstrated the presence of a long-lived population of NK cells with enhanced recall potential in CMV infection [87] or during homeostatic proliferation [115]. The persistence of “memory-like” NK cells in murine models is encouraging and the technique is quite simple. We and others have discovered similar phenotypes in humans infected with CMV, hantavirus, or Chickungunya virus [85, 86, 116–119]. To investigate the influence of these findings on possible graft versus tumor effects, Romee and colleagues at Washington University have initiated a clinical trial using cytokine-induced memory-like NK cells (see ClinicalTrials.gov and search NCT01898793).
5.2 Enhancement of NK cell activity using antibodies
Use of monoclonal antibodies to enhance immune cell recognition of tumor cells ranks among the major cancer treatment advances of the past decade [4, 6]. Monoclonal antibodies function in a several ways that include complement fixation, induction of antibody dependent cytotoxicity (ADCC) and induction of aberrant signaling. The isotype IgG1 subclass is most commonly used for antibody therapeutics because it has proven highly effective at stimulating activating Fc receptors on NK cells, neutrophils and macrophages (i.e., ADCC). Currently, 13 FDA approved monoclonal antibodies are available for cancer therapy. That list continues to expand [120]. Technology enhancements are focused primarily upon improving their ability to stimulate cytotoxic effector cells through modification of the Fc region, decreasing immunogenicity or reducing the level of complement activation [120]. Monoclonal antibodies have been generated against a variety of tumor antigens and tested in clinical trials in AML (CD33), breast cancer (CEA,TAG-72, ERBB2/3), ovarian cancer (mesothelin, MUC1), neuroblastoma (GD2), lymphoma (CD20, CD30), and others [55, 120, 121]. In hematological malignancy, the most effective monoclonal antibody is rituximab, which targets the B cell antigen CD20. Rituximab is now a standard of care for patients with CD20-expressing malignancies, including non-Hodgkin’s lymphoma (NHL) and chronic lymphocytic leukemia (CLL). Other antibodies typically used to treat solid tumors are directed at aberrantly expressed tyrosine kinase receptors such as HER2 or the epidermal growth factor receptor (EGFR) [6, 122]. In those few instances when such antibodies are used following HSCT, timing of administration is important because the first NK cells to recover are similar to CD56bright cells and have lower levels of CD16 expression [57].
In addition to utilizing antibodies to stimulate NK cells through ADCC, several groups have been interested in using monoclonal antibodies to enhance NK cell activity by blocking inhibitory KIR receptors (see ClinicalTrials.gov and search NCT01687387) [5, 123]. These antibodies appear safe and do not cause autoimmunity. Many tumors, particularly solid tumors, are resistant to NK cell-mediated killing because they express high levels of MHC class I molecules on their surface. Because ligation of KIR receptors delivers a powerful inhibitory signal to the NK cell, blocking this interaction could help NK cells overcome inhibition mediated by HLA class I molecules. When used in combination therapy, as discussed below, potential effects may include utilization of anti-KIR monoclonal antibodies, tumor- targeted antibodies and other checkpoint blockade therapeutics to mobilize endogenous NK cells for optimal therapy. At the appropriate stage of immune reconstitution or in the setting of adoptive transfer, co-administration of these reagents and cytokines may enhance the graft-vs-tumor effect [122, 124, 125].
5.3 Enhancement of antibody activity by preserving CD16 expression
Our group recently demonstrated that activated NK cells lose expression of CD16 and the homing receptor CD62L through a disintegrin and metalloprotease-17 (ADAM17) [126]. This CD16 receptor shedding is mediated by ADAM17 cleavage between Ala195 and Val196 [127]. Pharmacologic inhibition of ADAM17 enhanced CD16-mediated NK cell function by preserving CD16 on the surface, thus intensifying killing of rituximab coated lymphoma cells. Notably, ADAM family enzymes including ADAM10 and ADAM17 are highly expressed in lymphoma tumor stroma. Lymphoma-associated stress ligands (such as ULB, MICA, MICB and B7-H6) are also ADAM17 protease targets [128]. Findings suggest that novel therapeutic targets such as ADAM17 merit clinical investigation to assess augmentation of the efficacy of monoclonal antibody- dependent NK cell tumor cell killing.
5.4 Antibodies to redirect “unlicensed” NK cells
Recent data supports the importance of monoclonal antibodies to activate “unlicensed” NK cells. Hsu et. al. reported on a novel observation in a cohort of patients with neuroblastoma being treated with an anti-GD2 monoclonal antibody targeting the disialoganglioside surface antigen. Typically, “unlicenced” NK cells (i.e., those lacking self HLA ligands for their inhibitory KIR receptors) are thought to be hyporesponsive and developmentally immature [56]. The Hsu study showed that neuroblastoma patients lacking HLA class I ligands for inhibitory KIR receptors had significantly higher survival rates when treated with the anti-GD2 antibody. This effect was mediated through cytokine-mediated upregulation of self HLA molecules on the tumor cells and blockade of NK cells expressing inhibitory KIR. This observation suggests that the clinical benefit of the endogenous NK cell population was being mediated by the unlicensed portion of NK cells following crosslinking of CD16. It is likely that this situation may be present in other malignancies, including those treated with rituximab, which have been shown to have differing responses to NK cell-mediated ADCC dependent on the level of HLA class I expression [129]. Alternatively, the addition of monoclonal antibodies directed against the inhibitory KIR receptors may prove to block this attenuated response [5, 130].
6.0 Future perspectives
6.1 Genetic modification and alternative sources of NK cell products
To overcome limitations of the donor-derived NK cell therapies, several groups have investigated alternative donor sources including UCB, NK cell lines and pluripotent stem cells. If cryopreservation can be optimized, the prompt availability of an off-the-shelf product represents a significant step forward. Additional advantages include the ability to perform preclinical testing and to select for donors based on favorable characteristics including optimal KIR-genotype [131].
6.2 UCB-derived NK cells
UBC progenitors provide a rich source of hematopoietic progenitor cells and serve as an important in vitro system for studying the development of human NK cells [132]. Clinically appropriate doses of UCB-derived NK cells can be generated without the use of feeder cells [106, 133]. NK cells generated from UCB contain a mixture of immature and mature cells that produce cytokines and demonstrate cytotoxicity [116]. Development of functional NK cells (e.g. CD34 isolation, in vitro expansion) takes up to 4 weeks and requires processing in a GMP facility. Studies are ongoing and preliminary data is insufficient to assess comparative advantages. Yoon, et. al, have tested an approach using CD34+ cells from adult donors. Fourteen patients received donor-derived NK cells that were differentiated in vitro in the presence of stem cell factor, FLT3 ligand, IL7 and hydrocortisone (HDC), followed by IL-7, IL-15 and HDC. The infusions were given ~ 6–7 weeks after transplant in the outpatient setting [134]. Infusions were tolerated and no toxicity was observed except occasional alanine aminotransferase elevation (grade III) in two patients and development of grade II skin GVHD in one patient, although the concurrent discontinuation of immunosuppression suggests that the NK cells were not responsible.
6.3 NK cell lines
Several research teams have investigated the use of cell lines derived from malignant NK cell clones (i.e. NK-92, NKL, KYHG-1, YT, NKG). NK cell lines maintain some level of direct cytotoxic function and usually lack expression of inhibitory KIR. Because they can be grown in culture, genetic modification with different cytokine genes or chimeric antigen receptors is easily accomplished. Among the lines, NK-92 cells remain the most established and have been tested in clinical trials that include patients with renal cell carcinoma and malignant melanoma [135]. In a phase I dose escalation study treating 12 patients, investigators reported only transient toxicities and stable disease in 33% of patients. However, the in vivo activity of the NK-92 cells was difficult to establish [100]. Additional data are needed concerning the in vivo persistence of these infused lines. Because of their amenability to ex vivo manipulation, these cell lines may provide an important platform to facilitate whole-body in vivo imaging of infused cells. Appropriate technology remains to be developed.
6.4 NK cells derived from pluripotent stem cells
Pluripotent stem cells provide an additional source of NK cells. These include human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) [131, 136]. New methods of iPSC generation have approached 100% efficiency, thus bringing closer the day that hematopoietic-based therapies derived from these lines become available for clinical use. A defined method for producing NK cells from hESCs and iPSCs amenable to clinical translation has been recently established [137]. By adapting a feeder-free differentiation system, mature and functional NK cells can be generated in a system amenable to clinical scale-up. Significantly, in contrast to UCB-CD34+ derived NK cells or NK cell lines, the iPSC-derived NK cells maintained high levels of KIR and CD16 expression. If KIR expression does indeed dictate acquisition of final effector function, some of the relative advantages of using iPSC-derived NK cells for anti-cancer therapies are clarified. Using this improved differentiation method, it is estimated that one 6-well plate of hESCs or iPSCs could provide enough NK cells to treat several patients at the PB-NK doses currently used [89, 137]. Other advantages include: 1) unlimited source of KIR-typed NK cells for adoptive immunotherapy, 2) high level of function in preclinical animal models and, 3) a platform genetically amenable to tailor the therapy based on the patient’s cancer via tumor-specific receptors (TCRs or CARs) [138]. At the present, however, using iPSCs on a patient-specific basis is impractical. Third party iPSC-derived NK cells are subject to immune rejection in the recipient. To circumvent this limitation, specific genetic modulation must be used to decrease immunoreactivity of the infused cells [139]. Recently, Schwartz et.al. have shown the use of hESC-derived retinal pigmented epithelial cells to be safe and potentially effective in treating patients with macular degeneration, thus providing proof of concept for this cell source type [140].
6.5 Bi- and Tri-specific antibodies
Improvements in recombinant technology and antibody production have led to a new class of therapeutics which use either all, or part, of the antibody structure to mediate enhanced effector activity at the tumor site [120]. These include the fusion of two (bi-specific) or three (tri-specific) portions of the fragment of antigen-binding (Fab) region of a traditional antibody. These reagents maintain a high level of antigen specificity, but are derived from a relatively small segment of DNA and therefore offer the significant flexibility of swapping different reagents. The reagents serve to crosslink specific tumor antigens (e.g. CD19, CD20, CD33) with a potent stimulator on the effector cell (e.g. CD3, CD16, TCR) [120, 141, 142]. The major advantage of this technology is flexibility in selecting from a number of immune effector cells (CD16 on NK cells, CD3 on T cells) as well as from a variety of tumor antigens (CD19, EpCAM, Her2/neu, EGFR, CEA, CD33, EphA2, and MCSP).
We have focused on a platform using bispecific killer engagers (BiKEs) constructed with a single-chain Fv against CD16 and a single-chain Fv against a tumor-associated antigen [143–145]. Using CD16 ×19 BiKEs and a trispecific CD16 ×19 ×22 (TriKE), we have shown that CD16 signaling is potent and delivers a different signal comparable with natural recognition of rituximab, especially in regard to cytokine production. Flexibility and ease of production are important advantages of the BiKE and TriKE platform. We have recently developed a CD16 × 33 BiKE to target myeloid malignancies (AML and myelodysplastic syndrome). One of the most remarkable properties of this drug is its potent signaling. In refractory AML, we found that CD16 × 33 BiKE overcomes inhibitory KIR signaling, leading to potent killing and production of cytokines by NK cells [144]. Interestingly, ADAM17 inhibition enhances CD16 × 33 BiKE responses against primary AML targets. These immunotherapeutic approaches will be developed for clinical testing for hematologic malignancies and will allow for NK cell activation via CD16 while approximating NK cells in direct contact with targeted tumor cells
In contrast to other therapies aimed at redirecting immune cells, such as chimeric antigen receptor (CARs), the effect of bi-specific antibodies can be titrated while maintaining specificity. Thus, the likelihood of persistent B cell aplasia, which occurs with CD19 CAR-T cells, is reduced, decreasing the risk of lifelong hypogammaglobulinemia. One limitation of this therapeutic approach is the very short half-life of bi and tri –specific antibodies, which potentially limits trafficking to all tissues [142, 146].
6.6 Drugs to enhance tumor cell visibility
Sensitizing target cells to immune-mediated killing is an attractive way to enhance the immune response. Broad sensitization through high-dose radiation and chemotherapy likely contributes to some of the successful outcomes achieved by immunotherapy. We and others have studied the proteosome inhibitor bortezomib. Classically used to treat multiple myeloma and mantle cell lymphoma, bortezomib inhibition of the proteosome sensitizes tumor cells to TRAIL-mediated killing by NK cells [110]. The TNF-superfamily ligands FASL and TRAIL are found on cytotoxic NK cells and induce target cell apoptosis through ligation of death receptors on target cells [147]. This effect appears to break self-tolerance to tumor cells and could overcome the KIR-mediated inhibition of autologous NK cells. In combination with additional agents, including anti-KIR antibodies and IL-15, optimal balance of in vivo manipulation of a patients’ endogenous NK cells is theoretically possible [123]. Currently, clinical trials using lenalidomide and anti-KIR blockade in the treatment of multiple myeloma are in progress [123, 148]. Lenalidomide has previously been shown to up-regulate ligands on multiple myeloma cells for activating receptors on NK cells. Building upon this knowledge, these trials aim to tip the balance of both activating and inhibitory signals to optimize the anti-tumor effect. Additionally, drugs such as doxorubicin and the histone deacetylase inhibitor depsipeptide (FK228) have been shown to upregulate conserved death receptors Fas, TRAIL-R1/DR4, TNFR1, DR3 and DR6 [149–151]. Consequently, tumor cells are a better target for cytotoxic NK cells expressing FASL and TRAIL. While preclinical mouse studies have demonstrated efficacy using these agents, we combined bortezomib with adoptively transferred NK cells in AML cohort. We did not see significant changes in efficacy or NK cell cytotoxicity. Currently, some investigators have initiated a trial of adoptively transferred NK cells (up to 1 × 108/kg) with or without bortezomib and Treg depletion [102]. In the setting of either autologous or allogeneic transfer of natural killer cells, these studies demonstrate the flexibility and importance of considering the entire immune environment.
6.7 In vivo expansion and mechanism of homeostatic proliferation
Depletion of host lymphocytes is thought to make space and cytokines available, thereby optimizing the homeostatic proliferation of NK cells driven mainly by the cytokine IL-15 [1, 115]. As serum levels of both IL-15 and IL-7 rise, this depletion allows for saturating levels on the surface of NK cells and CD8 T cells. Both are populations required for optimal tumor clearance. It remains to be shown in humans how homeostatic proliferation may promote a long-lived population of NK cells [115]. Whether these NK cells share any phenotypic or functional properties with NK cells responding to CMV reactivation is also unclear [86]. Although the non-myeloblative conditioning regimen results in serum increases of IL-15 and IL-7, the response is limited and the levels quickly drop after 1 week [89]. Because of side effects and expansion of Tregs that accompanies systemic IL-2 therapy, alternative cytokines have been sought to effectively expand lymphocytes in vivo.
The most recent advance in allogeneic NK cell therapy for AML includes an exogenous IL-15 currently being tested in Phase 1 dose escalation trials at the University of Minnesota (see ClinicalTrials.gov and search NCT01385423). Patients with refractory AML are treated with lymphodepleting chemotherapy, allogeneic NK cells and daily infusion of IL-15 for 10 days. An IL-15 dose has been identified for further study. Results are expected within 1 year.
6.8 Role for check point blockade in NK cells?
Another important of focus which may advance the field of cellular therapy is the “check-point” blockade. To achieve effective immune surveillance, the immunosuppressive signals generated in tumor environment and host need to be interrupted. Strategies include blocking Treg signals (TGF-β), elimination of Treg and myeloid suppressor cells (MDSCs), use of anti-KIR antibodies [5, 120, 123, 148], or removing the “brakes” from costimulatory molecules such as programmed cell death 1 pathway (PD-1) [152]. Tumors are known to secrete cytokines, including TGFβ and IL-18, that are suppressive to both T cells and NK cells. Previous murine studies have shown that low levels of circulating IL-18 derived from tumor cells act to suppress the anti-tumor activity of NK cells. In this circumstance blockade of IL–18 through neutralizing antibody may circumvent its upregulation of PD-1 on NK cells. Others have found that IL-15 has a reciprocal function that decreases PD-1 expression in NK cells [137]. In addition to removing inhibitory signals through KIR blockade, these data suggest a role for checkpoint blockade in enhancing NK cell activity in vivo [120].
7.0 Conclusion
Clinical use of NK cells has been inspired by recognition of their potent anticancer activity. The studies discussed above provide a solid basis for development of future NK cell trials for cancer therapy while minimizing risks and toxicities (Figure 1). Important questions remain to be answered including, most urgently, determination of minimum in vivo NK cell expansion needed for clinically effective anti-tumor activity. At present, outcomes involving NK cell expansion interventions remain unpredictable. Furthermore, NK therapy for solid tumors is limited by uncertain homing and domination by an immunosuppressive, tumor-induced microenvironment which may interfere with immune responses. To advance NK cell therapies, both further study of basic NK biology as well as a better understanding of interactions with other immune cells will be required. NK cell product characteristics and effective cytokine cocktail proportions will likely vary for different tumor types and patient populations. Targeting through CD16 remains a powerful and attractive way to increase specificity, rivaling that of genetically modified T cells. Future clinical trials will be designed to exploit strategies to overcome the host immune barriers. Likewise, strategies to explore ex vivo NK cell expansion from blood, lymphoid progenitors, or pluripotent progenitor cells are being tested. In HCT, prospective studies are currently evaluating donor NK cell immunogenetics. Strategies to apply CMV-induced shaping of the immune response to enhance NK cell function are in development.
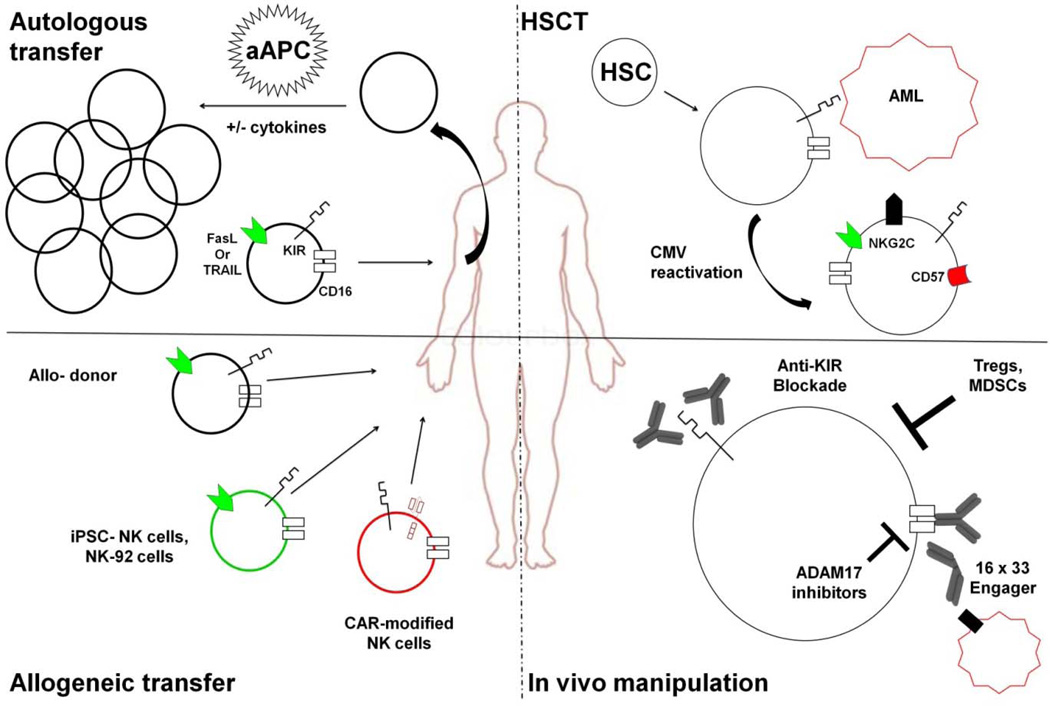
Figure 1. Schema for NK cell manipulation in HCT and immunotherapy.
Autologous or allogeneic NK cells can be collected from peripheral blood. Those cells can be enriched and infused or expanded with cytokines ± aAPCs prior to infusion into patients. Alternative sources of allogeneic NK cells include NK cell lines or NK cells derived from pluripotent stem cells. These NK cells can be further modified with chimeric antigen receptors specific for a patient’s tumor. In hematopoietic stem cell transplant (HSCT), NK cells can be transferred with the graft or develop from hematopoietic stem cells (HSCs). These alloreactive cells express inhibitory KIR and effector molecules that allow clearance of leukemic cells such as AML. If the patient reactivates CMV, a clonal expansion of NKG2C+CD57+KIR+ NK cells occurs, which have enhanced activity against AML cells. Finally, NK cells can be manipulated in vivo. These modalities include monoclonal antibodies to block inhibitory KIR or stimulation of ADCC through crosslinking of CD16. CD16 can also be stimulated by using bi- or tri-specific antibodies. ADCC can further be enhanced by blocking ADAM17 activity. To further enhance NK cell expansion and activity, Tregs and myeloid derived suppressor cells (MDSCs) must be eliminated.
Highlights.
NK cells have the capacity to acquire function through NK cell education
NK cell education/licensing occurs through inhibitory receptor recognition of HLA
Highly function NK cells can kill cancer targets and react to human CMV
Donor KIR B genes interact with recipient HLA-C1/x to improve transplant outcomes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Waldmann Ta, Lugli E, Roederer M, Perera LP, Smedley JV, Macallister RP, et al. Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood. 2011;117:4787–4795. doi: 10.1182/blood-2010-10-311456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105–110. [PubMed] [Google Scholar]
- 3.Becknell B, Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol. 2005;86:209–239. doi: 10.1016/S0065-2776(04)86006-1. [DOI] [PubMed] [Google Scholar]
- 4.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nature Reviews Immunology. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romagne F, Andre P, Spee P, Zahn S, Anfossi N, Gauthier L, et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood. 2009;114:2667–2677. doi: 10.1182/blood-2009-02-206532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science. 2013;341:1192–1198. doi: 10.1126/science.1241145. [DOI] [PubMed] [Google Scholar]
- 7.Ljunggren H-G, Malmberg K-J. Prospects for the use of NK cells in immunotherapy of human cancer. Nature Reviews Immunology. 2007;7:329–339. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nature Reviews Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor–modified T cells in chronic lymphoid leukemia. New England Journal of Medicine. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.June CH, Blazar BR, Riley JL. Engineering lymphocyte subsets : tools, trials and tribulations. Nature Reviews Immunology. 2009;9:704–716. doi: 10.1038/nri2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiessling R, Klein E, Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 13.Rezaei N, Mahmoudi E, Aghamohammadi A, Das R, Nichols KE. X-linked lymphoproliferative syndrome: a genetic condition typified by the triad of infection, immunodeficiency and lymphoma. Br J Haematol. 2011;152:13–30. doi: 10.1111/j.1365-2141.2010.08442.x. [DOI] [PubMed] [Google Scholar]
- 14.Mace EM, Hsu AP, Monaco-Shawver L, Makedonas G, Rosen JB, Dropulic L, et al. Mutations in GATA2 cause human NK cell deficiency with specific loss of the CD56bright subset. Blood. 2013;121:2669–2677. doi: 10.1182/blood-2012-09-453969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 16.Freud AG, Caligiuri MA. Human natural killer cell development. Immunol Rev. 2006;214:56–72. doi: 10.1111/j.1600-065X.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- 17.Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, et al. IL-15 trans-presentation promotes human NK cell development and diff erentiation in vivo. Cell Differentiation. 2009;206:25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farag SS, Caligiuri Ma. Human natural killer cell development and biology. Blood reviews. 2006;20:123–137. doi: 10.1016/j.blre.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Vacca P, Vitale C, Montaldo E, Conte R, Cantoni C, Fulcheri E, et al. CD34+ hematopoietic precursors are present in human decidua and differentiate into natural killer cells upon interaction with stromal cells. Proceedings of the National Academy of Sciences. 2011;108:2402–2407. doi: 10.1073/pnas.1016257108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huntington ND, Vosshenrich CA, Di Santo JP. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat Rev Immunol. 2007;7:703–714. doi: 10.1038/nri2154. [DOI] [PubMed] [Google Scholar]
- 21.Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115:2167–2176. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 24.Imada K, Bloom ET, Nakajima H, Horvath-Arcidiacono JA, Udy GB, Davey HW, et al. Stat5b is essential for natural killer cell-mediated proliferation and cytolytic activity. J Exp Med. 1998;188:2067–2074. doi: 10.1084/jem.188.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eidenschenk C, Jouanguy E, Alcaïs A, Mention J-J, Pasquier B, Fleckenstein IM, et al. Familial NK Cell Deficiency Associated with Impaired IL-2- and IL-15-Dependent Survival of Lymphocytes. The Journal of Immunology. 2006;177:8835–8843. doi: 10.4049/jimmunol.177.12.8835. [DOI] [PubMed] [Google Scholar]
- 26.Vilches C, Parham P. KIR: Diverse, Rapidly Evolving Receptors of Innate and Adaptive Immunity. Annual Review of Immunology. 2002;20:217–251. doi: 10.1146/annurev.immunol.20.092501.134942. [DOI] [PubMed] [Google Scholar]
- 27.Moretta A, Vitale M, Bottino C, Orengo AM, Morelli L, Augugliaro R, et al. P58 molecules as putative receptors for major histocompatibility complex (MHC) class I molecules in human natural killer (NK) cells. Anti-p58 antibodies reconstitute lysis of MHC class I-protected cells in NK clones displaying different specificities. J Exp Med. 1993;178:597–604. doi: 10.1084/jem.178.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moretta A, Sivori S, Vitale M, Pende D, Morelli L, Augugliaro R, et al. Existence of both inhibitory (p58) and activatory (p50) receptors for HLA-C molecules in human natural killer cells. J Exp Med. 1995;182:875–884. doi: 10.1084/jem.182.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moretta A, Bottino C, Vitale M, Pende D, Biassoni R, Mingari MC, et al. Receptors for HLA class-I molecules in human natural killer cells. Annu Rev Immunol. 1996;14:619–648. doi: 10.1146/annurev.immunol.14.1.619. [DOI] [PubMed] [Google Scholar]
- 30.Valiante NM, Uhrberg M, Shilling HG, Lienert-Weidenbach K, Arnett KL, D'Andrea A, et al. Functionally and structurally distinct NK cell receptor repertoires in the peripheral blood of two human donors. Immunity. 1997;7:739–751. doi: 10.1016/s1074-7613(00)80393-3. [DOI] [PubMed] [Google Scholar]
- 31.Chan HW, Kurago ZB, Stewart CA, Wilson MJ, Martin MP, Mace BE, et al. DNA methylation maintains allele-specific KIR gene expression in human natural killer cells. J Exp Med. 2003;197:245–255. doi: 10.1084/jem.20021127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan HW, Miller JS, Moore MB, Lutz CT. Epigenetic control of highly homologous killer Ig-like receptor gene alleles. J Immunol. 2005;175:5966–5974. doi: 10.4049/jimmunol.175.9.5966. [DOI] [PubMed] [Google Scholar]
- 33.Cooley S, Trachtenberg E, Bergemann TL, Saeteurn K, Klein J, Le CT, et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009;113:726–732. doi: 10.1182/blood-2008-07-171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pyo C-W, Guethlein La, Vu Q, Wang R, Abi-Rached L, Norman PJ, et al. Different patterns of evolution in the centromeric and telomeric regions of group A and B haplotypes of the human killer cell Ig-like receptor locus. PloS one. 2010;5:e15115. doi: 10.1371/journal.pone.0015115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belanger S, Tu MM, Rahim MM, Mahmoud AB, Patel R, Tai LH, et al. Impaired natural killer cell self-education and "missing-self" responses in Ly49-deficient mice. Blood. 2012;120:592–602. doi: 10.1182/blood-2012-02-408732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orr MT, Wu J, Fang M, Sigal LJ, Spee P, Egebjerg T, et al. Development and Function of CD94-Deficient Natural Killer Cells. PLoS One. 2010;5:e15184. doi: 10.1371/journal.pone.0015184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 39.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 40.Pende D, Parolini S, Pessino A, Sivori S, Augugliaro R, Morelli L, et al. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. Journal of Experimental Medicine. 1999;190:1505–1516. doi: 10.1084/jem.190.10.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baychelier F, Sennepin A, Ermonval M, Dorgham K, Debre P, Vieillard V. Identification of a cellular ligand for the natural cytotoxicity receptor NKp44. Blood. 2013;122:2935–2942. doi: 10.1182/blood-2013-03-489054. [DOI] [PubMed] [Google Scholar]
- 42.Vitale M, Bottino C, Sivori S, Sanseverino L, Castriconi R, Marcenaro E, et al. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. Journal of Experimental Medicine. 1998;187:2065–2072. doi: 10.1084/jem.187.12.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 44.Sivori S, Vitale M, Morelli L, Sanseverino L, Augugliaro R, Bottino C, et al. p46, a novel natural killer cell-specific surface molecule that mediates cell activation. Journal of Experimental Medicine. 1997;186:1129–1136. doi: 10.1084/jem.186.7.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cosman D, Fanger N, Borges L, Kubin M, Chin W, Peterson L, et al. A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules. Immunity. 1997;7:273–282. doi: 10.1016/s1074-7613(00)80529-4. [DOI] [PubMed] [Google Scholar]
- 46.Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206:1495–1503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pogge von Strandmann E, Simhadri VR, von Tresckow B, Sasse S, Reiners KS, Hansen HP, et al. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity. 2007;27:965–974. doi: 10.1016/j.immuni.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 48.Rosental B, Brusilovsky M, Hadad U, Oz D, Appel MY, Afergan F, et al. Proliferating cell nuclear antigen is a novel inhibitory ligand for the natural cytotoxicity receptor NKp44. J Immunol. 2011;187:5693–5702. doi: 10.4049/jimmunol.1102267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Claus M, Meinke S, Bhat R, Watzl C. Regulation of NK cell activity by 2B4, NTB-A and CRACC. Front Biosci. 2008;13:956–965. doi: 10.2741/2735. [DOI] [PubMed] [Google Scholar]
- 50.de Andrade LF, Smyth MJ, Martinet L. DNAM-1 control of natural killer cells functions through nectin and nectin-like proteins. Immunol Cell Biol. 2013;17:95. doi: 10.1038/icb.2013.95. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Wallace DL, de Lara CM, Ghattas H, Asquith B, Worth A, et al. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology. 2007;121:258–265. doi: 10.1111/j.1365-2567.2007.02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 53.Anfossi N, André P, Guia S, Falk CS, Roetynck S, Stewart CA, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 54.Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tarek N, Le Luduec JB, Gallagher MM, Zheng J, Venstrom JM, Chamberlain E, et al. Unlicensed NK cells target neuroblastoma following anti-GD2 antibody treatment. J Clin Invest. 2012;122:3260–3270. doi: 10.1172/JCI62749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cooley S, Xiao F, Pitt M, Gleason M, McCullar V, Bergemann TL, et al. A subpopulation of human peripheral blood NK cells that lacks inhibitory receptors for self-MHC is developmentally immature. Blood. 2007;110:578–586. doi: 10.1182/blood-2006-07-036228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, et al. NK cell education after allogeneic transplantation: dissociation between recovery of cytokine-producing and cytotoxic functions. Blood. 2011;118:2784–2792. doi: 10.1182/blood-2011-04-347070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yawata M, Yawata N, Draghi M, Little AM, Partheniou F, Parham P. Roles for HLA and KIR polymorphisms in natural killer cell repertoire selection and modulation of effector function. J Exp Med. 2006;203:633–645. doi: 10.1084/jem.20051884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mamessier E, Sylvain A, Thibult M-L, Houvenaeghel G, Jacquemier J, Castellano R, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. The Journal of Clinical Investigation. 2011;121:3609–3622. doi: 10.1172/JCI45816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doubrovina ES, Doubrovin MM, Vider E, Sisson RB, O’Reilly RJ, Dupont B, et al. Evasion from NK Cell Immunity by MHC Class I Chain-Related Molecules Expressing Colon Adenocarcinoma. The Journal of Immunology. 2003;171:6891–6899. doi: 10.4049/jimmunol.171.12.6891. [DOI] [PubMed] [Google Scholar]
- 61.Mapara MY, Sykes M. Tolerance and Cancer: Mechanisms of Tumor Evasion and Strategies for Breaking Tolerance. Journal of Clinical Oncology. 2004;22:1136–1151. doi: 10.1200/JCO.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 62.Carson WE, Ross ME, Baiocchi RA, Marien MJ, Boiani N, Grabstein K, et al. Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-gamma by natural killer cells in vitro. J Clin Invest. 1995;96:2578–2582. doi: 10.1172/JCI118321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smyth MJ, Cretney E, Kershaw MH, Hayakawa Y. Cytokines in cancer immunity and immunotherapy. Immunol Rev. 2004;202:275–293. doi: 10.1111/j.0105-2896.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- 64.Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, et al. Cytokine activation induces human memory-like NK cells. Blood. 2012;120:4751–4760. doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975;16:216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- 66.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 67.Farag SS, Bacigalupo A, Eapen M, Hurley C, Dupont B, Caligiuri MA, et al. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant. 2006;12:876–884. doi: 10.1016/j.bbmt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 68.Davies SM, Ruggieri L, DeFor T, Wagner JE, Weisdorf DJ, Miller JS, et al. Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Killer immunoglobulin-like receptor. Blood. 2002;100:3825–3827. doi: 10.1182/blood-2002-04-1197. [DOI] [PubMed] [Google Scholar]
- 69.Lowe EJ, Turner V, Handgretinger R, Horwitz EM, Benaim E, Hale GA, et al. T-cell alloreactivity dominates natural killer cell alloreactivity in minimally T-cell-depleted HLA-non-identical paediatric bone marrow transplantation. Br J Haematol. 2003;123:323–326. doi: 10.1046/j.1365-2141.2003.04604.x. [DOI] [PubMed] [Google Scholar]
- 70.Kroger N, Binder T, Zabelina T, Wolschke C, Schieder H, Renges H, et al. Low number of donor activating killer immunoglobulin-like receptors (KIR) genes but not KIR-ligand mismatch prevents relapse and improves disease-free survival in leukemia patients after in vivo T-cell depleted unrelated stem cell transplantation. Transplantation. 2006;82:1024–1030. doi: 10.1097/01.tp.0000235859.24513.43. [DOI] [PubMed] [Google Scholar]
- 71.Foley B, De Santis D, Lathbury L, Christiansen F, Witt C. KIR2DS1-mediated activation overrides NKG2A-mediated inhibition in HLA-C C2-negative individuals. Int Immunol. 2008;20:555–563. doi: 10.1093/intimm/dxn013. [DOI] [PubMed] [Google Scholar]
- 72.Venstrom JM, Pittari G, Gooley TA, Chewning JH, Spellman S, Haagenson M, et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med. 2012;367:805–816. doi: 10.1056/NEJMoa1200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McQueen KL, Dorighi KM, Guethlein LA, Wong R, Sanjanwala B, Parham P. Donor-recipient combinations of group A and B KIR haplotypes and HLA class I ligand affect the outcome of HLA-matched, sibling donor hematopoietic cell transplantation. Hum Immunol. 2007;68:309–323. doi: 10.1016/j.humimm.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stringaris K, Adams S, Uribe M, Eniafe R, Wu CO, Savani BN, et al. Donor KIR Genes 2DL5A, 2DS1 and 3DS1 are associated with a reduced rate of leukemia relapse after HLA-identical sibling stem cell transplantation for acute myeloid leukemia but not other hematologic malignancies. Biol Blood Marrow Transplant. 2010;16:1257–1264. doi: 10.1016/j.bbmt.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nguyen S, Dhedin N, Vernant JP, Kuentz M, Al Jijakli A, Rouas-Freiss N, et al. NK-cell reconstitution after haploidentical hematopoietic stem-cell transplantations: immaturity of NK cells and inhibitory effect of NKG2A override GvL effect. Blood. 2005;105:4135–4142. doi: 10.1182/blood-2004-10-4113. [DOI] [PubMed] [Google Scholar]
- 76.Vago L, Forno B, Sormani MP, Crocchiolo R, Zino E, Di Terlizzi S, et al. Temporal, quantitative, and functional characteristics of single-KIR-positive alloreactive natural killer cell recovery account for impaired graft-versus-leukemia activity after haploidentical hematopoietic stem cell transplantation. Blood. 2008;112:3488–3499. doi: 10.1182/blood-2007-07-103325. [DOI] [PubMed] [Google Scholar]
- 77.Kaminski BA, Kadereit S, Miller RE, Leahy P, Stein KR, Topa DA, et al. Reduced expression of NFAT-associated genes in UCB versus adult CD4+ T lymphocytes during primary stimulation. Blood. 2003;102:4608–4617. doi: 10.1182/blood-2003-05-1732. [DOI] [PubMed] [Google Scholar]
- 78.Nguyen S, Kuentz M, Vernant JP, Dhedin N, Bories D, Debre P, et al. Involvement of mature donor T cells in the NK cell reconstitution after haploidentical hematopoietic stem-cell transplantation. Leukemia. 2008;22:344–352. doi: 10.1038/sj.leu.2405041. [DOI] [PubMed] [Google Scholar]
- 79.Shilling HG, McQueen KL, Cheng NW, Shizuru JA, Negrin RS, Parham P. Reconstitution of NK cell receptor repertoire following HLA-matched hematopoietic cell transplantation. Blood. 2003;101:3730–3740. doi: 10.1182/blood-2002-08-2568. [DOI] [PubMed] [Google Scholar]
- 80.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 81.Lopez-Verges S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, et al. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2011;108:14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Elmaagacli AH, Steckel NK, Koldehoff M, Hegerfeldt Y, Trenschel R, Ditschkowski M, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 2011;118:1402–1412. doi: 10.1182/blood-2010-08-304121. [DOI] [PubMed] [Google Scholar]
- 83.Green ML, Leisenring WM, Xie H, Walter RB, Mielcarek M, Sandmaier BM, et al. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood. 2013;122:1316–1324. doi: 10.1182/blood-2013-02-487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manjappa S, Bhamidipati PK, Stokerl-Goldstein KE, Dipersio JF, Uy GL, Westervelt P, et al. Protective effect of cytomegalovirus reactivation on relapse after allogeneic hematopoietic cell transplantation in acute myeloid leukemia patients is influenced by conditioning regimen. Biol Blood Marrow Transplant. 2014;20:46–52. doi: 10.1016/j.bbmt.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119:2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, et al. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol. 2012;189:5082–5088. doi: 10.4049/jimmunol.1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 90.van der Most RG, Currie AJ, Mahendran S, Prosser A, Darabi A, Robinson BW, et al. Tumor eradication after cyclophosphamide depends on concurrent depletion of regulatory T cells: a role for cycling TNFR2-expressing effector-suppressor T cells in limiting effective chemotherapy. Cancer Immunol Immunother. 2009;58:1219–1228. doi: 10.1007/s00262-008-0628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baba J, Watanabe S, Saida Y, Tanaka T, Miyabayashi T, Koshio J, et al. Depletion of radio-resistant regulatory T cells enhances antitumor immunity during recovery from lymphopenia. Blood. 2012;120:2417–2427. doi: 10.1182/blood-2012-02-411124. [DOI] [PubMed] [Google Scholar]
- 92.Burns LJ, Weisdorf DJ, DeFor TE, Vesole DH, Repka TL, Blazar BR, et al. IL-2-based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: a phase I/II trial. Bone Marrow Transplant. 2003;32:177–186. doi: 10.1038/sj.bmt.1704086. [DOI] [PubMed] [Google Scholar]
- 93.Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28:955–959. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Curti A, Ruggeri L, D'Addio A, Bontadini A, Dan E, Motta MR, et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood. 2011;118:3273–3279. doi: 10.1182/blood-2011-01-329508. [DOI] [PubMed] [Google Scholar]
- 95.Sim GC, Martin-Orozco N, Jin L, Yang Y, Wu S, Washington E, et al. IL-2 therapy promotes suppressive ICOS+ Treg expansion in melanoma patients. J Clin Invest. 2013;2 doi: 10.1172/JCI46266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matsuoka K, Koreth J, Kim HT, Bascug G, McDonough S, Kawano Y, et al. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med. 2013;5:3005265. doi: 10.1126/scitranslmed.3005265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13:98–107. doi: 10.3109/14653249.2010.515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Iliopoulou EG, Kountourakis P, Karamouzis MV, Doufexis D, Ardavanis A, Baxevanis CN, et al. A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol Immunother. 2010;59:1781–1789. doi: 10.1007/s00262-010-0904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rizzieri DA, Storms R, Chen DF, Long G, Yang Y, Nikcevich DA, et al. Natural killer cell-enriched donor lymphocyte infusions from A 3-6/6 HLA matched family member following nonmyeloablative allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16:1107–1114. doi: 10.1016/j.bbmt.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013;10:230–252. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bachanova V, Burns LJ, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lindgren BR, et al. Allogeneic natural killer cells for refractory lymphoma. Cancer Immunol Immunother. 2010;59:1739–1744. doi: 10.1007/s00262-010-0896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lundqvist A, Berg M, Smith A, Childs RW. Bortezomib Treatment to Potentiate the Anti-tumor Immunity of Ex-vivo Expanded Adoptively Infused Autologous Natural Killer Cells. J Cancer. 2011;2:383–385. doi: 10.7150/jca.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Skeate R, Singh C, Cooley S, Geller M, Northouse J, Welbig J, et al. Hemolytic anemia due to passenger lymphocyte syndrome in solid malignancy patients treated with allogeneic natural killer cell products. Transfusion. 2013;53:419–423. doi: 10.1111/j.1537-2995.2012.03942.x. [DOI] [PubMed] [Google Scholar]
- 104.Rodella L, Zamai L, Rezzani R, Artico M, Peri G, Falconi M, et al. Interleukin 2 and interleukin 15 differentially predispose natural killer cells to apoptosis mediated by endothelial and tumour cells. Br J Haematol. 2001;115:442–450. doi: 10.1046/j.1365-2141.2001.03055.x. [DOI] [PubMed] [Google Scholar]
- 105.Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shah N, Martin-Antonio B, Yang H, Ku S, Lee DA, Cooper LJ, et al. Antigen presenting cell-mediated expansion of human umbilical cord blood yields log-scale expansion of natural killer cells with anti-myeloma activity. PLoS One. 2013;8:0076781. doi: 10.1371/journal.pone.0076781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Denman CJ, Senyukov VV, Somanchi SS, Phatarpekar PV, Kopp LM, Johnson JL, et al. Membrane-Bound IL-21 Promotes Sustained Ex Vivo Proliferation of Human Natural Killer Cells. PLoS One. 2012;7:e30264–e30264. doi: 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Somanchi SS, Somanchi A, Cooper LJ, Lee DA. Engineering lymph node homing of ex vivo-expanded human natural killer cells via trogocytosis of the chemokine receptor CCR7. Blood. 2012;119:5164–5172. doi: 10.1182/blood-2011-11-389924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fujisaki H, Kakuda H, Imai C, Mullighan CG, Campana D. Replicative potential of human natural killer cells. British Journal of Haematology. 2009;145:606–613. doi: 10.1111/j.1365-2141.2009.07667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Berg M, Lundqvist A, McCoy P, Jr, Samsel L, Fan Y, Tawab A, et al. Clinical-grade ex vivo-expanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy. 2009;11:341–355. doi: 10.1080/14653240902807034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.North J, Bakhsh I, Marden C, Pittman H, Addison E, Navarrete C, et al. Tumor-primed human natural killer cells lyse NK-resistant tumor targets: evidence of a two-stage process in resting NK cell activation. J Immunol. 2007;178:85–94. doi: 10.4049/jimmunol.178.1.85. [DOI] [PubMed] [Google Scholar]
- 112.Sabry M, Tsirogianni M, Bakhsh IA, North J, Sivakumaran J, Giannopoulos K, et al. Leukemic priming of resting NK cells is killer Ig-like receptor independent but requires CD15-mediated CD2 ligation and natural cytotoxicity receptors. J Immunol. 2011;187:6227–6234. doi: 10.4049/jimmunol.1101640. [DOI] [PubMed] [Google Scholar]
- 113.Sabry M, Lowdell MW. Tumor-Primed NK Cells: Waiting for the Green Light. 2013 doi: 10.3389/fimmu.2013.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cooper MA, Yokoyama WM. Memory-like responses of natural killer cells. Immunol Rev. 2010;235:297–305. doi: 10.1111/j.0105-2896.2010.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sun JC, Beilke JN, Bezman NA, Lanier LL. Homeostatic proliferation generates long-lived natural killer cells that respond against viral infection. J Exp Med. 2011;208:357–368. doi: 10.1084/jem.20100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gumá M, Angulo A, Vilches C, Gómez-Lozano N, Malats N, López-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104:3664–3671. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- 117.Gumá M, Budt M, Sáez A, Brckalo T, Hengel H, Angulo A, et al. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 2006;107:3624–3631. doi: 10.1182/blood-2005-09-3682. [DOI] [PubMed] [Google Scholar]
- 118.Bjorkstrom NK, Lindgren T, Stoltz M, Fauriat C, Braun M, Evander M, et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med. 2011;208:13–21. doi: 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Petitdemange C, Becquart P, Wauquier N, Beziat V, Debre P, Leroy EM, et al. Unconventional repertoire profile is imprinted during acute chikungunya infection for natural killer cells polarization toward cytotoxicity. PLoS Pathog. 2011;7:22. doi: 10.1371/journal.ppat.1002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 121.Hsi ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res. 2008;14:2775–2784. doi: 10.1158/1078-0432.CCR-07-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kohrt HE, Houot R, Weiskopf K, Goldstein MJ, Scheeren F, Czerwinski D, et al. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest. 2012;122:1066–1075. doi: 10.1172/JCI61226. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 123.Benson DM, Bakan CE, Zhang S, Collins SM, Liang J, Srivastava S, et al. IPH2101, a novel anti-inhibitory KIR antibody, and lenalidomide combine to enhance the natural killer cell versus multiple myeloma effect. Blood. 2011;118:6387–6391. doi: 10.1182/blood-2011-06-360255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Parihar R, Nadella P, Lewis A, Jensen R, De Hoff C, Dierksheide JE, et al. A phase I study of interleukin 12 with trastuzumab in patients with human epidermal growth factor receptor-2-overexpressing malignancies: analysis of sustained interferon gamma production in a subset of patients. Clin Cancer Res. 2004;10:5027–5037. doi: 10.1158/1078-0432.CCR-04-0265. [DOI] [PubMed] [Google Scholar]
- 125.Parihar R, Dierksheide J, Hu Y, Carson WE. IL-12 enhances the natural killer cell cytokine response to Ab-coated tumor cells. J Clin Invest. 2002;110:983–992. doi: 10.1172/JCI15950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Romee R, Foley B, Lenvik T, Wang Y, Zhang B, Ankarlo D, et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17) Blood. 2013;121:3599–3608. doi: 10.1182/blood-2012-04-425397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lajoie L, Congy-Jolivet N, Bolzec A, Gouilleux-Gruart V, Sicard E, Sung HC, et al. ADAM17-Mediated Shedding of FcγRIIIA on Human NK Cells: Identification of the Cleavage Site and Relationship with Activation. The Journal of Immunology. 2013 doi: 10.4049/jimmunol.1301024. [DOI] [PubMed] [Google Scholar]
- 128.Arribas J, Esselens C. ADAM17 as a therapeutic target in multiple diseases. Curr Pharm Des. 2009;15:2319–2335. doi: 10.2174/138161209788682398. [DOI] [PubMed] [Google Scholar]
- 129.Borgerding A, Hasenkamp J, Engelke M, Burkhart N, Trumper L, Wienands J, et al. B-lymphoma cells escape rituximab-triggered elimination by NK cells through increased HLA class I expression. Exp Hematol. 2010;38:213–221. doi: 10.1016/j.exphem.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 130.Kohrt HE, Thielens A, Marabelle A, Sagiv-Barfi I, Sola C, Chanuc F, et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood. 2013;10:10. doi: 10.1182/blood-2013-08-519199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Knorr DA, Kaufman DS. Pluripotent stem cell-derived natural killer cells for cancer therapy. Translational Research. 2010;156:147–154. doi: 10.1016/j.trsl.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grzywacz B, Kataria N, Sikora M, Oostendorp RA, Dzierzak EA, Blazar BR, et al. Coordinated acquisition of inhibitory and activating receptors and functional properties by developing human natural killer cells. Blood. 2006;108:3824–3833. doi: 10.1182/blood-2006-04-020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Spanholtz J, Preijers F, Tordoir M, Trilsbeek C, Paardekooper J, de Witte T, et al. Clinical-grade generation of active NK cells from cord blood hematopoietic progenitor cells for immunotherapy using a closed-system culture process. PLoS One. 2011;6:16. doi: 10.1371/journal.pone.0020740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yoon SR, Lee YS, Yang SH, Ahn KH, Lee JH, Kim DY, et al. Generation of donor natural killer cells from CD34(+) progenitor cells and subsequent infusion after HLA-mismatched allogeneic hematopoietic cell transplantation: a feasibility study. Bone Marrow Transplant. 2010;45:1038–1046. doi: 10.1038/bmt.2009.304. [DOI] [PubMed] [Google Scholar]
- 135.Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J, et al. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy. 2008;10:625–632. doi: 10.1080/14653240802301872. [DOI] [PubMed] [Google Scholar]
- 136.Woll PS, Grzywacz B, Tian X, Marcus RK, Knorr DA, Verneris MR, et al. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood. 2009;113:6094–6101. doi: 10.1182/blood-2008-06-165225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Knorr DA, Ni Z, Hermanson D, Hexum MK, Bendzick L, Cooper LJ, et al. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med. 2013;2:274–283. doi: 10.5966/sctm.2012-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Themeli M, Kloss CC, Ciriello G, Fedorov VD, Perna F, Gonen M, et al. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat Biotechnol. 2013;31:928–933. doi: 10.1038/nbt.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Torikai H, Reik A, Soldner F, Warren EH, Yuen C, Zhou Y, et al. Toward eliminating HLA class I expression to generate universal cells from allogeneic donors. Blood. 2013;122:1341–1349. doi: 10.1182/blood-2013-03-478255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schwartz SD, Hubschman J-P, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. The Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 141.Baeuerle PA, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 2009;69:4941–4944. doi: 10.1158/0008-5472.CAN-09-0547. [DOI] [PubMed] [Google Scholar]
- 142.Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 143.Vallera DA, Zhang B, Gleason MK, Oh S, Weiner LM, Kaufman DS, et al. Heterodimeric Bispecific Single-Chain Variable-Fragment Antibodies Against EpCAM and CD16 Induce Effective Antibody-Dependent Cellular Cytotoxicity Against Human Carcinoma Cells. Cancer Biother Radiopharm. 2013;23:23. doi: 10.1089/cbr.2012.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wiernik A, Foley B, Zhang B, Verneris MR, Warlick E, Gleason MK, et al. Targeting natural killer cells to acute myeloid leukemia in vitro with a CD16 × 33 bispecific killer cell engager and ADAM17 inhibition. Clin Cancer Res. 2013;19:3844–3855. doi: 10.1158/1078-0432.CCR-13-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gleason MK, Verneris MR, Todhunter DA, Zhang B, McCullar V, Zhou SX, et al. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol Cancer Ther. 2012;11:2674–2684. doi: 10.1158/1535-7163.MCT-12-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Topp MS, Kufer P, Gökbuget N, Goebeler M, Klinger M, Neumann S, et al. Targeted Therapy With the T-Cell–Engaging Antibody Blinatumomab of Chemotherapy-Refractory Minimal Residual Disease in B-Lineage Acute Lymphoblastic Leukemia Patients Results in High Response Rate and Prolonged Leukemia-Free Survival. Journal of Clinical Oncology. 2011;29:2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 147.Zamai L, Ahmad M, Bennett IM, Azzoni L, Alnemri ES, Perussia B. Natural Killer (NK) Cell–mediated Cytotoxicity: Differential Use of TRAIL and Fas Ligand by Immature and Mature Primary Human NK Cells. The Journal of Experimental Medicine. 1998;188:2375–2380. doi: 10.1084/jem.188.12.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Benson DM, Hofmeister CC, Padmanabhan S, Suvannasankha A, Jagannath S, Abonour R, et al. A phase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood. 2012;120:4324–4333. doi: 10.1182/blood-2012-06-438028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sayers TJ, Brooks AD, Koh CY, Ma W, Seki N, Raziuddin A, et al. The proteasome inhibitor PS-341 sensitizes neoplastic cells to TRAIL-mediated apoptosis by reducing levels of c-FLIP. Blood. 2003;102:303–310. doi: 10.1182/blood-2002-09-2975. [DOI] [PubMed] [Google Scholar]
- 150.Lundqvist A, Abrams SI, Schrump DS, Alvarez G, Suffredini D, Berg M, et al. Bortezomib and depsipeptide sensitize tumors to tumor necrosis factor-related apoptosis-inducing ligand: a novel method to potentiate natural killer cell tumor cytotoxicity. Cancer Res. 2006;66:7317–7325. doi: 10.1158/0008-5472.CAN-06-0680. [DOI] [PubMed] [Google Scholar]
- 151.Wennerberg E, Sarhan D, Carlsten M, Kaminskyy VO, D'Arcy P, Zhivotovsky B, et al. Doxorubicin sensitizes human tumor cells to NK cell- and T-cell-mediated killing by augmented TRAIL receptor signaling. Int J Cancer. 2013;133:1643–1652. doi: 10.1002/ijc.28163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Terme M, Ullrich E, Aymeric L, Meinhardt K, Desbois M, Delahaye N, et al. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res. 2011;71:5393–5399. doi: 10.1158/0008-5472.CAN-11-0993. [DOI] [PubMed] [Google Scholar]