Abstract
The present study investigates the analgesic effect of minocycline, a semi-synthetic tetracycline antibiotic, in a rat model of inflammation-induced visceral pain. Inflammation was induced in male rats by intracolonic administration of tri-nitrobenzenesulphonic acid (TNBS). Visceral hyperalgesia was assessed by comparing the viscero-motor response (VMR) to graded colorectal distension (CRD) prior and post 7 days after TNBS treatment. Electrophysiology recordings from CRD-sensitive pelvic nerve afferents (PNA) and lumbo-sacral (LS) spinal neurons were performed in naïve and inflamed rats. Colonic inflammation produced visceral hyperalgesia characterized by increase in the VMRs to CRD accompanied with simultaneous activation of microglia in the spinal cord and satellite glial cells (SGCs) in the dorsal root ganglions (DRGs). Selectively inhibiting the glial activation following inflammation by araC (Arabinofuranosyl Cytidine) prevented the development of visceral hyperalgesia. Intrathecal minocycline significantly attenuated the VMR to CRD in inflamed rats, whereas systemic minocycline produced a delayed effect. In electrophysiology experiments, minocycline significantly attenuated the mechanotransduction of CRD-sensitive PNAs and the responses of CRD-sensitive LS spinal neurons in TNBS-treated rats. While the spinal effect of minocycline was observed within 5 min of administration, systemic injection of the drug produced a delayed effect (60 min) in inflamed rats. Interestingly, minocycline did not exhibit analgesic effect in naïve, non-inflamed rats. The results demonstrate that intrathecal injection of minocycline can effectively attenuate inflammation-induced visceral hyperalgesia. Minocycline might as well act on neuronal targets in the spinal cord of inflamed rats, in addition to the widely reported glial inhibitory action to produce analgesia.
Keywords: microglia, satellite glial cell, minocycline, TNBS, visceral pain
1. Introduction
Chronic visceral pain, observed in several gastrointestinal (GI) disorders, is a multifaceted problem and remains poorly understood (Smith, 2010). Despite conventional belief that visceral pain is a variant of somatic pain, it significantly differs in neurological mechanisms and transmission pathways (Cervero and Laird, 1999). Unfortunately, there are very few specific analgesics for visceral pain and therapies commonly used are extensions of those used for general pain management (Cervero and Laird, 1999). Currently available treatments for visceral pain are unsatisfactory due to their adverse effects like altered GI mucosal homeostasis, motility, nausea, constipation, irritation and ulceration. Activation of glial cells and neuro-glial interactions are emerging as key mechanisms underlying chronic pain (DeLeo and Yezierski, 2001; Suter et al., 2007). Accumulating evidence has implicated activation of glial cells in the development and maintenance of chronic pain: microglia and astrocytes of the central nervous system (CNS) and satellite glial cells (SGCs) of the dorsal root ganglia (Takeda et al., 2009; Ji et al., 2013). Robust glial activation mediated pain has been reported in several models of pain including sciatic inflammatory neuropathy (Ledeboer et al., 2005), chronic constriction nerve injury (Stuesse et al., 2000), partial sciatic nerve ligation (Coyle, 1998), spinal nerve ligation (Jin et al., 2003), spinal nerve transection (Raghavendra et al., 2003) and peripheral inflammation (Cho et al., 2006). Given their involvement in several pathological conditions, activated glial cells are being considered as a potential pharmacological target for treating various forms of pain.
Minocycline, a second-generation, broad spectrum, semi-synthetic tetracycline antibiotic is of particular interest as an analgesic, in addition to its anti-microbial effect. Minocycline effectively crosses the blood-brain barrier (Aronson, 1980) and has a proven safety record in humans (Thomas and Le, 2004). Minocycline mediated inhibition of microglial activation has been reported to reduce nociception in inflammation-evoked pain (Hua et al., 2005), spinal cord contusion injury (Hains and Waxman, 2006) and spinal nerve ligation (Lin et al., 2007). While the majority of these studies attribute the analgesic effect of minocycline to inhibition of microglial activation (Tikka et al., 2001; Garrido-Mesa et al., 2013), some studies have also reported on its action on other targets like astrocytes (Zhang et al., 2012) and neurons (Gonzalez et al., 2007). Extensive literature published in the last decade indicates an essential role of glial cells in the development and maintenance of hyperalgesia. However, very little is known about the involvement of activated glial cells in visceral pain. Further, there is no information on the efficacy of minocycline as a potential analgesic for inflammation-induced visceral pain. The objective of this study was to determine 1) whether visceral hyperalgesia caused by inflammation of the colon is due to activation of the microglia and SGCs and 2) whether this visceral hyperalgesia can be attenuated by administration of minocycline. The study evaluates the effect of minocycline on both the DRG neurons and lumbar spinal cord neurons using a combined approach involving behavioral and electrophysiology experiments.
2. Materials and Methods
2.1. Animals
Adult male Sprague Dawley rats (Taconic, Indianapolis, IN, USA) with an average weight of 400 g (range: 350-450 g) were used for this study. Rats were kept in controlled conditions with a 12 h light/dark schedule and had access to both food and water ad libitum. Twenty-four hours before behavioral and electrophysiological studies, the animals were placed in a wire-bottom cage and access to food, but not water, was denied in order to empty the colon. All experiments were performed according to the approved guidelines of the Institutional Animal Care and Use committee (AUA # 355) at the Medical College of Wisconsin and The International Association for the Study of Pain (IASP).
2.2. Drugs and chemicals
TNBS (tri-nitrobenzene sulfonic acid) was purchased from Sigma-Aldrich, USA. Minocycline (Sigma-Aldrich, USA) was freshly dissolved in sterile distilled water before administration and heated briefly in a water bath until completely dissolved and the solution was clear. Minocycline was administered intraperitoneally (i.p.) at a dose of 50mg/kg, intrathecally (i.t.) at dose of 50μg/animal and intravenously (i.v.) at dose of 25mg/kg based on previously published reports (Cho et al., 2012; Liu et al., 2012). AraC (Arabinofuranosyl Cytidine; Sigma-Aldrich, USA) dissolved in saline was administered at a dose of 10μg/animal (i.t.) for 7 days (van der Kogel and Sissingh, 1985). Intrathecal (i.t) drugs were administered in a volume of 5μL followed by 5-10μL saline flush depending on the length of the catheter.
2.3.1 Induction of inflammation-induced visceral hyperalgesia
TNBS (tri-nitrobenzene sulfonic acid)-induced colonic inflammation was used as a model for visceral hyperalgesia and performed as previously described (Morris et al., 1989). Briefly, overnight fasted rats were anesthetized with pentobarbital sodium (50 mg/kg, i.p.) and 0.5mL of 50% TNBS (dissolved in ethanol, 1:1) was slowly injected into the descending colon using a 16 gauge gavage needle. Intracolonic TNBS-induced colon inflammation is considered a model for Crohn’s disease (Kruschewski et al., 2001). Onset of inflammation in TNBS-treated rats were confirmed biochemically by measuring the myeloperoxidase (MPO) activity in the colon and pathologically by staining the colon tissue with hematoxylin and eosin (H and E) stain using previously standardized procedures (Banerjee et al., 2009).
2.4. Recording of viscero-motor response (VMR)
2.4.1. Surgical procedure
Rats were initially anesthetized by injecting pentobarbital sodium (50 mg/kg, ip). Teflon coated electrodes (Cooner Wire, Part No. A5631, Chatsworth, CA, USA) were implanted in the external oblique muscle of the abdomen for electromyography (EMG) recordings. For intrathecal (i.t.) drug administration, the rats were placed in a stereotactic head holder under pentobarbital anesthesia and a polyethylene catheter (PE-10) was inserted through an incision in the atlanto-occipital membrane. The catheter was advanced caudally 7 or 7.5 cm from the incision site to the lumbar enlargement of the spinal cord. The external end of the catheter (PE-50) was tunneled subcutaneously to exit at the top of the head. The electrode and the catheter were externalized dorsally near the neck and secured in place with silastic tubing and the skin was closed using 3-0 silk suture. The position of the catheter was confirmed via infusion of 20μL sterile 2% lidocaine. Animals that exhibited transient hind limb paralysis due to the lidocaine injection alone were used for the study. The position of the catheter was also checked visually during laminectomy. Post-surgery, the rats were closely monitored and euthanized (Beuthanasia-D, Schering-Plough Animal Health Corp, NJ, USA) if they showed any signs motor abnormalities or paralysis.
2.4.2. VMR recordings
No less than 72 h after surgery, rats were placed inside the plexiglass restraining tubes for 2 hours/day for three consecutive days in order to acclimatize them to experimental conditions. On the day of VMR recordings (6-7 days after electrode/catheter implantation), rats were placed in the restraining tube and a highly compliant, flaccid latex balloon (6 cm long and 3.5 cm OD) coated with non-reactive bacteriostatic lubricant (Surgilube, Savage Laboratories, Melville, USA) was inserted into the descending colon and taped to the tail. Rats were allowed to rest inside the tube for at least 30 min before testing the VMR to colorectal distension (CRD). The EMG signal was amplified using the amplifier (A-M System, model 1700, Carlsborg, WA, USA). A stimulus-response function (SRF) to graded CRD (10, 20, 30, 40, 60 mmHg) was recorded. The duration of distension was 30s with a 180s inter-stimulus interval between the distension. Data were recorded real-time using the Spike 4/CED 1401 data acquisition program (CED 1401; Cambridge Electronic Design, Cambridge, UK). Following a baseline SRF, rats were lightly anesthetized with pentobarbital sodium and TNBS was administered as described above. Rats were then allowed to recover for 7 days in case of TNBS prior to repeating the VMR.
2.5. Electrophysiology
2.5.1. Recording from CRD-sensitive Pelvic nerve afferent (PNA) fiber
PNA fiber recordings were performed both in naïve and TNBS-treated rats. For PNA fiber recordings, a 3-4 cm long incision was made in the lower abdomen after anesthesia and the prostate lobe was reflected laterally to access the major pelvic ganglion (MPG), pelvic, and hypogastric nerves. The pelvic nerve was isolated from the surrounding fatty tissues and a pair of Teflon-coated stainless wires was placed around it proximal to the MPG to deliver electrical stimulation to pelvic nerve in order to confirm that recordings were made from the S1 sacral dorsal root. The electrodes were secured in place by draping a piece of peritoneal fat tissue around it. The wound was closed in layers using 3-0 silk suture. Following this, a laminectomy was performed to expose the lumbo-sacral (T13-S2) spinal cord and the rat was stabilized by clamping the thoracic vertebra and hip joint. The dorsal skin was opened, reflected laterally and tied to make a pool for mineral oil. The dura was carefully removed and the spinal cord was covered with warm mineral oil (37°C). Recordings were made from the distal cut end of the central processes of the sacral S1 dorsal root as previously described (Sengupta et al., 2002). The identified fiber was then tested to CRD (30mmHg) and once confirmed as a CRD-responsive fiber, a SRF was constructed to graded CRD (10-60 mmHg, 30s, 3min inter-stimulus interval). Action potentials were processed through a window discriminator and the frequency of impulses was counted (1s binwidth) on-line using the spike2/CED 1401 data acquisition program.
2.5.2 Recording from CRD-sensitive lumbo-sacral (LS) spinal neurons
Electrophysiological recordings from lumbo-sacral (LS) spinal neurons were performed both in naïve and TNBS-treated rats. Rats were anesthetized initially with sodium pentobarbital (50mg/kg, i.p) and maintained with supplemental doses of 5mg/kg/h. The trachea was cannulated to mechanically ventilate the rat. The femoral artery was cannulated for recording blood pressure and the femoral vein was catheterized for injection of anesthetic and muscle relaxant. The rat was paralyzed with gallamine triethiodide (1mg/kg, i.v.) and ventilated with room air (55-60 strokes/min and 3-4 mL stroke volume). Additional doses of gallamine triethiodide were given to maintain paralysis during the course of the experiment. For spinal transection, a laminectomy was performed to expose the cervical (C1-C2) spinal cord. The dura membrane was gently removed and 10-15μl of 2% lidocaine was applied to the dorsal surface of the exposed spinal cord. After 10 min, the spinal cord was completely transected. The transected area was covered with a small piece of gelfoam soaked in warm saline. Animals exhibited a vasodepressor response immediately after spinal transection that recovered in 15-30 min. All spinal recordings were made at least one hour following spinal transection. The LS spinal cord was exposed by laminectomy (T13-S2) and the rat was suspended from the thoracic vertebral and ischial spinal clamps. The dorsal skin was reflected laterally and tied to make a pool for mineral oil. The dura was carefully removed and a piece of gelfoam was placed on the spinal cord. The pool was fully covered with 1.75% agar in saline and after hardening of the agar a small window was cut to expose the spinal cord. Stainless steel microelectrodes (8-10 MΩ, FHC, Bowdoinham, ME) were used for extracellular recording. The placement of the electrode was 0.1-0.5mm lateral from the spinal midline and recordings were done between 900-1200μm from the dorsal surface of the spinal cord. The SRF for CRD was constructed following the method used for PNA recordings. The action potentials were amplified through a low-noise AC differential amplifier (model 3000; A-M Systems) and continuously monitored and displayed on an oscilloscope. Post experiments, data were analyzed using the Wave-mark analysis method of the Spike 4 software (CED, Cambridge, UK) to distinguish individual action potentials.
2.6. Experimental protocol
2.6.1. Behavioral study
The effect of minocycline was tested on the VMR to CRD of non-inflamed and TNBS-treated rats. The drug was administered either intraperitoneally (i.p.) or intrathecally (i.t.) and an SRF to graded CRD (10–60mmHg) was recorded before and after minocycline administration. In a separate group of rats, following a baseline VMR recording, araC was administered 1h before intracolonic TNBS instillation. AraC treatment was continued daily for 7 days after which the VMR was recorded to test the development of visceral hyperalgesia. Control rats were treated with araC for 7 days and the VMR was recorded before and after 7 days to determine the effect of the drug in non-inflamed rats.
2.6.2. Electrophysiology study
A baseline SRF to graded CRD of PNA neurons was constructed in naïve, TNBS and araC + TNBS-treated rats and repeated 30 and 60 min after injection of minocycline (25mg/kg, i.v.). Similarly, in a separate set of animals, CRD-sensitive LS spinal neurons from naïve and TNBS-treated rats were identified and SRFs to graded CRD were constructed before and 5, 30 and 60 min after injection of minocycline (25mg/kg, i.v.). All recordings from LS spinal neurons were conducted in spinal transected (C1-C2) rats.
2.7. Immunohistochemical (IHC) studies
For immunohistochemistry, rats were deeply anesthetized with sodium pentobarbital (50mg/kg, i.p.) and the chest was opened by mid-sternal incision. Animals were perfused transcardially with 300 mL of ice-cold 4% paraformaldehyde in 0.1 M PBS (pH 7.4). A small piece of distal colon was cut and stored in paraformaldehyde for H and E staining. Following a laminectomy, spinal cord (L4-S1) and DRGs (L6 and S1) were collected and incubated in 4% paraformaldehyde at 4° C overnight, washed with PBS and incubated in 30% sucrose at 4° C until sectioning. The tissues were embedded in cryo-embedding media (OCT, Fisher Scientific, Pittsburgh, PA, USA) and sectioned using a cryostat. Twenty five micron thick spinal cord sections were cut and collected in a 24-well plate containing the cryoprotectant. The sections were incubated for antigen retrieval using sodium citrate buffer (10mM, pH 8.5) at 81° C for 30 minutes. For DRGs, 5 micron thick sections were cut and collected on to sterile glass slide. The sections were allowed to reach room temperature and washed twice with 1X PBS for 5 min each. The sections were then incubated in blocking solution (0.1 M PBS + 0.25% Triton-X-100 + 10% normal goat serum) at room temperature for 1h followed by incubation with Iba-1 (Ionized calcium binding adaptor molecule 1) antibodies (Wako Chemicals; Cat # 019-19741) at a dilution of 1:250 for 48 hrs at 4° C. For DRG sections, the slides were incubated overnight at 4 °C in a humid chamber with primary polyclonal antibody for Glial fibrillary acidic protein (Anti-GFAP (rabbit polyclonal antibody, Thermo Scientific; Cat # PA1-10019) at a dilution of 1:250. Thereafter, the sections were washed (15 min/wash × 6) with wash buffer and incubated with goat anti-rabbit secondary antibody (Alexa Fluor 568, Invitrogen # A11036, 1:4000) at RT for 2h. The sections were washed as mentioned above and mounted using mounting medium (VECTASHIELD®; Vector Laboratories, Inc, CA, USA). Slides were examined under a fluorescence microscope (Nikon Eclipse 50i) using narrow band cubes Alexa 568 (DM 568, excitation filter 540-560, barrier filter 575-645 nm). Images were captured with a Spot II high-resolution digital camera (Diagnostic Instruments Inc., Sterling Heights, MI, USA) and were processed using Adobe Photoshop program. To maintain the consistency of image capturing, we used the same time exposure, gain and gamma adjustment for the all samples. Tissue sections obtained from 3 separate animals from each treatment groups were examined for alterations in the morphology of the microglia stained for the Iba-1 marker. Similarly, the activation of SGCs was quantified by measuring the fluorescent intensity of GFAP-ir cells from the DRGs.
2.8. Data analysis
An SRF to graded CRD was constructed to test the intensity dependent increase in EMG activity. The EMG response to each distension pressure was calculated and was normalized to the EMG response to the highest distension pressure (60 mmHg). Therefore, each animal served as its own control. For analysis of PNA fibers and LS spinal neuron’s responses to CRD, the total number of action potentials over a 60 s resting period prior to colon distension and during the distension period (30 s) was counted and represented as impulses/s. To measure the actual changes in response of the neurons to CRD, the mean firing frequency during the resting period (60s pre-distension) was subtracted from the mean firing frequency during colon distension (30s distension). The difference was calculated for each distension pressure tested (5-60 mmHg) and the resultant response was divided by the response of the neuron to the highest distension pressure (60 mmHg) in order to normalize the values to 1. Statistical analysis was determined by ANOVA followed by Student-Newman-Keuls test for multiple comparisons. Values are expressed as mean ± S.E.M. and P<0.05 were considered to be significant.
All image analysis, cell counts and fluorescence measurements were performed “off-line” on captured images taken from stained sections. The total numbers of positively identified activated microglia expressing Iba-1 were counted manually in individual spinal cord sections from 3 rats in each treatment group. Microglia were defined as activated if they displayed a clearly swollen cell body with reduced processes, these differ from normal or resting microglia where cell bodies were largely absent and large ramified processes were displayed. Assessment was performed by independent blinded investigators who quantified the total number of activated microglia. The numbers of activated microglia in each treatment group were expressed as a percentage of the total number of cells counted. For analysis of GFAP-immunoreactivity (GFAP-ir) expression in the L6-S1 DRG of naïve and inflamed rats, one section at the same magnification from different treatment groups (n=3/group) were chosen to calculate the average gray intensity value. The average intensity is defined as the difference of the average gray intensity value of a chosen field and the background (Luo et al., 2004). The images were converted to grey scale and the intensity of immunostaining around the neuron was measured by selecting the areas using a region of interest. Background fluorescence was measured by taking an image of an area within the sample, using the parameters described above, which contained no labeled structures which was then subtracted using IMAGE J (NIH open software). Following background subtraction, mean fluorescence grey intensity was then determined.
3. Results
3.1 Induction of colonic inflammation and visceral hyperalgesia
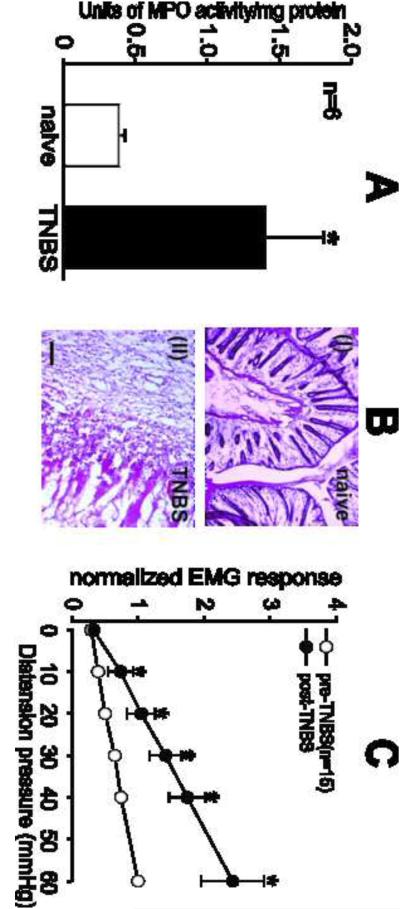
Inflammation of the colon after 7 days following instillation of TNBS was confirmed by comparing the activity of colonic MPO and the histology of the distal colon from naïve rats. TNBS-treated rats showed a significant (P<0.05) 3 fold increase in the colonic MPO activity compared to naïve rats (Fig.1A). While naïve rats exhibited normal colonic architecture, the distal colon of TNBS-treated rats showed severe inflammatory infiltration, edema, loss of the mucosal architecture, high level of vascular density, increase in the thickness of muscular wall and the width of the submucosal spaces (Fig.1B). The VMRs to CRD of the rats were measured before and after 7 days of TNBS instillation into the colon. When compared with baseline VMR, a significant increase in the VMRs to graded CRD at all distension pressures was observed post 7 days of TNBS treatment (Fig. 1C).
Fig.1.
Inflammation was confirmed by assessing the activity of MPO in the colon and by observing the pathology of the colon tissue. MPO activity was significantly (P<0.05) elevated in TNBS-treated rats compared to naïve rats; (A). H and E stained colon section from TNBS-treated rats (BI) exhibited significant inflammation characterized by complete loss of colonic architecture compared to naïve colon (BII). Scale bar represents 50 μm. Viscero-motor response (VMR) is represented as normalized electromyographic (EMG) activities to contraction of external abdominal muscle to graded (10–60mmHg) colorectal (CRD) distension. (C) Intracolonic administration of TNBS significantly (P<0.05 vs pre-TNBS) increased the VMR to colonic distension from 20 mmHg pressure onwards. Values expressed as mean ± S.E.M. of ‘n’ animals in each group. P<0.05 was considered significant. * compared with pre-TNBS.
3.2 Status of spinal microglia and SGCs in naïve and inflamed rats
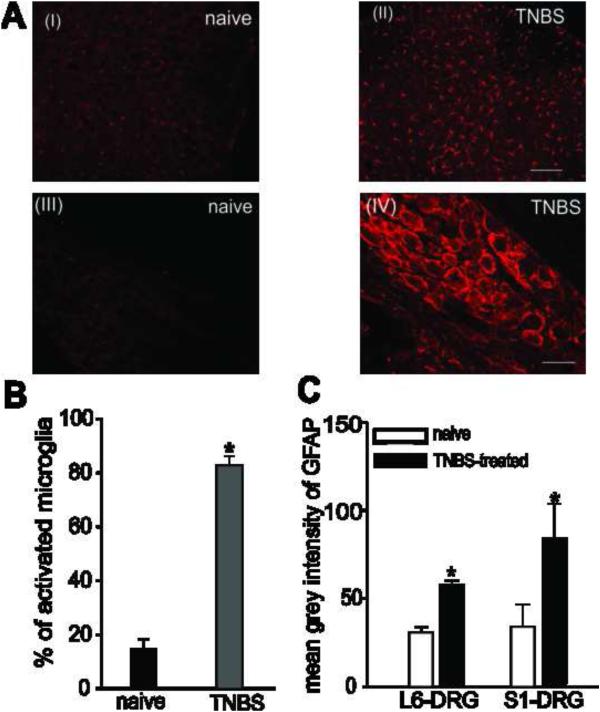
Immunohistochemical studies showed a very high level of Iba-1 positive, morphologically identified activated microglial cells in the LS spinal cord of rat post 7 days of TNBS-treatment, when compared to naïve non-inflamed rats (Fig. 2AI-II & B). Similarly, compared to naïve rats, TNBS-treatment produced significant activation of SGCs observed by increase in the average intensity of GFAP-ir in both the L6 and S1 DRGs (Fig.2AIII-IV &2C). This increase in the activation of microglia in the LS spinal cord and SGCs in the DRGs is in agreement with visceral hyperalgesia observed following TNBS treatment.
Fig.2.
Photomicrograph of Iba-1 positive stained microglial from the LS spinal cord and GFAP-ir SGCs from the L6 and S1 DRG from naïve and inflamed rats. The number of activated microglia was increased in the LS spinal cord of TNBS-treated rats (AII) post 7 days, when compared to naïve rats (AI). Scale bars represent 200 μm. Distribution of activated SGCs surrounding DRG neurons was determined by measuring the grey intensity of GFAP-ir SGCs. Intense GFAP-ir indicating activation of SGCs was seen around neurons from S1 DRGs post 7 days of TNBS treatment compared to naïve rats (AIII-IV). Scale bars represent 50μm. (B) Quantitative analysis showed that the number of activated microglia was found to be significantly (P<0.05) increased following TNBS-treated rats compared to naïve rats. Values expressed as mean % of activated cells ± S.E.M. (C) Similarly, the mean grey intensity indicating GFAP-ir was significantly higher in both L6 and S1 DRGs in TNBS-treated rats compared to naïve rats indicating activation of SGCs following inflammation of the colon. Values expressed as mean grey intensity of GFAP ± S.E.M. P<0.05 was considered significant. * compared with naïve rat.
3.3. Effect of araC treatment on the VMRs in TNBS-treated rats
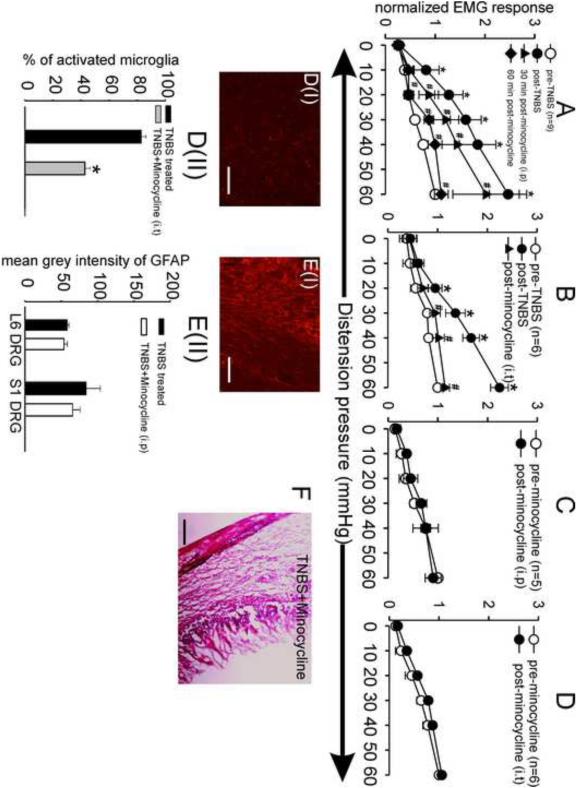
To further confirm the role of activated glial cells in the initiation and maintenance of visceral hyperalgesia following inflammation of colon, we investigated whether inhibiting the proliferation of glial cells following intracolonic administration of TNBS still produces visceral hyperalgesia. AraC is an anti-mitotic drug used to achieve complete ablation of proliferating cells in the CNS. It is reported to interfere with DNA replication resulting in DNA damage and cell death. Rapidly dividing cells, which require DNA replication for mitosis, are therefore most affected. AraC also inhibits both DNA and RNA polymerases and nucleotide reductase enzymes which are needed for DNA synthesis. AraC caused a significant 1.5 folds decrease in the number of Iba1 positive cells (i.e., microglia) in the lumbar spinal cord of AraC treated rats compared to vehicle treated controls (Audet et al., 2012). Additional experiments were performed wherein, we injected araC (10μg, i.t.), an antimitotic agent into the LS spinal cord to effectively prevent the proliferation in the microglia. Following a baseline VMR recording, TNBS was instilled into the colon to produce inflammation. AraC was administered intrathecally 1h prior and for 7 days post-TNBS treatment. While TNBS instillation into the colon produced visceral hyperalgesia in rats at day 7, treatment with araC prevented the development of this visceral hyperalgesia in following TNBS treatments as the VMRs did not show any significant increase (Fig. 3A). Intrathecal injection of araC for 7 days did not produce any marked alteration in the VMRs suggesting that the drug did not produce any neurotoxicity in the spinal cord (Fig. 3B). The potential activation of microglia in the LS spinal cord and SGCs in the L6-S1 DRGs were also investigated by IHC in this experiment. TNBS-treated rats that received araC (i.t) exhibited marked morphological changes in the microglia, characterized with a higher percentage of resting microglial cells compared to TNBS-treated rats. Although the cell body of the microglia in these rats was enlarged, it showed extensive branching characteristic to resting microglia (Fig 3C & E). Similarly, in both the L6 and S1 DRGs, GFAP-ir was significantly decreased in araC + TNBS-treated rats, compared with TNBS-treated rats (Fig 3D & F). The effect of araC treatment on the status of glial cells in naïve rats was not performed; however it did not produce any significant alteration in the VMR as shown in Fig 3C.
Fig.3.
Summary data of effects of araC treatment (10μg/animal for 7 days; i.t.) on VMR to CRD in naïve rats and TNBS-treated rats. (A) While TNBS-treated rats exhibited significant visceral hyperalgesia (shown in blue; data extrapolated from Fig. 1C), rats treated with araC for 7 days post-TNBS administration into the colon failed to develop visceral hyperalgesia, observed with no significant (P<0.05) change in their VMR (shown in red). (B) In naïve, non-inflamed rats, araC treatment for 7 days did not cause any significant alteration in the VMRs and were comparable to the baseline values. Values expressed as mean ± S.E.M. of ‘n’ animals in each study group. (C) Iba-1 positive stained sections of LS spinal cord from araC + TNBS-treated rat. Scale bar represent 200μm. (DI) GFAP-ir sections from S1 DRG of araC + TNBS-treated rats. Scale bar represent 50μm. (E) The number of activated microglial was significantly decreased in araC + TNBS-treated rats compared to TNBS-treated rats (TNBS data reused from Fig. 2B for comparison). Values are expressed as mean % of activated cells ± S.E.M. (F) The mean grey intensity from GFAP-ir sections from both L6 and S1 DRGs was found to be significantly (P<0.05) decreased when compared to TNBS-treated rats (TNBS data reused from Fig. 2C for comparison). Values expressed as mean grey intensity of GFAP ± S.E.M. P<0.05 was considered significant. * compared with TNBS-treated rats.
3.4 Effect of systemic and intrathecal minocycline on the VMR of naïve and inflamed rats
Systemic injection (i.p.) of minocycline (50mg/kg) produced analgesic effect in TNBS (Fig. 4A) model of inflammation-induced visceral hyperalgesia. Minocycline demonstrated a time dependent analgesic effect against TNBS-induced visceral hyperalgesia, with the maximum inhibitory effect being observed post 60 min of drug administration. In contrast, intrathecal injection of minocycline decreased the VMR to CRD within 5 min of administration (Fig. 4B). In naïve non-inflamed rats, both systemic and intrathecal administration of minocycline did not produce any attenuation of VMR (Fig. 4C & D), although its effect on the status of glial cells in naïve rats was not performed. This decrease in VMR following minocycline administration (i.t) is in agreement with the significantly decreased number of activated microglia in the LS spinal cord compared to TNBS-treated rats (Fig. 4DI-II). Similarly, minocycline also produced a slight but not significant decrease in the average intensity of GFAP-ir in the L6 and S1 DRGs of TNBS-treated rats (Fig. 4EI-II). In the present study, light microscopic observation of H and E stained colon tissue sections from TNBS-treated rats that received a single dose (i.p) of minocycline did not exhibit any significant improvement in the inflammation status (Fig. 4F).
Fig.4.
Summary data of the effects of minocycline (i.p. & i.t.) on VMR to CRD in naïve and TNBS-treated rats. Rats exhibiting significant visceral hyperalgesia following TNBS treatment (Figure 1C) were injected with minocycline by either systemic (n=9) or intrathecal (n=6) route. (A) Systemic administration (i.p.) of minocycline (50mg/kg) significantly attenuated the VMR in TNBS-treated rats. Minocycline produced a highly pronounced decrease in the VMR post 60 min of administration in TNBS-treated rats. (B) Intrathecal administration of minocycline also significantly (P<0.05 vs post-TNBS) attenuated the VMR in these rats to distending pressure ≥ 30 mmHg. (C) Systemic administration of the same dose of minocycline in naïve, non-inflamed rats did not alter the VMR. (D) Similarly, injection of the same dose of minocycline in non-inflamed rats also did not alter the VMR. Values expressed as mean ± S.E.M. P<0.05 was considered significant. * compared with pre-TNBS; # compared with post-TNBS. Intrathecal minocycline resulted in a significantly decreased number of activated microglia in the LS spinal cord (DI-II) compared to TNBS-treated rats (TNBS data reused from Fig. 2B for comparison). Scale bar represents 200μm. Values are expressed as mean % of activated cells ± S.E.M. * compared with TNBS-treated rats. However, the mean grey intensity from GFAP-ir sections from both L6 and S1 DRGs was not found to be significantly decreased when compared to TNBS-treated rats (EI-II) (TNBS data reused from Fig. 2C for comparison). Scale bar represents 50 μm. Values expressed as mean grey intensity of GFAP ± S.E.M. P<0.05 was considered significant. (F) H and E stained colon section from TNBS-treated rats administered a single systemic injection of minocycline exhibited significant inflammation indicating no significant improvement in the status of inflammation.
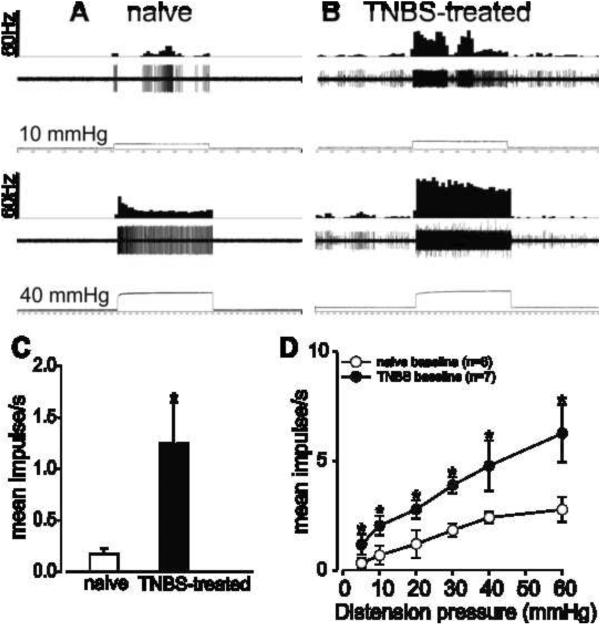
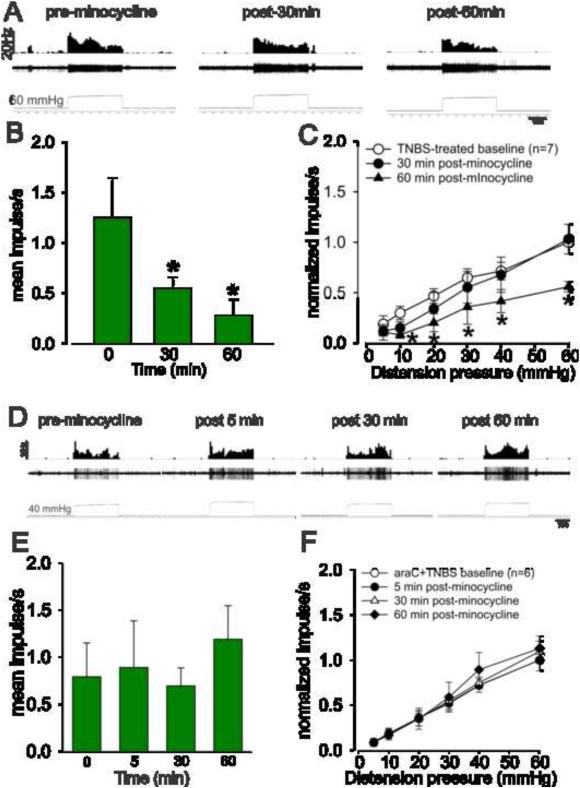
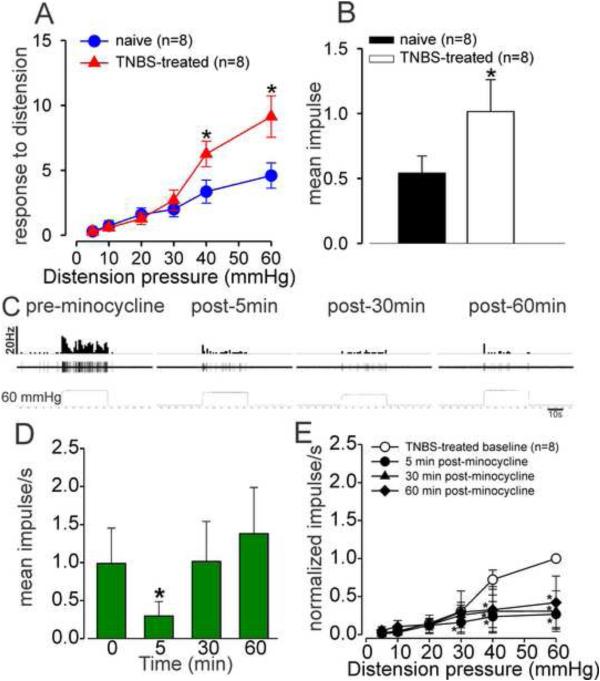
3.5 Effect of minocycline on the response of PNAs in inflamed and naïve rats
Figures 5A and 5B show the typical responses of a CRD-sensitive PNA fiber from a naïve and TNBS-treated rats. TNBS-treatment significantly (P<0.05 vs naïve) increased the spontaneous firing (Fig. 5C) and also increased the response of PNA fibers to CRD compared to naïve non-inflamed rats (Fig. 5D). Fig. 6A shows an example of typical response characteristic of PNA fiber before and after (30 and 60 min) injection of minocycline (25mg/kg, i.v.) in a TNBS-treated rat. Results showed that in inflamed rats, minocycline significantly decreased the spontaneous firing of these PNA fibers at both 30 and 60 min post administration (P<0.05 vs pre-minocycline; Fig. 6B). In TNBS-treated rats, there was a significant (P<0.05) reduction in the firing frequency of PNA fibers to CRD only after 60 min following administration of minocycline (Fig. 6C). Minocycline had no effect on the spontaneous firing and on the responses to CRD post 5 min of administration (data not presented). Fig. 6D illustrates the responses of a typical CRD-sensitive PNA fiber from araC + TNBS-treated rats before and after minocycline administration. Unlike in TNBS-treated rats, minocycline did not produce any significant decrease in the spontaneous firing (Fig. 6E) or in the responses (Fig. 6F) of the CRD-sensitive PNA fibers to CRD in araC + TNBS-treated rats.
Fig.5.
Example of typical response of a CRD-sensitive PNA fiber from a naive rat (A) and TNBS-treated rats to 40 mmHg distension (B). In all panels, the top trace shows the response to CRD represented as a frequency histogram (1s binwidth), the middle trace is the neuron action potential and the bottom trace is the distension pressure. (C) The spontaneous firing of the PNAs from TNBS-treated rats was significantly increased compared to naïve rats. (D) Intracolonic administration of TNBS also significantly increased the response of these fibers to graded CRD. Values expressed as mean impulse ± S.E.M of ‘n’ neurons in each treatment group *P<0.05 vs naïve baseline.
Fig.6.
Effect of minocycline on the mechanotransduction of PNA fibers from TNBS-treated rats. A: Example of the typical response of a CRD-sensitive PNA fiber from a TNBS-treated rat. In all panels, the top trace shows the response to CRD represented as a frequency histogram (1s binwidth), the middle trace is the neuron action potential and the bottom trace is the distension pressure. B: administration of minocycline (25mg/kg, i.v.) significantly decreased the spontaneous firing of these fibers. C: the drug also significantly attenuated the mechanotransduction of PNA at 60 min, but not at 30 min after injection. Values expressed as normalized mean impulse ± S.E.M of ‘n’ neurons. *P<0.05 vs TNBS-treated baseline. Effect of minocycline on the mechanotransduction of PNA fibers from araC + TNBS-treated rats. (D) Example of the typical response of a CRD-sensitive PNA fiber from araC + TNBS-treated rat. In all panels, the top trace shows the response to CRD represented as a frequency histogram (1s binwidth), the middle trace is the neuron action potential and the bottom trace is the distension pressure. (E) Administration of minocycline (25mg/kg, i.v.) did not alter the spontaneous firing of these fibers. (F) Minocycline also did not attenuate the mechanotransduction of PNA at time points investigated. . Values expressed as normalized mean impulse ± S.E.M of ‘n’ neurons.
3.6. Effect of minocycline on CRD-sensitive LS spinal neurons
TNBS-treatment significantly (P<0.05 vs naïve) increased the response to CRD (Fig. 7A) and spontaneous firing (Fig. 7B) of the LS spinal neurons compared to naïve non-inflamed rats . Fig. 7C illustrates the responses of a CRD-sensitive LS spinal neuron from a TNBS-treated rat before and after 5, 30 and 60 min of minocycline (25mg/kg, i.v.) administration. Minocycline produced a significant decrease in the spontaneous firing of these LS neurons post- 5 min of administration. However, at 30 and 60 min post-administration, minocycline did not inhibit the spontaneous firing of the neurons (Fig. 7D). The drug produced a significant (P<0.05) reduction in the responses of these neurons at all intensities of distending pressures ≥ 20 mmHg (Fig. 7E). This effect was observed at all-time points (5, 30 and 60min).
Fig.7.
(A) LS spinal neurons from TNBS-treated rats exhibited significantly increased response to CRD compared to naïve rats. (B) The spontaneous firing of the PNAs from TNBS-treated rats was significantly increased compared to naïve rats. Values expressed as mean impulse/s ± S.E.M of ‘n’ neurons. *P<0.05 vs TNBS-treated baseline. (C) an example of the typical response pattern of a CRD-sensitive LS spinal neuron from a TNBS-treated rat before and after 5, 30 and 60 min of minocycline injection. In all panels, the top trace shows the response to CRD represented as a frequency histogram (1s binwidth), the middle trace is the neuron action potential and the bottom trace is the distension pressure. (D) Administration of minocycline (25mg/kg, i.v.) significantly attenuated the spontaneous firing of LS spinal neuron at 5 min post-injection. (E) The neurons exhibited significant (P<0.05) inhibition to CRD when recorded 5 min after drug injection and the inhibition persisted when tested at 30 and 60 min. Values expressed as normalized mean impulse ± S.E.M of ‘n’ neurons. *P<0.05 vs TNBS-treated baseline.
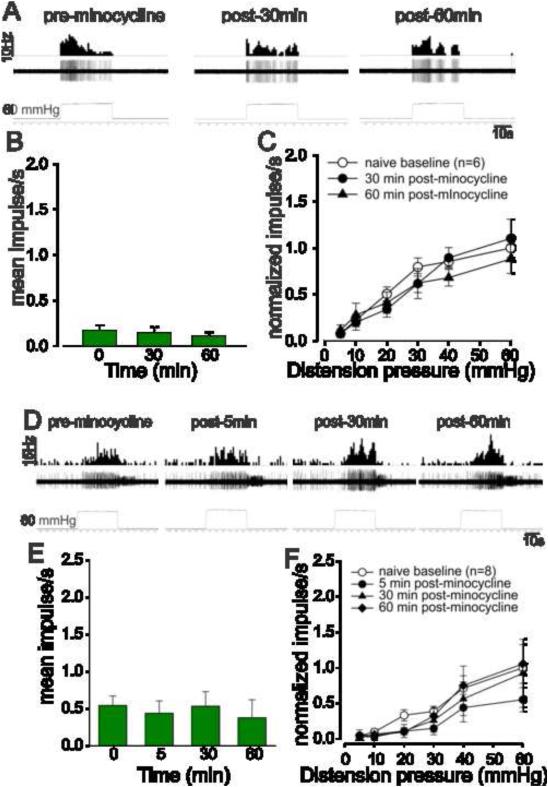
3.7. Effect of minocycline on CRD-sensitive PNAs and LS spinal neurons in naïve rats
The effect of minocycline on the responses of CRD-sensitive PNAs and LS spinal neurons to graded colorectal distension is presented in Fig. 8. Unlike in TNBS-treated inflamed rats, minocycline did not alter either the spontaneous firing or the response to CRD of both PNAs (Figs. 8A-C) and LS spinal neurons (Figs. 8D-F) in non-inflamed rats.
Fig.8.
The mean SRFs of PNA fibers in naïve, non-inflamed rats before and after administration of minocycline. (A) An example of the typical response characteristics of a CRD-sensitive PNA fiber from a naive rat. In all panels, the top trace shows the response to CRD represented as a frequency histogram (1s binwidth), the middle trace is the neuron action potential and the bottom trace is the distension pressure. (B) Administration of minocycline (25mg/kg, i.v.) did not significantly alter the spontaneous firing rate of these fibers. (C) Similarly, the SRFs recorded at 30 and 60 min indicate that the drug does not attenuate the mechanotransduction of PNA fibers in naïve rats. Values expressed as normalized mean impulse ± S.E.M of ‘n’ neurons. (D) An example of the typical response of a CRD-sensitive LS spinal neuron from a non-inflamed rat before and after 5, 30 and 60 min of minocycline injection. In all panels, the top trace shows the response to CRD represented as a frequency histogram (1s binwidth), the middle trace is the neuron action potential and the bottom trace is the distension pressure. (E) Administration of minocycline (25mg/kg, i.v.) did not alter the spontaneous firing rate of these neurons. (F) Similarly, the SRFs recorded at 5, 30 and 60 min indicate that minocycline does not alter the firing response of these LS spinal neurons in naïve rats. Values expressed as normalized mean impulse ± S.E.M of ‘n’ neurons.
4. Discussion
Recent studies with several pain models have indicated that minocycline could potentially be used as an analgesic drug in pain management (Kettenmann et al., 2011). The present study involved behavioral measurement of the visceral pain and electrophysiological recordings from PNA fibers and LS spinal cord neurons to investigate the analgesic effect of minocycline in a rat model of inflammation-induced chronic visceral pain. Although, large body of literature suggests that microglia is involved in the development of chronic pain after peripheral injury (Cao and Zhang, 2008), the role of microglia in the initiation and maintenance of chronic visceral pain has not been widely investigated. Similarly, while the antinociceptive effects of minocycline have been shown in several models of nociception (Stirling et al., 2005), the present study is the first report that investigates its effect on the LS spinal neurons and PNA fibers in a model of inflammation-induced visceral hyperalgesia.
Instillation of TNBS into the colon produced significant visceral hyperalgesia characterized by heightened EMG response to graded CRD. Consistent with other studies, TNBS-treated rats exhibited marked weight loss, decreased food intake and diarrhea compared to non-inflamed rats. Light microscopic images of the colon of TNBS-treated rats revealed severe inflammation and complete loss of the mucosal architecture, in addition to elevated MPO levels in the colon tissue. IHC studies on the LS spinal cord and DRGs showed activation of the microglia in the spinal cord and SGCs in the DRG. These observations are indicative of the fact that inflammation of the colon transforms these glial cells from resting state to active state. The resultant visceral hyperalgesia might be due to these activated glial cells that are reported to release pronociceptive cytokines which causes neuronal hyper-excitability (Hains and Waxman, 2006; Kettenmann et al., 2011).
To further confirm whether activation of glial cells following inflammation of the colon is directly responsible for the development of visceral hyperalgesia, we selectively inhibited the activation of spinal microglia and SGCs using araC, an antimitotic drug, reported to inhibit the activation of microglia (Sura, 2007). AraC was administered (i.t) for 7 days following TNBS treatment to inhibit the activation of both the microglia in the LS spinal cord and SGCs in the DRGs. Unlike TNBS-treated rats, which developed visceral hyperalgesia post 7 days, araC + TNBS-treated rats did not exhibit hyperalgesia even though their colon exhibited marked inflammation (data not presented). IHC studies from these TNBS-treated rats that received the araC showed a significantly lower number of activated microglia in the LS spinal cord and activated SGCs in the DRGs when compared to TNBS-treated rats. Administration of araC immediately following TNBS instillation significantly prevented the activation of microglia and SGCs post inflammation of the colon which correlates well with the failure to develop hyperalgesia in these rats. Continuous infusion of the araC over the CNS lesion site for 7 days immediately following a unilateral lesion of the nigrostriatal tract has been reported to significantly reduce the number of microglia around the lesion resulting in improved axonal regeneration (Rhodes et al., 2003). Similarly, early intrathecal treatment with low-dose of methotrexate (inhibitor of dihydrofolate reductase) reduced pain-like behavior after spared nerve injury by inhibiting microglial activation and p38 phosphorylation (Scholz et al., 2008). These reports further support our finding that inhibition of glial activation can successfully prevent the development of visceral hyperalgesia.
Intrathecal administration of minocycline produced an immediate analgesic effect in this model of inflammation-induced visceral hyperalgesia. In contrast, systemic administration of minocycline was significantly effective as an analgesic in TNBS-treated rats only at 60 min. Several studies have reported that minocycline inhibits microglia under various pathological conditions and reverses neuronal sensitization (Tikka et al., 2001; Raghavendra et al., 2003). While some studies have also reported on the direct action of minocycline on the neurons and astrocytes, the possibility of minocycline acting directly on the neurons in the LS spinal cord and DRG in our study is highly unlikely due to the fact that minocycline did not produce any significant change in the VMRs of naïve, non-inflamed rats. The time difference in action of minocycline observed in our study can be attributed to 1) the route of administration and 2) different targets of minocycline at the spinal cord and DRG. Minocycline when injected i.t has faster access to the LS spinal cord thereby, resulting in an immediate effect (5 min), probably due to a direct inhibitory action on spinal microglia. In contrast, access to the DRGs following systemic injection (i.p) of minocycline is greatly reduced due to several factors including the location of the DRGs, encapsulation of the DRGs in collagen barrier and diffusion of the drug to other non-specific regions. In addition, access to the DRGs following a single injection (i.t) of minocycline is also unlikely due to the small volume of the drug injected. It should be noted that the spinal microglia and the SGCs in the DRGs are phenotypically different type of cells although they are classified as glial cells. SGCs are very different in their morphology from either astrocytes or microglia, as they usually wrap around individual sensory neurons, thus forming a complete envelope (Hanani, 2005). This envelope acts as a mechanical barrier that can slow down the access of chemicals to the DRG neurons. Further, it is also highly plausible, that minocycline might have a different mechanism of action on the spinal microglia and SGCs in the DRG which might be reason for the observed difference in its time-course of action.
The results of the electrophysiological recordings also support the findings of the behavioral studies. TNBS-treatment significantly increased the spontaneous firing and the response of PNA fibers to CRD compared to naïve non-inflamed rats. A similar increase in the number of DRG neurons displaying spontaneous spikes leading to augmented neuronal excitability leading to reduced pain threshold following colon obstruction or peripheral inflammation has been reported (Huang and Hanani, 2005; Huang et al., 2010). In the present study, minocycline decreased the spontaneous firing and the responses of CRD sensitive PNAs and LS spinal neurons in TNBS-treated rats, although its effect on spinal neurons was much faster (5 min) than on the PNAs (60 min). Since all spinal recordings were done on spinal transected rats, the observed effect of minocycline is predominantly attributed to its inhibitory effect at the spinal level rather than its action on supra-spinal sites. Interestingly, minocycline also did not decrease the response of PNAs to CRD from araC + TNBS-treated rats. In non-inflamed rats minocycline did not affect either the spontaneous firing or the responses to CRD of both PNAs and LS spinal neurons. Taken together, these data suggests that the analgesic effect of minocycline results from its effect on microglia in the LS spinal cord and SGCs in the DRGs. The microglial inhibitory effect of minocycline is reported to be associated with attenuation of p38 mitogen-activated protein kinase (MAPK) activation in the spinal microglia (Hua et al., 2005).
While reports indicate that minocycline has no direct effect on the neurons, Gonzalez et al (2007) have reported that minocycline is associated with the mitigation of neuronal excitability, glutamate release, and Ca2+ overloading in hippocampal neurons. In our study, direct inhibitory action of minocycline on the LS spinal and PNA neurons is highly unlikely as minocycline was found to be ineffective on these neurons in naïve rats. However, the direct effect of minocycline on the LS spinal neurons and PNA neurons in inflamed rats cannot be ruled out, as inflammation has been reported to induce long-term alterations in the signaling processes either in the GI tract, spinal cord and brain (Delgado-Aros and Camilleri, 2005). This is further supported by the observed immediate inhibitory effect of minocycline on the LS spinal neurons (5 min) in TNBS-treated rats. The majority of the studies that report on the analgesic effect of minocycline have used a chronic treatment protocol which is in sharp contrast to our study where only a single injection of minocycline was administered. It is highly possible that the reversal of neuronal sensitization by inhibiting spinal microglia and blunting the expression of pro-inflammatory agents are less likely to occur within the short time frame (5 min) of our experiments. This is indicative of the possibility that minocycline might as well have other pain modulating mechanisms in addition to the widely accepted glial inhibitory effect. Recent reports suggests that minocycline inhibits Na+ channels in the primary afferent neurons (Kim et al., 2011) and also inhibits phosphorylated extracellular signal-regulated kinase (p-ERK) expression in the spinal cord (Cho et al., 2012), supporting a neuronal target for minocycline. Therefore, it is possible that the observed analgesic effect of minocycline in this study might not be exclusively through glial inhibition, but rather a combined effect on glial cells and neuronal targets. However, further experiments are warranted to identify these targets. In conclusion, the results from these experiments strongly indicate that activation of glial lead to visceral hyperalgesia and minocycline can be effectively used to attenuate inflammation-induced chronic visceral pain. Minocycline might as well act on neuronal targets in the spinal cord of inflamed rats, in addition to the widely reported glial inhibitory action to produce analgesia.
Acknowledgment
This work was supported by an NIH 1R56DK089493-01 grant awarded to Drs. J. N. Sengupta and B. Banerjee. No conflicts of interest exist with any of the authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics approval:
All experiments were performed according to the approved guidelines of the Institutional Animal care and Use committee at the Medical college of Wisconsin (approval # AUA355) and International Association for the Study of Pain (IASP) for humane use of laboratory animals.
References
- Audet JN, Gowing G, Paradis R, Soucy G, Julien JP. Ablation of proliferating cells in the CNS exacerbates motor neuron disease caused by mutant superoxide dismutase. PLoS One. 2012;7:e34932. doi: 10.1371/journal.pone.0034932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson AL. Pharmacotherapeutics of the newer tetracyclines. J Am Vet Med Assoc. 1980;176:1061–1068. [PubMed] [Google Scholar]
- Banerjee B, Medda BK, Zheng Y, Miller H, Miranda A, Sengupta JN, Shaker R. Alterations in N-methyl-D-aspartate receptor subunits in primary sensory neurons following acid-induced esophagitis in cats. Am J Physiol Gastrointest Liver Physiol. 2009;296:G66–77. doi: 10.1152/ajpgi.90419.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Zhang YQ. Spinal glial activation contributes to pathological pain states. Neuroscience Biobehavioral Rev. 2008;32:972–983. doi: 10.1016/j.neubiorev.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Cervero F, Laird JMA. Visceral Pain. The Lancet. 1999;353:2145–2148. doi: 10.1016/S0140-6736(99)01306-9. [DOI] [PubMed] [Google Scholar]
- Cho IH, Chung YM, Park CK, Park SH, Li HY, Kim D, Piao ZG, Choi SY, Lee SJ. Park, K., Kim JS, Jung SJ, Oh SB. Systemic administration of minocycline inhibits formalin-induced inflammatory pain in rat. Brain Res. 2006;1072:208–214. doi: 10.1016/j.brainres.2005.12.039. [DOI] [PubMed] [Google Scholar]
- Cho IH, Lee MJ, Jang M, Gwak NG, Lee KY, Jung HS. Minocycline markedly reduces acute visceral nociception via inhibiting neuronal ERK phosphorylation. Mol Pain. 2012;8:1–13. doi: 10.1186/1744-8069-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle DE. Partial peripheral nerve injury leads to activation of astroglia and microglia which parallels the development of allodynic behavior. Glia. 1998;23:75–83. [PubMed] [Google Scholar]
- DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- Delgado-Aros S, Camilleri M. Visceral Hypersensitivity. J Clin Gastroenterol. 2005;39:S194–203. doi: 10.1097/01.mcg.0000156114.22598.1b. [DOI] [PubMed] [Google Scholar]
- Garrido-Mesa N, Zarzuelo A, Gálvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol. 2013;169:337–352. doi: 10.1111/bph.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González JC, Egea J, Del Carmen Godino M, Fernandez-Gomez FJ, Sánchez-Prieto J, Gandía L, García AG, Jordán J, Hernández-Guijo JM. Neuroprotectant minocycline depresses glutamatergic neurotransmission and Ca2+ signaling in hippocampal neurons. Eur J Neurosci. 2007;26:2481–2495. doi: 10.1111/j.1460-9568.2007.05873.x. [DOI] [PubMed] [Google Scholar]
- Hains BC, Waxman SG. Activated microglia contributes to the maintenance of chronic pain after spinal cord injury. J Neurosci. 2006;26:4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Rev. 2005;48:457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Hua XY, Svensson CI, Matsui T, Fitzsimmons B, Yaksh TL, Webb M. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur J Neurosci. 2005;22:2431–2440. doi: 10.1111/j.1460-9568.2005.04451.x. [DOI] [PubMed] [Google Scholar]
- Huang TY, Hanani M. Morphological and electrophysiological changes in mouse dorsal root ganglia after partial colonic obstruction. Am J Physiol Gastrointest Liver Physiol. 2005;289:G670–G678. doi: 10.1152/ajpgi.00028.2005. [DOI] [PubMed] [Google Scholar]
- Huang TY, Belzer V, Hanani M. Gap junctions in dorsal root ganglia: possible contribution to visceral pain. Eur J Pain. 2010;14:e1–11. doi: 10.1016/j.ejpain.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Ji RR, Berta T, Nedergaard M. Glia and pain: Is chronic pain a gliopathy? Pain. 2013:2013. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of Microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kim TH, Kim HI, Kim J, Park M, Song JH. Effects of minocycline on Na+ currents in rat dorsal root ganglion neurons. Brain Res. 2011;1370:34–42. doi: 10.1016/j.brainres.2010.11.038. [DOI] [PubMed] [Google Scholar]
- Kruschewski M, Foitzik T, Perez-Canto A, Hubotter A, Buhr HJ. Changes of colonic mucosal microcirculation and histology in two colitis models: an experimental study using intravital microscopy and a new histological scoring system. Dig Dis Sci. 46:2336–2343. doi: 10.1023/a:1012334727509. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Lin CS, Tsaur ML, Chen CC, Wang TY, Lin CF, Lai YL, Hsu TC, Pan YY, Yang CH, Cheng JK. Chronic intrathecal infusion of minocycline prevents the development of spinal-nerve ligation-induced pain in rats. Reg Anesth Pain Med. 2007;32:209–216. doi: 10.1016/j.rapm.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Liu PY, Lu CL, Wang CC, Lee IH, Hsieh JC, Chen CC, Lee HF, Lin HC, Chang FY, Lee SD. Spinal microglia initiate and maintain hyperalgesia in a rat model of chronic pancreatitis. Gastroenterology. 2012;142:165–173. doi: 10.1053/j.gastro.2011.09.041. [DOI] [PubMed] [Google Scholar]
- Luo H, Cheng J, Han JS, Wan Y. Change of vanilloid receptor 1 expression in dorsal root ganglion and spinal dorsal horn during inflammatory nociception induced by complete Freund's adjuvant in rats. Neuro report. 2004;15:655–658. doi: 10.1097/00001756-200403220-00016. [DOI] [PubMed] [Google Scholar]
- Morris G, Beck P, Herridge M, Depew W, Szewczuk M, Wallace J. Hapten-induced model of colonic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- Rhodes KE, Moon IDF, Fawcett JW. Inhibiting cell proliferation during formation of the glial scar: effects on axon regeneration in the CNS. Neuroscience. 2003;120:41–56. doi: 10.1016/s0306-4522(03)00285-9. [DOI] [PubMed] [Google Scholar]
- Scholz J, Abele A, Marian C, Häussler A, Herbert TA, Woolf CJ, Tegeder I. Low-dose methotrexate reduces peripheral nerve injury-evoked spinal microglial activation and neuropathic pain behavior in rats. Pain. 138:130–142. doi: 10.1016/j.pain.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta JN, Medda BK, Shaker R. Effect of GABA(B) receptor agonist on distension-sensitive pelvic nerve afferent fibers innervating rat colon. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1343–1351. doi: 10.1152/ajpgi.00124.2002. [DOI] [PubMed] [Google Scholar]
- Smith HS. Activated microglia in nociception. Pain Physician. 2010;13:295–304. [PubMed] [Google Scholar]
- Stirling DP, Koochesfahani KM, Steeves JD, Tetzlaff W. Minocycline as a neuroprotective agent. Neuroscientist. 2005;11:308–322. doi: 10.1177/1073858405275175. [DOI] [PubMed] [Google Scholar]
- Stuesse SL, Cruce WL, Lovell JA, McBurney DL, Crisp T. Microglial proliferation in the spinal cord of aged rats with a sciatic nerve injury. Neurosci Lett. 2000;287:121–124. doi: 10.1016/s0304-3940(00)01142-3. [DOI] [PubMed] [Google Scholar]
- Sura J. Microglial cells in astroglial cultures: a cautionary note. J Neuroinflammation. 2007;4:26. doi: 10.1186/1742-2094-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter MR, Wen YR, Decosterd I, Ji RR. Do glial cells control pain? Neuron Glia Biol. 2007;3:255–268. doi: 10.1017/S1740925X08000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Takahashi M, Matsumoto S. Contribution of the activation of satellite glia in sensory ganglia to pathological pain. Neuroscience Biobehavioral Rev. 2009;33:784–792. doi: 10.1016/j.neubiorev.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Thomas M, Le WD. Minocycline: neuroprotective mechanisms in Parkinson’s disease. Curr Pharm Des. 2004;10:679–686. doi: 10.2174/1381612043453162. [DOI] [PubMed] [Google Scholar]
- Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21:2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kogel AJ, Sissingh HA. Effects of intrathecal methotrexate and cytosine arabinoside on the radiation tolerance of the rat spinal cord. Radio Ther Oncol. 1985;4:239–251. doi: 10.1016/s0167-8140(85)80089-x. [DOI] [PubMed] [Google Scholar]
- Zhang H, Yoon SY, Zhang H, Dougherty PM. Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of Paclitaxel-induced painful neuropathy. J Pain. 2012;13:293–303. doi: 10.1016/j.jpain.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]