Abstract
The impact of human leukocyte antigen (HLA) donor-specific antibodies (DSA) upon cord blood (CB) engraftment is controversial. We evaluated the influence of pre-existing HLA-antibodies (HLA-Abs) on engraftment in 82 double-unit CB recipients (median age 48 years) transplanted for hematologic malignancies. Of 28 patients (34%) with HLA-Abs, 12 had DSA (median MFI 5,255, range 1,057–9,453). DSA patients had acute leukemia (n = 11) or myelodysplasia (n = 1) and all received either high-dose or reduced intensity (but myeloablative) conditioning. After myeloablative CBT (n = 67), sustained donor engraftment was observed in 95% without HLA-Abs (median 23 days), 100% with non-specific HLA-Abs (median 23 days), and 92% with DSA (median 31 days, p = 0.48). Of 6 patients with HLA-Abs to one unit, 3 engrafted with that unit and 3 with the other. Of 6 patients with HLA-Abs against both units, one had graft failure despite being 100% donor, and 5 engrafted with one unit. Successful donor engraftment is possible in patients with DSA after myeloablative double-unit CBT. Our data suggest potential deleterious effects of DSA can be abrogated in patients with hematologic malignancies.
Introduction
Hematopoietic stem cell (HSC) allograft recipients are often allo-immunized. This sensitization may include antibodies (Abs) directed against mismatched HLA of potential donors. Animal models suggest that such Abs may be a barrier to allogeneic engraftment1,2. Moreover, graft failure is observed in approximately 5% of unrelated allograft recipients3, and analyses have suggested this may relate, at least in part, to pre-existing donor-specific Abs (DSA)4–7. In cord blood (CB) transplantation (CBT), marked donor-recipient HLA disparity and low cell dose are additional risk factors for graft failure. The ≥ 20% graft failure rates following single-unit CBT8,9 have been reduced by new conditioning and immunosuppression and the introduction of double-unit CBT10,11. Nonetheless, graft failure has not been eliminated and DSA is an accepted additional risk factor for graft failure in single-unit CBT12,13. However, double-unit CBT studies have yielded conflicting results14–16. While some investigators have recommended avoiding units against which the recipient has DSA12–14,16,17, this practice is controversial. We, therefore, analyzed the influence of HLA-Ab on the likelihood of engraftment and unit dominance in 82 double-unit CBT recipients. Our hypothesis was that the combination of immunosuppressive conditioning, lack of ATG, and double-unit grafts in patients with hematologic malignancies may abrogate the adverse effects of DSA upon engraftment described in CBT recipients in the literature.
Methods
Patient and Graft Characteristics
Consecutive first allograft recipients transplanted with double-unit CB grafts for the treatment of hematologic malignancies consenting to pre-transplant HLA-Abs analysis were analyzed. Patients/ guardians also provided informed consent to transplantation and outcome analysis. Patients were transplanted during the period 7/2008–7/2012. Patients received high-dose conditioning (n = 21), reduced intensity but functionally myeloablative conditioning (n = 46, predominantly with cyclophosphamide 50 mg/kg, fludarabine 150 mg/m2, thiotepa 10 mg/kg, and total body irradiation 400 cGy11, Cy 50/ Flu 150/ Thio 10/ TBI 400), or non-myeloablative conditioning (n = 15). Immunosuppression was with a calcineurin inhibitor and mycophenolate mofetil, and no patient received anti-thymocyte globulin (ATG). All patients received post-transplant granulocyte colony-stimulating factor.
CB units were selected based on 4-6/6 HLA-A,-B antigen, -DRB1 allele match to the recipient, cryopreserved total nucleated cell (TNC) dose ≥ 1.5 × 107/kilogram (kg)/unit (increased to 2.0 in 2011)18 and CB Bank. Above the TNC dose threshold, HLA-match was given priority. Patients and CB units were also typed at HLA-A,-B,-C and -DQ alleles, but high resolution match grade at 10 alleles was not used in unit selection during this period. Patients and units were not typed for HLA-DP. Additionally, HLA-Abs screening results were not available at the time of unit selection and were therefore not considered in selecting the graft.
HLA-Abs Screening
HLA-Abs screening was performed using LABScreen Mixed beads (One Lambda Inc, CA, USA) that detect Class I/II Abs with a panel coated with purified HLA-antigens according to manufacturer’s instructions. Test serum (20 μL) and controls were incubated with LABScreen beads (5 μl) in the dark at room temperature for 30 minutes. After 3 washes, R-Phycoerythrin-conjugated goat anti-human IgG was added followed by incubation and wash. Data acquisition and analysis was performed using Luminex 100. Sample reactivity was corrected for non-specific binding (One Lambda’s HLA Fusion software). Positive samples were further tested using LABScreen Single Antigen Class I/II beads. Positivity was defined as adjusted mean fluorescence intensity (MFI) ≥ 1000 and derived per laboratory validations19–21 and external proficiency programs. HLA-Abs profiles were compared with mismatched CB HLA-antigens to identify DSA.
Assessment of Donor Engraftment and Statistical Analyses
Donor chimerism was determined serially on marrow and blood after transplantation as previously described10. Sustained engraftment was defined as sustained donor-derived neutrophil recovery with total donor chimerism ≥ 90%. The dominant unit was the only one detected, or the unit contributing > 50% total chimerism in serial testing. Neutrophil recovery was the first of 3 consecutive days with a count ≥ 0.5 × 109/L.
Patient and graft characteristics were compared according to HLA-Abs presence using a Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables. Cumulative incidence functions estimated engraftment incidence according to HLA-Abs and Gray’s test was used to compare engraftment incidence. All tests were considered statistically significant based on a two-sided test at alpha level 0.05. All analyses were done using R statistical software (Vienna, Austria).
Results
Patient and Graft Demographics According to HLA-Abs
Twenty-eight patients (34%) had pre-existing HLA-Ab. HLA-Ab patients were predominantly female and more likely to be cytomegalovirus (CMV) sero-positive (Table 1). Otherwise there were no differences. Of the 28 patients with HLA-Abs, 26 received myeloablative conditioning (9 high-dose and 17 Cy 50/ Flu 150/ Thio 10/ TBI 40011), and 2 received non-myeloablative conditioning.
Table 1.
Patient and graft characteristics according to presence of HLA-antibodies (n = 82).
| Characteristics | Total | No Antibody | Antibody | p value |
|---|---|---|---|---|
|
| ||||
| N of patients | 82 | 54 (66%) | 28 (34%) | |
|
| ||||
| Median (range) age, years | 48 (2–69) | 48 (2–69) | 49 (10–64) | 0.53 |
|
| ||||
| Median (range) weight, kg | 70 (15–125) | 76 (15–125) | 67 (37–93) | 0.09 |
|
| ||||
| Gender, n (%) female | 34 (41%) | 14 (26%) | 20 (71%) | < 0.01 |
|
| ||||
| Diagnosis, n (%) | 0.06 | |||
| AML | 33 (40%) | 17 (31%) | 16 (57%) | |
| ALL | 14 (17%) | 9 (17% ) | 5 (18%) | |
| MDS & MPD | 9 (11%) | 6 (11%) | 3 (11%) | |
| Lymphoma & CLL | 26 (32%)3 | 22 (41%)3 | 4 (14%)3 | |
|
| ||||
| Recipient CMV+ | 47 (57%) | 26 (48%) | 21 (78%) | 0.03 |
|
| ||||
| Conditioning, n (%) | ||||
| High dose myeloablative | 21 (26%) | 12 (22%) | 9 (32%) | 0.16 |
| Reduced intensity* | 46 (56%) | 29 (54%) | 17 (61%) | |
| Non-myeloablative | 15 (18%) | 13 (24%) | 2 (7%) | |
|
| ||||
| Number of units | 164 | 108 | 56 | |
|
| ||||
| Unit-recipient HLA-A, -B antigen, -DRB1 allele match | ||||
| 6/6 | 7 (4%) | 4 (4%) | 3 (5%) | 0.12 |
| 5/6 | 78 (48%) | 45 (42%) | 33 (59%) | |
| 4/6 | 79 (48%) | 59 (55%) | 20 (36%) | |
|
| ||||
| Inf. TNC × 107/kg | ||||
| Larger | 2.9 (1.5–5.6) | 2.9 (1.5–5.6) | 2.9 (1.5–5.5) | 0.73 |
| Smaller | 2.0 (1.1–4.5) | 2.0 (1.4–4.5) | 2.0 (1.1–2.8) | 0.46 |
| Inf. CD34+ cell × 105/kg | ||||
| Larger | 1.4 (0.3–4.5) | 1.5 (0.3–4.5) | 1.3 (0.4–3.0) | 0.22 |
| Smaller | 0.7 (0.2–1.6) | 0.7 (0.2–1.6) | 0.6 (0.2–1.5) | 0.43 |
N indicates number; kg, kilogram; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; MPD, myeloproliferative disorder; CLL, chronic lymphocytic leukemia; CMV, cytomegalovirus; Inf, infused; TNC, total nucleated cells.
Reduced intensity conditioning was predominantly Cy 50/ Flu 150/ Thio 10/ TBI 400 in 44/46 patients. This regimen is functionally myeloablative.
HLA-Abs were donor-specific in 12 patients. These patients had a median age of 51 years (range 24–64), a median weight of 67 kilograms (range 51–93), 10 were female, 11 had acute leukemia and one had myelodysplasia, and all received myeloablative conditioning that was of high dose [cyclophosphamide 120 mg/kg, fludarabine 75 mg/m2, and total body irradiation TBI 1375 cGy (n = 2)] or of reduced intensity but functionally myeloablative [Cy 50/ Flu 150/ Thio 10/ TBI 40011 n = 10)]. DSA patients received a median infused TNC dose (x 107/kg) of 2.4 (larger unit, range 1.5–3.4) and 2.0 (smaller unit, range 1.4–2.5).
Nature of HLA-Abs
The nature of HLA-Abs is summarized in Table 2. Fifteen patients (54%) had class I Abs, 2 (7%) had class II Abs, and 11 (39%) had both. Eight patients had HLA-Abs to one locus only (4 HLA-A, 3 HLA-B, 1 HLA-DRB1), and the remaining 20 patients had Abs to combinations of HLA-A,-B,-C, and/or -DRB1,-DQ. Overall, 22 patients had HLA-A Abs, 18 had HLA-B Abs, 8 had HLA-C Abs, 10 had HLA-DRB1 Abs, and 8 had HLA-DQ Abs. Additionally, 7 patients also had Abs against HLA-DP but the potential contribution to engraftment outcome of such Abs was not analyzed as patients and CB units were not typed for DP.
Table 2.
Nature of HLA-antibodies (n = 28 patients).
| Characteristic | N (%) |
|---|---|
|
| |
| HLA-Specificity: | |
| Class I Ab | 15 (54%) |
| Class II Ab | 2 (7%) |
| Both Class I & II | 11 (39%) |
|
| |
| Donor Specificity: | |
| Ab: not specific for graft | 16 (57%) |
| Donor-specific Ab (DSA) | 12 (43%) |
| Specific for 1 unit | 6 |
| Specific for both units | 6 |
|
| |
| Median (range) MFI | |
| All Ab (n = 28) | 4,510 (1003–18479) |
| DSA (n = 12) | 5,255 (1,057–9,453) |
Ab indicates antibody; DSA, donor-specific Ab; MFI, mean fluorescence intensity.
Sixteen patients had Abs without specificity for either unit of the graft, and 12 had DSA [median 1.5/patient (range 1–4), 6 against one unit and 6 against both]. The median MFI of all HLA-Abs was 4,510, and the median MFI of DSA was 5,255.
Engraftment According to the Presence of HLA Abs
Overall, the cumulative incidence of engraftment for myeloablative CBT recipients (n = 67) was 95% (95%CI:87–98) at 42 days post-CBT. In those with DSA, sustained engraftment was observed in 11 of 12 patients for a cumulative incidence of 92% with neutrophil recovery occurring at a median of 31 days (range 13–40, Figure 1). The engraftment in DSA patients was not significantly different to that of patients without HLA-Abs (95% incidence at a median of 23 days, range 12–38) and patients with non-specific HLA-Abs (100% incidence at a median of 23 days, range 12–37) (Figure 1, P = 0.48 by Gray’s test). When patients with DSA (n = 12) and those without DSA (n = 55) were compared there was also no difference in the overall speed and success of engraftment (P = 0.25).
Figure 1. Cumulative incidence of sustained donor engraftment according to the presence of HLA-Abs after myeloablative conditioning (n = 67).
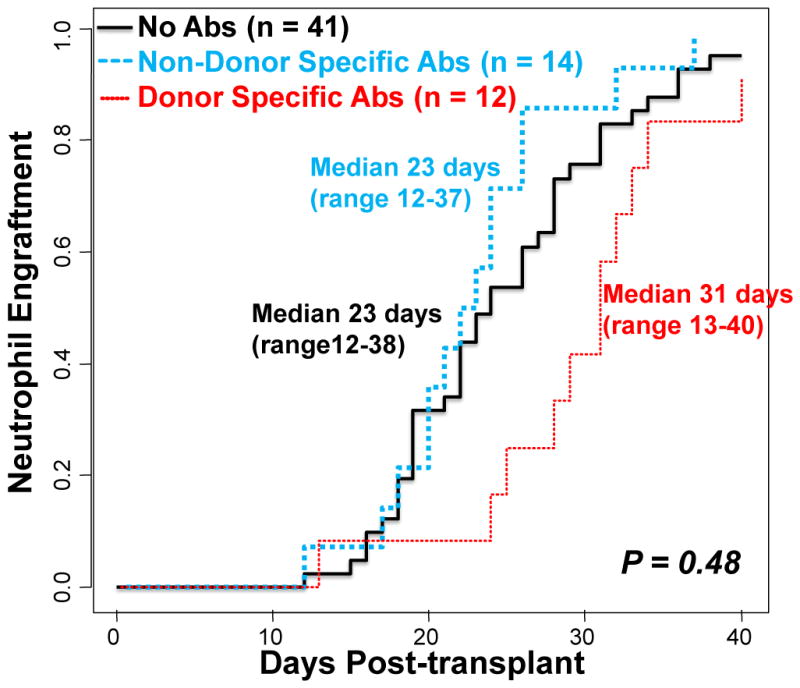
The presence of pre-existing HLA-Abs (non-specific or donor-specific) did not influence the cumulative incidence of sustained donor neutrophil engraftment.
One unit predominated in engraftment after myeloablative conditioning in all patients. Of the 6 DSA patients with HLA-Abs to only one unit of the graft, 3 engrafted with that unit and 3 with the other. Of 6 patients with HLA-Abs against both units, one had graft failure despite being 100% donor with one unit in the day 21 bone marrow and died in the setting of early onset multi-organ failure, and 5 engrafted with one unit. There were no differences in total donor chimerism according to the presence of non-specific Abs or DSA with each of the patient groups (no HLA-Abs, non-specific Abs, and DSA) having a median total donor chimerism of 100% as from 21 days after CBT.
As the infused viable CD34+ cell dose of the dominant unit is a critical determinant of engraftment after double-unit CBT22,23, the cell doses in patients with and without DSA were compared. The mean dominant unit infused viable CD34+ cell dose in engrafting DSA patients was lower at 0.74 × 105/kg (range 0.2–1.61) than that of the 53 without Abs or with non-specific Abs (1.22 × 105/kg, range 0.2–4.08, p = 0.02).
Of the recipients of non-myeloablative conditioning (n = 15), 14 engrafted. However, lack of DSA precluded their evaluation in non-myeloablative CBT recipients.
Discussion
Multiple reports have suggested an adverse effect of DSA upon engraftment after CBT. However, in this study we observed a high incidence of sustained engraftment in DSA patients with 11/12 patients engrafting. We hypothesize that this is attributed to the nature of the conditioning and/or immune suppression delivered to DSA patients in combination with double-unit grafts10,11, although the exact contributions of each of these components cannot be discerned individually. While 10/12 DSA patients were not appropriate for high-dose myeloablation, they were treated with a reduced intensity but functionally myeloablative and highly immunosuppressive regimen of cyclophosphamide 50 mg/kg, fludarabine 150 mg/m2, thiotepa 10 mg/kg, and total body irradiation 400 cGy11 (Cy 50/ Flu 150/ Thio 10/ TBI 400) supplemented with double-unit grafts. While no conclusion can be made about recipients of non-myeloablative conditioning, our findings suggest that when combined with a double-unit graft, intensified suppression of the recipient’s ability to reject the graft with Cy 50/ Flu 150/ Thio 10/ TBI 400 may be able to abrogate any potential deleterious effects of donor-specific HLA-Abs. It is also possible that omission of ATG could facilitate engraftment in this setting given that a T-replete CB graft may be better able to overcome negative recipient immunologic factors such as HLA-Ab.
Interpretation of the single case of graft failure in the CBT recipient with DSA is confounded by this patient’s early onset multi-organ failure that could have contributed to poor graft function despite being 100% donor. In addition, while there was no difference in the overall engraftment according to DSA, it was notable that the median time to recovery in DSA patients was 31 days. By chance, however, these patients had a significantly lower infused viable CD34+ cell dose in their dominant units. Dominant unit infused viable CD34+ cell dose is a critical determinant of engraftment speed in double-unit CBT recipients22,23. Thus, although a contribution of DSA to delayed engraftment cannot be excluded, the lower infused viable CD34+ cell dose could explain this observation. This is an important question as the speed of neutrophil engraftment is a critical determinant of TRM after double-unit CBT as shown in University of Minnesota/ Fred Hutchinson Cancer Research Center24 and unpublished MSKCC (J.Barker, 2013) analyses. Multivariate analysis of large numbers of patients controlling for the infused cell dose of the engrafting unit is the only method by which a potential further additional contribution of DSA to slowing engraftment could be further investigated. Furthermore, in our study, there was no indication that DSA influenced unit dominance. Unit dominance is likely related to CB hematopoietic potential25, and immune-mediated graft-versus-graft reactions10,22,26–28.
Our report adds to the controversy concerning the relevance of HLA-Abs in CBT in patients with hematologic malignancies. Notably, our findings are consistent with the Brunstein double-unit CBT study (n = 126) that reported a comparable cumulative incidence of engraftment in DSA and non-DSA patients and no association with unit dominance15. However, our findings differ from the Cutler study14. These investigators found a negative effect of DSA on double-unit CB engraftment in 73 patients (most of whom received ATG). Our results are also in contrast to the registry report of Ruggeri et al who observed a 44% incidence of engraftment in 14 patients with DSA who were transplanted after reduced intensity conditioning16. Of note, while the median DSA MFI in the Ruggeri study (3900) and ours (5,255) was similar, there are important differences between the patients in that report and the MSKCC experience. For example, of the 14 patients with DSA in the Ruggeri series, 4 had bone marrow failure syndromes, 7 received single-unit grafts, and 8 received a 200 cGy TBI-based preparative regimen that is of low intensity and has been associated with graft rejection in the absence of recent prior combination chemotherapy29. In addition to differences in patient populations, unit selection, conditioning, immunosuppression, and ATG inclusion, conflicting results may also be explained by variations in HLA-Ab assays. Assays could vary in sensitivity and/or what threshold constitutes a positive test.
Overall, our data suggest that the negative effect of HLA-Abs described in CBT literature can be abrogated with appropriate conditioning, immunosuppression and double-unit grafts. However, there are many unanswered questions. For example, no statement can be made about the ability to engraft patients with DSA undergoing double-unit CBT after non-myeloablative conditioning. Additionally, even if adverse effects of low and intermediate intensity DSA can be abrogated, it is not known if the same would be true for high intensity DSA. Clearly further investigation is warranted to determine when HLA-Abs may be prohibitive and how they can be overcome. A further question is how our findings should influence clinical practice. Incorporation of HLA-Ab testing into unit selection algorithms for double-unit CBT in patients with hematologic malignancies could dictate the selection of less desirable, lower dosed and/ or lesser HLA-matched units which could increase the risk of TRM30. If multiple comparable units are available, it is reasonable to avoid those against which the recipient has antibodies, especially if the MFI is very high. However, if the choice of units is limited, our data suggest that engraftment after double-unit CBT is possible with the conditioning regimens described in this report in patients with hematologic malignancies.
Acknowledgments
This work was supported in part by the Gabrielle’s Angel Foundation for Cancer Research, the Memorial Sloan-Kettering Cancer Center Society, the Translational and Integrative Medicine Research Grant, and P01 CA23766 from the National Cancer Institute, National Institutes of Health.
Footnotes
Author Contributions
P.B.D. and J.B. interpreted the data and wrote the manuscript, S.D performed the statistics and wrote the manuscript, C.B and M.L collected the data and wrote the manuscript, D.P, S.G, N.A.K and A.S wrote the manuscript. J.N.B. designed the study, interpreted the data, and wrote the manuscript.
Disclosure of Conflicts of Interest
The authors have no relevant conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Taylor PA, Ehrhardt MJ, Roforth MM, et al. Preformed antibody, not primed T cells, is the initial and major barrier to bone marrow engraftment in allosensitized recipients. Blood. 2007;109:1307–1315. doi: 10.1182/blood-2006-05-022772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Storb R. B cells versus T cells as primary barrier to hematopoietic engraftment in allosensitized recipients. Blood. 2009;113:1205. doi: 10.1182/blood-2008-09-177436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pulsipher MA, Chitphakdithai P, Logan BR, et al. Donor, recipient, and transplant characteristics as risk factors after unrelated donor PBSC transplantation: beneficial effects of higher CD34+ cell dose. Blood. 2009;114:2606–2616. doi: 10.1182/blood-2009-03-208355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anasetti C, Amos D, Beatty PG, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. N Engl J Med. 1989;320:197–204. doi: 10.1056/NEJM198901263200401. [DOI] [PubMed] [Google Scholar]
- 5.Ottinger HD, Rebmann V, Pfeiffer KA, et al. Positive serum crossmatch as predictor for graft failure in HLA-mismatched allogeneic blood stem cell transplantation. Transplantation. 2002;73:1280–1285. doi: 10.1097/00007890-200204270-00016. [DOI] [PubMed] [Google Scholar]
- 6.Spellman S, Bray R, Rosen-Bronson S, et al. The detection of donor-directed, HLA-specific alloantibodies in recipients of unrelated hematopoietic cell transplantation is predictive of graft failure. Blood. 2010;115:2704–2708. doi: 10.1182/blood-2009-09-244525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciurea SO, Thall PF, Wang X, et al. Donor-specific anti-HLA Abs and graft failure in matched unrelated donor hematopoietic stem cell transplantation. Blood. 2011;118:5957–5964. doi: 10.1182/blood-2011-06-362111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker JN, Scaradavou A, Stevens CE. Combined effect of total nucleated cell dose and HLA-match on transplant outcome in 1061 cord blood recipients with hematological malignancies. Blood. 2010;115:1843–1849. doi: 10.1182/blood-2009-07-231068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010;11:653–660. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of two partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 11.Ponce DM, Sauter C, Devlin S, et al. A novel reduced-intensity conditioning regimen induces a high incidence of sustained donor-derived neutrophil and platelet engraftment after double-unit cord blood transplantation. Biol Blood Marrow Transplant. 2013;19:799–803. doi: 10.1016/j.bbmt.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takanashi M, Fujiwara K, Tanaka H, Satake M, Nakajima K. The impact of HLA antibodies on engraftment of unrelated cord blood transplants. Transfusion. 2008;48:791–793. doi: 10.1111/j.1537-2995.2008.01678.x. [DOI] [PubMed] [Google Scholar]
- 13.Takanashi M, Atsuta Y, Fujiwara K, et al. The impact of anti-HLA antibodies on unrelated cord blood transplantations. Blood. 2010;116:2839–2846. doi: 10.1182/blood-2009-10-249219. [DOI] [PubMed] [Google Scholar]
- 14.Cutler C, Kim HT, Sun L, et al. Donor-specific anti-HLA antibodies predict outcome in double umbilical cord blood transplantation. Blood. 2011;118:6691–6697. doi: 10.1182/blood-2011-05-355263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunstein CG, Noreen H, DeFor TE, Maurer D, Miller JS, Wagner JE. Anti-HLA antibodies in double umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2011;17:1704–1708. doi: 10.1016/j.bbmt.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruggeri A, Rocha V, Masson E, et al. Impact of donor-specific anti-HLA antibodies on graft failure and survival after reduced intensity conditioning-unrelated cord blood transplantation: a Eurocord, Societe Francophone d'Histocompatibilite et d'Immunogenetique (SFHI) and Societe Francaise de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC) analysis. Haematologica. 2013;98:1154–1160. doi: 10.3324/haematol.2012.077685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocha V, Gluckman E. Improving outcomes of cord blood transplantation: HLA matching, cell dose and other graft- and transplantation-related factors. Br J Haematol. 2009;147:262–274. doi: 10.1111/j.1365-2141.2009.07883.x. [DOI] [PubMed] [Google Scholar]
- 18.Barker JN, Byam C, Scaradavou A. How I treat: the selection and acquisition of unrelated cord blood grafts. Blood. 2011;117:2332–2339. doi: 10.1182/blood-2010-04-280966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizutani K, Terasaki P, Hamdani E, et al. The importance of anti-HLA-specific antibody strength in monitoring kidney transplant patients. Am J Transplant. 2007;7:1027–1031. doi: 10.1111/j.1600-6143.2006.01721.x. [DOI] [PubMed] [Google Scholar]
- 20.Domen R, Casey H, Gaspari J. Correlation of HLA donor specific antibody (DSA) titers with the luminex mean fluorescence intensity (MFI) Hum Immunol. 2008;69:S35. [Google Scholar]
- 21.Vassallo RR, Hsu S, Einarson M, Barone J, Brodsky J, Moroff G. A comparison of two robotic platforms to screen plateletpheresis donors for HLA antibodies as part of a transfusion-related acute lung injury mitigation strategy. Transfusion. 2010;50:1766–1777. doi: 10.1111/j.1537-2995.2010.02626.x. [DOI] [PubMed] [Google Scholar]
- 22.Avery S, Shi W, Lubin M, et al. Influence of infused cell dose and HLA match on engraftment after double-unit cord blood allografts. Blood. 2011;117:3277–3285. doi: 10.1182/blood-2010-08-300491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purtill D, Smith KA, Tonon J, et al. Analysis of 402 cord blood units to assess factors influencing infused viable CD34+ cell dose: the critical determinant of engraftment. Blood. 2013;122:296. [Google Scholar]
- 24.Brunstein CG, Gutman JA, Weisdorf DJ, et al. Allogeneic hematopoietic cell transplantation for hematological malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116:4693–4699. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scaradavou A, Smith KM, Hawke R, et al. Cord blood units with low CD34+ cell viability have a low probability of engraftment after double unit transplantation. Biol Blood Marrow Transplant. 2010;16:500–508. doi: 10.1016/j.bbmt.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Georges GE, Lesnikov V, Baran SW, et al. A pre-clinical model of double versus single unit unrelated cord blood transplantation. Biol Blood Marrow Transplant. 2010;16:1090–1098. doi: 10.1016/j.bbmt.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutman JA, Turtle CJ, Manley TJ, et al. Single-unit dominance after double-unit umbilical cord blood transplantation coincides with a specific CD8+ T-cell response against the nonengrafted unit. Blood. 2010;115:757–765. doi: 10.1182/blood-2009-07-228999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eldjerou LK, Chaudhury S, Baisre-de Leon A, et al. An in vivo model of double unit cord blood transplantation that correlates with clinical engraftment. Blood. 2010;116:3999–4006. doi: 10.1182/blood-2010-03-276212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS, Wagner JE. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003;102:1915–1919. doi: 10.1182/blood-2002-11-3337. [DOI] [PubMed] [Google Scholar]
- 30.Eapen M, Klein JP, Ruggeri A, et al. Impact of allele-level HLA matching on outcomes after myeloablative single unit umbilical cord blood transplantation for hematologic malignancy. Blood. 2014;123:133–140. doi: 10.1182/blood-2013-05-506253. [DOI] [PMC free article] [PubMed] [Google Scholar]


