Abstract
This article examines a likely basis of the tenacity of biofilm infections that has received relatively little attention: the resistance of biofilms to mechanical clearance. One way that a biofilm infection persists is by withstanding the flow of fluid or other mechanical forces that work to wash or sweep microorganisms out of the body. The fundamental criterion for mechanical persistence is that the biofilm failure strength exceeds the external applied stress. Mechanical failure of the biofilm and release of planktonic microbial cells is also important in vivo because it can result in dissemination of infection. The fundamental criterion for detachment and dissemination is that the applied stress exceeds the biofilm failure strength. The apparent contradiction for a biofilm to both persist and disseminate is resolved by recognizing that biofilm material properties are inherently heterogeneous. There are also mechanical aspects to the ways that infectious biofilms evade leukocyte phagocytosis. The possibility of alternative therapies for treating biofilm infections that work by reducing biofilm cohesion could: 1) allow prevailing hydrodynamic shear to remove biofilm, 2) increase the efficacy of designed interventions for removing biofilms, 3) enable phagocytic engulfment of softened biofilm aggregates, and 4) improve phagocyte mobility and access to biofilm.
Keywords: cohesion, viscoelastic, failure, mechanical, material, neutrophil, detachment
Introduction
Bacteria or fungi that aggregate in biofilms can cause persistent infections (Costerton et al, 1999). These infections are typically localized, slow-moving, recurrent, and poorly controlled by antibiotics or antiseptics (Parsek & Singh, 2003). The host immune response to the continued presence of microorganisms can give rise to collateral damage to neighboring healthy tissue, perpetuating a degradative, non-healing state. A few well-known examples of biofilm-associated infections are cystic fibrosis pneumonia, catheter-associated urinary tract infection, prosthetic joint infection, periodontitis, and chronic dermal wounds.
One of the main reasons that biofilm infections are so persistent is the reduced susceptibility of microorganisms in biofilms to killing by antimicrobials including systemic antibiotics, topical antiseptics, and antimicrobial components of the host defense (Stewart & Costerton, 2001; Fux et al. 2005). Biofilm tolerance to antimicrobial agents has been extensively researched and discussed. The purpose of this article is to examine another likely basis of the tenacity of biofilm infections that has received much less attention: the resistance of biofilms to mechanical clearance. This topic requires engagement with structure, hydrodynamics, material properties, polymer gel physics, and adhesive and cohesive failure. The intent of this article is to outline the application of elementary biophysics concepts to the resilience of biofilm infections.
Structure
When thinking about mechanics, it is important to have a good grasp on the structure and geometry of the system. Much has been written about the three-dimensional architecture of biofilms formed in in vitro reactors and environmental settings (Costerton et al., 1995; O’Toole et al., 2000). In vivo, however, biofilm structures are often quite different as has been recently articulated by Bjarnsholt et al. (2013). The celebrated mushroom-shaped biofilm cluster observed in flow cells in the laboratory is not seen in vivo. Instead, relatively small aggregates of biofilm cells are found intermixed or covered with extensive host-derived material (Gristina et al., 1985; Baltimore et al., 1989; Hall-Stoodley et al., 2006; James et al., 2008; Stoodley et al., 2008; Bjarnsholt et al., 2009; Stoodley et al., 2010). In other niches thick biofilms form, but often contain substantial amounts of host or precipitated mineral particulates (Wright & Kirschner, 1979; Friskopp 1983; Marrie and Costerton, 1984; Marrie et al., 1987; Stickler et al., 1993; Tan et al., 2004). Conceptual models of in vivo biofilm structure are diagrammed in Figure 1.
Figure 1.
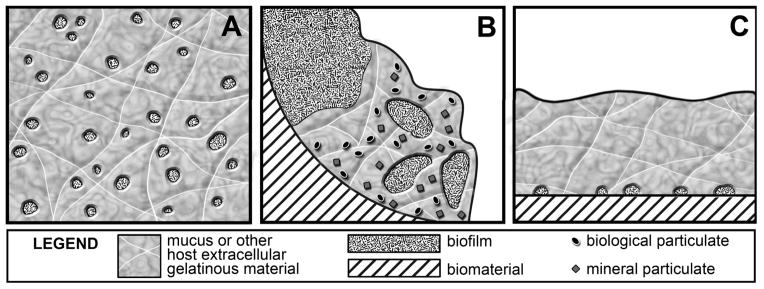
Conceptual models of in vivo biofilm structures. A, Small microbial aggregates (e.g., 5–50 microns in size) are distributed in a gel-like matrix which may be composed of host extracellular matrix material, dead neutrophils and released neutrophil DNA, and microbial extracellular polymeric substances (EPS). This model could apply to biofilms in the cystic fibrosis lung or in chronic wounds. B, Large aggregates of microorganisms comingle with precipitated mineral phases or host-derived material such as platelets and fibrinogen. This model could apply to biofilms such as dental plaque, the infectious vegetation on a heart valve, or the encrustation in a urinary catheter. In these examples, the biofilm/host material/mineral aggregation is attached to a surface. C, Small aggregates of surface-attached microrganisms are covered by a secondary layer primarily host-derived material.
Biofilm composition and mechanical properties
As a material, a biofilm can be conceptualized as a dispersion of colloidal particles (microbial cells, mineral precipitates, host biological debris) in a hydrogel (microbial extracellular polymeric substances (EPS) and host extracellular polymers such as mucus, collagen, or released DNA). In the biofilm literature, the constituents of EPS have been identified as polysaccharides, proteins, and extracellular DNA (Branda et al., 2005; Flemming and Wingender, 2010). There has been less attention to understanding the composition of admixed host polymers and particulates, but these components will clearly be important in the mechanics of the in vivo biofilm.
Biofilms typically exhibit viscoelastic behavior when mechanically stressed (Klapper et al., 2002; Stoodley et al., 2002; Wilking et al., 2011; Böl et al., 2013). That is, they can deform in both an elastic, reversible manner and in a viscous, irreversible manner. Most biological materials, such as mucus or tissue, are also soft and viscoelastic (Levental et al., 2007). There are numerous parameters that can be appropriately used to characterize the mechanical behavior of these materials. In this article I will mention only two: G, the elastic modulus, which can be thought of as a spring constant, and σB, the biofilm failure strength, which characterizes the applied force per area that will cause the biofilm to break. Both of these parameters characterize the response of a material to an applied force, but they capture qualitatively different aspects. G describes the reversible stretching of the material under tension and can be thought of as the stiffness of the material. Materials with larger values of G are harder to deform. σB describes the breaking of the material and can be thought of as its overall strength. Materials with larger values of σB are harder to break. It is clearly simplistic to think of these properties as single values in the context of the systems begin discussed. The reality of a viscoelastic material is that the measured failure strength will depend on how quickly or slowly stress is applied. In addition, we know that biofilms are heterogeneous with regions that are stronger or weaker.
Biofilms resist mechanical clearance
One way that a biofilm infection persists is by withstanding the flow of fluid or other mechanical forces that work to wash or sweep microorganisms out of the body (Seymour et al., 2004; Stewart, 2012). Here are four examples.
Consider the lumen of a central venous catheter, where there are typically periods of no flow punctuated by intervals of significant flow. For a biofilm to accumulate in these locations, it is critical that the shear stress applied during peak fluid flow is insufficient to remove the biofilm. In the familiar example of dental plaque, a spectrum of forces are exerted on the oral biofilm. These forces range from mild shear associated with the flow of saliva (weak but continuous), to contact with moving tongue, cheeks, or food (intermediate and frequent), to water jets and toothbrushing (occasional but intense.) Biofilm will only build up if it is strong enough to resist disruption by the frequent forces or is able to regrow in the intervals between the intense but infrequent forces. Toothbrushing is effective precisely because it is sufficiently powerful to remove much of the biofilm. To be effective, it must be repeated regularly on a cycle that is comparable to or shorter than the biofilm regrowth time. Of course there are locations in the dentition, such as interproximal zones, that are sheltered from the forces delivered by toothbrush bristles. These areas are prone to greater plaque accumulation.
Airborne particles, including microorganisms, are continuously deposited in the lungs. In healthy individuals, these particles are swept out of the lung by the mucociliary escalator. Beating cilia steadily push the fluid mucus layer, along with entrapped particles, up and out of the lungs. In individuals with cystic fibrosis, the mucus is thickened by the disease and the motion of the escalator is much impaired. The residence time of bacteria in the mucus layer is long enough for them to grow into biofilm aggregates and exert pathogenic effects.
In endocarditis, the biofilm attached to a heart valve persists in a turbulent, high shear environment. In this case, the flow of fluid never ceases so the intrinsic mechanical strength of the biofilm/vegetation has to exceed the maximum stress developed by the blood flow.
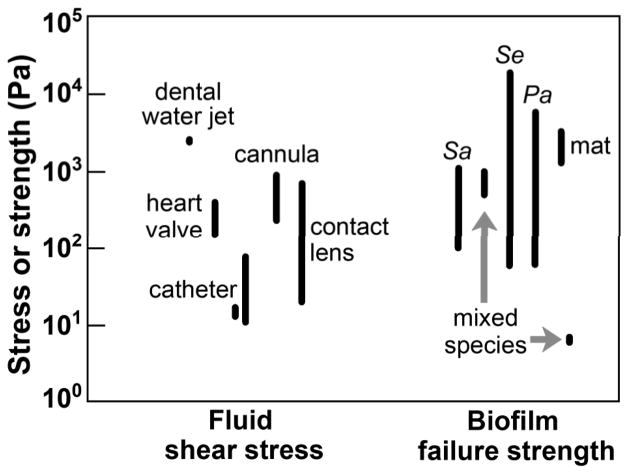
One can begin to develop a quantitative basis for analyzing the interactions described above by comparing the shear stress applied to the biofilm with the biofilm cohesive strength (Figure 2). Though measurements of biofilm cohesive strength range over orders of magnitude, this comparison allows us to appreciate that biofilms are strong enough to mechanically persist in many settings. The fundamental criterion for mechanical persistence is: τF < σB where τF is the applied shear stress and σB is the biofilm failure strength. Put another way, when the force applied is not enough to overcome biofilm cohesiveness, the biofilm persists.
Figure 2.
Magnitude of calculated shear stresses in the human body and measured biofilm failure strengths. Sources: Day, 1997; Ohashi & Harada, 1999; Bundy et al., 2001; Mareels et al., 2007; Möhle et al., 2007; Dasi et al., 2009; Aggarwal & Hozalski, 2010; De Bartolo et al., 2011; Clark et al., 2012; Vignaga et al., 2012; Rmaile et al, 2013. Sa, Staphylococcus aureus, Se, Staphylococcus epidermidis, Pa, Pseudomonas aeruginosa.
Dissemination of infection
Though the discussion thus far has focused on how biofilms stay stuck in place, we also know that biofilms can shed planktonic cells or aggregates of cells. This is important in the context of infection because planktonic release events can disseminate the infection to other parts of the body or spawn episodes of acute infection (Fux et al., 2004; Hall-Stoodley & Stoodley, 2005). For example, oral streptococci are sometimes recovered from heart valves. The bacteria are thought to have travelled to the heart, where they attached, following a transient bacteremia associated with teeth cleaning. The biofilm on a central venous catheter can be asymptomatic until enough microorganisms are released at once to trigger a bloodstream infection. In cystic fibrosis pneumonia, acute exacerbations may result from biofilm dispersion events.
There is an apparent contradiction in the requirement for biofilms to be both strong enough to resist detachment and weak enough to permit release of planktonic cells. This contradiction is resolved by recognizing that biofilm material properties, like other chemical and biological properties (Stewart & Franklin, 2008), are inherently heterogeneous. Aggarwal et al. (2009) have reported repeated microscale measurements of cohesive strength of Staphylococcus epidermidis and Pseudomonas aeruginosa biofilms; they find these values are distributed over more than two orders of magnitude (see Figure 2). In other words, there are parts of a biofilm that are strong enough to remain attached even during high shear stress events and there can also be parts of the same biofilm that are weak enough to readily detach.
The fundamental criterion for detachment (dissemination) is: τF > σB, which says that for detachment to occur the applied force must exceed biofilm cohesiveness.
Biofilms resist phagocytosis
Microorganisms interact with and evade phagocytic leukocytes via a complex exchange of biochemical signals and toxins (Nizet, 2007; Sarantis & Grinstein, 2012). Biofilms may additionally thwart phagocytosis by simple mechanical means. Here I consider this possibility. The ability of a phagocyte to engulf a biofilm aggregate likely depends on both the size of the aggregate and on its material properties, adhesive strength and cohesive strength in particular (Möller et al., 2013). For example, a neutrophil may be able to engulf a sufficiently small biofilm aggregate even if it is mechanically rigid and unbreakable. The critical threshold size for engulfment of a biofilm cluster would logically be similar to the size of the phagocyte itself. Neutrophils are approximately 10 to 12 μm in diameter. We can therefore speculate that any biofilm aggregate less than about 10 μm in size may be vulnerable to phagocytosis. Experimentally, neutrophils are able to phagocytose 3 μm and 6 μm diameter polystyrene beads, but exhibit frustrated phagocytosis when presented with 11 μm diameter beads, engulfing only about 50% of the bead circumference (Herant et al., 2006). If a biofilm aggregate is larger than 10 μm, it may still be subject to phagocytosis, but only if it is soft enough to be fragmented by the forces applied by the engulfing cell. In other words, there is likely a critical threshold value of biofilm cohesive strength above which a biofilm is protected from phagocytic engulfment. Using a mathematical model of the phagocytosis process, Herant et al. (2006) suggest that the attractive force between a neutrophil and its target body could be of the order of magnitude of 103 Pa. Referring to Figure 2, this could be sufficient to allow phagocytosis of some, but not all biofilms.
There is a second physical consideration in the phagocytic attack on a biofilm. This has to do with the mobility of the phagocyte. Phagocytes respond to attractant molecules released by microorganisms and migrate toward them. Such migration requires a permissive matrix. It is possible that some biofilm affected milieus, for example, the thickened mucus of the cystic fibrosis lung, are so viscous that they retard or prevent leukocyte chemotaxis. This could tip the balance in favor of the biofilm. In fact, Matsui et al. (2005) reported exactly this effect. Neutrophils migrated in mucus constituted at concentrations in the normal range (1.5 to 2.5% dry weight) but exhibited little migration in any direction in thickened (6.5%) mucus representative of the airway mucus in the CF lung. Parkhurst & Saltzman (1994) measured leukocyte migration in cervical mucus and concluded that neutrophils move effectively in normal mucus. These authors also measured human neutrophil motility in well-defined collagen gels and found that motility decreased with increasing collagen concentrations above 0.2 mg mL−1 (Parkhurst & Saltzman, 1992). A conservative extrapolation of their data suggests that neutrophils would be completely immobile in a collagen gel of 1 mg mL−1 (0.1%).
The elementary biophysics of hydrogels suggests that modest increases in local polymer concentration, C, either biofilm extracellular polymeric substances of host extracellular matrix materials, could lead to very large increases in the matrix strength. For example, the elastic modulus, G, of a gel increases approximately as: G ~ C2.25 (De Gennes, 1979). In other words, a doubling of matrix polymer concentration could increase gel modulus by nearly a factor of five. Such increases in gel rigidity could obviously reduce phagocyte mobility.
Together these observations suggest that the ability of leukocytes to effectively penetrate and police mucus layers can be strongly related to the extracellular polymer concentration because the polymers determine the viscoelastic properties of the gelatinous matrix. The matrix polymers may derive from secreted mucus, necrotic tissue, DNA released from dead neutrophils or neutrophil extracellular traps, as well as from the EPS produced by microbial biofilm.
This argument, formulated here in terms of leukocyte migration in mucus, may extend to the limited penetration of leukocytes to the surface of an encapsulated implant. After implantation of a biomaterial, it is common for chronic inflammation and a foreign body reaction to ultimately conclude with fibrous encapsulation of the device (Anderson et al., 2008; Bryers et al., 2012). The capsule consists largely of collagen deposited by fibroblasts, and it effectively walls off the implant surface. If there is a biofilm on this surface, this process may effectively construct a fortress in which bacterial biofilm is shielded from host cellular defenses.
Of course the EPS of a microbial biofilm itself may serve the function of excluding phagocytes. In the cystic fibrosis lung, there is a common selection over time for Pseudomonas aeruginosa mutants that overproduce the polysaccharide alginate. Does the copious alginate gel limit physical access of neutrophils to bacterial cells? This function has been postulated (Mai et al., 1993; Bjarnsholt et al., 2009).
Treatments based on weakening biofilm
The discussion of issues above naturally leads to the possibility of alternative therapies for treating biofilm infections based on weakening biofilm cohesion. Reducing biofilm cohesive (or adhesive) strength could: 1) allow prevailing hydrodynamic shear to remove biofilm, 2) increase the efficacy of designed interventions for removing biofilms, 3) enable phagocytic engulfment of softened biofilm aggregates, and 4) improve phagocyte mobility and access to biofilm.
A wide variety of chemical, biochemical, and enzymatic strategies can be envisioned for effecting biofilm weakening and dispersion (Chen & Stewart, 2000; Landini et al., 2010; Bjarnsholt et al., 2013; Kostakioti et al., 2013). I present a sampling of such approaches here for sake of illustration; this listing is far from comprehensive. The examples below focus on targeting the biofilm extracellular matrix.
A direct chemical attack on the biofilm extracellular matrix may be behind the relative success of halogens and other oxidizing biocides as anti-biofilm disinfectants. Free chlorine caused erosion of a S. epidermidis biofilm that was not observed with other antimicrobials (Davison et al., 2010). The strong oxidant periodate is sometimes used to diagnose the presence of polysaccharides in the biofilm matrix based on its ability to oxidize and degrade these macromolecules (Chaignon et al., 2007).
Enzymatic degradation of biofilm extracellular polymeric substances holds promise as a biofilm removal approach (Johansen et al., 1997; Marcato-Romain et al., 2011). For example, an enzyme discovered based on its involvement in the natural dispersion of aggregates of Actinobacillus actinomycetemcomitans (Kaplan et al., 2003), Dispersin B, cleaves a linear polymer of N-acetylglucosamine, a common biofilm extracellular polysaccharide. Treatment with this enzyme can remove biofilms of multiple bacterial species (Itoh et al., 2005) and has been shown to alter the mechanical stability of S. epidermidis biofilm under hydrodynamic challenge (Brindle et al., 2011). The intriguing strategy of delivering the coding potential for this same enzyme in the DNA of an engineered bacteriophage has been demonstrated (Lu & Collins, 2007).
Because biofilm EPS often contains proteins and extracellular DNA, proteases and DNAases are also candidate enzymes for breaking down biofilms (Whitchurch et al., 2002; Chaignon et al., 2007; Boles & Horswill, 2008; Hall-Stoodley et al., 2008; Lequette et al., 2010; Nijland et al., 2010).
It should also be possible to alter biofilm matrix cohesion by interfering with crosslinking interactions between matrix polymers. Chelants that compete for multivalent cations such as calcium or iron have been shown to remove biofilms (Turakhia et al., 1983; Banin et al., 2006; Raad et al., 2008). The efficacy of these agents may derive from disrupting electrostatic interactions between bridging cations and strands of negatively charged polymers (Chen & Stewart, 2002). Concentrated urea, which disrupts hydrogen bonding has been shown to facilitate removal of S. epidermidis biofilm (Brindle et al., 2011). Thus, hydrogen bonding may contribute to matrix integrity.
One can also imagine a new class of drugs that inhibit the biosynthesis, export, or anchoring of extracellular polymeric substances important for biofilm cohesion. These drugs, which are mostly hypothetical, would likely not be bactericidal. One potential example of such a strategy is a cocktail of chemistries reported to suppress exopolysaccharide synthesis in the cariogenic organism Streptococcus mutans (Falsetta et al., 2012).
A fourth way to target the biofilm matrix is through the regulatory mechanisms that control elaboration of EPS. The leading example of this possibility involves interfering with a regulatory mechanism now understood to be common in bacteria centered on the secondary messenger molecule cyclic di-GMP (Hengge, 2009; Römling et al., 2013). In overly simplistic yet consistent terms, high levels of cyclic di-GMP induce synthesis of EPS constituents and promote biofilm formation whereas low levels of cyclic di-GMP lead to breakdown of the matrix and stimulation of motility and cell release. Chemical inhibitors of cyclic di-GMP metabolism might someday be useful in forcing the transition toward dissolution of matrix polymers and upregulation of motility (Sambanthamoorthy et al., 2012). Other chemical signals tied in with cyclic di-GMP, such as nitric oxide, might also induce this switch (Barraud et al., 2009; Li et al., 2013)
One caution with these approaches is that they must be done in such a way so as to avoid dissemination of large numbers of planktonic microorganisms. Bacteria detached from a catheter would probably need to be deliberately withdrawn from the device and captured rather than allowed to escape into the bloodstream, for example. It may be necessary to simultaneously treat with antibiotics to control dispersed cells. It is also possible that in some instances the immune defenses may be adequate to neutralize the released microorganisms on their own.
Conclusion
It should not be surprising that existing antimicrobials fail to remove or weaken biofilms: these agents have been discovered and developed based on their ability to kill germs without consideration or measurement of biofilm removal. Some biocides and antiseptics, such as glutaraldehyde or chlorhexidine, may even crosslink biofilm and make it less prone to removal (Simões et al., 2005, Brindle et al., 2011). Progress in developing new anti-biofilm therapies will follow when the biofilm is better understood as a mechanical structure and measurement of biofilm material properties and biofilm removal becomes more routine (Jones et al., 2011; Lieleg et al., 2011; Böl et al., 2013). Mathematical and computer modeling of biofilm fluid-structure interactions also has an important role to play (Duddu et al., 2009; Taherzadeh et al., 2010; Vo et al., 2010; Lindley et al., 2012).
Acknowledgments
PSS acknowledges support from NIH award R01GM109452 and NSF award 0728621.
References
- Aggarwal S, Hozalski RM. Determination of biofilm mechanical properties from tensile tests performed using a micro-cantilever method. Biofouling. 2010;26:479–486. doi: 10.1080/08927011003793080. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore RS, Christie CD, Smith GJ. Immunohistopathologic localization of Pseudomonas aeruginosa in lungs from patients with cystic fibrosis. Implications for the pathogenesis of progressive lung deterioration. Am Rev Respir Dis. 1989;140:1650–1661. doi: 10.1164/ajrccm/140.6.1650. [DOI] [PubMed] [Google Scholar]
- Banin E, Brady KM, Greenberg EP. Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Appl Environ Microbiol. 2006;72:2064–2069. doi: 10.1128/AEM.72.3.2064-2069.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraud N, Schleheck D, Klebensberger J, Webb JS, Hassett DJ, Rice SA, Kjelleberg S. Nitric oxide signaling in Pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic di-GMP levels, and enhanced dispersal. J Bacteriol. 2009;191:7333–7342. doi: 10.1128/JB.00975-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnsholt T, Jensen PØ, Fiandaca MJ, Pedersen J, Hansen CR, Andersen CB, Pressler T, Givskov M, Høiby N. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr Pulmonol. 2009;44:547–558. doi: 10.1002/ppul.21011. [DOI] [PubMed] [Google Scholar]
- Bjarnsholt T, Alhede M, Alhede M, Eickhardt-Sørensen SR, Moser C, Kühl M, Jensen PO, Høiby N. The in vivo biofilm. Trends Microbiol. 2013;21:466–474. doi: 10.1016/j.tim.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Bjarnsholt T, Ciofu O, Molin S, Givskov M, Høiby N. Applying insights from biofilm biology to drug development - can a new approach be developed? Nat Rev Drug Discov. 2013;12:791–808. doi: 10.1038/nrd4000. [DOI] [PubMed] [Google Scholar]
- Böl M, Ehret AE, Albero AB, Hellriegel J, Krull R. Recent advances in mechanical characterization of biofilm and their significance for material modelling. Crit Rev Biotechnol. 2013;33:145–171. doi: 10.3109/07388551.2012.679250. [DOI] [PubMed] [Google Scholar]
- Boles BR, Horswill AR. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 2008;4:e1000052. doi: 10.1371/journal.ppat.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda SS, Vik A, Friedman L, Kolter R. Biofilms: the matrix revisited. Trends Microbiol. 2005;13:20–26. doi: 10.1016/j.tim.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Brindle ER, Miller DA, Stewart PS. Hydrodynamic deformation and removal of Staphylococcus epidermidis biofilms treated with urea, chlorhexidine, iron chloride, or DispersinB. Biotechnol Bioeng. 2011;108:2968–2977. doi: 10.1002/bit.23245. [DOI] [PubMed] [Google Scholar]
- Bryers JD, Giachelli CM, Ratner BD. Engineering biomaterials to integrate and heal: the biocompatibility paradigm shifts. Biotechnol Bioeng. 2012;109:1898–1911. doi: 10.1002/bit.24559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy KJ, Harris LG, Rahn BA, Richards RG. Measurement of fibroblast and bacterial detachment from biomaterials using jet impingement. Cell Biol Int. 2001;25:289–307. doi: 10.1006/cbir.2000.0648. [DOI] [PubMed] [Google Scholar]
- Chaignon P, Sadovskaya I, Ragunah C, Ramasubbu N, Kaplan JB, Jabbouri S. Susceptibility of staphylococcal biofilms to enzymatic treatments depends on their chemical composition. Appl Microbiol Biotechnol. 2007;75:125–132. doi: 10.1007/s00253-006-0790-y. [DOI] [PubMed] [Google Scholar]
- Chen X, Stewart PS. Biofilm removal caused by chemical treatments. Water Res. 2000;34:4229–4233. [Google Scholar]
- Chen X, Stewart PS. Role of electrostatic interactions in cohesion of bacterial biofilms. Appl Microbiol Biotechnol. 2002;59:718–720. doi: 10.1007/s00253-002-1044-2. [DOI] [PubMed] [Google Scholar]
- Clark TWI, Van Canneyt K, Verdonck P. Computational fluid dynamics and preclinical assessment of a novel hemodialysis catheter. Sem Dialysis. 2012;25:574–581. doi: 10.1111/j.1525-139X.2012.01052.x. [DOI] [PubMed] [Google Scholar]
- Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- Dasi LP, Simon HA, Sucosky P, Yoganathan AP. Fluid mechanics of artificial heart valves. Clin Exp Pharmacol Physiol. 2009;36:225–237. doi: 10.1111/j.1440-1681.2008.05099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison WM, Pitts B, Stewart PS. Spatial and temporal patterns of biocide action against Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother. 2010;54:2920–2927. doi: 10.1128/AAC.01734-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day KD. MSc Thesis. Massachusetts Institute of Technology; Cambridge, MA: 1997. The mechanics of a hydrogel contact lens on the human eye with a lubricating tear layer. [Google Scholar]
- De Bartolo C, Nigro A, Fragomeni G, Colacino FM, Wang D, Jones CC, Zwischenberger J. Numerical and experimental flow analysis of the Wang-Zwische double-lumen cannula. ASAIO J. 2011;57:318–327. doi: 10.1097/MAT.0b013e31821c08bc. [DOI] [PubMed] [Google Scholar]
- De Gennes PG. Scaling Concepts in Polymer Physics. Cornell University Press; Ithaca, NY: 1979. [Google Scholar]
- Duddu R, Chopp DL, Moran B. A two-dimensional continuum model of biofilm growth incorporating fluid flow and shear stress based detachment. Biotechnol Bioeng. 2009;103:92–104. doi: 10.1002/bit.22233. [DOI] [PubMed] [Google Scholar]
- Falsetta ML, Klein MI, Lemos JA, Silva BB, Agidi S, Scott-Anne KK, Koo H. Novel antibiofilm chemotherapy targets exopolysaccharide synthesis and stress tolerance in Streptococcus mutans to modulate virulence expression in vivo. Antimicrob Agents Chemother. 2012;56:6201–6211. doi: 10.1128/AAC.01381-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- Friskopp J. Ultrastructure of nondecalcified supragingival and subgingival calculus. J Periodontol. 1983;54:542–550. doi: 10.1902/jop.1983.54.9.542. [DOI] [PubMed] [Google Scholar]
- Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005;13:34–40. doi: 10.1016/j.tim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Fux CA, Wilson S, Stoodley P. Detachment characteristics and oxacillin resistance of Staphyloccocus aureus biofilm emboli in an in vitro catheter infection model. J Bacteriol. 2004;186:4486–4491. doi: 10.1128/JB.186.14.4486-4491.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gristina AG, Oga M, Webb LX, Hobgood CD. Adherent bacterial colonization in the pathogenesis of osteomyelitis. Science. 1985;228:990–993. doi: 10.1126/science.4001933. [DOI] [PubMed] [Google Scholar]
- Hall-Stoodley L, Stoodley P. Biofilm formation and dispersal and the transmission of human pathogens. Trends Microbiol. 2005;13:7–10. doi: 10.1016/j.tim.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Hall-Stoodley L, Hu FZ, Gieseke A, et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296:202–211. doi: 10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L, Nistico L, Sambanthamoorthy K, Dice B, Nguyen D, Mershon WJ, Johnson C, Hu FZ, Stoodley P, Ehrlich GD, Post JC. Characterization of biofilm matrix, degradation by DNase treatment and evidence of capsule downregulation in Streptococcus pneumoniae clinical isolates. BMC Microbiol. 2008;8:173. doi: 10.1186/1471-2180-8-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengge R. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol. 2009;7:263–273. doi: 10.1038/nrmicro2109. [DOI] [PubMed] [Google Scholar]
- Herant M, Heinrich V, Dembo M. Mechanics of neutrophil phagocytosis: experiments and quantitative models. J Cell Sci. 2006;119:1903–1913. doi: 10.1242/jcs.02876. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Wang X, Hinnebusch BJ, Preston JF, Romeo T. Depolymerization of β-1,6-N-acetyl-d-glucosamine disrupts the integrity of diverse bacterial biofilms. J Bacteriol. 2005;187:382–387. doi: 10.1128/JB.187.1.382-387.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James GA, Swogger E, Wolcott R, Pulcini Ed, Secor P, Sestrich J, Costerton JW, Stewart PS. Biofilms in chronic wounds. Wound Repair Regen. 2008;16:37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- Johansen C, Falholt P, Gram L. Enzymatic removal and disinfection of bacterial biofilms. Appl Environ Microbiol. 1997;63:3724–3728. doi: 10.1128/aem.63.9.3724-3728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones WL, Sutton MP, McKittrick L, Stewart PS. Chemical and antimicrobial treatments change the viscoelastic properties of bacterial biofilms. Biofouling. 2011;27:207–215. doi: 10.1080/08927014.2011.554977. [DOI] [PubMed] [Google Scholar]
- Kaplan JB, Ragunath C, Ramasubbu N, Fine DH. Detachment of Actinobacillus actinomycetemcomitans biofilm cells by an endogenous beta-hexosaminidase activity. J Bacteriol. 2003;185:4693–4698. doi: 10.1128/JB.185.16.4693-4698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapper I, Rupp CJ, Cargo R, Purevdorj B, Stoodley P. Viscoelastic fluid description of bacterial biofilm material properties. Biotechnol Bioeng. 2002;80:289–296. doi: 10.1002/bit.10376. [DOI] [PubMed] [Google Scholar]
- Kostakioti M, Hadjifrangiskou M, Hultgren SJ. Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb Perspect Med. 2013;3:a010306. doi: 10.1101/cshperspect.a010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landini P, Antoniani D, Burgess JG, Nijland R. Molecular mechanisms of compounds affecting bacterial biofilm formation and dispersal. Appl Microbiol Biotechnol. 2010;86:813–823. doi: 10.1007/s00253-010-2468-8. [DOI] [PubMed] [Google Scholar]
- Lequette Y, Boels G, Clarisse M, Faille C. Using enzymes to remove biofilms of bacterial isolates sampled in the food-industry. Biofouling. 2010;26:421–431. doi: 10.1080/08927011003699535. [DOI] [PubMed] [Google Scholar]
- Levental I, Georges PC, Janmey PA. Soft biological materials and their impact on cell function. Soft Matter. 2007;3:299–306. doi: 10.1039/b610522j. [DOI] [PubMed] [Google Scholar]
- Li Y, Heine S, Entian M, Sauer K, Frankenberg-Dinkel N. NO-induced biofilm dispersion in Pseudomonas aeruginosa is mediated by an MHYT domain-coupled phosphodiesterase. J Bacteriol. 2013;195:3531–3542. doi: 10.1128/JB.01156-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieleg O, Caldara M, Baumgartel R, Ribbeck K. Mechanical robustness of Pseudomonas aeruginosa biofilms. Soft Matter. 2011;7:3307–3314. doi: 10.1039/c0sm01467b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindley B, Wang Q, Zhang T. Multicomponent hydrodynamic model for heterogeneous biofilms: two-dimensional numerical simulations of growth and interaction with flows. Phys Rev E Stat Nonlin Soft Matter Phys. 2012;85:031908. doi: 10.1103/PhysRevE.85.031908. [DOI] [PubMed] [Google Scholar]
- Lu TK, Collins JJ. Dispersing biofilms with engineered enzymatic bacteriophage. Proc Natl Acad Sci USA. 2007;104:11197–11202. doi: 10.1073/pnas.0704624104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai GT, Seow WK, Pier GB, McCormack JG, Thong YH. Suppression of lymphocyte and neutrophil functions by Pseudomonas aeruginosa mucoid exopolysaccharide (alginate): reversal by physicochemical, alginase, and specific monoclonal antibody treatments. Infect Immun. 1993;61:559–564. doi: 10.1128/iai.61.2.559-564.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcato-Romain CE, Pechaud Y, Paul E, Girbal-Neuhauser E, Dossat-Létisse V. Removal of microbial multi-species biofilms from the paper industry by enzymatic treatments. Biofouling. 2012;28:305–314. doi: 10.1080/08927014.2012.673122. [DOI] [PubMed] [Google Scholar]
- Mareels G, Kaminsky R, Eloot S, Verdonck PR. Particle image velocimetry-validated, computational fluid dynamics-based design to reduce shear stress and residence time in central venous hemodialysis catheters. ASAIO J. 2007;53:438–446. doi: 10.1097/MAT.0b013e3180683b7c. [DOI] [PubMed] [Google Scholar]
- Marrie TJ, Cooper JH, Costerton JW. Ultrastructure of cardiac bacterial vegetations on native valves with emphasis on alterations in bacterial morphology following antibiotic treatment. Can J Cardiol. 1987;3:275–280. [PubMed] [Google Scholar]
- Matsui H, Verghese MW, Kesimer M, Schwab UE, Randell SH, Sheehan JK, Grubb BR, Boucher RC. Reduced three-dimensional motility in dehydrated airway mucus prevents neutrophil capture and killing bacteria on airway epithelial surfaces. J Immunol. 2005;175:1090–1099. doi: 10.4049/jimmunol.175.2.1090. [DOI] [PubMed] [Google Scholar]
- McDougald D, Rice SA, Barraud N, Steinberg PD, Kjelleberg S. Should we stay or should we go: mechanisms and ecological consequences for biofilm dispersal. Nat Rev Microbiol. 2011;10:39–50. doi: 10.1038/nrmicro2695. [DOI] [PubMed] [Google Scholar]
- Möhle RB, Langemann T, Haesner M, Augustin W, Scholl S, Neu TR, Hempel DC, Horn H. Structure and shear strength of microbial biofilms as determined with confocal laser scanning microscopy and fluid dynamic gauging using a novel rotating disc biofilm reactor. Biotechnol Bioeng. 2007;98:747–755. doi: 10.1002/bit.21448. [DOI] [PubMed] [Google Scholar]
- Möller J, Lühmann T, Chabria M, Hall H, Vogel V. Macrophages lift off surface-bound bacteria using a filopodium-lamellipodium hook-and-shovel mechanism. Sci Rep. 2013;3:2884. doi: 10.1038/srep02884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijland R, Hall MJ, Burgess JG. Dispersal of biofilms by secreted, matrix degrading, bacterial DNase. PLoS One. 2010;14:e15668. doi: 10.1371/journal.pone.0015668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizet V. Understanding how leading bacterial pathogens subvert innate immunity to reveal novel therapeutic targets. J Allergy Clin Immunol. 2007;120:13–22. doi: 10.1016/j.jaci.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Ohashi A, Koyama T, Syutsubo K, Harada H. A novel method for evaluation of biofilm tensile strength resisting erosion. Water Sci Technol. 1999;39:261–268. [Google Scholar]
- O’Toole GA, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu Rev Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- Parkhurst MR, Saltzman WM. Quantification of human neutrophil motility in three-dimensional collagen gels. Biophys J. 1992;61:306–315. doi: 10.1016/S0006-3495(92)81838-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst MR, Saltzman WM. Leukocytes migrate through three-dimensional gels of midcycle cervical mucus. Cell Immunol. 1994;156:77–94. doi: 10.1006/cimm.1994.1154. [DOI] [PubMed] [Google Scholar]
- Parsek MR, Singh PK. Bacterial biofilms: An emerging link to disease pathogenesis. Annu Rev Microbiol. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- Raad II, Fang X, Keutgen XM, Jiang Y, Sherertz R, Hachem R. The role of chelators in preventing biofilm formation and catheter-related bloodstream infections. Curr Opin Infect Dis. 2008;21:385–392. doi: 10.1097/QCO.0b013e32830634d8. [DOI] [PubMed] [Google Scholar]
- Rmaile A, Carugo D, Capretto L, Aspiras M, De Jager M, Ward M, Stoodley P. Removal of interproximal dental biofilms by high-velocity water microdrops. J Dent Res. 2013 doi: 10.1177/0022034513510945. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römling U, Galperin MY, Gomelsky M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev. 2013;77:1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambanthamoorthy K, Sloup RE, Parashar V, Smith JM, Kim EE, Semmelhack MF, Neiditch MB, Waters CM. Identification of small molecules that antagonize diguanylate cyclase enzymes to inhibit biofilm formation. Antimicrob Agents Chemother. 2012;56:5202–5211. doi: 10.1128/AAC.01396-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarantis H, Grinstein S. Subversion of phagocytosis for pathogen survival. Cell Host Microbe. 2012;12:419–431. doi: 10.1016/j.chom.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Seymour JD, Codd SL, Gjersing EL, Stewart PS. Magnetic resonance microscopy of biofilm structure and impact on transport in a capillary bioreactor. J Magn Reson. 2004;167:322–327. doi: 10.1016/j.jmr.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Simões M, Pereira MO, Vieira MJ. Effect of mechanical stress on biofilms challenged by different chemicals. Wat Res. 2005;39:5142–5152. doi: 10.1016/j.watres.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Stewart PS. Convection around biofilms. Biofouling. 2012;28:187–198. doi: 10.1080/08927014.2012.662641. [DOI] [PubMed] [Google Scholar]
- Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- Stewart PS, Franklin MJ. Physiological heterogeneity in biofilms. Nat Rev Microbiol. 2008;6:199–210. doi: 10.1038/nrmicro1838. [DOI] [PubMed] [Google Scholar]
- Stickler D, Ganderton L, King J, Nettleton J, Winters C. Proteus mirabilis biofilms and the encrustation of urethral catheters. Urol Res. 1993;21:407–411. doi: 10.1007/BF00300077. [DOI] [PubMed] [Google Scholar]
- Stoodley P, Cargo R, Rupp CJ, Wilson S, Klapper I. Biofilm material properties as related to shear-induced deformation and detachment phenomena. J Ind Microbiol Biotechnol. 2002;29:361–367. doi: 10.1038/sj.jim.7000282. [DOI] [PubMed] [Google Scholar]
- Stoodley P, Nistico L, Johnson S, Lasko LA, Baratz M, Gahlot V, Ehrlich GD, Kathju S. Direct demonstration of viable Staphylococcus aureus biofilms in an infected total joint arthroplasty. A case report. J Bone Joint Surg Am. 2008;90:1751–1758. doi: 10.2106/JBJS.G.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley P, Braxton EE, Jr, Nistico L, Hall-Stoodley L, Johnson S, Quigley M, Post JC, Ehrlich GD, Kathju S. Direct demonstration of Staphylococcus biofilm in an external ventricular drain in a patient with a history of recurrent ventriculoperitoneal shunt failure. Pediatr Neurosurg. 2010;46:127–132. doi: 10.1159/000319396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B, Gillam DG, Mordan NJ, Galgut PN. A preliminary investigation into the ultrastructure of dental calculus and associated bacteria. J Clin Periodontol. 2004;31:364–369. doi: 10.1111/j.1600-051X.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- Taherzadeh D, Picioreanu C, Küttler U, Simone A, Wall WA, Horn H. Computational study of the drag and oscillatory movement of biofilm streamers in fast flows. Biotechnol Bioeng. 2010;105:600–610. doi: 10.1002/bit.22551. [DOI] [PubMed] [Google Scholar]
- Turakhia MH, Cooksey KE, Characklis WG. Influence of a calcium-specific chelant on biofilm removal. Appl Environ Microbiol. 1983;46:1236–1238. doi: 10.1128/aem.46.5.1236-1238.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignaga E, Haynes H, Sloan WT. Quantifying the tensile strength of microbial mats grown over noncohesive sediments. Biotechnol Bioeng. 2012;109:1155–1164. doi: 10.1002/bit.24401. [DOI] [PubMed] [Google Scholar]
- Vo GD, Brindle E, Heys J. An experimentally validated immersed boundary model of fluid-biofilm interaction. Water Sci Technol. 2010;61:3033–3040. doi: 10.2166/wst.2010.213. [DOI] [PubMed] [Google Scholar]
- Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295:1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- Wilking JN, Angelini TE, Seminara A, Brenner MP, Weitz DA. Biofilms as complex fluids. MRS Bull. 2011;36:385–391. [Google Scholar]
- Wright JP, Kirschner RH. Scanning electron microscopy of infective endocarditis. Scan Electron Microsc. 1979;(3):793–9. [PubMed] [Google Scholar]