Abstract
The antiproliferative and antioxidant potential of Cymbopogon citratus (Lemon grass) extracts were investigated. The extracts were isolated by solvent maceration method and thereafter subjected to antiproliferative activity test on five different cancer cells: human colon carcinoma (HCT-116), breast carcinoma (MCF-7 and MDA-MB 231), ovarian carcinoma (SKOV-3 and COAV), and a normal liver cell line (WRL 68). The cell viability was determined using MTT assay. The DPPH radical scavenging assay revealed a concentration dependent trend. A maximum percentage inhibition of 45% and an IC50 of 278 μg/mL were observed when aqueous extract was evaluated. In contrast, 48.3% and IC50 of 258.9 μg/mL were observed when 50% ethanolic extract was evaluated. Both extracts at concentration of 50 to 800 μg/mL showed appreciative metal chelating activity with IC50 value of 172.2 ± 31 μg/mL to 456.5 ± 30 μg/mL. Depending on extraction solvent content, extract obtained from 50% ethanolic solvent proved to be more potent on breast cancer MCF-7 cell line (IC50 = 68 μg/mL). On the other hand, 90% ethanolic extract showed a moderate potency on the ovarian cancer (COAV) and MCF-7 cells having an IC50 of 104.6 μg/mL each. These results suggested antiproliferative efficacy of C. citratus ethanolic extract against human cancer cell lines.
1. Introduction
Cancer is among the leading causes of mortality among human population of all ages. In fact, it is responsible for 7.6 million deaths in 2008 [1]. It has been projected that the cancer mortality rate will extend to about 30.1 million by 2030 [1]. Current therapeutic interventions mostly involve malign surgery, radiotherapy, and chemotherapy, and at times the therapeutic efficiency is very low. This incurs the current increase in research on mild alternative cancer therapy. Bioactive phytochemicals exhibiting the ability to inhibit cancer cytogenesis by suppressing the tumor initiation, promotion, and progression are being considered as potential biocompatible anticancer agents. In this regard, the antiproliferative activity of several phytochemical extracts was reported [2–4].
Among the medicinal plants used, Cymbopogon citratus (lemon grass) is prominent and commonly explored in folk alternative medicine for the treatment of diverse ailments. Although, several bioactive compounds were reported to be isolated from C. citratus; among them is the acyclic monoterpene aldehydes described as citral that comprises of isomeric geranial and neral. Citral was reported to be the major bioactive component that incurs most of the plant's bio efficacy [5]. Popularly, the aqueous infusion of this plant is called “abafado” by Portuguese, and was said to have bioactive efficacy against nervous and gastrointestinal disturbances when administered orally [6]. In addition, it is also reported to be a potent free radical scavenger of reactive oxygen species [7]. Furthermore, citral was shown to possess activities like anti mutagenicity [8], antiproliferative effect against Trypanosoma cruzi [9], and antinociceptive and[10], antiparasitic effects against leishmaniasis [11, 12].
The efficient potency of C. citratus on free radical scavenging and antioxidation ability led us to evaluate the effect of its aqueous ethanolic extracts on proliferation and cell growth of several human cancer cell lines such as those of breast cancer [MDA-MB 231 and MCF-7], ovarian cancer [SKOV-3 and COAV], and colon cancer [HCT-116]. In addition, the phytochemical content analysis of the extracts is also reported.
2. Materials and Methods
2.1. Plant Materials, Phytochemicals Extraction and GCMS Analysis
C. citratus leaves were identified and obtained by MABLEAJAZ chair for Scientific Research in Prophetic Medicine, Faculty of Medicine, Taibah University, Saudi Arabia (Specimen Voucher Number: TU/JX03/SP2765). The leaves were cleaned, dried under shade for 7 days then grounded, weighed, homogenized in water (Ew), 50% ethanol (E50), and 90% ethanol (E90) at a ratio of 1 : 10 of plant powder to solvent, and left to macerate for 5 days at ambient temperature (25 ± 1°C) with occasional shaking and stirring. The mixture was then filtered and the resulting liquid was concentrated under reduced pressure at 40°C in an EYELA rotary evaporator yielding a dark brown to green extracts. The concentrated extracts were then kept in vacuo at 45°C for 3 days to evaporate the residual solvents resulting in the respective dried crude extract of either Ew, E50, or E90, respectively. Extracts were then dissolved in 0.5% DMSO before being used in the cell cultures at concentrations of 3, 6, 12, 25, 50, and 200 µg/mL.
Another portion of the extract (1 mg) was dissolved in 1 mL methylene chloride in a screw capped test tube. To this mixture, 1 mL of acidified methanol (methanol containing 15% H2SO4) was added, then tightly capped and incubated at 100°C for 2 hours. At the end of heating, the reaction mixture was allowed to cool down to room temperature, followed by addition of 1 mL of deionized water and vortex to induce phase separation, and to stand for a minute. Using Pasteur pipette, about 1 mL of the organic phase was carefully withdrawn into GC vials for GCMS phytochemical content analysis.
The GCMS analysis was conducted on Agilent 7000B triple quadrupole GCMSMS machine carrying triple axis detector and Agilent HP-5 MS separation column that has been impregnated with 5% phenyl methyl silox (30 m long × 0.25 mm internal diameter × 0.25 µm film thickness). A sample (1 µL) was automatically injected into the machine at a split ratio of 1 : 20. The injection temperature was set at 280°C. The oven ramping temperature profile was as follows: 40°C for 2 min then increased to 140°C at 3°C min−1, held at 140°C for 2 min then increased to 250°C at 10°C min−1, and then held at 250°C for 5 min. Helium was used as the carrier gas at a flow rate of 14 mL min−1. Mass spectra were acquired at 1250 scan speed using electron impact energy of 70 eV at 230°C ion-source temperature and 250°C interface temperature.Corresponding spectrum for each chromatogram peak was compared with deposited spectra in NIST database for compound identification.
2.2. DPPH Assay
Radical scavenging activities of the extracts were determined by a spectrophotometric assay using alcoholic solution of 1,1-diphenyl-2-picrylhydrazyl (DPPH) as reported somewhere else [7]. Briefly, different concentrations of extracts-DMSO solutions (15.6–250 µg/mL) are added to a solution of DPPH (200 mM) in absolute ethanol and incubated in dark for 30 min at room temperature (25 ± 1°C). The change in chrometric status of DPPH from purple to yellow upon reduction was measured spectrophotometrically at 517 nm for each sample after incubation against a control solution of DPPH and DMSO alone. Ascorbic acid was used as standard control. The free radical scavenging activities of the extracts were calculated as a percentage of radical reduction in (1). All experiments were performed in triplicates, and the IC50 values were determined from a calibration curve for each extract:
| (1) |
2.3. Metal Chelating Assay
The metal chelating abilities of the extracts were determined according to the method of Wang et al. [35] with minor modifications. The stock solutions of the extracts (100 µL of 5 mg/mL) were mixed with 135 µL of distilled water and 5 µL of 2 mM FeCl2 in a microplate. The reaction was initiated by the addition of 10 µL of 5 mM ferrozine. Thereafter solutions were mixed and allowed to stand for 10 min at room temperature (25 ± 1°C). After incubation, the absorbance was measured at 562 nm with a microplate reader (GF-M3000, UNICOM-OPTICS). Distilled water (100 µL) instead of sample solution was used as a control. Distilled water (10 µL) in place of ferrozine solution was used as a blank, which is used for error correction because of unequal colour of the sample solutions. EDTA-Na2 was used as reference standard. All measurements were performed in triplicate, and the ferrous ion-chelating ability was calculated according to (2):
| (2) |
2.4. FRAP Assay
The ferric reducing antioxidant powers (FRAP) of the extracts were assayed according to the previously described method [36] with slight modification. In brief, the FRAP reagent was prepared by adding 300 mM acetate buffer (3.1 mg sodium acetate/mL, pH 3.6) to 10 mM 2,4,6-tripyridyl-S-triazine (TPTZ) solution and 20 mM FeCl3·H2O (5.4 mg/mL). A portion of TPTZ reagent (290 μL) was added to each well of 96-well titre plate in triplicate, and aliquot sample (10 μL) of 1 mg/mL of prepared C. citratus extracts was used to read the absorbance at 593 nm in ELISA reader (Shimadzu, Japan) after every 4 min for 2 h.
2.5. Nitric Oxide Assay
Nitric oxide (NO) scavenging activity of the extracts was determined using Griess reaction. According to this reaction, when sodium nitro-prusside is used in aqueous solution at physiological pH, it generated NO• radicals that react with oxygen to produce nitrite ions. The produced ions are then quantified via spectrophotometric analysis as reported by Nagmoti et. al. [37]. In brief, 1 µL of 10 mM sodium nitro-prusside was mixed with 1 mL of test extracts or curcumin (as a reference control) at various concentrations (400–1600 µg/mL) dissolved in methanol and a control without test extracts, but only with an equivalent amount of methanol. The mixture was then incubated for 30 min at room temperature (25°C). After 30 min of incubation, 1 mL of Griess reagent (1% sulphanilamide, 2% phosphoric acid, and 0.1% naphthyl ethylenediamine dihydrochloride) was added to 1 mL of the incubated solution and vortex. The absorbance of the pink coloration during the diazotization of the nitrite with sulphanilamide and the subsequent coupling with naphthyl ethylenediamine dihydrochloride was measured at 546 nm. All the tests were performed in triplicate. Percentage inhibition was calculated using (3):
| (3) |
2.6. Evaluation of C. citratus Cytotoxicity by MTT Assay
Three different extracts were prepared for studying the antiproliferative effect of C. citratus extracts against the human cancer cell lines in reference to normal liver cell line WRL 68, these were Ew, E50, and E90 representing aqueous and 50% and 90% ethanol, respectively. Both the normal cell line (WRL 68) and the human cancer cell lines were used (breast cancer [MDA-MB 231 and MCF-7], ovarian cancer [SKOV-3 and COAV], and colon cancer cell lines [HCT-116]) were obtained from American Type Culture Collection and supplied by Department of Molecular Medicine, University of Malaya. Extract's antiproliferative effect by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was evaluated according to modified protocol [38]. Briefly, cell lines were cultured in RPMI-1640 growth medium and supplemented with C. citratus extracts at different concentrations (3–200 µg/ml), 10% (v/v) sterile fetal bovine serum (FBS, PAA Lab, Austria), 100 mg/mL streptomycin, 100 U/mL penicillin (PAA Lab, Austria) and 50 mg/mL fungizone (Sigma Aldrich). Cultures were incubated in 5% CO2 incubator at 37°C in a humidified atmosphere. The cells were harvested by detaching the cells from the culture flask using trypsin after the flask get confluent enough with the cells. The harvested cells were then aseptically introduced into 50 mL sterile falcon tube and washed with physiological buffer (pH 7.2) under spinning at 1200 rpm for 10 minutes. The supernatant was discarded, and the cells pellets were mixed with 1 mL of sterile media to form a cell suspension. The viable cells count was determined using trypan blue assay. About 10 µL of the cell suspension was mixed with 10 µL of trypan blue, and aliquot sample (10 µL) of this mixture was used to count the cells in Neo Bar chambers.
The Harvested cells were then seeded into 96-well culture plates at 5000 cells/well and allowed to adhere overnight. Both the water and alcoholic extracts of the C. citratus were dissolved in 5% dimethyl sulphoxide (DMSO) with final concentration of 0.5% and diluted to different concentration spanning from 3–200 µg/mL. Blank 5% DMSO was used as a control. Cells were incubated with the samples (three wells on a plate for each concentration) from 48 to 72 h. Thereafter, 10 µL of MTT (5 mg/mL) (Sigma) was added to each well and the plates were incubated at 37°C for 4 h. The media was then gently aspirated, and about 200 mL of DMSO was added to dissolve the formazan crystals. The amount of formazan product was measured spectrophotometrically at 570 nm using a microplate reader (GF-M3000). The percentage cell viability was calculated according to (4):
| (4) |
2.7. Statistical Analysis
The obtained experimental data were evaluated statistically as mean ± S.E.M. (standard error mean). The statistical differences between the groups were determined based on 95% confidence intervals using one-way ANOVA in SPSS program (SPSS Inc., USA). All obtained data were analyzed using Analysis of Variance (ANOVA). A probability value of P < 0.05 was considered as significant between the measurements of the two compared groups.
3. Results and Discussion
3.1. Analyses of Phytochemical Extracts, Radical Scavenging, and Antioxidant Efficacy
In this research, maceration using aqueous-solvent extraction of lemon grass yielded 5.4 g/100 g on dry weight basis. Based on GCMS qualitative analysis, eighteen (18) phytochemicals compounds from the extracts were identified (Table 1). The analysis revealed that the constituents of lemon grass extracts mostly belong to monoterpene, sesquiterpene, and phenolic acids. This phytochemical composition analysis was found to be in accord with previously reported studies [39, 40]. The antioxidants potential of C. citratus leaves extracts on the DPPH radical scavenging were determined by their hydrogen donating ability. Evaluating the DPPH radical scavenging activity of both Ew and E50 extracts against ascorbic acid as a standard control (Figure 1(a)), it is observed that at lower concentrations (100–200 µg/mL) both extracts revealed almost similar radical scavenging activity (Figure 1(a)). However, as the concentration increases, the Ew extract seems to have a logarithmic increase in percentage inhibition over the concentration range, achieving a maximum inhibition of about 45% and an IC50 value of about 278 as shown in Figure 1(a). On the other hand, increasing the concentration of E50 extract to 750 µg/mL revealed an increase in percentage inhibition. Beyond this value, the percentage inhibition appeared to approach plateau with the increment in the extract's concentration attaining a maximum percent inhibition of 48.3% with corresponding IC50 of 258.90 (Figure 1(a)). This could be due to the difference in the extracted terpenes content. When compared to the ascorbic acid, it takes about 1500 µg/mL of the extract to achieve the percentage inhibition of the standard control at a concentration about 20 µg/mL with corresponding IC50 of 6.21 µg/mL. These observations were found to be in agreement with similar observations on a higher IC50 values for C. citratus extracts [41–43].
Table 1.
Phytochemical composition of aqueous extract of Cymbopogon citratus.
| Number | Compound | Extract | Reported application | Reference | |
|---|---|---|---|---|---|
| Ew | E50 | ||||
| % | % | ||||
| 1 | Hydroquinone | 0.8 | ND | Treatment of melasma | [13] |
| 2 | Nerolidol | 24 | ND | Anticancer | [14] |
| 3 | β-Elemene | 42 | ND | Anticancer | [15] |
| 4 | β-Eudesmol | 12 | ND | Anticancer | [16] |
| 5 | Myrtenal | 4 | 0.6 | (i) Inhibits Alzheimer's acetylcholinesterase (ii) Anticancer (iii) Insect repellent |
[17] [18] [19] |
| 6 | Piperitone | 2.1 | 4.5 | Antimicrobial | [20] [21] |
| 7 | Nonadiyne | ND | 0.6 | Inhibits the release of endogenous nitric oxide | [22] |
| 8 | α-Cubebene | ND | 0.5 | Cytotoxic and antimicrobial | [23] |
| 9 | α-Copaene | ND | 0.3 | Antidiabetic | [24] |
| 10 | L-calamenene | ND | 0.2 | Anticancer | [25] |
| 11 | Elemol | ND | 41 | Antimosquitoes | [26] |
| 12 | Humulene | ND | 4 | Anti-inflammatory and inhibits the generation of tumor necrosis factor-α (TNF-α) and interleukin-1 β (IL1β) | [27] |
| 13 | Caryophyllene | ND | 0.9 | Antiulcerogenic and anti-inflammatory | [28] |
| 14 | Cubenol | ND | 2 | Cytotoxic effect | [29] |
| 15 | Cubebol | ND | 4.7 | Mosquito larvicidal activity | [30] |
| 16 | Carvone | 0.7 | 2 | Sprout suppression and antifungal activity in potato | [31] |
| 17 | β-Eudesmol | ND | 45 | Antimutagenic Antiangiogenic |
[32] [33] |
| 18 | Nonanenitrile | ND | 0.9 | Antipancreatitis and antiulcerogenic | [34] |
*ND: not detected.
Figure 1.
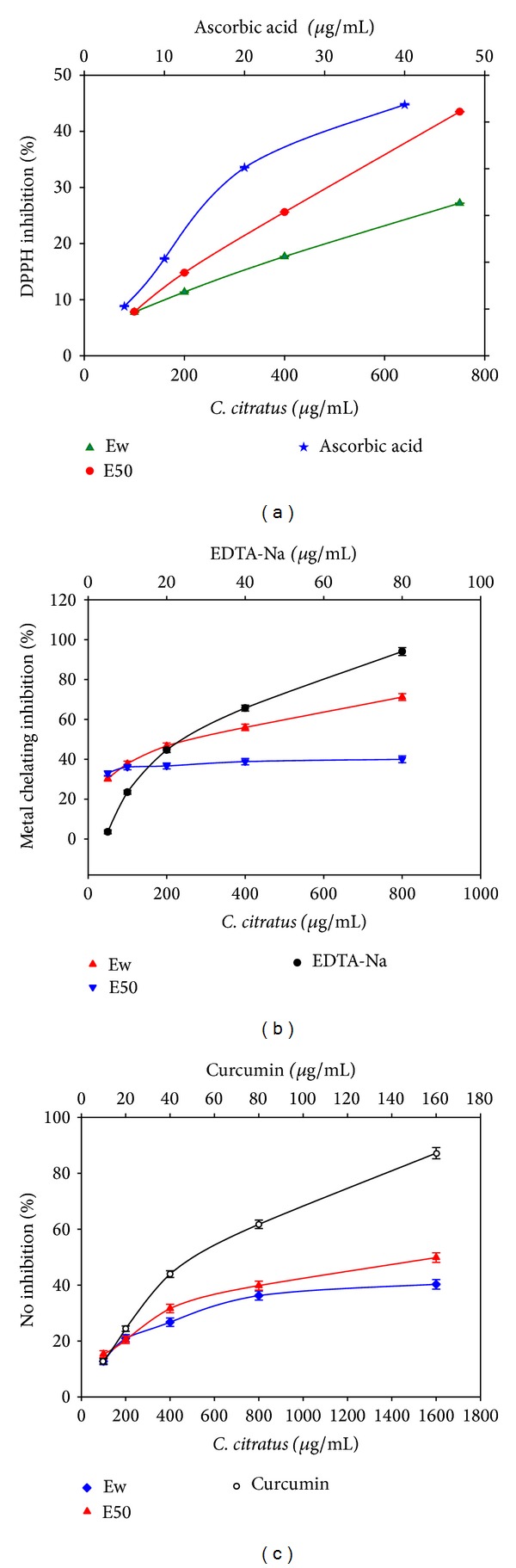
Antioxidant and radical scavenging property of C. citratus aqueous (Ew) and 50% ethanolic (E50) extracts. (a) DPPH radical scavenging activity. (b) Metal chelating capacity. (c) NO inhibition activity. All values are expressed as the means ± S.E.M. Statistical difference is significant at the P < 0.05 level.
The antioxidant results presented so far indicated that the phytochemical extracts of C. citratus leaves had both proton and electron donating abilities of primary antioxidant efficacy. However, it has been reported that secondary antioxidants serve as effective ligands in chelating metal ions, thereby suppressing the formation of hydroxyl radicals by Fenton's reaction [44]. Interestingly, in this assay, both the Ew and E50 extracts at concentrations of 50 to 800 µg/mL showed appreciative metal chelating activity (Figure 1(b)). Among the extracts studied, the highest activity was observed in Ew with corresponding metal chelating inhibition of 71.2% and an IC50 value of 172.2 ± 31 µg/mL, while the E50 extract showed the chelating inhibition of 40% and a corresponding IC50 value of 456.5 ± 30 µg/mL. In contrast to the standard control (EDTA-Na), the observed results were found to equal the standard's chelating performance of about 40 µg/mL (Figure 1(b)).
The ferric ion reducing capacity (FRAP) showed both the extracts to possess almost similar activity. The assay activity trend revealed an increase in value from 0.21 Fe2+/g in the Ew extract to 0.3 Fe2+/g in the E50 extract, signifying the effectiveness of the E50 extract as compared to the Ew extract. Runnie et al. [45] reported similar observation on the FRAP activity of C. citratus leaf extract. Within the animal cells, it is a fact that most inflammatory responses were associated with nitric oxide [46]. In this study, both the Ew and E50 extracts were checked for their inhibitory effect against nitric oxide production (Figure 1(c)). Among the analyzed samples and reference to the standard curcumin (IC50 = 10.8 ± 1.2 µg/mL), the E50 extract showed the highest nitric oxide inhibitory activity of 49.3% (IC50 462.9 ± 29 µg/mL) as compared to the Ew extract of 40.1% (432.4 ± 24 µg/mL). In fact, the extracts were found to have a nitric oxide scavenging activity equivalent to about 40 µg/mL of the standard curcumin (Figure 1(c)).
3.2. Antiproliferative Effect of C. citratus Extract on Different Cancer Cell Lines
The effects of extracts concentrations on the viability and growth of tumor cell lines in reference to the normal cell line have been observed (Figures 2(a)–2(f)). Generally, when the extracts treatments in cancer cell lines (Figures 2(a)–2(e)) were compared with that of the normal cell line WRL 68 (Figure 2(f)), the antiproliferative effect of the extract could be observed to be exerted more on the cancer cell lines, the extract was observed to cause an inhibition of less than 50% even at higher dosage (200 µg/mL). On the other hand, using the extract even at lower concentrations <50 µg/mL caused marked inhibition in the cell growth. At the lower concentrations (i.e. <50 µg/mL), it is observed that E50 extract has the highest inhibition activity. However, as the treatment concentration increases beyond 50 µg/mL, E90 showed the highest efficacy (Figures 2(a)–2(e)). Among the breast cancer cell lines tested, E90 showed lower inhibition in MDA-MB 231 cell line with corresponding 40% inhibition (Figure 2(a)) and IC50 >200 µg/mL as compared to observed inhibition activity on MCF-7 cell line (Figure 2(b)), which showed percent inhibition of 82.9% and IC50 of 104.6 µg/mL. In comparison to E90 extract, E50 was observed to be more potent on MCF-7 cell line (IC50 = 68 µg/mL) than on MDA-MB 231 cell line (IC50 ≥ 200 µg/mL). It has been reported that the antiproliferative activities of phytochemical extracts could be due to their influence on incurring increase expression of kinase and their activities of positive G1/S and G2/M regulators with simultaneous expression of p21 in presence of high level of p27 and p53 [4]. These effects caused a blockade in cell cycle, thus inducing the apoptosis in MCF-7 cells.
Figure 2.
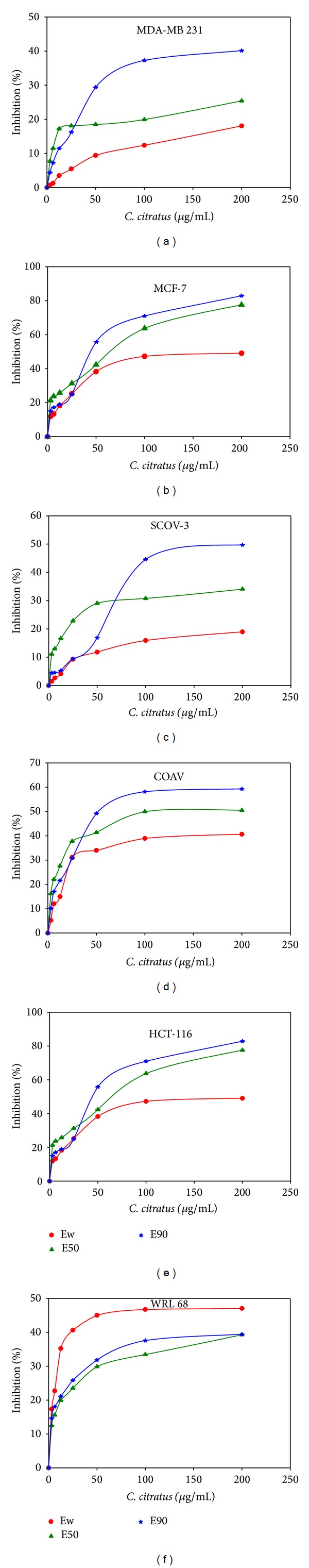
Antiproliferative efficacies of C. citratus extracts by MTT assay on (a) MDA-MB 231 (b) MCF-7, (c) SCOV-3, (d) COAV, (e) HCT-116, and (f) WRL 68 cell lines. Ew, E50, and E90 represent the aqueous, 50% and 90% ethanolic extracts, respectively. All values are expressed as the means ± S.E.M. Statistical difference is significant at the P < 0.05 level.
Evaluating the extract's antiproliferative effect on SCOV-3 (Figure 2(c)) and COAV (Figure 2(d)) cell lines, increasing the E90 concentration to 100 µg/mL resulted in a logarithmic increase in percentage inhibition with an observed IC50 of 200 and 104.6 µg/mL in SCOV-3 and COAV, respectively. Thereafter, increasing the concentration above 100 µg/mL resulted in minimal increase in the percentage inhibition achieving a maximum inhibition of about 50% and 59% in both SCOV-3 and COAV, respectively. This stabilization in the percentage inhibition at higher concentration could be attributed to the major death of the cell population. Similar observation has been reported by Konrad et al. [47], who observed a reduction in growth inhibition with increasing Urtica dioica root extract concentration on human prostate cancer (LNCaP) cell line. Analyzing the extract's efficacy on colon cancer cell line HCT-116 (Figure 2(e)), revealed a different trend, since the percentage inhibition appeared to continue to increase with increasing concentration up to 200 µg/mL resulting in maximum inhibition of about 83% and IC50 > 200 µg/mL. According to Dudai et al. [48] the mechanism of the inhibition is mostly accompanied by DNA fragmentation and caspase-3 catalytic activity induction. Although at higher ethanolic content extraction there is an appreciative inhibitory activity, the extract performance was found to be lower than that of aqueous extracts. In this extract all samples tested were found to have an IC50 > 200 µg/mL. In general, the results presented here on cell growth inhibition by C. citratus extract are further supported in the light of the antiproliferative effects of the plant's phytochemical constituents that have been published before as shown in Table 1.
4. Conclusions
The oxidative radical scavenging and chemo-preventive efficacy of C. citratus extracts were evaluated. Ew and E50 extracts have shown DPPH antioxidant activities. In addition, the extracts were found to show an appreciative iron chelating and nitric oxide scavenging activities. On evaluating the antiproliferative effect, five (5) human cancer cell lines were compared in this analysis. The E50 extract proved to be more potent on breast cancer MCF-7 cell line. On the other hand, E90 extract showed a moderate potency on the ovarian cancer (COAV) and MCF-7 cell lines. Compared to extracts from higher ethanolic content, aqueous extracts were observed to show lower inhibitory activity with a generalized IC50 > 200 µg/mL in all samples tested. In general, the observed efficacy could probably be due to the phytochemical constituents of this plant.
Acknowledgment
This research was gratefully supported by MABL EAJAZ chair for Scientific Research in Prophetic Medicine, Faculty of Medicine, Taibah University, Saudi Arabia (Grant no. SMPM1434/A0102).
Conflict of Interests
The authors declare that there is no conflict of interests in this paper.
References
- 1.WHO. Cancer Fact Sheet No. 297. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 2.Trouillas P, Calliste C-A, Allais D-P, et al. Antioxidant, anti-inflammatory and antiproliferative properties of sixteen water plant extracts used in the Limousin countryside as herbal teas. Food Chemistry. 2003;80(3):399–407. [Google Scholar]
- 3.Hofer D, Schwach G, Ghaffari Tabrizi-Wizsy N, Sadjak A, Sturm S, et al. Christia vespertilionis plant extracts as novel antiproliferative agent against human neuroendocrine tumor cells. Oncology Reports. 2013;29(6):2219–2226. doi: 10.3892/or.2013.2367. [DOI] [PubMed] [Google Scholar]
- 4.Manosroi J, Dhumtanom P, Manosroi A. Anti-proliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines. Cancer Letters. 2006;235(1):114–120. doi: 10.1016/j.canlet.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 5.Lewinsohn E, Dudai N, Tadmor Y, et al. Histochemical localization of citral accumulation in lemongrass leaves (Cymbopogon citratus (DC.) Stapf., Poaceae) Annals of Botany. 1998;81(1):35–39. [Google Scholar]
- 6.Carlini E, Contar DD, Silva-Filho AR, da Silveira-Filho NG, Frochtengarten ML, Bueno OF. Pharmacology of lemongrass (Cymbopogon citratus Stapf). I. Effects of teas prepared from the leaves on laboratory animals. Journal of Ethnopharmacology. 1986;17(1):37–64. doi: 10.1016/0378-8741(86)90072-3. [DOI] [PubMed] [Google Scholar]
- 7.Cheel J, Theoduloz C, Rodríguez J, Schmeda-Hirschmann G. Free radical scavengers and antioxidants from lemongrass (Cymbopogon citratus (DC.) Stapf.) Journal of Agricultural and Food Chemistry. 2005;53(7):2511–2517. doi: 10.1021/jf0479766. [DOI] [PubMed] [Google Scholar]
- 8.Vinitketkumnuen U, Puatanachokchai R, Kongtawelert P, Lertprasertsuke N, Matsushima T. Antimutagenicity of lemon grass (Cymbopogon citratus Stapf) to various known mutagens in salmonella mutation assay. Mutation Research/Genetic Toxicology. 1994;341(1):71–75. doi: 10.1016/0165-1218(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 9.Santoro G, Cardoso M, Guimarães L, Freire J, Soares M. Anti-proliferative effect of the essential oil of Cymbopogon citratus (DC) Stapf (lemongrass) on intracellular amastigotes, bloodstream trypomastigotes and culture epimastigotes of Trypanosoma cruzi (Protozoa: Kinetoplastida) Parasitology. 2007;134(11):1649–1656. doi: 10.1017/S0031182007002958. [DOI] [PubMed] [Google Scholar]
- 10.Viana G, Vale T, Pinho R, Matos F. Antinociceptive effect of the essential oil from Cymbopogon citratus in mice. Journal of Ethnopharmacology. 2000;70(3):323–327. doi: 10.1016/s0378-8741(99)00168-3. [DOI] [PubMed] [Google Scholar]
- 11.Santin MR, dos Santos AO, Nakamura CV, Dias Filho BP, Ferreira ICP, Ueda-Nakamura T. In vitro activity of the essential oil of Cymbopogon citratus and its major component (citral) on Leishmania amazonensis . Parasitology Research. 2009;105(6):1489–1496. doi: 10.1007/s00436-009-1578-7. [DOI] [PubMed] [Google Scholar]
- 12.Oliveira VC, Moura DM, Lopes JA, de Andrade PP, da Silva NH, Figueiredo RCBQ. Effects of essential oils from Cymbopogon citratus (DC) Stapf., Lippia sidoides Cham., and Ocimum gratissimum L. on growth and ultrastructure of Leishmania chagasi promastigotes. Parasitology Research. 2009;104(5):1053–1059. doi: 10.1007/s00436-008-1288-6. [DOI] [PubMed] [Google Scholar]
- 13.Baliña LM, Graupe K. The treatment of melasma: 20% azelaic acid versus 4% hydroquinone cream. International Journal of Dermatology. 1991;30(12):893–895. doi: 10.1111/j.1365-4362.1991.tb04362.x. [DOI] [PubMed] [Google Scholar]
- 14.Sperotto A, Moura D, Péres V, et al. Cytotoxic mechanism of Piper gaudichaudianum Kunth essential oil and its major compound nerolidol. Food and Chemical Toxicology. 2013;57:57–68. doi: 10.1016/j.fct.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Ying S, Xiao M, Xianghong Y, Yunpeng L. Effect of β-elemene on proliferation, cell cycle and angiogenesis of endothelial progenitor cells. Chongqing Medicine. 2012;6, article 008 [Google Scholar]
- 16.Bomfim DS, Ferraz RP, Carvalho NC, et al. Eudesmol isomers induce caspase-mediated apoptosis in human hepatocellular carcinoma HepG2 cells. Basic & Clinical Pharmacology & Toxicology. 2013;113(5):300–306. doi: 10.1111/bcpt.12097. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann D, Dogra AK, Wink M. Myrtenal inhibits acetylcholinesterase, a known Alzheimer target. Journal of Pharmacy and Pharmacology. 2011;63(10):1368–1371. doi: 10.1111/j.2042-7158.2011.01344.x. [DOI] [PubMed] [Google Scholar]
- 18.Babu LH, Perumal S, Balasubramanian MP. Myrtenal attenuates diethylnitrosamine-induced hepatocellular carcinoma in rats by stabilizing intrinsic antioxidants and modulating apoptotic and anti-apoptotic cascades. Cellular Oncology. 2012;35(4):269–283. doi: 10.1007/s13402-012-0086-4. [DOI] [PubMed] [Google Scholar]
- 19.Hardie J, Isaacs R, Pickett JA, Wadhams LJ, Woodcock CM. Methyl salicylate and (−)-(1R, 5S)-myrtenal are plant-derived repellents for black bean aphid, Aphis fabae Scop. (Homoptera: Aphididae) Journal of Chemical Ecology. 1994;20(11):2847–2855. doi: 10.1007/BF02098393. [DOI] [PubMed] [Google Scholar]
- 20.Şarer E, Toprak SY, Otlu B, Durmaz R. Composition and antimicrobial activity of the essential oil from Mentha spicata L. subsp. Spicata . Journal of Essential Oil Research. 2011;23(1):105–108. [Google Scholar]
- 21.Shahverdi A, Rafii F, Tavassoli F, Bagheri M, Attar F, Ghahraman A. Piperitone from Mentha longifolia var. chorodictya Rech F. reduces the nitrofurantoin resistance of strains of enterobacteriaceae. Phytotherapy Research. 2004;18(11):911–914. doi: 10.1002/ptr.1566. [DOI] [PubMed] [Google Scholar]
- 22.Doković D, Bulatović V, Božić B, Kataranovski MV, Zrakić T, Kovačević NN. 3,5-nonadiyne isolated from the rhizome of Cachrys ferulacea inhibits endogenous nitric oxide release by rat peritoneal macrophages. Chemical and Pharmaceutical Bulletin. 2004;52(7):853–854. doi: 10.1248/cpb.52.853. [DOI] [PubMed] [Google Scholar]
- 23.Compagnone RS, Chavez K, Mateu E, Orsini G, Arvelo F, Suárez AI. Composition and cytotoxic activity of essential oils from Croton matourensis and Croton micans from Venezuela. Records of Natural Products. 2010;4(2):101–108. [Google Scholar]
- 24.Ping H, Zhang G, Ren G. Antidiabetic effects of cinnamon oil in diabetic KK-Ay mice. Food and Chemical Toxicology. 2010;48(8-9):2344–2349. doi: 10.1016/j.fct.2010.05.069. [DOI] [PubMed] [Google Scholar]
- 25.Dai Y, Harinantenaina L, Brodie PJ, et al. Isolation and synthesis of two antiproliferative calamenene-type sesquiterpenoids from Sterculia tavia from the Madagascar Rain Forest. Bioorganic & Medicinal Chemistry. 2012;20(24):6940–6944. doi: 10.1016/j.bmc.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carroll J, Paluch G, Coats J, Kramer M. Elemol and amyris oil repel the ticks Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in laboratory bioassays. Experimental and Applied Acarology. 2010;51(4):383–392. doi: 10.1007/s10493-009-9329-0. [DOI] [PubMed] [Google Scholar]
- 27.Fernandes ES, Passos GF, Medeiros R, et al. Anti-inflammatory effects of compounds alpha-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea . European Journal of Pharmacology. 2007;569(3):228–236. doi: 10.1016/j.ejphar.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 28.Tambe Y, Tsujiuchi H, Honda G, Ikeshiro Y, Tanaka S. Gastric cytoprotection of the non-steroidal anti-inflammatory sesquiterpene, β-caryophyllene. Planta Medica. 1996;62(5):469–470. doi: 10.1055/s-2006-957942. [DOI] [PubMed] [Google Scholar]
- 29.Chen C-C, Wu J-H, Yang N-S, et al. Cytotoxic C35 terpenoid cryptotrione from the bark of Cryptomeria japonica . Organic Letters. 2010;12(12):2786–2789. doi: 10.1021/ol1009027. [DOI] [PubMed] [Google Scholar]
- 30.Gu H-J, Cheng S-S, Huang C-G, Chen W-J, Chang S-T. Mosquito larvicidal activities of extractives from black heartwood-type Cryptomeria japonica . Parasitology Research. 2009;105(5):1455–1458. doi: 10.1007/s00436-009-1550-6. [DOI] [PubMed] [Google Scholar]
- 31.Hartmans KJ, Diepenhorst P, Bakker W, Gorris LG. The use of carvone in agriculture: sprout suppression of potatoes and antifungal activity against potato tuber and other plant diseases. Industrial Crops and Products. 1995;4(1):3–13. [Google Scholar]
- 32.Miyazawa M, Shimamura H, Nakamura S-I, Kameoka H. Antimutagenic activity of (+)-β-eudesmol and paeonol from Dioscorea japonica . Journal of Agricultural and Food Chemistry. 1996;44(7):1647–1650. [Google Scholar]
- 33.Tsuneki H, Ma E-L, Kobayashi S, et al. Antiangiogenic activity of β-eudesmol in vitro and in vivo. European Journal of Pharmacology. 2005;512(2-3):105–115. doi: 10.1016/j.ejphar.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 34.Shiokawa Y, Akahane A, Katayama H, Mitsunaga T. Use of adenosine antagonists in the prevention and treatment of pancreatitis and ulcer. EP Patent 0497258 B1, 2002.
- 35.Wang T, Jonsdottir R, Ólafsdóttir G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chemistry. 2009;116(1):240–248. [Google Scholar]
- 36.Salama SM, Abdulla MA, AlRashdi AS, Ismael S, Alkiyumi SS, Golbabapour A. Hepatoprotective effect of ethanolic extract of Curcuma longa on thioacetamide induced liver cirrhosis in rats. BMC Complementary and Alternative Medicine. 2013;13, article 56 doi: 10.1186/1472-6882-13-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagmoti DM, Khatri DK, Juvekar PR, Juvekar AR. Antioxidant activity free radical-scavenging potential of Pithecellobium dulce Benth seed extracts. Free Radicals and Antioxidants. 2012;2(2):37–43. [Google Scholar]
- 38.Khaledi H, Alhadi AA, Yehye WA, Ali HM, Abdulla MA, Hassandarvish P. Antioxidant, cytotoxic activities, and structure–activity relationship of gallic acid-based indole derivatives. Archiv der Pharmazie. 2011;344(11):703–709. doi: 10.1002/ardp.201000223. [DOI] [PubMed] [Google Scholar]
- 39.Barbosa LCA, Pereira UA, Martinazzo AP, Maltha CRÁ, Teixeira RR, Melo EDC. Evaluation of the chemical composition of Brazilian commercial Cymbopogon citratus (D.C.) Stapf samples. Molecules. 2008;13(8):1864–1874. doi: 10.3390/molecules13081864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Negrelle R, Gomes E. Cymbopogon citratus (DC.) Stapf: chemical composition and biological activities. Revista Brasileira de Plantas Medicinais. 2007;9(1):80–92. [Google Scholar]
- 41.Piaru SP, Perumal S, Cai LW, et al. Chemical composition, anti-angiogenic and cytotoxicity activities of the essential oils of Cymbopogan citratus (lemon grass) against colorectal and breast carcinoma cell lines. Journal of Essential Oil Research. 2012;24(5):453–459. [Google Scholar]
- 42.Sah SY, Sia CM, Chang SK, Ang YK, Yim HS. Antioxidant capacity and total phenolic content of lemon grass (Cymbopogon citratus) leave. Annals Food Science and Technology. 2012;13(2):150–155. [Google Scholar]
- 43.Koh PH, Mokhtar RAM, Iqbal M. Antioxidant potential of Cymbopogon citratus extract: alleviation of carbon tetrachloride-induced hepatic oxidative stress and toxicity. Human & Experimental Toxicology. 2012;31(1):81–91. doi: 10.1177/0960327111407226. [DOI] [PubMed] [Google Scholar]
- 44.Deetae P, Parichanon P, Trakunleewatthana P, Chanseetis C, Lertsiri S. Antioxidant and anti-glycation properties of Thai herbal teas in comparison with conventional teas. Food Chemistry. 2012;133(3):953–959. [Google Scholar]
- 45.Runnie I, Salleh MN, Mohamed S, Head RJ, Abeywardena MY. Vasorelaxation induced by common edible tropical plant extracts in isolated rat aorta and mesenteric vascular bed. Journal of Ethnopharmacology. 2004;92(2-3):311–316. doi: 10.1016/j.jep.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 46.Saha K, Lajis N, Israf D, et al. Evaluation of antioxidant and nitric oxide inhibitory activities of selected Malaysian medicinal plants. Journal of Ethnopharmacology. 2004;92(2-3):263–267. doi: 10.1016/j.jep.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Konrad L, Müller H-H, Lenz C, Laubinger H, Aumüller G, Lichius JJ. Antiproliferative effect on human prostate cancer cells by a stinging nettle root (Urtica dioica) extract. Planta Medica. 2000;66(1):44–47. doi: 10.1055/s-2000-11117. [DOI] [PubMed] [Google Scholar]
- 48.Dudai N, Weinstein Y, Krup M, Rabinski T, Ofir R. Citral is a new inducer of caspase-3 in tumor cell lines. Planta Medica. 2005;71(5):484–488. doi: 10.1055/s-2005-864146. [DOI] [PubMed] [Google Scholar]


