Abstract
Synchronized neuronal activity in the cortex generates weak electric fields that are routinely measured in humans and animal models by electroencephalography and local field potential recordings. Traditionally, these endogenous electric fields have been considered to be an epiphenomenon of brain activity. Recent work has demonstrated that active cortical networks are surprisingly susceptible to weak perturbations of the membrane voltage of a large number of neurons by electric fields. Simultaneously, noninvasive brain stimulation with weak, exogenous electric fields (transcranial current stimulation, TCS) has undergone a renaissance due to the broad scope of its possible applications in modulating brain activity for cognitive enhancement and treatment of brain disorders. This review aims to interface the recent developments in the study of both endogenous and exogenous electric fields, with a particular focus on rhythmic stimulation for the modulation of cortical oscillations. The main goal is to provide a starting point for the use of rational design for the development of novel mechanism-based TCS therapeutics based on transcranial alternating current stimulation, for the treatment of psychiatric illnesses.
Keywords: feedback electric field, magnetic resonance imaging, neuronal network, transcranial alternating current stimulation
Abstract
La actividad sincronizada de las neuronas corticales genera campos eléctricos débiles que se miden rutinariamente en humanos y en modelos animales mediante la electroencefalografía y los potenciales de campo locales. Tradicíonalmente, estos campos eléctricos endógenos se han considerado un epifenómeno de la actividad cerebral. Trabajos recientes han demostrado con sorpresa que las redes corticales activas son sensibles a pequeños cambios del voltaje de la membrana de un gran número de neuronas mediante campos eléctricos. Al mismo tiempo, la estimulación cerebral no invasora con campos eléctricos exógenos débiles (estimulación transcraneal con corriente, ETC) ha experimentado un renacimíento debído al amplio alcance de sus posibles aplicaciones en la modulación de la actividad cerebral para el mejoramiento cognitivo y el tratamiento de los trastornos cerebrales. Esta revisión tiene como objetivo relacionar los desarrollos recientes en el estudio de los campos eléctricos endógenos y exógenos con especial atención a la estimulación rítmica para la modulación de las oscilaciones corticales. El propósito principal es proporcionar el punto de partida para el empleo de un diseño racional para el desarrollo de nuevas terapias basadas en el mecanismo de la ETC mediante la estimulación transcraneal con corriente alterna para el tratamiento de enfermedades psiquiátricas.
Abstract
L'activité neuronale synchronisée dans le cortex génère de faibles champs électriques mesurés couramment dans des modèles humains et animaux sous forme d'électroencéphalographie et de potentiels de champs locaux. Traditionnellement, ces champs électriques endogènes sont considérés comme un épiphénomène de l'activité cérébrale. D'après des travaux récents, des réseaux corticaux actifs sont étonnamment sensibles à de petites perturbations du voltage de la membrane d'un grand nombre de neurones par des champs électriques. Simultanément, la stimulation cérébrale non invasive avec de faibles champs électriques exogènes (stimulation transcrânienne par un courant, TCS) a pu renaître grâce à l'importance de ses applications possibles pour moduler l'activité cérébrale dans la stimulation cognitive et le traitement des troubles cérébraux. Cet article a pour but de relier les avancées récentes de l'étude des champs électriques endogènes et exogènes avec un regard particulier sur la stimulation rythmique pour la modulation des oscillations corticales. Le but principal est de fournir le point de départ d'une procédure rationnelle de développement de traitements nouveaux, dont les mécanismes sont élucidés, fondés sur la stimulation transcrânienne par courant alternatif, pour le traitement des maladies psychiatriques.
Introduction
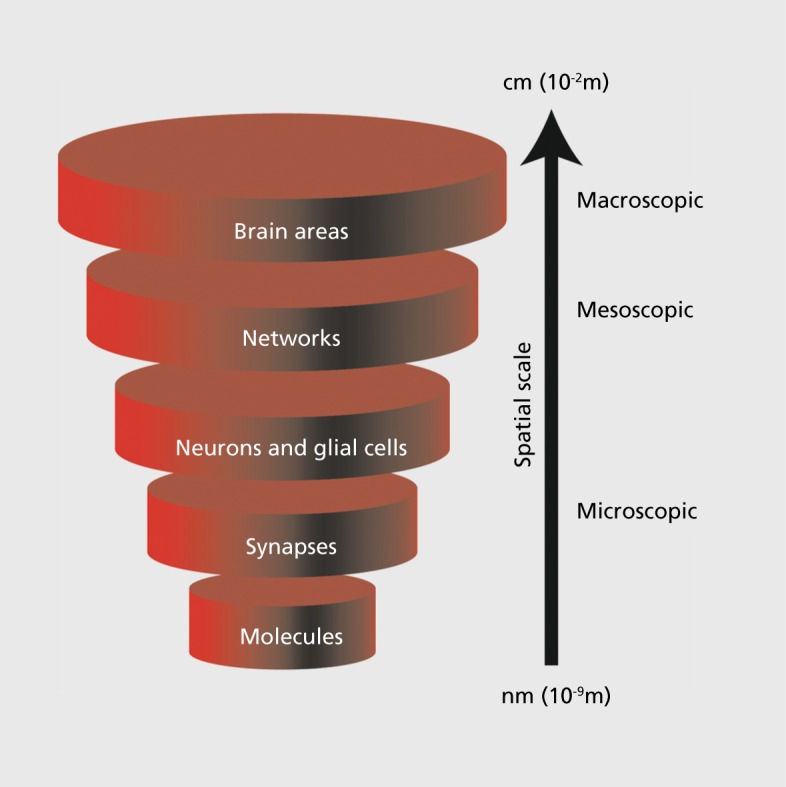
The brain, like any complex system, can be studied and modeled at different levels defined by the spatial scale of interest Figure 1.1 For example, brain function can be investigated at the microscopic, molecular scale by performing cell biology assays to understand the function of a specific signaling molecule involved in neuronal function. Alternatively, the brain can be studied at the level of entire brain areas by conducting noninvasive functional magnetic resonance imaging (fMRI) to measure blood oxygen level changes as indirect markers of neuronal activity.2,3 These two examples, one microscopic and one macroscopic, illustrate not only the differences in scientific methods and techniques, but also the differences in spatial scale that distinguish these (equally important) levels of investigation. Only integration of investigations across all spatial scales will likely enable us to fundamentally understand how the brain works (ie, by “vertical integration”).
Figure 1. Vertical integration of spatial scales from molecules (nanometer scale) all the way to the whole brain (centimeter scale). Integration of findings from the study of the brain at these different levels may represent the most promising approach to understand how neural activity gives rise to behavior and how impaired neural activity causes disease. This review focuses on the network level (at the mesoscopic scale) that is sandwiched between the microscopic and the macroscopic levels.
Given the immense burden of psychiatric illnesses on patients and their families, it is imperative to discover and develop novel treatments that surpass the existing therapeutic approaches in terms of efficacy and safety—even in light of the relative absence persisting today of a fundamental understanding of how the brain works. Importantly, recent advances in neuroscience research will enable the use of rational design, and the development of new treatments based on a mechanistic understanding of the underlying disease processes. In particular, we postulate here that at the network level, an intermediate (mesoscopic) level between the microscopic scale of molecules and the macroscopic scale of brain areas, represents a very attractive target for such an approach. This article will discuss the underlying basic science and sketch out a path forward that will fuse neuroscience research at the network level, engineering principles such as feedback control and dynamic systems theory, and medical sciences to advance rational design as a promising principle for the design of the next generation of neurotherapeutics for the treatment of mental illness. In particular, noninvasive brain stimulation offers a convenient tool to directly target the network level by altering the temporal structure of neuronal activity. With the exception of a few novel and rather poorly understood brain stimulation approaches such as ultrasound4,5 and laser,6 the vast majority of noninvasive brain stimulation is based on the application of electric and magnetic fields to modulate neuronal activity. Yet, since the development of the electroencephalogram (EEG) early in the 20th century,7 we know that network activity in brains also generates its own, endogenous, electric fields.8 In this review, we will discuss both endogenous and exogenous electric fields and will highlight the promising opportunities for the rational design of noninvasive brain stimulation approaches for the treatment of psychiatric disorders. In particular, there will be a focus on the modulation of cortical oscillations, a hallmark of physiological and pathological brain function.9,10
Ubiquitous neuronal network signal as a convenient epiphenomenon?
Neuronal signaling relies on the generation and transmission of transient electric impulses that represent the fundamental information unit in the brain.11,12 The canonical model of neural information processing is based on the notion that changes of the electric potential inside neurons relative to the constant electric potential outside of the neuron determines the membrane voltage, and therefore the functional state of individual neurons.13 Yet, the vast majority of neurophysiology studies are based on measurements of changes in the extracellular voltage such as the classical EEG, broadly used in both clinical and basic science settings, and the local field potential (LFP), an invasive recording of the extracellular voltage routinely performed in neuroscience animal studies.14 These fluctuations of extracellular voltage represent the endogenous electric field and reflect the activity of a large number of (synchronized) neurons; they have provided the basis for numerous discoveries about physiological and pathological states in the brain. These electric signals have routinely been considered an epiphenomenon in neuroscience, in the sense that the endogenous electric field plays no functional (”active“) role per se, but rather, represents a convenient side product of neuronal network activity to the benefit of the researcher or clinician who wants to measure brain activity. This view was supported by the realization that endogenous electric fields were comparably low in magnitude (around 1 V/m) and therefore unlikely to be powerful enough to directly modulate neuronal signaling. Studies which demonstrated that such weak electric fields change the membrane voltage only by an amount much smaller (typically about 0.5 to 1 mV)11,16 than what would be needed to activate a neuron (ie, bring it to firing threshold from rest, typically around 20 mV) further supported the assumed implausibility of endogenous electric fields to represent anything but an epiphenomenon.
Neuronal network sensitivity to weak electric fields
Historically, the effects of exogenous electric fields (“polarization”) were assessed in vivo. The field amplitudes used were typically on the order of 10 to 100 V/m, for which prominent effects at the single-cell level were found.17 Importantly, the effects found for these high stimulation amplitudes do not preclude the occurrence of more subtle yet functionally relevant changes of cortical network activity in response to weaker stimulation, as recently demonstrated in rats18 and ferrets.19 The establishment of the slice preparation, an in vitro assay of cellular and local network activity, has dramatically increased understanding of the mechanisms by which cellular and synaptic properties interact to form functional circuits in the brain.20 In this approach, thin sections of live brain tissue are maintained in vitro for targeted electrophysiological recordings using glass electrodes for intracellular studies and metal electrodes for extracellular measurements of neuronal activity. Use of this method to study the role of electric fields has proven fruitful and has led to a series of studies that demonstrated that weak electric fields modulate neuronal activity.16,21-23 In particular, the slice preparation has allowed: (i) relatively precise dosimetry to measure the strength of the applied electric field; (ii) reliable recording of small changes to the membrane voltage of individual neurons; and (iii) perhaps most importantly, the relative isolation of the effect of electric fields from other confounding factors inherent to the intact animal preparation. Interestingly, these studies mostly focused on the rodent hippocampus, a popular brain area for slice electrophysiology due to the relative simplicity of the circuitry. The main concern about the choice of this model system in the context of electric fields is the very high cell density in the rodent hippocampus where, as a result, the extracellular volume fraction is exceptionally low.24 Therefore, the extracellular resistivity and the effects of extracellular current/ voltage flow are potentially unique. The translation of these findings to the neocortex and other higher mammals such as ferrets, cats, nonhuman primates, and humans with lower cell densities in the hippocampus and neocortex remained in question. Nevertheless, these studies offered important evidence that weak electric fields can have a pronounced effect on neural activity as long as neurons are close to the threshold (either by current injection or by intrinsic network activity). In particular, the important concept that perturbations of membrane voltage by electric fields modulate spike timing instead of overall activity levels (for which stronger perturbations are needed) emerged.21-23 These studies also facilitated the “rebirth” of modern transcranial current stimulation (TCS, mostly transcranial direct current stimulation, tDCS).25 In TCS, a weak electric field is generated by exogenous current application to modulate neuronal activity (discussed below). Yet, the most challenging aspect of the question about the possible role of endogenous electric fields beyond a simple epiphenomenon remained unaddressed: what are the effects of weak activity-dependent electric fields (ie, “feedback”) such as the ones that occur in vivo during synchronized activity?
Feedback electric fields
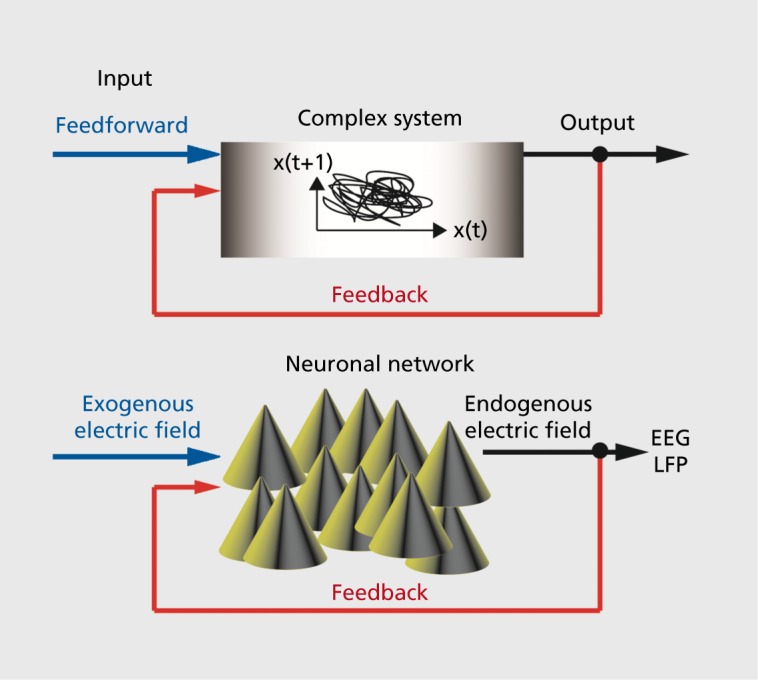
Feedback refers to any system that receives input that is not predefined (ie, “feedforward”), but rather depends on the behavior of the system itself (Figure 2). Control engineering is a highly effective branch of engineering that develops algorithms for feedback control of complex systems such as airplanes and chemical plants. In essence, these algorithms process real-time measurements such as velocity or temperature and decide what the best input (“control signal”) is to achieve a given behavior of the system, such as smoothly landing an airplane or inducing a specific chemical reaction in a production plant. Such feedback control systems are also omnipresent in biology as feedback represents a fundamental approach to maintain homeostasis (here, broadly defined). Regulation of insulin to control blood sugar is one of the numerous examples of such feedback regulation in biological systems. Returning to electric fields generated by neuronal activity, the question arises whether the “feedback” electric fields have similar effects as the “feedforward” electric fields used in the studies discussed above. In other words, does an endogenous electric field that tracks the endogenous network activity (ie, that occurs in vivo in the intact brain) also enhance these dynamics? Studying feedback systems is an experimentally difficult task that is often achieved by a so-called “separation of time scales” approach where the system is essentially studied without the feedback signal and a range of feedforward signals are individually evaluated. The behavior of the feedback system is then reconstructed by forming a composite of the feedforward responses of the system. For example, extracellular potassium concentration in the extracellular space fluctuates with neuronal activity, but the potassium concentration changes on a much slower time scale than the neuronal activity due to buffering and reuptake mechanisms.26 However, in the case of endogenous electric fields, this approach is not appropriate since the electric field varies on the same time scale as neuronal activity. As a result of these technical and conceptual difficulties, the possible role of endogenous electric fields in shaping neuronal network activity has remained unclear.27 A recently developed technical solution to overcome these hurdles enabled the direct assessment of the role of feedback on endogenous electric fields in cortical network dynamics.28 Specifically, a hybrid approach that combined biological slice preparation and analog electronics was employed to provide activity-dependent (feedback) electric fields by exogenous stimulation. Briefly, multiunit spiking activity was processed in real time to generate a low-pass filtered waveform that tracked the spiking activity (“simulated endogenous electric field”). Basing the signal on the multiunit activity was crucial, since multiunit activity (in contrast to LFP) can be recorded in the presence of low-frequency electric stimulation that tracks network activity. When such feedback electric fields were applied, spontaneous rhythmic activity in the slice was enhanced. Importantly, when the same system was used to suppress the activitydependent electric field, a reduction in the oscillatorystructure was found. Together these experiments provided strong support for endogenous feedback electric fields playing an active role in shaping (synchronized) cortical network dynamics. Detailed biophysical modeling of such cortical networks exposed to activity-dependent electric fields further validated these findings. Therefore, the endogenous electric fields generated by structured cortical network activity may be more than an epiphenomenon, but rather may play an active role as a neuronal communication mechanism.
Figure 2. Schematic representation of feedforward and feedback control of complex systems. Feedforward input is predetermined and independent of the response of the system to the input. Examples of feedforward signals in the context of this review are exogenous electric fields that are applied to animal preparations or humans in the form of TCS. Feedback is defined as input that depends of the state or output of the system to be controlled. Endogenous electric fields fall in this category since the neuronal activity of a network generates an electric field that in turn targets again the same neurons that generate the activity in the first place. EEG, electroencephalogram; LFP, local field potential; TCS, transcranial current stimulation.
Possible functional roles of endogenous electric fields
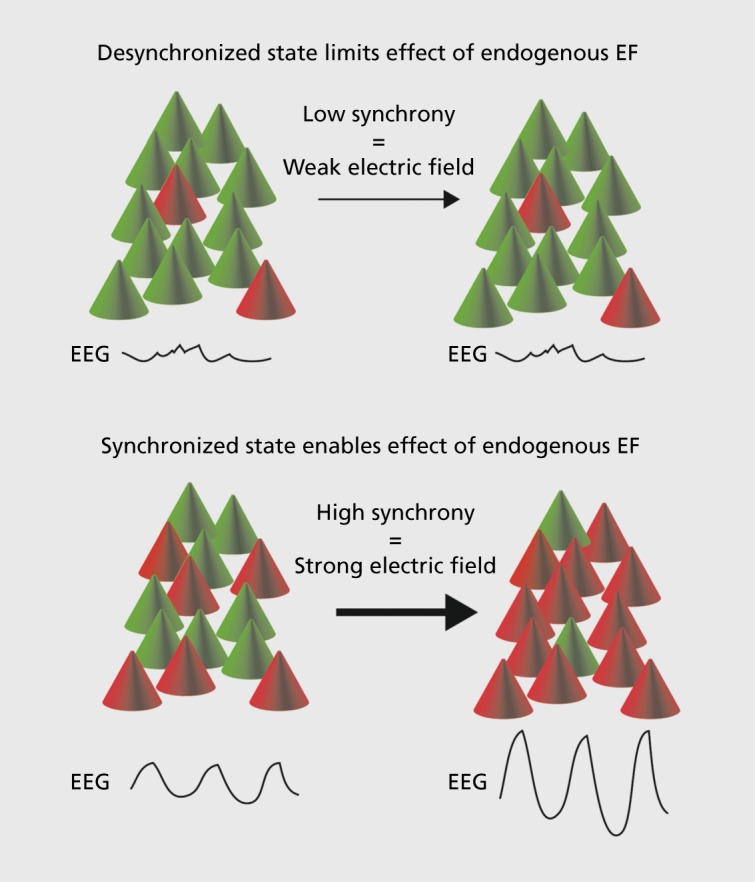
Given the finding that endogenous electric fields can enhance rhythmic cortical network dynamics, the functional roles served by this neuronal communication mechanism become an open question. When considering this question, we are left to hypothesize in the absence of experimental data. This is due to the (presumed) impossibility of isolating the feedback signal in intact brains, as opposed to slice preparations, where the relative lack of an endogenous field allows for simulation of endogenous fields by application of exogenous fields. From a conceptual viewpoint, it may be informative to consider the unique properties of such a neural communication mode, particularly in contrast to the canonical chemical synaptic transmission. First, communication by endogenous electric fields requires networkwide temporal organization of activity, such as oscillatory activity patterns, for electric fields to be of sufficient amplitude to modulate neuronal membrane voltage. Therefore, activity enhancement by an endogenous electric field is very likely limited to a subset of activity patterns that the cortex generates. In essence, a substantial number of neurons within a local network (mesoscopic scale, typically <100 μm) need to synchronize to generate a strong enough electric field (Figure 3). From a dynamic systems theory point of view, this indicates nonlinearity, since a critical number of neurons need to organize their activity for an effect to occur. As a result, it can be hypothesized that endogenous electric fields are particularly important for helping groups of neurons maintain synchronized activity once they have entered such a state (where endogenous electric fields can have an effect, Figure 3). Such a mechanism would, therefore, increase the stability of rhythmic cortical activity states. Second, endogenous electric fields may contribute to organizing cortical activity in space since electric fields can enhance activity in neighboring areas with hotspots of synchronized activity. Therefore, electric fields may expand areas of synchronized cells and increase information flow between spatially more distant sites.
Figure 3. Illustration of how sparse, nonsynchronized activity does not generate a pronounced electric field and therefore is likely unaltered by the proposed feedback between neuronal activity and electric fields. Synchronized activity generates a more pronounced electric field and is therefore able to use the resulting endogenous electric field to further sustain and amplify the ongoing synchronized activity. EEG, electroencephalogram; EF, electric field.
Rational design of noninvasive brain stimulation
Given the pronounced effects of very weak endogenous electric fields on cortical network dynamics, it is clear that application of external electric fields may represent a promising brain stimulation modality. In fact, the last decade has seen the (re-) emergence of TCS,29,30 most often referred to as tDCS due to the constant stimulation waveform typically used. TCS is a noninvasive brain stimulation modality where a weak electric current (typically 1 to 2 mA) is applied to the scalp by two saline-soaked sponge electrodes.31-35 Detailed modeling of the electric properties of the head and the brain have determined the resulting electric field to be around 1 V/m36-37 and thus comparable in amplitude to the endogenous electric fields discussed above. Importantly, TCS differs in many important aspects from transcranial magnetic stimulation (TMS), which applies spatially localized, suprathreshold perturbations by a stimulation current mediated by a time-varying magnetic field.38 Very little is known about the underlying mechanisms by which TCS alters brain function. The convergence of bottomup (effects of electric fields on neuronal activity) and top-down (develop clinically effective TCS paradigms) studies represents the basis for the rational design of novel stimulation paradigms. Indeed, one of the most major recent developments in TCS is the use of temporally structured waveforms such as in transcranial alternating current stimulation (tACS, sine-wave stimulation current, recently reviewed in ref 39) and transcranial random noise stimulation (tRNS, bandpass filtered noise).40 Therefore, the use of mechanistic insights on the action of electric fields in the nervous system is proposed for the development of next-generation TCS paradigms with higher efficacy and more long-lasting treatment benefits. This strategy is inspired by the rational design approach prevalent in modern research and development of novel pharmaceutical agents.
Transcranial alternating current stimulation
It remains unclear how exactly weak electric fields can modulate the mesoscopic and macroscopic dynamics of cortical networks. However, one inroad to our understanding resulted from the application of a conceptual approach that originated in physics. Given the often periodic (ie, rhythmic) structure of cortical network activity, the intuitive choice of stimulation waveform is one that is equally rhythmic. This reasoning has led to the early use of transcranial alternate current stimulation (t ACS), which has shown very interesting neurobiological effects despite a lack of understanding regarding if and how such periodic stimulation interacts with the intrinsic oscillators of cortical networks. The most prominent, paradigm-changing study employed tACS (in combination with a DC offset, technically so-tDCS) to enhance slow oscillatory activity (<1 Hz, the so-called slow oscillation, originally described in studies on cats by Steriade and colleagues)41,42 during slow-wave sleep.43 It has long been hypothesized for a long time that slowwave sleep is crucial for sleep-dependent learning and memory, and it was thus a very significant finding that tACS at 0.75 Hz (the stimulation signal also included a DC bias) enhanced memory consolidation in healthyhuman study participants. However, although the EEG confirmed an enhancement of slow rhythmic activity, the stimulation was not quite as specific in its effects, since it also enhanced activity signatures in higher frequency bands (sleep spindles, 10-16 Hz) that have also been associated with learning and memory.44,45 Interestingly, the same authors did not find a similar effect for the same stimulation paradigm in awake subjects,46 suggesting that the state of the brain may contribute to the response to stimulation (and its behavioral outcomes). Nevertheless, this study provided very strong motivation for the subsequent use of tACS to manipulate cortical oscillations, with the hope for a frequency-specific, noninvasive stimulation modality. Indeed, a-band tACS selectively enhanced a oscillations in occipital cortex47 and differed in its effect on spontaneous EEG activity depending on the brain state as defined by whether the subjects' eyes were open or closed.48 Additionally, tACS has been demonstrated to alter visual detection performance.49 Tactile sensations were elicited by tACS over the primary somatosensory cortex, but only for α and high-γ stimulation frequencies.50 Interestingly, stimulation in the α-band modulated γ-oscillations in the motor system, suggesting that stimulation of a given frequency band can also affect other frequency bands and therefore provide a counterargument to the idea of frequency-specific stimulation effects.51 Furthermore, α-band tACS over the dorsolateral prefrontal cortex (DL-PFC) failed to reduce tinnitus symptoms.52 Bilateral 8 band stimulation of DL-PFC and phase-synchronizing dual-channel frontoparietal stimulation both enhanced working memory performance.53,54 Phase-desynchronizing γ stimulation (180-degree phase offset) of occipital-parietal areas affected bistable motion perception.55 tACS also appears to modulate motor output; feedback tACS, based on measured tremors, in patients reduced tremor symptoms and therefore suggests that the phase of tACS plays an important role.56 α and β stimulation of the primary motor cortex had differential effects on motor performance.51 In particular, β-stimulation slowed movement,57 but increased corticospinal excitability measured by TMS.58,59 Similarly, the excitability of the occipital cortex was selectively increased by β-band tACS.60,61 γ-frequency tACS over the middle frontal gyrus enhanced fluid intelligence, while other frequencies failed to show an effect.62 High β-frequency tACS improved contrast perception, but did not modulate spatial attention.63 Even higher-frequency stimulation (in the so-called ripple range, 140 Hz)64 enhanced excitability in the motor cortex.65 Likely, these effects of tACS crucially depend on the total dose which involves session duration, amplitude, electrode size and position, and number of sessions. For example, an initial tACS study with short stimulation durations failed to show modulation of excitability in any stimulation frequency band.66 Due to the lack of standardization of stimulation parameters, the direct comparison between studies is not feasible, and the field of tACS is in its infancy due to the lack of commonly accepted stimulation effects. Nevertheless, it has become clear that tACS can elicit electrophysiological and behavioral effects that depend on the stimulation frequency. Understanding the underlying mechanism will enable the targeted choice of stimulation frequency to treat specific network deficits that may vary from patient to patient. The putative mechanism of frequency-specific effects as a starting point for such rational design is discussed below.
Network mechanisms of tACS
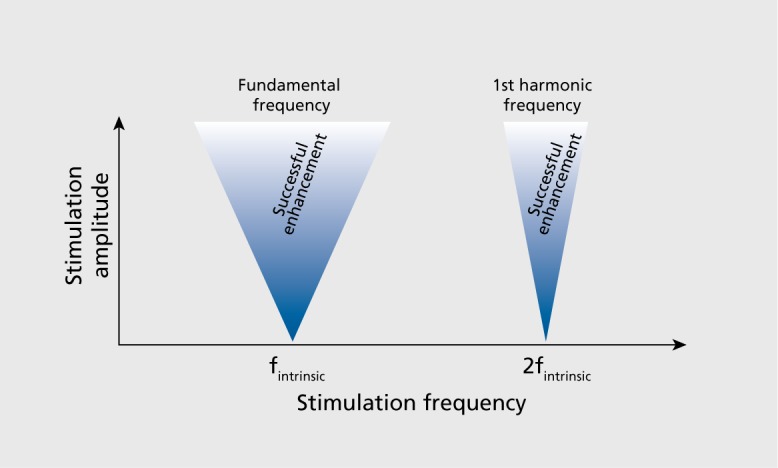
From the perspective of dynamic systems theory, tACS corresponds to a periodically forced intrinsic oscillator. The periodic force corresponds to the applied sine-wave stimulation current, and the endogenous network oscillations represent the intrinsic oscillator. It is well known that stimulation of intrinsic oscillators at different frequencies has different effects. Most prominently and implicitly assumed in the abovementioned studies, stimulation at the endogenous or intrinsic frequency is, in general, an effective way to enhance that oscillation. However, the question arises as to what extent the intrinsic oscillator rejects stimulation at other frequencies. This is fundamentally important for the design of brain stimulation to manipulate cortical oscillations. In the case where the intrinsic oscillator can easily be driven (entrained) at any given frequency, the design of effective and targeted tACS paradigms is comparatively trivial. However, if the intrinsic oscillator prefers certain frequencies, the rational design of tACS paradigms is more complex (and interesting). The selective preference of certain stimulation frequencies is called resonance, a well-known phenomenon that can be observed in many physical and biological systems. Technically, resonance can be easily determined by application of periodic stimulation with identical amplitude, but different frequencies. Any measure of oscillatory structures will reveal the degree to which the system prefers a given frequency. In fact, one can look for two fundamental properties that delineate the resonance properties of the system: (i) the presence of so-called “Arnold tongues”; and (ii) the presence of harmonics. Arnold tongues delineate the areas of entrainment for parameter pairs of stimulation amplitude and frequency. The tongueshaped areas derive from the fact that the stronger the amplitude of the periodic stimulation, the broader the range of frequencies to which an intrinsic oscillator can be entrained (Figure 4.) This corresponds to the intuitive concept that weak forces can only amplify the intrinsically present dynamics whereas stronger forces can—to a certain extent—override/modulate the frequency of the intrinsic oscillator. Harmonic frequencies refer to the phenomenon that stimulation at multiples of the intrinsic frequencies has a privileged effect on the amplitude of the ongoing oscillation. Computational simulations of large-scale cortical networks demonstrated that such resonance effects indeed mediate the modulatory effects of tACS19; yet detailed experimental demonstration of resonance at the network level has remained elusive. Likely, the discovery of such a phenomenon will build on the well-documented intrinsic resonance of individual neurons,67,68 especially layer V pyramidal cells that are interestingly very sensitive to electric fields due to their elongated somatodendritic axis.69
Figure 4. Arnold's tongues. Effects of periodic perturbations are limited to stimulation frequencies close to the intrinsic (fundamental) frequency and its harmonics. Inverted triangles (“tongues”) delimit areas where for increasing stimulation amplitude, a broader range of stimulation frequencies are effective. f, frequency.
Feedback stimulation: the future?
In this review, I have discussed the recent evidence for: (i) a modulatory effect of endogenous electric fields that likely provide a synchronizing network signal by feedback; and (ii) network resonance as the putative mechanistic principle by which rhythmically active neuronal networks are sensitive to periodic perturbations by both endogenous and exogenous electric fields. Together, these recent discoveries provide fertile grounds for the development of novel noninvasive brain stimulation paradigms. In particular, the use of periodic stimulation patterns that match and mismatch the endogenous activity structure for enhancement of physiological activity and suppression of pathological activity, respectively, appears as one of the most promising approaches that blend feedback stimulation with exploitation of the resonance structure of cortical networks. Treatment of psychiatric illnesses, such as depression and schizophrenia, with tACS has not yet been evaluated. However, preliminary success with tDCS further supports the pursuit of noninvasive, therapeutic brain stimulation for depression70-72 and other psychiatric illnesses.73 In contrast to today's brain stimulation, where the choice of stimulation waveforms and parameters are most often determined by clinical intuition and historical practices, tomorrow's brain stimulation will be more targeted and therefore more individualized by being based on the emerging mechanistic understanding of how networks generate activity patterns and how they are susceptible to applied perturbations. Such rational design brings the hope for novel, effective, and safe treatments for severe mental illnesses such as depression and schizophrenia.
Acknowledgments
The author thanks his mentors, in particular Drs Terry Sejnowski, David McCormick, David Rubinow, John Gilmore, and Steven Schiff. Funding from the UNC Department of Psychiatry, the Foundation of Hope, and the NIMH are gratefully acknowledged. Preparation of this publication was partially supported by the National Institute of Mental Health of the National Institutes of Health, under Award Number R01MH101547 (PI: Frohlich). The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Churchland PS., Sejnowski TJ. The Computational Brain. Cambridge, MA: MIT Press; 1992 [Google Scholar]
- 2.Ogawa S., Lee TM., Kay AR., Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raichle ME., MacLeod AM., Snyder AZ., Powers WJ., Gusnard DA., Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoo SS., Bystritsky A., Lee JH., et al Focused ultrasound modulates region-specific brain activity. Neuroimage. 2011;56:1267–1275. doi: 10.1016/j.neuroimage.2011.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tufail Y., Matyushov A., Baldwin N., et al Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 2010;66:681–694. doi: 10.1016/j.neuron.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Wells J., Kao C., Mariappan K., et al Optical stimulation of neural tissue in vivo. Opt Lett. 2005;30:504–506. doi: 10.1364/ol.30.000504. [DOI] [PubMed] [Google Scholar]
- 7.Berger H. Hans Berger on the Electroencephalogram of Man. The Fourteen Original Reports on the Human Electroencephalogram. Amsterdam, the Netherlands; New York, NY: Elsevier Publishing Company; 1969 [Google Scholar]
- 8.Nunez PL., Srinivasan R. Electric Fields of the Brain: the Neurophysics of EEG. 2nd ed. New York, NY: Oxford University Press; 2006 [Google Scholar]
- 9.Wang XJ. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev. 2010;90:1195–1268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buzsáki G. Rhythms of the Brain. New York, NY: Oxford University Press; 2006 [Google Scholar]
- 11.Adrian ED., Bronk DW. The discharge of impulses in motor nerve fibres: Part I. Impulses in single fibres of the phrenic nerve. J Physiol. 1928;66:81–101. doi: 10.1113/jphysiol.1928.sp002509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rieke F. Spikes: Exploring the Neural Code. Cambridge, MA: MIT Press; 1997 [Google Scholar]
- 13.Hodgkin AL., Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buzsáki G., Anastassiou CA., Koch C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 2012;13:407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tranchina D., Nicholson C. A model for the polarization of neurons by extrinsically applied electric fields. Biophys J. 1986;50:1139–1156. doi: 10.1016/S0006-3495(86)83558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deans JK., Powell AD., Jefferys JG. Sensitivity of coherent oscillations in rat hippocampus to AC electric fields. J Physiol. 2007;583:555–565. doi: 10.1113/jphysiol.2007.137711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purpura DP., McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol. 1965;28:166–185. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- 18.Ozen S., Sirota A., Belluscio MA., et al Transcranial electric stimulation entrains cortical neuronal populations in rats. J Neurosci. 2010;30:11476–11485. doi: 10.1523/JNEUROSCI.5252-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali MM., Sellers KK., Frohlich F. Transcranial alternating current stimulation modulates large-scale cortical network activity by network resonance. J Neurosci. 2013;33:11262–11275. doi: 10.1523/JNEUROSCI.5867-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dingledine R., Dodd J., Kelly JS. The in vitro brain slice as a useful neurophysiological preparation for intracellular recording. J Neurosci Methods. 1980;2:323–362. doi: 10.1016/0165-0270(80)90002-3. [DOI] [PubMed] [Google Scholar]
- 21.Reato D., Rahman A., Bikson M., Parra LC. Low-intensity electrical stimulation affects network dynamics by modulating population rate and spike timing. J Neurosci. 2010;30:15067–15079. doi: 10.1523/JNEUROSCI.2059-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radman T., Su Y., An JH., Parra LC., Bikson M. Spike timing amplifies the effect of electric fields on neurons: implications for endogenous field effects. J Neurosci. 2007;27:3030–3036. doi: 10.1523/JNEUROSCI.0095-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bikson M., Inoue M., Akiyama H., et al Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J Physiol. 2004;557:175–190. doi: 10.1113/jphysiol.2003.055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McBain CJ., Traynelis SF., Dingledine R. Regional variation of extracellular space in the hippocampus. Science. 1990;249:674–677. doi: 10.1126/science.2382142. [DOI] [PubMed] [Google Scholar]
- 25.Nitsche MA., Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frohlich F., Bazhenov M., Iragui-Madoz V., Sejnowski TJ. Potassium dynamics in the epileptic cortex: new insights on an old topic. Neuroscientist. 2008;14:422–433. doi: 10.1177/1073858408317955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jefferys JG. Nonsynaptic modulation of neuronal activity in the brain: electric currents and extracellular ions. Physiol Rev. 1995;75:689–723. doi: 10.1152/physrev.1995.75.4.689. [DOI] [PubMed] [Google Scholar]
- 28.Fröhlich F., McCormick DA. Endogenous electric fields may guide neocortical network activity. Neuron. 2010;67:129–143. doi: 10.1016/j.neuron.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terzuolo CA., Bullock TH. Measurement of imposed voltage gradient adequate to modulate neuronal firing. Proc Natl Acad Sci U S A. 1956;42:687–694. doi: 10.1073/pnas.42.9.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guleyupoglu B., Schestatsky P., Edwards D., Fregni F., Bikson M. Classification of methods in transcranial Electrical Stimulation (tES) and evolving strategy from historical approaches to contemporary innovations. J Neurosci Methods. 2013;219:297–311. doi: 10.1016/j.jneumeth.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaghi S., Acar M., Hultgren B., Boggio PS., Fregni F. Noninvasive brain stimulation with low-intensity electrical currents: putative mechanisms of action for direct and alternating current stimulation. Neuroscientist. 2010;16:285–307. doi: 10.1177/1073858409336227. [DOI] [PubMed] [Google Scholar]
- 32.Tadini L., El-Nazer R., Brunoni AR., et al Cognitive, mood, and electroencephalographic effects of noninvasive cortical stimulation with weak electrical currents. J ECT. 2011;27:134–140. doi: 10.1097/YCT.0b013e3181e631a8. [DOI] [PubMed] [Google Scholar]
- 33.Paulus W. Transcranial electrical stimulation (tES - tDCS; tRNS, tACS) methods. Neuropsychol Rehabil. 2011;21:602–617. doi: 10.1080/09602011.2011.557292. [DOI] [PubMed] [Google Scholar]
- 34.Huang YZ., Sommer M., Thickbroom G., et al Consensus: new methodologies for brain stimulation. Brain Stimul. 2009;2:2–13. doi: 10.1016/j.brs.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunoni AR., Nitsche MA., Bolognini N., et al Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 2012;5:175–195. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bikson M., Datta A. Guidelines for precise and accurate computational models of tDCS. Brain Stimul. 2012;5:430–431. doi: 10.1016/j.brs.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Neuling T., Wagner S., Wolters CH., Zaehle T., Herrmann CS. Finite-element model predicts current density distribution for clinical applications of tDCS and tACS. Front Psychiatry. 2012;3:83. doi: 10.3389/fpsyt.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barker AT., Jalinous R., Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 39.Herrmann CS., Rach S., Neuling T., Struber D. Transcranial alternating current stimulation: a review of the underlying mechanisms and modulation of cognitive processes. Front Hum Neurosci. 2013;7:279. doi: 10.3389/fnhum.2013.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terney D., Chaieb L., Moliadze V., Antal A., Paulus W. Increasing human brain excitability by transcranial high-frequency random noise stimulation. J Neurosci. 2008;28:14147–14155. doi: 10.1523/JNEUROSCI.4248-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steriade M., Timofeev I., Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- 42.Steriade M., McCormick DA., Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 43.Marshall L., Helgadottir H., Molle M., Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 44.Clemens Z., Fabo D., Halasz P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132:529–535. doi: 10.1016/j.neuroscience.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 45.Holz J., Piosczyk H., Feige B., et al EEG Sigma and slow-wave activity during NREM sleep correlate with overnight declarative and procedural memory consolidation. J Sleep Res. 2012;21:612–619. doi: 10.1111/j.1365-2869.2012.01017.x. [DOI] [PubMed] [Google Scholar]
- 46.Kirov R., Weiss C., Siebner HR., Born J., Marshall L. Slow oscillation electrical brain stimulation during waking promotes EEG theta activity and memory encoding. Proc Natl Acad Sci U S A. 2009;106:15460–15465. doi: 10.1073/pnas.0904438106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaehle T., Rach S., Herrmann CS. Transcranial alternating current stimulation enhances individual alpha activity in human EEG. PLoS One. 2010;5:e13766. doi: 10.1371/journal.pone.0013766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neuling T., Rach S., Herrmann CS. Orchestrating neuronal networks: sustained after-effects of transcranial alternating current stimulation depend upon brain states. Front Hum Neurosci. 2013;7:161. doi: 10.3389/fnhum.2013.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brignani D., Ruzzoli M., Mauri P., Miniussi C. Is transcranial alternating current stimulation effective in modulating brain oscillations? PLoS One. 2013;8:e56589. doi: 10.1371/journal.pone.0056589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feurra M., Paulus W., Walsh V., Kanai R. Frequency specific modulation of human somatosensory cortex. Front Psychol. 201 1;2:13. doi: 10.3389/fpsyg.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wach C., Krause V., Moliadze V., Paulus W., Schnitzler A., Pollok B. The effect of 10 Hz transcranial alternating current stimulation (tACS) on corticomuscular coherence. Front Hum Neurosci. 2013;7:511. doi: 10.3389/fnhum.2013.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vanneste S., Walsh V., Van De Heyning P., De Ridder D. Comparing immediate transient tinnitus suppression using tACS and tDCS: a placebo-controlled study. Exp Brain Res. 2013;226:25–31. doi: 10.1007/s00221-013-3406-7. [DOI] [PubMed] [Google Scholar]
- 53.Meiron O., Lavidor M. Prefrontal oscillatory stimulation modulates access to cognitive control references in retrospective metacognitive commentary. Clin Neurophysiol. 2014;125:77–82. doi: 10.1016/j.clinph.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 54.Polania R., Nitsche MA., Korman C., Batsikadze G., Paulus W. The importance of timing in segregated theta phase-coupling for cognitive performance. Curr Biol. 2012:1314–1318. doi: 10.1016/j.cub.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 55.Struber D., Rach S., Trautmann-Lengsfeld SA., Engel AK., Herrmann CS. Antiphasic 40 Hz oscillatory current stimulation affects bistable motion perception. Brain Topogr. 2014;27:158–171. doi: 10.1007/s10548-013-0294-x. [DOI] [PubMed] [Google Scholar]
- 56.Brittain JS., Probert-Smith P., Aziz TZ., Brown P. Tremor suppression by rhythmic transcranial current stimulation. Curr Biol. 2013;23:436–440. doi: 10.1016/j.cub.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pogosyan A., Gaynor LD., Eusebio A., Brown P. Boosting cortical activity at beta-band frequencies slows movement in humans. Curr Biol. 2009;19:1637–1641. doi: 10.1016/j.cub.2009.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feurra M., Bianco G., Santarnecchi E., et al Frequency-dependent tuning of the human motor system induced by transcranial oscillatory potentials. J Neurosci. 2011;31:12165–12170. doi: 10.1523/JNEUROSCI.0978-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schutter DJ., Hortensius R. Brain oscillations and frequency-dependent modulation of cortical excitability. Brain Stimul. 2011;4:97–103. doi: 10.1016/j.brs.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 60.Kanai R., Paulus W., Walsh V. Transcranial alternating current stimulation (tACS) modulates cortical excitability as assessed by TMS-induced phosphene thresholds. Clin Neurophysiol. 2010;121:1551–1554. doi: 10.1016/j.clinph.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 61.Kanai R., Chaieb L., Antal A., Walsh V., Paulus W. Frequency-dependent electrical stimulation of the visual cortex. Curr Biol. 2008;18:1839–1843. doi: 10.1016/j.cub.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 62.Santarnecchi E., Polizzotto NR., Godone M., et al Frequency-dependent enhancement of fluid intelligence induced by transcranial oscillatory potentials. Curr Biol. 2013;23:1449–1453. doi: 10.1016/j.cub.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 63.Laczo B., Antal A., Niebergall R., Treue S., Paulus W. Transcranial alternating stimulation in a high gamma frequency range applied over V1 improves contrast perception but does not modulate spatial attention. Brain Stimul. 2012;5:484–491. doi: 10.1016/j.brs.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 64.Buzsaki G., Silva FL. High frequency oscillations in the intact brain. Prog Neurobiol. 2012;98:241–249. doi: 10.1016/j.pneurobio.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moliadze V., Antal A., Paulus W. Boosting brain excitability by transcranial high frequency stimulation in the ripple range. J Physiol. 2010;588:4891–4904. doi: 10.1113/jphysiol.2010.196998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Antal A., Boros K., Poreisz C., et al Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimul. 2008;1:97–105. doi: 10.1016/j.brs.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 67.Dwyer J., Lee H., Martell A., van Drongelen W. Resonance in neocortical neurons and networks. Eur J Neurosci. 2012;36:3698–3708. doi: 10.1111/ejn.12001. [DOI] [PubMed] [Google Scholar]
- 68.Ulrich D. Dendritic resonance in rat neocortical pyramidal cells. J Neurophysiol. 2002;87:2753–2759. doi: 10.1152/jn.2002.87.6.2753. [DOI] [PubMed] [Google Scholar]
- 69.Radman T., Ramos RL., Brumberg JC., Bikson M. Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul. 2009;2:215–228. doi: 10.1016/j.brs.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brunoni AR., Valiengo L., Baccaro A., et al The sertraline vs. electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry. 2013;70:383–391. doi: 10.1001/2013.jamapsychiatry.32. [DOI] [PubMed] [Google Scholar]
- 71.Kalu UG., Sexton CE., Loo CK., Ebmeier KP. Transcranial direct current stimulation in the treatment of major depression: a meta-analysis. Psychol Med. 2012;42:1791–1800. doi: 10.1017/S0033291711003059. [DOI] [PubMed] [Google Scholar]
- 72.Berlim MT., Van den Eynde F., Daskalakis ZJ. Clinical utility of transcranial direct current stimulation (tDCS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and shamcontrolled trials. J Psychiatr Res. 2013;47:1–7. doi: 10.1016/j.jpsychires.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 73.Brunelin J., Mondino M., Gassab L., et al Examining transcranial directcurrent stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry. 2012;169:719–724. doi: 10.1176/appi.ajp.2012.11071091. [DOI] [PubMed] [Google Scholar]


