Abstract
Context
Knee osteoarthritis (OA), a disorder of cartilage and periarticular bone, is a public health problem without effective medical treatments. Some studies have suggested that vitamin D may protect against structural progression.
Objective
To determine if vitamin D supplementation reduces symptom and structural progression of knee OA
Design, Setting and Patients
The study was an NIH-funded, randomized, placebo-controlled, double-blind, clinical trial of 146 participants (mean age 62.4 (SD 8.5), 57 (61%) females, 115 (79%) whites) with symptomatic knee OA, performed at Tufts Medical Center between March 2006 – June 2009, testing a 2-year vitamin D intervention.
Intervention
Participants were randomized to either placebo, or oral cholecalciferol 2000 IU daily with dose escalation to elevate serum levels to >36 ng/ml.
Main Outcome Measures
Primary outcomes were knee pain severity (WOMAC pain, 0–20, 0=no pain and 20=extreme pain), and cartilage volume loss measured by magnetic resonance imaging. Secondary endpoints included physical function, knee function (WOMAC function, 0–68, 0=no difficulty and 68=extreme difficulty), cartilage thickness, bone marrow lesions and radiographic joint space width.
Results
Completion rate was 85%. Serum 25-hydroxyvitamin-D (25OHD) increased in the treatment arm (mean increase 16.1 ng/ml [95% CI, 13.7, 18.6]) vs. placebo (mean 2.1, [95% CI, 0.5, 3.7]), p<0.0001. Baseline knee pain was slightly worse in the treatment group (mean 6.9, [95% CI, 6.0, 7.7]) vs. placebo (mean 5.8, [95% CI, 5.0, 6.6], p=0.08). Baseline knee function was significantly worse in the treatment group (mean 22.7, [95% CI, 19.8, 25.6] than placebo (mean 18.5, [95% CI 15.8, 21.2], p=0.04). Knee pain decreased in both groups (vitamin D (mean, −2.3 [95% CI, −3.2, −1.4]); placebo mean (−1.5 [95% CI, −2.3, −0.6])) with no significant differences at any time point. Cartilage volume (%) decreased by the same extent in both groups (mean −4.3 [95% CI, −5.5, −3.1] vs. −4.3 [95% CI, −6.1, −2.4], p = 0.96). There were no differences in any of the secondary clinical endpoints.
Conclusions
Vitamin D supplementation for 2 years at a dose sufficient to elevate plasma levels of 250HD to > 36 ng/ml, when compared with placebo, did not reduce knee pain or cartilage volume loss in patients with symptomatic knee osteoarthritis.
Trial Registration
The study was registered on ClinicalTrials.gov (NCT00306774).
Introduction
Knee osteoarthritis (OA) is a common age-related musculoskeletal disorder that has significant functional impact and has considerable societal costs through work loss, early retirement and arthroplasty1–4. Despite its impact, there are no medical treatments established to influence the course of the disease.
Pathological changes in subchondral and periarticular bone, ranging from trabecular thickening to gross pathological disruption5, are prominent in OA and participate in disease progression6. Since the periarticular bone is a primary contributor to dispersion of loading forces across the joint5, 7–9, such changes likely further predispose to OA progression10.
The basis for considering that vitamin D might influence the course of knee OA arose from its known role in bone health, the importance of systemic11 and local bone changes in OA, and epidemiologic observations from some studies suggesting slower rates of OA progression among those with higher vitamin D levels12, 13.
Therefore, our goal was to determine through performance of a clinical trial if vitamin D supplementation reduces symptomatic and structural progression of knee OA.
Methods
Overview
This was a single center, randomized, placebo-controlled, double-blind, clinical trial with a planned enrollment of 144 participants with symptomatic knee OA, testing the efficacy of a 2-year vitamin D intervention strategy for knee pain and cartilage loss, measured by magnetic resonance imaging (MRI). The study was performed at Tufts Medical Center between March 2006 and June 2009. It was funded by a grant from the National Institutes of Health (R01 AR51361) and approved by the Institutional Review Board of Tufts Medical Center (protocol #7336) and registered on ClinicalTrials.gov (NCT00306774). All patients provided written informed consent for participation in the trial.
Sample
We recruited from among patients at Tufts Medical Center, and through advertisements in local newspapers, public transportation systems and radio stations. A sequential method of screening was implemented. A telephone pre-screen assessed knee pain, planned knee or hip surgery, participation in another study, and comorbidities. Subsequent screening involved a visit that included knee radiographs and a blood test. Eligible individuals were aged 45 years or older with symptomatic knee OA, based on an affirmative response to a standardized question about chronic knee pain14 and the presence of at least one osteophyte on a recent knee radiograph (equivalent to Kellgren and Lawrence grade 215). Individuals meeting these criteria fulfill American College of Rheumatology classification criteria for knee OA16. They also had to report at least mild pain on one of the weight-bearing questions of the Western Ontario and McMaster Universities (WOMAC) pain subscale17, and have knee pain or discomfort referable to the knee joint confirmed on a physical examination.
Exclusion criteria included daily supplemental intake of vitamin D >800 IU, serum calcium >10.5 mg/dL, hypercalcuria (spot urine calcium: creatinine ratio of >0.4), use of supplements or medications with purported effects on cartilage (e.g. glucosamine), intra-articular therapies within 3 months, and chronic oral corticosteroid use. Exclusionary comorbidities included lymphoma, sarcoidosis, tuberculosis, hyperparathyroidism, malabsorption disorders, glomerular filtration rate <30, history of inflammatory joint disease, pregnancy and any that precluded MRI.
Participants self-identified their race and ethnicity using the United States Census Bureau system.
Study Knee
We chose the knee with more severe disease based on the WOMAC pain score and radiographic grade, or, if these were identical, by randomization.
Randomization
We operated a stratified randomization system by KL grade (2, 3, 4), with 1:1 assignments permuted in blocks of 6. The randomization list was generated by the study statistician (ML) using SAS version 9.1 (SAS Institute Inc., Cary, NC), and provided to the research pharmacy at TMC. This list was concealed from the investigative team.
Study Intervention and Dose Adjustment Protocol
We purchased cholecalciferol 2,000 IU and identical placebo capsules from Tishcon Corporation, NY. The pills were made according to Good Manufacturing Principles and subjected to quality assurance testing. The initial dose was 2,000 IU daily, with subsequent adjustment in 2000 IU increments at the 4, 8, and 12 months for a target 25OHD level between 36 – 100 ng/ml, the lower level based on the cutpoint in prior epidemiologic studies at which vitamin D appeared to have an effect12, 13. Participants were not given calcium; however, they were given advice on optimal calcium intake.
Toxicity Monitoring and Safety Procedures
Oversight was provided by a Data and Safety Monitoring Board whose members were appointed by NIAMS. We obtained serum and urinary calcium levels and 25OHD levels at each visit. We performed surveillance for hypercalcemia (calcium≥10.5 mg/dl), hypercalcuria (calcium: creatinine ratio < 0.4) and hypervitaminosis D (>100 ng/ml). This permitted dose adjustment for hypercalcuria or hypervitaminosis D, but mandated withdrawal for hypercalcemia.
Masking of Treatment Assignment
Labeling and dispensation of study pills was performed by the TMC research pharmacy. Monitoring of vitamin D and calcium laboratory test results was performed by TMC Pathology Department staff, independently of the clinical team. Their reporting relationship was confined to the study statistician (ML), who was located at another Institution and was responsible for coordinating actions triggered by abnormal results. In order to maintain blinding in the event that a participant required a dose adjustment, we operated a double-dummy protocol, in which the statistician (ML) would select a control participant to form a vitamin D-placebo pair for a simultaneous dose change.
Adherence and concomitant analgesic use
We provided pill diaries to participants and performed pill counts at each visit. The use of concomitant nonsteroidal anti-inflammatory agents and analgesics was allowed and was recorded at each visit and in the daily logs.
Study assessments
Assessments occurred at a baseline/randomization visit and at months 2, 4, 8, 12, 16, 20, and 24. The clinical assessments included a musculoskeletal examination, WOMAC questionnaire (pain subscale range 0–20, 0=no pain, minimal clinically important improvement 3.9418; function subscale 0 – 68, 0 = no difficulty with daily activities, minimal clinically important improvement 6.66), adverse event ascertainment, pill counts, serum calcium and 25OHD, and spot urinary calcium: creatinine ratio. Physical function tests (timed 20-meter walk and chair rise test), and short form (SF)-36 questionnaire were collected at baseline, as well as 12- and 24-months.
Imaging included standardized semiflexed postero-anterior knee radiographs19 at baseline and 24 months, knee and hip dual x-ray absorptiometry (DXA; GE Lunar Prodigy Scanner) and magnetic resonance imaging (MRI) scans of the study knee at baseline, 12, and 24 months. The MRIs were obtained on a Siemens Avanta 1.5 Tesla scanner using a transmit-receive extremity coil, according to a standardized protocol that included a foot-positioning device. The sequences of relevance for bone marrow lesion (BML) assessment were sagittal, coronal, and axial intermediate-weighted (IW) fat-suppressed (FS) images with time to recovery (TR) of 2950 ms, time to echo (TE) of 31 ms, slice thickness of 3 mm, space thickness of 0.5 mm, and field of view (FOV) of 140 mm. The sequences of relevance for cartilage volume assessment were 3-dimensional sagittal water excitation dual echo steady state (DESS WE) images with TR of 18.2 ms, TE of 5.28 ms, slice thickness of 1.3 mm, and FOV of 140 mm. Finally, sagittal and coronal IW sequences were collected with TR of 2500 ms, TE of 40 ms, slice thickness of 3 mm, space thickness of 0.5 mm, and FOV of 140 mm.
MRI Cartilage Analysis
We measured cartilage parameters in the tibia and femur within the index compartment of each knee, defined as the compartment with predominant pathology.
We delineated the 3-dimensional cartilage segments using ANALYZE™ (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN) and eFilm™ (Merge Healthcare, Milwaukee, WI) and then used a customized program in MatLab (The MathWork, Natick, MA) to compute the cartilage metrics. In order to optimize sensitivity to change, we registered the baseline and follow-up images and specifically evaluated cartilage loss (not gain).
The reliability of knee cartilage volume measurements using MRI has been well documented20. In our hands, the intra-acquisition CVs were 1.7% for medial tibial and 1.4% for medial femoral cartilage, and the inter-acquisition CVs were 3.9% for medial tibial and 1.3% for medial femoral cartilage, which is within the range of reproducibility documented by other investigators20. We also tested the segmentation-resegmentation reproducibility for measurement of longitudinal cartilage volume loss on a convenience sample of 10 baseline and 2-year follow-up knee MRI pairs (20 image sets). The intraclass correlation coefficients between the first and second analyses of cartilage loss were excellent (0.96 for medial femoral and 0.93 for medial tibial).
MRI Bone Marrow Lesion (BML) Measurements
We measured manually the dimensions of each BML using the sagittal and coronal intermediate-weighted fat-suppressed sequences according to a method we previously validated21. The intra-tester reliability (intraclass correlations [3,1 model])22 for this approach were 0.90 to 0.96 for volume and volume change.
Peri-articular tibial bone mineral density (BMD) measurement
We performed Dual X-Ray absorptiometry of the knees (GE Lunar Prodigy) and defined tibial subchondral regions of interest (ROIs) according to a standardized protocol and calculated a medial:lateral tibial BMD ratio23. The reproducibility of this measurement was good (scan-rescan intra-class correlation coefficient (ICC) 0.96; coefficient of variation (CV) 1.46%).
Evaluation of radiographic severity
We evaluated knee radiographs for global severity using the KL scale15, operated as follows: grade 1: doubtful joint space narrowing (JSN) and possible osteophytes; 2: definite JSN (<50%) and osteophytes; 3: moderate JSN (50%), osteophytes, sclerosis and possible deformity of bone contour; 4: severe JSN (>50%), sclerosis and deformity of bone contour. We measured radiographic knee joint space width (JSW) using semi-automated software24 and static alignment according to a validated method25.
Vitamin D Analyses
Plasma 25OHD was measured at Tufts Medical Center by liquid chromatography, tandem mass spectrometry (LC/MS/MS) (Waters Acquity UPLC with TQD triple quadrupole mass spectrometer). In quality control testing, our measurements correlated at 0.994 with the National Institute of Standards and Technology (NIST) external standards. This assay’s sensitivity is <2.0 ng/mL and inter-assay CVs are 6.5–11% for 25-hydroxyvitamin D3.
Analytic plan
Our co-primary outcomes were the WOMAC knee pain subscale and MRI cartilage volume loss. We analyzed the WOMAC pain scores across time using mixed effects regression models for longitudinal repeated measures 26, after first evaluating the effect of time and correlation26. Likelihood ratio tests exhibited significant improvement in goodness-of-fit when a quadratic term for time was included in the model. Therefore, the repeated measures model ultimately included baseline KL grade, a quadratic time effect, treatment, and the interaction between time and treatment; the correlation within the repeated WOMAC scores was addressed by the random intercept. In the repeated measures models, the effect of treatment is captured by the time by treatment interaction and likelihood ratio (LR) tests were used to test for the significance of this.
For structural endpoints, we analyzed the difference between baseline and follow-up using general linear models. Models were adjusted by KL score since randomization had been stratified by KL score. Multiple imputation was used to evaluate the changes from baseline to study end in clinical and structural outcomes using the MI and MIANALYZE procedures in SAS. Imputations were performed separately for each treatment group and each outcome, using the baseline and 2 year measured outcome values, KL score, gender, age, race, and baseline values of body mass index and serum vitamin D. We performed secondary subgroup analyses among those with (i) low baseline 25OHD (≤15 ng/ml) (ii) sustained vitamin D response (25OHD level >40 ng/ml at both 12 and 24 months), (iii) normal knee alignment, and (iv) mild OA (Kellgren-Lawrence grade 2).
To compare the number of adverse events across treatment groups, allowing for multiple events and clustering by subjects, we used the negative binomial model, which can be formulated as a Poisson regression with a random effect for subjects27.
All analyses were performed 27SAS 9 (Cary, NC). Two-sided p-values <0.05 were considered statistically significant, and were not adjusted to account for multiple comparisons.
Power Computations
This study was designed to enroll 144 subjects at baseline (72 per arm), anticipating 20% dropout and 114 subjects completing the study. We estimated the potential effect of vitamin D on cartilage loss by modeling the rates of progression observed in the Framingham cohort13 and extrapolating this to cartilage loss using equivalence data generated by Cicuttini et al28. This allowed us to translate radiographic measures of progression into cartilage volume loss. Thus, we expected 201 μm3 cartilage loss per year in placebo (corresponding to a 5.3% reduction) and 115 μm3 (3% reduction) in vitamin D subjects13, 29, corresponding to a 43% reduction in the percent cartilage loss. In simulations with 114 subjects we obtained 80% power to detect this difference between groups in a random effects analysis. For change in WOMAC pain, measured on a scale from 0 to 20, we anticipated a standard deviation of 4.130. With 114 subjects a difference between groups of 2.2 units on the scale (or an effect size of 0.54) is detectable with 80% power.
Results
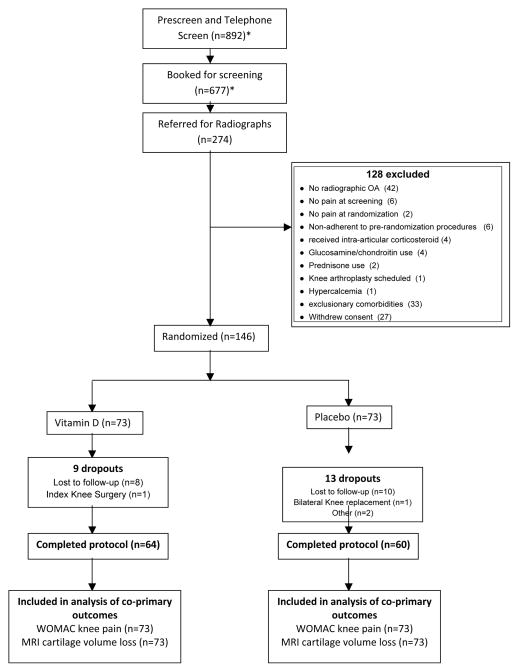
We randomized 146 participants from 274 in-person screens (Figure 1), exceeding our targeted recruitment by 2 due to timing of enrollment. The group assigned to vitamin D had slightly more severe disease, with higher scores for WOMAC pain (6.9 vs. 5.8, [95% CI of difference, −0.1, 2.2], p=0.08) and WOMAC function (22.7 vs. 18.5, [95% CI of difference, 0.3, 8.1], p=0.04), and less femoral cartilage volume (Table 1).
Figure 1. Flow of Participant Screening, Enrollment, and Participation.
*Reasons for failing the prescreen and the screening visits were not systematically recorded
Table 1.
Participant Characteristics at Baseline
| Vitamin D (N=73) | Placebo (N=73) | p-value | |
|---|---|---|---|
| Age (y), mean (SD) | 61.8 (7.7) | 63.0 (9.3) | 0.41 |
| Gender (female), n (%) | 49 (67%) | 40 (54%) | 0.13 |
| Race, n (%) | 0.07 | ||
| White | 52 (71%) | 63 (86%) | |
| Asian | 2 (3%) | 2 (3%) | |
| Black | 16 (22%) | 8 (11%) | |
| Other | 3 (4%) | 0 (0%) | |
| Taking vitamin D supplements, n (%) | 42 (59%) | 41 (56%) | 0.72 |
| WOMAC pain, mean (SD) | 6.9 (3.8) | 5.8 (3.4) | 0.08 |
| WOMAC function, mean (SD) | 22. 7 (12.3) | 18.5 (11.7) | 0.04 |
| BMI, kg/m2, mean (SD) | 30.5 (5.0) | 30.8(6.4) | 0.73 |
| Femoral neck BMD, mean (SD) | 0.9 (0.1) | 1.0 (0.1) | 0.43 |
| K/L Score, n (%) | 0.93 | ||
| 2 | 36 (49%) | 37 (51%) | |
| 3 | 22 (30%) | 20 (27%) | |
| 4 | 15 (21%) | 16 (22%) | |
| Malalignment, n (%) | 49 (67%) | 49 (67%) | 0.99 |
| Chair stand, mean seconds (SD) | 19.8 (7.2) | 18.6 (6) | 0.26 |
| 20-m walk, mean seconds (SD) | 16.8 (4.8) | 16.4 (4.4) | 0.67 |
| Tibial cartilage volume (mm3), mean (SD) | 1010 (437) | 1147.8 (472.8) | 0.09 |
| Tibial cartilage thickness (mm2), mean (SD) | 1.2 (0.4) | 1.2 (0.4) | 0.58 |
| Tibial BML (cm3) | 15.8 (28.0) | 13.2 (19.0) | 0.56 |
| Tibial BML (cm3) median, [25th,75th percentile] | 1.5 [0.0, 14.3] | 4.4 [0.0, 18.3] | 0.69 |
| Femur cartilage volume (mm3), mean (SD) | 4212 (1349) | 4740 (1273) | 0.03 |
| Femur cartilage thickness (mm2), mean (SD) | 1.8 (0.4) | 1.8 (0.3) | 0.28 |
| Femur BML (cm3) | 8.5 (15.0) | 9.6 (14.0) | 0.68 |
| Femur BML (cm3) median, [25th,75th percentile] | 1.3 [0.0, 10.7] | 2.3 [0.0, 15.2] | 0.75 |
| Plasma 25OHD, ng/ml, mean (SD) | 22.7 (11.4) | 21.9 (8.3) | 0.62 |
| JSW mean (SD) | 5.0 (1.8) | 5.1 (1.7) | 0.66 |
The WOMAC questionnaire asks questions about knee-specific pain (range 0–20, 0=no pain) and knee-specific function (range 0 – 68, 0 = no pain with activity)
Eighty-eight percent of the vitamin D arm and 82% of the placebo arm completed the intervention. Twenty-four participant pairs received vitamin D dose changes as follows: 18 pairs to 4000 IU per day, 4 pairs to 6000 IU, 1 pair to 8000 IU. One participant pair received a dose reduction to 0 IU. The mean plasma 25OHD level rose in the treatment group from 22.7 to 38.5 ng/ml at 24 months compared to 21.9 to 24.7 in the placebo arm (mean change 16.1 ng/ml [95% CI, 13.7, 18.6] vs. placebo (mean 2.1, [95% CI, 0.5, 3.7], p<0.0001). Overall, 61.3% of the treatment group and 8.3% of the placebo group reached the target level of 36 ng/ml by month 24 ([95% CI of difference, 39.3%, 66.7%], p<0.0001). Based on pill counts during the time the participant was active in the study, mean adherence was 96% for treatment and 97% for placebo.
Knee pain fell by about 2 units in both groups (Table 2) and the effect of treatment over time was not significant in the quadratic mixed effects model (LR chi-square statistic = 2.8 on 2 degrees of freedom [DF], p=0.22) (Figure 2). Results were similar for the effect of treatment in the secondary models using a linear time trend (p=0.65 LR chi-square =0.2 on 1 DF), and with visit as a categorical factor (p=0.56 LR chi-square =4.9 on 6 DF). Similarly, there were no evident differences between groups in the secondary clinical endpoints (Table 2)
Table 2.
Two year changes in the clinical outcomes (mean (95% CI))
| Vitamin D | Placebo | Between-group difference | p-value | |
|---|---|---|---|---|
| WOMAC Pain [0 – 20] | −2.31 (−3.24, −1.38) | −1.46 (−2.33, −0.60) | −0.87 (−2.12, 0.38) | 0.17 |
| WOMAC Function [0 – 68] | −6.97 (−9.76, −4.18) | −3.82 (−5.96, −1.68) | −3.11 (−6.52, 0.30) | 0.07 |
| Chair Stand, seconds | −1.25 (−2.74, 0.24) | −0.93 (−2.77, 0.92) | −0.32 (−2.87, 2.23) | 0.80 |
| 20 Meter Walk, seconds | 0.09 (−0.56, 0.75) | −0.24 (−1.03, 0.55) | 0.34 (−0.69, 1.37) | 0.52 |
All analyses are comparing baseline vs. 2 year outcomes. The results in this table were generated from mixed models on an imputed dataset, adjusted for Kellgren-Lawrence score.
Figure 2.

Plot of average WOMAC pain at study visits by treatment group. Results for the vitamin D group are shown by the red curve, and placebo group results by the black. 95% confidence intervals for the averages are shown by vertical bars. Numbers of subjects are shown at the bottom of the plotting area in red for the vitamin D group and in black for the placebo group. Visit weeks are slightly offset horizontally to allow viewing results for both groups.
There was about 4% loss of cartilage volume over the 2-year period in both groups and this was consistent for the tibial and femoral segments and similar in both arms (Table 3). There was also no significant between-group difference in change in cartilage thickness, BML size, or radiographic JSW.
Table 3.
Two year changes in structural outcomes in the index compartment of the study knee (mean (95% CI))
| Vitamin D | Placebo | Between-group difference | p | |
|---|---|---|---|---|
| Combined Cartilage Volume (mm3) | −205.83 (−253.58, −158.08) | −222.98 (−269.54, −176.43) | 17.15 (−52.26, 86.56) | 0.61 |
| Combined Cartilage Volume (%) | −4.30 (−5.48,−3.12) | −4.25 (−6.12, −2.39) | −0.05 (−1.91, 1.82) | 0.96 |
| Tibial Cartilage Volume (mm3) | −39.38 (−47.76, −31.00) | −41.66 (−51.02, −32.29) | 2.28 (−9.99, 14.55) | 0.71 |
| Tibial Cartilage Volume (%) | −4.62 (−5.67, −3.57) | −4.35 (−5.41, −3.28) | −0.27 (−1.62, 1.08) | 0.69 |
| Tibial Cartilage Thickness (mm2) | −0.05 (−0.07, −0.04) | −0.05 (−0.07, −0.03) | −0.01 (−0.03, 0.01) | 0.45 |
| Tibial BML size (cm3) | −0.65 (−5.43, 4.13) | −3.04 (−10.17, 4.10) | 2.38 (−4.03, 8.80) | 0.46 |
| Femur Cartilage Volume (mm3) | −168.05 (−199.76, −136.33) | −178.11 (−218.21, −138.02) | 10.07 (−44.38, 64.51) | 0.71 |
| Femur Cartilage Volume (%) | −3.91 (−5.45, −2.38) | −3.93 (−5.14, −2.72) | 0.02 (−1.62, 1.66) | 0.98 |
| Femur Cartilage Thickness (mm2) | −0.06 (−0.08, −0.05) | −0.06 (−0.07, b−0.05) | −0.00 (−0.02, 0.02) | 0.78 |
| Femur BML size (cm3) | −0.39 (−3.96, 3.19) | −2.33 (−5.60, 0.94) | 1.95 (−2.78, 6.68) | 0.42 |
| JSW (mm) | −0.35 (−0.54, −0.15) | −0.22 (−0.42, −0.03) | −0.12 (−0.38, 0.14) | 0.35 |
All analyses are comparing baseline vs. 2 year outcomes. The results in this table were generated from mixed models on an imputed dataset, adjusted for Kellgren-Lawrence score.
In the subset analyses for the WOMAC pain outcome, the effects were generally similar, and non-significant, albeit slightly larger among those with a low baseline 25OHD (change in pain −2.7 vs. −1.0, [95% CI of difference, −5.3, 1.9], p = 0.36, effect size = 0.4) and those with normal knee alignment (−1.9 vs. −0.1, [95% CI of difference, −4.2, 0.5], p=0.13, effect size = 0.5). For the cartilage volume outcome, results of the subset analyses were also non-significant, albeit with slightly greater effects among those with low baseline vitamin D (change in cartilage volume −170 vs. −264, 95% CI of difference, −59, 246], p=0.35, effect size = 0.5) and those who had a sustained response in the vitamin D level (−155 vs. −225, [95% CI of difference, −23, 165], p=0.15, effect size = 0.5).
There were 31 serious adverse events in the vitamin D and 23 in the placebo group but the number of participants who experienced an event was 16 in each group. All except one were considered unrelated, a ‘possibly related’ hip fracture. There were no episodes of hypercalcemia, and the numbers hypercalcuria or kidney stones were comparable (6 vs. 4 and 1 vs. 1). The number of participants with adverse events in each group was similar (64 vs. 63%). There were more endocrine (6 vs. 1 participants) and musculoskeletal (41 vs. 30) events in the vitamin D group. However, after accounting for clustering within participants, the differences in adverse event rates were not significant (beta estimate= −0.12, [95% CI, −0.26, 0.03], p=0.1).
The percentage of participants reporting use of nonsteroidal anti-inflammatory analgesics (NSAIDs) and opioids at any visit was 54% and 6%, respectively. For each visit, the participants in the treatment arm reported higher use of NSAIDs, but this reached statistical significance only at the 16-month visit (40% vs. 22%, [95% CI of difference 0.02. 0.34], p=0.02). There were no significant differences in opioid use at any visit.
Discussion
This study was predicated on the prominent participation of periarticular bone in OA, the known benefits of vitamin D on bone health, and epidemiologic studies that suggested that individuals with knee or hip OA and low levels of vitamin D have increased risk for structural progression12, 13. However, additional results from epidemiologic studies that emerged during the course of this study have been mixed demonstrating positive31, 32 and negative associations33. Two studies appeared to show strong associations of bone density with the development of knee OA11, 31, but some of those investigators later published concerns about the possibility of such associations arising as a result of contingent confounding34. Therefore, together with the results of this clinical trial, the overall data suggest that vitamin D supplementation at a dose sufficient to elevate 25OHD levels to >36 ng/ml does not have major effects on clinical or structural outcomes in knee OA, at least in a U.S. sample.
One concern in inferring a negative result is the possibility of type 2 error. While our measurement precision was good, the amount of cartilage loss we observed was smaller than expected, and this may have impaired out ability to detect a difference. Also, there was a small difference in change in pain that favored the treated group (effect size ~0.2), which was of larger magnitude among those with low vitamin D levels at baseline, and with normal mechanical knee alignment (effect size = ~0.4). However, these effects are much smaller than the study was originally designed to detect, and could be just be due to chance..
Other possible explanations for a negative result may be that individuals in the source population were replete in vitamin D, or that the intervention was insufficient, or that placebo participants took supplements. However, the levels of 25OHD in our participants were similar to prior samples12, 13, 33. Further, the mean vitamin D level did not rise in the placebo group, in contrast with the treated group. The cutpoint of 36 ng/ml was based on observational studies that had shown effects above this level12, 13, and 60% of our participants in the treatment arm achieved this target. A sensitivity analysis confined to the subset that exhibited a sustained response in vitamin D levels did not find a significant difference between groups. Thus, although there is a theoretical possibility that greater doses (or higher blood levels) of vitamin D are needed to exert a therapeutic effect, our data do not support this supposition.
Another question is whether a 2-year duration was sufficient. The original epidemiologic studies had observation periods of up to 8 years and so it is possible that small incremental benefits could take more time to accrue into a measurable outcome. Indeed, it may be informative that the observational studies with a negative result for knee OA were of shorter duration, and that even in osteoporosis studies the effect of vitamin D on whole body bone loss is extremely modest35.
We included individuals with KL grade 4 knee OA, which indicates fairly severe structural damage. This was intended to extend generalizability of our results to a stratum of the OA population who experience the greatest level of pain and health burden, however, there is also a risk of biasing results to the null through ceiling effects, or if therapeutic intervention is futile in this subset. Note, however, that we did not find evidence for this in stratified analyses.
Although MRI has provided a breakthrough in evaluation of OA structural pathology36, the post-acquisition image analysis is highly burdensome, and so we confined the segmentation to the involved compartment of the knee. This eliminated an opportunity to observe changes in other locations, but the clinical relevance of changes in those locations in the absence of a signal in the involved compartment would be difficult to interpret. It is reassuring in this regard that other knee OA clinical trials that utilized whole joint cartilage measurements exhibited little gain in statistical power for total versus medial compartment cartilage volume change37.
The optimal cartilage measurement approaches are still a topic of research and discussion38, with more recent work indicating that cartilage loss may be highly focal, favoring thickness and denudation measurements over total cartilage volume39. Furthermore, non-cartilaginous pathologies, such as bone marrow lesions, appear to relate more strongly to symptomatology. However, our secondary analyses using quantitative measurements of these features did not reveal any differences between the groups. With respect to measurement of more global aspects of knee OA structural damage, we had initially proposed to use a semi-quantitative visual rating scale, however, in preliminary analyses of our data we found that those instruments had substantially inferior sensitivity to change. Therefore, we opted for quantitative measurements of cartilage and bone marrow lesions.
In summary, the results of this trial together with recent observational data indicate that vitamin D does not have a major effect on knee OA symptoms or progression among individuals who have a 25OHD level over 15 ng/ml.
Acknowledgments
Timothy McAlindon had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Michael LaValley and Lori Lyn Price performed and are responsible for the statistical analyses for this study. We would like to thank the participants who made this study possible; also the following professionals for their uncompensated help with this study - Gayle Lester PhD, NIAMS for guidance and advice, Arthur Rabson MD, TMC, for surveillance of safety laboratory test monitoring, Cheryl Garganta MD PhD, TMC, for plasma vitamin D analyses, Eric Miller PhD, Tufts University School of Engineering, for image analysis advice, Jeff Duryea PhD, Brigham and Women’s Hospital, for radiographic measurements, and the Data and Safety Monitoring Board for their assistance with and oversight of this trial. The MRIs were performed under a contract by Longwood MRI. This study was performed with funding support from the National Institute for Arthritis (R01 AR051361) and Musculoskeletal Disorders, and the Office for Dietary Supplements, and was supported by grant number UL1 RR025752 from the National Center for Research Resources (NCRR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The study was registered on ClinicalTrials.gov (NCT00306774).
The funding sponsors played no part in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Sponsors had no access to the data and did not perform any of the study analysis.
Contributor Information
Timothy McAlindon, Email: tmcalindon@tuftsmedicalcenter.org, Division of Rheumatology, Tufts Medical Center, Box 406, 800 Washington Street, Boston, MA 02111, Phone: 617 636 5645, Fax: 617 636 5219.
Michael LaValley, Boston University School of Public Health, 801 Massachusetts Avenue, Crosstown Center, Boston MA 02118.
Erica Schneider, Imaging Institute, Cleveland Clinic Foundation, Cleveland, Ohio.
Melynn Nuite, Division of Rheumatology, Tufts Medical Center, Box 406, 800 Washington Street, Boston, MA 02111.
Ji Yeon Lee, Division of Rheumatology, Tufts Medical Center, Box 406, 800 Washington Street, Boston, MA 02111.
Lori Lyn Price, Biostatistics Research Center in the Institute for Clinical Research and Health Policy Studies (ICRHPS), Tufts Medical Center, Box 63, 800 Washington Street, Boston, MA 02111.
Grace Lo, Michael E. DeBakey Veterans Affairs Medical Center, Medical Care Line and Research Care Line; Houston Health Services Research and Development (HSR&D) Center of Excellence, Department of Medicine, Baylor College of Medicine, Houston, TX, Baylor College of Medicine, 1 Baylor Plaza, BCM 285 Houston, TX 77030.
Bess Dawson-Hughes, Jean Mayer USDA HNRCA at Tufts University, 711 Washington Street, Boston, MA 02111.
References
- 1.Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis and Rheumatism. 2008;59:1207–13. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Incidence and Prevalence Database for Procedures. Sunnyvale, CA: Timely Data Resources; 1995. The Incidence and Prevalence Database for Procedures; pp. 592–6. [Google Scholar]
- 3.Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84:351–8. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kosorok MR, Omenn GS, Diehr P, Koepsell TD, Patrick DL. Restricted activity days among older adults. Am J Public Health. 1992;82:1263–7. doi: 10.2105/ajph.82.9.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burr DB. The importance of subchondral bone in the progression of osteoarthritis. J Rheumatol Suppl. 2004;70:77–80. [PubMed] [Google Scholar]
- 6.Dieppe P, Cushnaghan J, Young P, Kirwan J. Prediction of the progression of joint space narrowing in osteoarthritis of the knee by bone scintigraphy. Ann Rheum Dis. 1993;52:557–63. doi: 10.1136/ard.52.8.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radin EL, Martin RB, Burr DB, Caterson B, Boyd RD, Goodwin C. Effects of mechanical loading on the tissues of the rabbit knee. J Orthop Res. 1984;2:221–34. doi: 10.1002/jor.1100020303. [DOI] [PubMed] [Google Scholar]
- 8.Clark JM, Huber JD. The structure of the human subchondral plate. J Bone Joint Surg Br. 1990;72:866–73. doi: 10.1302/0301-620X.72B5.2211774. [DOI] [PubMed] [Google Scholar]
- 9.Hoshino A, Wallace WA. Impact-absorbing properties of the human knee. J Bone Joint Surg Br. 1987;69:807–11. doi: 10.1302/0301-620X.69B5.3680348. [DOI] [PubMed] [Google Scholar]
- 10.Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res. 1986:34–40. [PubMed] [Google Scholar]
- 11.Zhang Y, Hannan MT, Chaisson CE, McAlindon TE, Evans SR, Aliabadi P, et al. Bone mineral density and risk of incident and progressive radiographic knee osteoarthritis in women: the Framingham Study. J Rheumatol. 2000;27:1032–7. [PubMed] [Google Scholar]
- 12.Lane NE, Gore LR, Cummings SR, Hochberg MC, Scott JC, Williams EN, et al. Serum vitamin D levels and incident changes of radiographic hip osteoarthritis: a longitudinal study. Study of Osteoporotic Fractures Research Group. Arthritis Rheum. 1999;42:854–60. doi: 10.1002/1529-0131(199905)42:5<854::AID-ANR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 13.McAlindon TE, Felson DT, Zhang Y, Hannan MT, Aliabadi P, Weissman B, et al. Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham Study. Ann Intern Med. 1996;125:353–9. doi: 10.7326/0003-4819-125-5-199609010-00001. [DOI] [PubMed] [Google Scholar]
- 14.O’Reilly SC, Muir KR, Doherty M. Screening for pain in knee osteoarthritis: which question? Annals of the Rheumatic Diseases. 1996;55:931–3. doi: 10.1136/ard.55.12.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kellgren JH, Lawrence JS. The epidemiology of chronic rheumatism: atlas of standard radiographs. Oxford, UK: Blackwell Scientific; 1962. [Google Scholar]
- 16.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 17.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 18.Stratford PW, Kennedy DM, Woodhouse LJ, Spadoni GF. Measurement properties of the WOMAC LK 3. 1 pain scale. Osteoarthritis Cartilage. 2007;15:266–72. doi: 10.1016/j.joca.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Buckland-Wright JC, Wolfe F, Ward RJ, Flowers N, Hayne C. Substantial superiority of semiflexed (MTP) views in knee osteoarthritis: a comparative radiographic study, without fluoroscopy, of standing extended, semiflexed (MTP), and schuss views. J Rheumatol. 1999;26:2664–74. [PubMed] [Google Scholar]
- 20.Eckstein F, Cicuttini F, Raynauld JP, Waterton JC, Peterfy C. Magnetic resonance imaging (MRI) of articular cartilage in knee osteoarthritis (OA): morphological assessment. Osteoarthritis Cartilage. 2006;14 (Suppl A):A46–75. doi: 10.1016/j.joca.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 21.Driban JB, Lo GH, Lee JY, Ward RJ, Miller E, Pang J, et al. Quantitative bone marrow lesion size in osteoarthritic knees correlates with cartilage damage and predicts longitudinal cartilage loss. BMC musculoskeletal disorders. 2011;12:217. doi: 10.1186/1471-2474-12-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 23.Lo GH, Hunter DJ, Zhang Y, McLennan CE, Lavalley MP, Kiel DP, et al. Bone marrow lesions in the knee are associated with increased local bone density. Arthritis Rheum. 2005;52:2814–21. doi: 10.1002/art.21290. [DOI] [PubMed] [Google Scholar]
- 24.Neumann G, Hunter D, Nevitt M, Chibnik LB, Kwoh K, Chen H, et al. Location specific radiographic joint space width for osteoarthritis progression. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2009;17:761–5. doi: 10.1016/j.joca.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraus VB, Vail TP, Worrell T, McDaniel G. A comparative assessment of alignment angle of the knee by radiographic and physical examination methods. Arthritis Rheum. 2005;52:1730–5. doi: 10.1002/art.21100. [DOI] [PubMed] [Google Scholar]
- 26.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer-Verlag; 2000. [Google Scholar]
- 27.Kirkwood BR, Sterne JAC. Essential Medical Statistics. 2. Oxford, England: Blackwell Science Ltd; 2003. p. 361. [Google Scholar]
- 28.Cicuttini FM, Wluka AE, Stuckey SL. Tibial and femoral cartilage changes in knee osteoarthritis. Ann Rheum Dis. 2001;60:977–80. doi: 10.1136/ard.60.10.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wluka AE, Davis SR, Bailey M, Stuckey SL, Cicuttini FM. Users of oestrogen replacement therapy have more knee cartilage than non-users. Ann Rheum Dis. 2001;60:332–6. doi: 10.1136/ard.60.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fransen M, Crosbie J, Edmonds J. Physical therapy is effective for patients with osteoarthritis of the knee: a randomized controlled clinical trial. J Rheumatol. 2001;28:156–64. [PubMed] [Google Scholar]
- 31.Bergink AP, Uitterlinden AG, Van Leeuwen JP, Buurman CJ, Hofman A, Verhaar JA, et al. Vitamin D status, bone mineral density, and the development of radiographic osteoarthritis of the knee: The Rotterdam Study. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2009;15:230–7. doi: 10.1097/RHU.0b013e3181b08f20. [DOI] [PubMed] [Google Scholar]
- 32.Heidari B, Heidari P, Hajian-Tilaki K. Association between serum vitamin D deficiency and knee osteoarthritis. International orthopaedics. 2011;35:1627–31. doi: 10.1007/s00264-010-1186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Felson DT, Niu J, Clancy M, Aliabadi P, Sack B, Guermazi A, et al. Low levels of vitamin D and worsening of knee osteoarthritis: results of two longitudinal studies. Arthritis and Rheumatism. 2007;56:129–36. doi: 10.1002/art.22292. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Niu J, Felson DT, Choi HK, Nevitt M, Neogi T. Methodologic challenges in studying risk factors for progression of knee osteoarthritis. Arthritis care & research. 2010;62:1527–32. doi: 10.1002/acr.20287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dawson-Hughes B, Dallal GE, Krall EA, Harris S, Sokoll LJ, Falconer G. Effect of vitamin D supplementation on wintertime and overall bone loss in healthy postmenopausal women. Ann Intern Med. 1991;115:505–12. doi: 10.7326/0003-4819-115-7-505. [DOI] [PubMed] [Google Scholar]
- 36.Guermazi A, Roemer FW, Burstein D, Hayashi D. Why radiography should no longer be considered a surrogate outcome measure for longitudinal assessment of cartilage in knee osteoarthritis. Arthritis research & therapy. 2011;13:247. doi: 10.1186/ar3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raynauld JP, Martel-Pelletier J, Bias P, Laufer S, Haraoui B, Choquette D, et al. Protective effects of licofelone, a 5-lipoxygenase and cyclo-oxygenase inhibitor, versus naproxen on cartilage loss in knee osteoarthritis: a first multicentre clinical trial using quantitative MRI. Annals of the Rheumatic Diseases. 2009;68:938–47. doi: 10.1136/ard.2008.088732. [DOI] [PubMed] [Google Scholar]
- 38.Conaghan PG, Hunter DJ, Maillefert JF, Reichmann WM, Losina E. Summary and recommendations of the OARSI FDA osteoarthritis Assessment of Structural Change Working Group. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2011;19:606–10. doi: 10.1016/j.joca.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wirth W, Hellio Le Graverand MP, Wyman BT, Maschek S, Hudelmaier M, Hitzl W, et al. Regional analysis of femorotibial cartilage loss in a subsample from the Osteoarthritis Initiative progression subcohort. Osteoarthritis Cartilage. 2009;17:291–7. doi: 10.1016/j.joca.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]