Abstract
Background
Recent work suggests that a subset of individuals with posttraumatic stress disorder (PTSD) exhibit marked dissociative symptoms, as defined by derealization and depersonalization. A dissociative subtype of PTSD was added to the diagnostic criteria listed in the DSM-5 to capture this presentation of PTSD. This study examined genetic polymorphisms for association with the symptoms that define the dissociative subtype of PTSD using a genome-wide approach.
Methods
The sample was comprised of 484 white, non-Hispanic, trauma-exposed veterans and their partners who were assessed for lifetime PTSD and dissociation using a structured clinical interview. The prevalence of PTSD was 60.5%. Single nucleotide polymorphisms (SNPs) from across the genome were obtained from a 2.5 million SNP array.
Results
Ten SNPs evidenced suggestive association with dissociation (p < 10−5). No SNPs met genome-wide significance criteria (p < 5 × 10−8). The peak SNP was rs263232 (β = 1.4, p = 6.12 × 10−7), located in the adenylyl cyclase 8 (ADCY8) gene; a second SNP in the suggestive range was rs71534169 (β = 1.63, p = 3.79 × 10−6), located in the dipeptidyl-peptidase 6 (DPP6) gene.
Conclusions
ADCY8 is integral for long term potentiation and synaptic plasticity and is implicated in fear-related learning and memory and long term memory consolidation. DPP6 is critical for synaptic integration and excitation. These genes may exert effects on basic sensory integration and cognitive processes that underlie dissociative phenomena.
Keywords: dissociative subtype, dissociation, posttraumatic stress disorder, genome-wide, gene, ADCY8, DPP6
Introduction
Recent research on the relationship between symptoms of dissociation and posttraumatic stress disorder (PTSD) has identified a distinct subset of individuals with the diagnosis who manifest a dissociative subtype of the disorder.[1–6] The subtype is defined primarily by symptoms of derealization (i.e., feeling as if the world is not real) and depersonalization (i.e., feeling as if oneself is not real) and is manifested in approximately 15–30% of individuals with PTSD.[3–6] In some investigations, DSM-IV PTSD criteria B3 (flashbacks) and C3 (psychogenic amnesia) have also been associated with the subtype.[3,6] Evidence for the subtype comes from latent profile analyses,[4–6] taxonometric analyses,[7] signal detection analyses,[8] and evaluation of the distribution of dissociative symptoms in large samples,[3,9] and has been replicated in veteran,[5,6] civilian,[4] and cross-cultural samples.[3] As a result of this psychometric and neuroimaging research (reviewed below), the dissociative subtype of PTSD was added to the Diagnostic and Statistical Manual of Mental Disorders, Version 5 (DSM-5[10]).
Evaluation of the dissociative subtype has important implications for both clinical practice and research. Clinically, dissociation is generally thought to interfere with PTSD treatment and is an important phenomenon to attend to in its own right. From a research standpoint, dissociation represents a source of heterogeneity in the clinical presentation of PTSD that may complicate the search for biomarkers, clinical correlates, and effective treatments if not taken into account. For example, findings of recent functional neuroimaging studies suggest that individuals with the dissociative subtype exhibit a different pattern of brain activation (e.g., hypoactivation of limbic brain regions and increased activation in pre-frontal regions) during exposure to traumatic memories than individuals with PTSD who do not dissociate.[1,2]
Although severity of trauma exposure is a predictor of the dissociative subtype of PTSD,[3,4,6] it is also possible that the capacity for dissociation is influenced by pre-existing biological vulnerabilities. Several studies suggest an association between stress-linked dissociative symptoms and blunted neurobiological reactivity.[11–17] In addition, a handful of small genetic studies have reported associations between candidate genes and broadly defined dissociative symptomatology. Specifically, single nucleotide polymorphisms (SNPs) in FK506 binding protein 5 (FKBP5), a gene involved in regulating the hypothalamic-pituitary-adrenal axis, were associated with dissociative symptoms in hospitalized children with medical injuries[18] and in women with a history of childhood trauma.[19] The serotonin transporter gene (SLC6A4) has been implicated in dissociation among individuals with obsessive-compulsive disorder[20] and trauma-exposed individuals.[21] Finally, the catechol-O-methyltransferase (COMT) gene has been associated with dissociation among children with a history of abuse.[22] These studies suggest the possibility of a genetic contribution to dissociative symptoms, but to date, genetic associations with the dissociative subtype of PTSD have not been evaluated. The aim of this study was to conduct a genome-wide search for polymorphisms associated with the symptoms of dissociation that define the subtype in a sample of trauma-exposed veterans and non-veterans. The phenotype was defined by severity scores on lifetime symptoms of derealization and depersonalization; this dimensional approach was expected to yield enhanced statistical power compared to use of a categorical designation of the phenotype because of the low prevalence of the subtype. The use of a genome-wide approach also allowed for a test of the replicability of the results of prior genetic association studies of dissociation.
Methods
Participants and Procedure
Participants were drawn from a trauma-exposed sample, as described by Logue et al.[23] Briefly, the study included 852 U.S. military veterans and their intimate partners who participated in one of two studies on the genetics of posttraumatic psychopathology and couple conflict behavior. Ancestry was determined using 10,000 randomly chosen polymorphisms with minor allele frequency (MAF) > .05 using the program STRUCTURE.[24,25] which uses a Bayesian clustering analysis to determine ancestry. The largest subpopulation identified through this procedure (n = 540) self-reported their racial background as white, non-Hispanic. Of these, 491 participants (364 veterans and 127 non-veteran partners) had been exposed to a DSM-IV Criterion A[26] trauma, as determined by the Clinician Administered PTSD Scale (CAPS), [27] a validated, semi-structured PTSD interview; 484 had scores on the CAPS interview items assessing derealization and depersonalization and were included in these analyses. In this final sample, 170 were women and 314 were men with a mean age of 52 (range: 21–75); 60.5% of the sample met criteria for a lifetime diagnosis of PTSD (247 veterans and 45 non-veteran partners). All interviews were administered by clinicians with either an MA or PhD in psychology, and were digitally recorded for the purposes of evaluating diagnostic reliability (see below) and maintaining quality control. The Principal Investigator of the study (MWM) oversaw weekly meetings of interviewers to review videotapes, discuss diagnostic concerns, and prevent rater drift. Veterans and their non-veteran partners took part in identical but separate assessment procedures. Partners included in this study had trauma exposure were not necessarily “controls.”
Measures
The Clinician Administered PTSD Scale (CAPS)
The CAPS,[27] the gold-standard structured diagnostic interview for PTSD, was used to assess PTSD diagnostic status, PTSD severity, and dissociation severity. Items measure the frequency and intensity of each of the 17 DSM-IV PTSD symptoms on a 0–4 scale for a total possible item severity score of 0–8 for each symptom. In addition, the CAPS includes items that assess associated features of PTSD, as listed in the DSM-IV. These include two items that assess derealization and depersonalization, which have been used previously to define the dissociative subtype of PTSD.[4–6] For the purposes of this study, the severity scores for each of these two items were combined to form a dimensional dissociation severity score with a possible range of 0–16. Inter-rater agreement (intra-class correlation coefficients) between primary and secondary ratings of ~30% of the diagnostic interview video recordings was high for the dimensional lifetime PTSD severity scores (ICC = .97) and for the dimensional lifetime dissociation severity scores (ICC = .93). Kappa for the lifetime PTSD diagnosis was .87.
Genotyping
DNA was isolated from peripheral blood samples on a Qiagen AutoPure instrument with Qiagen reagents and normalized using PicoGreen assays (Invitrogen, Grand Island, NY, USA). Genotyping was performed on an Illumina OMNI 2.5--8 array and scanned using an Illumina HiScan System (San Diego, CA, USA) according to the manufacturer’s specifications. All SNP locations were from the hg19 human-genome assembly (February 2009). Details on call rates, quality control, and elimination of samples are provided in Logue et al.[23] SNPs were eliminated from the analysis if their MAF was < 5% or if they failed a test of Hardy Weinberg equilibrium (p ≤ .000001) leaving a total of 1,197,702 SNPs for analysis. We also evaluated the possibility of population substructure within the Caucasian sample by using principal components (PC) analysis of 10,000 randomly selected genetic markers (with MAF > .05) in the program EIGENSTRAT.[28] We then took the top 10 PCs from that analysis and entered them into a multiple regression predicting dissociation severity and found no evidence for dissociation-associated population substructure (overall model F = 1.26, p = .25).
Statistical Analyses
All genetic association analyses were performed using the program PLINK.[29] Quantitative trait analysis was conducted, using the PLINK --linear option, and asymptotic p-values were obtained via a Wald test. Genome-wide level of significance was set at p < 5 × 10−8, and suggestive evidence for association was defined by p < 10−5. Follow-up analyses that examined the possible moderating role of biological sex were conducted via linear regression and the --interaction command in PLINK. In addition, we followed up on our main results by controlling for the effects of PTSD severity. Finally, to permit comparison with prior work, we examined if SNPs in any genes previously associated with dissociation (FKBP5, SLC6A4, COMT) reached gene-wide level of significance using the max(T) permutation procedure with 5000 replications to calculate p-values corrected for the number of SNPs evaluated on each gene (to allow for a more equitable comparison to prior candidate gene studies). Linkage disequilibrium (LD) was evaluated for pairs of SNPs in PLINK.
Results
The mean dissociation severity score was 1.13 (SD = 2.59, range: 0 – 15). The majority of participants (78%) evidenced no symptoms of derealization or depersonalization. The number of participants with lifetime PTSD who met presumptive criteria for the dissociative subtype was 19%, as defined by a CAPS frequency score of one or greater and intensity score of two or greater on either the derealization or depersonalization item; these cut-points are frequently used to determine the presence of each PTSD symptom on the CAPS.[30] A chi-square analysis revealed that the prevalence of the subtype did not differ between men (20.3%) and women (18.0%; p = .55).
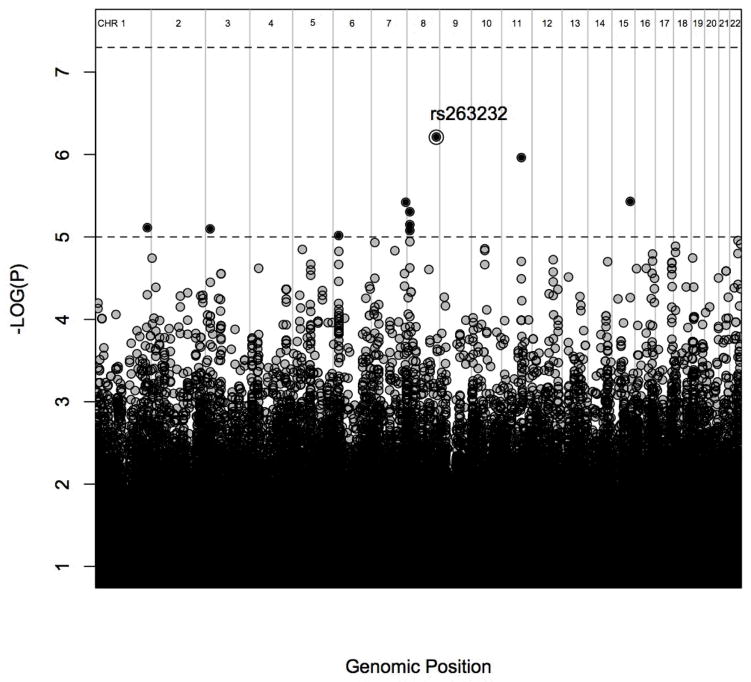
Ten SNPs showed associations with dissociative symptom severity in the suggestive range of p < 10−5. No SNPs were genome-wide significant (p < 5 × 10−8). The Manhattan Plot showing the negative log-transformed p-values from the genome-wide analysis of dissociation symptom severity is shown in Figure 1; additional details are provided in Table 1.
Figure 1.
Manhattan plot of genome-wide association results for dissociation severity. The bottom dashed lined represents suggestive level of statistical significance (p < 10−5) and the top dotted lined represents genome-wide level of statistical significance (p < 5 × 10−8). The peak SNP is circled.
Table 1.
SNPs showing association with Dissociative Symptom Severity at p < 10−5
| CHR | SNP | bp | BETA | SE | R2 | p | Gene | Other Info |
|---|---|---|---|---|---|---|---|---|
| 1 | rs9725031 | 232444455 | 1.13 | 0.25 | 0.04 | 7.72E−06 | intergenic | Closest gene is SIPA1L2 |
| 3 | rs1915919 | 20083881 | 0.74 | 0.16 | 0.04 | 7.99E−06 | KAT2B | |
| 6 | rs77006546 | 25219582 | 1.70 | 0.38 | 0.04 | 9.60E−06 | intergenic | Near CMAHP |
| 7 | rs71534169 | 154592866 | 1.63 | 0.35 | 0.04 | 3.79E−06 | DPP6 | |
| 8 | rs62488971 | 12808108 | 1.58 | 0.35 | 0.04 | 8.37E−06 | KIAA1456 | |
| 8 | rs2460905 | 12834600 | 1.38 | 0.30 | 0.04 | 4.95E−06 | KIAA1456 | |
| 8 | rs2466273 | 12866004 | 1.31 | 0.29 | 0.04 | 7.10E−06 | KIAA1456 | |
| 8 | rs263232 | 131808169 | 1.43 | 0.28 | 0.05 | 6.12E−07 | ADCY8 | |
| 11 | rs682457 | 87949532 | 1.11 | 0.22 | 0.05 | 1.09E−06 | MIR3166 | microRNA gene |
| 15 | rs77963519 | 82193846 | 1.56 | 0.33 | 0.04 | 3.71E−06 | intergenic | Closest gene is MEX3B |
Note. SNPs are listed by chromosome and bp. CHR = chromosome, SNP = single nucleotide polymorphism, bp = base pair, SE = standard error, Info = information, SIPA1L2 = signal-induced proliferation-associated 1 like 2; KAT2B = K(lysine) acetyltransferase 2B, CMAHP = cytidine monophospho-N-acetylneuraminic acid hydroxylase, pseudogene, DPP6 = dipeptidyl-peptidase 6, KIAA1456 = chromosome 8 open reading frame 79, ADCY8 = adenylate cyclase 8, MIR3166 = microRNA 3166, MEX3B = mex-3 homolog B.
The SNP with the strongest association with dissociation, rs263232 (β = 1.4, p = 6.12 × 10−7), is located at 131,808,169 base pair (bp) on chromosome 8 in the adenylyl cyclase 8 (ADCY8) gene at 131,792,547 – 132,054,672 bp. In our sample, the MAF of this SNP was 8.9%. Evaluation of the distribution of dissociation severity scores as a function of genotype yielded no evidence of outliers. Approximately 17% of individuals with zero copies of the risk allele had positive scores on dissociation compared to approximately 34% of individuals with one or two copies of the risk allele. The effect of the SNP remained suggestive (p = 5.66 × 10−7) after controlling for PTSD severity and was not significantly modified by sex (p = .09). Follow-up evaluation of ADCY8 revealed four other SNPs (rs4339660, rs7015079, rs263238, rs263234) that were nominally (p < .05) associated with dissociation severity (smallest p = .01).
A second SNP in the suggestive range was rs71534169 (β = 1.63, p = 3.79 × 10−6) located at 154,592,866 bp on chromosome 7 in the dipeptidyl-peptidase 6 (DPP6) gene located at 153,584,182–154,685,995 bp. In our sample, the MAF of this SNP was 6.5%. Thirty-eight additional SNPs in the gene evidenced nominally significant (p < .05) associations with dissociation (details from first author). Approximately 82% of individuals with no copies of the risk allele scored 0 on dissociation severity compared to 67% of individuals with 1 copy of the risk allele (no participants had two copies of the risk allele). The effect of the SNP remained significant (p = 3.96 × 10−5) after controlling for PTSD severity, and there was a significant SNP X sex interaction (β = −2.34, p = .002). Stratifying by sex showed the effect to be stronger among women (β = 3.26, p = 6.96 × 10−7) than men (β = .93, p = .03).
A number of other SNPs showed suggestive evidence of association with dissociation (see Table 1): three in chromosome 8 open reading frame 79 (KIAA1456), which ranged in their degree of linkage disequlibrium with each other (r2 range: .32 – .95, D’ range: .74 – 1.0); one in K(lysine) acetyltransferase 2B (KAT2B) on chromosome 3; one in microRNA 3166 (MIR3166); and three that were intergenic. In follow-up analyses, the association between these eight SNPs and dissociation severity remained significant after covarying for PTSD severity (with PTSD severity in the model, the largest SNP p-value was p = .0002 for rs77006546 and the smallest SNP p-value was p = 6.99 × 10−7 for rs682457). Four of these SNPs showed suggestive evidence of an association with sex, such that the effects were greater in women compared to men. Specifically, suggestive sex X SNP effects were found for rs9725031 (βinteraction = −1.48, p = .004; βmen = 0.57, p = .07; βwomen = 2.05, p = 1.17 × 10−6), and for the three SNPs in KIAA1456: rs62488971 (βinteraction = −2.44, p = .0008; βmen = 0.70, p = .11; βwomen = 3.14, p = 2 × 10−7); rs2460905 (βinteraction = −1.72, p = .005; βmen = .75, p = .05; βwomen = 2.46, p = 1.44 × 10−6); and rs2466273 (βinteraction = −1.63, p = .007; βmen = .74, p = .04; βwomen = 2.36, p = 3.00 × 10−6).1
Finally, we evaluated SNPs in the three genes that have shown association with dissociation previously. There were 30 SNPs included in the analysis of FKBP5 and of these, three were nominally significant (smallest uncorrected p-value = .01) but none withstood gene-wide permutation correction. Further, these three did not overlap with FKBP5 SNPs that have previously shown association with dissociation[18] or PTSD.[32] Thirteen SNPSs in SLC6A4 were evaluated and none showed significant association with dissociation (all p > .05). There were 29 SNPs in COMT that were evaluated and one (rs9306235) showed a nominally significant association (p = .04) that did not withstand gene-wide permutation testing (p = .42).
Discussion
We conducted a genome-wide association study to evaluate genetic predictors of derealization and depersonalization symptoms in a trauma-exposed sample with a high prevalence of PTSD. To our knowledge, this is the first genome-wide association test for dissociation symptom severity, as prior work has used a candidate gene approach to evaluate genes commonly examined across psychiatric phenotypes.[18–22] Although no SNPs in this study met standard criteria for genome-wide level of statistical significance, ten SNPs were in the “suggestive” range. Two SNPs were located in genes that have clear potential functional significance for dissociative symptomatology and have been associated with other psychiatric conditions in the past.
The peak SNP, rs263232, was located in ADCY8 on chromosome 8. ADCY8 has also been associated with bipolar disorder[33–35] (see also Avramopoulos et al.[36]), and depression comorbid with alcohol dependence in women.[37] ADCY8 is expressed pre-snyaptically in many areas of the brain, including the cortex, hippocampus, amygdala, thalamus, hypothalamus, and cerebellum.[38] It produces the enzyme adenylyl cyclase (AC8), one of two Ca2+/calmodulin sensitive adenylyl cyclase isoforms that catalyze the conversion of ATP to cyclic AMP, and thus impacts the same intracellular second messenger system as the pituitary adenylate cyclase-activating polypeptide (PACAP) gene recently associated with PTSD in women.[39]
Cyclic AMP is integral to long term potentiation, synaptic plasticity, and hence learning and memory. In the hippocampus, levels of cyclic AMP determine the basal balance between silenced and active synapses; and in AC8 deficient mice, rapid resetting of strongly depolarized presynaptic terminals is prevented. The adenylate cyclase signaling cascade is also critical to central and peripheral hypothalamic-pituitary-adrenal (HPA) axis regulation.
The functional impact of AC8 deficiency in rodents appears to be consistent with these mechanisms (Table 2; also see review by Wang & Zhang [40]). Such rodents are hyper-locomotive and appear to be insensitive to novelty, risk, context, and experience. They also show marked deficits in retention of conditioned fear, reductions in phosphorylated cyclic AMP response element binding protein (pCREB), differences in gene expression at time points relevant to memory consolidation and retention, and dysregulation of the HPA axis.
Table 2.
Functional Characteristics ADCY8 Knock-Out Mice
| Study | Pertinent Findings |
|---|---|
| Wong et al., (1999)[60] | ADC8 DKO mice exhibited impaired long-term fear memory. |
| Schaefer et al., (2000)[61] | ADC8 KO mice showed increased locomotion and more risk taking behavior in a plus maze, even after restraint stress, compared to WT mice. They failed to demonstrate longterm depression (LTD) and exhibited decreased pCREB expression after restraint stress in the CA1 field of the hippocampus. They were lighter than WT mice but consumed a comparable number of calories. After chronic stress, they showed increased basal corticosterone levels and blunted corticosterone responses to a stressor. |
| Li et al., (2006)[62] | ADC8 KO mice showed diminished morphine tolerance; ADC8/ADC1 DKO mice showed decreased morphine tolerance, withdrawal, hyperlocomotion, conditioned place preference, and pCREB in VTA. |
| Krishnan et al., (2008)[63] | ADC8 KO mice habituated more quickly to a novel environment, swam longer in a forced swim test, and were more socially interactive with unfamiliar rodents than WT mice. Paradoxically, they showed reduced body weight and decreased sucrose preference and fluid intake. They had lower pERK1/2 in hippocampus, but increased BDNF and neurotrophin signaling in nucleus accumbens. |
| de Mooij-van Malsen et al., (2009)[34] | A chromosome substitution mouse strain showed increased avoidance behavior linked to chromosome 15 upon which the AC8 gene is located and manifest increased AC8 expression in the hypothalamus and piriform cortex. |
| Razzoli et al., (2010)[64] | ADC8 KO mice showed increased home box locomotion and climbing during the dark period, were slower to enter a dark compartment after restraint stress, and swam longer in the forced swim test. They also had adrenal hypertrophy, decreased leptin, and otherwise lighter organ weights. |
| Wieczorek et al., (2010)[65] | ADC8/ADC1 DKO mice showed marked deficits in long-term, but not short term conditioned fear, and marked changes in gene expression at time points relevant to memory consolidation and retention. Selective restoration of forebrain AC8 expression restored memory in DKO animals. |
| Wieczorek et al., (2012)[66] | ADC8/ADC1 DKO mice evidenced memory deficits that were experience/context independent. They also showed reductions in proteins that are markers for neurotransmission and synaptic plasticity after conditioned fear trials. |
| Zhang et al., (2011)[67] | ADC8 DKO mice exhibited impaired spatial memory. |
Note. ADCY8: Adenylyl cyclase isoform 8 gene; AC8: adenylyl cyclase isoform 8; KO: knock-out; DKO: ADC8/ADC1 double knock-out; WT: wild-type; pCREB: phosphorylated cyclic-AMP response element binding protein; phospoERK1/2: phosphorylated extracellular-signal-regulated kinase type 1/2.
Failure to encode memories associated with intense emotion or stress has clear relevance to the dissociative subtype of PTSD as the DSM-IV PTSD criterion C3, psychogenic amnesia, has been uniquely associated with the subtype.[3,6] Some conceptualize dissociation in PTSD as an unconscious form of emotion avoidance that occurs in response to overwhelming fear, hyperarousal, or anxiety; although speculative, it is also conceivable that dissociation could occur as a neurobiological consequence of hyperarousal in the context of an AC8 deficiency that prevents the encoding and consolidation of contextual information.
Another SNP in the suggestive range of association with dissociation was rs71534169, which is located in the DPP6 gene on chromosome 7. This effect was stronger in women relative to men, suggesting possible sex-specific pathways in the development and manifestation of dissociative symptoms. DPP6 has most frequently been studied in relation to multiple sclerosis[41] and amyotrophic lateral sclerosis.[42,43] It has also shown association with autism.[44] The DPP6 protein is an auxillary subunit of voltage-gated potassium-4 channels that influence neuronal excitability and communication of excitability to distal dendrites. DPP6 is involved in regulating the Atype K+ current gradient, which regulates dendritic excitability. Hippocampal recordings from DPP6 knock-out mice demonstrate a decrease in this gradient and increased dendritic excitability.[45] Given the role of DPP6 in synaptic integration, it is possible that this protein also plays a role in dissociation, a state defined by poor integration of incoming sensory experiences and problems with region-specific cognitive processes that are ordinarily organized dynamically across time.
In addition, three SNPs in KIAA1456 and one in KAT2B showed suggestive evidence of association with dissociation; the effect of the KIAA1456 SNPs was stronger in women relative to men and was weakened when exposure to childhood sexual abuse was controlled. The KIAA1456 gene encodes a methyltransferase, thus potentially implicating dysregulation of epigenetic processes in the dissociative subtype of PTSD. In a recent in situ hybridization gene expression study, the density of KIAA1456 was found to be selectively lower in layers I, II, III, and V of Brodman’s area 9, but not area 46, of the dorsolateral prefrontal cortex in individuals with schizophrenia compared to controls.[46] KAT2B encodes a histone/protein acetylase and has been implicated in DNA repair, replication, and transcription,[47–49] global metabolic regulation,[50] and autophagy,[51] a cellular process by which cells recycle or dispose of internal constituents to maintain normal structure and function under conditions of stress (e.g., nutrient deprivation, hypoxia, and oxidative stress).[52] To our knowledge, KAT2B has not previously been associated with a psychiatric phenotype. More extensive study of both KIAA1456 and KAT2B is needed to confirm their role and specific mechanistic contributions to dissociation following trauma exposure.
The association between these SNPs and dissociation remained strong even after controlling for PTSD severity, suggesting that the association was not driven by the core set of PTSD symptoms. The genes implicated here are also distinct from those identified previously in studies of PTSD. The first genome-wide association study of PTSD[23] (evaluated in this same sample) suggested that the retinoid-related orphan receptor alpha (RORA) gene was associated with PTSD at a genome-wide level of significance and a subsequent genome-wide investigation implicated the tolloid-like 1 gene in PTSD.[53] Prior candidate gene work in PTSD in adults has implicated a variety of other genes including steroid 5-α-reductase type 2 (SRD5A2[54]), SLC6A4,[55–57] pituitary adenylate cyclase-activating polypeptide and its receptor (ADCYAP1 and ADCYAP1R1, respectively[39]), FKBP5,[32] and catechol-o-methyltransferase (COMT[58]), among others (for a recent review see Cornelis et al.[59]).
Some of these genes implicated in PTSD have also been implicated in candidate gene studies of dissociation. We did not replicate prior findings in smaller samples on the role of SLC6A4, FKBP5, or COMT in dissociation.[18–22] Sample size, age and developmental stage of participants, differences in the measurement of dissociation, differences in trauma exposure history, potential exposure to injury or oxidative stress due to substance abuse or other factors, and use of medications that may compensate or correct for particular genetic contributions to the dissociative phenotype could contribute to discrepancies in findings across studies.
Limitations
The results of this study should be interpreted in light of its limitations. First, the sample size of 484 was relatively small for a genome-wide association study, and there is always the risk that the results obtained here will not replicate in future research. Further, none of the SNPs met the strict level of significance needed to claim genome wide significance; they were all in the “suggestive” range and should be considered as such. Future work will need to evaluate the replicability of these results. In addition, our investigation was limited to white, non-Hispanic, trauma-exposed individuals, and it is unclear if these findings will generalize to other racial or demographic groups. We also were under-powered to fully evaluate potential sex differences in the SNP-dissociation relationships, and these results should be interpreted with caution. We did not examine potential SNP by trauma (i.e., gene by environment) effects given our focus on posttraumatic dissociation in a traumatized sample; it is unknown if these results generalize to dissociative disorders among non-traumatized populations or to trait dissociation. Related to this, it is possible that the assessment of dissociation was subject to recall bias in that participants may have attributed their dissociative symptoms to trauma even if they pre-dated trauma exposure. We focused on a dimensional phenotype, rather than a categorical one, in order to maximize the likelihood of having adequate power to detect small effects. Not all participants met full criteria for a lifetime diagnosis of PTSD, which could obscure findings between those with the dissociative subtype of PTSD from those without. Finally, it is not possible to know from this research whether the SNPs identified in association with the dissociative phenotype increase or decrease function of the genes in which they are embedded. Future basic and clinical studies will be needed to define their precise physiological and clinical impact.
Concerns about the lack of a replication sample are offset by the strengths of the study including our focus on a novel and highly specific psychiatric phenotype that has not been evaluated in genetic investigations to date. In addition, prior work examining the genetics of dissociation has been based on very small sample sizes. The current study represented an improvement upon prior ones as the sample size was larger and the genetic analyses were genome-wide. An additional strength of the study was its use of structured diagnostic interviews conducted by highly trained interviewers who showed good inter-rater reliability.
Conclusions
In conclusion, this was the first genetic association study of the primary symptoms that define the dissociative subtype of PTSD. Results revealed that SNPs in two genes of interest that are relevant to fear-related memory formation and processing (rs263232 in ADCY8), and synaptic integration (rs71534169 in DPP6) reached a “suggestive” level of genome-wide significance in their association with symptoms of derealization and depersonalization. These SNPs were still strongly associated with dissociative phenomena after PTSD severity was controlled for, suggesting that the effects were specific to dissociation. Failure to identify individuals with the dissociative subtype of PTSD may hinder efforts to identify the biological and etiological underpinnings of PTSD. Results of this study highlight the importance of using empirically refined phenotypes in genetic association studies.
Acknowledgments
Funding for this study was provided by National Institute on Mental Health award RO1 MH079806 and by a VA CSR&D Merit Award to Mark W. Miller. Erika J. Wolf’s contribution to this work was supported by a VA CSR&D Career Development Award. Karen S. Mitchell’s contribution to this work was supported by K01MH093750.
Footnotes
As dissociation is thought to be related to severe and early childhood trauma, we also examined the main effect of each of the 10 SNPs in the suggestive range of association while controlling for the number of reported instances of childhood sexual trauma occurring before age 13 as assessed by the Traumatic Life Events Questionnaire.[31] The main effect of all SNPs remained suggestive (i.e., p < 10−5) with the exception of the three SNPS in KIAA1456 and the intergenic SNPs rs77006546 and rs9725031. In these cases, the p-value for the SNP main effect fell just outside the cut-off for a suggestive association (p-value ranged from 1.04 × 10−5 to 2.01 × 10−5).
References
- 1.Lanius RA, Brand B, Vermetten E, et al. The dissociative subtype of posttraumatic stress disorder: Rationale, clinical and neurobiological evidence, and implications. Depress Anxiety. 2012;29:1–8. doi: 10.1002/da.21889. [DOI] [PubMed] [Google Scholar]
- 2.Lanius RA, Vermetten E, Loewenstein RJ, et al. Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatry. 2010;167:640–647. doi: 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein DJ, Koenen KC, Friedman MJ, et al. Dissociation in posttraumatic stress disorder: Evidence from the World Mental Health Surveys. Biol Psychiatry. 2012;73:302–312. doi: 10.1016/j.biopsych.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steuwe C, Lanius RA, Frewen PA. The role of dissociation in civilian posttraumatic stress disorder - Evidence for a dissociative subtype by latent class and confirmatory factor analysis. Depress Anxiety. 2012;29:689–700. doi: 10.1002/da.21944. [DOI] [PubMed] [Google Scholar]
- 5.Wolf EJ, Lunney CA, Miller MW, et al. The dissociative subtype of PTSD: A replication and extension. Depress Anxiety. 2012;29:679–688. doi: 10.1002/da.21946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf EJ, Miller MW, Reardon AF, et al. A latent class analysis of dissociation and posttraumatic stress disorder: evidence for a dissociative subtype. Arch Gen Psychiatry. 2012;69:698–705. doi: 10.1001/archgenpsychiatry.2011.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waelde LC, Silvern L, Fairbank JA. A taxometric investigation of dissociation in Vietnam veterans. J Trauma Stress. 2005;18:359–369. doi: 10.1002/jts.20034. [DOI] [PubMed] [Google Scholar]
- 8.Ginzburg K, Koopman C, Butler LD, et al. Evidence for a dissociative subtype of post-traumatic stress disorder among help-seeking childhood sexual abuse survivors. J Trauma Dissociation. 2006;7:7–27. doi: 10.1300/J229v07n02_02. [DOI] [PubMed] [Google Scholar]
- 9.Putnam FW, Carlson EB, Ross CA, et al. Patterns of dissociation in clinical and nonclinical samples. J Nerv Ment Dis. 1996;184:673–679. doi: 10.1097/00005053-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 10.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington DC: Author; 2013. [Google Scholar]
- 11.Dimoulas E, Steffian L, Steffian G, et al. Dissociation during intense military stress is related to subsequent somatic symptoms in women. Psychiatry. 2007;4:66–73. [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan CA, 3rd, Wang S, Rasmusson A, et al. Relationship among plasma cortisol, catecholamines, neuropeptide Y, and human performance during exposure to uncontrollable stress. Psychosom Med. 2001;63:412–422. doi: 10.1097/00006842-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Morgan CA, 3rd, Rasmusson AM, Wang S, et al. Neuropeptide-Y, cortisol, and subjective distress in humans exposed to acute stress: replication and extension of previous report. Biol Psychiatry. 2002;52:136–142. doi: 10.1016/s0006-3223(02)01319-7. [DOI] [PubMed] [Google Scholar]
- 14.Morgan CA, 3rd, Southwick S, Hazlett G, et al. Relationships among plasma dehydroepiandrosterone sulfate and cortisol levels, symptoms of dissociation, and objective performance in humans exposed to acute stress. Arch Gen Psychiatry. 2004;61:819–825. doi: 10.1001/archpsyc.61.8.819. [DOI] [PubMed] [Google Scholar]
- 15.Morgan CA, 3rd, Rasmusson A, Pietrzak RH, et al. Relationships among plasma dehydroepiandrosterone and dehydroepiandrosterone sulfate, cortisol, symptoms of dissociation, and objective performance in humans exposed to underwater navigation stress. Biol Psychiatry. 2009;66:334–340. doi: 10.1016/j.biopsych.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Simeon D, Knutelska M, Yehuda R, et al. Hypothalamic-pituitary-adrenal axis function in dissociative disorders, post-traumatic stress disorder, and healthy volunteers. Biol Psychiatry. 2007;61:966–973. doi: 10.1016/j.biopsych.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simeon D, Knutelska M, Smith L, et al. A preliminary study of cortisol and norepinephrine reactivity to psychosocial stress in borderline personality disorder with high and low dissociation. Psychiatry Res. 2007;149:177–184. doi: 10.1016/j.psychres.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Koenen KC, Saxe G, Purcell S, et al. Polymorphisms in FKBP5 are associated with peritraumatic dissociation in medically injured children. Mol Psychiatry. 2005;10:1058–1059. doi: 10.1038/sj.mp.4001727. [DOI] [PubMed] [Google Scholar]
- 19.Dackis MN, Rogosch FA, Oshri A, Cicchetti D. The role of limbic system irritability in linking history of childhood maltreatment and psychiatric outcomes in low-income, high-risk women: moderation by FK506 binding protein 5 haplotype. Dev Psychopathol. 2012;24:1237–1252. doi: 10.1017/S0954579412000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lochner C, Seedat S, Hemmings SM, et al. Investigating the possible effects of trauma experiences and 5-HTT on the dissociative experiences of patients with OCD using path analysis and multiple regression. Neuropsychobiology. 2007;56:6–13. doi: 10.1159/000109971. [DOI] [PubMed] [Google Scholar]
- 21.Pieper S, Out D, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Behavioral and molecular genetics of dissociation: the role of the serotonin transporter gene promoter polymorphism (5-HTTLPR) J Trauma Stress. 2011;24:373–380. doi: 10.1002/jts.20659. [DOI] [PubMed] [Google Scholar]
- 22.Savitz JB, van der Merwe L, Newman TK, et al. The relationship between childhood abuse and dissociation. Is it influenced by catechol-O-methyltransferase (COMT) activity? Int J Neuropsychopharmacol. 2008;11:149–161. doi: 10.1017/S1461145707007900. [DOI] [PubMed] [Google Scholar]
- 23.Logue MW, Baldwin C, Guffanti G, et al. A genome-wide association study of posttraumatic stress disorder identifies the retinoid-related orphan receptor alpha (RORA) gene as a significant risk locus. Mol Psychiatry. 2013;18:937–942. doi: 10.1038/mp.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington DC: Author; 1994. [Google Scholar]
- 27.Blake DD, Weathers FW, Nagy LM, et al. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 28.Price AL, Patterson NJ, Plenge RM, et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 29.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weathers FW, Ruscio AM, Keane TM. Psychometric properties of nine scoring rules for the Clinician-Administered Posttraumatic Stress Disorder Scale. Psychol Assess. 1999;11:124–133. [Google Scholar]
- 31.Kubany ES, Haynes SN, Leisen MB, et al. Development and preliminary validation of a brief-broad-spectrum measure of trauma exposure: The Traumatic Life Events Questionnaire. Psychol Assess. 2000;12:411–424. doi: 10.1037//1040-3590.12.2.210. [DOI] [PubMed] [Google Scholar]
- 32.Binder EB, Bradley RG, Liu W, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zandi PP, Zöllner S, Avramopoulos D, et al. Family-based SNP association study on 8q24 in bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:612–618. doi: 10.1002/ajmg.b.30651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Mooij-van Malsen AJ, van Lith HA, Oppelaar H, et al. Interspecies trait genetics reveals association of Adcy8 with mouse avoidance behavior and a human mood disorder. Biol Psychiatry. 2009;66:1123–1130. doi: 10.1016/j.biopsych.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 35.Zhang P, Xiang N, Chen Y, et al. Family-based association analysis to finemap bipolar linkage peak on chromosome 8q24 using 2,500 genotyped SNPs and 15,000 imputed SNPs. Bipolar Disord. 2010;12:786–792. doi: 10.1111/j.1399-5618.2010.00883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Avramopoulos D, Willour VL, Zandi PP, et al. Linkage of bipolar affective disorder on chromosome 8q24: follow-up and parametric analysis. Mol Psychiatry. 2004;9:191–196. doi: 10.1038/sj.mp.4001388. [DOI] [PubMed] [Google Scholar]
- 37.Procopio DO, Saba LM, Walter H, et al. Genetic markers of comorbid depression and alcoholism in women. Alcohol Clin Exp Res. 2013;37:896–904. doi: 10.1111/acer.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muglia LM, Schaefer ML, Vogt SK, et al. The 5′-flanking region of the mouse adenylyl cyclase type VIII gene imparts tissue-specific expression in transgenic mice. J Neurosci. 1999;19:2051–2058. doi: 10.1523/JNEUROSCI.19-06-02051.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ressler KJ, Mercer KB, Bradley B, et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, Zhang M. The role of Ca2 -stimulated adenylyl cyclases in bidirectional synaptic plasticity and brain function. Rev Neurosci. 2012;23:67–78. doi: 10.1515/revneuro-2011-0063. [DOI] [PubMed] [Google Scholar]
- 41.Brambilla P, Esposito F, Lindstrom E, et al. Association between DPP6 polymorphism and the risk of progressive multiple sclerosis in Northern and Southern Europeans. Neurosci Lett. 2012;530:155–160. doi: 10.1016/j.neulet.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Blauw HM, Al-Chalabi A, Andersen PM, et al. A large genome scan for rare CNVs in amyotrophic lateral sclerosis. Hum Mol Genet. 2010;19:4091–4099. doi: 10.1093/hmg/ddq323. [DOI] [PubMed] [Google Scholar]
- 43.Kwee LC, Liu Y, Haynes C, Gibson JR, et al. A high-density genome-wide association screen of sporadic ALS in US veterans. PLoS One. 2012;7:e32768. doi: 10.1371/journal.pone.0032768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marshall CR, Noor A, Vincent JB, et al. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82:477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun W, Maffie JK, Lin L, et al. DPP6 establishes the A-type K(+) current gradient critical for the regulation of dendritic excitability in CA1 hippocampal neurons. Neuron. 2011;71:1102–1115. doi: 10.1016/j.neuron.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guillozet-Bongaarts AL, Hyde TM, Dalley RA, et al. Altered gene expression in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. doi: 10.1038/mp.2013.30. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakaguchi K, Herrera JE, Saito S, et al. DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 1998;12:2831–2841. doi: 10.1101/gad.12.18.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho H, Orphanides G, Sun X, et al. A human RNA polymerase II complex containing factors that modify chromatin structure. Mol Cell Biol. 1998;18:5355–5363. doi: 10.1128/mcb.18.9.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tini M, Benecke A, Um S-J, et al. Association of CBP/p300 acetylase and thymine DNA glycosylase links DNA repair and transcription. Mol Cell. 2002;9:265–77. doi: 10.1016/s1097-2765(02)00453-7. [DOI] [PubMed] [Google Scholar]
- 50.Nojima K, Sugimoto K, Ueda H, et al. Analysis of hepatic gene expression profile in a spontaneous mouse model of type 2 diabetes under a high sucrose diet. Endocrine J. 2013;60:261–274. doi: 10.1507/endocrj.ej12-0258. [DOI] [PubMed] [Google Scholar]
- 51.Yi C, Yu L. How does acetylation regulate autophagy? Autophagy. 2012;8:1529–1530. doi: 10.4161/auto.21156. [DOI] [PubMed] [Google Scholar]
- 52.Kroemer G, Marino G, Levine G. Autophagy and the integrated stress response. Mol Cell. 2010;22:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie P, Kranzler HR, Yang C, Zhao H, Farrer LA, Gelertner J. Genome-wide association study identifies new susceptibility loci for posttraumatic stress disorder. Bio Psychiatry. doi: 10.1016/j.biopsych.2013.04.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gillespie CF, Almli LM, Smith AK, et al. Sex dependent influence of a functional polymorphism in steroid 5-α-reductase type 2 (SRD5A2) on post-traumatic stress symptoms. Am J Med Genet B Neuropsychiatr Genet. 2013;162:283–292. doi: 10.1002/ajmg.b.32147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kilpatrick DG, Koenen KC, Ruggiero KJ, et al. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane exposed adults. Am J Psychiatry. 2007;164:1693–1699. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- 56.Koenen KC, Aiello AE, Bakshis E, et al. Modification of the association between serotonin transporter genotype and risk of posttraumatic stress disorder in adults by county-level social environment. Am J Epidemiol. 2009;169:704–711. doi: 10.1093/aje/kwn397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Z, Baker DG, Harrer J, et al. The relationship between combat-related posttraumatic stress disorder and the 5-HTTLPR/rs25531 polymorphism. Depress Anxiety. 2011;28:1067–1073. doi: 10.1002/da.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kolassa IT, Kolassa S, Ertl V, et al. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-o-methyltransferase Val(158)Met polymorphism. Biol Psychiatry. 2010;67:304–308. doi: 10.1016/j.biopsych.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 59.Cornelis MC, Nugent NR, Amstadter AB, Koenen KC. Genetics of post-traumatic stress disorder: review and recommendations for genome-wide association studies. Curr Psychiatr Rep. 2010;12:313–326. doi: 10.1007/s11920-010-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong ST, Athos J, Figueroa XA, et al. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron. 1999;23:787–798. doi: 10.1016/s0896-6273(01)80036-2. [DOI] [PubMed] [Google Scholar]
- 61.Schaefer ML, Wong ST, Wozniak DF, et al. Altered stress-induced anxiety in adenylyl cyclase type VIII-deficient mice. J Neurosci. 2000;20:4809–4820. doi: 10.1523/JNEUROSCI.20-13-04809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li S, Lee ML, Bruchas MR, et al. Reduction in morphine responses in mice lacking calmodulin-stimulated adenylyl cyclases. Molecular Pharmacology. 2006;70:1742–1749. doi: 10.1124/mol.106.025783. [DOI] [PubMed] [Google Scholar]
- 63.Krishnan V, Graham A, Mazei-Robison MS, et al. Calcium-sensitive adenylyl cyclases in depression and anxiety: behavioral and biochemical consequences of isoform targeting. Biol Psychiatry. 2008;64:336–343. doi: 10.1016/j.biopsych.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Razzoli M, Andreoli M, Maraia G, et al. Functional role of Calcium-stimulated adenylyl cyclase 8 in adaptations to psychological stressors in the mouse: implications for mood disorders. Neuroscience. 2010;170:429–440. doi: 10.1016/j.neuroscience.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 65.Wieczorek L, Maas JW, Jr, Muglia LM, et al. Temporal and regional regulation of gene expression by calcium-stimulated adenylyl cyclase activity during fear memory. PLoS One. 2010;5:e13385. doi: 10.1371/journal.pone.0013385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wieczorek L, Majumdar D, Wills TA, et al. Absence of Ca2+-stimulated adenylyl cyclases leads to reduced synaptic plasticity and impaired experience-dependent fear memory. Transl Psychiatry. 2012;2:e126. doi: 10.1038/tp.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang M, Moon C, Chan GC, et al. Ca-stimulated type 8 adenylyl cyclase is required for rapid acquisition of novel spatial information and for working/episodic-like memory. J Neurosci. 2008;28:4736–4744. doi: 10.1523/JNEUROSCI.1177-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]