Abstract
Objective
To study the efficacy and safety of combining mifepristone before misoprostol use in second trimester to considerably reduce the induction–abortion interval with the lowest possible dose and adverse reaction.
Material and methods
A prospective study was conducted which included 60 patients visiting the antenatal OPD for elective abortions between 13 and 20 weeks of gestation as per the MTP act. They were randomly divided into two groups of 30 each—the study group received mifepristone 200 mg orally before misoprostol, whereas the control group was induced with misoprostol alone. The results were analyzed.
Observation
Statistical analysis of the study was done using χ2 test. The induction–abortion interval was significantly shorter in the study group, thereby decreasing the side-effects of the drug as well as duration of hospital stay.
Conclusion
This study, like many others, offers a reliable, safe, and cost-effective option by combining mifepristone before misoprostol to decrease the induction–abortion interval.
Keywords: Second trimester abortion, Termination of pregnancy, Mifepristone, Misoprostol
Introduction
The efficacy of misoprostol in second trimester abortions has been reported [1–3] Misoprostol, a synthetic prostaglandin E, is an effective abortifacient and uterotonic drug. It is used in the first trimester in conjunction with mifepristone (RU-486), an orally active antiprogesterone, which has been approved by the FDA. During pregnancy, just as in the first trimester, this antiprogesterone drug blocks the progesterone receptors, causes estrogen dominance, and sensitizes the myometrium to the contraction-inducing activity of prostaglandins [4] Therefore, if mifepristone is given prior to induction with misoprostol, then there is disruption of pregnancy causing decidual necrosis, myometrial contractions, and cervical softening resulting in earlier and complete second trimester abortion. Though this combination is not currently FDA-approved, in certain circumstances, the Food and Drug administration Act recognizes that off-label uses of approved products are appropriate, rational, and accepted in medical practice, if based on sound scientific evidence [4].
We undertook this trial to study the efficacy and safety of combining mifepristone before misoprostol use in second trimester to considerably reduce the induction–abortion interval with the lowest possible dose and adverse reaction.
Materials and Methods
A total of 150 cases of second trimester abortions took place during the period of 15 months between May 2011 to July 2012. Out of these, 90 cases were spontaneous abortions, i.e., patients admitted with leaking or bleeding per vaginum or with pain as inevitable abortions.
Ours was a prospective study which included 60 patients visiting the antenatal OPD for elective abortions between 13 and 20 weeks of gestation. They were randomly divided into two groups of 30 each.
The criteria for second trimester MTP were as per the Medical Termination of Pregnancy Act of 1971 revised guidelines. The opinion of two consulting Gynaecologists regarding reasons for MTP was taken and necessary documentation was followed.
Exclusion Criteria
Previous scar on uterus;
Patients with leaking or bleeding per vaginum;
Patients with inevitable or incomplete abortion;
Intrauterine foetal death; and
Previously failed MTP with drugs (considered as incomplete abortion).
Proper counseling was done, and a written informed consent obtained prior to starting the treatment regimens.
Patients in the study group (Group 1): given 200 mg of mifepristone in the OPD on day 1 and advised admission on day 3 or as soon as pain or bleeding started. On admission (48 h) after mifepristone, 400 mg misoprostol was inserted vaginally followed by 200 mg 6 h till abortion occurred.
In the control group-2, only misoprostol was instilled vaginally in a similar dose: 400 mcg followed by 200 mcg 6 h till abortion. Patients were monitored regarding onset of contractions, bleeding, side-effects like fever and shivering, and completion of abortion.
Intravenous antibiotics were administered on admission to all patients after instilling vaginal misoprostol. Check curettage was done post abortion in both the groups as a standard protocol. Inj. Anti-D immunoglobulin (150 mcg) was administered to all those with negative blood group within 72 h of first dose.
Observation
-
A)
The age of patients in both groups ranged between 20 and 38 years, the average being 29.5 years.
-
B)
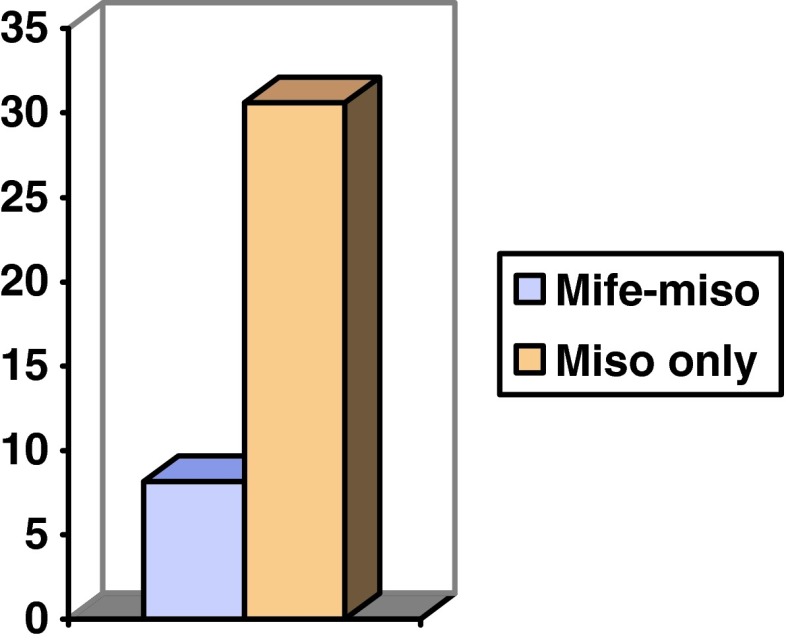
93 % of females from the trial group aborted within 16 h as against 26 % in the control group (Fig. 1). The mean induction–abortion interval was 8 h 15 min in the study group, whereas in the control group, it was more than 24 h. About 16 patients (>50 %), aborted between 24 and 36 h after the first dose (Fig. 2).
-
C)
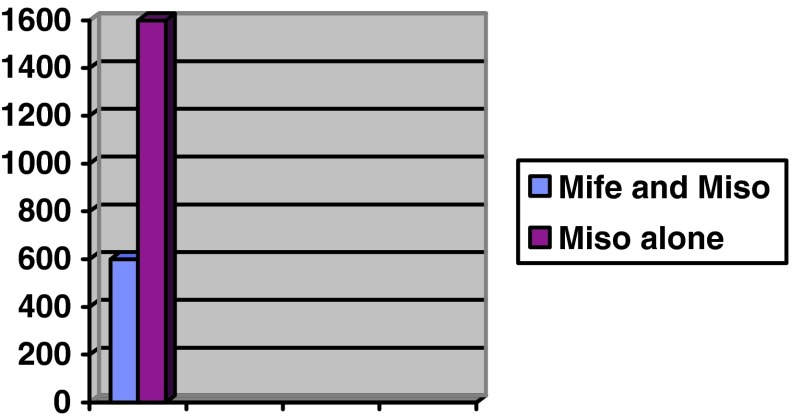
The average dose of misoprostol required in group 1 was 600 mcg, whereas in group 2 it was 1,600 microgram misoprostol (Fig. 3).
-
D)
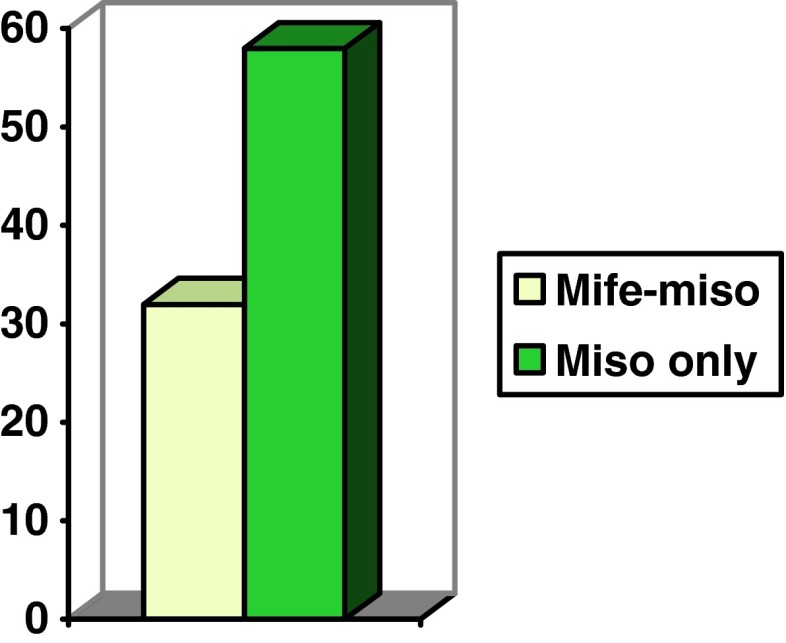
The mean duration of hospital stay (in hours) was 32 h in the study group, whereas it ranged between 36 and 72 h in the control (Fig. 4).
Fig. 1.
Completion of procedure with drugs versus time
Fig. 2.
Comparing induction–abortion interval in hours
Fig. 3.
Dose of Misoprostol reqd
Fig. 4.
Duration of hospital stay in hours in both groups
-
E)The mean parity in
- Study group 60 % were G3 or more, 40 % were primis, or with one previous child.
- Control group 66 % were G3 or more, 34 % were primis, or with one living issue (Fig. 5).
-
F)
The mean period of gestation in both the groups was between 16 and 17 weeks.
-
G)
The amount of blood lost was directly proportional to the period of gestation. Yet, none of the patients required a blood transfusion. Those patients with pre-op or post-op Hb <8 g % (total 6 patients) were given intravenous iron–sucrose post-procedure and discharged on double-oral iron therapy for 3 weeks.
Fig. 5.
Gravidity statistics
-
H)The complications noted were:
- Fever (>2 spikes till abortion): 7 in study group and 19 in control group;
- Shivering: 3 in study group and 17 in control; and
- Failure of MTP (failure to abort >24 h. after first dose of misoprostol): None in study group and 16 in control group. However, only 3 patients failed to abort after 48 h of misoprostol alone, in which case, prostaglandin PGF2α, was used to complete the procedure.
Statistical analysis of the study showed that χ2 test was positive, i.e., The induction–abortion interval was significantly shorter in the study group, thereby decreasing the side-effects of the drug as well as duration of hospital stay.
Conclusion
The incidence of second trimester abortions has significantly reduced off-late, thanks to the PNDT act. Yet, when the condition does not have a favorable outcome, i.e., hazardous to the life of either the foetus or mother, the benefit of pregnancy termination outweighs the risk of continuation. This process, rightly known as mini-labor, is not only painful physically, but also impacts psychologically. It is our concern to reduce this stressful period to the shortest. This study, like many others, offers a reliable, safe, and cost-effective option by combining mifepristone before misoprostol to decrease the induction–abortion interval.
References
- 1.El Rafaey H, Templeton A. Induction of abortion in second trimester by mifepristone and misoprostol: RCT betn 2 misoprostol regimens. Hum Reprod. 1995;10:475–478. doi: 10.1093/oxfordjournals.humrep.a135965. [DOI] [PubMed] [Google Scholar]
- 2.Webster D, Penny GC, Templeton A. A comparison of 600 and 200 mg mifepristone prior to second trimester abortion with PG misoprostol. Br J Obstet Gynaecol. 1996;103:706–709. doi: 10.1111/j.1471-0528.1996.tb09842.x. [DOI] [PubMed] [Google Scholar]
- 3.Nagaria T, Sirmor N. Misoprostol vs mifepristone and misoprostol in second trimester termination of pregnancy. J Obstet Gynecol India. 2012;61:659–662. [DOI] [PMC free article] [PubMed]
- 4.Allen R, O’Brien BM. Uses of misoprostol in obstetrics and gynecology. Rev Obstet Gynecol. 2009;2:159–168. [PMC free article] [PubMed]