Abstract
Carica papaya is widely used in folk medicine as herbal remedy to prevent, protect against, and cure several diseases. These curative properties are based on the presence in different parts of the plant of phytochemical nutrients with antioxidant effect. Seeds are the less exploited part; thus this study is aimed at assessing the antioxidant activities of the C. papaya seeds water extract against hydrogen peroxide (H2O2) oxidative stress in human skin Detroit 550 fibroblasts. C. papaya seeds water extract is not toxic and acts as a potent free radical scavenger, providing protection to Detroit 550 fibroblasts that underwent H2O2 oxidative stress. Data show that (i) the maximum protective effect is achieved by the simultaneous administration of the extract with 1 mM H2O2; (ii) the extract in presence of an oxidative stress does not increase catalase activity and prevents the release of cytochrome C and the inner mitochondrial transmembrane potential (Δψ m) loss; (iii) the extract is more efficient than vitamin C to hamper the oxidative damage; (iv) the purified subfractions of the seeds water extract exert the same antioxidant effect of whole extract. In conclusion, C. papaya seeds water extract is potentially useful for protection against oxidative stress.
1. Introduction
Oxidative stress, based on imbalance between prooxidants production and antioxidant defences, is involved in aging process and in several human chronic diseases, for example, cancer, atherosclerosis, coronary heart disease, macular degeneration, Alzheimer's disease, inflammation, and emphysema [1]. Vitamins C and E, β-carotene, flavonoids, tannins, anthocyanins, and other phenolic compounds are plant derived compounds with antioxidant activities by scavenging free radicals and represent a special group of nutritional supplements. Food rich in these antioxidants plays a key role in the prevention of oxidative stress based diseases [2, 3].
Papaya (Carica papaya), a member of the family Caricaceae, is a tropical fruit rich in dietary antioxidants (vitamin C, tocopherols, total phenols, and β-carotene) [4] and bioactive phytochemicals with antioxidant activity (benzyl isothiocyanate) [5]. Different parts of C. papaya (leaves, barks, roots, latex, fruit, flowers, and seeds) are used in folk medication to treat a broad range of diseases [6]. Several scientific studies validate many of these traditional uses by demonstrating that C. papaya displays a wide range of therapeutic activities (i.e., antiprotozoal, antifungal, antibacterial, antiviral, anti-inflammatory, antihypertensive, hypoglycemic and hypolipidemic, wound healing, antitumor, neuroprotective, diuretic, abortifacient, and antifertility) [6, 7]. These properties mainly depend on the antioxidant activity of some secondary metabolites present in the C. papaya organs. The studies of the antioxidant nutrients in C. papaya have led to the identification of the main compounds that differ in the different organs. Whole fruit extract contains ferulic, p-coumaric, and caffeic acid, carotenoids, and vitamin C that collectively protect human cells from oxidative stresses [4] and promote wound healing and skin repair [8, 9]; leaves extract contains folic acid, vitamins B12, A, and C, alkaloids, saponins, glycosides, tannins, and flavonoids [10] with anticancer activity [11, 12] and protection against the alcohol-induced oxidative damage to the gastric mucosa [13]; seeds extract contains different phenolic compounds, vanillic acid, and vitamin C with antioxidant [14–17] and anticancer [5] activities. Thus, C. papaya extracts may act as a synergistic therapeutic dietary supplement in patients with oxidative stress related diseases or could be added to formulations to promote wound healing. Even if literature data reporting on papaya fruit protection against H2O2-induced oxidative damage are conspicuous, up to date, no researches have explored similar antioxidant activity of papaya seeds water extract. Indeed, water extract has the advantage to prevent the use of harmful chemical compounds. It is worth noting to mention that seeds represent the waste of the consumption of papaya fruits, not only as food but also as raw material for medical preparations. Therefore, the aim of this study was to investigate the antioxidant activity of C. papaya seeds water extract in human skin Detroit 550 fibroblasts in which the oxidative stress was induced by hydrogen peroxide (H2O2). Special attention was paid to the modality of administration and to evaluate if the highest antioxidant effect of C. papaya seeds water extract depends on the whole crude extract or on specific subfractions.
2. Materials and Methods
2.1. Chemicals
All chemicals were of analytical grade and provided by Sigma-Aldrich (St. Louis, MO, USA) unless otherwise indicated.
2.2. C. papaya Seeds Water Extract Preparation and Fractionation
Seeds were removed from mature C. papaya fruits, rinsed with water, and left to dry at room temperature (RT). 10 g of seeds were ground in a mortar, and the powder was left in infusion for 24 h (hours) in 500 mL of sterile distilled water at RT. The crude extract was subjected to a short run (800 g) to obtain a pellet representing the seeds wastes. Unless otherwise indicated, fibroblasts were treated for 1, 2, 4, 8, or 24 h with 1 or 2 mg/mL (w/v, in culture medium) of supernatant in the presence or absence of a prooxidant (1 mM H2O2) with or without a known antioxidant (50, 100, 150, 200, and 250 μM vitamin C).
To test also the efficacy of the extract subfractions, the crude whole extract was subjected to short runs by Centrifuge 4236 A (Beckman Coulter Inc., Brea, CA, USA). In particular, the last supernatant obtained at 100000 g was centrifuged at 4750 g by using a Centricon centrifugal filter device with an Ultracel YM-10 (Merck Millipore, Billerica, MA, USA) regenerated cellulose membrane that permits the ultrafiltration into the lower chamber of substances with molecular weight (MW) < 10 kDa, while substances with MW > 10 kDa are collected in the upper chamber. The pellet obtained was added to fibroblasts in order to obtain 1 mg/mL (w/v, in culture medium) final concentration. In Figure 1, the detailed fractioning procedure is illustrated.
Figure 1.
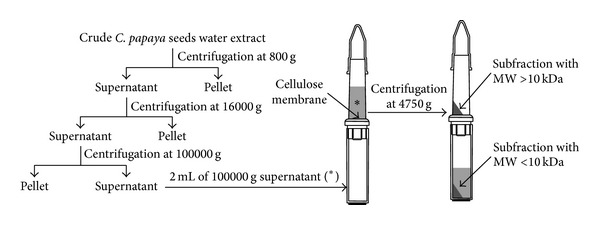
Differential centrifugation of crude C. papaya seeds water extract. The crude extract was subjected to a short run at 800 g by Centrifuge 4236 A (Beckman Coulter Inc., Brea, CA, USA) to obtain a first pellet and a supernatant that was further centrifuged at 16000 g to obtain a second pellet and supernatant. The second supernatant was centrifuged at 100000 g to obtain a third pellet and supernatant. This last supernatant was centrifuged at 4750 g by using a Centricon centrifugal filter device with an Ultracel YM-10 regenerated cellulose membrane that permits the ultrafiltration into the lower chamber of substances with molecular weight (MW) < 10 kDa, while substances with MW > 10 kDa are collected in the upper chamber (Merck Millipore, Billerica, MA, USA).
2.3. Cell Cultures and Treatments
Human skin Detroit 550 fibroblasts were purchased from Centro Substrati Cellulari (Istituto Zooprofilattico Sperimentale, Brescia, Italy) and cultured in 75 cm2 flasks (Iwaki, Tokyo, Japan) at a cell density of 106 fibroblasts/mL in Eagle's minimum essential medium (EMEM) with Earle's Balanced Salts (Cambrex BioScience, Verviers, Belgium), supplemented with 10% (v/v) heat-inactivated fetal calf serum (Cambrex), 2 mM L-glutamine (Cambrex), 100 IU/mL penicillin-streptomycin, and 10000 IU/mL nystatin (antimycotic solution) (Cambrex) in a humidified atmosphere with 5% CO2 at 37°C. Culture medium was changed every 2 days.
The prooxidative stress was induced by incubating the fibroblasts with 1 mM H2O2 for 1 h at 37°C either alone or in combination with 1 or 2 mg/mL (w/v, in culture medium) of C. papaya seeds water extract or vitamin C (50, 100, 150, 200, and 250 μM). C. papaya extract or vitamin C was added to the culture medium simultaneously with H2O2 and/or during the recovery (1 h) in fresh medium at 37°C.
2.4. Cell Viability and Morphology
MTT Assay. Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye mitochondria reduction in living fibroblasts according to Sladowski et al. [18]. Briefly, 5 × 105 fibroblasts were incubated with 1 mg/mL of MTT prepared in supplemented EMEM culture medium, for 2 h at 37°C and 5% CO2; fibroblasts were washed three times with 0.2 M Phosphate Buffer Saline (PBS) pH 7.4, and the reduced MTT formazan crystals were solubilised with DiMethyl SulfOxide (DMSO) (Carlo Erba, Milano, Italy). The optic density (OD) was read at the spectrophotometer (Ultrospec 4000 UV/Visible Spectrophotometer, Pharmacia Biotech, Stockholm, Sweden) at 570 nm.
Light Microscopy. Morphological changes of living fibroblasts were investigated with an inverted light microscope (LM) Eclipse TS100 (Nikon, Kawasaki, Kanagawa Prefecture, Japan). Fibroblasts, washed with 0.2 M PBS pH 7.4 and fixed with formalin 4% (v/v, in 0.2 M PBS pH 7.4), were rubber scraped and deposited on slides. Haematoxylin-eosin (H-E) staining slides were examined under a LM Eclipse 80i (Nikon) for the scoring of apoptosis, necrosis, or mitosis by counting at least 500 fibroblasts in 50–100 high-power microscopic fields (0.25 mm2).
Electron Microscopy. Ultrastructure of cell was obtained by conventional transmission (TEM) and scanning electron microscopy (SEM). 10 × 106 fibroblasts washed with 0.2 M PBS pH 7.4 and rubber scraped were fixed with 2.5% glutaraldehyde (v/v, in 0.1 M cacodylate buffer pH 7.4) for 1 h at ice temperature. After an extensive washing, fibroblasts were postfixed with 1% OsO4 (w/v, in 0.1 M cacodylate buffer pH 7.4) for 1 h at 4°C. Fibroblasts were dehydrated, embedded in Spurr's resin (TAAB Laboratories Equipment Ltd, Aldermaston, England), and examined under a CM12 TEM (Philips, Amsterdam, Netherlands) operating at 80 kV. An aliquot of 1% OsO4 postfixed fibroblasts was dehydrated by using a Critical Point Dryer 020 (Balzer, Balzers, Liechtenstein), stub-mounted, and gold-coated by using a Balzer Sputter Coater 040 before observation under a XL20 SEM (Philips) operating at 15 kV.
2.5. Catalase Activity
For catalase (EC 1.11.1.6.; 2H2O2 oxidoreductase) activity assay, fibroblasts were rinsed three times with 0.2 M PBS pH 7.4, rubber scraped, centrifuged at 450 g for 7–10 min at 4°C, and suspended in 1 mL of cold buffer (50 mM potassium phosphate pH 7, containing 1 mM EDTA). 3–5 × 106 fibroblasts were sonicated for four cycles on ice (40% of amplitude, 10 s of sonication, and 5 s of pause) (Sonoplus Ultrasonic homogenizer HD 2070, Bandelin electronic, Berlin, Germany) and then centrifuged at 10000 g for 15 min at 4°C. Catalase activity was determined in the supernatants by using the FR20 kit following the manufacturer's instructions (Oxford Biomedical research, Oxford, USA).
2.6. Induction of Apoptosis
Fibroblasts were induced to apoptosis with 10 μg/mL puromycin (PMC, in culture medium) for 6 h at 37°C in a 5% CO2 humidified atmosphere or with 10−2 M cycloheximide (CHX, in culture medium) for 24 h followed by recovery in fresh medium for an additional 1 h at 37°C in a 5% CO2 humidified atmosphere, in the absence or in the presence of 1 or 2 mg/mL (w/v, in culture medium) of C. papaya seeds water extract.
2.7. [Ca2+]
5 × 107 fibroblasts washed twice with loading buffer (107 mM NaCl, 5 mM KCl, 7 mM NaHCO3, 3 mM CaCl2, 1 mM MgSO4 × 6H2O, 20 mM Hepes, 10 mM glucose, and 1% bovine serum albumin (BSA)) were incubated at the concentration of 106 fibroblasts/mL with 4 mM 1-(2-(4-carboxyphenyl)-6-amino-benzofuran-5-oxy)-2-(2′-amino-5′-methylphenoxy)ethane-N,N,N′,N′-tetraacetic acid (Fura-2) acetoxymethyl ester for 45 min at 37°C in a 5% CO2 humidified atmosphere. After two washes with the loading buffer, fibroblasts were resuspended in the same freshly made buffer at a final concentration of 7 × 106 fibroblasts/mL and stored at RT until use. 2 mL of cell suspension, prewarmed at 37°C for 20 min and adjusted at a final concentration of 1.4 × 105 fibroblasts/mL, was placed in a glass cuvette for Fura-2 fluorescence measure with a FP-750 spectrofluorometer (Jasco Europe s.r.l., Lecco, Italy) equipped with an electronic stirring system and a thermostabilised (37°C) cuvette holder and monitored by a personal computer running the Jasco Spectra Manager software for Windows 95 (Jasco Europe s.r.l.). The excitation wavelengths were 340 and 380 nm and the emission wavelength was 510 nm; the slit widths were set to 10 nm. Fluorescence values were converted to [Ca2+]i values according to Grynkiewicz et al. [19].
2.8. HSP-70
The inducible cytosolic Heat Shock Protein 70 (HSP-70) protein was detected by Western blot from the total cytosolic proteins of Detroit 550 fibroblasts. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) by using 12.5% acrylamide gels according to Laemmli [20] and then electrotransferred onto nitrocellulose paper sheets (Hybond-C extra, Amersham, UK) according to Towbin et al. [21]. Before electrophoresis, proteins (25 μg) were denaturized with 2% SDS, 10% glycerol, 5% β-mercaptoethanol, and 0.05% bromophenol blue in 62.5 mM Tris-HCl (pH 6.8) for 4 min at 95°C. After blockage with 25 mM Tris-HCl (pH 8.0) containing 3% BSA, 127 mM NaCl, and 2.7 mM KCl (TBS buffer) and washing with TBS containing 0.05% Tween 20, nitrocellulose sheets were incubated with monoclonal anti-HSP-70 (1 μg/mL) for 2 h at RT. Specific antibody binding was detected using goat anti-mouse immunoglobulin G (IgG) conjugated to biotin (1 μg/mL), streptavidin conjugated to peroxidase (1 : 1,500 dilution), and 3-3′diaminobenzidine (DAB) as a substrate. Biotinylated SDS molecular weight (MW) standards were run in parallel to samples and stained. β-actin Western blots were used as controls. Quantification was performed by using a GS-700 Imaging densitometer (Bio-Rad, Hercules, CA, USA).
2.9. Mitochondrial Transmembrane Potential
Changes in the inner mitochondrial transmembrane potential (Δψ m) were determined by incubating fibroblasts with the fluorescent probe 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) (Cell Technology, JC-1 Kit, Mountain View, CA, USA) for 15 min at 37°C in the dark. JC-1 was dissolved in DMSO, stored, and used according to the manufacturer's instruction. The monomeric form of the lipophilic cation JC-1 remains in the cytoplasm and stains it in green, while the dimeric form enters the mitochondria with an intact membrane potential and stains them in red. The percentage of the organic solvent in the samples never exceeded 1% (v/v). Fibroblasts grown on coverslips were rapidly washed with 0.2 M PBS pH 7.4, drained to prevent buffer salt crystals formation, and air-dried. Once completely dried, coverslips were mounted with Histovitrex mounting medium (Carlo Erba, Milan, Italy) and observed with an epifluorescence LM Eclipse 80i (Nikon, Kawasaki, Kanagawa Prefecture, Japan) equipped with a C-HGFIE Hg precentered fiber illuminator (130 W) and a digital camera DXM 1200F by setting a FITC or TRITC filter.
2.10. Cytochrome C Quantification
Quantitative determination of cytosolic and mitochondrial cytochrome C was performed with an Assay Designs' human cytochrome C TiterZyme Enzyme Immunometric Assay (EIA) kit (Assay Designs Inc., Ann Arbor, MI, USA). Fibroblasts rinsed twice with 0.2 M PBS pH 7.4 were suspended in digitonin cell permeabilization buffer (250 nM sucrose, 137 mM NaCl, 70 mM KCl, 1.3 mM Na2HPO4, 1.4 mM K2HPO4, 0.2 mg/mL digitonin, and 0.1% hydorol M) to obtain a cytosolic fraction of cytochrome C and then in a radio-immunoprecipitation assay (RIPA) cell lysis buffer (50 mM Tris HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% SDS) to obtain a mitochondrial fraction of cytochrome C. Both fractions were incubated with a biotinylated monoclonal antibody to cytochrome C immobilized on a microtiter plate for 1 h. The excess of antibody was washed out and streptavidin conjugated to alkaline phosphatase was added for 30 min before the addition of pNPP (p-nitrophenyl phosphate) substrate for additional 45 min. The colour generated by the reaction was read at 405 nm, using the ETI-SYSTEM Fast Reader (Sorin Biomedica, Vicenza, Italy). The values were expressed as pg of cytochrome C of μg total proteins. The protein concentration was measured by using a Bio-Rad Protein assay kit.
2.11. Statistical Analysis
Data were analysed by performing one-way analysis of variance (ANOVA) at the 95% confidence level. P values less than 0.05 were considered significant. Data are the means ± standard errors (SEs) of six independent experiments each done in duplicate.
3. Results
3.1. Cytotoxicity of C. papaya Seeds Water Extract
C. papaya seeds water extract was preliminary tested for cytotoxicity (Figure 2). The two concentrations of extract used in the present work (1 and 2 mg/mL) were never toxic at all incubation times. Indeed, 1 mg/mL extract increased cell viability of about 43% over the control value at 4 h of incubation (P < 0.05, Figure 2(a)). Likewise, H-E stained control (Figure 2(b), (A)) and 1 mg/mL extract 4 h treated Detroit 550 fibroblasts (Figure 2(b), (D)) exhibited the same spindle shape with a central large ovoid nucleus and many randomly distributed filopodia and microvilli that were better seen at SEM (Figure 2(b), (B)–(E)) and TEM (Figure 2(b), (C)–(F)) level. The TEM analysis allowed observing that these cytoplasmic protrusions were particularly abundant in fibroblasts incubated with 1 mg/mL of C. papaya seeds water extract (Figure 2(b), (C)–(F), insert). The percentages of apoptotic, necrotic, and mitotic cells were not modified between control versus treated fibroblasts during 8 h of culture (Figure 2(c)).
Figure 2.
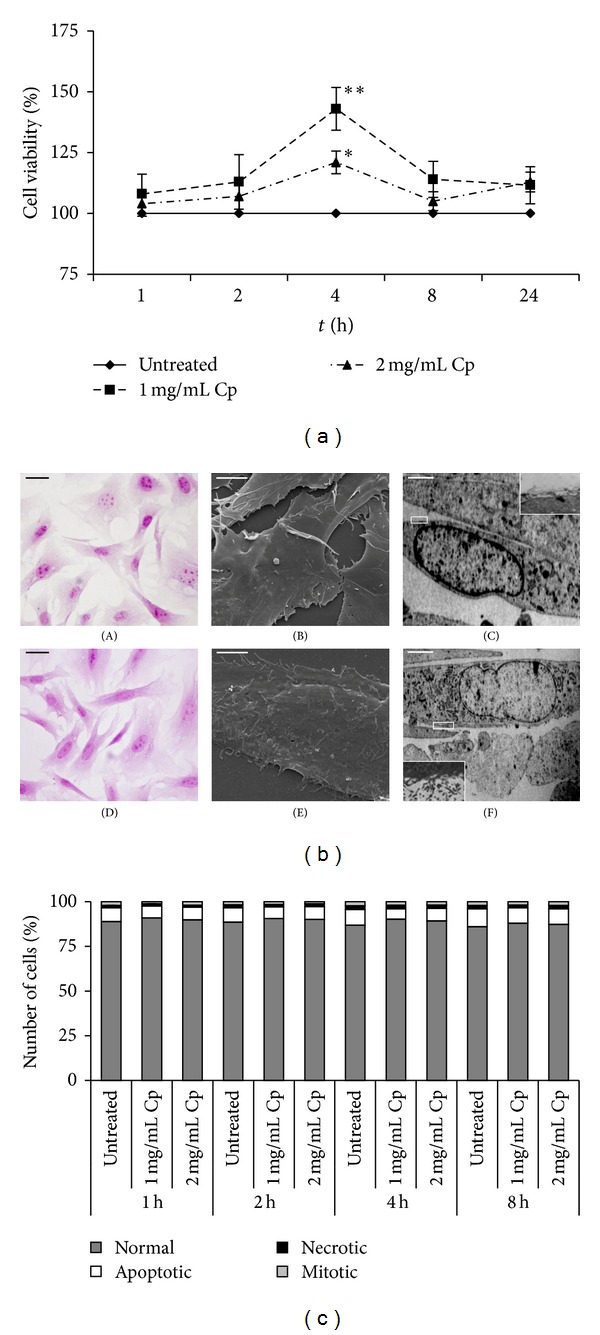
Viability and morphology of Detroit 550 fibroblasts treated with C. papaya seeds water extract. (a) Viability was assessed by MTT assay. Detroit 550 fibroblasts were treated with 1 or 2 mg/mL C. papaya (Cp) seeds water extract for 1, 2, 4, 8, and 24 hours (h). The values are reported as percentage of the control untreated fibroblasts considered as 100%. Each value represents the mean ± SE of six independent experiments, each done in duplicate. Single star indicates value significantly different from untreated control fibroblasts (P < 0.05). Two stars indicate value significantly different from the one star value (P < 0.05). (b) LM (A, D), SEM (B, E), and TEM (C, F) micrographs of Detroit 550 fibroblasts untreated (A–C) or treated with 1 mg/mL of C. papaya seeds water extract for 4 h (D–F). (A, D): H-E stained fibroblasts; bars = 20 μm. (B, E): bars = 5 μm. (C, F): the inset is a magnification showing microvilli; bars = 5 μm. (c) Percentages of normal, apoptotic, necrotic, and mitotic Detroit 550 fibroblasts after treatment with 1 or 2 mg/mL C. papaya (Cp) seeds water extract for 1, 2, 4, and 8 h. At least 500 fibroblasts for each treatment were scored on H-E stained slides.
3.2. Antioxidant Activity of C. papaya Seeds Water Extract
The antioxidant activity of C. papaya seeds water extract was assayed on fibroblasts in which H2O2-mediated oxidative stress was induced. C. papaya seeds water extract protected fibroblasts from the oxidative stress only when added simultaneously with H2O2 (Figure 3(a)). In fact, cell viability was increased on extract-treated fibroblasts with respect to the fibroblasts incubated only with H2O2, thus suggesting an antioxidant activity. However, the extract was unable to protect fibroblasts when added during the recovery (Figure 3(a)). No differences in the antioxidant efficacy were found between the two extract concentrations.
Figure 3.
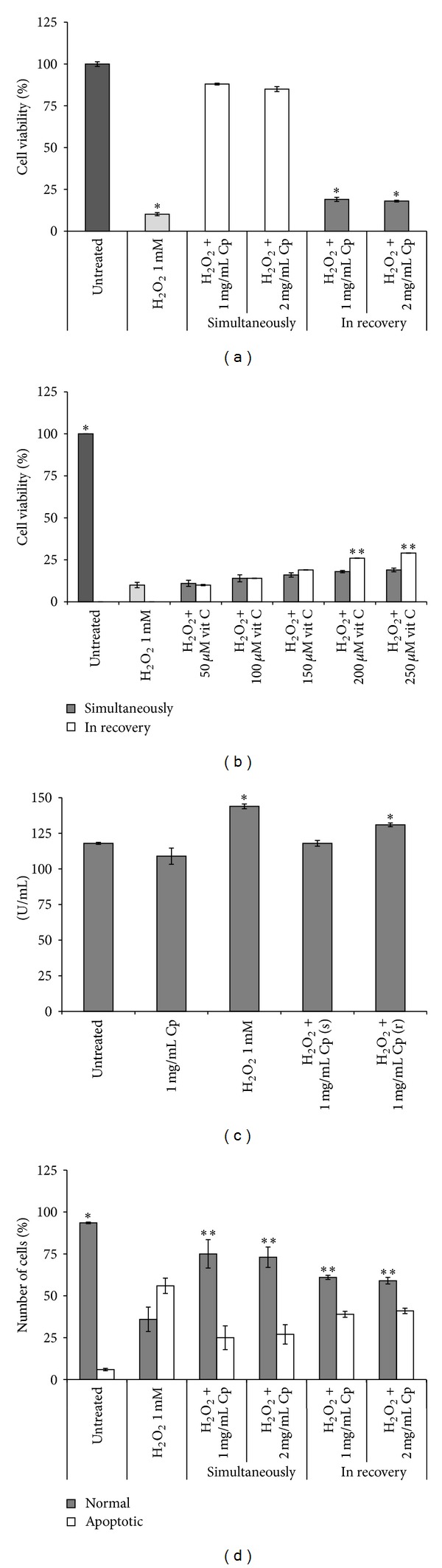
Antioxidant activity of C. papaya seeds water extract on Detroit 550 fibroblasts. (a-b) Viability was assessed by MTT assay. The values are reported as percentage of the control untreated fibroblasts considered as 100%. (a) Detroit 550 fibroblasts were treated with 1 or 2 mg/mL C. papaya (Cp) seeds water extract simultaneously with 1 mM H2O2 for 1 h or during the recovery. Single star indicates values significantly different from untreated control fibroblasts (P < 0.05). (b) Detroit 550 fibroblasts were treated with different concentrations of vitamin C (vit C) simultaneously with 1 mM H2O2 for 1 h or during the recovery. Single star indicates that the value is significantly different from all others (P < 0.05). Two stars indicate values significantly different from those marked with one star and from the simultaneous treatment value (P < 0.05). (c) Catalase enzyme activity (U/mL) measured in Detroit 550 fibroblasts treated with 1 mg/mL of Cp seeds water extract added simultaneously (s) with 1 mM H2O2 for 1 h or during recovery (r). Single star shows values significantly different from all others (P < 0.05). (d) Percentages of normal and apoptotic Detroit 550 fibroblasts after treatment with 1 mM H2O2 administrated either alone or in combination with 1 or 2 mg/mL of Cp extract or with Cp extract added during 1 h recovery. At least 500 fibroblasts for each treatment were scored on H-E stained slides. Single star shows a value significantly different from all others and from the corresponding apoptotic untreated control fibroblasts (P < 0.05). Two stars indicate values significantly different from the untreated control fibroblasts and from the corresponding apoptotic ones (P < 0.05). Each value represents the mean ± SE of six independent experiments, each done in duplicate.
The antioxidant activity of C. papaya seeds water extract was compared with a well-known antioxidant, vitamin C (Figure 3(b)). No toxic concentrations of vitamin C (50, 100, 150, 200, and 250 μM) were added to the culture medium simultaneously with H2O2 or during recovery. Antioxidant vitamin C effect was differently exerted than extract. In fact, vitamin C was more efficient to hamper the oxidative stress when added after oxidative stress ignition than simultaneously with H2O2 in a concentration dependent manner (Figure 3(b)). Interestingly, C. papaya seeds water extract was, in absolute, more efficient than vitamin C to protect Detroit 550 fibroblasts against the oxidative stress. In fact, cell viability increased about 9 times with 1 mg/mL C. papaya seeds water extract (added during 1 h H2O2 incubation) and about 3 times with the most efficient concentration of vitamin C (200 μM added during recovery) with respect to H2O2 treatment alone (please compare Figures 3(a) and 3(b)).
3.3. Catalase Activity
Catalase enzyme is one of the most efficient antioxidant molecules of eukaryotic living cells by hydrolysing H2O2 to water and oxygen. The H2O2-induced oxidative stress increased catalase activity of about 1.2-fold over the control (P < 0.05, Figure 3(c)). When the extract was added simultaneously to the oxidative stress, catalase enzyme activity was similar to the control value (Figure 3(c)). The reduction of the catalase activity was only partially observed (−10% than H2O2 treatment alone) when the extract was added during the recovery (P < 0.05, Figure 3(c)).
3.4. Antiapoptotic Activity of C. papaya Seeds Water Extract
H2O2 represents also an inducer of apoptosis through the production of reactive oxygen species (ROS) [22]; thus the ability of the C. papaya seeds water extract to reduce the percentage of apoptotic fibroblasts was assayed. Data are reported in Figure 3(d). Both concentrations of C. papaya seeds water extract very efficiently decreased the number of apoptotic fibroblasts of about 30% when added during the H2O2-mediated oxidative stress (P < 0.05, Figure 3(d)). In fact, cell viability was comparable to untreated ones (Figure 3(a)). Conversely, any antiapoptotic effect of both concentrations of C. papaya seeds water extract was observed when added during recovery, as indicated by the unchanged viability (Figure 3(a)) and apoptotic cells values (Figure 3(b)) versus H2O2 treatment alone. To verify if C. papaya seeds water extract exerts protection from apoptosis induced through pathways different from oxidative stress, fibroblasts were incubated with CHX and PMC that are able to induce apoptosis by inhibiting protein synthesis. Data of Figure 4 show that induction of apoptosis was unaffected by the presence of C. papaya seeds water extract added simultaneously to CHX or PMC treatments (Figures 4(a) and 4(b)). Comparable results were obtained by biochemical and morphological analysis.
Figure 4.
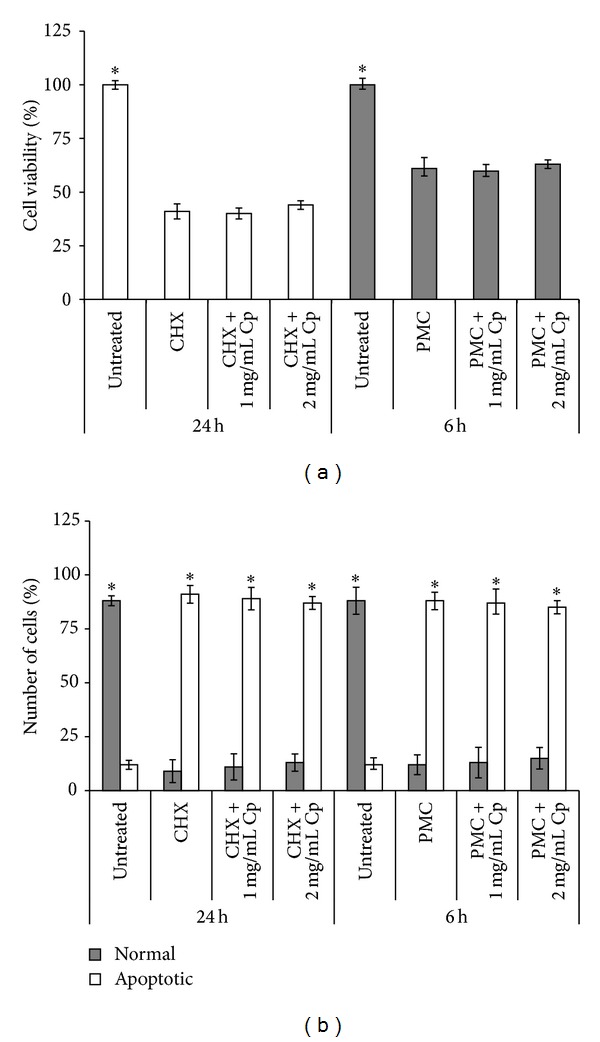
Antiapoptotic activity of C. papaya seeds water extract on Detroit 550 fibroblasts. Detroit 550 fibroblasts were treated with 10−2 M cycloheximide (CHX, in culture medium) or 10 μg/mL puromycin (PMC, in culture medium) either alone or in combination with 1 or 2 mg/mL of C. papaya (Cp) extract for 24 or 6 hours (h), respectively. (a) Viability was assessed by MTT assay. Stars show significant value with the corresponding 24 or 6 h values (P < 0.05). (b) Percentages of normal and apoptotic fibroblasts. At least 500 fibroblasts for each treatment were scored on H-E stained slides. Single star indicates values that differ significantly from the corresponding normal or apoptotic ones (P < 0.05). Each value represents the mean ± SE of six independent experiments, each done in duplicate.
Altogether these data suggest that inhibition of H2O2-mediated apoptosis is not exerted on the apoptotic process itself but it is the result of the antioxidant activity of C. papaya seeds water extract. In fact, no inhibition of H2O2-mediated apoptosis was observed when the extract was added while oxidative stress is already ignited.
3.5. Mitochondrial Δψ m and Cytochrome C
Oxidative stress induces the loss of Δψ m and the release of cytochrome C into the cytoplasm. These two events trigger the onset of apoptosis via the intrinsic pathway. The Δψ m was evaluated by the high sensitive fluorescent probe JC-1. The monomeric form of JC-1 labels the cytoplasm of fibroblasts in brilliant green, while the dimeric form can enter into mitochondria with a normal Δψ m and stain them in red. Data are shown in Figures 5 and 6. JC-1 labelled fibroblasts treated with 1 mg/mL of C. papaya seeds water extract did not alter Δψ m (Figures 5(e) and 5(f)), while 1 mM of H2O2 induced the loss of Δψ m, thus preventing the passage of JC-1 dimers into the mitochondria (Figures 5(g) and 5(h)). When 1 mg/mL of C. papaya seeds water extract was added simultaneously to the oxidative stress, it prevented the Δψ m loss and, thus, mitochondria were red stained (Figures 5(i) and 5(j)). The extract was unable to hamper Δψ m loss when added during recovery (Figures 5(k) and 5(l)).
Figure 5.
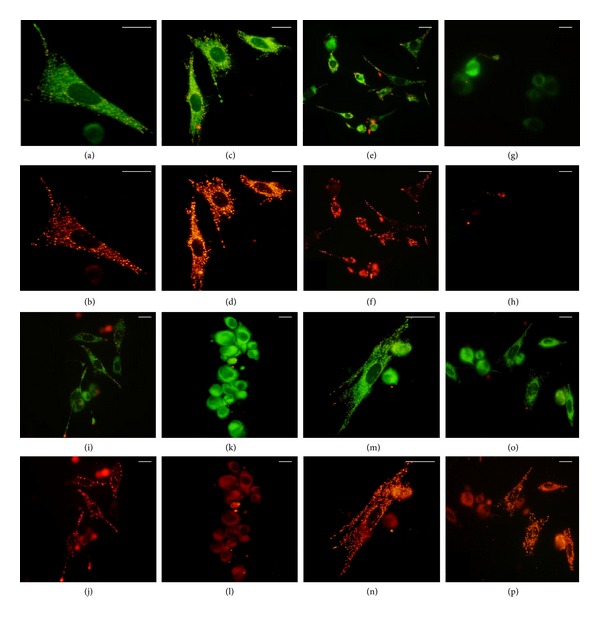
Detroit 550 fibroblasts labelled with the fluorescent probe JC-1 after the treatment with the C. papaya seeds water extract and/or with H2O2. Fibroblasts were labelled with JC-1 (1% v/v) and green or red fluorescence was observed with an epifluorescence LM by setting a FITC (a, c, e, g, i, k, m, and o) or TRITC filter (b, d, f, h, j, l, n, and p). (a)–(d): control untreated fibroblasts at T0 (a, b) and at 4 h (c-d); (e)-(f): fibroblasts treated with 1 mg/mL of C. papaya (Cp) extract for 4 h; (g)-(h): fibroblasts incubated with H2O2 1 mM for 1 h; ((i)-(j)): fibroblasts incubated with 1 mM H2O2 and 1 mg/mL of Cp simultaneously for 1 h; (k)-(l): fibroblasts incubated with 1 mM H2O2 for 1 h and with 1 mg/mL of Cp extract added during recovery; (m)-(n): cell treated with 1 mg/mL of the subfraction with MW > 10 kDa (see Figure 1); (o)-(p): fibroblasts simultaneously incubated with 1 mg/mL of the subfraction with MW > 10 kDa and 1 mM H2O2 for 1 h. Bars = 20 μm.
Figure 6.
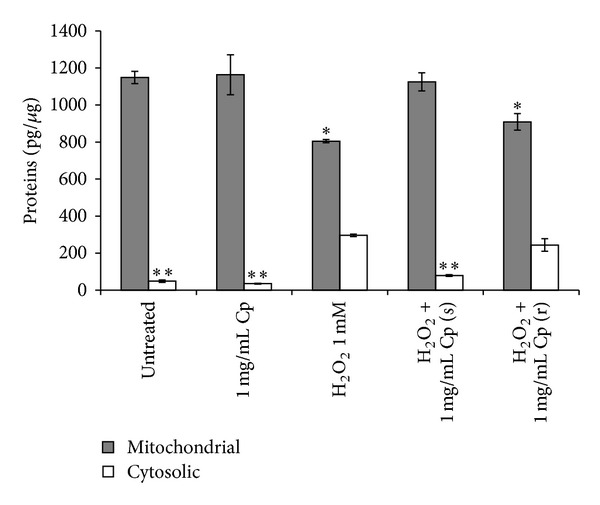
Cytoplasmic and mitochondrial cytochrome C levels. Detroit 550 fibroblasts incubated with 1 mM H2O2 for 1 h simultaneously (s) or during recovery (r) with 1 mg/mL of C. papaya (Cp) seeds water extract were harvested for the content of mitochondrial and cytosolic cytochrome C by ELISA assay. Concentrations are expressed as pg/μg of total proteins. Single star indicates a significant value versus the untreated control fibroblasts (P < 0.05); two stars indicate a significant value versus all the others values relative to the cytosolic cytochrome C (P < 0.05). Each value represents the mean ± SE of six independent experiments, each done in duplicate.
Mitochondrial Δψ m loss causes in turn changes in the permeability of the outer mitochondrial membrane and provokes the release of cytochrome C, a well-known apoptotic-inducing factor. The cytosolic concentration of cytochrome C increased 6-fold than the control value during oxidative stress, that is, 300 pg versus 50 pg, respectively (P < 0.05, Figure 6). The addition during the oxidative stress of 1 mg/mL C. papaya seeds water extract prevented the release of cytochrome C. No effect has been observed upon the administration of the extract after the oxidative stress induction, as indicated by the unchanged cytochrome C concentration values with respect to H2O2 damaged fibroblasts (Figure 6).
3.6. [Ca2+] Ions
Oxidative stress induces Ca2+ ions influx into the cytoplasm, which in turn causes disruption of the normal physiological pathways and leads to cell death. Ca2+ ions influx into the cytoplasm was prevented by 1 mg/mL of C. papaya seeds water extract when added simultaneously to H2O2 (Figure 7). The same behaviour was observed with addition of 200 μM vitamin C either alone or simultaneously with 1 mM H2O2 added for 1 h. [Ca2+] ions concentration was 160 nM in H2O2 treated fibroblasts and 100 nM in control and extract or vitamin C treated ones (P < 0.05, Figure 7).
Figure 7.
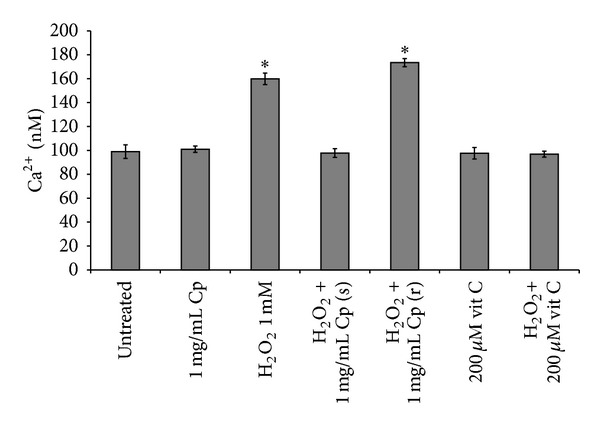
Ca2+ ions. Ca2+ ions concentration (nM) found after incubation of Detroit 550 fibroblasts with 1 mg/mL C. papaya (Cp) seeds water extract or with 200 μM vitamin C (vit C) added simultaneously (s) or during recovery (r) with 1 mM H2O2 either for 1 h. The values indicated with the single star differ significantly from all the others (P < 0.05). Each value represents the mean ± SE of six independent experiments, each done in duplicate.
3.7. HSP-70
HSPs protect cells against a plethora of stresses, including oxidative stress. A reduction of 7% of the amount of HSP-70 was observed when fibroblasts were treated for 1 h with 1 mg/mL C. papaya seeds water extract (P < 0.05, Figure 8(a)). Overexpression of HSP-70 protein was found upon 1 mM H2O2 incubation of Detroit 550 fibroblasts (P < 0.05, Figure 8(b)). C. papaya seeds water extract, added during the oxidative stress but not during the recovery, hampered the stress by decreasing the amount of HSP-70 to control value (Figure 8(b)).
Figure 8.
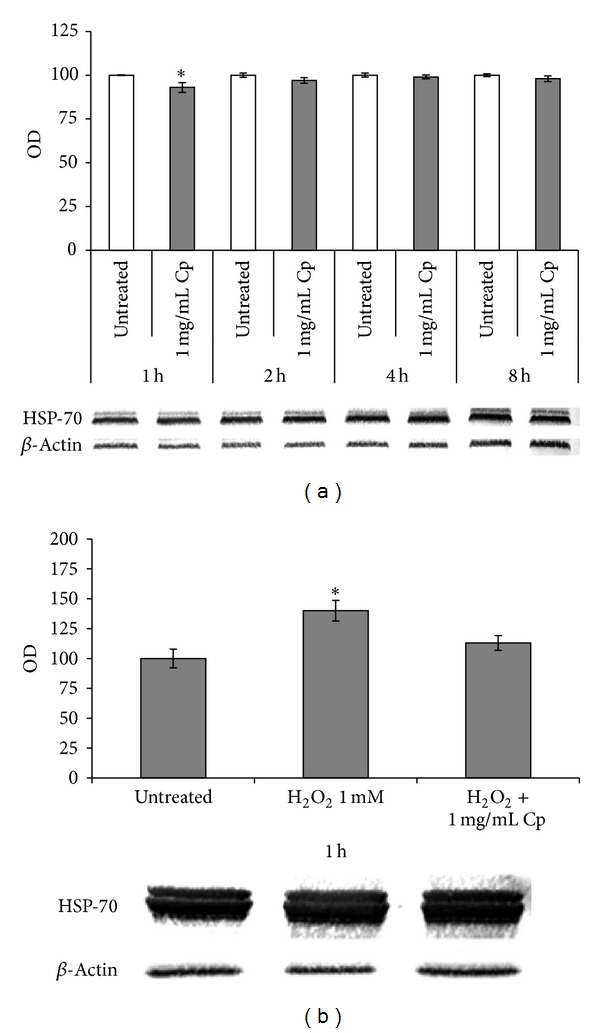
Western blot of HSP-70. (a) Western blots and optic densities (OD) of inducible cytosol HSP-70 of control untreated Detroit 550 fibroblasts or treated with 1 mg/mL of C. papaya (Cp) seeds water extract for 1, 2, 4, and 8 hours (h); (b) Western blots and OD of inducible cytosol HSP-70 of Detroit 550 fibroblasts incubated with 1 mM H2O2 for 1 h in absence or in presence of 1 mg/mL of Cp seeds water extract. Values are expressed as percentage of the control untreated fibroblasts value taken as 100%. The star shows a significant value with respect to all the others without star (P < 0.05). Each value represents the mean ± SE of six independent experiments, each one done in duplicate.
3.8. Antioxidant Activity of C. papaya Seeds Water Extract Subfractions
The fractionation of C. papaya seeds water extract by differential centrifugation (Figure 1) allowed us to evaluate if the antioxidant effect of extract was an exclusive property of the whole crude extract or was mainly related to a specific class of compounds. Any of the different subfractions (1 mg/mL final concentration) was cytotoxic as shown with MTT assay and JC1 staining (Figures 9 and 5, resp.). Figure 5 shows red stained mitochondria of Detroit 550 fibroblasts treated with MW > 10 kDa subfraction alone (Figures 5 (m) and 5(n)) and simultaneously with H2O2 (Figures 5(o) and 5(p)). The subfractions increased viability of Detroit 550 fibroblasts: the maximum increment was measured with the supernatant obtained by 100000 g centrifugation of 16000 g supernatant (P < 0.05, Figure 9), especially with the subfraction containing substances with MW < 10 kDa (Figure 9). Regarding the antioxidant activity, the different subfractions were not significantly different than the whole extract when added simultaneously with the oxidative stress (Figure 9). Any of the subfractions could reduce the oxidative stress when added during recovery.
Figure 9.
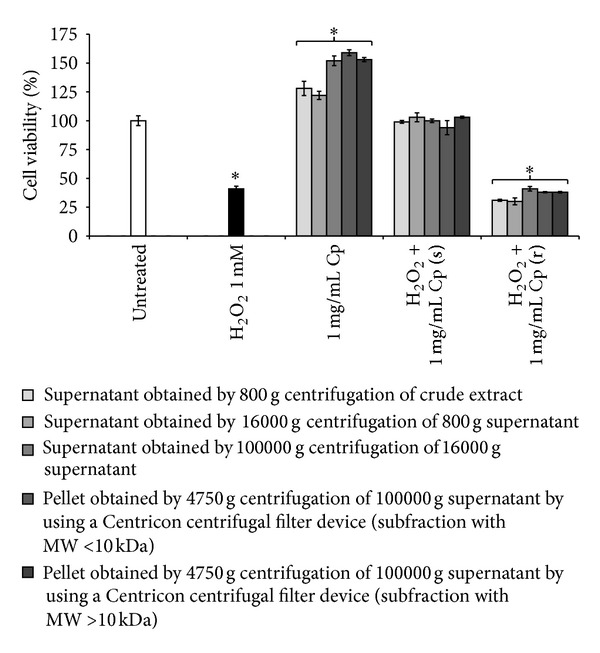
Viability of Detroit 550 fibroblasts treated with different subfractions of C. papaya seeds water extract. Viability of Detroit 550 fibroblasts treated with 1 mg/mL (w/v, in the culture medium) of different subfractions obtained from the C. papaya (Cp) extract (see Figure 1) administrated either alone or with 1 mM H2O2 added simultaneously (s) or during 1 h recovery (r) was evaluated by MTT test. One star indicates values significantly different from control untreated fibroblasts (P < 0.05). Each value represents the mean ± SE of six independent experiments done in duplicate. For the preparation of subfractions, see Figure 1 and Materials and Methods section.
4. Discussion
The present study shows that C. papaya seeds water extract has a potent antioxidant activity in H2O2 oxidative stress-induced human skin Detroit 550 fibroblasts. Our results suggest that the extract is not toxic, decreases cell death, ensures Ca2+ homeostasis, and counteracts mitochondrial dysfunctionality in oxidative stress-damaged Detroit 550 fibroblasts. The best effect primarily depends on the modality of administration and to a lesser extent on the dose and composition, that is, whole extract versus subfractions. We found that (i) the protective effect was exerted when the extract was added concomitantly to the oxidative stress, suggesting that the bioactive compounds of C. papaya extract are not inactivated by H2O2 (as it is for vitamin C) but, conversely, can scavenge H2O2 but not free radical; (ii) the sufficient dose to induce antioxidant effect in our experimental design is 1 mg/mL, likely due to the concentration of the diverse bioactive molecules; (iii) the crude whole extract is effective as much as the subfractions.
Thus, our data strongly highlight the antioxidant role of the molecules contained in C. papaya seeds water extract, encouraging their exploitation. In fact, substances with antioxidant properties have recently received unprecedented attention as possible therapeutic and preventive agents in consideration of the role of ROS in health and diseases. In addition, due to the augmented recycling interest of the agrofood industry, the numbers of studies on residual sources in replacing synthetic antioxidants with natural ones are increasing. The wastes or byproducts from food processing such as seeds and peels contain higher source of potential antioxidant compounds than the edible portion [23]; conversely, byproduct-derived antioxidants successfully developed are very limited. Grape seed and olive waste extract in the European food processing industry represent these exceptions [24]. In this context, C. papaya seeds water extract could be a source to exploit. In fact, papaya seeds contain several molecules, like fatty acids, crude protein, crude fiber, papaya oil, carpaine, benzyl isothiocyanate, glucotropaeolin, benzyl thiourea, hentriacontane, β-sitosterol, caricin, and an enzyme myrosin [7] that confer vermifuge [25, 26], abortifacient [27, 28], antifertility [29–32], wound-healing [33], anticancer [34], antibacterial [35], and antifungal [36] properties as well as antioxidant activities for the presence of secondary metabolites, such as phenolic compounds, vanillic acid, flavonoids, α-tocopherol, and vitamin C. Despite the wide and historical use of C. papaya in the traditional management of many diseases [4], the scientific validation of the use of papaya seeds as antioxidant is scarce. Therefore, in the present work we tested C. papaya seeds water extract that conforms to the use in folk medicine and ensures no toxicity related to chemicals used during the preparation. In our hands, C. papaya seeds water extract is not toxic and, as a whole, it is more efficient than vitamin C to hamper the H2O2 oxidative damage.
The choice of the methodology for extract preparation is very important to preserve antioxidant activity [17, 37]. The antioxidant activity of seed extract could be further improved, taking into account that the comparison of five extract fractions, that is, ethanol, petroleum ether, ethyl acetate, n-butanol, and water, demonstrated the strongest antioxidant activity of the ethyl acetate and n-butanol fractions, whereas the water extract had always the weakest antioxidant activity [17]. Despite this data, we tested the water extract because it is edible, thus compatible with a putative human administration. Here, we also demonstrated that the modality of administration is crucial as well. Since experimental H2O2 oxidative stress is based on two-step procedure, that is, 1 mM H2O2 for 1 h followed by 1 h of recovery in fresh medium, we found that the best modality of papaya extract administration is when it is added simultaneously with the oxidative stress inducer. This suggests that the extract acts directly with H2O2. In addition, C. papaya seeds water extract was, as a whole, much more efficient than vitamin C, a well-known antioxidant agent. In fact, vitamin C had its highest antioxidant activity when added in the recovery. H2O2 must be firstly converted by fibroblasts to free radicals that can be then neutralized by vitamin C. In fact, ascorbic acid or vitamin C is a water soluble antioxidant that delays or inhibits cellular damage mainly through free radical scavenging. Vitamin C belongs to primary antioxidants group that are able to donate electrons to free radical, scavenging them to compounds more stable and not harmful to cells [38].
The robust antioxidant activity of C. papaya seed extract can protect from cell death. In fact, we showed the ability of the extract to prevent H2O2-induced apoptosis. However, the extract does not affect directly the apoptotic process but indirectly by hampering the oxidative stress-induced apoptosis. In fact, C. papaya extract has no efficacy against CHX- and PMC-induced apoptosis, inhibitors of protein synthesis. Therefore, the protection from the oxidative-stress-induced apoptosis is an indirect consequence of the ability of the extract to prevent the production or to block the activity of harmful metabolites, like free radicals.
Antioxidant function in biological systems is much more complicated than a simple free radical scavenging process. An antioxidant may affect biological system by (i) suppressing the formation of ROS and reactive nitrogen species (RNS); (ii) affecting enzyme activities [39]; (iii) inducing de novo biosynthesis of defence enzymes and thereby affecting other endogenous antioxidants [40]; (iv) preserving NO activity [41], or (v) sequestering transition metal ions. It is obvious that some compounds use more than one mechanism for their antioxidants effect on biological systems. Our data show that the antioxidant effect of C. papaya seeds water extract did not increase scavenging enzymes activity, as catalase. When added simultaneously to the oxidative stress, the extract reduces the catalase activity to normal level. This suggests that the antioxidant effects of the C. papaya seeds water extract are exerted not via modulation of catalase enzyme activity but by (i) scavenging and chelating the oxidative molecules (H2O2) or (ii) reestablishing or maintaining the reduction/oxidation balance inside the cell.
Oxidative stress causes mitochondrial dysfunction via loss of Δψ m, accumulation of mitochondrial DNA mutations, and increment of the level of oxidative damage to DNA, proteins, and lipids [1]. Yakes and Van Houten [42] demonstrated that H2O2 treatment affects mainly mitochondria rather than DNA in human cells. In our system, C. papaya seeds water extract prevents the Δψ m loss and the consequent release of cytochrome C into the cytosol.
Cytochrome C is normally bound to the inner mitochondrial membrane by association with cardiolipin, whose peroxidation leads to cytochrome C and release through the outer mitochondrial membrane into the cytosol. Once in the cytoplasm, cytochrome C triggers the activation of caspase-9 that activates the intrinsic pathway of apoptosis [43]. Since C. papaya seeds water extract prevents oxidative-induced cell death by blocking the cytochrome C release from mitochondria to cytosol, by reducing the mitochondrial ROS and by inhibiting the mitochondrial permeability transition, we speculate that it is cell permeable and able to target mitochondria.
Oxidative stress burst can disrupt normal physiological pathways that, in turn, trigger the Ca2+ signaling dependent cell death. In fact, oxidative stress causes Ca2+ influx into the cytoplasm from the extracellular environment and from the endoplasmic reticulum (ER) through, respectively, cell membrane and ER channels [44]. C. papaya seeds water extract elicits antioxidant effect also by preventing the H2O2-induced alteration of Ca2+ homeostasis. The significant cytosolic HSP-70 induction occurred after exposure to H2O2 was decreased by C. papaya seeds water extract to normal level. These results point to the possible involvement of redox mechanisms in the heat shock signal transduction pathway, which may play an important regulatory role in the genetic mechanisms of tolerance to oxidative stress.
The antioxidant properties of five subfractions of the water crude extract obtained by differential centrifugation were tested in the same conditions of the whole extract. Each subfraction showed activities comparable to those tested for the whole extract, thus indicating that the antioxidant activity of the C. papaya seeds water extract is not entirely attributable to only one molecule or few association of molecules and that there is no synergistic activity of the bioactive compounds. It is worth noting that one of the five subfractions tested expressed a property that was not shown by the other subfractions. In fact, the subfraction with MW > 10 kDa was able to counteract the loss of Δψ m. The reason of such behavior needs further investigations.
Altogether our data are in favour of a beneficial effect of the exogenous supplementation of C. papaya seeds water extract (as whole or as MW > 10 kDa subfraction) representing an efficient tool to minimize free radical-induced damage.
5. Conclusions
In conclusion, this study indicates that also nonedible parts of C. papaya, in particular seeds water extract, may be a promising source of antioxidants, which may have therapeutic implications. In fact, we showed that C. papaya seeds protect fibroblasts from H2O2-induced stress due to the antioxidant activity of the water extract. All together our results gave indication on the benefits, on the modality of administration, and on the extraction of C. papaya seeds that are important for the extent of the antioxidant activity and open a new perspective in the biotechnological utilization of the C. papaya. Indeed, this aspect could be very important to recycle the waste of papaya fruit, playing an important role to improve the complete utilization of its nonedible byproducts.
Conflict of Interests
Elisa Panzarini, Majdi Dwikat, Stefania Mariano, Cristian Vergallo, and Luciana Dini have no conflict of interests regarding the publication of this paper.
References
- 1.Brieger K, Schiavone S, Miller FJ, Krause KH. Reactive oxygen species: from health to disease. Swiss Medical Weekly. 2012;142:1–14. doi: 10.4414/smw.2012.13659. [DOI] [PubMed] [Google Scholar]
- 2.Stanner SA, Hughes J, Kelly CN, Buttriss J. A review of the epidemiological evidence for the ‘antioxidant hypothesis’. Public Health Nutrition. 2004;7(3):407–422. doi: 10.1079/phn2003543. [DOI] [PubMed] [Google Scholar]
- 3.Mursu J, Robien K, Harnack LJ, Park K, Jacobs DR., Jr. Dietary supplements and mortality rate in older women: The Iowa Women’s Health Study. Archives of Internal Medicine. 2011;171(18):1625–1633. doi: 10.1001/archinternmed.2011.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali A, Devarajan S, Waly M, Essa MM, Rahman MS. Nutritional and medicinal value of papaya (Carica papaya L.) In: Essa MM, Manickavasagan A, Sukumar E, editors. Natural Products and Bioactive Compounds in Disease Prevention. New York, NY, USA: Nova Science Publishers; 2011. pp. 34–42. [Google Scholar]
- 5.Nakamura Y, Yoshimoto M, Murata Y, et al. Papaya seed represents a rich source of biologically active isothiocyanate. Journal of Agricultural and Food Chemistry. 2007;55(11):4407–4413. doi: 10.1021/jf070159w. [DOI] [PubMed] [Google Scholar]
- 6.Jaiswal P, Kumar P, Singh VK, Singh DK. Carica papaya Linn: a potential source for various health problems. Journal of Pharmacy Research. 2010;3(5):998–1003. [Google Scholar]
- 7.Krishna KL, Paridhavi M, Patel JA. Review on nutritional, medicinal and pharmacological properties of Papaya (Carica papaya Linn.) Natural Product Radiance. 2008;7(4):364–373. [Google Scholar]
- 8.Anuar NS, Zahari SS, Taib IA, Rahman MT. Effect of green and ripe Carica papaya epicarp extracts on wound healing and during pregnancy. Food and Chemical Toxicology. 2008;46(7):2384–2389. doi: 10.1016/j.fct.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 9.Ajlia SASH, Majid FAA, Suvik A, Effendy MAW, Serati Nouri H. Efficacy of papain-based wound cleanser in promoting wound regeneration. Pakistan Journal of Biological Sciences. 2010;13(12):596–603. doi: 10.3923/pjbs.2010.596.603. [DOI] [PubMed] [Google Scholar]
- 10.Imaga NA, Gbenle GO, Okochi VI, et al. Phytochemical and antioxidant nutrient constituents of Carica papaya and parquetina nigrescens extracts. Scientific Research and Essays. 2010;5(16):2201–2205. [Google Scholar]
- 11.Morimoto C, Dang NH, Dang N. Patent. WO2006004226-A1, EP1778262-A1, JP2008505887-W, US2008069907-A1. YS Therapeutic Co Ltd (YSTH-Non-standard) Toudai Tlo Ltd (TODNon- standard) Morimoto C (MORI-Individual) Dang NH (DANG-Individual); 2008. Cancer prevention and treating composition for preventing, ameliorating, or treating solid cancers, e.g. lung, or blood cancers, e.g. lymphoma, comprises components extracted from brewing papaya. [Google Scholar]
- 12.Otsuki N, Dang NH, Kumagai E, Kondo A, Iwata S, Morimoto C. Aqueous extract of Carica papaya leaves exhibits anti-tumor activity and immunomodulatory effects. Journal of Ethnopharmacology. 2010;127(3):760–767. doi: 10.1016/j.jep.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Indran M, Mahmood AA, Kuppusamy UR. Protective effect of Carica papaya L leaf extract against alcohol induced acute gastric damage and blood oxidative stress in rats. West Indian Medical Journal. 2008;57(4):323–326. [PubMed] [Google Scholar]
- 14.Kothari V, Seshadri S. Antioxidant activity of seed extracts of Annona squamosa and Carica papaya. Nutrition and Food Science. 2010;40(4):403–408. [Google Scholar]
- 15.Norshazila S, Zahir IS, Suleiman KM, Aisyah MR, Rahim KK. Antioxidant levels and activities of selected seeds of Malaysian tropical fruits. Malaysian Journal of Nutrition. 2010;16(1):149–159. [PubMed] [Google Scholar]
- 16.Contreras-Calderón J, Calderón-Jaimes L, Guerra-Hernández E, García-Villanova B. Antioxidant capacity, phenolic content and vitamin C in pulp, peel and seed from 24 exotic fruits from Colombia. Food Research International. 2011;44(7):2047–2053. [Google Scholar]
- 17.Zhou K, Wang H, Mei W, Li X, Luo Y, Dai H. Antioxidant activity of Papaya seed extracts. Molecules. 2011;16(8):6179–6192. doi: 10.3390/molecules16086179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sladowski D, Steer SJ, Clothier RH, Balls M. An improved MTT assay. Journal of Immunological Methods. 1993;157:203–207. doi: 10.1016/0022-1759(93)90088-o. [DOI] [PubMed] [Google Scholar]
- 19.Grynkiewicz G, Poenie M, Tsien RJ. A new generation of Ca2+ indicators with greatly improved fluorescence properties. The Journal of Biological Chemistry. 1985;260(6):3440–3450. [PubMed] [Google Scholar]
- 20.Laemmli UK. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrilamide gels to nitrocellulose sheets: procedure and some applications. Proceedings of the National Academy of Sciences of the United States of America. 1979;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Formichi P, Radi E, Battisti C, et al. Human fibroblasts undergo oxidative stress-induced apoptosis without internucleosomal DNA fragmentation. Journal of Cellular Physiology. 2006;208(2):289–297. doi: 10.1002/jcp.20662. [DOI] [PubMed] [Google Scholar]
- 23.Okonogi S, Duangrat C, Anuchpreeda S, Tachakittirungrod S, Chowwanapoonpohn S. Comparison of antioxidant capacities and cytotoxicities of certain fruit peel. Food Chemistry. 2007;103(3):839–846. [Google Scholar]
- 24.Peschel W, Sánchez-Rabaneda F, Diekmann W, et al. An industrial approach in the search of natural antioxidants from vegetable and fruit wastes. Food Chemistry. 2006;97(1):137–150. [Google Scholar]
- 25.Okeniyi JAO, Ogunlesi TA, Oyelami OA, Adeyemi LA. Effectiveness of dried Carica papaya seeds against human intestinal parasitosis: a pilot study. Journal of Medicinal Food. 2007;10(1):194–196. doi: 10.1089/jmf.2005.065. [DOI] [PubMed] [Google Scholar]
- 26.Sapaat A, Satrija F, Mahsol HH, Ahmad AH. Anthelmintic activity of papaya seeds on Hymenolepis diminuta infections in rats. Tropical Biomedicine. 2012;29(4):508–512. [PubMed] [Google Scholar]
- 27.Oderinde O, Noronha C, Oremosu A, Kusemiju T, Okanlawon OA. Abortifacient properties of aqueous extract of Carica papaya (Linn) seeds on female Sprague-Dawley rats. The Nigerian Postgraduate Medical Journal. 2002;9(2):95–98. [PubMed] [Google Scholar]
- 28.Adebiyi A, Adaikan PG, Prasad RNV. Tocolytic and toxic activity of papaya seed extract on isolated rat uterus. Life Sciences. 2003;74(5):581–592. doi: 10.1016/j.lfs.2003.06.035. [DOI] [PubMed] [Google Scholar]
- 29.Chinoy NJ, D’Souza JM, Padman P. Effects of crude aqueous extract of Carica papaya seeds in male albino mice. Reproductive Toxicology. 1994;8(1):75–79. doi: 10.1016/0890-6238(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 30.Lohiya NK, Goyal RB, Jayaprakash D, Ansari AS, Sharma S. Antifertility effects of aqueous extract of Carica papaya seeds in male rats. Planta Medica. 1994;60(5):400–404. doi: 10.1055/s-2006-959518. [DOI] [PubMed] [Google Scholar]
- 31.Udoh P, Essien I, Udoh F. Effects of Carica papaya (paw paw) seeds extract on the morphology of pituitary-gonadal axis of male Wistar rats. Phytotherapy Research. 2005;19(12):1065–1068. doi: 10.1002/ptr.1388. [DOI] [PubMed] [Google Scholar]
- 32.Ortega-Pacheco A, Jimenez-Coello M, Acosta-Viana KY, et al. Effects of papaya seeds extract on the sperm characteristics of dogs. Animal Reproduction Science. 2011;129(1-2):82–88. doi: 10.1016/j.anireprosci.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Nayak BS, Ramdeen R, Adogwa A, Ramsubhag A, Marshall JR. Wound-healing potential of an ethanol extract of Carica papaya (Caricaceae) seeds. International Wound Journal. 2012;9(6):650–655. doi: 10.1111/j.1742-481X.2011.00933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen TT, Shaw PN, Parat MO, Hewavitharana AK. Anticancer activity of Carica papaya: a review. Molecular Nutrition & Food Research. 2013;57(1):153–164. doi: 10.1002/mnfr.201200388. [DOI] [PubMed] [Google Scholar]
- 35.Dawkins G, Hewitt HH, Wint Y, Obiefuna PCM, Wint B. Antibacterial effects of Carica papaya fruit on common wound organisms. West Indian Medical Journal. 2003;52(4):290–292. [PubMed] [Google Scholar]
- 36.Chávez-Quintal P, González-Flores T, Rodríguez-Buenfil I, Gallegos-Tintoré S. Antifungal activity in ethanolic extracts of Carica papaya L. cv. Maradol leaves and seeds. Indian Journal of Microbiology. 2011;51(1):54–60. doi: 10.1007/s12088-011-0086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ang YK, Sia WCM, Khoo HE, Yim HS. Antioxidant potential of Carica papaya peel and seed. Focusing on Modern Food Industry. 2012;1(1):11–16. [Google Scholar]
- 38.Vaya J, Aviram M M. Nutritional antioxidants: mechanisms of action, analyses of activities and medical application. Current Medicinal Chemistry—Immunology, Endocrine & Metabolic Agents. 2001;1(1):99–117. [Google Scholar]
- 39.Tasinato A, Boscoboinik D, Bartoli G-M, Maroni P, Azzi A. d-α-tocopherol inhibition of vascular smooth muscle cell proliferation occurs at physiological concentrations, correlates with protein kinase C inhibition, and is independent of its antioxidant properties. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(26):12190–12194. doi: 10.1073/pnas.92.26.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radical Biology and Medicine. 2000;28(10):1456–1462. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 41.Stewart-Lee AL, Forster LA, Nourooz-Zadeh J, Ferns GAA, Anggard EE. Vitamin E protects against impairment of endothelium-mediated relaxations in cholesterol-fed rabbits. Arteriosclerosis and Thrombosis. 1994;14(3):494–499. doi: 10.1161/01.atv.14.3.494. [DOI] [PubMed] [Google Scholar]
- 42.Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(2):514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szeto HH. Cell-permeable, mitochondrial-targeted, peptide antioxidants. The AAPS Journal. 2006;8(2):E277–E283. doi: 10.1007/BF02854898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ermak G, Davies KJA. Calcium and oxidative stress: from cell signaling to cell death. Molecular Immunology. 2002;38(10):713–721. doi: 10.1016/s0161-5890(01)00108-0. [DOI] [PubMed] [Google Scholar]


