Abstract
Major depressive disorder (MDD) is a major public health concern. Despite tremendous advances, the pathogenic mechanisms associated with MDD are still unclear. Moreover, a significant number of MDD subjects do not respond to the currently available medication. MicroRNAs (miRNAs) are a class of small noncoding RNAs that control gene expression by modulating translation, messenger RNA (mRNA) degradation, or stability of mRNA targets. The role of miRNAs in disease pathophysiology is emerging rapidly. Recent studies demonstrating the involvement of miRNAs in several aspects of neural plasticity, neurogenesis, and stress response, and more direct studies in human postmortem brain provide strong evidence that miRNAs can not only play a critical role in MDD pathogenesis, but can also open up new avenues for the development of therapeutic targets. Circulating miRNAs are now being considered as possible biomarkers in disease pathogenesis and in monitoring therapeutic responses because of the presence and/or release of miRNAs in blood cells as well as in other peripheral tissues. In this review, these aspects are discussed in a comprehensive and critical manner.
Keywords: biomarker, depression, miRNA, neural plasticity, neurogenesis, stress
Abstract
El trastorno depresivo mayor (TDM) es un importante tema de salud pública. A pesar de los enormes avances, aun no están aclarados los mecanismos patogénicos asociados con el TDM. Además, un número significativo de sujetos con TDM no responden a los medicamentos actualmente disponibles. Los microARNs (miARNs) constituyen una clase de ARNs pequeños no codificantes que controlan la expresión génica al modular la translación, la degradación del ARN mensajero (ARNm) o la estabilidad de los blancos del ARNm. Rápidamente está apareciendo un nuevo papel para los miARNs en la fisiopatología de la enfermedad. Hay estudios recíentes que demuestran la participación de los miARNs en diversos aspectos de la plasticidad neural, la neurogénesis y la respuesta al estrés. Estudios más específicos en cerebro humano postmortem aportan importante evidencia relacionada con el papel crítico que pueden jugar los miARNs en la patogénesis del TDM, y también en la apertura de nuevos caminos para el desarrollo de blancos terapéuticos. Los miARNs circulantes se están considerando actualmente como posibles biomarcadores en la patogénesis de la enfermedad y en el monitoreo de las respuestas terapéuticas dada la presencia ylo liberación de los miARNs en las células sanguíneas como en otros tejidos periféricos. En esta revisión se discuten estos aspectos de una forma crítica y comprensible.
Abstract
L'épisode dépressif caracterisé (EDC) est un problème majeur de santé publique. Malgré des progrès considérables, les mécanismes pathogènes associés à l'EDC restent obscurs. De plus, un nombre significatif de personnes atteintes d'EDC ne répond pas aux traitements actuellement disponibles. Les microARN (miARN) sont une classe de petits ARN non codants qui contrôlent l'expression du gène en modulant la translation, la dégradation de l'ARN messager (ARNm) ou la stabilité des cibles de l'ARNm. La connaissance du rôle des miARN dans la physiopathologie des maladies prend rapidement de l'essor. Des études récentes démontrant l'implication des miARN dans plusieurs aspects de plasticité neuronale, de neurogenèse, de réponse au stress et d'études plus directes dans le cerveau humain postmortem affirment que les miARN non seulement jouent un rôle décisif dans la pathogenèse de l'EDC, mais qu'ils peuvent aussi inaugurer de nouvelles voies pour le développement de cibles thérapeutiques. Les miARN circulants sont maintenant considérés comme des biomarqueurs possibles dans la pathogenèse de la maladie et dans les réponses thérapeutiques de contrôle à cause de la présence et/ou de la libération des miARN dans les cellules sanguines comme dans d'autres tissus périphériques. Nous analysons dans cet article ces différents aspects de façon complète et critique.
Introduction
Major depressive disorder (MDD) is one of the most prevalent psychiatric disorders, it affects about 17% of Americans during their lifetime1 and is associated with psychosocial impairment, poor quality of life, and significant disability,2 morbidity, and mortality.3-5 MDD is being diagnosed at early ages, and about 25% of people diagnosed with MDD are under 19 years old. Although much work has been done to characterize MDD, about 40% of MDD patients do not respond to the currently available medications.6 This is partially a result of poor understanding of the molecular pathophysiology underlying MDD.
As is well known, compromised neural and structural plasticity are intimately associated with MDD.7,8 This is evident from studies in MDD subjects showing altered structural and functional plasticity,9-12 changes in the synaptic circuitry,13 decreased dorsolateral prefrontal cortical activity,14,15 impaired synaptic connectivity between the frontal lobe and other brain regions,16,17 changes in number and shape of dendritic spines,18,19 the primary location of synapse formation, altered dendritic morphology of neurons in the hippocampus, decreased length and number of apical dendrites,20 neuronal atrophy and decreased volume of the hippocampus,21,22 decreased number of neurons and glia in cortical areas,23 and spatial cognition deficits.24. In addition, MDD is associated with a negative impact on learning and memory,25,26 while stress, a major factor in depression, hinders performance of hippocampal-dependent memory tasks and impairs the induction of hippocampal long-term potentiation (LTP).27
The cellular mechanisms that underlie such compromised neural plasticity and structural impairments in MDD are not clearly understood, and no single mechanism appears to be responsible for its etiopathogenesis; however, it is becoming increasingly evident that MDD may result from disruptions across whole cellular networks, leading to aberrant information processing in the circuits that regulate mood, cognition, and neurovegetative functions.7 In fact, evidence demonstrating impaired cellular networks that regulate neural plasticity has reshaped our views about the neurobiological underpinnings of MDD.28
In recent years, the emergence of small noncoding RNAs as a mega-controller and regulator of gene expression has gained much attention in various disease pathophysiologies. These small noncoding RNAs regulate gene expression by several mechanisms, including ribosomal RNA modifications, repression of mRNA expression by RNA interference, alternative splicing, and regulatory mechanisms mediated by RNA-RNA interactions. The group of small noncoding RNAs include: miRNAs, small nucleolar RNAs, small interfering RNAs, piwi-interacting RNAs, splisosomal RNAs, and p/MRP RNase genes.29,30 Among them, miRNAs are the most studied and well characterized; they have emerged as a major regulator of neural plasticity and higher brain functioning,31,32 regulate about 60% of total mammalian RNAs, and are involved in virtually all biological functions. By modulating translation and/or stability of mRNA targets in a coordinated and cohesive fashion, they are able to regulate entire genetic circuitries.33 It has been shown that a combination of miRNAs is a much more powerful regulator than individual miRNAs. Interestingly, differential coexpression of a group of miRNAs has not only been shown to play a direct role in human disease pathogenesis, but can also help in identifying the nature of disordered pathways implicated in such pathogenesis.34-37
miRNAs are expressed highly in neurons, and because they can regulate the expression of a large number of target mRNAs, neuronal miRNA pathways can create an extremely powerful mechanism to dynamically adjust the protein content of neuronal compartments, even without the need for new gene transcription.38,39 miRNAs have been extensively studied in cancer biology; however, a large body of evidence demonstrates their role in several neuropsychiatric diseases, such as schizophrenia, autism, Parkinson's disease, Huntington's disease, Tourette's syndrome, Fragile X syndrome, DiGeorge syndrome, Down syndrome, and Alzheimer's disease. Studies are now being geared to examine if mutations in genes that encode miRNAs or various components of miRNA biogenesis machinery can lead to aberrant miRNA synthesis and target genes that can be linked to specific disease pathophysiology. Knowledge of the role of miRNAs in MDD is still in its infancy; however, several lines of evidence clearly demonstrate that miRNAs may play a major role in the development of stress-related disorders, including MDD. The aim of this review is to critically evaluate the role of miRNAs in MDD pathogenesis and examine whether miRNAs can be developed as biomarkers for depression.
miRNA biogenesis and regulation of target mRNA expression
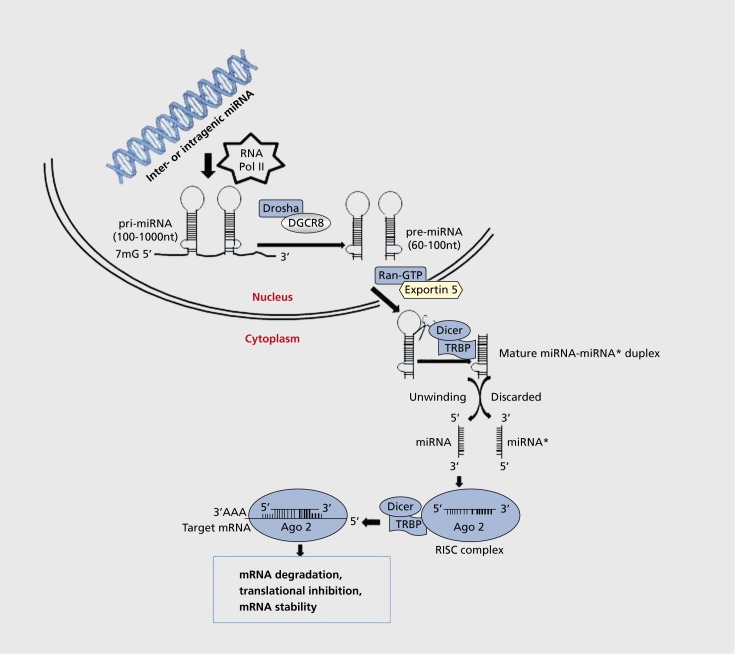
An overview of miRNA biogenesis is depicted in Figure 1. As shown, miRNA biogenesis occurs in the nucleus. miRNAs are encoded within primary miRNA (primiRNA) gene transcripts that may be intergenic (away from known protein-coding genes) or may be located within introns of protein-coding host genes (intragenic).
Figure 1. miRNA biogenesis. microRNAs (miRNAs) are encoded in the genome (inter or intragenis) and transcribed by RNA polymerase II (RNA pol II) to generate primary microRNA (pri-miRNA). These pri-miRNAs are taken up by the RNA II enzyme Drosha/DiGeorge syndrome critical region 8 (DGCR8) protein, which catalyzes the formation of precursor miRNA (pre-miRNA). Pre-miRNA is then exported to cytoplasm by ExportinB in conjunction with Ras-related nuclear protein, RanGTR In the cytoplasm, pre-miRNA is cleaved into miRNA:miRNA* duplex by the Rnase III enzyme Dicer/Tar RNA-binding protein (TRBP) or PKR activating protein (PACT). One of these miRNA/miRNA* duplexes is discarded and the other one is loaded onto an Argonaute (Ago) homologue protein (isoforms of eukaryotic translation initiation factor [eIF] 2c) to generate the effector complex, known as RNA-induced silencing complex (RISC). The RISC complex directs miRNA to specific “short-seed” sequences located predominantly within the 3' UTR region of the target messenger RNA (mRNA). This leads to degradation or translational inhibition. nt, nucleotides.
miRNA genes are transcribed to long primary miRNA by RNA polymerase II or III. The pri-miRNAs are then processed further within the nucleus to form one or a series of small hairpin miRNA precursors, or precursor miRNAs (pre-miRNAs), that are generally 60 to 100 nt long and fold into a stem-loop structure. The protein that converts pri-miRNA into pre-miRNA is an RNase III enzyme, Drosha. Generally, Drosha requires the DiGeorge syndrome critical region 8 (DGCR8) protein as a cefaclor for activation. Together with DGCR8, Drosha forms a large complex known as the “microprocessor complex.” Drosha removes the flanking segments and ≈ 11 base pair (bp) stem region of the pri-miRNA. The pre-miRNAs are then transported out of the nucleus via the exportin transfer system, which consists of Exportin 5 and guanosine triphosphate -bound Ran (RanGTP). Pre-miRNA is released into the cytoplasm upon hydrolysis of GTP to GDP. The premiRNAs are further processed in the cytoplasm by the RNase III enzyme Dicer, which coverts pre-miRNA into double-stranded mature small RNA (miRNA/miRNA* duplexes) of approximately 22 nucleotides (nt) long.40 Dicer requires cofactors such as HIV-1 transactivating response (TAR) RNA-binding protein (TRBP) or protein kinase R (PKR)-activating protein (PACT). One of the miRNA/miRNA* duplexes is loaded onto an Argonaute (Ago) homologue protein (isoform of the eukaryotic translation initiation factor [eIF] 2C) to generate the effector complex, known as RNA-induced silencing complex (RISC). The other miRNA* strand is degraded.
miRNA-mediated regulation of target mRNAs and expression
RISC binds to specific “short-seed” sequences located predominantly within the 3' untranslated region (3' UTR) of target mRNAs, and can interfere with the translation of mRNA and/or reduce mRNA levels. miRNA-mediated translational inhibition also depends upon the 5' cap region of the target mRNA. Ago proteins can stimulate miRNA-dependent translation inhibition by competing with efF4E for the 5' cap binding site, thus preventing circularization of mRNA and lowering initiation efficiency.41 Although miRNAs target transcripts through imperfect base-pairing to multiple sites in 3' UTRs, Watson-Crick base-pairing to the 5' end of miRNAs, especially to the so-called “seed” that comprises nucleotides 2 to 7, is also crucial for targeting.42 This provides a mechanism by which one miRNA can target several mRNAs. RISC can also associate with both the 60S ribosome and eIF6.43 eIF6 regulates the formation of the translationally active SOS subunit. By regulating eIF6, miRNAs can modify polysome formation and expose target mRNAs for degradation.43 In addition to the direct sequence-specific interaction of RISC with mRNAs, other proteins that bind nearby sites within the 3' UTR (eg, fragile X mental retardation protein [FMRP] homologues, Hu protein B [HuB] family members, and other adenylate-uridylate-rich element [ARE]-binding proteins) may control the magnitude and even the direction of miRNA effects. In certain circumstances (eg, depending on the phase of the cell cycle in dividing cells, which possibly reflects reversible phosphorylation or methylation of FMRP homologues), miRNAs may actually enhance, rather than inhibit, translation.44 miRNA-mediated regulation of mRNA stability is another mechanism by which miRNAs suppress expression of specific mRNA. Using miR-125b and let-7 as representative miRNAs, Wu et al45 showed that in mammalian cells the reduction in mRNA abundance is a consequence of accelerated deadenylation, which leads to rapid mRNA decay. Besides regulating translational processes, it has been shown that miRNA can also regulate gene transcription by targeting transcription factors. In this case, levels of transcription factors are downregulated by miRNAs, which in turn cause less expression of mRNA, leading to reduced protein synthesis.46,47
Recent evidence suggests that miRNA biogenesis can be regulated at the epigenetic level.48 For example, inhibitors of DNA methylation and histone deacetylases can affect expression of several miRNAs.49 On the other hand, a subset of miRNAs can control the expression of epigenetic regulators, such as DNA methyltransferases, histone deacetylases, and polycomb group genes, leading to transcriptional activation of numerous protein coding gene sequences, thereby contributing to gene expression. This network of feedback between miRNAs and epigenetic pathways appears to form an epigenetics-miRNA regulatory circuit, and to organize the whole gene expression profile.50
The expression of miRNAs is tissue-specific51-53 and, in some cases, even cell-type-specific.54-56 In addition, some of the miRNAs are expressed specifically at the developmental stages.57,58 Approximately 20% to 40% of miRNAs in the brain are developmentally regulated.59,60 For example, miR-124a, which is conserved at the nucleotide level and is important for neuronal differentiation, neurite outgrowth, and glucocorticoid receptor (GR)—mediated functions, is expressed throughout embryonic and adult brain.61,62 There are studies which suggest that miRNAs, such as miR-124 and miR-128, are primarily expressed in neurons, whereas miR-23, miR-26, and miR-29 are expressed in high amounts in astrocytes.63 A recent study by He et al64 suggests that a large number of miRNAs show distinct profiles in glutamatergic and GABAergic neurons and subtypes of GABAergic neurons. Even within neurons, it has been demonstrated that some of the pre-miRNAs are highlyexpressed in the dendrites where they can be locally transcribed into mature miRNAs65 and can locally regulate mRNA translation. These include synaptically enriched miRNAs: miR-200c, miR-339, miR-332, miR-318, miR-29a, miR-7, and miR-137.65,66 Several of the miRNAs are also expressed in the exons and presynaptic nerve terminals; some of them (miR-16, miR-221, miR-204, miR-15b) are highly expressed in distal axons compared with cell bodies.67 Moreover, a number of miRNAs encoded by a common pri-miRNA were differentially expressed in the distal axons, suggesting that there is a differential subcellular transport of miRNAs derived from the same coding region of the genome.67 Interestingly, the entire transcriptional machinery involved in the synthesis of mature miRNA has been shown to be localized in the synaptic fraction.65,68,69
Given their differential nature of expression and because of the local regulation of mRNA translation, the role of miRNAs in numerous biological phenomena, including neuronal development, cell proliferation, cell cycle, neurogenesis, synaptic development, axon guidance, and neuronal plasticity, have been studied.70-73
miRNAs: potential regulators of neurogenesis and neural plasticity
miRNAs in neurogenesis
Adult neurogenesis, the process of generating new neurons from neural stem cells, plays a critical role in synaptic plasticity, learning and memory, and mood regulation.74,75 In the mammalian brain, neurogenesis occurs throughout adulthood in the hippocampus (subgranular zone [SGZ] of the dentate gyrus) and olfactory bulb (subventricular zone [SVZ]). Neurogenesis in the SVZ is important for olfactory learning,76 whereas hippocampal neurogenesis is involved in memory and spatial learning.77 Numerous studies suggest that stress and MDD are associated with decreased hippocampal neurogenesis.78-80 Additionally, disturbed adult neurogenesis, possibly resulting in a malfunctioning of hippocampus, may contribute to cognitive deficits.81 Conversely, hippocampal neurogenesis buffers stress responses and depressive behavior.82 Enriched environment, exercise, electroconvulsive therapy, deep brain stimulation, and antidepressants increase hippocampal neurogenesis.83 The regulatory factors that control adult neurogenesis are currently under investigation; however, recent studies demonstrate that miRNAs play a role in both embryonic as well as adult neurogenesis.84 For example, Choi et al56 demonstrated that olfactory tissues express more than 100 distinct miRNAs, the most abundant being the miR-124a and let-7 variants and the family of miR-200. To determine whether miRNAs are required during olfactory neuronal development, these investigators analyzed embryonic tissues in which Dicer function was specifically ablated in olfactory progenitor cells. They showed that the loss of miRNA function from olfactory progenitor cells produced no alterations in patterning. In contrast, they noted that terminal differentiation of the olfactory progenitor pool into mature olfactory neurons does not occur and that the olfactory precursor cell population is not maintained. Dicer depletion also impacts proliferation and cell death, migration, and differentiation during corticogenesis as assessed by McLoughlin et al85 in the developing brain. Using markers for proliferation and in vivo labeling, they showed reduced numbers of proliferating cells, altered cell cycle kinetics from embryonic day 15.5 (E15.5), distributed progenitor cells throughout the cortex (rather than restricted to the SVZ and ventricular zones), and increased cortical cell death as early as E15.5. DGCR8 heterozygous mice also show reduced cell proliferation and neurogenesis in adult hippocampus.86 Pathania et al87 recently showed that timely directed overexpression of miR-132 at the onset of synaptic integration using an inducible approach leads to a significant increase in the survival of newborn neurons in SVZ. These effects are mirrored with respective changes in the frequency of γ-aminobutyric acid (GABA)ergic and glutamatergic synaptic inputs reflecting altered synaptic integration. The results suggest that miR-132 forms the basis of a structural plasticity program seen in SVZ-olfactory bulb postnatal neurogenesis. Cheng et al88 showed that miR-124 is an important regulator of the temporal progression of adult neurogenesis. They found that knockdown of endogenous miR-124 maintained purified SVZ stem cells as dividing precursors, whereas ectopic expression led to precocious and increased neuron formation in mice. They identified the SRY-box transcription factor Sox9 as a physiological target of miR-1 24 during the transition from the transit-amplifying cell to the neuroblast stage. The overexpression of Sox9 abolished neuronal differentiation, whereas Sox9 knockdown led to increased neuron formation. Thus, miR-124-mediated repression of Sox9 is important for progression along the SVZ stem cell lineage to neurons. Bruno et al89 identified brain-specific miRNA: miR-128, that represses nonsense-mediated RNA decay machinery, which controls transcripts of a battery of target genes to regulate neurogenesis and neural differentiation. More recently, Åkerblom et al,90 using a transgenic reporter mouse, found that miR-124 expression is initiated in the rapidly amplifying progenitors and remains expressed in the resulting neurons. Inhibition of miR-124 in vivo results in the blockade of neurogenesis, leading to the appearance of ectopic cells with astrocyte characteristics in the olfactory bulb. Conversely, neural stem cells are not maintained in the SVZ, when miR-124 is overexpressed, resulting in a loss of neurogenesis. These results suggest that miR-124 is a neuronal fate determinant in the SVZ. miR-137, which is epigenetic ally regulated by DNA methyl-CpG-binding protein, can modulate proliferation and differentiation of adult neural stem cells such that overexpression of miR-137 promotes, whereas its reduction enhances proliferation of adult neural stem cells.91 Recently, Zhang et al92 identified a new cerebellum-enriched rno-miR-592, which plays an important role in embryonic neurogenesis and/or astrogliogenesis. By using gain-/loss-of-function approaches, they demonstrated that rno-miR-592 could change the balance between neuron- and astrocyte-like differentiation and neuronal morphology. miR-592 could induce astrogliogenesis differentiation arrest and/or enhance neurogenesis in vitro, whereas silencing of miR-592 was not beneficial for neuronal maturation. They also identified LRRC4C and NFASC as miR-592 target genes, suggesting that these two target genes may be involved in miR-592 regulative function in neuronal progenitor cell differentiation and neuronal maturation.
miRNAs in neural plasticity
As mentioned earlier, miRNAs play a critical role in regulating synaptic and neural plasticity. It has been shown that by knocking down components of the miRNA synthesis machinery such as Dicer leads to a reduction in neuronal size and branching as well as aberrant axonal pathfinding.93-95 On the other hand, DGCR8 knockout mice show loss of synaptic connectivity and reduced number and size of the dendritic spines.96,97 At the behavioral level, these mice display impaired spatial working memory-dependent tasks.97 FMRP, which regulates protein synthesis in dendritic spines after binding to specific sites within the 3'UTR of certain mRNAs in concert with RISC components Agol and Dicer,98,99 is associated with learning, and LTP. Both FMRP and RISC complex components are localized in the somatodendritic compartment100,101 and FMRP associates strongly with several components of the miRNA machinery, including mature miRNAs, pre-miRNAs, Dicer, and eIF2c. One of the RISC proteins, armitage, is essential for LTP and synaptic protein synthesis and is cleaved during the learning process.102 FMRP is also associated with miR-125b and miR-132 in the brain. miR-132 overexpression increases dendritic protrusion as well as branching,103 whereas miR-125b targets NR2A mRNA and regulates synaptic plasticity in a negative fashion.104 Similar negative regulation of the size of dendritic spines in rat hippocampal neurons has been shown to be associated with miR-138 as well as miR-134. miR-138 controls the expression of acyl-protein thioesterase 1 (APT1), an enzyme regulating the palmitoylation status of proteins that are known to function at the synapse.66 On the other hand, miR-134 inhibits translation of Lim-domain-containing protein kinase 1 (Limkl),105 a protein that regulates dendritic spine growth.106 Exposure to brain-derived neurotrophic factor (BDNF) relieves Limkf translation suppression caused by miR-134. miR-134 can also promote dendritogenesis by inhibiting the translational repressor Pumilio 2.107 Interestingly, BDNF is lower in the brain of depressed subjects.108,109 Recently, Cohen et al110 showed that miR-485 regulates dendritic spine number in an activity-dependent manner, in conjunction with synaptic vesicle protein SV2A. miRISC protein Mov 10, an RNA helicase that associates with the Argonaute protein, is present at synapses and regulates synaptic expression of calmodulin (CaM) kinase II and Limkl.111
Several studies demonstrate that Creb, a key transcription factor regulating synaptic plasticity and whose expression is lower in specific brain regions of MDD subjects,112 is one of the major targets of a number of miRNAs. Conversely, many miRNAs have binding sites in their promoter regions for Crebl.114 Expression of miR-132, which enhances neurite outgrowth, dendritic morphogenesis, and spine formation,103,113,115 is induced by BDNF via Creb. Another miRNA, miR-124, which plays a critical role in maintaining neuronal cell identity, is a major target of Creb. Interestingly, miR-124 is rapidly and robustly regulated by serotonin, which selectively affects mature miR-124 levels, without affecting its precursor, suggesting that the miR-1 24 level may be regulated during Dicer processing or RISC incorporation and stabilization by Ago.116 miR-124 responds to serotonin by de-repressing Creb and thereby enhances serotonin-dependent long-term facilitation.116 More recently, it has been shown that expression of SIRT1, which modulates synaptic plasticity and memory formation, is regulated via Creb, which itself is translationally repressed by miR-134.117 On the other hand, SIRT1 inhibits the expression of miR-134 via a repressor complex containing the transcription factor YY1. Unchecked miR-134 expression after SIRT1 deficiency may result in reduced expression of Creb and BDNF, whereas knocking down miR-134 rescues LTP and memory impairment caused by SIRT1 deficiency.117
Impey et al115 have shown that CREB- and activity regulated miR-132 is necessary and sufficient for hippocampal spine formation. Expression of the miR-132 target, p250GAP, is inversely correlated with miR-132 levels and spinogenesis. Furthermore, knockdown of p250GAP increases spine formation while introduction of a p250GAP mutant that is unresponsive to miR-132 attenuates this activity. Inhibition of miR-132 decreases both miniature excitatory postsynaptic current (mEPSC) frequency and the number of glutamate receptor 1 (GluRl)-positive spines, while knockdown of. p250GAP has the opposite effect. Additionally, the miR-132/p250GAP circuit regulates Ras-related C3 botulinum toxin substrate 1 (Rac1) activity and spine formation bymodulating synapse-specific Kalirin7-Rac1 signaling. These results suggest that neuronal activity regulates spine formation, in part, by increasing transcription of miR-132, which in turn activates a Rac1-Pak actin remodeling pathway.115 Behaviorally, it has been shown that overexpression of miR-132 in the rat perirhinal cortex impairs short-term recognition memory, which is associated with a reduction in both long-term depression and long-term potentiation, and could be predicted from the excitatory and dendritogenic effects of mir132.118
Long-lasting changes at synapses are at the core of brain activities, which primarily rely on enduring changes in synaptic efficacy.119,120 Such synaptic efficacy is critically dependent upon the regulation of specific protein synthesis near or within the synapse.121-123 In this regard, miRNAs provide fascinating mechanisms to modulate synaptic efficacy and plasticity by regulating translational control of protein synthesis locally at the synapse. Lugli et al65 determined that there was a synaptic enrichment of miRNAs in mouse forebrain synaptosomes and found that significant subsets of forebrain-expressed miRNAs are highly enriched in synaptic fractions relative to total forebrain homogenate. These synaptic enriched miRNAs were biologically distinct from synaptic-depleted miRNAs, both in their expression patterns (synaptic-enriched miRNAs are expressed predominantly in pyramidal neurons, whereas synaptic-depleted miRNAs tend to have widespread and abundant tissue expression) and in their evolutionary histories (synaptic-enriched miRNAs tend to be evolutionary new and often mammalian-specific, whereas the synaptic depleted miRNAs tend to be highly conserved across vertebrates). Interestingly, they found that the synaptic enrichment was not simply related to the specificity of miRNA expression within neurons, but they arise from precursors that are expressed in the synaptic fractions and associated tightly with postsynaptic density (PSD).65 Furthermore, the synaptic enrichment of miRNAs was related to structural features of their precursors, suggesting a basis by which pre- or pri-miRNA may be selectively and stably transported to dendrites.124 Since both Dicer and pre-RNAs are expressed in synaptic fractions and are strongly associated with PSD, it suggests that at least a portion of the mature miRNAs are locally processed near synapses. Dicer is released from PSD and its RNase III activity is markedly enhanced following stimuli such as N-methyl-D-aspartate (NMDA) that can cause an increase in local calcium and activation of calpain. Dicer is expressed in PSDs, but is enzymatically inactive until conditions that activate calpain cause its liberation.65,68 These findings suggest that miRNAs are formed, at least in part, by the processing of miRNA precursors locally within dendritic spines, and synaptic stimulation may lead to local processing of miRNA precursors in proximity to the synapse. Synaptic efficacy can be regulated by modulating miRNA functions at the synapse and consequently synaptic plasticity due to the critical feature of miRNAs to regulate gene circuitry locally at the synapse in an activity-dependent fashion. This may provide a unique opportunity at the therapeutic level, where regulation of miRNA can be used to control plasticity at the synapse.
miRNAs in MDD pathogenesis and treatment
The diagnostic and prognostic values of miRNA have been established in various types of cancer.125 The potential of miRNAs as diagnostic markers for psychiatric and neurodegenerative diseases has been advancing rapidly.31,126-128 Both preclinical and clinical evidence demonstrates that miRNAs can be extensively involved in stress-related disorders and MDD, as well as the antidepressant response.
Coping response to stress and miRNAs
An individual's ability to cope with stress is critical in the development of MDD. We recently examined miRNA expression in both the frontal cortex of rats who developed behavior (learned helpless [LH]) that resembles stress-induced depression and those who did not develop depression (nonlearned helpless [NLH]), even though they received similar inescapable shocks (Table I).129 In this manner, we were able to distinguish the factors associated with the development of the depression phenotype. We found that NLH rats showed a robust adaptive miRNA response to inescapable shocks whereas LH rats showed a markedly blunted miRNA response. One set of miRNAs showed large, significant, and consistent alterations in NLH rats, consisting of miR-96, miR-141, miR-182, miR-183, miR-183*, miR-198, miR-200a, miR-200a*, miR-200b, miR-200b*, miR-200c, and miR-429. All were downregulated in NLH rats relative to tested controls (no shock group), and all showed a blunted response in LH rats (more like tested controls). These miRNAs were encoded at a few shared polycistronic loci, suggesting that their downregulation was coordinately controlled at the level of transcription. Most of these miRNAs have previously been shown to be enriched in synaptic fractions.65 Moreover, almost all of these miRNAs share 5'-seed motifs with other members of the same set, suggesting that they will hit similar or overlapping sets of target mRNAs. Interestingly, half of this set are predicted to hit Crebl as a target, and binding sites for CREB lie upstream of miR-96, miR-182, miR-183, miR-200a, miR200b, miR-200c, miR-220a*, and miR-200b*. This suggests that a similar feedback loop arrangement may also exist for Creb, similar to what has been described for other Creb-stimulated miRNAs and target genes.114 Since these miRNAs are downregulated in NLH rats, but not LH rats, this can be interpreted as a homeostatic response intended to minimize the repressive effects on Crebl. In addition, we identified a large core coexpression module, consisting of miRNAs that are strongly correlated with each other across individuals of the LH group, but not with either the NLH or tested control group. The presence of such a module implies that the normal homeostatic miRNA response to repeated inescapable shock is not merely absent or blunted in LH rats; rather, gene expression networks are actively reorganized in LH rats, which may support their distinctive persistent phenotype.
Another piece of evidence comes from studies of stress-sensitive F344 rats (which show a higher stress response to restraint stress) compared with Sprague-Dawley rats (which show lower hypothalamic-pituitary adrenal axis activity over a period of time). In this context, it is important to mention that glucocorticoids regulate the hypothalamic-pituitary adrenal axis through a negative feedback mechanism while binding to soluble GRs in the pituitary and the hypothalamus and inhibit the release of corticotropin-releasing factor and adrenocorticotropic hormone. Several studies have reported that the GR expression of is downregulated in depressed individuals.130 The GR protein is under constant miRNA regulation.131 More specifically, miR-124a and miR-18a bind to the 3' UTR of GR and downregulate its expression.131 Overexpression of miR-18a attenuates the glucocorticoid -induced leucine zipper, a gene induced by stress-like levels of glucocorticoid. It is possible that higher expression of miR-18a and consequent downregulation of GR could be responsible for the genetic susceptibility to stress in F344 rats.132 Turner et al133 recently predicted several possible miRNA binding sites within the GR first exon, suggesting further regulation of GR genes by miRNAs.
TABLE I. miRNAs implicated in stress and depression.
| miRNAs | Effects | Références |
| Restreint stress | Frontal cortex: miR-9, miR-9*, miR-26b, miR-29b, miR-30b, miR-30c, rniR-30e, miR-125a, miR-126-3p, miR-129-3p, miR-207, miR-212, miR-351, miR-423, miR-487b, miR-494, miR-690, miR-691, miR-709, miR-711, and Let-7 a-e let-7a, miR-9, miR-26a/b, miR-30b/c, miR-125a | Rinaldi et al124 |
| Immobilization stress | Hippocampus CA1, amygdala: miR-134, miR-183, miR-132, Let-7a-1, miR-9-1, miR-124a-1 | Meerson et al135 |
| Unpredictable chronic mild stress | Hippocampus: miR-298, miR-130b, miR-135a, miR-323, miR-503, miR-15b, miR-532, and miR-125a and up-regulating miRNAs miR-7a, miR-212, miR-124, miR-139, miR-182 | Cao et al136 |
| Early life stress | MediaI prefrontal cortex: pre-miRs 132, 124-1, 9-1, 9-3, 212, 29a | Uchida et al140 |
| Animal model of dépression | Learned helpless vs control frontal cortex: mmu-miR-184, mmu-miR-197, mmu-miR-107, mmu-miR-329, mmu-miR-125a-5p, mmu-miR-872, mmu-miR-181c, mmu-miR-18a*, mmu-miR-29b*, mmu-let-7a*, rno-Iet-7e*, rno-miR-20a* | Smalheiser et al129 |
| Postmortem brain studies | Prefrontal cortex: hsa-miR-142-5p, hsa-miR-33a, hsa-miR-137, hsa-miR-489, hsa-miR-148b, hsa-miR-101, hsa-miR-324-5p, hsa-miR-301a, hsa-miR-146a, hsa-miR-335, hsa-miR-494, hsa-miR-20b, hsa-miR-376a*, hsa-miR-190, hsa-miR-155, hsa-miR-660, hsa-rniR-552, hsa-miR-453, hsa-miR-130a, hsa-miR-27a, hsa-miR-497, hsa-miR-10a, hsa-miR-20a, hsa-miR-142-3p | Smalheiser et al156 |
| Peripheral mononuclear cells | has-miR-107, miR-133a, miR-148a, miR-200c, miR-381, miR-425-3p, miR-494, miR-517b, miR-579, miR-589, miR-636, miR-652, miR-941, miR-1243 | Belzeaux et al179 |
| Whole blood cells (12 weeks of treatmentwith escitalopram) | hsa-miR-130b, hsa-miR-505, hsa-miR-29b-2 , hsa-miR-26b, hsa-miR-22, hsa-miR-26a, hsa-miR-664, hsa-miR-494, hsa-let-7d, hsa-let-7g, hsa-let-7e, hsa-miR-34c-5p, hsa-let-7f, hsa-miR-629, hsa-miR-106b, hsa-miR-103, hsa-miR-191, hsa-miR-128, hsa-miR-502-3p, hsa-miR-374b, hsa-miR-132, hsa-miR-30d, hsa-miR-500, hsa-miR-770-5p, has-miR-589, hsa-miR-183, hsa-miR-574-3p, hsa-miR-140-3p, hsa-miR-335, hsa-miR-361-5p | Bocchio-Chiavetto et al180 |
miRNAs in response to stress
Several types of stressors have been utilized to examine how miRNAs respond to stressors. Interestingly, acute and chronic restrained stress cause differential changes in miRNA expression in a brain region-specific manner (Table I). 134 For example, acute stress induces a transient increase in the expression of selected miRNAs (miR-9, miR-9*, miR-26b, miR-29b, miR-30b, miR-30c, miR-30e, miR-125a, miR-126-3p, miR-129-3p, miR-207, miR-212, miR-351, miR-423, miR-487b, miR-494, miR-690, miR691, miR-709, miR-711, and Let-7 a-e) in the frontal cortex, but not the hippocampus. Some of them (let-7a, miR-9, miR-26a/b, miR-30b/c, and miR-125a) show an increase in their expression 5 days after acute stress; however, their expression levels are not altered after repeated restraint. These results suggest that acute stress modulates miRNA expression quickly to external stimuli by changing their synaptic efficacy through regulation of localized mRNA translation.
Using psychological stress (acute or chronic immobilization), Meerson et al135 examined miRNA expression in the central amygdala and the Cornu Ammonis area 1 (CA1) region of the hippocampus of rats subjected to acute or chronic immobilization stress (Table I). They found that the expression of several miRNAs was differentially altered during acute and chronic stress, with chronic stress causing much larger changes than acute stress. Some of the miRNAs that were altered during acute and chronic stress include: miR-134, miR-183, miR-132, Let-7a-l, miR-9-1, and miR-124a-l. Interestingly, except for miR-Let-7a-l, the expression of stress-responsive miRNAs were different in the two analyzed brain regions. In the CA1 region, miR-376b and miR-208 increased whereas miR-9-1 decreased under both acute and chronic stress conditions. Stressresponsive miR-134 and miR-183 target many splicing factors, such as SC35, SRP46, and SFRSll. SC35 promotes the alternative splicing of acetylcholinesterase (AChE) from the synapse-associated isoform AChE-S to soluble AChE-R protein and the expression of SC35 is increased during stress. Thus, by regulating splicing factors and their targets, miR-183 and miR-134 may modify both alternative splicing and cholinergic neurotransmission in the stressed brain. In addition, one of the targets of miR-183 is profilin 2 mRNA, which regulates dendritic spine morphology in neurons. Interestingly, neurotransmitter homeostasis and behavior are severely affected in profilin 2 knockdown mice136 and prolifin 2 (PFN2) expression is increased in lymphoblastoid cell lines of monozygotic twin pairs discordant for bipolar disorder.137
Using unpredictable chronic mild stress combined with separation, Cao et al found changes in 13 specific miRNAs in the rat hippocampus (Table I).138 These include downregulating miRNAs (miR298, miR-130b, miR-135a, miR-323, miR-503, miR-15b, miR-532, and miR-125a) and upregulating miRNAs (miR7a, miR-212, miR-124, miR-139, and miR-182). Among these, miR125a and miR-182 recovered to normal after intervention with traditional herbal antidepressant medication.
The effect of early-life stress, which is one of the critical factors in the development of affective disorders,139 on miRNA expression, has also been studied. Uchida et al found that maternal separation enhanced stress vulnerability to repeated restraint stress exposure in adulthood (Table I).140 At the molecular level, maternal separation increased the expression of repressor element-f silencing transcription factor (REST) 4. Transient overexpression of REST4 in the medial prefrontal cortex of neonatal mice produced depression-like behaviors in adults after repeated exposure to restraint stress, suggesting that REST4 may play a role in the development of stress vulnerability. REST regulates the expression of several miRNAs141,142 that are involved in brain development and plasticity.46,113,116 For example, maternal separation increased the expression of REST-associated premiRs 132, 124-1, 9-1, 9-3, 212, and 29a in rat medial prefrontal cortex.140 miR-132 and miR-124 are involved in synaptic plasticity113,116 and cause decreased dendritic length,143 high synaptic density,144 and altered basal neuronal activity.145 Interestingly, Bai et al146 demonstrated that maternal deprivation not only led to the development of depressive behavior in rats and a subsequent decrease in BDNF expression, but decreased BDNF expression was negatively correlated with the expression of miR-16. miR-16 has been shown to be an important effector of antidepressant action in serotonergic raphe and noradrenergic locus coeruleus as well as adult neurogenesis in the hippocampus.147
miRNAs and antidepressants
Several recent studies show that miRNAs may be involved in the mechanisms of action of antidepressants. Baudry et al148 showed that the serotonin transporter is a target of miR-16, which has a higher expression pattern in noradrenergic neurons than in serotonergic neurons. A reduction of miR-f 6 in noradrenergic neurons caused de novo serotonin transporter expression.
Interestingly, long-term treatment with fluoxetine to mice increased miR-16 levels in serotonergic raphe nuclei, which, in turn, reduced serotonin transporter expression. Furthermore, raphe responded to long-term fluoxetine treatment by releasing S100β, which, in turn, acted on the noradrenergic neurons of the coeruleus locus. Thus, by lowering miR-16 levels, S100β unlocked the expression of serotonergic functions in the noradrenergic brain area. This study suggests that miR-16 acts as a central effector in regulating serotonin transporter expression and mediates the adaptive response of serotonergic and noradrenergic neurons to fluoxetine treatment. These investigators further showed that fluoxetine treatment induced the secretion of various signaling molecules such as BDNF, Wnt2, and 15d-PGJ2 from raphe serotonergic neurons and acted cooperatively on the hippocampus, whereas S100β controlled the locus coeruleus-dependent hippocampal response to fluoxetine.147 These signals relayed the action of fluoxetine on adult hippocampal neurogenesis by downregulating miR-16 in the hippocampus in a region-specific manner. In a complementary fashion, these signaling molecules were found to be increased in the cerebrospinal fluid of depressed patients upon fluoxetine treatment.147
O'Connor et al149 investigated whether early-life stress in rats induced changes in hippocampal miRNA levels and whether the rapidly acting NMDA receptor antagonist ketamine, electroconvulsive therapy (ECT), or fluoxetine treatment could reverse these changes. They found that early-life stress affected expression of multiple hippocampal miRNAs. Antidepressant treatments reversed some of these effects including a stress-induced change to miR-451. Ketamine and ECT possessed the highest number of common targets, suggesting convergence on common pathways. Interestingly, all three treatments possessed miR-598-5p as a common target. This demonstrates that changes to hippocampal miRNA expression may represent an important component of stress-induced pathology, and antidepressant action may reverse these. In this context, Ryan et al150 examined ECT-induced BDNF expression and BDNFassociated miRNAs. Following acute or chronic electroconvulsive stimulation (ECS), they found that the level of selective miR-212 was significantly increased in dentate gyrus. miR-2f 2 level was also increased in parallel in whole blood following chronic ECS and was positively correlated with miR-212 level in the dentate gyrus, suggesting that miR-212 may be crucial in the mechanism of action of ECT and that it can be used as a biomarker.
Using genome-wide expression profiling of human lymphoblastoid cell lines (LCLs), Morag et al151 identified CHL1 as a selective serotonin reuptake inhibitor (SSRI) sensitivity biomarker. The same group reported that specific miRNAs may be implicated in SSRI sensitivity of LCLs.152 They examined genome-wide expression profiling with miRNAs in LCLs exhibiting high or low sensitivities to paroxetine. They found that miR-1513p had a 6.7-fold higher basal expression in paroxetinesensitive LCLs, which was correlated with lower expression of CHL1, a target of miR-151-3p. The additional miRNAs miR-212, miR-132, miR-30b*, let-7b, and let-7c also differed by > 1.5-fold between the two LCL groups. These results suggest a possible therapeutic value of miRNAs in responders vs nonresponders to antidepressant treatment in MDD patients.
Schmidt et al153 found that the therapeutic action of fluoxetine is associated with a reduction in prefrontal cortical mir-1971 expression levels in a mouse model of post-traumatic stress disorder. Long-term lithium and valproate treatment have also been shown to alter a number of miRNAs; however, 9 miRNAs (let-7b, let-7c, miR-105, miR-128a, miR-24a, miR-30c, miR-34a, miR221, and miR-144) were regulated by both lithium and valproate. The most significant signaling pathways that are targeted by these miRNAs are the PKC, PTEN, ERK-MAP kinase, Wnt/β-catenin, and β-adrenergic pathways. Some of these have been shown to be altered in MDD and bipolar disorder.154 Lithium- and valproate induced downregulated miR-128a, miR-24, and miR-34a significantly upregulated hippocampal protein levels of DPP10, GRM7, and THRB. Among these proteins, GRM7 has emerged as a novel candidate gene for bipolar disorder.155
miRNAs in MDD subjects
One of the approaches that have been taken to directly assess the status of miRNAs in psychiatric illnesses is to examine the postmortem brain. Using this approach, we recently profiled miRNA expression in the prefrontal cortex of depressed subjects who had died by suicide (Table I).156 We took several different approaches to analyzing the data. When we analyzed miRNA expression globally, we found that 21 miRNAs were significantly downregulated in the MDD group. We also found that 24 miRNAs were downregulated by 30% (although not statistically significant), suggesting a global downregulation of miRNA levels in the MDD group. When analyzed individually, we found that almost half of the downregulated miRNAs were encoded at chromosomal loci near other miRNAs and are possibly transcribed by the same pri-miRNA gene transcripts (mir-1 42 -5p and 142-3p; mir-494, 376a*, 496, and 369-3p; mir-23b, 27b and 24-1*; mir-34b* and 34c; mir-17* and 20a). In addition, three pairs of miRNAs were encoded at distances greater than 100 kb, but still lie within the same chromosomal region (mir-424 and 20b at Xq26.2-3, 377 kb apart; mir-142 and 301a at 17q22, 820 kb apart; mir-3245p and 497 at 17pl3.1, 205 kb apart). This suggests that at least some of the downregulated miRNA expression is due to decreased transcription. Many of the downregulated miRNAs also shared 5' seed sequences that are involved in target recognition. For example, identical seed sequences are shared by: (i) mir-20a and 20b; (ii) mir-301 a and 130a; and (iii) mir-424 and 497. In addition, a 6-mer nucleotide motif is shared by mir-34a, 34b*, and 34c, and strikingly, a 5-mer motif (AGUGC) within the 5' seed is shared by 5 of the affected miRNAs (mir-148b, 301a, 130a, 20a, and 20b) that is predicted to bind Alu sequences within the 3* UTR region of target mRNAs. This suggests that the downregulated miRNAs should exhibit extensive overlap among their mRNA targets. When we did pair-wise correlation (a complementary method of analyzing the miRNA expression data is to identify pairs of miRNAs that are coregulated in their expression, up or down, across individuals within a single group) we found that a set of 29 miRNAs, none of whom were pairwise correlated in the normal control group, formed a very extensive interconnected network in the MDD group. Several of the miRNAs (let-7b, mir132, 181b, 338-3p, 486-5p, and 650) were “hubs” correlated with four to nine other miRNAs in the network. Target analysis revealed that many of the targets are transcription factors, and nuclear, transmembrane, and signaling proteins. Intriguingly, four different downregulated miRNAs target VEGFA (mir-20b, 20a, 34a, and 34b*), a molecule implicated in depression in both humans and animal models. Other validated targets include BCL2 (mir-34a), DNMT3B (mir-148b), and MYCN (mir-101, 34a). Among predicted targets, estrogen receptor a, ESR1, was predicted to be targeted by three different downregulated miRNAs (mir-148b, 301a, 496). Others targeted by three or more affected miRNAs include ubiquitin ligases (UBE2D1 and UBE2W); signal transduction mediators (CAMK2G, AKAP1); the splicing factor NOVA1 that regulates brain-specific alternative splicing; the GABA-A receptor subunit GABRA4; calcium channel CACNA1C; and brain-active transcription factors including SMAD5, MITF, BACH2, MYCN, and ARID4A. Several of these predicted targets interact with validated targets; for example, ARIA4A binds E2F1; SMAD5 binds RUNX1; and estradiol treatment decreases E2F1 levels in the prefrontal cortex.157 BACH2 transcription factor binding sites have been identified upstream of many brainexpressed miRNAs.114 Retinoblastoma binding protein 1 (ARIA4A) is of interest because it recruits histone deacetylases and regulates gene expression via chromatin-based silencing.
Recently, He et al158 studied an association between miRNA processing gene variants and depression. They genotyped three polymorphisms from three miRNA processing genes (DGCR8, AG01, and GEMIN4) in a case-control study including 314 patients and 252 matched healthy controls. Frequencies of genotypes and alleles showed a significant difference between patients with depression and healthy controls in DGCR8 rs3757 and AGOl rs636832. An allele frequency was significantly higher in rs3757 and lower in rs636832, respectively. Variant allele of DGCR8 rs3757 was associated with increased risk of suicidal tendency and improvement response to antidepressant treatment, whereas the variant of AGOl rs636832 showed decreased risk of suicidal tendency, suicidal behavior, and recurrence. Besides, allele frequency showed significant difference when comparing patients with remission with controls; no significant differences were found in GEMIN4 rs7813 between patients and healthy controls. DGCR8 rs3757 and AGOl rs636832 were found to have a significant association with depression, and GEMIN4 rs7813 did not affect susceptibility to depression. These observations suggested that miRNA processing polymorphisms may affect depression risk and treatment.
Circulating miRNAs: potential biomarker in depression and antidepressant response
miRNAs can be detected in circulating biological fluids such as serum, plasma, urine, saliva, and cerebrospinal fluid (CSF).159-161 Interestingly, it has been shown that miRNAs are endogenously expressed in plasma and their expression profile is similar in blood cells.162 More interestingly, under healthy conditions, these miRNAs are stably expressed in blood cells; however, under pathological conditions, the profile of miRNAs changes significantly, suggesting the possibility that peripheral miRNAs can be used as a reliable biomarker under disease conditions. Although the source of miRNAs in peripheral cells is not clear, it has been shown that miRNAs can be released actively or passively from tissues into the circulating blood.162,163 The actively secreted miRNAs are enclosed in exosomes164 and protected by RNA binding proteins including NPM1,165 HDL,166 or Argonaute2.167 Therefore, measuring miRNAs in blood cells is highly reproducible. Interestingly, it has been shown that exosomes containing miRNAs are excreted physiologically in response to stress or brain injury, suggesting that these miRNAs can be ideal biomarker candidates.168-170 Exosomal miRNAs are processed by the same machinery used in miRNA biogenesis and thus have widespread consequences within the cell by inhibiting the expression of target protein-coding genes.169
Over the past several years, attempts have been put forward towards developing circulating miRNAs as a potential biomarker for many diseases. These include cancer and cardiovascular, inflammatory, and neurodegenerative diseases.128,171-173 In cancer patients, miRNAs have not only been shown to be useful indicators of various types of cancer, but based on miRNA profiling, it can be shown which patient group responds better to a particular treatment regimen.125 In neurological disorders, there is a significant correlation between circulating miRNAs and brain disease and injury.174 For example, miR-9 is decreased in blood cells, and in the cortex and hippocampus of Alzheimer's disease patients.175 Similarly, circulating miR-34 serves as a novel biomarker for Huntington's disease.176 Identifying biomarkers in these diseases has been a challenging task due to the heterogeneity and complex nature of psychiatric illnesses. Nonetheless, emerging studies provide evidence that miRNAs can be used successfully as biomarkers in these illnesses. For example, in schizophrenia patients, several circulating miRNAs (miR-181b, miR-219-2-3p, miR-1308, let-7g, and miR-195) have been identified as potential disease biomarkers.177 Plasma miR-134 levels in drug-free, 2-week medicated, and 4-week medicated bipolar mania patients were significantly decreased when compared with controls, and its level increased following medication.178 Decreased circulating miR-134 level, both in drug-free and medicated patients, showed a negative correlation with the clinical scales, suggesting that a decrease in plasma miR-134 levels may be directly associated with the pathophysiology and severity of manic symptoms in bipolar disorder.
The use of miRNAs as a peripheral biomarker in MDD is gaining momentum. Belzeaux et al examined miRNA expression profiles in peripheral blood mononuclear cells (PBMCs) collected from 16 severe MDD patients and 13 matched controls at baseline, and 2 and 8 weeks after treatment (Table I). 179 A comparison of miRNA expression between MDD patients and controls at baseline and at 8 weeks showed a similar number of dysregulated miRNAs (14 miRNAs, with 9 miRNAs upregulated and 5 downregulated). miRNAs that showed changes between MDD and controls at base line included: has-miR-107, miR-133a, miR-148a, miR-200c, miR-381, miR-425-3p, miR-494, miR-517b, miR-579, miR-589, miR-636, miR-652, miR-941, and miR-1 243. Only two miRNAs showed stable overexpression in MDD patients during the 8-week follow-up compared with controls (miR-941 and miR-589). They also identified miRNAs exhibiting significant variations of expression among patients with clinical improvement (7 upregulated and 1 downregulated). Fourteen dysregulated miRNAs had putative mRNA targets that were differentially expressed in MDD, suggesting that a common RNA regulatory network functions in MDD. These results suggest the potential utility of miRNA signatures as markers of major depressive episode evolution.
Bocchio-Chiavetto et al conducted a whole-miRNA ome quantitative analysis in the blood of 10 MDD subjects after 12 weeks of treatment with escitalopram (Table I).180 They found that 30 miRNAs were differentially expressed after the escitalopram treatment: 28 miRNAs were upregulated, and two miRNAs were downregulated. Thirteen (let-7d, let-7e, miR-26a, miR-26b, miR-34c-5p, miR-103, miR-128, miR-132, miR-183, miR-192, miR-335, miR-494, and miR-22) play a role in neural plasticity and stress response and in the pathogenetic mechanisms of several neuropsychiatric diseases. miR-132 exerts critical functions in the biological circuits implicated in neurogenesis and synaptic plasticity, stimulating axonal and dendritic outgrowth in different brain areas.181 This miRNA, together with miR-26a, miR-26b, and miR-183, widely contributes to the action of the neurotrophin BDNF in the brain.103,134,182,183 miR-132, miR-26a, miR-26b, miR-183, let-7d, let-7e, miR-26b, miR-103, miR-128, miR-494, and miR-22 have been reported to play a role in the pathogenesis of psychiatric disorders and in the mechanism of action of antipsychotic drugs and mood stabilizers. Moreover, postmortem studies on the brains of bipolar disorder patients showed increased levels of miR-22* in the prefrontal cortex.184 On the other hand, miR-494 and miR-335 are downregulated in the prefrontal cortex of MDD patients.156 The target genes of these altered miRNAs include: BDNF, GR, NR3C1 and nitric oxide synthase NOS1, growth factors (IGF1, FGF1, FGFR1, VEGFα, and GDNF), calcium channels (CACN41C, CACNB4, SLC6A12, and SLC8A3), and neurotransmitter receptors (GABRA4 and 5-HT4), some of which have been implicated in MDD and in the mechanism of action of antidepressants. These studies suggest that miRNAs can not only be used to diagnose, but can also be used for treatment response.
Conclusion and future directions
Based on studies showing the involvement of miRNAs in neural plasticity, neurogenesis, and stress response, it is clear that miRNAs may participate in the pathogenesis of MDD. More direct evidence comes from human postmortem brain studies showing aberrant expression of miRNAs in the prefrontal cortical area. From these, as well as animal studies showing a blunted response in NLII rats, one can assume that miRNAs may actively participate in developing the MDD phenotype.
Despite these findings, one needs to find an integrated view of miRNA networks and the pathways that are affected by these miRNAs. It is well established that a combination of miRNAs is a much more powerful regulator than individual miRNAs. Interestingly, differential c-expression of a group of miRNAs has not only been shown to play a direct role in human disease pathogenesis, but they also help in identifying the nature of disordered pathways implicated in such pathogenesis.34-37 A set of miRNAs that are significantly affected in MDD, and the corresponding set of mRNAs that are affected in the same samples, will help resolve this issue. The affected miRNAs and mRNAs are likely to interact with and regulate each other, either directly as targets or indirectly as part of larger regulatory networks. One can also identify sets of miRNAs that are not correlated in expression across individuals in the control group, yet are positively correlated in the MDD group and vice versa. There is a possibility that the correlated miRNAs and mRNAs are likely to be driven in their expression by the same (possibly overlapping) set of transcription factors or epigenetic influences. If a given miRNA is driven by one transcription factor in the control group and by a different transcription factor in the MDD group, this may result in no change in its mean expression levels across groups, yet may be detectable by observing shifts in the miRNAs that are correlated across individuals. In addition, it is important to determine whether the changes in miRNA/mRNA network are similar or different across different brain areas and more so, whether they are cell type-specific and are reversible. Also important is to examine the potential reasons for altered miRNA expression. Is it because of genetic changes in the promoter region upstream of primary miRNA gene transcripts, the pre-miRNA hairpin, or the mature miRNA, or due to RNA editing of transcripts or epigenetic suppression of the chromosomal region encoding the miRNAs? A variety of enzymes are responsible for processing miRNAs. These include Drosha, Dicer and cofactors DGCR8, TRBR and PACT. Several of these proteins have been shown to be modified post-translationally in a dynamic manner. For example, altering the relative expression of eIF2c may change the efficiency of translational arrest produced by a given miRNA.185 Recently, it has been shown that Dicer is activated by proteolytic cleavage under conditions of elevated calcium levels68,124 and eIF2C undergoes reversible phosphorylation within cells, which is required for its translocation to processing bodies.186 The phosphorylation of eIF2C appears to be due to activation of ERK1/2.186 Since we have shown abnormalities in calcium-sensing proteins and ERK1/2 signaling in the brain of MDD subjects,187-190 it will be worthwhile to ask whether Dicer cleavage patterns or eIF2C phosphorylation are altered in the MDD group. One can also examine whether there is any genetic link between miRNA and MDD. Such genetic linkage has been reported in specific miRNAs in schizophrenia.191
The development of miRNAs as biomarkers for MDD pathogenesis will be an important step in detecting MDD pathogenesis. In this regard, blood cell studies will be critical. The miRNA findings in these cells of MDD subjects so far have been very exciting, and indicate the possibility of developing biomarkers in a noninvasive manner. Also, the blood expressed miRNAs can be expanded to monitor antidepressant response. Much work, however, is needed in this direction, since miRNAs may be expressed differently in various blood cell types. In addition, whether miRNAs in a specific blood cell-type truly represent brain-derived miRNAs, is unclear at the present time. In this regard, measuring miRNAs in exosomes may be an alternate and better approach.192,193
Acknowledgments
The research was supported by grants from National Institute of Mental Health (R01MH082802, R21MH081099, R21MH091509, 1R01MH101890, 1R01MH0100616), American Foundation for Suicide Prevention, and University of Illinois at Chicago Clinical and Translational Sciences National supported by the Center for Advancing Translational Sciences, National Institutes of Health (grant number UL1TR000050).
Selected abbreviations and acronyms
- Ago
Argonaute
- DGCR8
DiGeorge syndrome critical region 8
- eIF
eukaryotic translation initiation factor
- FMRP
fragile X mental retardation protein
- GR
glucocorticoid receptor
- HuB
Hu protein B
- LH
learned helplessness
- LTP
long-term potentiation
- MDD
major depressive disorder
- miRNA
microRNA
- NLH
nonlearned helplessness
- PACT
protein kinase R (PKR)-activating protein
- pre-miRNA
precursor miRNA
- pri-miRNA
primary miRNA
- RISC
RNA-induced silencing complex
- TRBP
HIV-1 transactivating response RNA-binding protein
REFERENCES
- 1.Kessler RC., McGonagle KA., Zhao S., et al Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 2.Broadhead WE., Blazer DG., George LK., et al Depression, disability days, and days lost from work in a prospective epidemiologic survey. JAMA. 1990;264:2524–2528. [PubMed] [Google Scholar]
- 3.Pompili M., Innamorati M., Rihmer Z., et al Cyclothymic-depressiveanxious temperament pattern is related to suicide risk in 346 patients with major mood disorders. J Affect Disord. 2012;136:405–411. doi: 10.1016/j.jad.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Serafini G., Pompili M., Innamorati M., et al Affective temperamental profiles are associated with white matter hyperintensity and suicidal risk in patients with mood disorders. J Affect Disord. 2011;129:47–55. doi: 10.1016/j.jad.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Innamorati M., Pompili M., Gonda X., et al Psychometric properties of the Gotland Scale for Depression in Italian psychiatric inpatients and its utility in the prediction of suicide risk. J Affect Disord. 2011;132:99–103. doi: 10.1016/j.jad.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Fava M., Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. 1996;19:179–200. doi: 10.1016/s0193-953x(05)70283-5. [DOI] [PubMed] [Google Scholar]
- 7.Leistedt SJ., Linkowski P. Brain, networks, depression, and more. Eur Neuropsychopharmacol. 2013;23:55–62. doi: 10.1016/j.euroneuro.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Ota KT., Duman RS. Environmental and pharmacological modulations of cellular plasticity: role in the pathophysiology and treatment of depression. Neurobiol Dis. 2013;57:28–37. doi: 10.1016/j.nbd.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ongur D., Drevets WC., Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajkowska G. Histopathology of the prefrontal cortex in major depression: what does it tell us about dysfunctional monoaminergic circuits? Prog Brain Res. 2010;126:397–412. doi: 10.1016/S0079-6123(00)26026-3. [DOI] [PubMed] [Google Scholar]
- 11.Cotter D., Mackay D., Landau S., Kerwin R., Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psych. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- 12.Miguel-Hidalgo J., Rajkowska G. Morphological brain changes in depression. Can antidepressants reverse them? CNS Drugs. 2002;16:361–372. doi: 10.2165/00023210-200216060-00001. [DOI] [PubMed] [Google Scholar]
- 13.Aganova EA., Uranova NA. Morphometric analysis of synaptic contacts in the anterior limbic cortex in the endogenous psychoses. Neurosci Behav Physiol. 1992;22:59–65. doi: 10.1007/BF01186670. [DOI] [PubMed] [Google Scholar]
- 14.Dolan RJ., Bench CJ., Liddle PF., et al Dorsolateral prefrontal cortex dysfunction in the major psychoses; symptom or disease specificity? J Neurol Neurosurg Psychiatry. 1993;56:1290–1294. doi: 10.1136/jnnp.56.12.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drevets WC., Ongür D., Price JL. Reduced glucose metabolism in the subgenual prefrontal cortex in unipolar depression. Mol Psych. 1998;3:190–191. doi: 10.1038/sj.mp.4000380. [DOI] [PubMed] [Google Scholar]
- 16.Andreasen NC. Linking mind and brain in the study of mental illnesses: a project for a scientific psychopathology. Science. 1997;275:1586–1593. doi: 10.1126/science.275.5306.1586. [DOI] [PubMed] [Google Scholar]
- 17.Honer WG. Assessing the machinery of mind: synapses in neuropsychiatry disorders. J Neurosci. 1999;24:116–121. [PMC free article] [PubMed] [Google Scholar]
- 18.Toni N., Buchs PA., Nikonenko I., Bron CR., Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- 19.Hajsza T., MacLusky NJ., Leranth C. Short-term treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. Eur J Neurosci. 2005;21:1299–1303. doi: 10.1111/j.1460-9568.2005.03968.x. [DOI] [PubMed] [Google Scholar]
- 20.McEwen BS. Effects of adverse experiences for brain structure and function. Biol Psych. 2000;48:713–714. doi: 10.1016/s0006-3223(00)00964-1. [DOI] [PubMed] [Google Scholar]
- 21.Sheline Yl. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol Psych. 2000;48:791–800. doi: 10.1016/s0006-3223(00)00994-x. [DOI] [PubMed] [Google Scholar]
- 22.Sala M., Perez J., Soloff P., et al Stress and hippocampal abnormalities in psychiatric disorders. Eur Neuropsychopharmacol. 2004;14:393–405. doi: 10.1016/j.euroneuro.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Rajkowska G., Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets. 2007;6:219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sackeim HA. Functional brain circuits in major depression and remission. Arch Gen Psychiatry. 2001;58:649–650. doi: 10.1001/archpsyc.58.7.649. [DOI] [PubMed] [Google Scholar]
- 25.Honer WG., Falkai P., Young C., et al Cingulate cortex synaptic terminal proteins and neural cell adhesion molecule in schizophrenia. Neuroscience. 1997;78:99–110. doi: 10.1016/s0306-4522(96)00489-7. [DOI] [PubMed] [Google Scholar]
- 26.Bearden CE., Glahn DC., Monkul ES., et al Patterns of memory impairment in bipolar disorder and unipolar major depression. Psychiatry Res. 2006;42:139–150. doi: 10.1016/j.psychres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Howland JG., Wang YT. Synaptic plasticity in learning and memory: stress effects in the hippocampus. Prog Brain Res. 2008;169:145–158. doi: 10.1016/S0079-6123(07)00008-8. [DOI] [PubMed] [Google Scholar]
- 28.Farazi TA., Juranek SA., Tuschl T. The growing catalog of small RNAs and their association with distinct Argonaute/Piwi family members. Development. 2008;135:1201–1214. doi: 10.1242/dev.005629. [DOI] [PubMed] [Google Scholar]
- 29.D'Sa C., Duman RS. Antidepressants and neuroplasticity. Bipolar Disord. 2002;4:183–94. doi: 10.1034/j.1399-5618.2002.01203.x. [DOI] [PubMed] [Google Scholar]
- 30.Schouten M., Buijink MR., Lucassen PJ., Fitzsimons CP. New neurons in aging brains: molecular control by small non-coding RNAs. Front Neurosci. 2012;6:25. doi: 10.3389/fnins.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dwivedi Y. Evidence demonstrating role of microRNAs in the etiopathology of major depression. J Chem Neuroanat. 2011;42:142–156. doi: 10.1016/j.jchemneu.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Im HI., Kenny PJ. MicroRNAs in neuronal function and dysfunction. Trends Neurosci. 2012;35:325–334. doi: 10.1016/j.tins.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malphettes L., Fussenegger M. Impact of RNA interference on gene networks. Metab Eng. 2006;8:672–683. doi: 10.1016/j.ymben.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Stäehler CF., Keller A., Leidinger P., et al Whole miRNome-wide differential co-expression of microRNAs. Genomics Proteomics Bioinformatics. 2012;10:285–294. doi: 10.1016/j.gpb.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu J., Li CX., Li YS., et al MiRNA-miRNA synergistic network: construction via co-regulating functional modules and disease miRNA topological features. Nucleic Acids Res. 2011;39:825–836. doi: 10.1093/nar/gkq832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi JK., Yu U., Yoo OJ., Kim S. Differential coexpression analysis using microarray data and its application to human cancer. Bioinformatics. 2005;21:4348–4355. doi: 10.1093/bioinformatics/bti722. [DOI] [PubMed] [Google Scholar]
- 37.Mo WJ., Fu XP., Han XT., et al A stochastic model for identifying differential gene pair co-expression patterns in prostate cancer progression. BMC Genomics. 2009;10:340. doi: 10.1186/1471-2164-10-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schratt G. Fine-tuning neural gene expression with microRNAs. Curr Opin Neurobiol. 2009;19:213–219. doi: 10.1016/j.conb.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Hussain MU. Micro-RNAs (miRNAs): genomic organisation, biogenesis and mode of action. Cell Tissue Res. 2012;349:405–413. doi: 10.1007/s00441-012-1438-0. [DOI] [PubMed] [Google Scholar]
- 40.Chendrimada TP., Gregory Rl., Kumaraswamy E., et al TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436:740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathonnet G., Fabian MR., Svitkin YV., et al MicroRNA inhibition of translation initiation in vitro by targeting the cap-binding complex elF4F. Science. 2007;317:1764–1767. doi: 10.1126/science.1146067. [DOI] [PubMed] [Google Scholar]
- 42.Brodersen P., Voinnet O. Revisiting the principles of microRNA target recognition and mode of action. Nat Rev Mol Cell Biol. 2009;10:141–148. doi: 10.1038/nrm2619. [DOI] [PubMed] [Google Scholar]
- 43.Chendrimada TP., Finn KJ., Ji X., et al MicroRNA silencing through RISC recruitment of elF6. Nature. 2007;447:823–828. doi: 10.1038/nature05841. [DOI] [PubMed] [Google Scholar]
- 44.Vasudevan S., Tong Y., Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 45.Wu L., Fan J., Belasco JG. MicroRNAs direct rapid deadenylation of mRNA. Proc Natl Acad Sci U S A. 2006;103:4034–4039. doi: 10.1073/pnas.0510928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kosik KS. The neuronal microRNA system. Nat Rev Neurosci. 2006;7:911–920. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- 47.Michalak P. RNA world - the dark matter of evolutionary genomics. J EvolBiol. 2006;19:1768–1774. doi: 10.1111/j.1420-9101.2006.01141.x. [DOI] [PubMed] [Google Scholar]
- 48.Tardito D., Mallei A., Popoli M. Lost in translation. New unexplored avenues for neuropsychopharmacology: epigenetics and microRNAs. Expert Opin Investig Drugs. 2013;22:217–233. doi: 10.1517/13543784.2013.749237. [DOI] [PubMed] [Google Scholar]
- 49.Chuang JC., Jones PA. Epigenetics and microRNAs. Pediatr Res. 2007;61:24R–29R. doi: 10.1203/pdr.0b013e3180457684. [DOI] [PubMed] [Google Scholar]
- 50.Sato F., Tsuchiya S., Meltzer SJ., Shimizu K. MicroRNAs and epigenetics. FEBS J. 2011;278:1598–1609. doi: 10.1111/j.1742-4658.2011.08089.x. [DOI] [PubMed] [Google Scholar]
- 51.Bak M., Silahtaroglu A., Møller M., et al MicroRNA expression in the adult mouse central nervous system. RNA. 2008;14:432–444. doi: 10.1261/rna.783108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He X., Zhang Q., Liu Y., Pan X. Cloning and identification of novel microRNAs from rat hippocampus. Acta Biochim Biophys Sin (Shanghai). 2007;39:708–714. doi: 10.1111/j.1745-7270.2007.00324.x. [DOI] [PubMed] [Google Scholar]
- 53.Olsen L., Klausen M., Helboe L., Nielsen FC., Werge T. MicroRNAs show mutually exclusive expression patterns in the brain of adult male rats. PLoS One. 2009;4:1–7. doi: 10.1371/journal.pone.0007225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Landgraf P., Rusu M., Sheridan R., et al A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kapsimali M., Kloosterman WP., de Bruijn E., et al MicroRNAs show a wide diversity of expression profiles in the developing and mature central nervous system. Genome. Biol. 2007;8:R173. doi: 10.1186/gb-2007-8-8-r173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi PS., Zakhary L., Choi WY., et al Members of the miRNA-200 family regulate olfactory neurogenesis. Neuron. 2008;57:41–55. doi: 10.1016/j.neuron.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 58.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 59.Miska EA., Alvarez-Saavedra E., Townsend M., et al Microarray analysis of microRNA expression in the developing mammalian brain. Genome Biol. 2004;5:R68. doi: 10.1186/gb-2004-5-9-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sempere LF., Freemantle S., Pitha-Rowe I., et al Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5:R13. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lagos-Quintana M., Rauhut R., Yalcin A., et al Identification of tissuespecific microRNAs from mouse. Curr Biol. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 62.Yu JY., Chung KH., Deo M., Thompson RC., Turner DL. MicroRNA miR-124 regulates neurite outgrowth during neuronal differentiation. Exp Cell Res. 2008;314:2618–2633. doi: 10.1016/j.yexcr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smirnova L., Gräfe A., Seiler A., et al Regulation of miRNA expression during neural cell specification. Eur J Neurosci. 2005;21:1469–1477. doi: 10.1111/j.1460-9568.2005.03978.x. [DOI] [PubMed] [Google Scholar]
- 64.He M., Liu Y., Wang X., et al Cell-type-based analysis of microRNA profiles in the mouse brain. Neuron. 2012;73:35–48. doi: 10.1016/j.neuron.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lugli G., Torvik VI., Larson J., Smalheiser NR. Expression of microRNAs and their precursors in synaptic fractions of adult mouse forebrain. J Neurochem. 2008;106:650–661. doi: 10.1111/j.1471-4159.2008.05413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Siegel G., Obernosterer G., Fiore R., et al A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nat Cell Biol. 2009;11:705–716. doi: 10.1038/ncb1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Natera-Naranjo O., Aschrafi A., Gioio AE., Kaplan BB. Identification and quantitative analyses of microRNAs located in the distal axons of sympathetic neurons. RNA. 2010;16:1516–1529. doi: 10.1261/rna.1833310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lugli G., Larson J., Martone ME., Jones Y., Smalheiser NR. Dicer and elF2c are enriched at postsynaptic densities in adult mouse brain and are modified by neuronal activity in a calpain-dependent manner. J Neurochem. 2005;94:896–905. doi: 10.1111/j.1471-4159.2005.03224.x. [DOI] [PubMed] [Google Scholar]
- 69.Dictenberg JB., Swanger SA., Antar LN., Singer RH., Bassell GJ. A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile X syndrome. Dev Cell. 2008;14:926–939. doi: 10.1016/j.devcel.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Presutti C., Rosati J., Vincenti S., Nasi S. Non coding RNA and brain. BMC Neurosci. 2006;(suppl 1):S5. doi: 10.1186/1471-2202-7-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fiore R., Siegel G., Schratt G. MicroRNA function in neuronal development, plasticity and disease. Biochim Biophys Acta. 2008;1779:471–478. doi: 10.1016/j.bbagrm.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 72.Liu C., Zhao X. MicroRNAs in adult and embryonic neurogenesis. Neuromolecular Med. 2009;11:141–152. doi: 10.1007/s12017-009-8077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vreugdenhil E., Berezikov E. Fine-tuning the brain: microRNAs. Front Neuroendocrine/. 2009;31:128–133. doi: 10.1016/j.yfrne.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 74.Jun H., Mohammed Qasim Hussaini S., Rigby MJ., Jang MH. Functional role of adult hippocampal neurogenesis as a therapeutic strategy for mental disorders. Neural Plast. 2012;2012:854285. doi: 10.1155/2012/854285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eisch AJ., Petrik D. Depression and hippocampal neurogenesis: a road to remission? Science. 2012;338:72–75. doi: 10.1126/science.1222941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alonso M., Viollet C., Gabellec MM., et al Olfactory discrimination learning increases the survival of adult-born neurons in the olfactory bulb. J Neurosci. 2006;26:10508–10513. doi: 10.1523/JNEUROSCI.2633-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dupret D., Revest JM., Koehl M., et al Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DeCarolis NA., Eisch AJ. Hippocampal neurogenesis as a target for the treatment of mental illness: a critical evaluation. Neuropharmacology. 2010;58:884–893. doi: 10.1016/j.neuropharm.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lucassen PJ., Meerlo P., Naylor AS., et al Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: Implications for depression and antidepressant action. Eur Neuropsychopharmacol. 2010;20:1–17. doi: 10.1016/j.euroneuro.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 80.Mendez-David I., Hen R., Gardier AM., David DJ. Adult hippocampal neurogenesis: an actor in the antidepressant-like action. Ann Pharm Fr. 2013;71:143–149. doi: 10.1016/j.pharma.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 81.Lee MM., Reif A., Schmitt AG. Major depression: a role for hippocampal neurogenesis? Curr Top Behav Neurosci. 2013;14:153–179. doi: 10.1007/7854_2012_226. [DOI] [PubMed] [Google Scholar]
- 82.Snyder JS., Soumier A., Brewer M., Pickel J., Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lazarov O., Mattson MP., Peterson DA., Plmpllkar SW., van Praag H. When neurogenesis encounters aging and disease. Trends Neurosci. 2010;33:569–579. doi: 10.1016/j.tins.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lang MF., Shi Y. Dynamic roles of microRNAs in neurogenesis. Front Neurosci. 2012;6:71. doi: 10.3389/fnins.2012.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McLoughlin HS., Fineberg SK., Ghosh LL., Tecedor L., Davidson BL. Dicer is required for proliferation, viability, migration and differentiation in corticoneurogenesis. Neuroscience. 2012;223:285–295. doi: 10.1016/j.neuroscience.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ouchi Y., Banno Y., Shimizu Y., et al Reduced adult hippocampal neurogenesis and working memory deficits in the DGCRS-deficient mouse model of 22q11.2 deletion-associated schizophrenia can be rescued by IGF2. J Neurosci. 2013;29:9408–9419. doi: 10.1523/JNEUROSCI.2700-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pathania M., Torres-Reveron J., Yan L., et al miR-132 enhances dendritic morphogenesis, spine density, synaptic integration, and survival of newborn olfactory bulb neurons. PLoS One. 2012;7:e38174. doi: 10.1371/journal.pone.0038174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cheng LC., Pastrana E., Tavazoie M., Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bruno Ig., Karam R., Huang L., et al Identification of a microRNA that activates gene expression by repressing nonsense-mediated RNA decay. Mol Cell. 2011;42:500–510. doi: 10.1016/j.molcel.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Åkerblom M., Sachdeva R., Barde I., et al MicroRNA-124 is a subventricular zone neuronal fate determinant. J Neurosci. 2012;32:8879–8889. doi: 10.1523/JNEUROSCI.0558-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Szulwach KE., Li X., Smrt RD., et al Crosstalk between microRNA and epigenetic regulation in adult neurogenesis. J Cell Biol. 2010;189:127–141. doi: 10.1083/jcb.200908151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang J., Zhang J., Zhou Y., et al Novel cerebellum-enriched mir-592 may mlay a role in neural progenitor cell differentiation and neuronal maturation through regulating Lrrc4c and Nfasc in rat. Curr Mol Med. 2013;13:1432–1445. doi: 10.2174/15665240113139990072. [DOI] [PubMed] [Google Scholar]
- 93.Davis TH., Cuellar TL., Koch SM., et al Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J Neurosci. 2008;28:4322–4330. doi: 10.1523/JNEUROSCI.4815-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.De Pietri Tonelli D., Pulvers JM., Haffner C., et al MiRNAs are essential for survival and differentiation of newborn neurons but not for expansion of neural progenitors during early neurogenesis in the mouse embryonic neocortex. Development. 2008;135:3911–3921. doi: 10.1242/dev.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schaefer A., O'Carroll D., Tan CL., et al Cerebellar neurodegeneration in the absence of microRNAs. J Exp Med. 2007;204:1553–1558. doi: 10.1084/jem.20070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Olde Loohuis NF., Kos A., Martens GJ., et al MicroRNA networks direct neuronal development and plasticity. Cell Mol Life Sci. 2012;69:89–102. doi: 10.1007/s00018-011-0788-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stark KL., Xu B., Bagchi A., et al Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11 -deletion mouse model. Nat Genet. 2008;40:751–760. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- 98.Jin P., Zarnescu DC., Ceman S., et al Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nat Neurosci. 2004;7:113–117. doi: 10.1038/nn1174. [DOI] [PubMed] [Google Scholar]
- 99.Qurashi A., Chang S., Peng J. Role of microRNA pathway in mental retardation. Scientific World Journal. 2007;7:146–154. doi: 10.1100/tsw.2007.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Caudy AA., Myers M., Hannon GJ., Hammond SM. Fragile X-related protein and VIG associate with the RNA interference machinery. Genes Dev. 2002;16:2491–2496. doi: 10.1101/gad.1025202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.lshIzuka A., Siomi MC., Siomi H. A Drosophila fragileX protein interacts with components of RNAi and ribosomal proteins. Genes Dev. 2002;16:2497–2508. doi: 10.1101/gad.1022002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ashraf SI., McLoon AL., Sclarsic SM., Kunes S. Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell. 2006;124:191–205. doi: 10.1016/j.cell.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 103.Wayman GA., Davare M., Ando H., et al An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proc Natl Acad Sci USA. 2008;105:9093–9098. doi: 10.1073/pnas.0803072105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Edbauer D., Neilson JR., Foster KA., et al Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron. 2010;65:373–384. doi: 10.1016/j.neuron.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schratt GM., Tuebing F., Nigh EA., et al A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- 106.Meng Y., Takahashi H., Meng J., et al Regulation of ADF/cofilin phosphorylation and synaptic function by LIM-kinase. Neuropharmacology. 2004;47:746–754. doi: 10.1016/j.neuropharm.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 107.Fiore R., Khudayberdiev S., Christensen M., et al Mef2-mediated transcription of the miR379-410 cluster regulates activity-dependent dendritogenesis by fine-tuning Pumilio2 protein levels. EMBOJ. 2009;28:697–710. doi: 10.1038/emboj.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dwivedi Y., Rizavi HS., Conley RR., et al Altered gene expression of brainderived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 109.Dwivedi Y., Rizavi H., Zhang H., et al Neurotrophin receptor activation and expression in human postmortem brain: effect of suicide. Biol Psychiatry. 2009;65:319–328. doi: 10.1016/j.biopsych.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cohen JE., Lee PR., Chen S., Li W., Fields RD. MicroRNA regulation of homeostatic synaptic plasticity. Proc Natl Acad Sci USA. 2011;108:11650–11655. doi: 10.1073/pnas.1017576108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Banerjee S., Neveu P., Kosik KS. A coordinated local translational control point at the synapse involving relief from silencing and MOV10 degradation. Neuron. 2009;64:871–884. doi: 10.1016/j.neuron.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 112.Dwivedi Y., Rao JS., Rizavi HS., et al Abnormal expression and functional characteristics of cyclic adenosine monophosphate response element binding protein in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:273–282. doi: 10.1001/archpsyc.60.3.273. [DOI] [PubMed] [Google Scholar]
- 113.Vo N., Klein ME., Varlamova O., et al A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc Natl Acad Sci USA. 2005;102:16426–16431. doi: 10.1073/pnas.0508448102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu J., Xie X. Comparative sequence analysis reveals an intricate network among REST, CREB and miRNA in mediating neuronal gene expression. Genome Biol. 2006;7:R85. doi: 10.1186/gb-2006-7-9-r85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Impey S., Davare M., Lasiek A., et al An activity-induced microRNA controls dendritic spine formation by regulating Rac1-PAK signaling. Mol Cell Neurosci. 2010;43:146–156. doi: 10.1016/j.mcn.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rajasethupathy P., Fiumara F., Sheridan R., et al Characterization of small RNAs in Aplysia reveals a role for miR-124 in constraining synaptic plasticity through CREB. Neuron. 2009;63:803–817. doi: 10.1016/j.neuron.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gao J., Wang WY., Mao YW., et al A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Scott HL., Tamagnini F., Narduzzo KE., et al MicroRNA-132 regulates recognition memory and synaptic plasticity in the perirhinal cortex. Eur J Neurosci. 2012;36:2941–2948. doi: 10.1111/j.1460-9568.2012.08220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eyman M., Cefaliello C., Ferrara E., et al Local synthesis of axonal and presynaptic RNA in squid model systems. Eur J Neurosci. 2007;25:341–350. doi: 10.1111/j.1460-9568.2007.05304.x. [DOI] [PubMed] [Google Scholar]
- 120.Ju W., Morishita W., Tsui J., et al Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci. 2004;7:244–253. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- 121.eiler IJ., Irwin SA., Klintsova AY., et al FragileX mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc Natl Acad Sci USA. 1997;94:5395–5400. doi: 10.1073/pnas.94.10.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Todd PK., Mack KJ., Malter JS. The fragile X mental retardation protein is required for type-l metabotropic glutamate receptor-dependent translation of PSD-95. Proc Natl Acad Sci U S A. 2003;100:14374–14378. doi: 10.1073/pnas.2336265100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sutton MA., Schuman EM. Local translational control in dendrites and its role in long-term synaptic plasticity. J Neurobiol. 2005;64:116. doi: 10.1002/neu.20152. [DOI] [PubMed] [Google Scholar]
- 124.Smalheiser NR. Synaptic enrichment of microRNAs in adult mouse forebrain is related to structural features of their precursors. Biol Direct. 2008;3:44. doi: 10.1186/1745-6150-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Budhu A., Ji J., Wang XW. The clinical potential of microRNAs. J Hematol Oncol. 2010;3:37. doi: 10.1186/1756-8722-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Serafini G., Pompili M., Innamorati M., et al The role of microRNAs in synaptic plasticity, major affective disorders and suicidal behavior. Neurosci Res. 2012;73:179–190. doi: 10.1016/j.neures.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 127.Chana G., Bousman CA., Money TT., et al Biomarker investigations related to pathophysiological pathways in schizophrenia and psychosis. Front Cell Neurosci. 2013;7:95. doi: 10.3389/fncel.2013.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dorval V., Nelson PT., Hébert SS. Circulating microRNAs in Alzheimer's disease: the search for novel biomarkers. Front Mol Neurosci. 2013;6:24. doi: 10.3389/fnmol.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Smalheiser NR., Lugli G., Rizavi HS., et al MicroRNA expression in rat brain exposed to repeated inescapable shock: differential alterations in learned helplessness vs. non-learned helplessness. int J Neuropsychopharmacol. 2011;14:1315–1325. doi: 10.1017/S1461145710001628. [DOI] [PubMed] [Google Scholar]
- 130.Pariante CM., Miller AH. Glucocorticoid receptors in major depression relevance to pathophysiology and treatment. Biol Psych. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 131.Vreugdenhil E., Verissimo CS., Mariman R., et al MicroRNA 18 and 124a down-regulate the glucocorticoid receptor: implications for glucocorticoid responsiveness in the brain. Endocrinology. 2009;150:2220–2228. doi: 10.1210/en.2008-1335. [DOI] [PubMed] [Google Scholar]
- 132.Uchida S., Nishida A., Hara K., et al Characterization of the vulnerability to repeated stress in Fischer 344 rats: possible involvement of microRNAmediated down-regulation of the glucocorticoid receptor. Eur J Neurosci. 2008;27:2250–2261. doi: 10.1111/j.1460-9568.2008.06218.x. [DOI] [PubMed] [Google Scholar]
- 133.Turner JD., Alt SR., Cao L., et al Transcriptional control of the glucocorticoid receptor: CpG islands, epigenetics and more. Biochem Pharmacol. 2010;80:1860–1868. doi: 10.1016/j.bcp.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 134.Rinaldi A., Vincenti S., De Vito F., et al Stress induces region specific alterations in microRNAs expression in mice. Behav Brain Res. 2010;208:265–269. doi: 10.1016/j.bbr.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 135.Meerson A., Cacheaux L., Goosens KA., et al Changes in brain microRNAs contribute to cholinergic stress reactions. J Mol Neurosci. 2010;40:47–55. doi: 10.1007/s12031-009-9252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Witke W. The role of profilin complexes in cell motility and other cellular processes. Trends Cell Biol. 2004;14:461–469. doi: 10.1016/j.tcb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 137.Matigian N., Windus L., Smith H., et al Expression profiling in monozygotic twins discordant for bipolar disorder reveals dysregulation of the WNT signalling pathway. Mol Psychiatry. 2007;12:815–825. doi: 10.1038/sj.mp.4001998. [DOI] [PubMed] [Google Scholar]
- 138.Cao MQ., Chen DH., Zhang CH., Wu ZZ. Screening of specific microRNA in hippocampus of depression model rats and intervention effect of Chaihu Shugan San. Zhongguo Zhong Yao Za Zhi. 2013;38:1585–1589. [PubMed] [Google Scholar]
- 139.Raabe FJ., Spengler D. Epigenetic risk factors in PTSD and depression. Front Psychiatry. 2013;4:80. doi: 10.3389/fpsyt.2013.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Uchida S., Hara K., Kobayashi A., et al Early life stress enhances behavioral vulnerability to stress through the activation of REST4-mediated gene transcription in the medial prefrontal cortex of rodents. J Neurosci. 2010;30:15007–15018. doi: 10.1523/JNEUROSCI.1436-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Conaco C., Otto S., Han JJ., Mandel G. Reciprocal actions of REST and a microRNA promote neuronal identity. Proc Natl Acad Sci U S A. 2006;103:2422–2427. doi: 10.1073/pnas.0511041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Otto SJ., McCorkle SR., Hover J., et al Anew binding motif for the transcriptional repressor REST uncovers large gene networks devoted to neuronal functions. J Neurosci. 2007;27:6729–6739. doi: 10.1523/JNEUROSCI.0091-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pascual R., Zamora-León P., Catalán-Ahumada M., Valero-Cabré A. Early social isolation decreases the expression of calbindin D-28k and dendritic branching in the medial prefrontal cortex of the rat. Int J Neurosci. 2007;117:465–476. doi: 10.1080/00207450600773459. [DOI] [PubMed] [Google Scholar]
- 144.Ovtscharoff W Jr., Braun K. Maternal separation and social isolation modulate the postnatal development of synaptic composition in the infralimbic cortex of Octodon degus. Neuroscience. 2001;104:33–40. doi: 10.1016/s0306-4522(01)00059-8. [DOI] [PubMed] [Google Scholar]
- 145.Stevenson CW., Marsden CA., Mason R. Early life stress causes FG-7142induced corticolimbic dysfunction in adulthood. Brain Res. 2008;1193:43–50. doi: 10.1016/j.brainres.2007.11.062. [DOI] [PubMed] [Google Scholar]
- 146.Bai M., Zhu X., Zhang Y., et al Abnormal hippocampal BDNF and miR-16 expression is associated with depression-like behaviors induced by stress during early life. PLoS One. 2012;7:e46921. doi: 10.1371/journal.pone.0046921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Launay JM., Mouillet-Richard S., Baudry A., Pietri M., Kellermann O. Raphe-mediated signals control the hippocampal response to SRI antidepressants via miR-16. Transl Psychiatry. 2011;22:1:e56. doi: 10.1038/tp.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Baudry A., Mouillet-Richard S., Schneider B., Launay JM., Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329:1537–1541. doi: 10.1126/science.1193692. [DOI] [PubMed] [Google Scholar]
- 149.O'Connor RM., Grenham S., Dinan TG., Cryan JF. microRNAs as novel antidepressant targets: converging effects of ketamine and electroconvulsive shock therapy in the rat hippocampus. Int J Neuropsychopharmacol. 2013;16:1885–1892. doi: 10.1017/S1461145713000448. [DOI] [PubMed] [Google Scholar]
- 150.Ryan KM., O'Donovan SM., McLoughlin DM. Electroconvulsive stimulation alters levels of BDNF-associated microRNAs. Neurosci Lett. 2013;549:125–129. doi: 10.1016/j.neulet.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 151.Morag A., Pasmanik-Chor M., Oron-Karni V., et al Genome-wide expression profiling of human lymphoblastoid cell lines identifies CHL1 as a putative SSRI antidepressant response biomarker. Pharmacogenomics. 2011;12:171–184. doi: 10.2217/pgs.10.185. [DOI] [PubMed] [Google Scholar]
- 152.Oved K., Morag A., Pasmanik-Chor M., et al Genome-wide miRNA expression profiling of human lymphoblastoid cell lines identifies tentative SSRI antidepressant response biomarkers. Pharmacogenomics. 2012;13:1129–1139. doi: 10.2217/pgs.12.93. [DOI] [PubMed] [Google Scholar]
- 153.Schmidt U., Herrmann L., Hagl K., et al Therapeutic action of fluoxetine is associated with a reduction in prefrontal cortical miR-1971 expression levels in a mouse model of posttraumatic stress disorder. Front Psychiatry. 2013;4:66. doi: 10.3389/fpsyt.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Machado-Vieira R., Salvadore G., DiazGranados N., et al New therapeutic targets for mood disorders. ScientificWorldJournal. 2010;10:713–726. doi: 10.1100/tsw.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Saus E., Brunet A., Armengol L., et al Comprehensive copy number variant (CNV) analysis of neuronal pathways genes in psychiatric disorders identifies rare variants within patients. J Psychiatr Res. 2010;44:971–978. doi: 10.1016/j.jpsychires.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 156.Smalheiser NR., Lugli G., Rizavi HS., et al MicroRNA expression is downregulated and reorganized in prefrontal cortex of depressed suicide subjects. PLoS One. 2012;7:e33201. doi: 10.1371/journal.pone.0033201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang J., Cheng CM., Zhou J., et al Estradiol alters transcription factor gene expression in primate prefrontal cortex. J Neurosci Res. 2004;76:306–314. doi: 10.1002/jnr.20076. [DOI] [PubMed] [Google Scholar]
- 158.He Y., Zhou Y., Xi Q., et al Genetic variations in microRNA processing genes are associated with susceptibility in depression. DNA Cell Biol. 2012;31:1499–1506. doi: 10.1089/dna.2012.1660. [DOI] [PubMed] [Google Scholar]
- 159.Cogswell JP., Ward J., Taylor IA., et al Identification of miRNA changes in Alzheimer's disease brain and CSF yields putative biomarkers and insights into disease pathways. J Alzheimers Dis. 2008;14:27–41. doi: 10.3233/jad-2008-14103. [DOI] [PubMed] [Google Scholar]
- 160.Hanke M., Hoefig K., Merz H., et al A robust methodology to study urine microRNA as tumor marker: microRNA-126 and microRNA-182 are related to urinary bladder cancer. Urol Oncol. 2010;28:655–661. doi: 10.1016/j.urolonc.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 161.Weber JA., Baxter DH., Zhang S., et al The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56:1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen X., Ba Y., Ma L., et al Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 163.Fichtlscherer S., De Rosa S., Fox H., et al Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 164.Gallo A., Tandon M., Alevizos I., lllei GG. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS ONE. 2012;7:e30679. doi: 10.1371/journal.pone.0030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang K., Zhang S., Weber J., Baxter D., Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010;38:7248–7259. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vickers KC., Palmisano BT., Shoucri BM., Shamburek RD., Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nature Cell Biology. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Arroyo JD., Chevillet JR., Kroh EM., et al Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hanson EK., Lubenow H., Ballantyne J. Identification of forensically relevant body fluids using a panel of differentially expressed microRNAs. Anal Biochem. 2009;387:303–314. doi: 10.1016/j.ab.2009.01.037. [DOI] [PubMed] [Google Scholar]
- 169.Cortez MA., Bueso-Ramos C., Ferdin J., et al MicroRNAs in body fluids - the mix of hormones and biomarkers. Nat Rev Clin Oncol. 2011;8:467–477. doi: 10.1038/nrclinonc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mendell JT., Olson EN. MicroRNAs in stress signaling and human disease. Cell. 2012;148:1172–1187. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang Q., Wei L., Guan X., et al Briefing in family characteristics of microRNAs and their applications in cancer research. Biochim Biophys Acta. 2014;1844:191–197. doi: 10.1016/j.bbapap.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 172.Piva R., Spandidos DA., Gambari R. From microRNA functions to microRNA therapeutics: Novel targets and novel drugs in breast cancer research and treatment (Review). Int J Oncol. 2013;43:985–994. doi: 10.3892/ijo.2013.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Saito Y., Saito H. MicroRNAs in cancers and neurodegenerative disorders. Front Genet. 2012;3:194. doi: 10.3389/fgene.2012.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ceman S., Saugstad J. MicroRNAs: Meta-controllers of gene expression in synaptic activity emerge as genetic and diagnostic markers of human disease. Pharmacol Ther. 2011;130:26–37. doi: 10.1016/j.pharmthera.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chan AW., Kocerha J. The path to microRNA: therapeutics in psychiatric and neurodegenerative disorders. Front Genet. 2012;17:3–82. doi: 10.3389/fgene.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gaughwin PM., Ciesla M., Lahiri N., et al Hsa-miR-34b is a plasma-stable microRNA that is elevated in pre-manifest Huntington's disease. Hum Mol Genet. 2011;20:2225–2237. doi: 10.1093/hmg/ddr111. [DOI] [PubMed] [Google Scholar]
- 177.Shi W., Du J., Qi Y., et al Aberrant expression of serum miRNAs in schizophrenia. J Psychiatr Res. 2012;46:198–204. doi: 10.1016/j.jpsychires.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 178.Rong H., Liu TB., Yang KJ., et al MicroRNA-1 34 plasma levels before and after treatment for bipolar mania. J Psychiatr Res. 2011;45:92–95. doi: 10.1016/j.jpsychires.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 179.Belzeaux R., Bergon A., Jeanjean V., et al Responder and nonresponder patients exhibit different peripheral transcriptional signatures during major depressive episode. Transl Psychiatry. 2012;2:e185. doi: 10.1038/tp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bocchio-Chiavetto L., Maffioletti E., Bettinsoli P., et al Blood microRNA changes in depressed patients during antidepressant treatment. Eur Neuropsychopharmacol. 2013;23:602–611. doi: 10.1016/j.euroneuro.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 181.Mellios N., Sugihara H., Castro J. miR-132, an experience-dependent microRNA, is essential for visual cortex plasticity. Nat Neurosci. 2011;14:1240–1242. doi: 10.1038/nn.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Caputo V., Sinibaldi L., Fiorentino A. Brain derived neurotrophic factor (BDNF) expression is regulated by microRNAs miR-26a and miR-26b allelespecific binding. PLoS One. 2011;6:e28656. doi: 10.1371/journal.pone.0028656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kawashima H., Numakawa T., Kumamaru E., et al Glucocorticoid attenuates brain-derived neurotrophic factor-dependent upregulation of glutamate receptors via the suppression of microRNA-132 expression. Neuroscience. 2010;165:1301–1311. doi: 10.1016/j.neuroscience.2009.11.057. [DOI] [PubMed] [Google Scholar]
- 184.Kim AH., Reimers M., Maher B., et al MicroRNA expression profiling in the prefrontal cortex of individuals affected with schizophrenia and bipolar disorders. Schizophr Res. 2010;124:183–191. doi: 10.1016/j.schres.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Azuma-Mukai A., Oguri H., Mituyama T., et al Characterization of endogenous human Argonautes and their miRNA partners in RNA silencing. Proc Natl Acad Sci USA. 2008;105:7964–7969. doi: 10.1073/pnas.0800334105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zeng Y., Sankala H., Zhang X., Graves PR. Phosphorylation of Argonaute 2 at serine-387 facilitates its localization to processing bodies. Biochem J. 2008;413:429–436. doi: 10.1042/BJ20080599. [DOI] [PubMed] [Google Scholar]
- 187.Dwivedi Y., Rizavi HS., Roberts RC., et al Reduced activation and expression of ERK1/2 MAP kinase in the post-mortem brain of depressed suicide subjects. J Neurochem. 2001;77:916–928. doi: 10.1046/j.1471-4159.2001.00300.x. [DOI] [PubMed] [Google Scholar]
- 188.Dwivedi Y., Zhang H., Pandey GN. Calcium sensing proteins in depressive disorder. Am Coll Neuropsychophramacol. 2011;36:S75–S197Abstract 29. [Google Scholar]
- 189.Dwivedi Y., Rizavi HS., Zhang H., et al Aberrant extracellular signal-regulated kinase (ERK)1/2 signalling in suicide brain: role of ERK kinase 1 (MEK1). Int J Neuropsychopharmacol. 2009;12:1337–1354. doi: 10.1017/S1461145709990575. [DOI] [PubMed] [Google Scholar]
- 190.Dwivedi Y., Rizavi HS., Conley RR., Pandey GN. ERK MAP kinase signaling in post-mortem brain of suicide subjects: differential regulation of upstream Raf kinases Raf-1 and B-Raf. Mol Psychiatry. 2006;11:86–98. doi: 10.1038/sj.mp.4001744. [DOI] [PubMed] [Google Scholar]
- 191.Mellios N., Sur M. The emerging role of microRNAs in schizophrenia and autism spectrum disorders. Front Psychiatry. 2012;3:39. doi: 10.3389/fpsyt.2012.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kalani A., Tyagi A., Tyagi N. Exosomes: Mediators of neurodegeneration, neuroprotection and therapeutics. Mol Neurobiol 2013. [Epub ahead of print] PMID:23999871. doi: 10.1007/s12035-013-8544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Corrado C., Raimondo S., Chiesi A., et al Exosomes as intercellular signaling organelles involved in health and disease: basic science and clinical applications. Int J Mol Sci. 2013;14:5338–5366. doi: 10.3390/ijms14035338. [DOI] [PMC free article] [PubMed] [Google Scholar]