Abstract
Purpose of review
Ciliopathies are genetic disorders caused by defects of primary ciliary structure and/or function and are characterized by pleiotropic clinical features. The ciliopathies include several partially overlapping syndromes such as Joubert syndrome, Bardet–Biedl syndrome and Meckel–Gruber syndrome, all of which have pronounced neurodevelopmental features. Here we focus on potential roles of cilia in central nervous system function, to explore how impairments may cause brain malformation and neurodevelopmental disease.
Recent findings
Cilia have long been considered as ‘sensory cellular antennae’, responding as chemo-sensors, mechano-sensors and thermo-sensors, although their roles in development were not well understood until recently. The surprising finding that disparate syndromes are all due to defects of the primary cilia, along with the recent advances in genetics, has helped elucidate further roles of primary cilia beyond sensory functions. Several molecules that are associated with key signaling pathways have been discovered in primary cilia. These include sonic hedgehog, wingless, planar cell polarity and fibroblast growth factor, which are essential for many cellular processes. Additionally, mutations in ‘ciliome’ genes have largely shown developmental defects such as abnormal body axis and brain malformation, implying disrupted cilia-related signaling pathways. Accordingly, the emerging theme is that primary cilia may play roles as modulators of signal transduction to help shape cellular responses within the environmental context during both development and homeostasis.
Summary
The link between cilia and signal pathways has become a framework for understanding the pathogenesis of ciliopathies. Despite recent progress in ciliary biology, fundamental questions remain about how cilia regulate neuronal function in the central nervous system. Therefore, investigation of ciliary function in the nervous system may reveal cilia-modulating mechanisms in neurodevelopmental processes, as well as suggest new treatments for disease.
Keywords: brain, central nervous system, cilia, ciliopathy, Joubert syndrome, neuron, signaling pathways
Introduction
The mechanisms associated with ciliopathies have been extensively studied in recent years and the research advances have identified many genes and molecular signals involved in ciliary formation and function. The range of clinical features of ciliopathies includes early fetal lethality, respiratory dysfunction, reproductive sterility, skeletal dysplasia including polydactyly, rib and bone defects, cardiac defects, cystic kidney, hepatic fibrosis, obesity, diabetes and deafness, accounting for almost every organ in the body. Notably, neurological deficits including hydrocephalus and structural brain defects are common features in Joubert syndrome (JBTS), Bardet–Biedl syndrome (BBS), Meckel–Gruber syndrome (MKS) and nephronophthisis (NPHP) [1–3]. Although critical in central nervous system (CNS) development, as well as neurogenesis [4–6], the role of cilia in brain development and neuronal function still remains largely unknown. Moreover, the multitude of affected organs and the variability in clinical presentation of these disorders challenges our understanding of the context-dependent role that cilia play in human health.
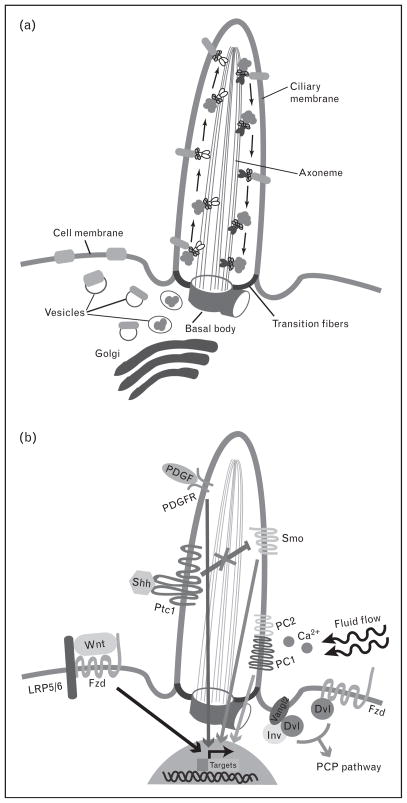
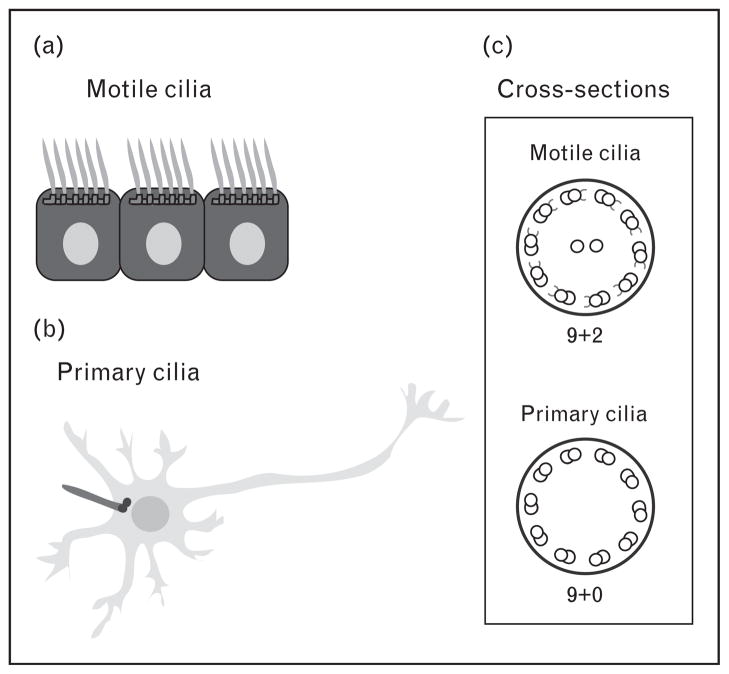
Cilia are hair-like extensions protruding from the surface from most types of cells including progenitor and differentiated cells. The cilium is composed of a microtubule cytoskeleton (the axoneme) and anchored to the cell by the basal body that is derived from the mother centriole (Fig. 1). There are two types of cilia: motile and non-motile (primary). Although there are some exceptions, motile cilia have nine doublet microtubules containing inner and outer dynein arms, as well as two central microtubules (9 + 2 axoneme). Primary cilia, on the contrary, have the nine doublet microtubules, but lack the two central microtubules and dynein arms (9 + 0 axoneme) (Fig. 2). In the brain, motile cilia are present only in specific type of cells: ependymal cells lining the ventricle and some choroid plexus (ChP) cells. On the contrary, primary cilia are present in most brain cells: some ChP cells, neural stem cells, neurons and astrocytes within the brain parenchyma. It was initially assumed that primary cilia were simply sensory organelles, responding to flow and receiving external signals during brain development. However, recent studies have demonstrated diverse roles of primary cilia, which include regulation of signal transduction that goes beyond their sensory roles [7••,8•]. Here, we review the predominant neurodevelopmental defects in ciliopathies and speculate on the relationship between ciliary functions and signaling pathways by highlighting newly identified roles of cilia in brain development.
Figure 1. Intraflagellar transporting and signaling pathways within the primary cilium.
(a) The protein cargos are transported from the Golgi to the basal body by vesicle trafficking in the cellular cytoplasm. In order to move ciliary cytosolic and membrane proteins, kinesin 2 and dynein 2, motor proteins associating with intraflagellar transport (IFT) complex, travel along the axoneme between the base and the tip of the cilium in an anterograde and retrograde direction respectively. (b) The primary cilium modulates several signal transduction pathways. Sonic hedgehog (Shh) signaling is activated by binding of Shh to patched (Ptc1), releasing smoothened (Smo) and the downstream target gene transcription from inhibition. Canonical wingless (Wnt) signaling through low-density lipoprotein receptor-related protein (LRP)5/6 and Frizzled (Fzd) is inhibited by the primary cilium, whereas noncanonical Wnt signaling [planar cell polarity (PCP) pathway] requires the primary cilium to be activated by Dishevelled (Dvl) interacting with either Fzd or Inversin (Inv)/other PCP components such as Vangl2. Platelet-derived growth factor (PDGF) signaling through PDGF receptor (PDGFR) requires ciliary localization of the receptor to activate downstream targets. In response to fluid flow, calcium signaling is activated through mechanosensing by polycystin-1 (PC1) and polycystin-2 (PC2) localized in the ciliary membrane.
 , ciliary membrane proteins;
, ciliary membrane proteins;
 , ciliary proteins;
, ciliary proteins;
 , IFT complex;
, IFT complex;
 , kinesin 2;
, kinesin 2;
 , dynein 2;
, dynein 2;
 , nonciliary proteins.
, nonciliary proteins.
Figure 2. Cilia in various types of cells in the brain.
(a) Motile cilia anchored to the basal body are present in the ependymal cells lining the ventricle. (b) Neurons contain primary cilia projecting from basal bodies, but the cilium appears buried among the dendrites and axons of adjacent neurons. (c) Schematic diagram of the cilia axoneme cross-section. Motile cilia have 9 + 2 microtubule pair ultrastructure, with inner and outer dynein arms, whereas primary cilia have 9 + 0 microtubule pair ultrastructure, without dynein arms.
Characteristics of ciliopathies
Many syndromes characterized as ciliopathies display important neurological features, most commonly mental retardation and a variety of structural deficits (Table 1). Apart from the phenotypic characterization of the disorders, identification of causative genes together with studies on gene function have provided new insight to connect ciliary function with key signaling pathways that are essential in development of the CNS. Here, we first focus on a prototypical ciliopathy, JBTS, as a canonical human neurodevelopmental ciliopathy.
Table 1.
Summary of the ciliopathies
| Disorders | CNS defects | Other defects | Signal pathways |
|---|---|---|---|
| JBTS | Cerebellar malformation | Cystic kidney | Shh, Wnt, PI, PCP |
| Oculomotor apraxia | Polydactyly | ||
| Encephalocele | Retinitis pigmentosa | ||
| Hydrocephalus | Ataxia, hypotonia | ||
| Mental retardation | Cardiac defects | ||
| MKS | Encephalocele | Cystic kidney | Shh, PCP |
| Hydrocephalus | Polydactyly | ||
| Mental retardation | Retinal degeneration | ||
| Hepatic fibrosis | |||
| Cardiac defects | |||
| NPHP | Cerebellar malformation | Hepatic fibrosis | Shh, Wnt, PCP |
| Oculomotor apraxia | Situs inversus | ||
| Mental retardation | Retinitis pigmentosa | ||
| BBS | Mental retardation | Obestiy, diabetes | Shh, Wnt, PCP |
| Cystic kidney | |||
| Polydactyly | |||
| Retinal degeneration | |||
| OFD1 | Cerebellar malformation | Craniofacial malformation | PCP |
| Hydrocephalus | Polydactyly | ||
| Mental retardation | Cystic kidney |
CNS, central nervous system; JBTS, Joubert syndrome; MKS, Meckel–Gruber syndrome; NPHP, nephronophthisis; OFD1, oral–facial–digital syndrome type 1; PCP, planar cell polarity; PI, phosphatidylinositol; Shh, sonic hedgehog; Wnt, wingless.
Joubert syndrome
JBTS is a genetically and phenotypically heterogeneous ciliopathy, and like the majority of these diseases, inherited in an autosomal recessive fashion. The key feature on axial brain MRI sections is the ‘molar tooth’ sign, a distinctive mid-hindbrain malformation useful to delineate JBTS from other cerebellar defects. The key neurological phenotypes include intellectual disability together with oculomotor apraxia and neonatal dysregulations of breathing patterns. It is characterized by not only neurological phenotypes but also other various symptoms: cystic kidney, liver fibrosis, polymicrogyria and polydactyly [9], characteristics overlapping with other ciliopathies (particularly with MKS), thus suggesting a common pathogenesis.
The inclusion of JBTS as a ciliopathy came after the realization that the genes mutated in JBTS patients encode proteins localized predominantly to the primary cilia. In fact, all of the causative JBTS genes identified to date, INPP5E, TMEM216, AHI1, NPHP1, CEP290, TMEM67, RPGRIP1L, ARL13B, CC2D2A and OFD1, encode proteins that are localized to the base or the axoneme of the cilium. Further, several of these proteins have been implicated in the modulation of signaling pathways, such as sonic hedgehog (Shh), wingless (Wnt) and phosphatidylinositol [10–13]. Although some genes make greater contribution to the overall disease burden than others, still genetic diagnosis is possible in less than half of patients. Some genotype–phenotype correlations have emerged: we now know that CEP290 mutations are present in about half of patients displaying disease involving brain, eye and kidney, whereas AHI1 mutations are present in about 20% of patients with brain and eye pathology and about 10% of patients with only brain involvement. On the contrary, several genes make minor contributions to disease, such as the ARL13B and the RPGRIP1L genes [9,14].
The mechanism inducing malformed cerebellum and brainstem in JBTS has not been delineated, although some mutant mice of the causative genes (INPP5E, CEP290, AHI1 and ARL13B) are available now to begin to address this question. Knockout mice for Inpp5e are lethal at birth with encephaloceles as a major pathological feature, whereas Arl13b mutants die at mid-gestation with neural tube defects (NTDs) [13,15]. Both of these probably represent complete null alleles because either it was engineered as a null (in the case of Inpp5e) or it phenocopies a null allele (in the case of the Arl13b). It is interesting that in humans with JBTS, only missense mutations in INPP5E and ARL13B have been reported, which probably represent partially inactive alleles [10,16]. Therefore, the specific brain phenotype of JBTS might result from partially disabled protein activity, rather than loss of protein for INPP5E and ARL13B. However, for other genes like CEP290 and AHI1, almost all JBTS mutations are null truncating mutations. Both Cep290 and Ahi1 knockout mice generally display features consistent with ciliopathies such as retinal degeneration and cystic kidney [12,17], but the severity does not correlate with the human disease, as neither mouse mutant has a striking cerebellar phenotype (unpublished observation). Importantly, there are no obvious differences in sequence conservation among these various genes across the mammalian lineage. Thus, the differences might relate to a background/modifier effect, might be due to differences between human and mouse brain, or might represent different requirements for gene function during development. Although it may be difficult to study JBTS using animal models for these reasons, the generation of cerebellar-specific conditional or double/triple-knockout mice might help overcome some of these hurdles.
Despite lack of striking phenotypes in the cerebellum of mutant mice, many JBTS-causing genes are expressed in the CNS, suggesting their potential functions during development. INPP5E, encoding inositol polyphosphate-5-phosphatase E, is expressed in the nervous system including brain, retina and spinal cord, and is localized in the primary cilia [10,15]. Furthermore, it has been suggested that INPP5E is involved in cilia stability by controlling phosphatidylinositol signaling [10,15]. AHI1 is also strongly expressed in the brain and retina [17], and plays a role as a positive regulator of canonical Wnt signaling [12]. ARL13B, a member of Ras GTPase family, is specifically localized in the cilia of cerebellar granule neurons and in the retinal photoreceptor connecting cilia [16]. ARL13B mutant mice have impaired neural tube patterning [13], which is also observed in the mutant mice for OFD1 [18], a JBTS gene coupled to Shh signaling. These molecular signaling pathways control diverse cellular processes such as cell proliferation, differentiation and migration, all of which are important aspects of neurodevelopment. These new data focused on studying JBTS disease mechanisms complement previous basic studies on primary cilia, supporting the crucial role of primary cilia in the regulation of signaling pathways during CNS development. Investigations of the JBTS causative genes in modulating mechanisms controlling signal transduction will shed light on the specified ciliary roles in the developing nervous system.
The role of cilia in the central nervous system
Consistent with various neurological symptoms detected in ciliopathy patients, most cells in the brain (neurons including neural progenitors and mature neurons, glial cells/astrocytes and ependymal cells) have primary cilia. However, the role of neuronal primary cilia has been largely ignored. Only now have studies started to explore the specific roles of the tiny and mysterious organelles in the nervous system.
The primary cilia in brain development
Brain patterning is controlled by morphogens such as Shh, Wnt and Fgf, which control key transcription factors, to progressively subdivide discrete germinal domains along the dorsal/ventral and anterior/posterior axes. Requirement of morphogen-mediated signaling in this process has implicated a pivotal role of primary cilia during brain patterning. Correspondingly, mutant mice for several intraflagellar transport (IFT) components including IFT88, IFT139 and IFT172, which are essential for ciliogenesis [7••,19–21], have shown malformed brains, revealing the relevance of primary cilia function in brain morphogenesis [21–23].
Shh signaling, among several signal pathways, has been extensively studied in both ciliary function and CNS development. The role of cilia-mediated Shh signaling, especially, was clearly demonstrated in development of specific CNS regions. The hippocampal dentate gyrus, one site where new neurons are continuously produced throughout life and controlling circuit plasticity, learning and memory [24], is under primary cilia-mediated Shh control. Notably, when the ciliary genes kif3a, ift88, ftm or stumpy are removed, not only is there a loss of cilia but also there are defects in hippocampal neurogenesis such as decreased proliferation of granule neuron precursors (GNPs) and absence of radial astrocytes in the dentate gyrus [4,5,25]. This suggests a possible connection between Shh signaling, which acts as a mitogen in the GNPs, and ciliary function during hippocampal neurogenesis. Indeed, mutant mice of these ciliary genes appear altered in responsiveness to Shh signal [4]. Interestingly, loss of either cilia or Shh signal (loss of Smo) has no effect on generation of other types of cells such as astrocytes in the hippocampus [4], suggesting that the role of cilia-mediated Shh signaling may depend on the cell type and brain region. In the future, it might be interesting to look for mutations in ‘ciliary’ genes in patients with cognitive or behavioral defects.
Shh signaling plays a critical role in the development and neurogenesis of the cerebellum. Similar to dentate gyrus granule neurons, the majority of cerebellar granule neurons are produced from cerebellar GNPs (CGNPs) at postnatal ages, under the control of Shh. The link between cilia-mediated Shh signaling and cerebellar development can be extended to the cerebellar hypoplasia observed in many ciliopathies such as JBTS. Accordingly, the conditional removal of ciliary genes (e.g. kif3a, ift88 or stumpy) in CGNPs gives rise to striking dysgenesis and abnormal foliation of the cerebellum, as well as decreased expression of Shh target genes and proliferation of CGNPs [5,6,26], indicating that cilia are essential for Shh-dependent proliferation of CGNPs during cerebellar development. Studies focusing on mutations of other ciliary genes (fantom, ofd1 and smo) have also shown cerebellar defects [6,18,27]. Here we have highlighted the role of Shh signaling in order to link ciliary function during brain development. However, we also speculate the potential roles of Wnt and Fgf signaling that may be modulated by cilia both in the cerebellum as well as in other regions of the brain.
Defects in axonal tract decussation within the brain, including a complete absence of the pyramidal decussation, have been frequently observed in JBTS patients [28,29]. These axonal deficits may underlie defective functional brain organization, which is associated with sensorimotor and cerebellar cortical activation [30], and underlie some of the motor and cognitive defects in ciliopathies. Concurrently, mental retardation is a fairly common neurological feature among various ciliopathies (Table 1). Although these phenotypes suggest the potential role of neuronal primary cilia in axonal growth and guidance, little is known about the relationship between ciliary and axonal behavior; thus, several questions remain unanswered. First, is the neuronal primary cilium essential for axonal growth or guidance? Second, how can the neuronal cilia regulate axonal behavior? Third, how can axonal defects link to motor and cognitive deficits of ciliopathies? Fourth, how can the tiny neuronal primary cilia lying buried near the cell body (Fig. 2) have such a pronounced effect on the much longer axon? Fifth, how are the spatial constraints overcome? It is tempting to speculate a role of the primary cilia in determining CNS laterality given the role of motile cilia in determining visceral laterality, but there is currently lack of direct evidence of a mechanism for the axonal defects observed in JBTS.
Roles of cilia in the brain: beyond neuronal function
Apart from neuronal primary cilia, motile cilia are evident on ependymal cells lining the lateral ventricular surface in the brain. These cells are polarized in the epithelial plane, and this planar polarity is essential for propelling cerebrospinal fluid (CSF) flow [31]. Defects in ependymal ciliary motility results in an excessive accumulation of CSF, thereby causing hydrocephalus [32–34], and an altered migration of neuroblasts toward the olfactory bulb [35]. Accordingly, hydrocephalus, one of the most common primary cilia-associated anomalies of the CNS [36], has been detected in several ciliopathies (Table 1). However, the cellular and molecular mechanisms for the planar polarity and coordinated ciliary movement have not been fully explored. Recently, several studies have provided insight into the mechanisms underlying planar polarity and the directionality of CSF flow [37•,38,39••]: the precursor cells of ependyma, termed radial glia, already establish orientation of cilia prior to the generation of ependymal cells in the ventricular wall [38]; a combination of planar polarity signaling and hydrodynamic forces (by the emerging fluid flow) regulates docking and rotational orientation of the ependymal motile cilia [39••]; and core planar polarity components such as Celsr1, 2 and 3 and Vangl2 are essential for ciliary orientation and function [37•]. Despite these remarkable findings, there are still unanswered questions. Guirao et al. [39••] suggest that existing CSF flow determines the directionality of ependymal cilia, but it is unclear what determines the initial fluid flow direction in the brain lateral ventricle, if this is purely random, or a response to pressure differences. Tissir et al. [37•] suggest the lack or dysfunction of ependymal motile cilia could be a contributing factor in the cause of hydrocephalus. They demonstrate that hydrocephalus is probably not due to a lack of ependymal cilia, as there is evidence of pathology prior to the development of motile cilia on these cells. Rather, they suggest that loss of cilia leads to altered function of the ChP epithelium, resulting in hydrocephalus [32].
Potential roles of cilia in the central nervous system: opportunities and challenges
As the cilium has emerged as a key organelle in development of the nervous system, many studies have focused on uncovering hidden mechanisms. By uncovering multi-functional roles and structure–function relationships, some answers have emerged, as well as further mysteries.
Reciprocal function between signaling and cilia
Linking ciliary function in the nervous system with signaling pathways such as planar cell polarity (PCP) and Shh has provided new insight to understanding the mechanism of neural tube closure. However, we still do not fully understand how cilia modulate signaling pathways and many open questions remain: first, which signaling pathways required for ciliogenesis? Disruption of the PCP effector proteins such as Inturned and Fuzzy resulted in loss of cilia, as well as NTDs, suggesting involvement of cilia in PCP-dependent neural tube closure [40]. Additionally, some mutant mice of Dishevelled (DVL), one of the PCP core proteins, demonstrated rostral NTDs, which are similar to those in IFT or Shh signaling-disrupted mice [41]. Although this suggests the requirement of PCP signaling in ciliogenesis, disruption of most PCP core proteins including DVL, Vangl, Frizzled and Flamingo has not demonstrated ciliary defects. Therefore, PCP proteins may regulate ciliogenesis and signal transduction in parallel. Studies determining genetic relationship between PCP proteins and ciliary proteins may address this possibility. Second, which pathways require the cilium for signal transduction? The interaction between PCP proteins and cilia-localized proteins such as Inversin, BBS4 and BBS10 [42–44] imply that the cilium plays important roles in signal transduction. In the kidney, ciliary protein polycystin 1 is involved in gene transcription to activate two signaling pathways: mTOR (mammalian target of rapamycin) and STAT6 (signal transducer and activator of transcription 6) [45]. However, it is still argued whether cilia themselves or ciliary proteins are necessary. It is noteworthy that zebrafish without cilia exhibit Shh signaling defects, but display normal Wnt and PCP signaling [46]. This suggests that the above two hypotheses may be differently applied in each signal pathway, implicating unique ciliary functions dependent on signaling.
Function of the neuronal primary cilia as sensors
Several sensory neurons in the olfactory, acoustico-vestibular and visual systems display highly modified specialized cilia. The symptoms of blindness and deafness in ciliopathy patients and models have prompted the question, ‘do primary cilia also play roles in sensory signal transduction?’ Interestingly, several G protein-coupled receptors such as somatostatin receptor type 3, serotonin receptor 6 (HTR6), melanin-concentrating hormone receptor 1, a subset of dopamine receptors are localized to the primary cilia of neurons [47–50]. These data suggest that neuronal primary cilia may be essential for activating signaling pathways responsible for several sensory inputs. Concurrently, a recent study has suggested that hypothalamic neuronal primary cilia may be responsible for obesity phenotype by controlling leptin signaling [51], although the ciliary localization of leptin receptors remains to be determined. Future studies aimed at disrupting either neuronal primary cilia or signaling molecules in the nervous system will be required to determine direct connection between neuronal primary cilia and sensory transductions.
Tubulin posttranslational modification of cilia in the nervous system
Microtubules, composed of heterodimers of α-tubulin and β-tubulin, are subject to diverse posttranslational modifications (PTMs), including acetylation, tyrosination/detyrosination, polyglutamylation, polyglycylation, palmitoylation and phosphorylation in the highly conserved C-terminal regions [52–54]. The existence of several PTMs has been demonstrated in neuronal and nonneuronal cells, since the first implication of a PTM in brain tubulin [55]. However, the properties of modified microtubules, as well as the connection to neuronal cilia, have remained unclear until now. Recently, several studies have suggested that these PTMs occur at the cilium and play roles in ciliary function [56–60], and have identified various PTM enzymes at the cilium including tubulin tyrosine ligase, polyglutamylase and depolyglutamylase [61–63]. It has been generally accepted that the modified microtubules may play a role in neurite outgrowth and maturation, as well as in maintaining neuronal morphology [64,65]. We thus speculate that the role of ciliary PTMs may be linked to the modified microtubule-based neuronal functions in helping the cell respond to extracellular signaling and/or in microtubule-associated protein-mediated intracellular signal transduction. Therefore, it will be essential to determine: whether the different distributions of tubulin modification enzymes and their regulators depend on cell types or subcellular organelles; how PTMs regulate the cytoskeletal network and dynamics including changes of neuronal morphology and motility; and whether depletion of ciliary genes causes tubulin PTM defects, thereby disrupting neuronal functions.
Conclusion
Dysplasia and dysfunction of primary cilia has been shown to cause defects in the CNS, as well as a number of organs such as heart and kidney in the ciliopathy spectrum. Primary cilia play critical roles in controlling neuronal development, modulating various signal transduction pathways. Despite the enormous amount of progress we have made studying signaling pathways influenced by primary cilia and related genes in the nervous system, we are still far from understanding their roles in classical neuronal behaviors such as neuronal migration and axonal guidance. It is possible that primary cilia in neurons may also act as mediators in signal transduction. Alternatively, they may be involved in cell–cell communication and/or cytoskeleton-associated processes. The field of ciliary biology still has many mysteries yet to be solved. The presence of severe structural multiorgan abnormalities suggests that treatment options in children may be limited. However, at the same time the involvement of ongoing signaling may contribute to the intellectual disabilities and other deficits, which might be amendable to treatments. The search for cilia-modulating pathways or mechanisms to bypass the requirement for cilia in specific pathways might represent one avenue of investigation.
Key points.
Defective primary cilia give rise to neurodevelopmental disorders now termed as ciliopathies, including Joubert syndrome.
Primary cilia in the central nervous system function as mediators of signal transduction during development.
Signaling pathways such as planar cell polarity involve the function of both motile and primary cilia in brain development.
Acknowledgments
J.E.L. is supported by the American Heart Association (09POST2250641). J.G.G. is a Howard Hughes Medical Institute investigator. The authors would like to thank Francis Lee, Jennifer Silhavy and Madeline Lancaster for stimulating scientific discussion.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 183).
- 1.Hildebrandt F, Attanasio M, Otto E. Nephronophthisis: disease mechanisms of a ciliopathy. J Am Soc Nephrol. 2009;20:23–35. doi: 10.1681/ASN.2008050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giordano L, Vignoli A, Pinelli L, et al. Joubert syndrome with bilateral polymicrogyria: clinical and neuropathological findings in two brothers. Am J Med Genet A. 2009;149A:1511–1515. doi: 10.1002/ajmg.a.32936. [DOI] [PubMed] [Google Scholar]
- 3.Rooryck C, Pelras S, Chateil JF, et al. Bardet-Biedl syndrome and brain abnormalities. Neuropediatrics. 2007;38:5–9. doi: 10.1055/s-2007-981466. [DOI] [PubMed] [Google Scholar]
- 4.Han YG, Spassky N, Romaguera-Ros M, et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- 5.Breunig JJ, Sarkisian MR, Arellano JI, et al. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Natl Acad Sci U S A. 2008;105:13127–13132. doi: 10.1073/pnas.0804558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spassky N, Han YG, Aguilar A, et al. Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev Biol. 2008;317:246–259. doi: 10.1016/j.ydbio.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7••.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. An excellent review assessing the role of primary cilia-mediated Shh signaling during development, as well as clarifying the connection between developmental signaling and cilia function in human diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. J Cell Sci. 2010;123:499–503. doi: 10.1242/jcs.050377. A review summarizing the molecular signaling pathways modulated through primary cilia and related ciliary proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parisi MA. Clinical and molecular features of Joubert syndrome and related disorders. Am J Med Genet C Semin Med Genet. 2009;151C:326–340. doi: 10.1002/ajmg.c.30229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bielas SL, Silhavy JL, Brancati F, et al. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet. 2009;41:1032–1036. doi: 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valente EM, Logan CV, Mougou-Zerelli S, et al. Mutations in TMEM216 perturb ciliogenesis and cause Joubert, Meckel and related syndromes. Nat Genet. 2010;42:619–625. doi: 10.1038/ng.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lancaster MA, Louie CM, Silhavy JL, et al. Impaired Wnt-beta-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nat Med. 2009;15:1046–1054. doi: 10.1038/nm.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caspary T, Larkins CE, Anderson KV. The graded response to sonic hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–778. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Doherty D. Joubert syndrome: insights into brain development, cilium biology, and complex disease. Semin Pediatr Neurol. 2009;16:143–154. doi: 10.1016/j.spen.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacoby M, Cox JJ, Gayral S, et al. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat Genet. 2009;41:1027–1031. doi: 10.1038/ng.427. [DOI] [PubMed] [Google Scholar]
- 16.Cantagrel V, Silhavy JL, Bielas SL, et al. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet. 2008;83:170–179. doi: 10.1016/j.ajhg.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louie CM, Caridi G, Lopes VS, et al. AHI1 is required for photoreceptor outer segment development and is a modifier for retinal degeneration in nephronophthisis. Nat Genet. 2010;42:175–180. doi: 10.1038/ng.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrante MI, Zullo A, Barra A, et al. Oral-facial-digital type I protein is required for primary cilia formation and left-right axis specification. Nat Genet. 2006;38:112–117. doi: 10.1038/ng1684. [DOI] [PubMed] [Google Scholar]
- 19.Pazour GJ, Dickert BL, Vucica Y, et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran PV, Haycraft CJ, Besschetnova TY, et al. THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nat Genet. 2008;40:403–410. doi: 10.1038/ng.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorivodsky M, Mukhopadhyay M, Wilsch-Braeuninger M, et al. Intraflagellar transport protein 172 is essential for primary cilia formation and plays a vital role in patterning the mammalian brain. Dev Biol. 2009;325:24–32. doi: 10.1016/j.ydbio.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stottmann RW, Tran PV, Turbe-Doan A, et al. Ttc21b is required to restrict sonic hedgehog activity in the developing mouse forebrain. Dev Biol. 2009;335:166–178. doi: 10.1016/j.ydbio.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willaredt MA, Hasenpusch-Theil K, Gardner HA, et al. A crucial role for primary cilia in cortical morphogenesis. J Neurosci. 2008;28:12887–12900. doi: 10.1523/JNEUROSCI.2084-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990;301:365–381. doi: 10.1002/cne.903010304. [DOI] [PubMed] [Google Scholar]
- 25.Town T, Breunig JJ, Sarkisian MR, et al. The stumpy gene is required for mammalian ciliogenesis. Proc Natl Acad Sci U S A. 2008;105:2853–2858. doi: 10.1073/pnas.0712385105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chizhikov VV, Davenport J, Zhang Q, et al. Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool. J Neurosci. 2007;27:9780–9789. doi: 10.1523/JNEUROSCI.5586-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vierkotten J, Dildrop R, Peters T, et al. Ftm is a novel basal body protein of cilia involved in Shh signalling. Development. 2007;134:2569–2577. doi: 10.1242/dev.003715. [DOI] [PubMed] [Google Scholar]
- 28.Yachnis AT, Rorke LB. Cerebellar and brainstem development: an overview in relation to Joubert syndrome. J Child Neurol. 1999;14:570–573. doi: 10.1177/088307389901400904. [DOI] [PubMed] [Google Scholar]
- 29.ten Donkelaar HJ, Hoevenaars F, Wesseling P. A case of Joubert’s syndrome with extensive cerebral malformations. Clin Neuropathol. 2000;19:85–93. [PubMed] [Google Scholar]
- 30.Parisi MA, Pinter JD, Glass IA, et al. Cerebral and cerebellar motor activation abnormalities in a subject with Joubert syndrome: functional magnetic resonance imaging (MRI) study. J Child Neurol. 2004;19:214–218. [PubMed] [Google Scholar]
- 31.Del Bigio MR. The ependyma: a protective barrier between brain and cerebrospinal fluid. Glia. 1995;14:1–13. doi: 10.1002/glia.440140102. [DOI] [PubMed] [Google Scholar]
- 32.Banizs B, Pike MM, Millican CL, et al. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development. 2005;132:5329–5339. doi: 10.1242/dev.02153. [DOI] [PubMed] [Google Scholar]
- 33.Ibanez-Tallon I, Pagenstecher A, Fliegauf M, et al. Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymal flow and reveals a novel mechanism for hydrocephalus formation. Hum Mol Genet. 2004;13:2133–2141. doi: 10.1093/hmg/ddh219. [DOI] [PubMed] [Google Scholar]
- 34.Lechtreck KF, Delmotte P, Robinson ML, et al. Mutations in Hydin impair ciliary motility in mice. J Cell Biol. 2008;180:633–643. doi: 10.1083/jcb.200710162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawamoto K, Wichterle H, Gonzalez-Perez O, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- 36.Bruni JE, Del Bigio MR, Clattenburg RE. Ependyma: normal and pathological. A review of the literature Brain Res. 1985;356:1–19. doi: 10.1016/0165-0173(85)90016-5. [DOI] [PubMed] [Google Scholar]
- 37•.Tissir F, Qu Y, Montcouquiol M, et al. Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nat Neurosci. 2010;13:700–707. doi: 10.1038/nn.2555. This study demonstrates that brain ependymal cilia are involved in the pathophysiology of hydrocephalus by regulating PCP signaling and identifies the genetic factors related to the mechanism. [DOI] [PubMed] [Google Scholar]
- 38.Mirzadeh Z, Han YG, Soriano-Navarro M, et al. Cilia organize ependymal planar polarity. J Neurosci. 2010;30:2600–2610. doi: 10.1523/JNEUROSCI.3744-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.Guirao B, Meunier A, Mortaud S, et al. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat Cell Biol. 2010;12:341–350. doi: 10.1038/ncb2040. This article gives one possible answer to how fluid flow-mediated morphogenesis is regulated by identifying the linked mechanisms between external hydrodynamic cues and PCP signaling in controlling motile cilia directionality. [DOI] [PubMed] [Google Scholar]
- 40.Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and hedgehog signaling. Nat Genet. 2006;38:303–311. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- 41.Hamblet NS, Lijam N, Ruiz-Lozano P, et al. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–5838. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- 42.Ross AJ, May-Simera H, Eichers ER, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 43.Simons M, Gloy J, Ganner A, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stoetzel C, Laurier V, Davis EE, et al. BBS10 encodes a vertebrate-specific chaperonin-like protein and is a major BBS locus. Nat Genet. 2006;38:521–524. doi: 10.1038/ng1771. [DOI] [PubMed] [Google Scholar]
- 45.Low SH, Vasanth S, Larson CH, et al. Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev Cell. 2006;10:57–69. doi: 10.1016/j.devcel.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Huang P, Schier AF. Dampened hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development. 2009;136:3089–3098. doi: 10.1242/dev.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Handel M, Schulz S, Stanarius A, et al. Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience. 1999;89:909–926. doi: 10.1016/s0306-4522(98)00354-6. [DOI] [PubMed] [Google Scholar]
- 48.Brailov I, Bancila M, Brisorgueil MJ, et al. Localization of 5-HT(6) receptors at the plasma membrane of neuronal cilia in the rat brain. Brain Res. 2000;872:271–275. doi: 10.1016/s0006-8993(00)02519-1. [DOI] [PubMed] [Google Scholar]
- 49.Berbari NF, Lewis JS, Bishop GA, et al. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci U S A. 2008;105:4242–4246. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marley A, von Zastrow M. DISC1 regulates primary cilia that display specific dopamine receptors. PLoS One. 2010;5:e10902. doi: 10.1371/journal.pone.0010902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davenport JR, Watts AJ, Roper VC, et al. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol. 2007;17:1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westermann S, Weber K. Posttranslational modifications regulate microtubule function. Nat Rev Mol Cell Biol. 2003;4:938–947. doi: 10.1038/nrm1260. [DOI] [PubMed] [Google Scholar]
- 53.Verhey KJ, Gaertig J. The tubulin code. Cell Cycle. 2007;6:2152–2160. doi: 10.4161/cc.6.17.4633. [DOI] [PubMed] [Google Scholar]
- 54.Hammond JW, Cai D, Verhey KJ. Tubulin modifications and their cellular functions. Curr Opin Cell Biol. 2008;20:71–76. doi: 10.1016/j.ceb.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barra HS, Arce CA, Rodriguez JA, et al. Some common properties of the protein that incorporates tyrosine as a single unit and the microtubule proteins. Biochem Biophys Res Commun. 1974;60:1384–1390. doi: 10.1016/0006-291x(74)90351-9. [DOI] [PubMed] [Google Scholar]
- 56.Ikegami K, Sato S, Nakamura K, et al. Tubulin polyglutamylation is essential for airway ciliary function through the regulation of beating asymmetry. Proc Natl Acad Sci U S A. 2010;107:10490–10495. doi: 10.1073/pnas.1002128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suryavanshi S, Edde B, Fox LA, et al. Tubulin glutamylation regulates ciliary motility by altering inner dynein arm activity. Curr Biol. 2010;20:435–440. doi: 10.1016/j.cub.2009.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kubo T, Yanagisawa HA, Yagi T, et al. Tubulin polyglutamylation regulates axonemal motility by modulating activities of inner-arm dyneins. Curr Biol. 2010;20:441–445. doi: 10.1016/j.cub.2009.12.058. [DOI] [PubMed] [Google Scholar]
- 59.Wloga D, Webster DM, Rogowski K, et al. TTLL3 Is a tubulin glycine ligase that regulates the assembly of cilia. Dev Cell. 2009;16:867–876. doi: 10.1016/j.devcel.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 60.Pathak NH, Drummond IA. Polyglutamylation and the fleer gene. Methods Cell Biol. 2009;94:317–332. doi: 10.1016/S0091-679X(08)94016-4. [DOI] [PubMed] [Google Scholar]
- 61.Ersfeld K, Wehland J, Plessmann U, et al. Characterization of the tubulin-tyrosine ligase. J Cell Biol. 1993;120:725–732. doi: 10.1083/jcb.120.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Dijk J, Rogowski K, Miro J, et al. A targeted multienzyme mechanism for selective microtubule polyglutamylation. Mol Cell. 2007;26:437–448. doi: 10.1016/j.molcel.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 63.Rogowski K, van Dijk J, Magiera MM, et al. A family of protein-deglutamylating enzymes associated with neurodegeneration. Cell. 2010;143:564–578. doi: 10.1016/j.cell.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 64.Fukushima N, Furuta D, Hidaka Y, et al. Posttranslational modifications of tubulin in the nervous system. J Neurochem. 2009;109:683–693. doi: 10.1111/j.1471-4159.2009.06013.x. [DOI] [PubMed] [Google Scholar]
- 65.Ikegami K, Setou M. Unique posttranslational modifications in specialized microtubule architecture. Cell Struct Funct. 2010;35:15–22. doi: 10.1247/csf.09027. [DOI] [PubMed] [Google Scholar]