Abstract
Mechanisms that modulate the generation of Th17 cells are incompletely understood. We report that the activation of CK2 by CD5 is essential for the efficient generation of Th17 cells in vitro and in vivo. The CD5-CK2 signaling pathway enhanced TCR induced activation of AKT and promoted the differentiation of Th17 cells by two independent mechanisms: inhibiting GSK3, and activating mTOR. Genetic ablation of the CD5-CK2 signaling pathway attenuated TCR induced AKT activation and consequently increased activity of GSK3 in Th17 cells. This resulted in Th17 cells being more sensitive to IFN-γ mediated inhibition. In the absence of CD5-CK2 signaling, we observed decreased activity of S6K and attenuated nuclear translocation of RORγt. These results reveal a novel and essential function of CD5-CK2 signaling pathway and GSK3-IFNγ axis in regulating Th differentiation and provide a possible means to dampen Th17 responses in autoimmune diseases.
Keywords: CD5, Th17, GSK3, AKT, cytokine receptor signaling
INTRODUCTION
CD5 is a surface glycoprotein expressed on T and B1a cells [1]. The endogenous ligand of CD5 has not been established, but its three extra cellular cysteine rich scavenge receptor domains suggest a possible role as a pattern recognition receptor. CK2, a serine threonine kinase central to cell survival, activation and cell-cycle regulation, constitutively associates with a carboxy-terminus proximal domain of CD5 [2–5]. Engagement of CD5 activates CK2 to promote survival in T cells. Cd5Δck2bd/Δck2bd (CD5ΔCK2bd), “knock in” mice were generated that lack the ability for CD5 to bind and activate CK2 [6]. As predicted, T cells from CD5ΔCK2bd mice exhibited increased levels of T cell activation induced cell death [5, 6]. Remarkably, the CD5ΔCK2bd developed less severe experimental autoimmune encephalomyelitis (EAE) that was associated with decreased generation of IL-17 expressing T cells.
Crosslinking of CD5 significantly enhances Th17 differentiation [7]. We subsequently showed that intact CD5-CK2 signaling was necessary for efficient generation of Th17 cells, in vitro, however the mechanism was not determined [6]. CK2 can enhance the activity of AKT directly as well as indirectly by inhibiting PTEN, the inhibitor of PI3K [8, 9]. CD5 has been shown to activate AKT in thymic T cells [10]. AKT enhances nuclear translocation of RORγt through activation of mTORC1 and S6K2, an essential step for Th17 differentiation [11]. AKT also phosphorylates and inhibits glycogen synthase kinase-3(GSK3), a kinase that enhances IFN-γ receptor induced STAT activation and NFκb activation [12–14].Thus the CD5-CK2 signaling axis in T cells may have function beyond cell activation and survival.
We sought to explain the mechanism by which CD5 alters Th17 cell differentiation. We determined that CD5 induced CK2 activation inhibited GSK3β, leading to decreased cell sensitivity to IFN-γ. Additionally, we found that CD5-CK2 signaling is necessary for efficient nuclear localization of RORγt. Our results show that CD5-CK2 signaling is necessary for efficient Th17 differentiation that is mediated through the AKT and GSK3 signaling pathways.
RESULTS AND DISCUSSION
CD5 increases Th17 polarization
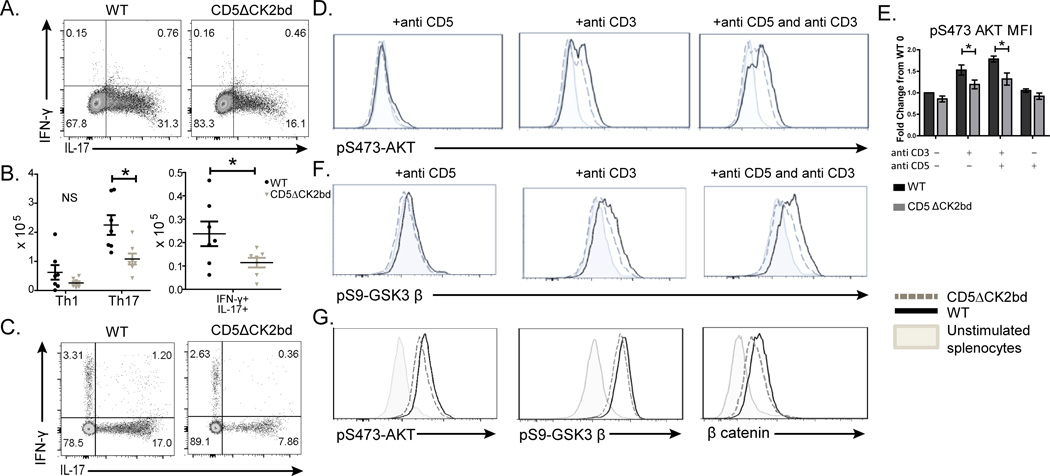
The differentiation of naïve CD4+ T cells, in vitro, into Th17 cells is reduced in the absence of CD5-CK2 activation, while Th1 differentiation was unaffected (Fig. 1A, [6]). To determine if intact CD5-CK2 signaling is necessary for the efficient differentiation of CD4+ T cells to Th17 cells in vivo we transferred CD45.2 B6.OT2 or CD45.2 CD5ΔCK2bd.OT2 TCR transgenic T cells into CD45.1 mice and then immunized the recipients with OVA323–339 peptide. We observed fewer CD45.2+ Th17 cells in proportion and absolute number in recipients that received CD5ΔCK2bd.OT2 T cells than B6.OT2 T cells (Fig. 1B, 1C). Moreover, numbers of IFN-γ+ IL-17+ OT2 T cells were lower in CD5ΔCK2bd recipients. This reduction in “double producer” cells may reflect deficiency in the generation of “precursor” IFN-γ− IL-17+ cell from which these double cytokine expressers arise [15, 16]. Although recipients of CD5ΔCK2bd.OT2 T cells contained fewer Th1 cells of donor origin, the difference from B6.OT2 recipients was not significant (Fig. 1B, 1C). These results establish that intact CD5-CK2 signaling is necessary for efficient generation of Th17 cells in vitro and in vivo.
Figure 1.
Role of CD5-CK2 signaling in Th17 differentiation in vitro and in vivo. A) IL-17A and IFN-γ expression in CD4 gated T cells from WT.OT2 and CD5ΔCK2bd.OT2 mice stimulated with OVA323–339 peptide and irradiated APC under Th17 polarizing conditions for three days. Dot plot is representative of 5 independent experiments. B) Absolute cell numbers and C) a representative dot plot of Th1, Th17 and IFN-γ+ IL-17+ cells from CD45.2 WT.OT2 or CD45.2 CD5ΔCK2bd.OT2 CD4+ T cells transferred i.p. into CD45.1 C57Bl/6 mice. Recipient mice were immunized 24hrs after transfer and spleens analyzed five days later. Each dot in B) represents an individual mouse (n=7); mean±SEM is shown. *p<0.05, Student’s t test. D) Representative histogram and E) fold change from unstimulated WT pooled from 4 independent experiments showing pS473-AKT in CD4+ T-cells from WT or CD5ΔCK2bd mice stimulated ex vivo for 5 minutes with 5µg/ml each of anti-CD5, anti-CD3 or anti-CD5 + anti-CD3. Cells were then stained for pS473-AKT using tyramide enhancement; mean±SEM *p<0.05, Student’s t test. F) pS9-GSK3β in CD4 gated T cells from WT or CD5ΔCK2bd mice stimulated as above and measured by flow cytometry. Cells stimulated as in D) and stained for pS9-GSK3β. Data is a representative histogram from 8 independent experiments. Levels of G) pS473-AKT, pS9-GSK3β and β-catenin in CD4 gated T cells after Th17 culture. pS473-AKT and pS9-GSK3β were analyzed on day 3 and β-catenin on day 5 of culture. Data is representative of 3 independent experiments
CD5-CK2 signaling enhances AKT activation and GSK3 phosphorylation
CK2 is a regulator of PI3K/AKT/GSK3 signaling pathways [8, 9]. Furthermore, AKT-activation is an essential component for the differentiation of naïve T cells to Th17 cells [14]. We hypothesized that the CD5-CK2 signaling pathway enhances Th17 differentiation through increased AKT activity. To test this, the phosphorylation of S473-AKT was measured in T cells from WT and CD5ΔCK2bd mice following engagement of TCR, co-engagement of TCR and CD5 or engagement of CD5 alone [17]. TCR engagement induced the phosphorylation of S473-AKT that was enhanced by co-engagement of CD5 in WT T cells (Fig. 1D, 1E). Anti-CD3 stimulation of CD5ΔCK2bd T cells induced phosphorylation of AKT to levels significantly lower than that in WT CD4+ T cells which was not further enhanced by co-engagement of CD3 and CD5 (Fig. 1D, 1E). CD5 engagement alone did not induce significant pS473-AKT in WT or CD5ΔCK2bd T cells. AKT phosphorylates the serine threonine kinase GSK3β at S9 and inhibits its activity [18]. Hence, GSK3β phosphorylation at S9 offers an intracellular readout for AKT activity. We found that pS9-GSK3β was significantly lower in CD4+ T cells from CD5ΔCK2bd mice than those from WT mice when stimulated with anti-CD3 alone or anti-CD3 and anti-CD5 (Fig. 1F, Supporting Information Fig 1C).
Next, we interrogated if CD5-CK2 signaling modulated AKT and GSK3β phosphorylation during Th17 differentiation. We found that Th17 cells generated with CD4+ T cells from WT mice had higher levels of pS473-AKT, which correlated with greater levels of pS9-GSK3β, than cells from CD5-CK2 signaling deficient mice (Fig. 1G). β-catenin is degraded following phosphorylation by GSK3 [19]. Consistent with this, we found that CD5ΔCK2bd Th17 cells had lower levels of β-catenin than WT Th17 cells (Fig. 1G). We previously reported that the loss of the CD5-CK2 signaling pathways leads to greater apoptosis [5, 6]. Since these analyses were performed three days after stimulation under Th17 polarizing conditions the viability was similar in WT and CD5ΔCK2bd T cell polarizing cultures (data not shown). Taken together, these data show that the CD5-CK2 signaling pathway enhances TCR-induced AKT activation leading to the attenuation of GSK3.
GSK3 regulates Th17 differentiation
On the basis of our recent report that GSK3 is essential for IFN-γR signaling in T cells, we predicted IFN-γ induced STAT1 activation would be enhanced in activated CD5ΔCK2bd T cells through increased GSK3 activity [13]. Th17 polarized CD5ΔCK2bd T cells had greater phosphorylation of Y701-STAT1 than in Th17 polarized WT T cells and consequently greater expression of Tbet (Fig. 2A). These observations suggest that enhanced sensitivity to IFN-γ may contribute to the reduced generation of Th17 cells in the absence of CD5-CK2 signaling [6]. To test for this, we assayed the generation of Th17 cells in cultures polarized in the presence or absence of anti-IFN-γ, or following the addition of exogenous IFN-γ. As we observed previously, fewer IL-17 expressing CD4+ T cells were generated from CD5-CK2 signaling deficient T cells in the presence of anti-IFN-γ (Fig. 2B, 2C, [6]). When anti-IFN-γ was excluded, the proportion of Th17 cells generated from CD5ΔCK2bd.OT2 T cells was significantly less than in the presence of anti-IFN-γ. In contrast, exclusion of anti-IFN-γ did not significantly affect the generation of Th17 cells from WT.OT2 T cells (Fig. 2B, 2C). The expression of IFN-γR1 on WT.OT2 and CD5ΔCK2bd.OT2 T cells and the levels of endogenous IFN-γ in the polarization cultures were equal, and therefore not responsible for the differences in IFN-γ sensitivity (Supporting Information Fig. 1D and 1E). These results indicate that Th17 cells generated from CD5ΔCK2bd T cells are more sensitive than WT T cells to inhibition from endogenously produced IFN-γ. In fact, the addition of 2ng/ml exogenous IFN-γ proportionally inhibited the generation of Th17 cells from both WT and CD5ΔCK2bd T cells (Fig. 2B, 2C).
Figure 2.
Impact of GSK3 activity on Th17 differentiation. WT.OT2 and CD5ΔCK2bd.OT2 CD4+ T cells were stimulated for 3 days with OVA323–339 and irradiated APC in Th17 conditions. A) T cell pY701-STAT1 and T bet levels determined by flow cytometry. Data is representative of 4 independent experiments. B) Representative dot plot and C) averaged percentage of IL-17+ T cells from 2–4 independent experiments measured by flow cytometry. CD4+ T cell were stimulated under Th17 polarizing conditions ± anti-IFN-γ, 2ng/ml or 20ng/ml IFN-γ. mean±SEM **p<0.001 *p<0.05, Mann Whitney test. Representative dot plots of D) WT.OT2 and CD5ΔCK2bd.OT2 Th17 polarized cells with and without the addition of the GSK3 inhibitor LiCl for 3 days. Data is representative of 4 independent experiments.
A previous study reported that pharmacological inhibition of GSK3 over a five day culture reduced Th17 differentiation, that we recapitulated (data not shown) [20]. In apparent contradiction, increased AKT activity or genetic ablation of GSK3α enhances Th17 differentiation [11, 14]. Concordant with these latter studies, our results indicate that CD5-CK2 signaling promotes Th17 differentiation by an AKT activation and GSK3 inhibition pathway. We further show that inhibition of GSK3 activity using 5 or 10 mM LiCl during a three day polarization markedly enhances Th17 differentiation (Fig. 2D, data not shown). Our finding is also concordant with the observation that Li treatment was ineffective in suppressing experimental autoimmune encephalomyelitis (EAE) induced by encephalitogenic Th17 cells [13]. These results reveal that GSK3 has opposing activity in Th17 biology, inhibitory during differentiation but necessary for long-term persistence in culture. The striking increase in Th17 cells in the presence of a GSK3-inhibitor indicates that GSK3 regulates Th17 differentiation by mechanisms beyond IFN-γR signaling.
In the context of CD5-CK2 signaling, the above results led us to ask if inhibition of GSK3 with LiCl would overcome the deficiency in CD5ΔCK2bd T cells to differentiate into Th17 cells. The addition of LiCl induced partial recovery in the generation of Th17 cells by CD5ΔCK2bd T cells (Fig. 2D). Our results show that CD5-CK2 signals inhibit IFN-γ responses. This regulation is likely through AKT and GSK3, however other mechanisms may be involved. Additionally, this effect on IFN-γ signaling may also have an impact on Th1 differentiation under certain conditions.
CD5 enhances nuclear localization of RORγt
The incomplete rescue of CD5ΔCK2bd Th17 and IFN-γ+IL-17+ differentiation by LiCl led us to consider the possibility of a mechanism independent of GSK3. We excluded deficits in IL-6 or IL-23 signaling as a mechanism since pY705-STAT3 was greater in CD5ΔCK2bd T cells than in WT T cells from Th17 polarizing cultures (Fig. 3A, [6]). The AKT-mTOR pathway activates the binding of RORγt to S6K2 and transport to the nucleus [11]. Consistent with reduced AKT-mTOR activation, CD5ΔCK2bd Th17 polarized cells had lower phosphorylation of the S6K substrate S6 (Fig. 3A). Concordantly, CD5ΔCK2bd Th17 polarized cells had lower levels of nuclear RORγt (Fig. 3B, 3C). We previously reported that expression of Rorc was equivalent between Th17 polarizing cultures from WT and CD5ΔCK2bd mice [6]. Therefore we infer that the reduced nuclear RORγt is due to inefficient translocation into the nucleus rather than lower expression. In fact, by flow cytometry we observed equivalent levels of cytoplasmic RORγt in CD4+ T cells Th17 polarizing cultures from WT.OT2 and CD5ΔCK2bd.OT2 mice. However, when the analysis of nuclear RORγt was included, a greater total level was observed in CD4+ T-cells from WT Th17 polarizing cultures (Fig. 3D). Overall these results show that functional CD5-CK2 signaling is necessary for efficient translocation of RORγt into the nucleus mediated in part through mTOR and S6K activity.
Figure 3.
CD5-CK2 signaling modulates nuclear levels of RORγt in Th17 polarized cells. WT.OT2 or CD5ΔCK2bd.OT2 CD4+ T cells were stimulated in vitro under Th17 polarizing conditions without anti-IFN-γ for 3 days with OVA323–339 and irradiated APC. A) Phosphorylation of Y705-STAT3 and S235/S236-S6 analyzed by flow cytometry. B) Representative photomicrographs with 20µm scale bars and C) quantification of nuclear levels of RORγt as a percent ratio ±SEM of RORγt to Hoechst staining from IHC staining of anti-RORγt (red), Hoechst (Blue), and anti-CD3 (green). Data is averaged from 4 independent experiments *p<0.05, Mann Whitney test. D) Cytosolic RORγt levels following permeablization with 40% methanol and stained for RORγt and gated Ku80− (nuclear antigen). Total RORγt levels were determined by flow cytometry. Data is representative of 4 independent experiments.
CONCLUDING REMARKS
In summary, our study demonstrates that the immunoreceptor CD5 by activating CK2 is necessary for the efficient generation of Th17 cells. Through two non-overlapping mechanisms CD5-CK2 signaling cooperate with TCR initiated signals to amplify AKT activation and promote Th17 generation. These mechanisms downstream of AKT are a GSK3-dependent mechanism that includes dampening IFN-γ STAT1 activation, and a mTOR dependent mechanism leading to enhanced nuclear transport of RORγt.
MATERIALS AND METHODS
Mice
C57BL/6 mice (CD45.1 and CD45.2) were from NCI–Frederick Cancer Research. C57BL/6 CD5ΔCK2bd “knock in” mice and OT2 TCROVA were described previously [6, 21] Animals were housed in specific pathogen free condition and according to National Institutes of Health and University of Alabama at Birmingham Institutional Animal Care and Use Committee guidelines.
Assay for in vivo Th17 generation
CD4+ cells were isolated using magnetic micro beads (Invitrogen) as per manufacturer’s instructions. 2×106 cells were in injected i.p. in PBS. Mice were immunized with 300µg OVA323–339 peptide in complete Freund’s adjuvant.
In vitro Th17 polarizations
Purified CD4+ T cells were stimulated with 10 µg/ml OVA323–339 peptide and cultured in Th17 polarizing conditions as described previously [6]. Thy-1 depleted irradiated spleen cells were used as antigen presenting cells (APC)
Flow cytometry
Intracellular cytokine staining was performed as previously described [5]. Cell viability was determined with fixable viability dye eF660 or eF780 from eBioscience. The following antibodies were used: anti-IFN-γ (XMG1.2), anti-IL-17a (TC11-18H10.1), anti-CD4 (RM4–5), and anti-CD4 (GK1.5) (Biolegend). Cells from OT2 TCROVA recipients were stained with anti-CD45.2 (104)(Biolegend) and anti-CD45.1 (A20) (BD).
For phospho flow cytometry, cells were fixed for 7 min in 0.4% paraformaldehyde at room temperature and stained with fixable viability dye. After at an additional 7 mins in 4% paraformaldehyde the cells were permeablized in 90% methanol for 20 mins at 4°C. Cells were then stained with antibodies to pY701-STAT1 (58D6), pY705-STAT3 (D3A7), pS9-GSK3β (D85E12), pS473-AKT (D9E), pS235/236-S6 (D57.2.2E), Ku80 and/or β catenin (L54E2)(from Cell Signaling) and anti-CD4. Other reagents used as needed were goat anti-rabbit 488 (Jackson Immunoresearch), anti-RORγ(AFKJS-9, eBioscience), anti-RORγ (B2D) (eBioscience), anti-Tbet (4B10)(Biolegend). Tyramide amplification of pS473-AKT was performed using the kit (Molecular Probes). All samples were analyzed using a BD Facs Calibur or BD LSR2 and analyzed with FlowJo software.
Immunohistochemistry
Th17 polarized cells were stained as described [11] with anti-RORγ (AFKJS-9) and anti-CD3 biotin (145-2C11) (eBioscience), Hoechst stain, streptavidin 488 (Invitrogen), and goat anti-rat 594. Images from 2 independent experiments were scored using Metamorph software from Molecular Devices. Signal colocalization was determined by signal integration comparison.
Supplementary Material
Supporting Information Figure 1. Representative gating scheme for A) in vivo polarization of donor CD45.2 OT2 T cells into CD45.1 recipient mice and B) all other in vitro assays. C) pS9-GSK3β expression levels in CD4 gated T cells from WT or CD5ΔCK2bd mice stimulated ex vivo for 5 minutes with 5µg/ml each of anti-CD5, anti-CD3 or anti-CD5 + anti-CD3 and measured by flow cytometry. n= 8 independent experiments, *p<0.05 paired Student’s t test. WT.OT2 and CD5ΔCK2bd.OT2 CD4+ T cells were stimulated with OVA323–339 and irradiated APC in Th17 conditions. D) Flow cytometry measurement of IFN-γR1 (CD119) surface levels. Results are representative of 3 independent experiments. E) IFN-γ ELISA performed on culture supernatants following 3 days stimulation. n= 4 independent experiments.
ACKNOWLEDGEMENTS
Supported by grants from the National Institutes of Health (NIH) T32-AI007051 (DM), R01 NS064261 (PD), and AI1076562 (CR), and by the National Multiple Sclerosis Society RG3891 (CR). We thank the University of Alabama at Birmingham Comprehensive Arthritis, Musculoskeletal and Autoimmunity Center (CAMAC) Analytic and Preparative Cytometry Facility (P30 AR048311 – John D. Mountz), (G20 RR025858), and the University of Alabama at Birmingham Animal Resources (G20 RR022807and G20 RR025858 – Samuel C. Cartner).
Abbreviations used
- EAE
experimental autoimmune encephalomyelitis
- GSK3
glycogen synthase kinase 3
- CK2
casein kinase
Footnotes
DISCLOSURES
The authors have no financial conflicts of interest.
REFERENCES
- 1.Soldevila G, Raman C, Lozano F. The immunomodulatory properties of the CD5 lymphocyte receptor in health and disease. Curr Opin Immunol. 2011;23:310–318. doi: 10.1016/j.coi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raman C, Kimberly RP. Cutting Edge: Differential CD5-Dependent Regulation of CD5-Associated CK2 Activity in Mature and Immature T Cells: Implication on TCR/CD3-Mediated Activation1. The Journal of Immunology. 1998;161:5817–5820. [PubMed] [Google Scholar]
- 3.Raman C, Kuo A, Deshane J, Litchfieldi DW, Kimberly RP. Regulation of Casein Kinase 2 by Direct Interaction with Cell Surface Receptor CD5. The Journal of Biological Chemistry. 1998;173:19183–19189. doi: 10.1074/jbc.273.30.19183. [DOI] [PubMed] [Google Scholar]
- 4.Calvo J, Vilda JM, Places L, Simarro M, Padilla O, Andreu D, Campbell KS, Aussel C, Lozano F. Human CD5 signaling and constitutive phosphorylation of C-terminal serine residues by casein kinase II. J Immunol. 1998;161:6022–6029. [PubMed] [Google Scholar]
- 5.Axtell RC, Xu L, Barnum SR, Raman C. CD5-CK2 Binding/Activation-Deficient Mice Are Resistant to Experimental Autoimmune Encephalomyelitis: Protection Is Associated with Diminished Populations of IL-17-Expressing T Cells in the Central Nervous System. Journal of Immunology. 2006;177:8542–8549. doi: 10.4049/jimmunol.177.12.8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sestero CM, McGuire DJ, De Sarno P, Brantley EC, Soldevila G, Axtell RC, Raman C. CD5-dependent CK2 activation pathway regulates threshold for T cell anergy. J Immunol. 2012;189:2918–2930. doi: 10.4049/jimmunol.1200065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Wit J, Souwer Y, van Beelen AJ, de Groot R, Muller FJ, Klaasse Bos H, Jorritsma T, Kapsenberg ML, de Jong EC, van Ham SM. CD5 costimulation induces stable Th17 development by promoting IL-23R expression and sustained STAT3 activation. Blood. 2011;118:6107–6114. doi: 10.1182/blood-2011-05-352682. [DOI] [PubMed] [Google Scholar]
- 8.Maira GD, Salvi M, Arrigoni G, Marin O, Sarno S, Brustolon F, Pinna L. Ruzzene, Protein kinase CK2 phosphorylates and upregulates Akt/PKB. Cell Death and Differentiation. 2005;12:668–677. doi: 10.1038/sj.cdd.4401604. [DOI] [PubMed] [Google Scholar]
- 9.Miller SJ, Lou DY, Seldin DC, Lane WS, Neel BG. Direct identification of PTEN phosphorylation sites. FEBS Lett. 2002;528:145–153. doi: 10.1016/s0014-5793(02)03274-x. [DOI] [PubMed] [Google Scholar]
- 10.Ordoñez-Rueda D, Lozano F, Sarukhan A, Raman C, Garcia-Zepeda EA, Soldevila G. Increased numbers of thymic and peripheral CD4+CD25+Foxp3+ cells in the absence of CD5 signaling. European Journal of Immunology. 2009;39:2233–2247. doi: 10.1002/eji.200839053. [DOI] [PubMed] [Google Scholar]
- 11.Kurebayashi Y, Nagai S, Ikejiri A, Ohtani M, Ichiyama K, Baba Y, Yamada T, Egami S, Hoshii T, Hirao A, Matsuda S, Koyasu S. PI3K-Akt-mTORC1-S6K1/2 axis controls Th17 differentiation by regulating Gfi1 expression and nuclear translocation of RORgamma. Cell Rep. 2012;1:360–373. doi: 10.1016/j.celrep.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Beurel E, Jope RS. Differential Regulation of STAT Family Members by Glycogen Synthase Kinase-3. Journal of Biological Chemistry. 2008;283:21934–21944. doi: 10.1074/jbc.M802481200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowse AL, Naves R, Cashman KS, McGuire DJ, Mbana T, Raman C, De Sarno P. Lithium Controls Central Nervous System Autoimmunity through Modulation of IFN-gamma Signaling. PLoS One. 2012;7:e52658. doi: 10.1371/journal.pone.0052658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulen MF, Bulek K, Xiao H, Yu M, Gao J, Sun L, Beurel E, Kaidanovich-Beilin O, Fox PL, DiCorleto PE, Wang JA, Qin J, Wald DN, Woodgett JR, Jope RS, Carman J, Dongre A, Li X. Inactivation of the enzyme GSK3alpha by the kinase IKKi promotes AKT-mTOR signaling pathway that mediates interleukin-1-induced Th17 cell maintenance. Immunity. 2012;37:800–812. doi: 10.1016/j.immuni.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 19.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 20.Beurel E, Yeh WI, Michalek SM, Harrington LE, Jope RS. Glycogen synthase kinase-3 is an early determinant in the differentiation of pathogenic Th17 cells. J Immunol. 2011;186:1391–1398. doi: 10.4049/jimmunol.1003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1. Representative gating scheme for A) in vivo polarization of donor CD45.2 OT2 T cells into CD45.1 recipient mice and B) all other in vitro assays. C) pS9-GSK3β expression levels in CD4 gated T cells from WT or CD5ΔCK2bd mice stimulated ex vivo for 5 minutes with 5µg/ml each of anti-CD5, anti-CD3 or anti-CD5 + anti-CD3 and measured by flow cytometry. n= 8 independent experiments, *p<0.05 paired Student’s t test. WT.OT2 and CD5ΔCK2bd.OT2 CD4+ T cells were stimulated with OVA323–339 and irradiated APC in Th17 conditions. D) Flow cytometry measurement of IFN-γR1 (CD119) surface levels. Results are representative of 3 independent experiments. E) IFN-γ ELISA performed on culture supernatants following 3 days stimulation. n= 4 independent experiments.