Abstract
Hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome is an autosomal dominant disorder in which germline mutations of fumarate hydratase (FH) gene confer an increased risk of cutaneous and uterine leiomyomas as well as renal cancer. HLRCC-associated renal cancer is highly aggressive, and frequently presents as a solitary mass. We reviewed the clinicopathologic features of 9 patients with renal tumors presenting as sporadic cases, but who were later proven to have FH germline mutations. Histologically, all tumors showed mixed architectural patterns, with papillary as the dominant pattern in only 3 cases. Besides papillary, tubular, tubulopapillary, solid and cystic elements, 6 of 9 tumors contained collecting duct carcinoma-like areas with infiltrating tubules, nests or individual cells surrounded by desmoplastic stroma. Prominent tubulocystic carcinoma-like component and sarcomatoid differentiation were identified. While all tumors exhibited the proposed hallmark of HLRCC (large eosinophilic nucleolus surrounded by a clear halo), this feature was often not uniformly present throughout the tumor. Prior studies have shown that high level of fumarate accumulated in HLRCC tumor cells causes aberrant succination of cellular proteins by forming a stable chemical modification, S-(2-succino)-cysteine (2SC), which can be detected by immunohistochemistry. We thus explored the utility of detecting 2SC by immunohistochemistry in the differential diagnosis of HLRCC tumors and other high-grade renal tumors, and investigated the correlation between 2SC staining and FH molecular alterations. All confirmed HLRCC tumors demonstrated diffuse and strong nuclear and cytoplasmic 2SC staining, while all clear cell (184/184, 100%), most high-grade unclassified RCC (93/97, 96%) and the large majority of type 2 papillary (35/45, 78%) cases showed no 2SC immunoreactivity. A subset of papillary (22%) and rare unclassified (4%) tumors showed patchy or diffuse cytoplasmic staining without nuclear labeling, unlike the pattern seen with confirmed HLRCC tumors. Sequencing revealed no germline or somatic FH alterations in 14 tumors that either exhibited only cytoplasmic 2SC staining (n=5) or were negative for 2SC (n=9), despite their HLRCC-like morphologic features. Our results emphasize the pivotal role of pathologic examination in the diagnosis of HLRCC patients, and indicate immunohistochemical detection of 2SC as a useful ancillary tool in the differentiation of HLRCC renal tumors from other high-grade renal cell carcinomas.
Keywords: renal cancer, HLRCC, fumarate hydratase, succination, 2SC, immunohistochemistry
Introduction
Hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome is an inherited autosomal dominant disorder in which germline mutations of fumarate hydratase (FH) gene confer an increased risk of cutaneous and uterine leiomyomas as well as renal cell carcinoma (RCC).(1-4) In affected families, leiomyomas of the skin or uterus often occur at relatively high frequency, reported to affect 76% of individuals or 100% women at a mean age of 25 or 30 years, respectively. In comparison, the penetrance for RCC is approximately 20-30%.(3, 5, 6) However, RCCs associated with HLRCC syndrome are clinically aggressive. Many patients present with local or distant metastasis and succumb to disease within less than 5 years from initial diagnosis.(3, 7) In addition, unlike other familial RCC syndromes typically developing multiple and bilateral tumors, RCCs in the HLRCC setting can be solitary and unilateral, providing little clue to their hereditary nature.
Histologically, while HLRCC tumors in the early literature were described mostly as type 2 papillary RCC and occasionally as collecting duct carcinoma (1-4), a spectrum of architectural patterns including papillary, tubulopapillary, tubular, solid and cystic elements have been described(5, 7). The seminal work by Merino et al. (7) proposed the morphologic hallmark of HLRCC renal tumors to be the characteristic nuclear features: a large nucleus with a very prominent inclusion-like eosinophilic nucleolus that is surrounded by a perinucleolar halo. Considering the architectural and nuclear features, the differential diagnosis of HLRCC renal tumors could include a variety of high-grade RCCs of different histologic subtypes, particularly the sporadic papillary RCC with type 2 features, collecting duct carcinoma (CDC) or high-grade RCC, unclassified. Immunohistochemistry employing common markers used for the classification of RCC have not identified a specific maker for HLRCC renal tumors. (7)
FH is one of the key enzymes in the Krebs cycle and catalyzes the conversion of fumarate to malate. In HLRCC-associated tumors, a “second-hit” or somatic inactivation of the remaining FH allele causes a functional loss of FH that leads to abnormal intracellular accumulation of fumarate. Interestingly, the increased level of fumarate in cells can spontaneously react with cysteine sulfhydryl group of proteins to form a stable chemical modification, S-(2-succino)-cysteine (2SC), by a process called protein succination.(8-10) This modification can be detected by a polyclonal antibody developed against 2SC in cells with increased levels of fumarate, such as adipocytes grown in high-glucose medium as a model of type 2 diabetes.(9) Based on this finding, Bardella et al. showed that the presence of 2SC positivity by immunohistochemistry predicted genetic alterations of FH gene in patients referred for genetic testing, and this immunoreactivity was absent in a diverse group of non-HLRCC related tumors (11). However, this study has not been independently validated.
In this study, we report our experience in HLRCC renal tumors with clinical presentations mimicking sporadic RCCs, and describe their expanding clinicopathologic spectrum. We also explore the 2SC immunohistochemical characteristics in HLRCC tumors as well as a variety of high grade RCCs that enter the differential diagnosis, particularly those with nuclear features imitating HLRCC. We further investigate the correlation between 2SC immunohistochemistry and FH molecular alterations.
Materials and Methods
Patients
Based on the observations of Merino et al. (7), since 2008 we have been raising the possibility of RCC being associated with the HLRCC syndrome in our pathology reports when a primary or metastatic renal tumor demonstrated the characteristic morphological features of HLRCC described in that study (7). Germline genetic testing triggered by this pathologic suspicion allowed us to identify 7 molecularly confirmed cases of HLRCC. We were also able to identify 2 additional cases that presented before 2008 in a retrospective histologic and molecular analysis of RCC, unclassified. All available archival material and gross pathologic reports of these 9 cases were reviewed. Two of the 9 cases were from the consultation files of one of the authors. Clinical data were obtained from a prospectively maintained institutional urology database, or from the submitting clinicians (consultation cases). Gross pathology reports and radiology findings provided data regarding tumor laterality, size and multifocality. Tumor stage was determined using all available clinical and pathologic information and was assigned using the 2010 TNM staging system of the American Joint Committee on Cancer. (12)
2SC Immunohistochemistry
The generation of the 2SC polyclonal antibody and confirmation of its specificity for succinated proteins has previously been described.(9) This antibody is not yet commercially available. Immunohistochemical staining for 2SC was performed using this polyclonal antibody following the method described by Bardella et al.(11). Briefly, antigen retrieval was performed by microwaving in citrate buffer, pH 6.0, for 15 minutes. The tissues were then blocked for endogenous peroxidase, incubated with 2SC polyclonal antibody (1:5000), washed in PBS (0.1% Tween20), and followed by incubation with anti-rabbit HRP polymer (Envision, Dako) for 30 minutes. Staining was assessed for intensity (1+ to 3+) and staining pattern (nuclear and cytoplasmic vs. cytoplasmic). Renal tumors from genetically confirmed HLRCC patients were used as positive controls in all experiments.
Besides confirmed HLRCC cases, 2SC immunohistochemistry was performed on multiple tissue microarrays and whole tissue sections representing184 clear cell, 45 papillary with type 2 features and 97 high-grade unclassified RCC tumors, as well as two Xp11.2 (TFE3) translocation-associated RCCs and two renal medullary carcinomas. Among these cases, except a portion of clear cell RCC, the majority of type 2 papillary and high-grade unclassified RCC cases had at least focally prominent nucleoli that could raise concern for HLRCC tumors. Selected cases of translocation-associated RCC and renal medullary carcinoma with very prominent nucleoli were included. The “type 2” features of papillary RCC were defined as larger tumor cells, with eosinophilic cytoplasm, nuclear pseudostratification, and high nuclear grade.(13, 14) The diagnosis of TFE3 translocation-associated RCC was confirmed by TFE3 immunohistochemistry and fluorescence in-situ hybridization (FISH) test. Both renal medullary carcinoma cases showed loss of INI1 by immunohistochemistry.
Molecular Analysis
Frozen or formalin-fixed, paraffin-embedded (FFPE) tissue samples were collected from 16 RCC patients undergoing radical or partial nephrectomy at our institution. All 16 renal tumors exhibited high grade nuclear features and very prominent nucleoli. All patients had signed informed consent for tissue utilization, and the study was approved by our institutional research board. Tissues were macro-dissected to ensure >70% tumor content. DNA was extracted from tumor or matched normal tissue using DNeasy Blood and Tissue Kit or QIAamp DNA FFPE Tissue Kit (Qiagen, Valencia, CA) for frozen or FFPE tissue, respectively, according to the manufacturer's instructions.
FH gene status was examined using the IMPACT assay (Integrated Mutation Profiling of Actionable Cancer Targets) (15, 16). This assay uses solution phase hybridization-based exon capture and massively parallel DNA sequencing to capture all protein-coding exons and selected introns of 230 oncogenes, tumor suppressor genes, and members of pathways deemed actionable by targeted therapies as previously described. Briefly, 93-500ng genomic DNA was used to construct barcoded sequence libraries (New England Biolabs, Kapa Biosystems, NEXTflex), pooled at 100ng/library, and subjected to exon capture by hybridization (Nimblegen SeqCap). Captured pools were subsequently sequenced on an Illumina HiSeq 2000 to generate paired-end 75-bp reads. Sequence data were analyzed to identify three classes of somatic alterations: single-nucleotide variants (MuTect(17)), small insertions/deletions (indels, GATK(18)), and copy number alterations. Copy number was computed using tumor:normal ratios of normalized coverage data to determine amplifications and deletions(16). All candidate FH somatic alterations and FH locus in paired normal controls were reviewed manually using the Integrative Genomics Viewer(19).
Results
Clinical Presentation of HLRCC Renal Tumors Encountered in a Sporadic Setting
Overall, we identified a group of 9 HLRCC patients with renal tumors who were subsequently confirmed to harbor FH germline mutations either by genetic testing or molecular analysis of their tumors. The clinical characteristics of these cases are summarized in Table 1. Briefly, there were 5 men and 4 women with a mean age of 36 years (range 24-61y). No skin leiomyomas were revealed in past history or documented by physical examination at the initial presentation. One patient had previously resected uterine fibroids at age 32, 5 years before presentation with her renal tumor.(20) Imaging studies of another 32-year-old patient showed uterine fibroids at the time of presentation. Two patients had a family history of RCC in first-degree relatives. Other types of cancer (ovarian, breast and lung) in parents were reported in 3 cases. None of the patients presented with a known family history of HLRCC or was specifically suspected for this syndrome by clinicians at the initial presentation. All patients underwent radical or partial nephrectomy, and were found to have at least a pT3 tumor and regional lymph node involvement. Seven out of the 9 patients developed distant metastases involving lung, liver, bone and other sites, and 5 patients died of the disease during a mean follow-up period of 15 months (median 10 m).
Table 1.
Clinical characteristics of 9 genetically confirmed HLRCC patients with renal tumors
| Pt | Sex | Age | Symptoms | Leiomyoma | Family Hx at Presentation | Kidney Mass | Size (cm) | pT | Metastasis | Follow-Up (Month) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 37 | Flank pain | Uterine at 32 | None | L /solitary | 4.9 | pT3a | RPLNs | DOD (44) |
| 2 | M | 38 | Flank pain, hematuria | None | None | R/solitary | 12.5 | pT3a | RPLNs, adrenal, liver, lung, bone | DOD (28) |
| 3 | F | 32 | Abdominal discomfort | Uterine (imaging only) | N/A (adopted) | R/ solitary | 9.2 | pT3a | RPLNs, lung, liver | DOD (23) |
| 4 | M | 24 | Flank pain | None | Mother ovarian ca | L /solitary | 5 | pT3a | RPLNs, lung | AWD (10) |
| 5 | M | 61 | Flank pain, hematuria | None | Father RCC, mother breast ca, daughter fibroid | L /two masses | 15; 3 | pT3a | RPLNs, adrenal, liver | DOD (8) |
| 6 | M | 42 | Flank pain, hematuria | None | None | L /solitary | 6.5 | pT3a | RPLNs, adrenal, liver | DOD (8) |
| 7 | M | 34 | Incidental renal mass | None | Father non-small cell lung ca | L /solitary | 12.2 | pT3a | RPLNs | AWD (10) |
| 8 | F | 30 | Flank pain | None | None | R /solitary | 6.7 | pT3a | RPLNs, adrenal | AWD (12) |
| 9 | F | 25 | Flank pain | Yes (but not identified initially) | Mother RCC | R/solitary | 11.5 | pT3a | RPLNs | NED (6) |
RPLNs: retroperitoneal lymph nodes; DOD: death of disease; AWD: alive with disease; RCC: renal cell carcinoma; ca:cancer
Based on histological features, the possibility of HLRCC was raised by pathologic examination at the time of diagnosis in 7 cases, which led to subsequent genetic counseling and identification of FH germline mutations. Compared to routine clinical encounters, genetic counseling in these patients revealed more evidence suggesting an association with the HLRCC syndrome. In 6 of these patients additional histories of prior cutaneous lesions, uterine fibroids and RCC in second- or third-degree relatives were elicited in 3, 1 and 2 cases, respectively.
HLRCC Renal Tumors Exhibit an Expanding Morphologic Spectrum
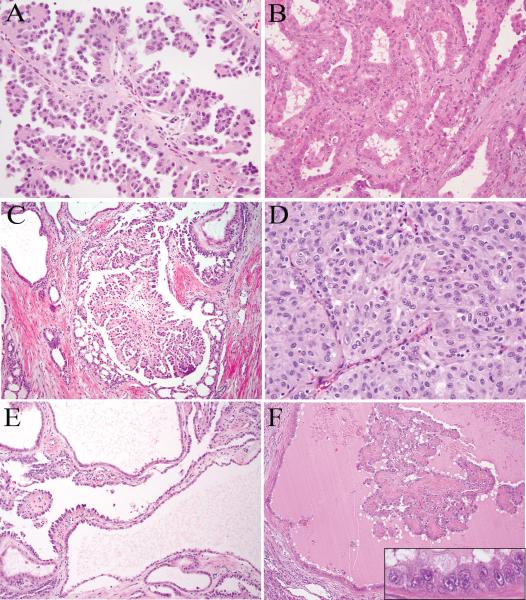
Histologically, these tumors displayed a spectrum of architectural patterns. As previously described by Merino et al.(7), papillary, tubular, tubulopapillary and solid patterns were frequently seen (Fig. 1A-D). Five cases had cystic areas that were often associated with intracystic papillary or tubulopapillary elements (Fig. 1E-F). Mixed architectural patterns were noted in all 9 tumors (Table 2). Papillary as the dominant pattern was seen in only 3 tumors. The dominant patterns in other tumors were tubulopapillary (n=5) and solid (n=1). The papillary, tubulopapillary, tubular and cystic areas were often intermixed. Notably, the fibrovascular cores in papillae were often hyalinized (Fig. 1F) or showed edematous changes, while only rarely containing foamy histiocytes. Micropapillary fronds were also frequently seen in papillary elements (Fig. 1A).
FIGURE 1.
HLRCC renal tumors show papillary (A), tubular (B), tubulopapillary (C) and solid (D) architectural patterns. Cysts are lined by single to multi-layered tumor cells, and often associated with intracystic papillary growth (E and F). The characteristic nuclear features of HLRCC tumors, a viral inclusion-like eosinophilic nucleolus surrounded by a clear halo, can be appreciated in cells lining the cysts at high magnification (F inset).
Table 2.
Architectural patterns of HLRCC renal tumors
| Pt | Papillary | Tubular | Tubulopapillary | Solid | Cystic | CDC-like infiltration | Tubulocystic | Sarcomatoid | Vacuolated/cribriform |
|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | + | + | + | + | |||
| 2 | + | + | + | + | |||||
| 3 | + | + | + | + | + | + | |||
| 4 | + | + | + | ||||||
| 5 | + | + | + | + | |||||
| 6 | + | + | + | ||||||
| 7 | + | + | + | ||||||
| 8 | + | + | + | + | + | + | + | + | |
| 9 | + | + | + | ||||||
| 7/9 | 4/9 | 5/9 | 5/9 | 6/9 | 6/9 | 3/9 | 1/9 | 3/9 | |
CDC: collecting duct carcinoma
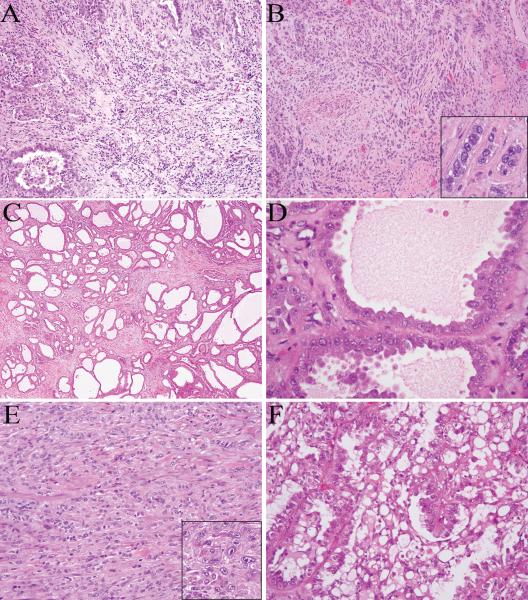
CDC-like areas were found to be common in this cohort, with tubules, solid nests or individual cells infiltrating a desmoplastic stroma (Fig. 2A-B). Intratumoral inflammatory infiltrates were also prominent in 3 of these 6 tumors. Additionally, some tumors contained a tubulocystic component that closely resembled tubulocystic carcinoma, with variably sized tubules, some cystically dilated, in a hypocellular and fibrotic stroma (Fig. 2C-D). The lining cells in the tubulocystic component showed prominent nucleoli and focally a hobnail appearance. Although this tubulocystic pattern was prominent and presented as distinct areas or tumor nodules, other growth patterns such as tubulopapillary, solid or infiltrating tubules were identified in the remaining portions of all 3 tumors. In addition, sarcomatoid growth was identified in one tumor, consisting of sheets of spindle cells (Fig. 2E). Moreover, 3 cases showed prominent intracytoplasmic and/or intercellular vacuoles, imparting a sieve-like or cribriform appearance (Fig. 2F). This sieve-like/cribriform pattern was seen in areas of all growth patterns, including tubular, papillary, solid and cystic.
FIGURE 2.
HLRCC renal tumors contain collecting duct carcinoma-like (A-B), tubulocystic (C-D), or sarcomatoid (E) areas. They also can show prominent intracytoplasmic and intercellular vacuoles, imparting a cribriform appearance (F). The characteristic nuclear features are present in scattered cells at high magnification (B and E insets).
The cytoplasm of the tumor cells was predominantly eosinophilic; however, focal areas containing more amphophilic or clear cytoplasm were also noted. While all tumors exhibited the proposed hallmark of HLRCC, a large viral inclusion-like eosinophilic nucleolus surrounded by a clear halo, this feature was often not uniformly present throughout the tumor (Fig. 1-2). Within areas of a given tumor, the characteristic nuclear finding could be apparent only in scattered cells, while other tumor cells displayed smaller nucleoli without a distinct perinucleolar halo, thus requiring careful microscopic evaluation in tumors with such focal changes.
2SC Immunoreactivity in HLRCC and Other High Grade RCCs with Prominent Nucleoli
Given this broad architectural and cytological spectrum noted in HLRCC tumors, the differential diagnosis could include a variety of high grade RCCs exhibiting prominent nucleoli. While the inclusion-like nucleoli and perinucleolar halo of HLRCC renal tumors were quite unique, in our experience, occasionally a non-HLRCC high-grade RCC can also focally show macro-nucleoli with peri-nucleolar clearing, mimicking the characteristics of HLRCC. To test the utility of 2SC immunohistochemistry in helping distinguish HLRCC renal tumors from other high grade RCCs, 2SC immunoreactivity was performed in HLRCC renal tumors and the results compared with 184 clear cell RCC, 45 papillary RCC with type 2 features and 97 high-grade unclassified RCC cases, as well as TFE3 translocation RCC and renal medullary carcinoma that showed nuclear features raising concern for HLRCC.
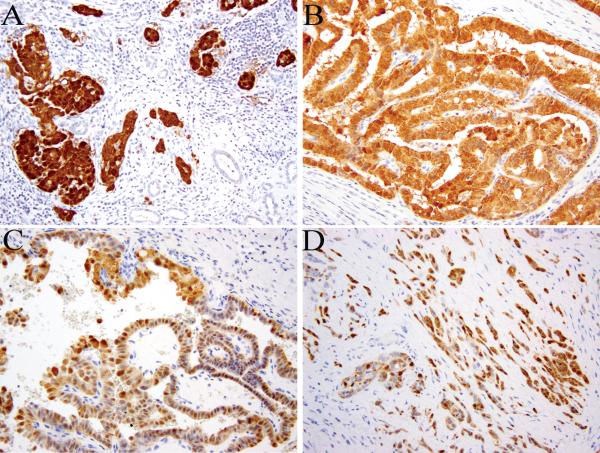
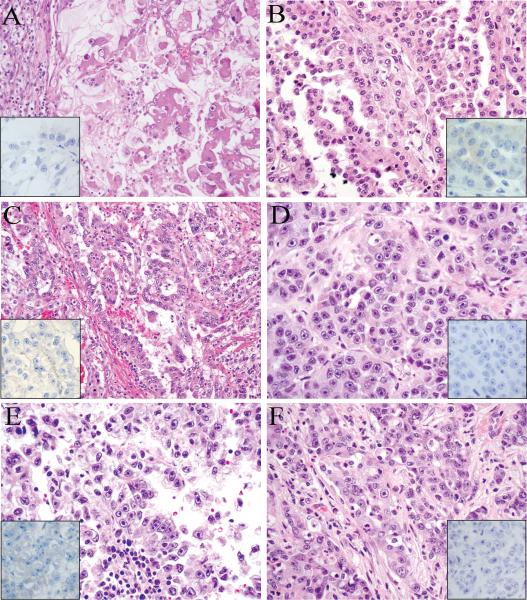
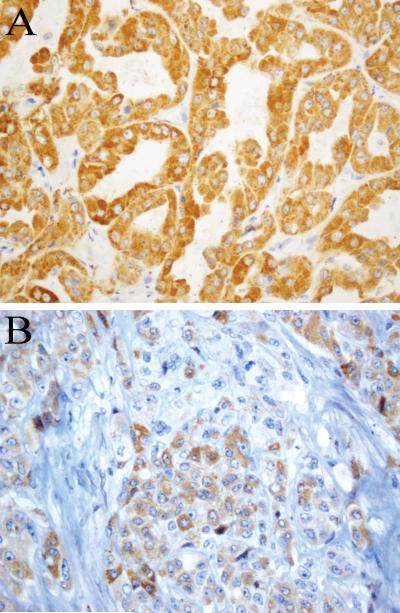
All confirmed HLRCC tumors (9/9, 100%) demonstrated diffuse and strong staining for 2SC, while the adjacent renal parenchyma was negative (Fig. 3). In contrast, all clear cell RCC cases (184/184, 100%), almost all high-grade unclassified RCC (93/97, 96%) and the large majority of type 2 papillary (35/45, 78%) cases showed no 2SC immunoreactivity. Two TFE3 translocation-associated tumors and two renal medullary carcinomas were also negative. This lack of 2SC immunoreactivity was observed for the majority of tumors in these groups that displayed nuclear features imitating HLRCC tumors (Fig. 4).
FIGURE 3.
2SC immunohistochemistry shows diffuse and strong reactivity in HLRCC renal cancer. The staining is both nuclear and cytoplasmic (A-D).
FIGURE 4.
Clear cell RCC (A), type 2 papillary RCC (B-C), unclassified RCC (D), TFE3 translocation RCC (E), and renal medullary carcinoma (F) with nuclear features mimicking HLRCC renal cancer. The 2SC immunohistochemistry in these cases are all negative (insets).
Only a subset of type 2 papillary (10/45, 22%) and high-grade unclassified (4/97, 5%) tumors showed patchy or diffuse 2SC immunostaining. Interestingly, unlike the nuclear and cytoplasmic staining pattern seen in all 9 confirmed HLRCC tumors, 2SC staining pattern in these cases was predominantly cytoplasmic (Fig. 5).
FIGURE 5.
2SC immunohistochemistry shows a cytoplasmic staining pattern in a few unclassified RCC (A) and type 2 papillary RCC (B) cases.
Correlation between 2SC Immunoreactivity and FH Molecular Aberrations
To further evaluate the correlation between 2SC immunohistochemistry and a diagnosis of HLRCC renal tumors or presence of other potential molecular aberrations of FH gene, we conducted molecular analysis of FH gene by targeted deep sequencing in 16 cases demonstrating HLRCC or HLRCC-like nuclear features and showing various 2SC staining patterns. Additionally, FH genetic testing results were available in another 7 cases. These results are summarized in Table 3.
Table 3.
2SC immunohistochemistry and FH molecular alterations detected in HLRCC and other renal tumors exhibiting HLRCC nuclear features
| Case | Diagnosis | HLRCC Nuclear Features | 2SC Intensity | 2SC Staining Pattern | FH Germline Alteration | FH Somatic Alteration |
|---|---|---|---|---|---|---|
| 1 | HLRCC | Yes | +++ | diffuse, nuclear and cytoplasmic | c.1189G>A (p.G397R) | p.S419* |
| 2 | HLRCC | Yes | +++ | diffuse, nuclear and cytoplasmic | c.568delAC (p.T190fs) | p.234_235insT |
| 3 | HLRCC | Yes | +++ | diffuse, nuclear and cytoplasmic | c.1431insAAA (p.477_478insK) | LOH |
| c.683T>A (p.I228N) | LOH | |||||
| 4 | HLRCC | Yes | +++ | diffuse, nuclear and cytoplasmic | c.1020T>A (p.N340K) | N/A |
| 5 | HLRCC | Yes | +++ | diffuse, nuclear and cytoplasmic | c.1138insA (p.M380fs) | N/A |
| 6 | HLRCC | Yes | +++ | diffuse, nuclear and cytoplasmic | c.1083-1086delTGAA (p.N361delEfs) | N/A |
| 7 | HLRCC | Yes | +++ | diffuse, nuclear and cytoplasmic | c.1189G>A (p.G397R) | N/A |
| 8 | HLRCC | Yes | +++ | diffuse, nuclear and cytoplasmic | c.698G>A (p.R233H) | N/A |
| 9 | HLRCC | Yes | +++ | diffuse, nuclear and cytoplasmic | p.R233H | N/A |
| 10 | Unclassified RCC | Yes | +++ | diffuse, cytoplasmic | None found | No mutation or loss |
| 11 | Unclassified RCC | Yes | +++ | diffuse, cytoplasmic | None found | No mutation or loss |
| 12 | Unclassified RCC | Yes | ++ | diffuse, cytoplasmic | None found | N/A |
| 13 | Type 2 PRCC | Yes | ++ | patchy, cytoplasmic | None found | No mutation or loss |
| 14 | Type 2 PRCC | Yes | + | focal, weak cytoplasmic | None found | No mutation or loss |
| 15 | Type 2 PRCC | Yes | - | - | None found | No mutation or loss |
| 16 | Unclassified RCC | Yes | - | - | None found | No mutation or loss |
| 17 | Unclassified RCC | Yes | - | - | None found | No mutation or loss |
| 18 | Unclassified RCC | Yes | - | - | None found | No mutation or loss |
| 19 | Unclassified RCC | Yes | - | - | None found | No mutation or loss |
| 20 | Unclassified RCC | Yes | - | - | None found | No mutation or loss |
| 21 | Unclassified RCC | Yes | - | - | None found | No mutation or loss |
| 22 | Unclassified RCC | Yes | - | - | None found | No mutation or loss |
| 23 | Unclassified RCC | Yes | - | - | None found | No mutation or loss |
PRCC: papillary renal cell carcinoma; LOH: loss of heterozygosity
For confirmed HLRCC cases, besides the germline FH mutations, a “second-hit”, either a second somatic mutation of FH gene or loss of heterozygosity (LOH) was identified in 3 cases for which we also analyzed somatic alterations in tumor tissue,. All other 14 cases sequenced, despite displaying nuclear features mimicking HLRCC, had no germline or somatic alterations of FH gene, nor did they show the same 2SC staining pattern as in confirmed HLRCC tumors. These included the 3 unclassified RCC and 2 type 2 papillary RCC cases that exhibited diffuse or patchy cytoplasmic 2SC immunoreactivity, as well as another 9 cases that were negative for 2SC staining.
Discussion
First described by Launonen et al. (1) in 2001, the HLRCC syndrome is now known to confer an increased risk of renal cell carcinomas and smooth muscle tumors of the skin and uterus (21). Within the spectrum of this syndromic disease, renal carcinoma is the most aggressive presentation and cause of mortality. Therefore, pathological suspicion or diagnosis of HLRCC-associated RCC is critical for appropriate clinical management and genetic counseling of the family members. Recently, HLRCC renal cancer has been recommended to be recognized as a distinct entity in the International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia.(22) However, these are often solitary tumors exhibiting a broad morphologic spectrum, posing a difficult diagnostic challenge. In our experience, it is not uncommon for a renal mass to be the first recognized manifestation that brings clinical attention to a patient's genetic syndrome, possibly due to the asymptomatic nature of many of the cutaneous leiomyomas and the relative frequency of uterine leiomyomas in the general population. Families presenting with RCC only have also been reported in the literature.(6)
In this study, we summarize our experience in diagnosing HLRCC renal tumors that presented as sporadic RCCs, and describe their expanding clinicopathologic features. All of the confirmed HLRCC renal tumor cases in our cohort had no known family history of HLRCC at their initial presentations. Consistent with the incomplete penetrance of renal cancers in this syndrome, only 2 of the 9 patients had a family history of RCC in first-degree relatives. Other types of cancer (e.g. lung, breast etc.) that have not been associated with HLRCC syndrome were seen in some family members. Although skin or uterine leiomyomas tend to have high penetrance compared to renal tumors, it was often difficult to elicit this history or evidence of these lesions in routine clinical encounters, particularly when there are no obvious symptoms. This finding is in line with the previous reports that about 40% HLRCC families only had either mild cutaneous presentations (< 5 leiomyomas) or lacked skin lesions. (3, 5) In cases when there was little or no clinical evidence suggesting HLRCC, a suspicion raised by pathologists based on histologic features was often the key step leading to additional clinical work-up and genetic counseling that established the diagnosis.
Compared to sporadic RCC in general, patients with HLRCC renal tumors presented at a younger age (mean 36 years). However, this disease is not limited to young patients: the oldest patient in our cohort was 61-year-old; and in a previous study (7), 9 out of 38 (24%) patients were older than 50. These renal tumors frequently presented at an advanced pathologic stage, with involvement of regional lymph nodes being a common finding at nephrectomy. Some authors have suggested that the morphology of these tumors is that of type 2 papillary RCC. In this cohort, we found that RCC-associated with this syndrome frequently displayed mixed architectural patterns, similar to that described by Merino et al.(7) In fact, patterns other than papillary were frequently the dominant component. Besides papillary, tubulopapillary, tubular, solid and cystic elements, CDC-like areas with infiltrating tubules, nests or individual cells in a desmoplastic stroma were identified in more than half of the cases, emphasizing the morphologic similarities between HLRCC renal tumors and CDCs.
Similarly, three cases contained tubulocystic areas closely resembling tubulocystic carcinoma, yet all containing other components (tubulopapillary and papillary patterns). This coexistence of tubulocystic carcinoma-like elements with other architectural patterns in HLRCC renal tumors suggests it being considered as an important differential diagnosis for cases described in the literature as tubulocystic carcinoma with mixed papillary, solid or dedifferentiated components. (23-26) It remains unknown whether HLRCC renal tumors could consist exclusively or almost exclusively of a tubulocystic element, although most of the tubulocystic carcinomas described are pure, well-circumscribed tumors with overall good prognosis.(27) In an array comparative genomic hybridization (aCGH) study of renal tumors associated with HLRCC(28), about 30-40% HLRCC tumors appeared to harbor chromosomal 7 or 17 gains, suggesting that trisomy 7 or 17 by FISH is not a reliable marker to separate HLRCC renal cancer from sporadic papillary RCC or tubulocystic carcinoma.
Additionally, we observed a cribriform pattern (some due to abundant intracytoplasmic or intercellular vacuoles), clear cell features and sarcomatoid differentiation within the morphologic spectrum of genetically confirmed HLRCC tumors. These features as well as the mixed architectural patterns raise multiple differential diagnostic possibilities, including renal medullary carcinoma, Xp11 or t (6, 11) translocation RCC, high-grade clear cell RCC and high-grade unclassified RCC.
While the most distinctive morphological feature of HLRCC tumors, a prominent eosinophilic nucleolus with a clear perinucleolar halo, was indeed present in all genetically confirmed cases, its presence was non-uniform. Confounding the diagnostic difficulty, other high-grade RCC in the differential diagnosis of HLRCC almost all had prominent nucleoli, occasionally showing HLRCC-like nuclear features (Fig. 4), although usually limited to focal areas or in scattered cells. With the exception of a few specific ancillary studies, such as INI1 (SMARCB1) loss by immunohistochemistry and TFE3/TFEB immunostaining or FISH tests, immunohistochemical panels used for the classification of renal neoplasms are generally not very helpful for distinguishing HLRCC tumors from these other high-grade RCC cases.
We investigated the utility of 2SC immunohistochemistry, a marker to detect the aberrant succination of proteins in cells with high levels of fumarate, in this challenging diagnostic scenario, and correlated the 2SC immunoreactivity to the presence of FH germline mutation. Among the various subtypes of RCC we tested, 2SC immunostaining appeared to be a very sensitive marker for HLRCC tumors. All confirmed cases showed diffuse and strong nuclear and cytoplasmic staining; conversely, none of the tumors with negative 2SC staining in the molecularly characterized subset was found to harbor FH germline alterations. This high sensitivity is in keeping with the finding reported by Bardella et al. (11). Additionally, we identified a small subset of unclassified and type 2 papillary RCC that exhibited immunoreactivity to 2SC, but did not harbor FH germline or somatic alterations. However, the 2SC immunoreactivity observed in this subset was only cytoplasmic staining, unlike that found in HLRCC tumors (nuclear and cytoplasmic). Although further validation is required, if one uses the latter pattern (nuclear and cytoplasmic labeling) as the criterion for judging 2SC in the setting of diagnosing HLRCC tumors, then 2SC immunohistochemistry supports a diagnosis of HLRCC in a highly specific manner.
In contrast to typical immunohistochemistry assays in which a peptide/protein is the specific antigen, 2SC immunohistochemistry stains all the proteins with the stable 2SC modification (succination). Therefore, in different cellular contexts, the staining pattern could vary. A recent study utilized a proteomic-based screen and identified 94 protein succination targets in cells and renal cysts of Fh1 (murine FH)-deficient mice.(29) These targets included a broad range of nuclear, cytoplasmic and mitochondrial proteins, suggesting nuclear protein succination could be a feature of HLRCC tumors. Whether the non-nuclear 2SC staining pattern we identified in the small number of non-HLRCC cases represents a different mechanism of fumarate accumulation or is due to other unknown causes, requires future investigation. In addition, while we found the 2SC immunohistochemistry to be a very helpful aid in diagnosing HLRCC tumors, the antibody is not yet commercially available and the assay is only being performed in a limited number of laboratories.
The molecular characterization we conducted revealed somatic inactivation of the remaining FH allele in HLRCC renal cancer, which is consistent with the role of FH as a tumor suppressor gene. Its loss of function in the tumor cells corresponded to strong 2SC staining, whereas adjacent renal parenchyma maintained a wild-type allele and did not show 2SC immunoreactivity. Based on a limited sample in this cohort, somatic mutation of FH seems to be a rare event in non-HLRCC renal tumors that showed nuclear features imitating HLRCC.
In summary, our results suggests that a combination of careful histological examination and 2SC immunohistochemistry can greatly increase our ability to recognize HLRCC-associated RCC, a group of tumors with an expanding histologic spectrum that mimic many other high-grade renal tumors. As a useful ancillary tool, 2SC immunohistochemistry helps us improve the specificity when we raise a suspicion for this aggressive hereditary condition mainly based on pathologic features. While the ultimate clinical diagnosis of a HLRCC syndrome still requires genetic testing, pathologic examination plays a pivotal role in facilitating the identification and clinical management of these patients.
Acknowledgments
The work received funding from Cycle for Survival of Memorial Sloan-Kettering Cancer Center (to Y.B.C.). A.R.B. is supported by National Cancer Institute of the National Institutes of Health under award number 5T32CA160001.
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Launonen V, Vierimaa O, Kiuru M, et al. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci U S A. 2001;98:3387–3392. doi: 10.1073/pnas.051633798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomlinson IP, Alam NA, Rowan AJ, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406–410. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 3.Toro JR, Nickerson ML, Wei MH, et al. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. American journal of human genetics. 2003;73:95–106. doi: 10.1086/376435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alam NA, Rowan AJ, Wortham NC, et al. Genetic and functional analyses of FH mutations in multiple cutaneous and uterine leiomyomatosis, hereditary leiomyomatosis and renal cancer, and fumarate hydratase deficiency. Hum Mol Genet. 2003;12:1241–1252. doi: 10.1093/hmg/ddg148. [DOI] [PubMed] [Google Scholar]
- 5.Wei MH, Toure O, Glenn GM, et al. Novel mutations in FH and expansion of the spectrum of phenotypes expressed in families with hereditary leiomyomatosis and renal cell cancer. J Med Genet. 2006;43:18–27. doi: 10.1136/jmg.2005.033506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardie B, Remenieras A, Kattygnarath D, et al. Novel FH mutations in families with hereditary leiomyomatosis and renal cell cancer (HLRCC) and patients with isolated type 2 papillary renal cell carcinoma. J Med Genet. 2011;48:226–234. doi: 10.1136/jmg.2010.085068. [DOI] [PubMed] [Google Scholar]
- 7.Merino MJ, Torres-Cabala C, Pinto P, et al. The morphologic spectrum of kidney tumors in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome. Am J Surg Pathol. 2007;31:1578–1585. doi: 10.1097/PAS.0b013e31804375b8. [DOI] [PubMed] [Google Scholar]
- 8.Alderson NL, Wang Y, Blatnik M, et al. S-(2-Succinyl)cysteine: a novel chemical modification of tissue proteins by a Krebs cycle intermediate. Archives of biochemistry and biophysics. 2006;450:1–8. doi: 10.1016/j.abb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Nagai R, Brock JW, Blatnik M, et al. Succination of protein thiols during adipocyte maturation: a biomarker of mitochondrial stress. J Biol Chem. 2007;282:34219–34228. doi: 10.1074/jbc.M703551200. [DOI] [PubMed] [Google Scholar]
- 10.Merkley ED, Metz TO, Smith RD, et al. The succinated proteome. Mass spectrometry reviews. 2013 doi: 10.1002/mas.21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bardella C, El-Bahrawy M, Frizzell N, et al. Aberrant succination of proteins in fumarate hydratase-deficient mice and HLRCC patients is a robust biomarker of mutation status. J Pathol. 2011;225:4–11. doi: 10.1002/path.2932. [DOI] [PubMed] [Google Scholar]
- 12.Edge SBBD, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. Springer; New York, NY: 2010. [Google Scholar]
- 13.Delahunt B, Eble JN. Papillary renal cell carcinoma: a clinicopathologic and immunohistochemical study of 105 tumors. Mod Pathol. 1997;10:537–544. [PubMed] [Google Scholar]
- 14.Eble JN, Sauter G, Epstein JI, et al., editors. Tumours of the urinary system and male genital organs. IARC Press; Lyon: 2004. [Google Scholar]
- 15.Iyer G, Hanrahan AJ, Milowsky MI, et al. Genome sequencing identifies a basis for everolimus sensitivity. Science. 2012;338:221. doi: 10.1126/science.1226344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Won HH, Scott SN, Brannon AR, et al. Detecting Somatic Genetic Alterations in Tumor Specimens by Exon Capture and Massively Parallel Sequencing. J Vis Exp. 2013:e50710. doi: 10.3791/50710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cibulskis K, Lawrence MS, Carter SL, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nature biotechnology. 2013;31:213–219. doi: 10.1038/nbt.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DePristo MA, Banks E, Poplin R, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson JT, Thorvaldsdottir H, Winckler W, et al. Integrative genomics viewer. Nature biotechnology. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garg K, Tickoo SK, Soslow RA, et al. Morphologic features of uterine leiomyomas associated with hereditary leiomyomatosis and renal cell carcinoma syndrome: a case report. Am J Surg Pathol. 2011;35:1235–1237. doi: 10.1097/PAS.0b013e318223ca01. [DOI] [PubMed] [Google Scholar]
- 21.Lehtonen HJ. Hereditary leiomyomatosis and renal cell cancer: update on clinical and molecular characteristics. Familial cancer. 2011;10:397–411. doi: 10.1007/s10689-011-9428-z. [DOI] [PubMed] [Google Scholar]
- 22.Srigley JR, Delahunt B, Eble JN, et al. The International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia. Am J Surg Pathol. 2013;37:1469–1489. doi: 10.1097/PAS.0b013e318299f2d1. [DOI] [PubMed] [Google Scholar]
- 23.Zhou M, Yang XJ, Lopez JI, et al. Renal tubulocystic carcinoma is closely related to papillary renal cell carcinoma: implications for pathologic classification. Am J Surg Pathol. 2009;33:1840–1849. doi: 10.1097/PAS.0b013e3181be22d1. [DOI] [PubMed] [Google Scholar]
- 24.Hora M, Urge T, Eret V, et al. Tubulocystic renal carcinoma: a clinical perspective. World J Urol. 2011;29:349–354. doi: 10.1007/s00345-010-0614-7. [DOI] [PubMed] [Google Scholar]
- 25.Deshmukh M, Shet T, Bakshi G, et al. Tubulocystic carcinoma of kidney associated with papillary renal cell carcinoma. Indian journal of pathology & microbiology. 2011;54:127–130. doi: 10.4103/0377-4929.77363. [DOI] [PubMed] [Google Scholar]
- 26.Al-Hussain TO, Cheng L, Zhang S, et al. Tubulocystic carcinoma of the kidney with poorly differentiated foci: a series of 3 cases with fluorescence in situ hybridization analysis. Hum Pathol. 2013;44:1406–1411. doi: 10.1016/j.humpath.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Amin MB, MacLennan GT, Gupta R, et al. Tubulocystic carcinoma of the kidney: clinicopathologic analysis of 31 cases of a distinctive rare subtype of renal cell carcinoma. Am J Surg Pathol. 2009;33:384–392. doi: 10.1097/PAS.0b013e3181872d3f. [DOI] [PubMed] [Google Scholar]
- 28.Koski TA, Lehtonen HJ, Jee KJ, et al. Array comparative genomic hybridization identifies a distinct DNA copy number profile in renal cell cancer associated with hereditary leiomyomatosis and renal cell cancer. Genes Chromosomes Cancer. 2009;48:544–551. doi: 10.1002/gcc.20663. [DOI] [PubMed] [Google Scholar]
- 29.Ternette N, Yang M, Laroyia M, et al. Inhibition of mitochondrial aconitase by succination in fumarate hydratase deficiency. Cell reports. 2013;3:689–700. doi: 10.1016/j.celrep.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]