Abstract
Background. Aryl hydrocarbon receptor (AhR), a multifunctional regulator that senses and responds to environmental stimuli, plays a role in normal cell development and immune regulation. Recent evidence supports a significant link between environmental exposure and AhR in the development of allergic diseases. We sought to investigate whether AhR plays a role in mediating cockroach allergen-induced allergic immune responses. Methods. AhR expression in human lung fibroblasts from asthmatic and healthy individuals and in cockroach extract (CRE) treated human lung fibroblasts (WI-38) was examined. The role of AhR in modulating CRE induced TGFβ1 production was investigated by using AhR agonist, TCDD, antagonist CH122319, and knockdown of AhR. The role of latent TGFβ1 binding protein-1 (LTBP1) in mediating TCDD induced active TGFβ1 release was also examined. Results. AhR expression was higher in airway fibroblasts from asthmatic subjects as compared to healthy controls. AhR in fibroblasts was activated by TCDD with an increased expression of cyp1a1 and cyp1b1. Increased AhR expression was observed in CRE-treated fibroblasts. Importantly, CRE induced TGFβ1 production in fibroblasts was significantly enhanced by TCDD but inhibited by CH122319. Reduced TGFβ1 production was further confirmed in fibroblasts with AhR knockdown. Moreover, AhR knockdown inhibited CRE induced fibroblast differentiation. Furthermore, TCDD induced active TGFβ1 release was significantly inhibited by LTBP1 knockdown. Conclusion. These results provide evidence for the role of AhR in modulating cockroach allergen-induced immune responses through controlling the active TGFβ1 release, suggesting a possible synergistic effect between exposure to allergens and environmental chemicals on the development of allergic diseases.
1. Introduction
Asthma is the most prevalent serious chronic illness of children in the U.S [1]. While it is generally accepted that environmental chemicals and pollutants can contribute to the occurrence and exacerbation of asthma [2–5], the mechanistic links remain largely unknown. Specifically, environmental chemicals and pollutants have been shown to modulate environmental allergen-induced allergic diseases like asthma [6–8]. AhR is a multifunctional regulator that senses and responds to environmental stimuli and plays a role in normal cell development and immune regulation. It is known that dioxins and dioxin-like compounds, TCDD, PAH, and particulate matter (PM), can activate AhR, which then translocates to the nucleus and dimerizes with AhR nuclear translocator (ARNT). Within the nucleus, the AhR/ARNT heterodimer binds to xenobiotic responsive element (XRE) sequences and leads to changes in gene transcription (e.g., cyp1a1, cyp1b1) and a variety of toxicological effects, such as ROS generation, cell differentiation, and inflammatory cytokine production [9, 10]. Recent discoveries regarding AhR and environmental toxicant interaction and its influence on immune responses [11–14] highlight the potential link between environmental exposure and AhR in modulating allergen-induced allergic diseases. One intriguing and plausible hypothesis is that the expression of allergic and inflammatory disease could be attributable to those new immune “adjuvant” factors, for example, environmental chemicals, which often coexist with allergens and contribute to progressive fibrosis and pathological remodeling in asthma.
Indeed, recent studies have changed our understanding of asthma from that of a purely inflammatory disease to a disease in which both inflammatory and structural components are equally involved [15]. It has been suggested that there is a strong link between allergen-induced allergic airway inflammation and remodeling [16, 17]. In particular, exposure to cockroach allergen in early life can lead to allergic airway inflammation and an increased risk of developing asthma [18, 19]. One of the central components of airway remodeling is subepithelial fibrosis caused by deposition of collagen in asthma, a process in which fibroblasts and myofibroblast are critical. It has been shown that there is a correlation between the number of myofibroblasts and the degree of subepithelial fibrosis in the airway of asthmatic patients [20]. Of interest to us, allergens can induce an increase in the number of myofibroblasts [21], which may contribute to the progression of subepithelial fibrosis [22]. Recruitment of fibroblasts to the airway in asthma has been suggested to be potentiated by IL13 through a mechanism involving transforming growth factor-beta1 (TGF-β1) and MMPs [23].
TGF-β1 is produced by many cells within the lung, including fibroblasts, and plays a critical role in cell growth, differentiation, and immune regulation, and has been considered a principal mediator of airway remodeling [24–27]. Recent studies have demonstrated that disruption in TGFβ1 signaling imposes a strong predisposition for human allergic diseases [28]. Specifically, increased active TGFβ1 has been observed in airways from asthmatic patients [29] and from experimental mice during allergic airway inflammation [30] (Gao et al. JI 2014 in revision). Furthermore, increased TGFβ1 activity appears to be controlled by latent TGFβ-binding protein-1 (LTBP-1) [31]. Interestingly, primary mouse embryo fibroblasts from AhR−/− mice had increased expression of LTBP1 and higher levels of total and active TGFβ1 that can be partially blocked by antibodies against LTBP1 [32], suggesting that AhR may control LTBP1 expression and subsequently activation of TGFβ1 signaling. Although TGFβ1 signaling and the AhR pathway have been well-studied, detailed information about the interaction between AhR and TGFβ1 signaling remains largely unknown. Several mechanisms have been suggested regarding the regulation of AhR on TGFβ1 signaling, including deregulation of TGFβ1 secretion, suppression of TGFβ1, or downregulation of the LTBP1 expression [11, 32, 33]. Furthermore, studies on ITE, an AhR agonist, have suggested that ITE can disrupt TGFβ1 signaling by inhibiting the nuclear translocation of Smad2/3/4 and block TGFβ1-induced myofibroblast differentiation and extracellular matrix production [34]. TGFβ1, in turn, can suppress the AhR mediated gene expression through deregulation of AhR expression and/or localization [35]. Thus, studies on the cross-regulation between AhR and TGFβ1 signaling might be essential for better understanding of the underlying mechanisms for the synergic effects between environmental chemicals and allergens on the development of allergic diseases. Specifically, we postulated that active TGFβ1, released from fibroblasts or epithelium damaged by repeated environmental exposure, induces cell differentiation and immune regulation and is regulated by AhR.
In the present study, we have specifically focused on the functional significance of AhR in modulating cockroach allergen-induced immune responses through the release of active TGFβ1. We found increased AhR expression in airways of asthmatic patients. Especially, AhR expression was increased in WI-38, a lung fibroblast cell line, after exposure to cockroach allergen. We then demonstrated that TCDD, an AhR agonist, can induce AhR specific downstream genes cyp1a1 and cyp1b1 expression. Importantly, we found the modulating effects of AhR on cockroach allergen-induced TGFβ1 production and fibroblast differentiation. Finally, we demonstrated that TCDD-induced active TGFβ1 was significantly blocked by LTBP knockdown. Taken together, we suggest that AhR plays a role in modulating cockroach allergen-induced immune responses by controlling the active TGFβ1 release, and there is a possible synergistic effect between exposure to allergens and environmental chemicals on the development of allergic diseases.
2. Materials and Methods
2.1. Assessment of AhR in Human Airway
Paraffin-embedded human airway sections (5 μm) from asthmatic (n = 4) and healthy individuals (n = 4) were used for immunofluorescence analysis. These human airway sections were provided by Dr. Allen (Johns Hopkins Asthma & Allergen Center). While it is not clear about the allergic status for those asthmatic subjects, all healthy individuals were nonallergic individuals. The human airway sections from three nonallergic heavy smokers also included controlling for AhR expression. In brief, nonspecific binding was blocked using 10% blocking serum in PBS for 1 hour. Sections were subsequently incubated with anti-human polyclonal antibody against Aryl hydrocarbon receptor (ab84833, 1 : 20, Abcam) and fibroblast marker antibody ER-TR7 (sc-73355, 1 : 50, Santa Cruz Biotechnology) overnight at 4°C. Normal rabbit IgG (Sigma-Aldrich) and rat IgG were used as a negative control. Sections were then incubated with Alexa Fluor 594-labeled goat anti-rabbit and FITC-labeled rabbit anti-rat secondary antibodies IgG at 1/100 dilutor for 2 hrs at RT. Nuclei were counterstained with 4′,6-diamidino-2-phenylin-dole, dihydrochloride (DAPI) (Sigma-Aldrich). Sections were subsequently dehydrated, mounted, and observed under the fluorescent microscope. The slides were evaluated using micrographs taken by a fluorescent microscope (Olympus BX-5). Imaging software (iVision; Biovision) was used to analyze areas of positive staining.
2.2. Flow Cytometry
WI-38 cells were fixed with BD Cytofix/Cytoperm solution for 30 min and then incubated with specific first antibody or isotype control for 30 min at 4°C in the dark. Then the cells were washed and incubated with fluorescent-conjugated second antibody. The following antibodies were used: anti-AhR (ab2770, Abcam) and anti-pSmad2/3(cell signaling). The samples were then analyzed on a FACSCalibur flow cytometer (BD Biosystems).
2.3. Immunocytochemical Analysis in WI 38 Cells
Cultured cells were fixed with 10% formalin at room temperature (RT) for 10 minutes and permeabilized for 5 mins with PBS containing Triton X100 and BSA buffer (0.3% TTX, 1% bovine serum albumin: TTX/BSA buffer). The cells were further blocked in 10% blocking serum for 30 min and then incubated with first antibody for 1 hour at RT. After washing with PBS, cells were incubated with fluorescent labelled secondary antibodies for 30 minutes at RT. Nuclei were counterstained with DAPI. Sections were subsequently dehydrated, mounted, and observed under the fluorescent microscope. The following antibodies were used: anti-AhR primary antibody (Abcam, ab2770, 1 : 20); anti-α-SMA (Abcam, ab32575); and anti-vimentin (eBioscience).
2.4. Quantitative Real-Time RT-PCR (qRT-PCR)
Total RNA from WI38 was extracted with RNAeasy kit (Qiagen, Valencia, CA). RT-PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems, USA) according to the manufacturer's protocol. The primers for each gene were designed on the basis of the respective mRNA sequences so that the targets were 100–200 bp in length. Relative mRNA expression was calculated by normalization of all expression levels to actinand then compared to untreated control cells by the ΔΔCT method as described previously [36]. The following primers were used:
Actin: F, AGAAAATCTGGCACCACACC; R, CAGAGGCGTACAGGGATAGC; AhR: F, GTCGTCTAAGGTGTCTGCTGGA; R, CGCAAACAAAGCCAACTGAGGTG; cyp1a1: F, GATTGAGCACTGTCAGGAGAAGC; R, ATGAGGCTCCAGGAGATAGCAG; cyp1b1: F, GCCACTATCACTGACATCTTCGG; R, CACGACCTGATCCAATTCTGCC; ltbp1: F, TGAATGCCAGCACCGTCATCTC; R, CTGGCAAACACTCTTGTCCTCC; ltbp-2: F, CTGCACAGATGACAACGAGTGTC; R, AGAGTGTAGCCAGGGTAGCAGA; ltbp-3: F, CGGTCACTACAAGTGCAACTGC; R, CTTGTTCTCGCATTTGCCATCCG; ltbp-4: F, TTCCAGTGCAGGACCTGTCCTT; R: GAAGGAGCCTTCGGTGTTAGTG.
2.5. ELISA
Supernatants from cultured fibroblasts (WI38) were collected and measured for active TGFβ1 by ELISA (eBiosciences), according to the manufacturer's instructions. Results were read with a Bio-Rad Bio-Plex instrument (Bio-Rad Laboratories, Hercules, CA).
2.6. Gene Knockdown by siRNA
Transcriptional knockdown was performed by transfection with siRNA oligonucleotide duplexes as a final concentration of 20 nM in DMEM using DharmaFECT transfection reagent (Thermo Scientific, Waltham, MA). A siRNA/transfection reagent complex was formed when siRNA and transfection reagent were mixed for 20 minutes. Then the transfection complex was added to each experimental well and incubated in the serum free media for 6 hours at 37°C. The transfection medium was replaced with complete medium and incubated at 37°C for an additional 24–48 hours. The gene knockdown was confirmed by qRT-PCR and western blotting. AhR specific siRNA was purchased from Thermo Scientific and LTBP1 specific siRNA was purchased from Sigma.
2.7. Western Blotting
Cells were washed twice with ice cold PBS and lysed in RIPA buffer containing protease and phosphatase inhibitor cocktails (Sigma). Protein content was measured with BCA reagent (Pierce). Equivalent protein samples were subjected to SDS-PAGE electrophoresis and then transferred to a polyvinylidene difluoride membrane (Millipore). After blocking with 5% nonfat dry milk in TBST, the membrane was incubated with primary anti-AhR (Abcam), anti-LTBP1 (GeneTex), anti-α-SMA (Abcam, ab32575), anti-vimentin (eBioscience), or anti-β-actin (clone C4, Santa Cruz) antibody as a loading control for normalization. Proteins reactive with primary Abs were visualized with HRP-conjugated secondary Ab and ECL reagents (Amersham). The levels of proteins were quantified by ImageJ (National Institutes of Health, USA) for the densitometric analysis of the band intensities and normalized to those of β-actin.
2.8. Statistical Analysis
Data are expressed as the means ± SEM for each group. Statistical significance for normally distributed samples was assessed using an independent two-tailed Student's t-test or with analysis of variance by using GraphPad Prism version 5.1 software (GraphPad Software, La Jolla, CA). Differences with P < 0.05 were considered statistically significant.
3. Results
3.1. Increased AhR Expression in Fibroblasts from Asthmatic Patients
To examine whether there was a differential expression for AhR in asthmatic and healthy individuals, we performed immunofluorescence analysis for both AhR and fibroblast marker ER-TR7 in human airway sections. Compared to healthy individuals (Figure 1, middle panel), the airway sections from asthmatic patients showed significant expression of AhR, increased fibroblasts marker ER-TR7, and thickening of basal membranes (Figure 1, top panel). Particularly, AhR was predominantly expressed in fibroblasts and basal membranes. Interestingly, significantly increased AhR expression was also observed in airway fibroblasts from heavy smokers (Figure 1, bottom panel). These findings suggest an increased AhR expression in fibroblasts from asthmatic patients and possibly from those who are repeatedly exposed to smoking.
Figure 1.
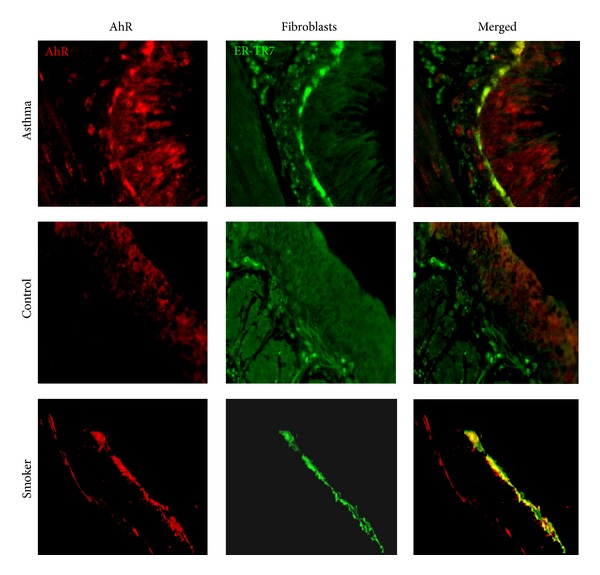
AhR expression in human airway. Immunofluorescence analysis of AhR expression in the airway, particularly fibroblasts from asthmatics (top), healthy individuals (middle), and heavy smokers (bottom), for antibodies against AhR (red) and fibroblasts marker (ER-TR-7, green). Figure 1 represents 4 individuals from each group.
3.2. Increased AhR Expression in CRE-Treated Human Lung Fibroblasts
To delineate the role of AhR in the regulation of fibroblast's function and its mechanisms, we used human lung fibroblast cell line as an in vitro model. To validate AhR expression in fibroblasts, we detected AhR expression in WI-38, a human lung fibroblast cell line, by flow cytometry and western blot (data not shown). We found that AhR was constitutively expressed in fibroblasts (Figure 2(a)). We next examined whether AhR is functional; we treated fibroblasts using different doses of TCDD known AhR ligands (0.1 nM and 1 nM) for 2 to 48 hours; expression of AhR downstream genes cyp1a1 (Figure 2(b)) and cyp1b1 (Figure 2(c)) was examined by RT-PCR. Compared to those untreated fibroblasts, an increased expression was noted in TCDD treated fibroblasts for cyp1a1 in a dose- and time-dependent manner. There was nearly a 2-fold increase in cyp1a1 expression after treatment with 1.0 nM TCDD for 48 hours. Similarly, an 18.5-fold increase was observed for cyp1b1 when 1.0 nM TCDD was used to treat fibroblasts for 48 hours, suggesting that TCDD can activate the AhR pathway in fibroblasts. Furthermore, to investigate whether CRE can induce AhR expression, we treated fibroblasts with 50 μg/mL CRE for 2–48 hours and AhR expression was examined by RT-PCR. An increased AhR expression was seen with a peak level at 4 hours for a 5.7-fold increase when compared with untreated cells (Figure 2(d)). Increased AhR expression in a dose-dependent manner was further observed by immunofluorescence analysis (Figure 2(e)), suggesting that cockroach allergen can induce AhR expression which may be critical in modulating allergen-induced immune responses.
Figure 2.
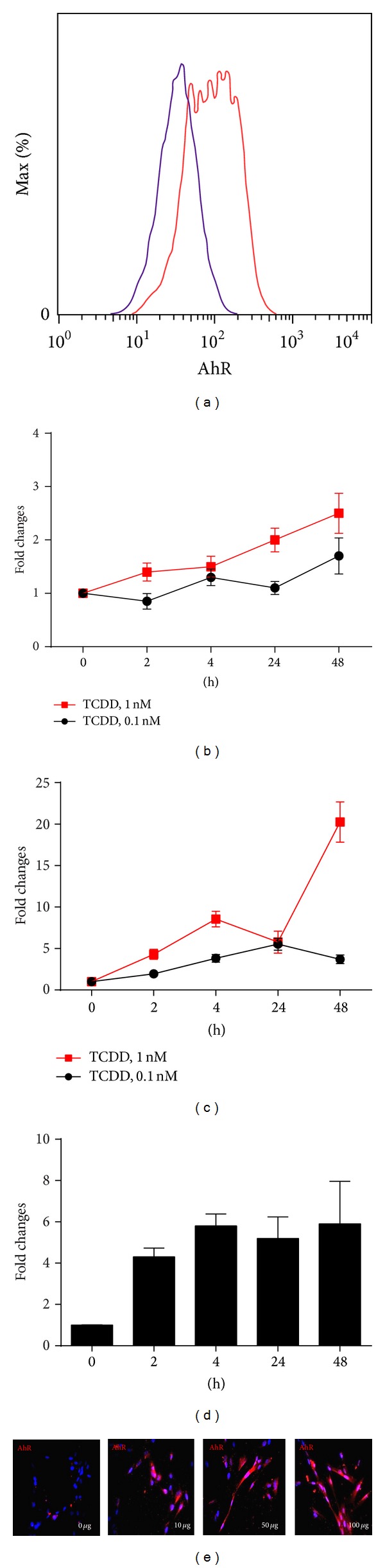
AhR expression in CRE-treated human lung fibroblasts. (a) AhR expression in WI-38 was detected using antibody against AhR (red line) and IgG2 (blue line) by flow cytometry. (b-c) Fibroblasts were treated with different doses of TCDD (0.1 nM and 1 nM) for 2 to 48 hours; expressions of cyp1a1 (b) and cyp1b1 (c) were examined by RT-PCR. (d) Fibroblasts were treated with 50 μg/mL CRE for 2–48 hours and AhR expression was examined by RT-PCR. (e) Immunofluorescence analysis of AhR expression in CRE-treated fibroblasts at various doses (0–100 μg/mL). Bars represent mean ± SEM of 3 independent experiments.
3.3. AhR Modulates CRE Induced TGFβ1 Production in Fibroblasts
To investigate whether AhR can modulate cockroach allergen-induced TGFβ1 production that may control cell differentiation and immune regulation, we treated fibroblasts with CRE (50 μg/mL) in the presence or absence of AhR agonist TCDD or antagonist CH122319 and detected the levels of active TGFβ1 in supernatants of cultured and treated fibroblasts. We found that TCDD induced a significant release of active TGFβ1 by fibroblasts that were treated with TCDD at various doses (1.0, 10 nM) and times (24, 48 hours, Figure 3(a)). Both cockroach allergen and TCDD as an individual induced increased levels of active TGFβ1 by fibroblasts. Interestingly, the increased TGFβ1 was further enhanced when both of them were combined (Figure 3(b)). In contrast, AhR antagonist CH122319 inhibited CRE+TCDD induced TGFβ1 production (Figure 3(b)). Cockroach allergen-induced active TGFβ1 release and the inhibition of CH122319 were further confirmed by using natural purified cockroach allergen, Bla g2 (Figure 3(c)). These findings suggest that AhR can modulate cockroach allergen-induced TGFβ1 production.
Figure 3.
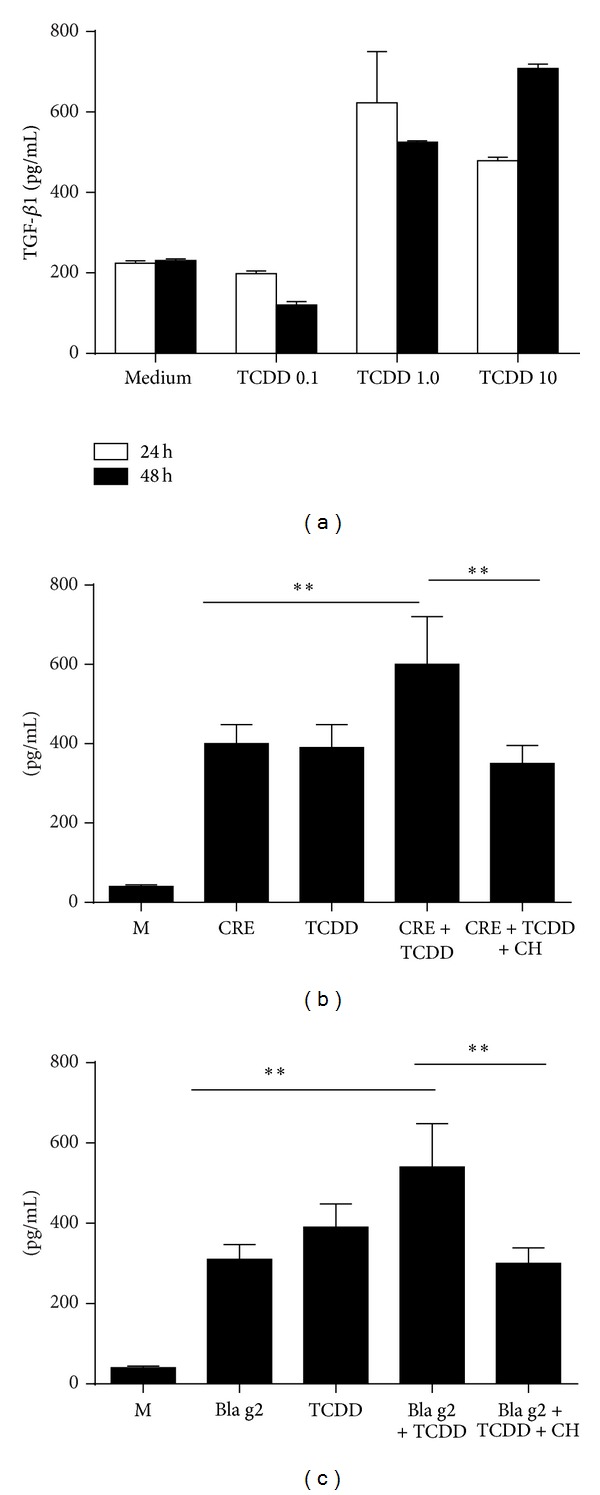
AhR modulates cockroach allergen-induced TGFβ1 production. (a) TCDD can induce TGFβ1 secretion by fibroblasts and (b) cockroach allergen-induced TGFβ1 secretion by fibroblasts can be further enhanced by TCDD but inhibited by AhR antagonist CH122319 (c). (c) Cockroach allergen-induced TGFβ1 production and the inhibitory role of CH122319 were further confirmed when Bla g2 was used. Bars represent mean ± SEM of 3 independent experiments. *P < 0.05, **P < 0.01.
3.4. Reduced Levels of Active TGFβ1 in Fibroblasts with AhR Knockdown
To further examine the modulation of AhR on cockroach allergen-induced TGFβ1 production, we knocked down AhR in fibroblasts using siRNAs. The AhR knockdown was validated by RT-PCR (Figure 4(a)), which showed at least 60% knockdown for si-RNA-2, and by western blotting (Figure 4(b)). AhR expression in Figure 4(b) was further quantified by ImageJ for the densitometric analysis of the band intensities and normalized to those of β-actin and then compared to scramble si-RNA treated group (Figure 4(c)). There was at least 70% knockdown for si-RNA-2. We next used fibroblasts with AhR knocked down by si-AhR-2 to detect TGFβ1 expression at the RNA levels by RT-PCR (Figure 4(d)) and active TGFβ1 secretion by ELISA (Figure 4(e)). We found that TGFβ1 expression at the RNA level or in secretion was significantly reduced for fibroblasts with AhR knockdown. To examine whether AhR knockdown can affect the activation of TGFβ signaling, we treated those fibroblasts with or without AhR knockdown with 5 ng/mL TGFβ1 and measured phosphorylated Smad2/3 (p-Smad2/3) at various times by flow cytometry (Figure 4(f)). We noted an increased activation of Smad2/3 at 15 min for all these treated cells but we noted a decline at 30 mins and 120 mins. Interestingly, fibroblasts with AhR knockdown showed remarkable reduction in the levels of p-Smad2/3 at 120 mins as compared to the control cells. These data suggest that there may be a crosstalk between AhR pathway and TGFβ1 pathway.
Figure 4.
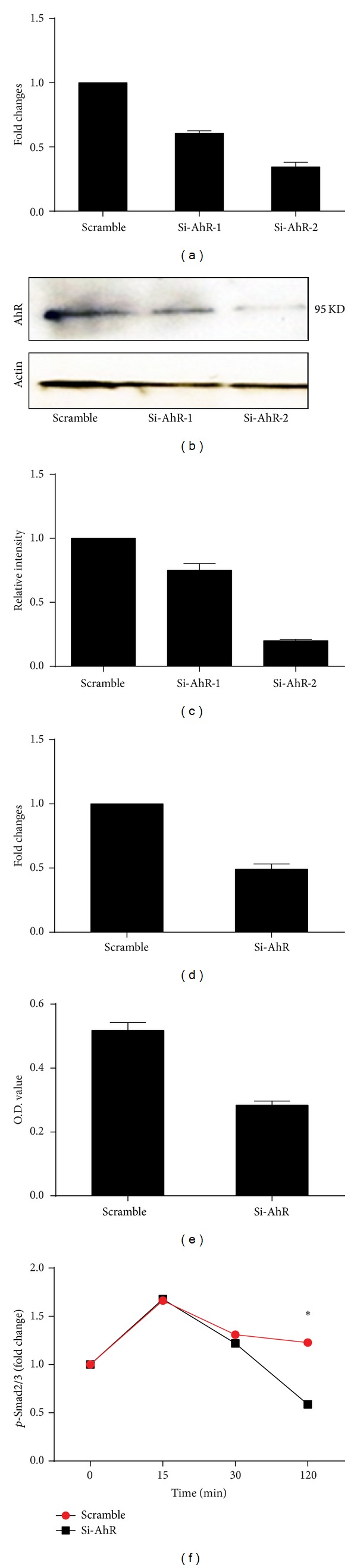
TGFβ1 production was reduced in fibroblasts with AhR knockdown by siRNA. (a–c) AhR knockdown by siRNAs was confirmed by RT-PCR (a) and western blotting (b). (c) AhR expression in (b) was quantified by ImageJ and normalized to those of β-actin and then compared to scramble si-RNA treated group. (d) TGFβ1 expression in fibroblasts treated with scrambled siRNA and si-AhR. (e) Levels of secreted TGFβ1 stimulated with CRE by fibroblasts treated with scramble si-RNA and si-AhR. (f) WI38 cells were stimulated with 5 ng/mL TGFβ1 for indicated time after AhR knockdown for 48 h; p-Smad2/3 was detected with flow cytometry. Bars represent mean ± SEM of 3 independent experiments. *P < 0.05, **P < 0.01.
3.5. AhR Modulates CRE Induced Fibroblast Differentiation
To examine whether AhR controls fibroblast differentiation induced by CRE, we cultured fibroblasts with and without AhR knockdown and treated with CRE (50 μg/mL) for 24 and 72 hours. Differentiation of fibroblasts was evaluated by the expression of α-SMA with DAPI for nuclei immune-staining. While no clear change was noted in the α-SMA expression for fibroblasts with and without AhR knockdown at basal levels and 24 hours, an increased expression was seen when treated with CRE for 72 hours for fibroblasts without AhR knockdown. The α-SMA positive staining in fibroblasts was quantified and analyzed by Imaging software (Figure 5(b)). A significantly greater expression of α-SMA was observed for fibroblasts with CRE treatment. Finally, reduced α-SMA expression was detected by RT-PCR in fibroblasts with AhR knockdown as compared to those without gene knockdown (Figure 5(c)), suggesting that AhR may control cockroach allergen-induced differentiation. CRE induced fibroblast differentiation was further confirmed by immune-staining (Figure 5(d)) and western blots (Figure 5(e)) with another myofibroblast marker, vimentin.
Figure 5.
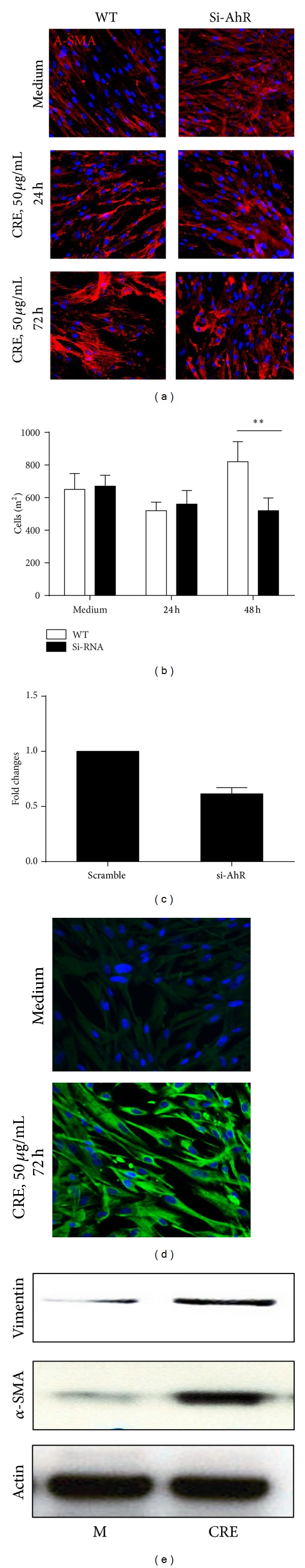
AhR controls fibroblast differentiation induced by CRE. (a) Differentiation of fibroblasts with and without AhR knockdown was evaluated by the expression of α-SMA with DAPI for nuclei immune-staining after cells were treated with CRE (50 μg/mL) for 24 and 72 hours. (b) Positive staining for α-SMA was analyzed by Imaging software (iVision; Biovision). (c) α-SMA expression was detected by RT-PCR in fibroblasts with or without AhR knockdown. Bars represent mean ± SEM of 3 independent experiments, **P < 0.01. (d) Vimentin was detected by immune-staining after cells were treated with CRE (50 μg/mL) for 72 hours. (e) Both α-SMA and vimentin expression were detected by western blots after WI38 cells were treated with 50 μg/mL CRE for 72 h.
3.6. Increased LTBP1 Expression in TCDD Treated Fibroblasts
TGFβ1 activity has been shown to be controlled by LTBP-1 [31]. Furthermore, it has been suggested that AhR may control LTBP1 expression, and subsequently activation of TGFβ1 signaling [32]. To investigate whether the AhR ligand can activate LTBP1 that may control TGFβ1 release, we treated fibroblasts using different doses of TCDD (0.1 nM and 10 nM) for different times (2 to 48 hours), and expression of LTBP1 to 4 at the RNA levels was measured by RT-PCR. Of these, increased LTBP1 was observed after treatment with TCDD (Figure 6(a)). The peak level was at 4 h for 0.1 nM and 24 h for 1 nM TCDD, respectively. Reduced expression of LTBP-2 was detected (Figure 6(b)), while no significant changes of LTBP-3 and 4 were detected (data not shown). To see the importance of increased LTBP-1 in AhR modulating active TGFβ1 release, we pretreated fibroblasts with LTBP-1 specific siRNA. We found that TCDD induced active TGFβ1 was significantly inhibited when LTBP-1 was knockdown (Figure 6(c)), suggesting that LTBP-1 may be critical in AhR controlling the release of active TGFβ1.
Figure 6.
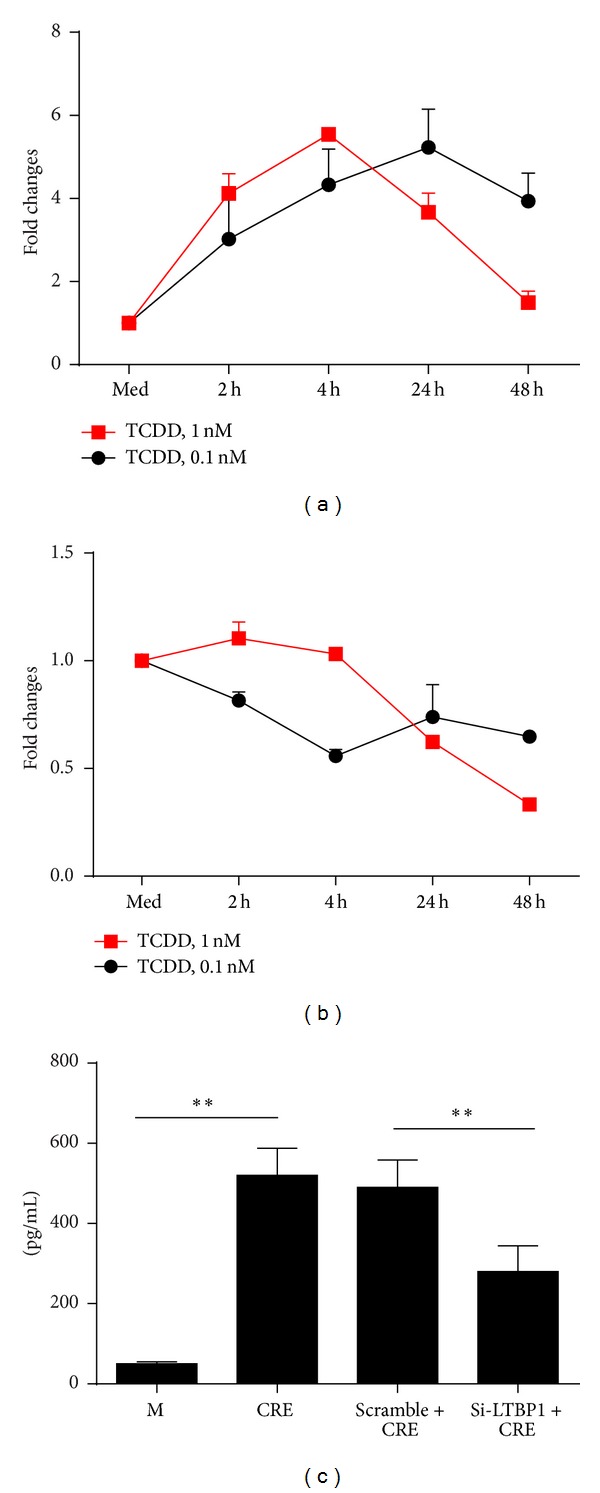
AhR controls LTBP-1 expression in fibroblasts. (a-b) Fibroblasts were treated with TCDD at 0.1 nM and 1 nM for 2 to 48 hours; expression of LTBP1 (a) and LTBP-2 (b) was detected by RT-PCR. Each point represents mean ± SEM of at least 3 independent experiments. (c) WI38 cells were treated with LTBP1 siRNA, scramble RNA, or medium control for 24 h and then treated with 50 μg/mL CRE for 24 h; active form TGFβ1 in the supernatant was detect with ELISA.
4. Discussion
In the present study, we investigated the functional significance of AhR in modulating cockroach allergen-induced immune responses by controlling the release of active TGFβ1. We found an increased AhR expression in the airways of asthmatic patients, mainly in airway epithelium and fibroblasts. While it is recognized that dioxins and dioxin-like compounds, TCDD, PAH, and PM, can activate AhR and lead to ROS generation, cell differentiation, and inflammatory cytokine production, we, for the first time, found that AhR ligands could enhance cockroach allergen-induced active TGFβ1 production. The findings may suggest a possible biological link between environmental exposure and AhR in modulating allergen-induced allergic diseases. Because of the increased expression of AhR in epithelium, and predominantly in fibroblasts of the thickening basal membrane from asthmatic patients, it is possible that AhR as a sensor for environmental chemicals and allergens contributes to progressive fibrosis and pathological remodeling in asthma.
TGFβ1 has been shown to be critical in cell growth, differentiation, and immune regulation, and a principal mediator of airway remodeling [24–27]. Our recent studies have observed an increased active TGFβ1 in airways from cockroach allergen-induced mouse models (Gao et al., submitted 2013). In this study, we found that cockroach allergen can induce an increased production of active TGFβ1 in fibroblasts. It is known that TGFβ1 and AhR signaling pathways can crossregulate each other in a cell-specific manner [37]. We found that TCDD as an AhR agonist can enhance cockroach allergen-induced TGFβ1 production, but CH122319 as an antagonist can inhibit allergen-induced TGFβ1 secretion. TCDD that we used in this study has been considered as the most potent AhR ligand known and has been shown to be only slightly metabolized and to be relatively slowly excreted [38, 39]. The role of AhR in regulating cockroach allergen-induced TGFβ1 release was further confirmed by using Bla g2, a purified cockroach allergen with undetectable endotoxin levels, and by using a human fibroblast cell line (WI38) with or without AhR knockdown. Particularly, the fibroblasts with AhR knockdown showed reduction of the levels of active TGFβ1 as compared to the control cells. Furthermore, those cells with AhR knockdown showed remarkable reduction in the levels of p-Smad2/3. These findings suggest that AhR may be a positive regulator of cockroach allergen-induced TGFβ1 signaling in human fibroblasts. Our findings seem to be contradictory to several previous studies suggesting a negative role of AhR in the initiation of food allergic responses [40, 41], regulation of TGFβ1 secretion, and LTBP1 expression [11, 32, 42]. For instance, ITE, an AhR agonist, has been demonstrated to disrupt TGFβ1 signaling by inhibiting the nuclear translocation of Smad2/3/4 and to block TGFβ1-induced myofibroblast differentiation and extracellular matrix production [34]. In contrast, some other studies suggest that constitutive AhR activity positively controls TGFβ1, TGFβ2, and LTBP-1 in malignant glioma cells [43]. Thus, we postulate that there may be significant complexity of the regulatory mechanisms of AhR on TGFβ1 signaling with coregulation of multiple other pathways.
A significant correlation has been observed between the number of myofibroblasts and the degree of subepithelial fibrosis in the airway of asthmatic patients [20], and fibroblasts have been recognized as major players by differentiation into myofibroblasts that may control the development subepithelial fibrosis in asthma. Further, TGF-β1 has been suggested to recruit fibroblasts to the airway in asthma [23]. We thus investigated whether AhR controls fibroblast differentiation after exposure to cockroach allergen. We found that, while there was no clear change in α-SMA expression (a marker for myofibroblasts) for fibroblasts with and without AhR knockdown at basal levels and at 24 hours, inhibited differentiation of fibroblasts was observed in AhR knocked-down fibroblasts with CRE treatment for 72 hours. This was further confirmed by showing a reduced α-SMA expression at the transcriptional level. Interestingly, a significant reduction in fibroblast proliferation was also noted at 72 hours (data not shown). Thus, we, for the first time, illustrate that AhR may control cockroach allergen-induced fibroblast differentiation that is critical in controlling subepithelial fibrosis and airway remodelling.
To explore the possible underlying mechanisms of AhR modulating allergen-induced TGFβ1 signaling, we investigated LTBP1, which has been shown to be critical in controlling TGFβ1 activity [31]. AhR has been shown to regulate Ltbp-1 transcription by a mechanism involving recruitment of coactivators such as CREB1 and corepressors such as HDAC2 to the Ltbp-1 promoter [44]. We thus hypothesized that AhR may regulate LTBP-1 transcription, control gene expression, and subsequently activate TGFβ1 signaling [32]. Indeed, an increased LTBP1 was observed after treatment with TCDD with the peak levels of expression at early time points (4 h). In contrast, LTBP-2 expression was reduced, and LTBP-3 and 4 remained unchanged after treatment with TCDD (data not shown). Most importantly, AhR modulating active TGFβ1 release was significantly blocked when LTBP-1 was knocked down. The findings support our initial hypothesis that LTBP-1 is critical in controlling AhR mediated TGFβ1 secretion. Although the mechanism is not clear regarding the regulation of active TGFβ1 by LTBP-1, studies have suggested that LTBP-1 contributes to TGF-beta1 activation, possibly through a process involving extracellular protease activities [31]. However, our findings in human fibroblasts showed that activation of AhR pathway can increase LTBP-1 expression, but in contrast, studies on the primary mouse embryo fibroblasts from AhR-/- mice also showed an increased expression of LTBP1 and higher levels of active TGFβ1 [32]. So far, the reasons for the discrepancies between studies from human and mouse fibroblasts with or without AhR knockdown are largely unknown, a subject which would be of interest to pursue in the future.
Taken together, this study provides evidence for the contribution of AhR to the mechanism regulating cockroach allergen-induced activation of TGFβ1. In particular, studies on the interplay between AhR and TGFβ1 pathways may better help us understand the potential mechanisms regarding the environmental chemical exposure modulating allergen-induced immune responses. As shown in Figure 7, we propose a model of the role of AhR in modulating environmental chemicals and allergen-induced activation of TGFβ1 signaling (partially modified based on the article by Starsichova et al. 2012) [35]. Briefly, epithelial cells damaged by repeated exposure to environmental chemicals and allergens can release active TGFβ1, which is largely controlled by LTBP-1. On the other hand, environmental chemicals bind AhR, leading to its activation, which plays a role in the control of LTBP-1 transcription, activation of TGFβ1 signaling, and subsequently the control of cell proliferation, differentiation, and airway remodeling. These studies provide an important basis for a further detailed investigation of the interaction between AhR and TGFβ1 signaling in environmental chemicals and allergens induced inflammation and repair/remodeling in asthma.
Figure 7.
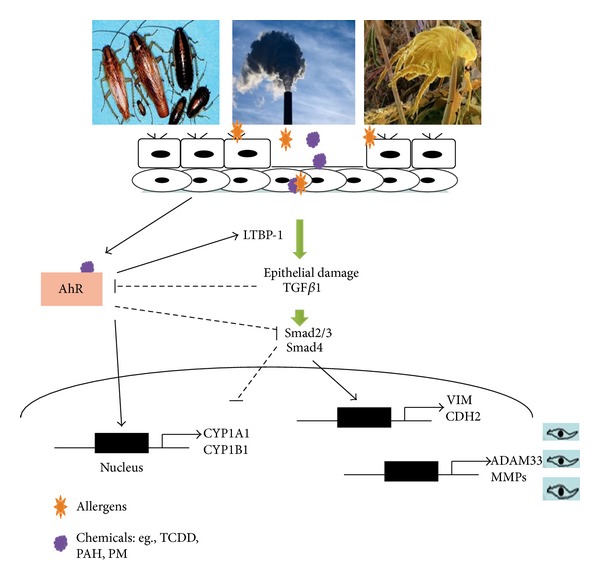
Proposed model of the role of AhR in modulating environmental chemicals and allergens induced activation of TGFβ1 signaling (modified from an article by Denison and Nagy 2003) [38].
Acknowledgments
This research was supported by the National Institutes of Health (NIH), Grants 1R21AI088406 and RO1ES021739. The authors thank Liang Yan for her excellent technical assistance.
Abbreviations
- CRE:
Cockroach extract
- AhR:
Aryl hydrocarbon receptor (AhR)
- TCDD:
2,3,7,8-Tetrachlorodibenzo-p-dioxin
- TGFβ:
Transforming growth factor β
- cyp1a1:
Cytochrome P450, family 1, subfamily B
- cyp1b1:
Cytochrome P450, family 1, subfamily B
- LTBP-1:
Latent TGFβ-binding protein-1 (LTBP-1)
- α-SMA:
α-Smooth muscle actin.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA dissemination committee report. Allergy. 2004;59(5):469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Jenerowicz D, Silny W, Danczak-Pazdrowska A, Polanska A, Osmola-Mankowska A, Olek-Hrab K. Environmental factors and allergic diseases. Annals of Agricultural and Environmental Medicine. 2012;19(3):475–481. [PubMed] [Google Scholar]
- 3.Hwang B-F, Lee Y-L, Lin Y-C, Jaakkola JJK, Guo YL. Traffic related air pollution as a determinant of asthma among Taiwanese school children. Thorax. 2005;60(6):467–473. doi: 10.1136/thx.2004.033977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansel NN, McCormack MC, Belli AJ, et al. In-home air pollution is linked to respiratory morbidity in former smokers with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2013;187(10):1085–1090. doi: 10.1164/rccm.201211-1987OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishimura KK, Galanter JM, Roth LA, et al. Early-life air pollution and asthma risk in minority children. The GALA II and SAGE II studies. American Journal of Respiratory and Critical Care Medicine. 2013;188(3):309–318. doi: 10.1164/rccm.201302-0264OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J-T, Son J-Y, Cho Y-S. The adverse effects of fine particle air pollution on respiratory function in the elderly. Science of the Total Environment. 2007;385(1–3):28–36. doi: 10.1016/j.scitotenv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Ito K, Mathes R, Ross Z, Nádas A, Thurston G, Matte T. Fine particulate matter constituents associated with cardiovascular hospitalizations and mortality in New York City. Environmental Health Perspectives. 2011;119(4):467–473. doi: 10.1289/ehp.1002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou J, Ito K, Lall R, Lippmann M, Thurston G. Time-series analysis of mortality effects of fine particulate matter components in Detroit and seattle. Environmental Health Perspectives. 2011;119(4):461–466. doi: 10.1289/ehp.1002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohtake F, Takeyama K-I, Matsumoto T, et al. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature. 2003;423(6939):545–550. doi: 10.1038/nature01606. [DOI] [PubMed] [Google Scholar]
- 10.Puga A, Tomlinson CR, Xia Y. Ah receptor signals cross-talk with multiple developmental pathways. Biochemical Pharmacology. 2005;69(2):199–207. doi: 10.1016/j.bcp.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 11.Quintana FJ, Basso AS, Iglesias AH, et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 12.Veldhoen M, Hirota K, Westendorf AM, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453(7191):106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 13.Masuda K, Kimura A, Hanieh H, et al. Aryl hydrocarbon receptor negatively regulates LPS-induced IL-6 production through suppression of histamine production in macrophages. International Immunology. 2011;23(10):637–645. doi: 10.1093/intimm/dxr072. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Tung HY, Tsai YM, et al. Aryl hydrocarbon receptor controls murine mast cell homeostasis. Blood. 2013;121(16):3195–3204. doi: 10.1182/blood-2012-08-453597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Muhsen S, Johnson JR, Hamid Q. Remodeling in asthma. Journal of Allergy and Clinical Immunology. 2011;128(3):451–462. doi: 10.1016/j.jaci.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 16.Phipps S, Benyahia F, Ou T-T, Barkans J, Robinson DS, Kay AB. Acute allergen-induced airway remodeling in atopic asthma. American Journal of Respiratory Cell and Molecular Biology. 2004;31(6):626–632. doi: 10.1165/rcmb.2004-0193OC. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd CM, Robinson DS. Allergen-induced airway remodelling. European Respiratory Journal. 2007;29(5):1020–1032. doi: 10.1183/09031936.00150305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. The New England Journal of Medicine. 1997;336(19):1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 19.Matsui EC, Wood RA, Rand C, et al. Cockroach allergen exposure and sensitization in suburban middle-class children with asthma. Journal of Allergy and Clinical Immunology. 2003;112(1):87–92. doi: 10.1067/mai.2003.1588. [DOI] [PubMed] [Google Scholar]
- 20.Brewster CE, Howarth PH, Djukanovic R, Wilson J, Holgate ST, Roche WR. Myofibroblasts and subepithelial fibrosis in bronchial asthma. American Journal of Respiratory Cell and Molecular Biology. 1990;3(5):507–511. doi: 10.1165/ajrcmb/3.5.507. [DOI] [PubMed] [Google Scholar]
- 21.Gizycki MJ, Ädelroth E, Rogers AV, O’Byrne PM, Jeffery PK. Myofibroblast involvement in the allergen-induced late response in mild atopic asthma. American Journal of Respiratory Cell and Molecular Biology. 1997;16(6):664–673. doi: 10.1165/ajrcmb.16.6.9191468. [DOI] [PubMed] [Google Scholar]
- 22.Holgate ST. Pathogenesis of asthma. Clinical and Experimental Allergy. 2008;38(6):872–897. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- 23.Ingram JL, Huggins MJ, Church TD, et al. Airway fibroblasts in asthma manifest an invasive phenotype. American Journal of Respiratory and Critical Care Medicine. 2011;183(12):1625–1632. doi: 10.1164/rccm.201009-1452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMillan SJ, Xanthou G, Lloyd CM. Manipulation of allergen-induced airway remodeling by treatment with anti-TGF-β antibody: effect on the Smad signaling pathway. Journal of Immunology. 2005;174(9):5774–5780. doi: 10.4049/jimmunol.174.9.5774. [DOI] [PubMed] [Google Scholar]
- 25.Alcorn JF, Rinaldi LM, Jaffe EF, et al. Transforming growth factor-β1 suppresses airway hyperresponsiveness in allergic airway disease. American Journal of Respiratory and Critical Care Medicine. 2007;176(10):974–982. doi: 10.1164/rccm.200702-334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halwani R, Al-Muhsen S, Al-Jahdali H, Hamid Q. Role of transforming growth factor-β in airway remodeling in asthma. American Journal of Respiratory Cell and Molecular Biology. 2011;44(2):127–133. doi: 10.1165/rcmb.2010-0027TR. [DOI] [PubMed] [Google Scholar]
- 27.Peng X, Mathai SK, Murray LA, et al. Local apoptosis promotes collagen production by monocyte-derived cells in transforming growth factor β1-induced lung fibrosis. Fibrogenesis and Tissue Repair. 2011;4(1, article 12) doi: 10.1186/1755-1536-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frischmeyer-Guerrerio PA, Guerrerio AL, Oswald G, et al. TGFbeta receptor mutations impose a strong predisposition for human allergic disease. Science Translational Medicine. 2013;5(195) doi: 10.1126/scitranslmed.3006448.195ra94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torrego A, Hew M, Oates T, Sukkar M, Kian FC. Expression and activation of TGF-β isoforms in acute allergen-induced remodelling in asthma. Thorax. 2007;62(4):307–313. doi: 10.1136/thx.2006.063487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosendahl A, Checchin D, Fehniger TE, Ten Dijke P, Heldin C-H, Sideras P. Activation of the TGF-β/activin-Smad2 pathway during allergic airway inflammation. American Journal of Respiratory Cell and Molecular Biology. 2001;25(1):60–68. doi: 10.1165/ajrcmb.25.1.4396. [DOI] [PubMed] [Google Scholar]
- 31.Gomez-Duran A, Mulero-Navarro S, Chang X, Fernandez-Salguero PM. LTBP-1 blockade in dioxin receptor-null mouse embryo fibroblasts decreases TGF-β activity: role of extracellular proteases plasmin and elastase. Journal of Cellular Biochemistry. 2006;97(2):380–392. doi: 10.1002/jcb.20637. [DOI] [PubMed] [Google Scholar]
- 32.Santiago-Josefat B, Mulero-Navarro S, Dallas SL, Fernandez-Salguero PM. Overexpression of latent transforming growth factor-β binding protein 1 (LTBP-1) in dioxin receptor-null mouse embryo fibroblasts. Journal of Cell Science. 2004;117(6):849–859. doi: 10.1242/jcs.00932. [DOI] [PubMed] [Google Scholar]
- 33.Chang X, Fan Y, Karyala S, et al. Ligand-independent regulation of transforming growth factor β1 expression and cell cycle progression by the aryl hydrocarbon receptor. Molecular and Cellular Biology. 2007;27(17):6127–6139. doi: 10.1128/MCB.00323-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehmann GM, Xi X, Kulkarni AA, et al. The aryl hydrocarbon receptor ligand ITE inhibits TGFβ1-induced human myofibroblast differentiation. American Journal of Pathology. 2011;178(4):1556–1567. doi: 10.1016/j.ajpath.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Starsichova A, Hruba E, Slabakova E, et al. TGF-beta1 signaling plays a dominant role in the crosstalk between TGF-beta1 and the aryl hydrocarbon receptor ligand in prostate epithelial cells. Cellular Signalling. 2012;24(8):1665–1676. doi: 10.1016/j.cellsig.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Gao P, Grigoryev DN, Rafaels NM, et al. CD14, a key candidate gene associated with a specific immune response to cockroach. Clinical and Experimental Allergy. 2010;40(9):1353–1364. doi: 10.1111/j.1365-2222.2010.03561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tritschler I, Gramatzki D, Capper D, et al. Modulation of TGF-β activity by latent TGF-β-binding protein 1 in human malignant glioma cells. International Journal of Cancer. 2009;125(3):530–540. doi: 10.1002/ijc.24443. [DOI] [PubMed] [Google Scholar]
- 38.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annual Review of Pharmacology and Toxicology. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 39.Kerkvliet NI, Steppan LB, Vorachek W, et al. Activation of aryl hydrocarbon receptor by TCDD prevents diabetes in NOD mice and increases Foxp3+ T cells in pancreatic lymph nodes. Immunotherapy. 2009;1(4):539–547. doi: 10.2217/imt.09.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulz VJ, Smit JJ, Willemsen KJ, et al. Activation of the aryl hydrocarbon receptor suppresses sensitization in a mouse peanut allergy model. Toxicological Sciences. 2011;123(2):491–500. doi: 10.1093/toxsci/kfr175. [DOI] [PubMed] [Google Scholar]
- 41.Schulz VJ, van Roest M, Bol-Schoenmakers M, et al. Aryl hydrocarbon receptor activation affects the dendritic cell phenotype and function during allergic sensitization. Immunobiology. 2013;218(8):1055–1062. doi: 10.1016/j.imbio.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Bussmann UA, Barañao JL. Interaction between the aryl hydrocarbon receptor and transforming growth factor-β signaling pathways: evidence of an asymmetrical relationship in rat granulosa cells. Biochemical Pharmacology. 2008;76(9):1165–1174. doi: 10.1016/j.bcp.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 43.Gramatzki D, Pantazis G, Schittenhelm J, et al. Aryl hydrocarbon receptor inhibition downregulates the TGF-Β/Smad pathway in human glioblastoma cells. Oncogene. 2009;28(28):2593–2605. doi: 10.1038/onc.2009.104. [DOI] [PubMed] [Google Scholar]
- 44.Gomez-Duran A, Ballestar E, Carvajal-Gonzalez JM, et al. Recruitment of CREB1 and histone deacetylase 2 (HDAC2) to the mouse Ltbp-1 promoter regulates its constitutive expression in a dioxin receptor-dependent manner. Journal of Molecular Biology. 2008;380(1):1–16. doi: 10.1016/j.jmb.2008.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]


