Abstract
The aim of this study was to determine the effect of Tai Chi on biological markers of oxidative stress in saliva and its relationship with periodontal disease (PD) in older adults. We carried out a quasi-experimental study with a sample of 71 sedentary volunteers with PD who were divided into a control group of 34 subjects and an experimental group of 37 subjects who performed Tai Chi 5 days a week for a period of 6 months. PD status was characterized using the Periodontal Disease Index (PDI). Superoxide dismutase (SOD), total antioxidant status (TAS), and TBARS levels of both groups were measured by spectrophotometric methods. In addition, inflammation markers (TNF-α, IL-1β, IL-6, IL-8, and IL-10) were measured by flow cytometry. We found a statistically significant increase in SOD activity (P < 0.001) and TAS concentration (P < 0.05), whereas levels of IL-1β were significantly lower (P < 0.01). Likewise, a statistically significant decrease in the PDI (P < 0.05) was observed in subjects who performed Tai Chi during a period of 6 months. Our findings suggest that the practice of Tai Chi has both antioxidant and anti-inflammatory effects that are linked to the improvement of PD in older adults.
1. Introduction
Tai Chi (TC) is a traditional Chinese exercise linked to martial arts that has been shown to have a positive effect on aerobic capacity, muscle strength, balance, and motor control [1]. The practice of TC involves exercises that promote posture, flexibility, relaxation, wellness, and mental concentration [2]. TC is characterized by extremely slow movements, absolute continuity without interruption or pause, and a total awareness and focus on its implementation [3]. Unlike many exercises that are characterized by muscle strength and effort, TC movements are slow, soft, and lightweight [1–3]. TC is classified as a moderate type of exercise, as its intensity does not exceed 55% of an individual's maximum oxygen expenditure and 60% of an individual's maximum heart rate [4].
Recently, the practice of TC has been promoted in the elderly population due to its beneficial health effects, including, among others, the prevention of falls, osteoporosis, hypertension, and diabetes mellitus as well as rheumatological and neurological disorders [2, 5, 6]. Our research group has shown that regular practice of TC increases superoxide dismutase activity and total antioxidant status in the serum of the elderly [7, 8]. We hypothesize that the antioxidant effect of TC may also be observed in saliva and could have a positive effect on the oral health of the elderly. Periodontal disease (PD) is one of the major chronic oral diseases in the elderly and is characterized by a destructive inflammatory process that affects the supporting tissues of the teeth, causing both alveolar bone resorption and formation of periodontal pockets and eventually leads to tooth loss [9, 10]. It has been demonstrated that oxidative stress in the saliva is an etiologic factor and pathophysiologic of PD [11–15]. Therefore, the aim of this study is to determine the effect of Tai Chi on biological markers of oxidative stress in the saliva as well as its relationship with periodontal disease in the elderly.
2. Methods
2.1. Design and Subjects
A quasi-experimental study was performed with a sample size of 71 sedentary volunteers with a clinical diagnostic of periodontal disease. The age range of the subjects was 60–74 years. Volunteers taking nutritional supplements or anti-inflammatory medications were excluded from the study (n = 10). All participants gave their written, informed consent for inclusion in the study. The investigation protocol was approved by the Ethics Committee of the Universidad Nacional Autónoma de México (UNAM), Zaragoza Campus (IN306213-2).
Subjects were divided into two groups: a control group (CG) with 30 subjects who did not exercise and an experimental group (EG) with 31 subjects who performed Tai Chi (Eight-Form) [16] 5 days a week for 60-minute sessions under the supervision of a qualified instructor for 6 months (Figure 1). Twelve subjects (5 from the experimental and 7 from the control group) were excluded from the study analysis as they were unable to complete the study.
Figure 1.
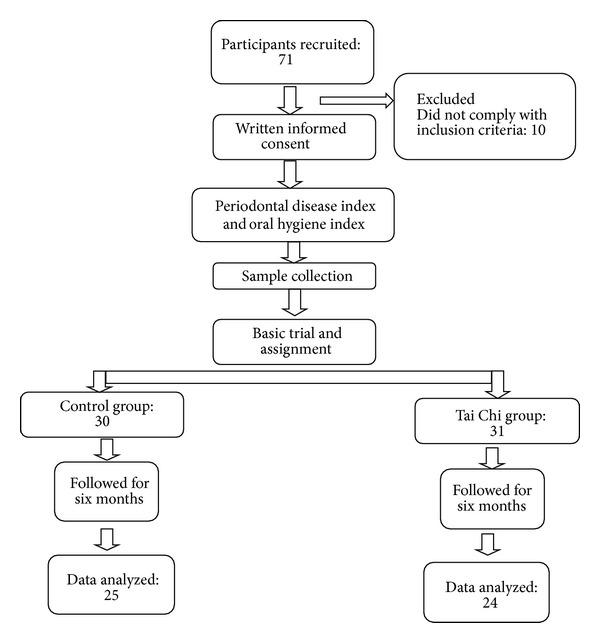
General scheme for study tracking.
2.2. Periodontal Health Status
The periodontal health status of each subject was measured using the Periodontal Disease Index (PDI). The examination procedure involved the insertion of a graduated periodontal probe between the subjects' teeth and gums at a standard force to measure pocket depth. These assessments were made for sextants of the dentition, with the third molars only included if the second molars were missing. The final periodontal disease score was determined by taking the mean of the sextant scores [17].
Oral cleanliness was measured using the Oral Hygiene Index-Simplified (OHI-S). Oral debris and calculus were estimated by running the side of an explorer along the surface of the examined teeth, including the upper first molars (teeth 16 and 26), the lingual faces of the lower first molars (teeth 36 and 46), and the labial faces of the upper right (tooth 11) and lower left (tooth 31) incisors [18].
2.3. Sample Collection and Preparation
Whole unstimulated saliva samples were collected from both groups (control and experimental) before (baseline) and after the six-month period. The samples were obtained one to two hours after an eight-hour fasting period and were collected in 15 mL polypropylene tubes. Saliva was allowed to pool in the bottom of the mouth and was drained into the collection tube. At the end of the collection period, saliva samples were centrifuged at 2,500 rpm for 10 minutes. The supernatant fraction was then aliquoted into storage vials and kept at −80°C until further analysis.
2.4. Saliva TBARS
The TBARS assay was performed using whole saliva, as described by Jentzsch et al. (1996) [19]. In the TBARS assay, one molecule of malondialdehyde reacts with two molecules of thiobarbituric acid (TBA), producing a pink pigment with an absorption peak of 535 nm. Amplification of peroxidation during the assay is prevented by the addition of the chain-breaking antioxidant butylated hydroxytoluene (BHT).
2.5. Saliva Total Antioxidant Status (TAS)
Antioxidant quantification was performed by monitoring 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS+) radical formation (Randox Laboratories, Ltd., Crumlin Co., UK). The antioxidants present suppressed the bluish-green staining of the ABTS+ cation, which is proportional to the antioxidant concentration level. The reaction kinetics were measured using a colorimetric technique in an Autoanalyzer Vitalab Eclipse Merck (Dieren, The Netherlands) [20].
2.6. Saliva Superoxide Dismutase (SOD)
Xanthine and xanthine oxidase (XOD) were used to generate superoxide radicals, which react with 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyltetrazolium chloride to produce a red formazan dye. SOD activity was assessed by measuring the degree of inhibition of the reaction (Randox Laboratories Ltd., Crumlin Co., UK). The kinetics of SOD activity were measured using a colorimetric technique in an Autoanalyzer Vitalab Eclipse Merck (Dieren, The Netherlands) [21].
2.7. Quantification of Cytokines
Aliquots of each saliva sample were assayed by flow cytometry (CBA Kit, Human Inflammatory Cytokine, BD Biosciences, Becton, Dickinson and Company, USA) to determine the levels of interleukin 1-beta (IL-1β), interleukin 6 (IL6), interleukin 8 (IL-8), interleukin 10 (IL-10), and tumor necrosis factor-alpha (TNF-α) [22].
2.8. Statistical Analysis
Data were analyzed using descriptive statistics, where we determined the mean and standard error (SE) and performed a repeated measures analysis of variance (repeated measures ANOVA). A P value of <0.05 was considered statistically significant. P values were determined using the statistical analysis program SPSS, version 16.0.
3. Results
3.1. Biochemical Characteristics
In Table 1, the biochemical values related to glycosylated hemoglobin, cholesterol, triglycerides, HDL, and Albumin for baseline and six months later are shown, revealing no statistically significant differences between the groups.
Table 1.
Biochemical parameters of the study population by group.
| Control (n = 25) | Tai Chi (n = 24) | |||
|---|---|---|---|---|
| Baseline | Six months | Baseline | Six months | |
| HbA1c (%) | 6.56 ± 1.80 | 8.96 ± 2.4 | 8.09 ± 2.2 | 7.29 ± 2.3 |
| Cholesterol (mg/dL) | 213.3 ± 39.2 | 209.6 ± 29.4 | 212.6 ± 44.2 | 209.5 ± 36.6 |
| Triglycerides (mg/dL) | 175.1 ± 38.5 | 207.6 ± 41.9 | 160.8 ± 76.4 | 195.8 ± 130 |
| HDL (mg/dL) | 39.33 ± 8.02 | 40.33 ± 3.5 | 44.375 ± 9.6 | 52.70 ± 11.98 |
| Albumin (g/dL) | 4.76 ± 0.05 | 4.77 ± 0.12 | 4.75 ± 0.20 | 4.69 ± 0.25 |
Values are means ± SE. ANOVA P > 0.05. HbA1c: glycosylated hemoglobin; HDL: High-density lipoproteins.
3.2. Changes in Oxidative Stress Markers by Intervention
With respect to the oxidative stress markers in saliva, a significant increase in the total antioxidant activity (0.53 ± 0.33 mmol/L at baseline versus 0.70 ± 0.35 mmol/L after intervention, P < 0.01) and in SOD activity (1.62 ± 0.83 UI/L at baseline versus 2.79 ± 1.6 UI/L after intervention, P < 0.001) was found in the group that practiced Tai Chi for six months. No significant differences were observed in the values of lipoperoxides (P > 0.05) (Table 2).
Table 2.
Oxidative stress markers, baseline, and postintervention by group.
| Control (n = 25) |
Tai Chi (n = 24) |
|||
|---|---|---|---|---|
| Baseline | Six months | Baseline | Six months | |
| TAS (mmol/L) | 0.72 ± 0.35 | 0.62 ± 0.29 | 0.53 ± 0.33 | 0.70 ± 0.35* |
| SOD (UI/L) | 2.63 ± 1.8 | 2.33 ± 1.1 | 1.62 ± 0.83 | 2.79 ± 1.6† |
| Lipoperoxides (μmol/L) | 0.14 ± 0.14 | 0.08 ± 0.09 | 0.11 ± 0.07 | 0.14 ± 0.09 |
Values are means ± SE. Repeated measures analysis of variance *P < 0.01; † P < 0.001. TAS: total antioxidant status, SOD: superoxide dismutase.
3.3. Changes in Inflammatory Markers by Intervention
A significant decrease in the concentration of interleukin 1β in saliva (783.62 ± 174.9 pg/mL at baseline versus 624.97 ± 196.7 pg/mL after intervention, P < 0.01) was found in the experimental group after practicing Tai Chi. Additionally, a borderline statistically significant decrease in the concentration of IL-6 was observed in the same group (18.66 ± 7.25 pg/mL at baseline versus 4.76 ± 1.93 pg/mL after intervention, P = 0.09) (Table 3).
Table 3.
Inflammatory markers, baseline, and postintervention by group.
| Control (n = 25) |
Tai Chi (n = 24) |
|||
|---|---|---|---|---|
| Baseline | Six months | Baseline | Six months | |
| TNF-α (pg/mL) | 2.004 ± 1.50 | 5.325 ± 2.23 | 0.5119 ± 0.009 | 4.2410 ± 0.435 |
| IL-1β (pg/mL) | 1180.18 ± 244 | 1353.37 ± 176 | 783.62 ± 174.9 | 624.97 ± 196.7* |
| IL-6 (pg/mL) | 20.80 ± 5.01 | 59.45 ± 13.8 | 18.66 ± 7.25 | 4.76 ± 1.93† |
| IL-8 (pg/mL) | 3560.53 ± 809 | 3215.66 ± 260 | 4971.24 ± 835 | 2252.42 ± 330 |
| IL-10 (pg/mL) | 3.15 ± 0.66 | 0.25 ± 0.21 | 0.21 ± 2.5 | 2.9 ± 1.5 |
Values are means ± SE. Repeated measures analysis of variance *P < 0.01; † P = 0.09. TNF-α: tumor necrosis factor alpha; IL-1β: interleukin 1β; IL-6: interleukin 6; IL-8: interleukin 8; IL-10: interleukin 10.
3.4. Changes in Periodontal Disease by Intervention
No significant differences were observed in the oral hygiene index-simplified in any of the groups (P > 0.05). However, a statistically significant decrease in the periodontal disease index was observed in the experimental group (3.62 ± 0.9 baseline versus 3.28 ± 0.8 after intervention, P < 0.05) (Table 4).
Table 4.
Oral hygiene index-simplified and periodontal disease index by group.
| Control (n = 25) | Tai Chi (n = 24) | |||
|---|---|---|---|---|
| Baseline | Six months | Baseline | Six months | |
| OHIS | 2.43 ± 0.3 | 2.45 ± 0.2 | 2.45 ± 0.3 | 2.46 ± 0.3 |
| PDI (mm) | 3.2188 ± 0.6 | 3.7960 ± 0.4 | 3.6267 ± 0.9 | 3.2813 ± 0.8* |
Values are means ± SE. Repeated measures analysis of variance *P < 0.05; OHIS: oral hygiene index-simplified; PDI: periodontal disease index.
4. Discussion
Oxidative stress (OxS) and chronic inflammation (CI) are biological changes inherent to aging and are risk factors for several chronic degenerative diseases such as periodontal disease (PD), one of the most prevalent aging-related diseases [23–25]. Several preventive and therapeutic options have been proposed to counteract these biochemical alterations, including, among others, dietary supplementation resulting in antioxidant effects, antioxidant vitamins, and administration of NSAIDs at low doses [26–29]. It has also recently been shown that a healthy diet and regular moderate physical exercise have antioxidant and anti-inflammatory effects, reducing the risk of chronic diseases or contributing to its treatment [30–32]. Walking and the practice of TC are among the modalities of moderate physical exercise recommended for the maintenance or improvement of health in the elderly [1, 33]. Some studies have shown that Tai Chi has positive effects on cardiorespiratory function and the musculoskeletal system, improving a person's ability to control their posture and balance which, consequently, decreases their frequency of falls [1–3]. Likewise, it has been shown that the practice of Tai Chi has a positive effect on the efficiency of the antioxidant system in adult subjects and the elderly. Thus, it has been proposed that the practice of Tai Chi could prevent and control chronic, degenerative diseases that occur with age [7, 8, 34, 35]. Additionally, it has been observed that the regular practice of physical exercise has been linked to a significantly lower frequency of periodontal disease in adult subjects [36–39].
Our results indicate a statistically significant decrease in the rate of periodontal disease in subjects practicing Tai Chi. These findings support the proposal that regular physical exercise promotes biological changes that positively impact the pathophysiological process of periodontal disease. A significant increase in the total antioxidant status and SOD activity in the experimental group were found, suggesting that one of the possible mechanisms involved in the improvement of periodontal disease is the antioxidant effect brought about by the practice of Tai Chi. Oxidative stress has been linked to the pathophysiology of PD, because reactive oxygen species (ROS) may selectively damage proteoglycans associated with soft periodontal tissues and the alveolar bone as well as chains of proline type 1 collagen, significantly altering fibroblast functions such as adhesion and proliferation as well as their half-life [40–43]. The excessive production of ROS by neutrophils and fibroblasts in periodontal tissues activates NF-κB and triggers the signaling cascade that activates osteoclasts, leading to inflammation [44]. Additionally, the generation of OxS causes an imbalance of metalloproteinases and their tissue inhibitors, leading to the degradation of periodontal tissue [45].
It has also been shown that physical exercise lowers the levels of markers of inflammation [30, 46, 47]. Thus, the decrease of IL-1β observed in saliva in the experimental group suggests that the practice of Tai Chi has an anti-inflammatory effect on periodontal tissue. The mechanisms of these changes have not been fully elucidated, but it has been proposed that this effect may result from the regulation of cytokine expression caused by muscle contractions during exercise [48].
Finally, it has been noted that the effect of moderate physical exercise, such as Tai Chi, on oxidative stress is linked to an adaptive process influenced by the change in the body's redox balance in favor of more alkaline conditions in the cell. The reactive species generated during physical activity act as the signal that is necessary for the activation of the MAPK proteins p38 and ERK1/ERK2, which, in turn, activate the transcription factor sensitive to the redox state, NF-κB, via activation of the kinase that phosphorylates the inhibitor of this factor (IκB). Once freed of its inhibitor, NF-κB is transported into the nucleus where it can promote the synthesis of various antioxidant enzymes, such as MnSOD [7, 8, 45, 49, 50]. Our findings suggest that the antioxidant effect of Tai Chi has a beneficial effect on oral health in the elderly.
5. Conclusions
Our results show that the practice of Tai Chi has an antioxidant and anti-inflammatory oral effect, supporting the proposal that the regular practice this type of exercise can contribute to the prevention and control of periodontal disease during the aging process. Thus, our findings support the proposal to recommend the regular practice of Tai Chi as a coadjuvant for the prevention and treatment of periodontal disease in older adults.
Acknowledgments
This work was supported by Dirección General de Asuntos del Personal Académico, Universidad Nacional Autónoma de México (DGAPA, UNAM), PAPIIT IN306213-2 and Posgrado en Ciencias Biológicas, UNAM.
Conflict of Interests
There are no financial conflict of interests.
References
- 1.Blake H, Hawley H. Effects of tai chi exercise on physical and psychological health of older people. Current Aging Science. 2012;5(1):19–27. doi: 10.2174/1874609811205010019. [DOI] [PubMed] [Google Scholar]
- 2.Lan C, Chen SY, Lai JS, Wong AM. Tai Chi Chuan in medicine and health promotion. Evidence-Based Complementary and Alternative Medicine. 2013;2013:17 pages. doi: 10.1155/2013/502131.502131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jahnke R, Larkey L, Rogers C, Etnier J, Lin F. A comprehensive review of health benefits of qigong and tai chi. The American Journal of Health Promotion. 2010;24(6):e1–e25. doi: 10.4278/ajhp.081013-LIT-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li JX, Hong Y, Chan KM. Tai chi: physiological characteristics and beneficial effects on health. British Journal of Sports Medicine. 2001;35(3):148–156. doi: 10.1136/bjsm.35.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang Y-K, Nien Y-H, Tsai C-L, Etnier JL. Physical activity and cognition in older adults: the potential of Tai Chi Chuan. Journal of Aging and Physical Activity. 2010;18(4):451–472. doi: 10.1123/japa.18.4.451. [DOI] [PubMed] [Google Scholar]
- 6.Li F, Fisher KJ, Harmer P, Irbe D, Tearse RG, Weimer C. Tai chi and self-rated quality of sleep and daytime sleepiness in older adults: a randomized controlled trial. Journal of the American Geriatrics Society. 2004;52(6):892–900. doi: 10.1111/j.1532-5415.2004.52255.x. [DOI] [PubMed] [Google Scholar]
- 7.Rosado-Pérez J, Santiago-Osorio E, Ortiz R, Mendoza-Núñez VM. Tai Chi improves oxidative stress in Mexican older adults. The Journal of Nutrition, Health & Aging. 2012;16:642–646. doi: 10.1007/s12603-012-0029-9. [DOI] [PubMed] [Google Scholar]
- 8.Rosado-Pérez J, Rocío Ortiz R, Santiago-Osorio E, Mendoza-Núñez VM VM. Effect of Tai Chi versus walking on oxidative stress in Mexican older adults. Oxidative Medicine and Cellular Longevity. 2013;2013:8 pages. doi: 10.1155/2013/298590.298590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Champagne CME, Buchanan W, Reddy MS, Preisser JS, Beck JD, Offenbacher S. Potential for gingival crevice fluid measures as predictors of risk for periodontal diseases. Periodontology 2000. 2003;31:167–180. doi: 10.1034/j.1600-0757.2003.03110.x. [DOI] [PubMed] [Google Scholar]
- 10.Tezal M, Uribe S. A lack of consensus in the measurement methods for and definition of periodontitis. Journal of the American Dental Association. 2011;142(6):666–667. doi: 10.14219/jada.archive.2011.0250. [DOI] [PubMed] [Google Scholar]
- 11.Dahiya P, Kamal R, Gupta R, Bhardwaj R, Chaudhary K, Kaur S. Reactive oxygen species in periodontitis. Journal of Indian Society of Periodontology. 2013;17(4):411–416. doi: 10.4103/0972-124X.118306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapple ILC, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontology 2000. 2007;43(1):160–232. doi: 10.1111/j.1600-0757.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 13.Chapple ILC, Mason GI, Garner I, et al. Enhanced chemiluminescent assay for measuring the total antioxidant capacity of serum, saliva and crevicular fluid. Annals of Clinical Biochemistry. 1997;34(4):412–421. doi: 10.1177/000456329703400413. [DOI] [PubMed] [Google Scholar]
- 14.Kim S-C, Kim O-S, Kim O-J, Kim Y-J, Chung H-J. Antioxidant profile of whole saliva after scaling and root planing in periodontal disease. Journal of Periodontal and Implant Science. 2010;40(4):164–171. doi: 10.5051/jpis.2010.40.4.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brock GR, Butterworth CJ, Matthews JB, Chapple ILC. Local and systemic total antioxidant capacity in periodontitis and health. Journal of Clinical Periodontology. 2004;31(7):515–521. doi: 10.1111/j.1600-051X.2004.00509.x. [DOI] [PubMed] [Google Scholar]
- 16.Li F, Fisher KJ, Harmer P, Shirai M. A simpler eight-form Easy Tai Chi for elderly adults. Journal of Aging and Physical Activity. 2003;11(2):206–218. [Google Scholar]
- 17.Ramfjord SP. The Periodontal Disease Index (PDI) Journal of Periodontology. 1967;38(6):602–610. doi: 10.1902/jop.1967.38.6.602. [DOI] [PubMed] [Google Scholar]
- 18.Greene JC, Vermillion JR. The simplified oral hygiene index. Journal of the American Dental Association. 1964;68:7–13. doi: 10.14219/jada.archive.1964.0034. [DOI] [PubMed] [Google Scholar]
- 19.Jentzsch AM, Bachmann H, Fürst P, Biesalski HK. Improved analysis of malondialdehyde in human body fluids. Free Radical Biology and Medicine. 1996;20(2):251–256. doi: 10.1016/0891-5849(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 20.Mendoza-Núñez VM, Ruiz-Ramos M, Sánchez-Rodríguez MA, Retana-Ugalde R, Muñoz-Sánchez JL. Aging-related oxidative stress in healthy humans. The Tohoku Journal of Experimental Medicine. 2007;231:216–268. doi: 10.1620/tjem.213.261. [DOI] [PubMed] [Google Scholar]
- 21.Cook EB, Stahl JL, Lowe L, et al. Simultaneous measurement of six cytokines in a single sample of human tears using microparticle-based flow cytometry: allergics vs. non-allergics. Journal of Immunological Methods. 2001;254(1-2):109–118. doi: 10.1016/s0022-1759(01)00407-0. [DOI] [PubMed] [Google Scholar]
- 22.Knight JA. Free Radicals, Antioxidants, Aging, & Disease. Washington, DC, USA: AACC PRESS; 1999. [Google Scholar]
- 23.Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 24.Cannizzo ES, Clement CC, Sahu R, Follo C, Santambrogio L. Oxidative stress, inflamm-aging and immunosenescence. Journal of Proteomics. 2011;74(11):2313–2323. doi: 10.1016/j.jprot.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Campisi G, Chiappelli M, De Martinis M, et al. Pathophysiology of age-related diseases. Immunity & Ageing. 2009;6(12) doi: 10.1186/1742-4933-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stahl W, Sies H. Antioxidant defense: vitamins E and C and carotenoids. Diabetes. 1997;46(2):S14–S18. doi: 10.2337/diab.46.2.s14. [DOI] [PubMed] [Google Scholar]
- 27.Cheng T-Y, Zhu Z, Masuda S, Morcos NC. Effects of multinutrient supplementation on antioxidant defense systems in healthy human beings. Journal of Nutritional Biochemistry. 2001;12(7):388–395. doi: 10.1016/s0955-2863(01)00153-x. [DOI] [PubMed] [Google Scholar]
- 28.Faizuddin M, Tarannum F, Korla N, Swamy S. Association between long-term aspirin use and periodontal attachment level in humans: a cross-sectional investigation. Australian Dental Journal. 2012;57(1):45–50. doi: 10.1111/j.1834-7819.2011.01650.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Zhang C. Vasoprotection by dietary supplements and exercise: role of TNFα signaling. Experimental Diabetes Research. 2012;2012:6 pages. doi: 10.1155/2012/972679.972679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosado-Pérez J, Santiago-Osorio E, Ortiz R, Mendoza-Núñez VM. Moderate physical activity diminishes oxidative stress and the inflammatory process in elderly. HealthMed Journal. 2011;5:173–179. [Google Scholar]
- 31.Battino M, Mezzetti B. Update on fruit antioxidant capacity: a key tool for Mediterranean diet. Public health nutrition. 2006;9(8):1099–1103. doi: 10.1017/S1368980007668554. [DOI] [PubMed] [Google Scholar]
- 32.El-Khoury F, Cassou B, Charles MA, Dargent-Molina P. The effect of fall prevention exercise programmes on fall induced injuries in community dwelling older adults: systematic review and meta-analysis of randomised controlled trials. British Medical Journal. 2013;347 doi: 10.1136/bmj.f6234.f6234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeom HA, Keller C, Fleury J. Interventions for promoting mobility in community-dwelling older adults. Journal of the American Academy of Nurse Practitioners. 2009;21(2):95–100. doi: 10.1111/j.1745-7599.2008.00390.x. [DOI] [PubMed] [Google Scholar]
- 34.Goon JA, Noor Aini AH, Musalmah M, Yasmin Anum MY, Wan Nazaimoon WM, Wan Ngah WZ. Effect of Tai Chi exercise on DNA damage, antioxidant enzymes, and oxidative stress in middle-age adults. Journal of Physical Activity and Health. 2009;6(1):43–54. doi: 10.1123/jpah.6.1.43. [DOI] [PubMed] [Google Scholar]
- 35.Palasuwan A, Suksom D, Margaritis I, Soogarun S, Rousseau AS. Effects of tai chi training on antioxidant capacity in pre- and postmenopausal women. Journal of Aging Research. 2011;2011:8 pages. doi: 10.4061/2011/234696.234696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merchant AT, Pitiphat W, Rimm EB, Joshipura K. Increased physical activity decreases periodontitis risk in men. European Journal of Epidemiology. 2003;18(9):891–898. doi: 10.1023/a:1025622815579. [DOI] [PubMed] [Google Scholar]
- 37.Sanders AE, Slade GD, Fitzsimmons TR, Bartold PM. Physical activity, inflammatory biomarkers in gingival crevicular fluid and periodontitis. Journal of Clinical Periodontology. 2009;36(5):388–395. doi: 10.1111/j.1600-051X.2009.01394.x. [DOI] [PubMed] [Google Scholar]
- 38.Bawadi HA, Khader YS, Haroun TF, Al-Omari M, Tayyem RF. The association between periodontal disease, physical activity and healthy diet among adults in Jordan. Journal of Periodontal Research. 2011;46(1):74–81. doi: 10.1111/j.1600-0765.2010.01314.x. [DOI] [PubMed] [Google Scholar]
- 39.Al-Zahrani MS, Borawski EA, Bissada NF. Increased physical activity reduces prevalence of periodontitis. Journal of Dentistry. 2005;33(9):703–710. doi: 10.1016/j.jdent.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Waddington RJ, Moseley R, Embery G. Reactive oxygen species: a potential role in the pathogenesis of periodontal diseases. Oral Diseases. 2000;6(3):138–151. doi: 10.1111/j.1601-0825.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 41.Rittié L, Monboisse J-C, Gorisse M-C, Gillery P. Malondialdehyde binding to proteins dramatically alters fibroblast functions. Journal of Cellular Physiology. 2002;191(2):227–236. doi: 10.1002/jcp.10093. [DOI] [PubMed] [Google Scholar]
- 42.Wei D, Zhang X-L, Wang Y-Z, Yang C-X, Chen G. Lipid peroxidation levels, total oxidant status and superoxide dismutase in serum, saliva and gingival crevicular fluid in chronic periodontitis patients before and after periodontal therapy. Australian Dental Journal. 2010;55(1):70–78. doi: 10.1111/j.1834-7819.2009.01123.x. [DOI] [PubMed] [Google Scholar]
- 43.Moore S, Calder KAC, Miller NJ, Rice-Evans CA. Antioxidant activity of saliva and periodontal disease. Free Radical Research. 1994;21(6):417–425. doi: 10.3109/10715769409056594. [DOI] [PubMed] [Google Scholar]
- 44.Jimi E, Aoki K, Saito H, et al. Selective inhibition of NF-κB blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nature Medicine. 2004;10(6):617–624. doi: 10.1038/nm1054. [DOI] [PubMed] [Google Scholar]
- 45.Pozo P, Valenzuela MA, Melej C, et al. Longitudinal analysis of metalloproteinases, tissue inhibitors of metalloproteinases and clinical parameters in gingival crevicular fluid from periodontitis-affected patients. Journal of Periodontal Research. 2005;40(3):199–207. doi: 10.1111/j.1600-0765.2005.00786.x. [DOI] [PubMed] [Google Scholar]
- 46.Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and c-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. Journals of Gerontology A. 2000;55(12):M709–M715. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- 47.Irwin MR, Olmstead R. Mitigating cellular inflammation in older adults: a randomized controlled trial of tai chi chih. The American Journal of Geriatric Psychiatry. 2012;20(9):746–772. doi: 10.1097/JGP.0b013e3182330fd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiological Reviews. 2008;88(4):1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 49.Gomez-Cabrera M-C, Domenech E, Viña J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radical Biology and Medicine. 2008;44(2):126–131. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 50.Leeuwenburgh C, Fiebig R, Chandwaney R, Ji LL. Aging and exercise training in skeletal muscle: responses of glutathione and antioxidant enzyme systems. The American Journal of Physiology—Regulatory Integrative and Comparative Physiology. 1994;267(2):R439–R445. doi: 10.1152/ajpregu.1994.267.2.R439. [DOI] [PubMed] [Google Scholar]


