Summary
Intercellular adhesion molecule-1 (ICAM-1) plays an important role in leukocyte trafficking, induction of cellular immune responses, and immunological synapse formation. As a member of the immunoglobulin superfamily of adhesion proteins, ICAM-1 is composed of repeating Ig-like domains, a transmembrane domain, and short cytoplasmic tail that participates in intracellular signaling events. At least seven ICAM-1 protein isoforms are generated by alternative splicing, however little is known regarding their immunobiology. We have previously shown using different lines of ICAM-1 mutant mice (Icam1tm1Jcgr and Icam1tm1Bay) that expression of alternatively spliced ICAM-1 isoforms can significantly influence the disease course during the development of EAE. In this study, we show using a newly developed transgenic mouse (CD2-Icam1D4del/Icam1null) that T cell-specific expression of a single ICAM-1 isoform composed of Ig domains 1, 2, 3 and 5, can mediate the initiation and progression of EAE. Our results indicate that the ICAM-1 isoform lacking Ig domain 4 can drive pathogenesis in demyelinating disease and may be a novel therapeutic target for treating multiple sclerosis.
Keywords: adhesion molecules, alternative splicing, central nervous system, demyelinating disease, transgenic animals
Introduction
Intercellular adhesion molecule-1 (ICAM-1, CD54), is a member of the immunoglobulin-like (Ig) superfamily of adhesion proteins [1]. The full-length form of ICAM-1 contains five extracellular Ig domains, although it’s now well-recognized that similar to several other Ig domain superfamily members, this adhesion molecule undergoes alternative splicing leading to the production of at least seven isoforms including a soluble protein [2–8]. Little is known about the function, expression, and ligand binding specificity of these ICAM-1 isoforms. Recent studies from our lab have demonstrated that ICAM–1 mutant mice expressing different combinations of alternatively spliced isoforms (Icam1tm1Jcgr and Icam1tm1Bay), develop experimental autoimmune encephalitomyelitis (EAE) with markedly different severity [9–12]. These data indicate that these different ICAM-1 molecules are functional and can play unique roles in inflammatory disease. Based on these findings, we reasoned that the increased severity of EAE observed in Icam1tm1Bay mice was primarily due to the expression of ICAM-1 isoforms lacking domain 4 [12]. To assess this possibility, we developed a transgenic mouse (CD2-Icam1D4del) with T cell-specific expression of an ICAM-1 isoform composed of Ig domains 1, 2, 3 and 5. This transgene was then crossed into the Icam1null background (CD2-Icam1D4del/Icam1null) allowing us to determine whether a single alternatively spliced ICAM-1 isoform can modulate the development and progression of EAE. Surprisingly, we found that CD2-Icam1D4del/Icam1null mice developed EAE with comparable disease onset and severity to that of wild type mice. Our results strongly suggest that the ICAM-1 isoform lacking Ig domain 4 can promote demyelinating disease pathogenesis and may be a novel therapeutic target for treating multiple sclerosis.
Results and discussion
Generation and characterization of CD2-Icam1D4del/Icam1null mice
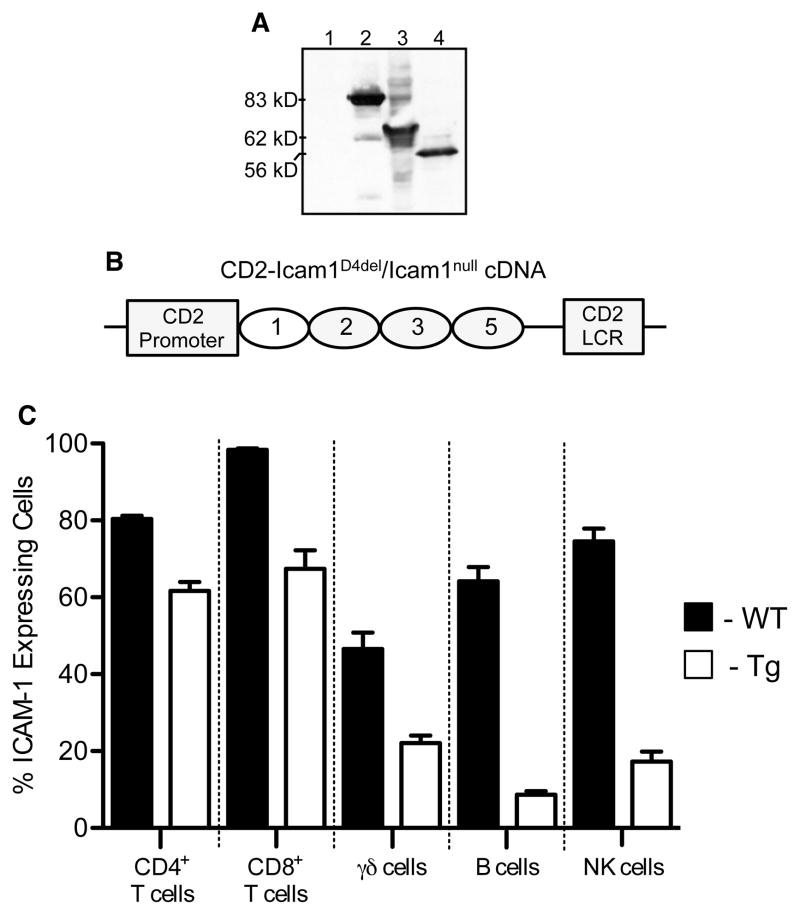
In previous studies using ICAM-1-deficient mice we found that alternatively spliced ICAM-1 isoforms, like the full-length molecule, were functional and could promote the development of EAE [12]. Furthermore, we observed markedly different EAE phenotypes among the different lines of ICAM-1 mutant mice that were dependent on the specific combinations of isoforms expressed [12]. Of particular interest was the observation that Icam1tm1Bay mice developed severe EAE and that adoptive transfer of encephalitogenic Icam1tm1Bay T cells induced fulminant disease even when transferred to Icam1null mice. These data suggested that T cell-specific expression of the ICAM-1 splice variant composed of Ig domains, 1, 2, 3, and 5 was critical for EAE development, although we could not rule out potential contributions from other isoforms in mediating CNS inflammation. To specifically analyze the T cell-specific roles of this ICAM-1 molecule and to determine it’s disease-inducing potential; we generated a transgenic mouse line in which expression of this isoform was driven by the CD2 promoter. First, a cDNA encoding for this isoform was generated (Icam1D4del), sequenced-verified, subcloned into a FLAG-tag vector, and expressed in 293 cells to verify the appropriate molecular weight (Fig. 1A). For comparison, a full-length ICAM-1 cDNA was also subcloned into the same vector and expressed in 293 cells. Next, the Icam1D4del cDNA was subcloned into a CD2 cassette as described in materials and methods (Fig. 1B) [13]. Transgenic mice were then generated (referred to as CD2-Icam1D4del) and crossed with Icam1null mutants to obtain CD2-Icam1D4del/Icam1null mice. Using flow cytometry we found that that 60–70% of CD8+ and CD4+ splenic T cells isolated from CD2-Icam1D4del/Icam1null mice expressed this ICAM-1 isoform with lesser expression on γδ T cells (22%) and minimal expression on B cells (~8%) and NK cells (~17%) (Fig. 1C). B cell expression of this isoform is most likely not critical to the disease phenotypes we report below since B cells are not required for disease development in MOG peptide-induced EAE in C57BL/6 mice [14]. The role of NK cells in EAE remains controversial with disease phenotypes in NK-depleted mice ranging from severe or fatal [15, 16] to mild EAE [17], neither of which is consistent with our results.
Figure 1.
Generation of CD2-Icam1D4del/Icam1null mice. (A) Western blot analysis showing expression of the CD2-Icam1D4del cDNA construct. Lane 1: control nontransfected 293 cells, lane 2: Flag tagged control, lane 3: full-length flagged, ICAM-1 isoform, lane 4: CD2-Icam1D4del flagged, isoform. (B) Schematic of the CD2-Icam1D4del/Icam1null construct showing the CD2 promoter and LCR locus and ICAM-1 immunoglobulin domains 1, 2, 3 and 5. (C) Flow cytometric analysis of splenocytes showing the percent ICAM-1-expressing CD4+ T cells, CD8+ T cells, γδ T cells, B cells, and NK cells from wild type and CD2-Icam1D4del/Icam1null mice. Shown is the mean ± SEM of three mice per group.
CD2-Icam1D4del/Icam1null mice develop robust active EAE
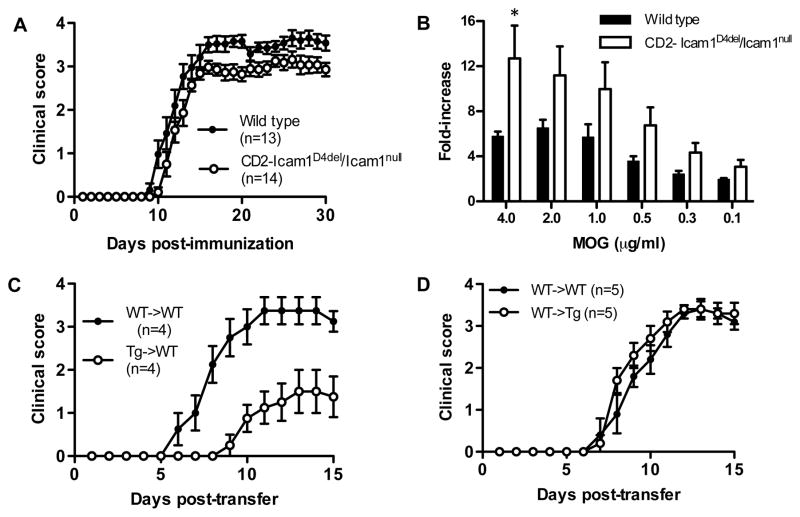
We first compared active EAE between wild type and CD2-Icam1D4del/Icam1null mice to examine for differences in initiation and progression of disease. We observed that CD2-Icam1D4del/Icam1null mice developed EAE almost identically to wild type mice (Fig. 2A and Table 1; 12.2d vs. 11.4d). Overall disease severity in CD2-Icam1D4del/Icam1null mice was less than that of controls, but the difference was not statistically significant (CDI of 53 vs. 65, ns, Wilcoxon signed rank test). There was no difference in disease incidence or mortality between the two groups of mice (Table 1). We also performed T cell in vitro proliferation assays to determine if the disease phenotype observed correlated with the proliferative ability of these cells. T cells from CD2-Icam1D4del/Icam1null mice proliferated to a greater extent than those derived from wild type mice, however the difference was not significant except at the highest dose of MOG used in the assay (Fig. 2B). These findings made in the CD2-Icam1D4del/Icam1null mice are similar to what we previously observed in Icam1tm1Bay mice, except that the EAE disease course observed in the latter mutants was more severe than controls throughout the chronic phase of disease. In addition, the proliferative capacity of Icam1tm1Bay mutant T cells was significantly higher than wild type cells [12, 18]. Taken together, our results suggest that T cell expression of the isoform lacking Ig domain 4 alone is sufficient for driving the development of active EAE and is likely a dominant disease-inducing isoform in wild type mice.
Figure 2.
EAE parameters in wild type and CD2-Icam1D4del/Icam1null mice. (A) Active EAE was induced in both groups of mice as described in the Materials and Methods and clinical signs were scored for 30 days. Results shown are the daily mean clinical score for wild type (n=13) and ICAM-1 mutant mice (n=14) from three experiments. There was no significant difference in onset or overall disease severity between the two groups. (B) CD2-Icam1D4del/Icam1null T cells proliferate to a greater extent than wild type T cells on recall challenge with MOG peptide35–55 (0.125–4 μg/ml). The results shown are expressed as the mean + SEM of fold-induction of wild type and CD2-Icam1D4del/Icam1null T cell proliferation relative to background proliferation (n=4). * p<0.05 (C) Transferred EAE was induced in wild type (n=4) mice by injecting encephalitogenic T cells (~5 × 106) derived from CD2-Icam1D4del/Icam1null mice with active EAE. As a control, transferred EAE was induced in wild type mice (n=4) using encephalitogenic T cells derived from wild type mice with EAE. Disease onset was significantly delayed on transfer of CD2-Icam1D4del/Icam1null T cells (p=0.03, unpaired t-test) and disease severity was significantly reduced from day 8 to day 15, (p<0.05, Mann Whitney test) compared to wild type to wild type transfers. (D) Transferred EAE was induced in wild type (n=5) and CD2-Icam1D4del/Icam1null (n=5) mice by injecting encephalitogenic T cells (~5 × 106) derived from wild-type mice with active EAE. There was no significant difference in disease onset or severity between the two groups.
Table I.
Active and transferred EAE signs in wild type and CD2-Icam1D4del/Icam1null mice.
| CDIA | Disease OnsetB | Disease IncidenceC | |
|---|---|---|---|
| Wild type (n=13) | 65 | 11.4d | 100 |
| CD2-Icam1D4del/Icam1null (mice n=14) | 53 | 12.2d | 100 |
|
| |||
| WT > WT n=5 | 20 | 9.2d | 100 |
| WT > CD2-Icam1D4del/Icam1null n=5 | 22 | 8.4d | 100 |
|
| |||
| WT > WT n=4 | 25 | 8.3d | 100 |
| CD2-Icam1D4del/Icam1null > WT n=4 | 7 | 17d (p=0.03) | 75 |
Cumulative Disease Index is the mean of the sum of daily clinical scores observed between days 7 and 30.
Disease onset is defined as the first day of two consecutive days with a clinical score of two or more.
Disease incidence is defined as the percent of mice that displayed any clinical signs of disease.
Divergent EAE phenotypes in adoptive transfer using CD2-Icam1D4del/Icam1null mice
We next performed transfers of encephalitogenic T cells derived from CD2-Icam1D4del/Icam1null mice to wild type mice and observed a significant delay in disease onset (17d vs. 8d, p=0.03, unpaired t-test) and in disease severity (CD1: 7 vs. 25, p<0.05, days 8 to 15, Mann Whitney test) compared to wild type to wild type transfer controls (Fig. 2C and Table 1). These results were surprising since in previous studies we showed that transfer of Icam1null T cells into wild type recipients resulted in little to no disease activity. In contrast, transfer of encephalitogenic T cells from Icam1tm1Bay mice produced robust EAE that was more severe than wild type transfers [12, 19]. The marked in vitro proliferation we observed for CD2-Icam1D4del/Icam1null T cells (Fig. 2B) indicates that although the cells have a strong proliferative capacity, additional signals they receive upon transfer to wild type mice expressing all ICAM-1 isoforms may inhibit their encephalitogenic potential. Alternatively, the CD2-Icam1D4del/Icam1null T cells, unlike the Icam1tm1Bay T cells, may be more susceptible to wild type T regulatory cell suppression following transfer, which may lead to lead an attenuated form of transferred EAE.
We next performed adoptive transfer studies in which wild type encephalitogenic T cells were transferred to CD2-Icam1D4del/Icam1null mice. We observed that wild type T cells were fully capable of inducing disease in CD2-Icam1D4del/Icam1null mice (Fig. 2D). There were no significant differences in disease onset or severity, or during the acute and chronic phases of disease between transfer of wild type cells to CD2-Icam1D4del/Icam1null mice and control transfers from wild type to wild type mice (Table 1). These results parallel our earlier findings in which transfer of wild type encephalitogenic T cells to Icam1tm1Bay mice induced EAE similar to wild type control transfers [12] but are dramatically different than in similar transfer experiments involving Icam1null mice as recipients [19]. These data provide additional support for the role of the ICAM-1 splice variant composed of Ig domains, 1, 2, 3, and 5 in disease induction and progression.
Here we demonstrate that a single alternatively spliced ICAM-1 isoform, expressed on T cells, can significantly modulate the development and severity of autoimmune demyelinating disease. These results are remarkable for several reasons. First, these data extend our previous findings that ICAM-1 expression on endothelial cells is not required for EAE development. In previous studies we transferred T cells from Icam1tm1Bay mice to Icam1null mice and observed EAE severity comparable to wild type transfers [12]. Thus other adhesion molecule receptor/ligand pairs such a α4β1/VCAM-1 and LFA-1/ICAM-2 may substitute for ICAM-1 interactions in mediating firm adhesion and transendothelial migration events that occur during disease development [20, 21]. However, this does not rule out a contribution for the β2-integrins and ICAM-1 in leukocyte recruitment in demyelinating disease since loss of expression of different β2-integrins has profound effects on EAE development (reviewed in [22]). For example, altered ICAM-1 expression on γδ T cells in the CD2-Icam1D4del/Icam1null mouse (Fig. 1C) may make an important contribution to the severity of the transferred EAE we report here since it is well-established that this T cell subset modulates disease development and progression [23]. Second, the distinct EAE phenotypes between CD2-Icam1D4del/Icam1null, Icam1tm1Jcgr, Icam1tm1Bay, and Icam1null mice further highlight the important roles of ICAM-1 isoform expression on leukocytes for EAE development. In addition, various combinations of the alternatively spliced forms of this molecule appear responsible for variations in disease severity [12, 19]. Most strikingly, our results demonstrate that isoforms other than the full length ICAM-1 molecule, including the isoform lacking domain 4 in particular, can drive disease pathogenesis. Collectively our data suggest that ICAM-1 isoforms have unique roles in EAE, although the specific ligands and intracellular signaling mechanisms employed by individual or combinations of isoforms remains to be elucidated. Nevertheless our results indicate that ICAM-1 isoform-specific therapeutic approaches may provide a new avenue in the treatment of demyelinating diseases.
Materials and methods
Generation and characterization of CD2-Icam1 isoform transgenic mice
Sequence-verified full-length and CD2-Icam1D4del cDNAs were first subcloned into the p3xFLAG-CMV-10 expression vector. To verify correct expression and molecular weight, the constructs were then transfected into 293 cells. Appropriately sized ICAM-1 proteins were detected by western blot analysis. Next the CD2-Icam1D4del cDNA was subcloned into a CD2 cassette containing the CD2 promoter and locus containing regions [13]. After purification and removal of the plasmid sequences, the construct was injected into fertilized eggs from C57BL/6 mice. A single transgenic line was obtained and subsequently bred with Icam1null mice. CD2-Icam1D4del/Icam1null mice are healthy, fertile, and require no special husbandry. Flow cytometry staining for ICAM-1 expression on T cell subsets (GK1.5 and 53–6.7), B cells (PE MB19-1) and NK cells (APC DX5) was performed on splenocytes of wild type and CD2-Icam1D4del/Icam1null mice as previously described [24]. All antibodies were from eBioscience. Stained cells were run on a FACSCalibur and data was analyzed with CellQuest software (BD biosciences). Inbred C57BL/6 mice were used as controls for all experiments and all studies were performed with approval from the UAB IACUC.
Induction of active and adoptive transfer EAE
For active EAE, control and CD2-Icam1D4del/Icam1null mice were immunized with myelin oligodendrocyte glycoprotein (MOG) peptide35–55 as previously described [19]. MOG peptide was synthesized by standard 9-fluorenyl-methoxycarbonyl chemistry and was >95% pure as determined by reversed phase-HPLC (Biosynthesis, Lewisville, TX). Onset and progression of EAE clinical signs was monitored daily (30 days) using a standard clinical scale ranging from 0 to 6 as follows: 0, asymptomatic; 1, loss of tail tone; 2, flaccid tail; 3, incomplete paralysis of one or two hind limbs; 4, complete hind limb paralysis; 5, moribund; 6, dead. Only mice with a score of at least 2 (flaccid tail) observed for 2 or more consecutive days were judged to have onset of EAE. A cumulative disease index (CDI) was calculated from the sum of the daily clinical scores observed between day 7 and day 30. For transferred EAE, spleens of control and CD2-Icam1D4del/Icam1null donors were removed two to three weeks following induction of active EAE, and prepared as previously described [25]. Adoptive transfer EAE was induced by injecting ~5×106 purified wild type T cells into CD2-Icam1D4del/Icam1null recipient mice as described in the Results section. Reciprocal transfers were performed by injecting CD2-Icam1D4del/Icam1null encephalitogenic T cells into wild type mice. Control experiments in which adoptive transfer of wild type encephalitogenic T cells into wild type recipients were performed simultaneously for all transfer experiments. Mice were scored daily for up to 19 days using the system described above.
T cell proliferation and cytokine production
Antigen-specific T cell proliferation assays were performed as previously described [25]. Single cell suspensions from spleens from wild type and CD2-Icam1D4del/Icam1null mice obtained 14 days after EAE induction were cultured in triplicate in 96-well plates at 5 × 105 cells/well with increasing concentrations of MOG35–55 peptide (0.125 to 4 μg/ml). After 48 h, cultures were pulsed with 3H-thymidine for 18 h and incorporation of thymidine was measured.
Statistical analyses
Statistical significance, with respect to disease onset and severity, between wild type and CD2-Icam1D4del/Icam1null mice for active and transferred EAE experiments was calculated using the Mann Whitney test (Prism 5.0, GraphPad Software, Inc., La Jolla, CA). For statistical significance between control and CD2-Icam1D4del/Icam1null mice in proliferation assays the unpaired student’s t-test was used.
Acknowledgments
The authors gratefully acknowledge the help of Dr. Robert Kesterson and the UAB Transgenic Mouse Facility, which is supported by grants from the NIH (P30 CA13148, P30 AR048311, P30 DK074038, P30 DK05336, and P60 DK079626), for help with the generation of the CD2-Icam1D4del/Icam1null transgenic mice. This work was also supported by grants from the National Multiple Sclerosis Society (NMSS PP1058) and from the NIH (NS46032) to S.R. Barnum and to D.C. Bullard and from the NIH (F31 NS077811) to T.N. Ramos.
Abbreviations
- MOG
myelin oligodendrocyte glycoprotein
Footnotes
Conflict of Interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Staunton DE, Marlin SD, Stratowa C, Dustin ML, Springer TA. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988;52:925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- 2.Giorelli M, De Blasi A, Defazio G, Avolio C, Iacovelli L, Livrea P, Trojano M. Differential regulation of membrane bound and soluble ICAM 1 in human endothelium and blood mononuclear cells: effects of interferon beta-1a. Cell Commun Adhes. 2002;9:259–272. doi: 10.1080/15419060216305. [DOI] [PubMed] [Google Scholar]
- 3.King PD, Sandberg ET, Selvakumar A, Fang P, Beaudet AL, Dupont B. Novel isoforms of murine intercellular adhesion molecule-1 generated by alternative RNA splicing. Journal of Immunology. 1995;154:6080–6093. [PubMed] [Google Scholar]
- 4.Ochietti B, Lemieux P, Kabanov AV, Vinogradov S, St-Pierre Y, Alakhov V. Inducing neutrophil recruitment in the liver of ICAM-1-deficient mice using polyethyleneimine grafted with Pluronic P123 as an organ-specific carrier for transgenic ICAM-1. Gene Ther. 2002;9:939–945. doi: 10.1038/sj.gt.3301716. [DOI] [PubMed] [Google Scholar]
- 5.Robledo O, Papaioannou A, Ochietti B, Beauchemin C, Legault D, Cantin A, King PD, Daniel C, Alakhov VY, Potworowski EF, St-Pierre Y. ICAM-1 isoforms: specific activity and sensitivity to cleavage by leukocyte elastase and cathepsin G. Eur J Immunol. 2003;33:1351–1360. doi: 10.1002/eji.200323195. [DOI] [PubMed] [Google Scholar]
- 6.Werner A, Martin S, Gutierrez-Ramos JC, Raivich G. Leukocyte recruitment and neuroglial activation during facial nerve regeneration in ICAM-1-deficient mice: effects of breeding strategy. Cell Tissue Res. 2001;305:25–41. doi: 10.1007/s004410100393. [DOI] [PubMed] [Google Scholar]
- 7.Wakatsuki T, Kimura K, Kimura F, Shinomiya N, Ohtsubo M, Ishizawa M, Yamamoto M. A distinct mRNA encoding a soluble form of ICAM-1 molecule expressed in human tissues. Cell Adhes Commun. 1995;3:283–292. doi: 10.3109/15419069509081014. [DOI] [PubMed] [Google Scholar]
- 8.van Den Engel NK, Heidenthal E, Vinke A, Kolb H, Martin S. Circulating forms of intercellular adhesion molecule (ICAM)-1 in mice lacking membranous ICAM-1. Blood. 2000;95:1350–1355. [PubMed] [Google Scholar]
- 9.Xu H, Gonzalo JA, St Pierre Y, Williams IR, Kupper TS, Cotran RS, Springer TA, Gutierrez-Ramos JC. Leukocytosis and resistance to septic shock in intercellular adhesion molecule 1-deficient mice. J Exp Med. 1994;180:95–109. doi: 10.1084/jem.180.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sligh JE, Ballantyne CM, Rich SS, Hawkins HK, Smith CW, Bradley A, Beaudet AL. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci. 1993;90:8529–8533. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunne JL, Collins RG, Beaudet AL, Ballantyne CM, Ley K. Mac-1, but not LFA-1, uses intercellular adhesion molecule-1 to mediate slow leukocyte rolling in TNF-alpha-induced inflammation. J Immunol. 2003;171:6105–6111. doi: 10.4049/jimmunol.171.11.6105. [DOI] [PubMed] [Google Scholar]
- 12.Hu X, Barnum SR, Wohler JE, Schoeb TR, Bullard DC. Differential ICAM-1 isoform expression regulates the development and progression of experimental autoimmune encephalomyelitis. Mol Immunol. 2010;47:1692–1670. doi: 10.1016/j.molimm.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhumabekov T, Corbella P, Tolani M, Kioussis D. Improved version of a human CD2 minigene based vector for T cell-specific expression in mice. J Immunol Meth. 1995;185:133–140. doi: 10.1016/0022-1759(95)00124-s. [DOI] [PubMed] [Google Scholar]
- 14.Hjelmstrom P, Juedes AE, Fjell J, Ruddle NH. B-cell-deficient mice develop experimental allergic encephalomyelitis with demyelination after myelin oligodendrocyte glycoprotein sensitization. J Immunol. 1998;161:4480–4483. [PubMed] [Google Scholar]
- 15.Zhang B, Yamamura T, Kondo T, Fujiwara M, Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J Exp Med. 1997;186:1677–1687. doi: 10.1084/jem.186.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto Y, Kohyama K, Aikawa Y, Shin T, Kawazoe Y, Suzuki Y, Tanuma N. Role of natural killer cells and TCR gamma delta T cells in acute autoimmune encephalomyelitis. Eur J Immunol. 1998;28:1681–1688. doi: 10.1002/(SICI)1521-4141(199805)28:05<1681::AID-IMMU1681>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 17.Winkler-Pickett R, Young HA, Cherry JM, Diehl J, Wine J, Back T, Bere WE, Mason AT, Ortaldo JR. In vivo regulation of experimental autoimmune encephalomyelitis by NK cells: alteration of primary adaptive responses. J Immunol. 2008;180:4495–4506. doi: 10.4049/jimmunol.180.7.4495. [DOI] [PubMed] [Google Scholar]
- 18.Samoilova EB, Horton JL, Chen Y. Experimental autoimmune encephalomyelitis in intercellular adhesion molecule-1-deficient mice. Cell Immunol. 1998;190:83–89. doi: 10.1006/cimm.1998.1395. [DOI] [PubMed] [Google Scholar]
- 19.Bullard DC, Hu X, Schoeb TR, Collins RG, Beaudet AL, Barnum SR. Intercellular adhesion molecule-1 expression is required on multiple cell types for the development of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:851–857. doi: 10.4049/jimmunol.178.2.851. [DOI] [PubMed] [Google Scholar]
- 20.Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against α4β1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 21.Soilu-Hanninen M, Roytta M, Salmi A, Salonen R. Therapy with antibody against leukocyte integrin VLA-4 (CD49d) is effective and safe in virus-facilitated experimental allergic encephalomyelitis. J Neuroimmunol. 1997;72:95–105. doi: 10.1016/s0165-5728(96)00158-0. [DOI] [PubMed] [Google Scholar]
- 22.Hu X, Wohler JE, Dugger KJ, Barnum SR. beta2-integrins in demyelinating disease: not adhering to the paradigm. J Leukoc Biol. 2010;87:397–403. doi: 10.1189/jlb.1009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wohler JE, Smith SS, Zinn KR, Bullard D, Barnum SR. gd T cells in experimental autoimmune encephalomyelitis: early trafficking events and cytokine requirements. Eur J Immunol. 2009;39:1516–1526. doi: 10.1002/eji.200839176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wohler JE, Barnum SR. Nylon wool purification alters the activation of T cells. Mol Immunol. 2008 doi: 10.1016/j.molimm.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szalai AJ, Nataf S, Hu XZ, Barnum SR. Experimental allergic encephalomyelitis is inhibited in transgenic mice expressing human C-reactive protein. J Immunol. 2002;168:5792–5797. doi: 10.4049/jimmunol.168.11.5792. [DOI] [PubMed] [Google Scholar]