SUMMARY
Hematopoietic stem cells (HSCs) are produced by a small cohort of hemogenic endothelial cells (ECs) during development through the formation of intra-aortic hematopoietic cell (HC) clusters (HCs). The Runx1 transcription factor plays a key role in the EC to HC and HSC transition. We show that Runx1 expression in hemogenic ECs and the subsequent initiation of HC formation are tightly controlled by the sub-aortic mesenchyme, although the mesenchyme is not a source of HCs. Runx1 and Notch signaling are involved in this process, with Notch signaling decreasing with time in HCs. Inhibiting Notch signaling readily increases HC production in mouse and chicken embryos. In the mouse however, this increase is transient. Collectively, we show complementary roles of hemogenic ECs and mesenchymal compartments in triggering aortic hematopoiesis. The sub-aortic mesenchyme induces Runx1 expression in hemogenic-primed endothelial cells and collaborates with Notch dynamics to control aortic hematopoiesis.
INTRODUCTION
In vertebrates, the aorta was shown to autonomously generate adult-type hematopoietic stem cells (HSCs) during development. Aortic hematopoiesis is characterized by the production of small clusters of hematopoietic cells (HCs) that accumulate in the lumen, closely associated with the endothelial floor (Dieterlen-Lièvre et al., 2006; Dzierzak and Speck, 2008). Polarization of hematopoiesis to the vessel floor in the avian embryo was shown to rely on the replacement of the initial aortic roof by somite-derived endothelial cells (ECs) (Pardanaud et al., 1996; Pouget et al., 2006). Polarization is under the control of a reciprocal Hedgehog-BMP molecular gradient in the zebrafish embryo (Wilkinson et al., 2009) and/or activated by a somitic Wnt16/Notch pathway (Clements et al., 2011). In the mouse, HCs are found both dorsally and ventrally in the aorta (Taoudi and Medvinsky, 2007; Yokomizo and Dzierzak, 2010) but HSCs are restricted to the ventral side, suggesting that underlying tissues influence hematopoietic production (Taoudi and Medvinsky, 2007).
Compelling evidence indicates that HCs are derived from specialized Endothelial Cells (ECs) endowed with a hemogenic potential in the avian (Jaffredo et al., 1998), mouse (de Bruijn et al., 2000; Zovein et al., 2008) and human (Oberlin et al., 2002) embryos, although a sub-aortic origin cannot be completely ruled out (Bertrand et al., 2005; Rybtsov et al., 2011). Live imaging techniques showed that embryonic stem cells generated ECs which, in turn, produced hematopoietic cells (Eilken et al., 2009; Lancrin et al., 2009). Finally time-lapse approaches showed that this production occurs in vivo in mouse aortic explants (Boisset et al., 2010) and in whole zebrafish embryos (Bertrand et al., 2010a; Kissa and Herbomel, 2010; Lam et al., 2010).
When and how the hemogenic program is induced is yet to be discovered. Several lines of evidence, however, indicate that local environmental signals influence hematopoiesis. For instance, an inductive/trophic effect of endoderm on mesoderm was shown to confer hemogenic potential to non-hemogenic ECs (Pardanaud and Dieterlen-Lièvre, 1999), or to influence HSC number in the aorta (Peeters et al., 2009). The presence of several molecules involved in hematopoiesis suggests that the ventral aortic mesenchyme may serve as a hematopoiesis-promoting microenvironment (Marshall et al., 2000). Moreover, cell lines isolated from the aortic region are potent supporters of embryonic and adult hematopoiesis (Oostendorp et al., 2002). However, the origin and role(s) of the sub-aortic mesenchyme are poorly understood. The problem lays primarily in the facts that: 1) due to specific embryological constraints in the mouse embryo, endothelium and sub-aortic mesenchyme are not amenable to physical separation, and 2) both endothelium and sub-aortic mesenchyme are reported to express the key transcription factor Runx1, making the situation difficult to analyze (Azcoitia et al., 2005; North et al., 1999). Runx1 is responsible for the production of HCs and HSCs in the aorta (North et al., 1999; North et al., 2002) and seems to be required for the earliest phases of hematopoietic cell formation from the endothelium, but dispensable for the later ones (Chen et al., 2009). Yet neither the precise time point at which Runx1 is expressed during aortic hematopoiesis nor the developmental events controlling its expression have been identified.
Considering that aortic hematopoiesis mostly originates from hemogenic ECs, it can be viewed as a cell fate change in which ECs loose their characteristics and acquire hematopoietic-specific markers (Jaffredo et al., 2010). This endothelial-to-hematopoietic transition is under the control of the Notch pathway. Notch regulates cell fate decisions in many developmental systems including hematopoiesis. Gene inactivation experiments showed that Notch signaling, and the Notch ligand Jagged1 are involved in embryonic hematopoiesis (Hadland et al., 2004; Kumano et al., 2003), Notch signaling activates Gata2 expression via RBPjκ (Robert-Moreno et al., 2005; Robert-Moreno et al., 2008). The Notch pathway was shown to be upstream the genetic cascade driving Runx1 expression during hematopoietic production in the zebrafish aorta (Burns et al., 2005) and specifically required for HSC formation (Bertrand et al., 2010b; Rowlinson and Gering, 2010) through a Wnt16-dependant mechanism (Clements et al., 2011).
Here we show that during aorta formation, Runx1 expression is a secondary event tightly controlled by the sub-aortic mesenchyme, but that mesenchyme is not a source of HCs. Absence of the sub-aortic mesenchyme prevents both Runx1 expression and HC formation, showing the interdependent roles of the hemogenic EC and mesenchyme. However, the sub-aortic mesenchyme has no influence on vessel identity. Neither peri-aortic smooth muscle cells nor the mesonephros influenced aortic hematopoiesis. Runx1 expression is accompanied by down-regulation of the Notch pathway in hemogenic ECs, a prerequisite to initiate hematopoiesis. This mechanism is conserved, but a few members of the Notch pathway display species-specific differences. Moreover, blocking Notch signaling results in overproduction of CD45-positive cells from the aorta. Taken together, our work opens the field for the future identification of critical regulators of aortic hematopoiesis and points to a necessary comparison between species for future biomedical applications.
RESULTS
Runx1 expression is spatially and temporally controlled during formation of the aorta
We established the expression patterns of runx1, pu-1 (a Runx1 target (Huang et al., 2008)) and c-myb (a Runx1 molecular partner (Hernandez-Munain and Krangel, 1994)) by in situ hybridization on adjacent sections at selected stages of aorta formation in the chick embryo. Expression was examined at stages representing pre-fusion paired aortas, and post-fusion aortas before the HC stage, at 48h (Figure 1A, A′ and S1A–D), 55h (Figure 1B, B′), 60h (Figure 1C, C′ and S1E–J), of development. Later stages displayed already reported runx1 expressions in the hemogenic endothelium and the hematopoietic clusters and were not included to the figures. Runx1 expression was found to initiate in the lateral aspect of the paired aortas one day before the HC stage, and to progressively extend ventrally while remaining confined to the endothelial layer marking the hemogenic endothelium. Pu-1 and c-myb mRNAs followed runx1 expression with a slight delay, and also marked the hemogenic endothelium (Figure S1A–J) and HCs (not shown). Contrary to the mouse expression pattern (Azcoitia et al., 2005; North et al., 1999; Zovein et al., 2008), no runx1 mesenchymal expression was found in the chicken embryo. This lateral to ventral pattern strongly suggested that runx1 expression was tightly controlled. We thus sought for tissues or cells whose association or migration to the floor of the aorta was contemporaneous with runx1 expression.
Figure 1. Runx1 patterns and dynamics of aorta formation.
(A–C′) Aortic runx1 expression. In situ hybridization.
(A). Early paired aortae stage; 48h. No conspicuous runx1 expression is present at that stage. Bar=50μm.
(A′) Magnification of the frame in A. A single runx1+ cell is present in the lateral endothelium (arrowhead). Bar=15μm.
(B) Late paired aortae stage; 55h. Runx1 is barely detectable. Bar=70μm. B′. Higher magnification of the frame in B. Runx1 expression is restricted to a few cells in the ventro-lateral endothelium (arrowheads). Bar=20μm.
(C) Single aorta stage, immediately after fusion; 60h.
Runx1 is expressed throughout the whole ventral endothelium except in its ventral most part. Bar=80μm. C′. Magnification of the frame in C showing the endothelial-specific expression of runx1. Bar=25μm.
(D–G) Fate mapping of the sub-aortic mesenchyme.
(D) Experimental design. The embryo is cultured ventral side up. Endoderm is opened and a DiI crystal (red arrow) is deposited on the splanchnopleural mesoderm.
(E) 10-somite stage. Bilateral deposition of crystals.
(F) Speed measure of a DiI crystal. Crystal progression is constant over time.
(G) Cross section through a 12-somite stage embryo bilaterally labeled with DiI. The DiI+ areas are immediately underneath the aorta. Bar=70μm.
(H) Splanchnopleural origin of the primitive aorta. In addition to the splanchnopleural mesoderm, the aorta was sometimes labeled indicating that both tissues have the same origin. Here, a single DiI crystal was deposited on one side resulting in the staining of the whole hemi aorta and associated mesenchyme.
Ao, aorta; C, coelom; N, notochord; NT, neural tube; S, somite.
See also Figure S1.
Mapping the origin of the sub-aortic mesenchyme
We focused on the sub-aortic mesenchyme, whose onset of formation and subsequent differentiation was coincident with runx1 expression pattern. Sub-aortic mesenchyme was recently shown to originate from the lateral plate mesoderm in mouse (Wasteson et al., 2008) and chicken embryos (Wiegreffe et al., 2009), but the precise location of the mesenchymal precursors was not defined. Based on the observation of E1.5 to E2 avian embryos, a splanchnopleural origin appeared likely.
We performed fate-mapping experiments using DiI labeling and quail/chicken grafts. In the first series of experiments, groups of cells in 10 to 13 somite stage embryos were labeled using DiI crystals that were inserted in the splanchnic mesoderm at the level of the last-formed somite, at different distances from the midline (n=22; Figure 1D, E). Six samples, recorded during 24h or 36h, showed very dynamic movements (supplementary movie 1). DiI+ cells that were lateral to the somites moved to the embryo midline. This movement is due to the formation of the lateral body folds that raises the embryo body and, at the same time, allows left and right splanchnic epithelial sheets to meet. Distance measurements showed that DiI+ cells moved at a constant speed of 11μm/hour, covering about 300 μm to reach the midline (Figure 1F and supplementary movie 1). Analysis of sections showed that DiI+ cells localized underneath the aorta, (Figure 1G), and never crossed the midline. In most cases ECs were not labeled. Placing the crystals more superficially, immediately underneath the endoderm, resulted in aortic endothelium staining (Figure 1H), demonstrating the close association between the splanchnic mesoderm and aortic rudiments and the ventral origin of the primitive aorta. In a second series of experiments, pieces of chicken splanchnic mesoderm were replaced by their quail counterparts (n=17 embryos), and the location of quail cells monitored with the quail-specific antibody QCPN (not shown). DiI and quail-chicken approaches yielded similar results. These approaches were completed with scanning electron microscopy studies or normal embryos during embryonic day 2. In Figure S1K (22 somite stage), the splanchnic epithelium began to wrap around the aortic rudiment. Slightly later on, cells reached the aortic floor (Figure S1L).
In conclusion, the sub-aortic tissue originated from a splanchnic mesoderm segment localized between 250 to 300μm from the embryo midline. Labeled cells lateral to this segment associated with the future gut (not shown), revealing a precise dorso ventral allocation of splanchnic mesodermal blocks according to their medio-lateral position.
Preventing migration of the sub-aortic mesenchyme inhibits runx1 expression and initiation of hematopoiesis without impairing vessel formation or arterio-venous identity
Fate mapping experiments prompted us to study the role of the sub-aortic mesenchyme in the initiation of hematopoiesis. Migration of mesenchyme to the midline was prevented by making a slit on one side of the embryo, either immediately lateral to the somite or in the intermediate mesoderm, that separated the embryo proper from the lateral plate (Figure 2A). The non-operated side served as control. Both experiments yielded similar results. The slit was made at E2 when the embryo was still flat and no contact between lateral plate and aortic anlagen had yet occurred (Figure 2A). The slit does not modify the dorso-ventral allocation of hemogenic and non-hemogenic ECs of the aorta since 1° it was made when the aortic anlagen were still formed and 2° the global shape of the aorta and the presence and correct position of the segmental arteries, derived from the somite, are not modified. Lack of lateral plate resulted in the absence of a coelom, fusion between ectoderm and endoderm, and formation of a hemi-digestive tube on the operated side (Figure 2B). One day after the slit was made, the paired aortas fused but no conspicuous sign of aortic hematopoiesis was yet visible (n=3). Runx1 was expressed by ventral aortic EC on the control side, but was totally absent on the operated side (Figure 2C). Vascular endothelial (Ve) cadherin was expressed normally, showing that vascular EC identity was not impaired by the operation (Figure 2D). Delta–like4 expression was maintained, demonstrating no change in arterio-venous identity (Figure S2A). Two days after the slit was made, runx1+ HCs were visible in the control but not in the operated side (Figure 2E) (n=4), consistent with the lack of runx1 endothelial expression one day earlier. Ve-cadherin remained expressed by ECs, but its expression on the control side was down-regulated in HCs (Figure 2F), in keeping with our previous data (Jaffredo et al., 2005). Delta-like4 was also maintained in ECs and down-regulated in HCs (Figure S2B). Formation of the sub-aortic mesenchyme thus appeared critical for the initiation of runx1 expression and the production of HCs. However, the absence of mesenchyme had no influence on the expression of arterial markers, indicating that the lack of runx1 expression and the absence of HCs on the operated side were not due to a loss of arterial identity.
Figure 2. Role of the sub-aortic mesenchyme in aortic hematopoiesis.
(A) Experimental scheme and further development. The cut separates somite and future kidney from the lateral plate. (upper scheme) preventing the splanchnopleural mesoderm to contribute to the aortic region (lower scheme). Ectoderm: white; endoderm: green, somites: dark pink and lateral plate: light pink.
(B) Schematic reprentation of normal and operated embryos 48h after the slit. Mid-trunk level.
Left hand scheme: normal embryo. Somite-derived structures are in dark pink. The aorta (red, median circle) has two rows of hematopoietic cells. The gut endoderm (green) is surrounded by the mesenchyme (light pink) are appended into the coelomic cavity.
Right hand scheme: operated embryo. Symmetry is not affected except in the ventral part of the embryo. Aortic hematopoiesis pattern is affected. Coelom on the operated side is lacking and gut is opened. Endoderm and ectoderm have fused
(C–F) Aortic region at 24h (C, D) and 48h (E, F) after the slit. In situ hybridization with runx1 (C, E) and ve-cadherin (D, F)
(C) Runx1 expression is present on the control side (left) but absent on the operated side (right).
(D) Ve-cadherin pattern is normal suggesting that no major modification of EC identity has occurred. Bar=130μm.
(E) Runx1+ HC clusters are visible on the control side (black arrow) but are lacking on the operated side. Note the presence of a loose ventral tissue on the operated side compared to the control side.
(F) Ve-cadherin expression is normal. Note the decrease of ve-cadherin expression mRNA in the HC clusters as reported (Jaffredo et al., 2005). Bar=150μm.
C, coelom, CV, cardinal vein.
See also Figure S2.
We used the same experimental approach to analyze the role of smooth muscle cells in the initiation and maintenance of aortic hematopoiesis. No difference was seen in smooth muscle actin expression in the control and operated sides (Figure S2C) demonstrating that this tissue is not sufficient by itself to induce runx1 expression and cluster formation in the absence of sub-aortic mesenchyme. Finally, since a role for mesonephros and Wolffian duct had been suggested, we selectively removed the intermediate mesoderm over the length of 4 somites on one side, with the non-operated side serving as the control. In addition to morphological criteria, cjagged2 was used to confirm the absence of mesonephros. One day after the ablation, runx1 was found in both the operated and non-operated sides, indicating that signaling from the mesonephros is not required for hematopoietic specification (Figure S2D, E).
Endothelial, but not mesenchymal origin of the hematopoietic clusters
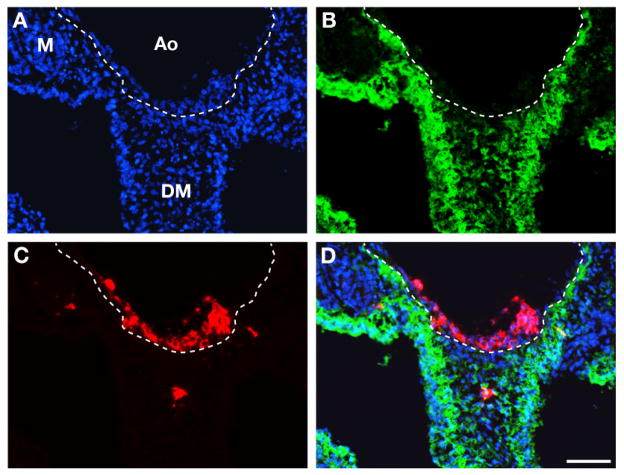
The absence of HCs following the block of sub-aortic mesenchyme migration could be due to either a lack of induction on the hemogenic endothelium, or an absence of hematopoietic cells carried by the mesenchyme. To discriminate between these possibilities we labeled the whole lateral plate mesoderm at the epithelial stage with the lipophilic dye 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFDA-SE; Invitrogen) and followed the labeled cells until the HC stage. Since the splanchnic epithelium gives rise to the sub-aortic mesenchyme, we expected the mesenchyme to be fluorescent. If HCs originate from this source, they should also be labeled. CFDA-SE was inoculated into the coelom, allowing cells lining the cavity to be labeled. Two-day embryos received 1–2μl of 5μM CFDA-SE and were further incubated for 24h. Seven embryos were analyzed; all displayed a similar staining. The sub-aortic mesenchymal cells were CFSE+ (Figure 3A, B), but CD45+ HC clusters were free of CFSE labeling (Figure 3C, D) indicating that they did not originate from the sub-aortic mesenchyme.
Figure 3. Tracing splanchnopleural mesoderm derivatives using CFDA-SE.
24h after CFDA-SE labeling. Same section triple stained with DAPI (A), CFDA-SE (B) and CD45 (C). The dotted line indicates the limit between the endothelium and the sub-aortic mesenchyme.
DAPI staining reveals the topography of the tissues. CFDA-SE stains the sub-aortic mesenchyme and the coelomic epithelium. CD45 stains the aortic clusters. (D) merge of DAPI, CFDA-SE and CD45 signals. The aortic HC clusters were never green in keeping with an endothelial origin of HC clusters and the complementary roles of aortic endothelium and sub-aortic mesenchyme in aortic hematopoiesis. Bar=80μm.
Interplay between Notch signaling and runx1 expression in aortic hematopoiesis
We investigated the role of Notch signaling during the early steps of aortic hematopoiesis. Serrate1 and serrate2 are avian orthologues of mouse Notch ligands Jag1 and Jag2 (and will hereafter be referred to as cjagged1 and 2, respectively). Both cjagged1 and cjagged2 displayed the same expression pattern by in situ hybridization during aortic development (not shown), but because cjagged2 yielded the better signal, it was chosen for further analysis. At the 29–32 somite stage, the time of aortic fusion for the level considered, cjagged2 was present throughout all aortic EC (Figure S3B), and runx1 expression intensified (Figure S3A). Immediately after fusion, cjagged2 expression decreased in the two ventral ridges (Figure 4B) where runx1 displayed the strongest expression (Figure 4A). At the cluster stage, cjagged2 and runx1 became mutually exclusive (Figure S3C, D). Thus runx1 expression is associated with down-regulation of the cjagged1 and 2 Notch ligands, which become restricted to ECs.
Figure 4. Analysis of the Notch pathway during aortic hematopoiesis.
(A, B) Mutual exclusion between cjagged2 and runx1 during aortic hematopoiesis. In situ hybridization, adjacent sections separated by 7μm.
(A) Runx1 expression extends to the whole floor (arrowheads) except to ECs in the middline.
(B) Cjagged2 expression is lost (arrowheads) in cells up-regulating runx1.
(C, D) Q-PCR analysis of the Notch pathway in AGM ECs at the hematopoietic stage in chicken (C) and mouse (D) embryos i.e. respectively E3.5 and E11.5. ECs (pink) were sorted on the basis of AcLDL upake (chicken) and CD144 (mouse) expression. HCs (green) were retreived by including the expression of CD45.
(E) Hes1 expression analysis by Q-PCR in the E11.5 Runx1+ hemogenic endothelium compared to increasing stages of hematopoietic maturation characterized by their combinatorial expression of CD41 and CD45. A decrease in Hes1 expression associated to HC maturation from the endothelium is clearly visible.
(F, G) Detection of Active Notch following pTP1-Venus reporter construct electroporation in the aorta.
(F) HC cluster stage. Two clusters are visible (white arrows). The green staining (active Notch) is restricted to ECs and absent from the clusters.
(G) Same section double stained for active Notch (green) and CD45 (red) to visualize the clusters. Bar=40μm
(H–L) Hematopoietic production analysis of E9.5 mouse P-Sps treated or not with DAPT.
(H) Number of cells in vehicle- and DAPT-treated aortas after 7 days of coculture on OP9 layer. Differences in cell number are not significant.
(I, J) Percentages of CD45+ cells produced by E9.5 mouse P-Sp after 3 (I) and 6 (J) days of culture with or without DAPT. A significant increase of CD45+ cell production is visible at 3 days in the DAPT-treated samples compared to the vehicle-treated samples. The inverse is observed at 6 days of culture. Error bars represent the results from four independent assays.
(K) Percentage of 7AAD+ cells at 3 (D3) and 6 (D6) days following mouse P-Sp culture incipience. NS: not significant
(L) Percentage of wells positive for D7-CAFCs. A significant decrease in CAFCs is observed in the DAPT-treated samples in keeping with previously published data (Robert-Moreno et al., 2008).
The error bars represent s.e.m. Three stars indicates p<0.01, two p<0.25, one p<0.5, NS, not significant
See also Figure S3.
Q-PCR analysis demonstrates down regulation of the Notch pathway during aortic hematopoiesis
Avian and mouse embryos at pre HC (E2.5 and E9) and HC (E3.5 and E11.5) stages were used to isolate ECs and HCs from the aortic region by flow cytometry, and expression of members of the Notch pathway and several Notch targets was probed by Q-PCR (Supplemental experimental procedure). Both avian and mouse embryos displayed a similar expression pattern of Notch signaling in ECs at the pre HC stage (Figure S3E, F). Expression of several Notch pathway members, including Jag2, Dll4 and Gata2 was enriched in the EC fraction compared to the non-endothelial fraction, whereas Notch1, Jagged1, RbpjK, Hes1, and Hey2 displayed weaker levels of expression relative to the non-EC fraction (Figure S3E, F). The EC-associated expression pattern remained unchanged at the time of HC production, but there was a strong decrease in Notch ligand expression (Jag1, Jag2, Dll4) and an increase in RbpjK expression in the HC as compared to the EC population (Figure 4C, D). Although changes were more visible in the chicken embryo, both species followed the same pattern, except for Jag2 in the mouse embryo, which showed no decrease in HCs relative to ECs (Figure 4D).
We also took advantage of the Runx1-GFP reporter mouse (Lorsbach et al., 2004) to analyze expression of the Notch target Hes1 in a purified population of hemogenic endothelium (Runx1-GFP+CD144+CD41−CD45−), an immature HC population (Runx1-GFP+CD144+CD41+CD45−), and two more mature HC populations (Runx1-GFP+CD144+CD41+CD45+ and Runx1-GFP+CD144+CD41−CD45+) at the 40–45 somite pair stage (E11.5). All three HC populations had a reduced level of Hes1 expression relative to ECs (Figure 4E). Together the data suggest that Notch signaling is reduced in HCs relative to hemogenic ECs.
Active Notch1 is expressed by both the aortic endothelium and the sub-aortic mesenchyme
As another approach to identify cells in the aortic region that contained activated Notch, we electroporated the Notch reporter plasmid pTP1-Venus (Kohyama et al., 2005) into the chick aorta (Rossello and Torres, 2010). We found GFP+ cells both in the endothelium (Figure S3G) and in the sub-aortic mesenchyme (Figure S3H) before the HC stage. However, during the HC stage Notch signaling remained present in ECs and in tissues surrounding the aorta, but disappeared from the HCs (Figure 4F, G) (n=15). In situ hybridization and immunohistochemistry demonstrated the presence of Notch1, but not Notch2 in the aortic region (not shown), suggesting that the active Notch signal was derived from Notch1.
Blocking gamma secretase activity promotes hematopoietic production in aorta organotypic culture and whole embryo culture
In order to evaluate the role of Notch signaling on aortic hematopoiesis, we employed the widely used chemical Notch inhibitor, DAPT, to block gamma-secretase activity and prevent cleavage of the Notch intracellular domain. We added 50μM DAPT to E9.5 (20–25 somite pairs) mouse P-Sp explant cultures. No significant difference in cell counts was found between control and DAPT-treated cultures after 7 days (4 independent experiments in the mouse; Figure 4H). We first examined the production of CD45+ HCs (which includes both committed HCs and progenitors) after 3 and 6 days. Three days after DAPT treatment, mouse P-Sp explants contained significantly more CD45+ cells compared to vehicle treated controls (Figure 4I), suggesting that the downregulation of Notch signaling that accompanies HC cluster formation augments HC production. After 6 days of explant culture, the inverse is observed (Figure 4J), indicating that the enhancing effect of DAPT on CD45+ cell number was transient. We checked whether this could be related to an increase of apoptosis or cell death during the culture. Flow cytometric analysis of the CD45+ fraction with Annexin V and 7AAD revealed that DAPT treatment did not alter the percentage of 7AAD+ cells in the CD45+ population of mouse AGM explant cultures at either 3 or 6 days (Figure 4K). In contrast, during this time course, the percentage of apoptotic Annexin V+ 7AAD− cells increased more in the DAPT-treated cultures than in controls (2 fold versus 1,7 fold – data not shown). Thus differences in the initial phases of CD45+ HC production and response of HC to apoptosis and cell death explain the differential effects of DAPT treatment at early and late time points in the explant culture period. We next quantified the number of hematopoietic progenitors after 7 days of explant culture with or without 50μM DAPT. Clonogenic progenitors were assayed in methylcellulose and in D7-cobblestone area forming cells (CAFC) assays. Mouse D7-CAFCs, as well as total CFCs (data not shown) were decreased 2–3 fold (p=0.05) in the presence of DAPT (Figure 4L), in keeping with previous data (Robert-Moreno et al., 2008), and consistent with the DAPT-induced decrease in CD45+ cells after 6 days of explant culture.
When chicken P-Sps at equivalent hematopoietic stages (26–30 somite pairs) were treated in the same conditions, no significant difference in cell counts was found between control and DAPT-treated cultures after 7 days (5 independent experiments; Figure S3I). However, CD45+ HCs produced in DAPT-treated samples were found to be less sensitive to cell death than their non-treated counterpart (FigureS3J). Again, hematopoietic progenitors were also quantified. Because not all hematopoietic cytokines for chick progenitors are commercially available, only D7-CAFCs assays were performed for chick aorta cells by co-culturing with MS5 stromal cells. In contrast to the mouse, the number of D7-CAFCs was increased in chick cultures in the presence of DAPT (p=0.016, Figure S3K, L). We also exposed whole chicken embryos to DAPT and examined CD45+ cells in the aorta. DAPT was delivered to the endoderm, close to the aortic anlagen. Treatment was performed at the 29 to 32-somite stage and lasted 24h (n=6). Application at earlier stages resulted in abnormalities and death. DMSO caused hemorrhages in the yolk sac but did not impair embryo viability (Figure 5A). Sections revealed a large increase in the number of CD45+ cells after 24 hours of DAPT exposure, in keeping with the increase seen in short term mouse P-Sps explant cultures. In the most dramatic cases, the aorta was filled with CD45+ cells that formed a giant cluster (Figure 5B, C). In some instances we found some dorsal ECs expressing CD45, but the majority of the CD45+ cells remained attached to the ventral side. No HC cluster was detected in the cardinal veins indicating that DAPT treatment did not change the identity of the vessels, as confirmed by the artery-specific marker delta-like4 (data not shown). We occasionally observed CD45+ HC clusters in the paired aorta (Figure 5D), which was never seen in vehicle-treated embryos, indicating that hematopoietic production was also accelerated. We believe that the difference in the response of chick and mouse HCs to DAPT is due 1° to a DAPT-induced increase of apoptosis at 6 days of culture, leading to a decrease in CD45+ cells in the mouse culture and 2° to a lower sensitivity to cell death of chick HC produced in DAPT-treated samples compared to their non-treated counterpart
Figure 5. Effect of whole embryo DAPT treatment on aortic hematopoiesis.
A, Representative embryo following DAPT treatment. No gross anomaly is visible but some blood lacunae have formed. Bar=1mm.
B, C. Cross sections through the aorta of a treated embryo after 24h. CD45 immunohistology. Numerous CD45+ cells are present in the aortic lumen. These structures form a large HC cluster attached to the ventral aortic side.
D. Ectopic hematopoiesis in the paired aortae. A ventral HC cluster on the left hand aorta and a dorsal cluster on the right hand aorta are clearly visible. HC are never seen at this level in normal embryos.
Bar=50μm.
DISCUSSION
Our study unravels the critical role of the sub-aortic mesenchyme in regulating Runx1 expression in the hemogenic endothelium and the role of the associated Notch pathway in these early events. Conserved Runx1 regulatory elements from chicken to human suggested common regulatory pathways between species (Bee et al., 2009; Ng et al., 2010; Nottingham et al., 2007). Despite these new insights, it was not clear whether Runx1 was constitutively expressed from the onset of aorta formation or secondarily regulated by developmental events. Here we show that ECs of the aortic anlage did not express runx1 nor pu1 and c-myb. Instead, runx1 and associated genes are secondarily expressed as aortas matured. In addition, pu1 and c-myb mRNAs are found expressed in the hemogenic endothelium earlier than expected further documenting the EC to HC switch.
Before the present study, the sub-aortic tissue was thought to originate from the lateral plate mesoderm (Wasteson et al., 2008; Wiegreffe et al., 2009). Using tracing techniques, we identified a band of splanchnic mesoderm giving rise to the sub-aortic tissue; cells lateral to this band contribute to the gut mesoderm revealing a specific allocation of splanchnopleural cells along the medio-lateral axis. The tracing techniques also demonstrate the initial splanchnopleural-associated origin of the aortic rudiments.
The experimental block in the migration of the sub-aortic mesenchyme demonstrates the critical role of this tissue for runx1 expression and aortic hematopoiesis. Whether the mesenchyme is required for the initiation or also for further hematopoietic steps needs to be determined. Our data also suggest that the splanchopleure-derived aortic hemogenic endothelium is primed to express runx1, but does not express it since it receives signal(s) from the mesenchyme that triggers the hematopoietic program. A supportive role for the sub-aortic mesenchyme has been proposed several years ago, but never experimentally demonstrated, based on the presence of TGFβ family molecules and tenascin, known to be key factors in hematopoietic development, thus constituting the earliest HSC niche (Cortes et al., 1999; Marshall et al., 2000; Marshall et al., 1999). Cell lines derived from this region exhibit a strong hematopoietic support and are phenotypically characterized as stromal cells (Durand et al., 2007; Oostendorp et al., 2002). BMP4 is present in the sub-aortic mesenchyme and plays a prominent role in promoting HSC survival and expansion (Durand et al., 2007). In zebrafish, sub-aortic BMP would trigger runx1 expression in the ventral aspect of the aorta (Wilkinson et al., 2009). Although we have not specifically addressed the molecular nature of the initiating signal, sub-mesenchymal BMP4 is clearly present in the avian embryo at the time of hematopoietic emergence and may play a role in runx1 induction. In the mouse embryo, the positive role of BMP appears to be precisely regulated by inhibitory Smads (Pimanda et al., 2007). However BMP signaling, testified by the expression of the phosphorylated Smads 1, 5, 8, is present from the earliest phases of chicken aorta formation before runx1 becomes expressed (Richard, Drevon, Jaffredo; unpublished data). Thus, if BMP4 is necessary for runx1 induction, it does not appear to be sufficient to trigger runx1 expression, and additional signals are required. In these “slitted” embryos, peri-aortic smooth muscle cells appear normal suggesting that this cell type is not involved in aortic hematopoiesis as previously proposed (Galmiche et al., 1993). This is also the case for the intermediate mesoderm-derivative, the absence of which has no role on runx1 expression.
Despite convincing reports on the central role of the aortic endothelium in generating hematopoiesis (Bertrand et al., 2010a; Boisset et al., 2010; Kissa and Herbomel, 2010; Lam et al., 2010), it was not clear whether the sub-aortic mesenchyme was also able to generate aortic clusters as proposed (Bertrand et al., 2005). CFDA-SE labeling demonstrates that HC clusters originate exclusively from the endothelium whereas the sub-aortic mesenchyme is a hematopoietic-supportive tissue that does not produce HC, hence demonstrating the complementary roles of these two aortic compartments. This approach has also been successfully used to study the formation of aortic vascular smooth muscle cells (Wiegreffe et al., 2009).
Notch expression in aortic ECs and HCs displays a conserved signature between species. HC cluster production is accompanied by the decrease of Notch ligands. This pattern is however more prominent in the chicken embryo than in the mouse embryo and takes places as early as runx1 expression initiates in the hemogenic endothelium. This pattern is also prominent as HCs mature from CD41+ to CD45+ cells. Our functional experiments also indicate that notch1 signaling is down regulated in HC clusters. During the early phases of endothelio-mesenchymal interactions, notch1 is however not expressed or expressed at low levels in the mesenchyme. Ligand expression being restricted to ECs, this pattern suggests a notch-independent mechanism of action. A requirement for Notch signaling and intra-embryonic HSC production has been shown for mouse and zebrafish embryos. Loss of function experiments demonstrates that ablating Notch signaling suppresses definitive (embryo-derived), but not primitive (yolk sac-derived) hematopoiesis (Burns et al., 2005; Kumano et al., 2003; Robert-Moreno et al., 2005; Robert-Moreno et al., 2008). Of note, in the mouse embryo, Notch1 and Jag1 are required for aortic hematopoiesis to occur (Robert Moreno et al., 2008). Here we show that suppression of Notch signaling enhances the production of CD45+ HC in mouse and chicken P-Sps. However, this production is transient and at 6 days after DAPT exposure, mouse P-Sp CD45+ cells are less numerous in the DAPT-treated samples than in the vehicle-treated sample. Moreover, chicken CD45+ HC appear less sensitive to cell death when treated by DAPT. In keeping with HC production, CAFC formation in the mouse aorta is strongly reduced in the mouse P-Sp treated by DAPT as previously reported (Kumano et al., 2003; Robert-Moreno et al., 2008) but is enhanced in chicken P-Sps. This in vitro effect is corroborated by the in vivo effect of DAPT on chicken embryos. We thus bring a new light on the apparent blockade of aorta hematopoiesis in the mouse embryo following Notch loss of function. Consistent with previous results (Burns et al., 2005; Robert-Moreno et al., 2008), manipulating the hematopoietic production in the aorta using the Notch pathway has no effect on arterial identity since overproduction of HC clusters remains restricted to the aorta. Down-regulation of Notch signaling in the hemogenic endothelium is thus required for aortic hematopoiesis to occur. Taken together our study clearly pleads for a thorough comparison between models especially if one aims at exploiting discoveries for future biomedical applications.
EXPERIMENTAL PROCEDURES
Embryos
Chicken (Gallus gallus JA57 strain) and quail (Coturnix coturnix japonica) eggs were incubated at 38±1°C in humidified atmosphere until embryos reached the appropriate stage. Embryos were either operated in ovo or cultured according to Chapman et al., (2001) and incubated at 37°C/5% CO2.
Pregnant C57Bl6 mice were purchased near Janvier (France). Females were killed by cervical dislocation.
Quail–chicken transplantations
Quail donnor splanchnopleural mesoderm posterior to the last formed somite was isolated and transplanted into chicken recipients of the same stage (10 to 13 somite) either in ovo or in culture. In ovo grafts were introduced throughout ectoderm and somatopleural layers into the splanchnopleural layer. In culture, grafts were inserted into a cut of approximately the same size performed ventrally. Grafted embryos were incubated for an additional 24 to 48hrs. Samples were fixed in 3,7% formaldehyde for 1h at room temperature (RT) embedded in paraffin, and processed for histochemistry.
In Ovo “Slits”
Sixteen-somite stage embryos received India ink (Pelikan)/PBS solution (50-50) into the sub-germinal cavity for visualization. A cut encompassing 10 somites and passing through the three germ layers was made with a microscalpel. The slit lined either immediately lateral to the paraxial mesoderm or the intermediate mesoderm including the mesonephros in that latter case. Embryos were sacrificed 24 to 48 h later.
Chicken embryo cultures and DAPT treatment
Ten-somite stage embryos were washed in saline and transferred ventral side up onto a 35mm dish according to Chapman et al., (2001). A drop of DAPT, N-(3,5-difluorophenylacetyl-L-alanyl)]-S-phenylglycine t-ButylEster (InSolution γ-Secretase Inhibitor IX, Calbiochem) at 2,5mM in DMSO was applied to the embryonic endoderm. Embryos were placed back in culture for 24h.
Labeling of the splanchnopleural mesoderm
Crystals of carbocyanine dye DiI were prepared according to (Kimura et al., 2006). After local endoderm removal, DiI crystals of 5–30μm in diameter were deposited with a glass micropipette onto the splanchnopleural mesoderm lateral to the last formed somite. Labeled embryos were incubated, photographed and processed for histology.
Image acquisition
Chicken embryos were cultured ventral side up in dishes with a glass bottom in a humidified atmosphere at 37.5°C. They were observed under an inverted microscope Leica DMIRBE with a 5X objective. Images were taken overnight using a COOL SNAP HQ2 camera. Stacks of twelve pictures were taken every ten minutes with visible light and fluorescence. Best focus images were compiled and analyzed with the Metamorph software.
Histological procedures
For cryostat and paraplast sections, embryos were fixed respectively in 4% paraformaldehyde or Formoy’s solution and processed as described in (Pouget et al., 2006).
Immunohistology
Antibodies
Sections were stained with: QCPN, developed by Carlson and Carlson, which recognizes all quail cell nuclei, was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242), anti α-Smooth Muscle Actin (αSMA; Sigma, clone 1A4), anti chicken CD45 antibody (HISC7; Cedi-Diagnostics B.V, The Netherlands) anti-GFP (Roche Applied Science). Secondary Goat Anti Mouse (GAM) antibodies were used coupled to biotin, Horse Radish Peroxydase (Southern Biotechnology Associated), Alexa Fluor 488 (Molecular Probes). Tyramide Signal Amplification Cyanin 3 (Perkin Elmer) was used to increase the signal. Sections were counterstained with DAPI.
In situ hybridization on sections
Hybridization was performed according to (Minko et al., 2003) and (Wilting et al., 1997).
RNA probes
The chicken cjagged1, cjagged2, notch2 and delta1 probes were gifts from Dr. R. Goitsuka, (Research Institute for Biological Sciences, Chiba, Japan.). Chicken notch1 extracellular domain was from Dr M. Marx, Institut Curie, Orsay, France. Chicken dll4 was kindly provided by Dr. M. Scaal (Freiburg, Germany) Chicken pu1 probe was from Dr Z. Kherrouche (Institut de Biologie de Lille, France). Chicken myb, runx1 and ve-cadherin probes were obtained as described (Bollerot et al., 2005). Sense and anti-sense RNA probes were synthesized using r-UTP-Digoxygenin (Roche).
CFDA-SE labeling
5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFDA-SE; Invitrogen) was used to label the splanchnic mesoderm. A 10-mM CFDA-SE stock solution in DMSO (Sigma) was diluted in PBS and inoculated in ovo into the coelomic cavities at the cervical levels of 19–22 somite-stage embryos with a borosilicated glass capillary. Inoculated embryos were checked under a UV-lamp and incubated for an additional 24hr period.
Aorta and cell cultures
In vitro aorta organotypic culture
E9.5 mouse or E3 chicken aortas were dissected out and submitted to organotypic cultures on OP9 cells (Nakano et al., 1994) as previously described (Kumano et al., 2003). Briefly, aorta explants were seeded on OP9 stromal cells in RPMI1640 (Invitrogene) with 10% fetal calf serum (FCS) supplemented with 50ng/ml Stem Cell Factor and 5ng/ml recombinant mouse Interleukin3 (Promocell) with or without 50μM DAPT (Sigma), and incubated at 37°C in 5%CO2 for 7 days. Half of the medium was renewed at day 1 and 4 of culture.
Hematopoietic cell assays
After the seven days of organotypic culture, aortas and OP9 cells were mechanically dissociated by pipetting and collagenase I treatment for 30 minutes at 37°C. After centrifugation, cell pellets were resuspended in RPMI1640 with 10% FCS and adherent cells allowed to attach on tissue culture plates for 40mn at 37°C. Non-adherent hematopoietic cells were then recovered, counted and submitted to methylcellulose CFC assay (10 000 cells plated) or day 7 cobblestone-area-forming cells assay (D7 CAFC - 2 000 to 20 000 cells plated), as previously described (Petit-Cocault et al., 2007)). Cultures were maintained at 37°C, and colonies or CAFCs scored at day 7.
Scanning electron microscopy
Avian embryos were fixed in 4% Glutararaldehyde/1X PBS for 1h at room temperature. Embryos were included in 1% low melting point Agarose (InVitrogen). Sections of 300μm thick were obtained on a Leica VT1000S vibratome. Samples were post-fixed in 1% OsO4 in 2X PBS for 1h at room temperature. Samples were dehydrated in successive ethanol bathes from 30% to 95% 10 min each and three bathes in 100% ethanol. Samples were dried by hexamethyldisilazane (Sigma-Aldrich), vacuum-desiccated overnight, mounted onto 12-mm SEM stubs (EM Sciences), and gold-palladium sputter coated. Coverslips were viewed on a Cambridge S220 scanning electron microscope at 12 kV and 15-mm working distance. Pictures were acquired with the Orion 6.60.4 software and colorized using Photoshop CS3.
Electroporation of DNA constructs into the endothelium of dorsal aorta in the chicken embryo
26–28 somite-stage embryos were used and processed as in (Rossello and Torres, 2010). RBPJ-k reporter pTP1-Venus construct (Kohyama et al., 2005; Sato et al., 2008) was diluted in RNase-free water (2 μg/μl) alongside with the construct pECFP-N1 (BD Biosciences) (6:1 ratio). Electroporated embryos were incubated for an additional 24hr period and checked under a UV-lamp before collection.
FACS sorting of EC and HC and flow cytometry analysis
Chicken ECs were metabolically stained using inoculation of Alexa 488 coupled Acetylated Low Density Lipoproteins into the heart of E2 or E3 embryos as described (Jaffredo et al., 1998). HC were labeled at E3 using the chicken-specific anti-CD45 (HISC7) coupled PE (SouthernBiotech, Birmingham; clone DT40).
E9 mouse ECs were isolated from their surface expression of CD31 (PECAM). E11.5 mouse EC and HC were sorted on the basis of respectively CD144+ CD45− and CD144+ CD45+ as described. E11.5 mouse Runx1gfp/gfp AGM regions (Lorsbach et al., 2004) were sorted into hemogenic endothelium, immature, and mature hematopoietic cluster cell fractions via FACS on a low speed FACSVantage SE. Dead cells were excluded by 7-Amino-Actinomycin D (BD Biosciences) and populations sorted based on expression of GFP, Alexa Fluor 647 anti-mouse CD144 (eBioscience), Phycoerythrin (PE) anti-mouse CD41, and APC-Cy7 Rat anti-mouse CD45 (BD Biosciences).
Cell staining of chick and mouse P-Sp cultures was done in PBS with 0.5% bovine serum albumine (BSA) using the following antibodies: Allophycocyanin (APC) anti-chick or anti-mouse CD45 (Southern Biotech and Biolegend). For Annexin V analysis, immuno-stained cells were resuspended in Annexin V buffer and stained with fluorescein isothiocyanate (FITC)-Annexin V (Biolegend) according to the manufacturer’s guidelines. Dead cells were excluded by 7AAD (Beckman Coulter) staining. FACS analysis was performed on a LSRII flow cytometer (BD Biosciences).
Supplementary Material
Acknowledgments
We thank Drs. Claire Pouget and Charles Durand for critical reading of the manuscript and V. Georget and R. Schwartzman from the cell imaging facility of the IFR83 for expert assistance on live imaging and J. Dumortier for help in chicken embryo culture. We are grateful to S. Gournet for excellent photographic assistance. C.R. is a recipient of the French Ministry of Research and Higher Education and Fondation pour la Recherche Médicale. This Research was funded by CNRS, UPMC, ARC-INCA and Fondation pour la Recherche Médicale Grants (TJ), and R01HL091724 (NAS).
Footnotes
Supplemental Information includes three figures, one movie, and Supplemental Experimental Procedures and can be found with this article online at doi:xxx.
References
- Azcoitia V, Aracil M, Martinez AC, Torres M. The homeodomain protein Meis1 is essential for definitive hematopoiesis and vascular patterning in the mouse embryo. Dev Biol. 2005;280:307–320. doi: 10.1016/j.ydbio.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Bee T, Ashley EL, Bickley SR, Jarratt A, Li PS, Sloane-Stanley J, Gottgens B, de Bruijn MF. The mouse Runx1 +23 hematopoietic stem cell enhancer confers hematopoietic specificity to both Runx1 promoters. Blood. 2009;113:5121–5124. doi: 10.1182/blood-2008-12-193003. [DOI] [PubMed] [Google Scholar]
- Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010a;464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand JY, Cisson JL, Stachura DL, Traver D. Notch signaling distinguishes 2 waves of definitive hematopoiesis in the zebrafish embryo. Blood. 2010b;115:2777–2783. doi: 10.1182/blood-2009-09-244590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand JY, Giroux S, Golub R, Klaine M, Jalil A, Boucontet L, Godin I, Cumano A. Characterization of purified intraembryonic hematopoietic stem cells as a tool to define their site of origin. Proc Natl Acad Sci U S A. 2005;102:134–139. doi: 10.1073/pnas.0402270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- Bollerot K, Romero S, Dunon D, Jaffredo T. Core binding factor in the early avian embryo: cloning of Cbfbeta and combinatorial expression patterns with Runx1. Gene Expr Patterns. 2005;6:29–39. doi: 10.1016/j.modgep.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Burns CE, Traver D, Mayhall E, Shepard JL, Zon LI. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev. 2005;19:2331–2342. doi: 10.1101/gad.1337005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SC, Collignon J, Schoenwolf GC, Lumsden A. Improved method for chick whole-embryo culture using a filter paper carrier. Dev Dyn. 2001;220:284–289. doi: 10.1002/1097-0177(20010301)220:3<284::AID-DVDY1102>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Clements WK, Kim AD, Ong KG, Moore JC, Lawson ND, Traver D. A somitic Wnt16/Notch pathway specifies haematopoietic stem cells. Nature. 2011;474:220–224. doi: 10.1038/nature10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes F, Debacker C, Peault B, Labastie MC. Differential expression of KDR/VEGFR-2 and CD34 during mesoderm development of the early human embryo. Mech Dev. 1999;83:161–164. doi: 10.1016/s0925-4773(99)00030-1. [DOI] [PubMed] [Google Scholar]
- de Bruijn MF, Speck NA, Peeters MC, Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. Embo J. 2000;19:2465–2474. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterlen-Lièvre F, Pouget C, Bollerot K, Jaffredo T. Are intra-aortic hemopoietic cells derived from endothelial cells during ontogeny? Trends Cardiovasc Med. 2006;16:128–139. doi: 10.1016/j.tcm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Durand C, Robin C, Bollerot K, Baron MH, Ottersbach K, Dzierzak E. Embryonic stromal clones reveal developmental regulators of definitive hematopoietic stem cells. Proc Natl Acad Sci U S A. 2007;104:20838–20843. doi: 10.1073/pnas.0706923105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilken HM, Nishikawa S, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457:896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- Galmiche MC, Koteliansky VE, Briere J, Herve P, Charbord P. Stromal cells from human long-term marrow cultures are mesenchymal cells that differentiate following a vascular smooth muscle differentiation pathway. Blood. 1993;82:66–76. [PubMed] [Google Scholar]
- Hadland BK, Huppert SS, Kanungo J, Xue Y, Jiang R, Gridley T, Conlon RA, Cheng AM, Kopan R, Longmore GD. A requirement for Notch1 distinguishes 2 phases of definitive hematopoiesis during development. Blood. 2004;104:3097–3105. doi: 10.1182/blood-2004-03-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Munain C, Krangel MS. Regulation of the T-cell receptor delta enhancer by functional cooperation between c-Myb and core-binding factors. Mol Cell Biol. 1994;14:473–483. doi: 10.1128/mcb.14.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Zhang P, Hirai H, Elf S, Yan X, Chen Z, Koschmieder S, Okuno Y, Dayaram T, Growney JD, et al. PU.1 is a major downstream target of AML1 (RUNX1) in adult mouse hematopoiesis. Nat Genet. 2008;40:51–60. doi: 10.1038/ng.2007.7. [DOI] [PubMed] [Google Scholar]
- Jaffredo T, Bollerot K, Sugiyama D, Gautier R, Drevon C. Tracing the hemangioblast during embryogenesis: developmental relationships between endothelial and hematopoietic cells. Int J Dev Biol. 2005;49:269–277. doi: 10.1387/ijdb.041948tj. [DOI] [PubMed] [Google Scholar]
- Jaffredo T, Gautier R, Eichmann A, Dieterlen-Lievre F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development. 1998;125:4575–4583. doi: 10.1242/dev.125.22.4575. [DOI] [PubMed] [Google Scholar]
- Jaffredo T, Richard C, Pouget C, Teillet MA, Bollerot K, Gautier R, Drevon C. Aortic remodelling during hemogenesis: is the chicken paradigm unique? Int J Dev Biol. 2010;54:1045–1054. doi: 10.1387/ijdb.103062tj. [DOI] [PubMed] [Google Scholar]
- Kimura W, Yasugi S, Stern CD, Fukuda K. Fate and plasticity of the endoderm in the early chick embryo. Dev Biol. 2006;289:283–295. doi: 10.1016/j.ydbio.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- Kohyama J, Tokunaga A, Fujita Y, Miyoshi H, Nagai T, Miyawaki A, Nakao K, Matsuzaki Y, Okano H. Visualization of spatiotemporal activation of Notch signaling: live monitoring and significance in neural development. Dev Biol. 2005;286:311–325. doi: 10.1016/j.ydbio.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Kumano K, Chiba S, Kunisato A, Sata M, Saito T, Nakagami-Yamaguchi E, Yamaguchi T, Masuda S, Shimizu K, Takahashi T, et al. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity. 2003;18:699–711. doi: 10.1016/s1074-7613(03)00117-1. [DOI] [PubMed] [Google Scholar]
- Lam EY, Hall CJ, Crosier PS, Crosier KE, Flores MV. Live imaging of Runx1 expression in the dorsal aorta tracks the emergence of blood progenitors from endothelial cells. Blood. 2010 doi: 10.1182/blood-2010-01-264382. [DOI] [PubMed] [Google Scholar]
- Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorsbach RB, Moore J, Ang SO, Sun W, Lenny N, Downing JR. Role of RUNX1 in adult hematopoiesis: analysis of RUNX1-IRES-GFP knock-in mice reveals differential lineage expression. Blood. 2004;103:2522–2529. doi: 10.1182/blood-2003-07-2439. [DOI] [PubMed] [Google Scholar]
- Marshall CJ, Kinnon C, Thrasher AJ. Polarized expression of bone morphogenetic protein-4 in the human aorta-gonad-mesonephros region. Blood. 2000;96:1591–1593. [PubMed] [Google Scholar]
- Marshall CJ, Moore RL, Thorogood P, Brickell PM, Kinnon C, Thrasher AJ. Detailed characterization of the human aorta-gonad-mesonephros region reveals morphological polarity resembling a hematopoietic stromal layer. Dev Dyn. 1999;215:139–147. doi: 10.1002/(SICI)1097-0177(199906)215:2<139::AID-DVDY6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Minko K, Bollerot K, Drevon C, Hallais MF, Jaffredo T. From mesoderm to blood islands: patterns of key molecules during yolk sac erythropoiesis. Gene Expr Patterns. 2003;3:261–272. doi: 10.1016/s1567-133x(03)00053-x. [DOI] [PubMed] [Google Scholar]
- Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265:1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- Ng CE, Yokomizo T, Yamashita N, Cirovic B, Jin H, Wen Z, Ito Y, Osato M. A Runx1 intronic enhancer marks hemogenic endothelial cells and hematopoietic stem cells. Stem Cells. 2010;28:1869–1881. doi: 10.1002/stem.507. [DOI] [PubMed] [Google Scholar]
- North T, Gu TL, Stacy T, Wang Q, Howard L, Binder M, Marin-Padilla M, Speck NA. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999;126:2563–2575. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- North TE, de Bruijn MF, Stacy T, Talebian L, Lind E, Robin C, Binder M, Dzierzak E, Speck NA. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16:661–672. doi: 10.1016/s1074-7613(02)00296-0. [DOI] [PubMed] [Google Scholar]
- Nottingham WT, Jarratt A, Burgess M, Speck CL, Cheng JF, Prabhakar S, Rubin EM, Li PS, Sloane-Stanley J, Kong ASJ, et al. Runx1-mediated hematopoietic stem-cell emergence is controlled by a Gata/Ets/SCL-regulated enhancer. Blood. 2007;110:4188–4197. doi: 10.1182/blood-2007-07-100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin E, Tavian M, Blazsek I, Peault B. Blood-forming potential of vascular endothelium in the human embryo. Development. 2002;129:4147–4157. doi: 10.1242/dev.129.17.4147. [DOI] [PubMed] [Google Scholar]
- Oostendorp RA, Harvey KN, Kusadasi N, de Bruijn MF, Saris C, Ploemacher RE, Medvinsky AL, Dzierzak EA. Stromal cell lines from mouse aorta-gonads-mesonephros subregions are potent supporters of hematopoietic stem cell activity. Blood. 2002;99:1183–1189. doi: 10.1182/blood.v99.4.1183. [DOI] [PubMed] [Google Scholar]
- Pardanaud L, Dieterlen-Lièvre F. Manipulation of the angiopoietic/hemangiopoietic commitment in the avian embryo. Development. 1999;126:617–627. doi: 10.1242/dev.126.4.617. [DOI] [PubMed] [Google Scholar]
- Pardanaud L, Luton D, Prigent M, Bourcheix LM, Catala M, Dieterlen-Lièvre F. Two distinct endothelial lineages in ontogeny, one of them related to hemopoiesis. Development. 1996;122:1363–1371. doi: 10.1242/dev.122.5.1363. [DOI] [PubMed] [Google Scholar]
- Peeters M, Ottersbach K, Bollerot K, Orelio C, de Bruijn M, Wijgerde M, Dzierzak E. Ventral embryonic tissues and Hedgehog proteins induce early AGM hematopoietic stem cell development. Development. 2009;136:2613–2621. doi: 10.1242/dev.034728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit-Cocault L, Volle-Challier C, Fleury M, Péault B, Souyri M. Dual role of Mpl receptor during the establishment of definitive hematopoiesis. Development. 2007;134:3031–3040. doi: 10.1242/dev.001818. [DOI] [PubMed] [Google Scholar]
- Pimanda JE, Ottersbach K, Knezevic K, Kinston S, Chan WY, Wilson NK, Landry JR, Wood AD, Kolb-Kokocinski A, Green AR, et al. Gata2, Fli1, and Scl form a recursively wired gene-regulatory circuit during early hematopoietic development. Proc Natl Acad Sci U S A. 2007;104:17692–17697. doi: 10.1073/pnas.0707045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouget C, Gautier R, Teillet MA, Jaffredo T. Somite-derived cells replace ventral aortic hemangioblasts and provide aortic smooth muscle cells of the trunk. Development. 2006;133:1013–1022. doi: 10.1242/dev.02269. [DOI] [PubMed] [Google Scholar]
- Robert-Moreno A, Espinosa L, de la Pompa JL, Bigas A. RBPj{kappa}-dependent Notch function regulates Gata2 and is essential for the formation of intra-embryonic hematopoietic cells. Development. 2005;132:1117–1126. doi: 10.1242/dev.01660. [DOI] [PubMed] [Google Scholar]
- Robert-Moreno A, Guiu J, Ruiz-Herguido C, Lopez ME, Ingles-Esteve J, Riera L, Tipping A, Enver T, Dzierzak E, Gridley T, et al. Impaired embryonic haematopoiesis yet normal arterial development in the absence of the Notch ligand Jagged1. Embo J. 2008;27:1886–1895. doi: 10.1038/emboj.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossello CA, Torres M. Gene transfer by electroporation into hemogenic endothelium in the avian embryo. Dev Dyn. 2010;239:1748–1754. doi: 10.1002/dvdy.22317. [DOI] [PubMed] [Google Scholar]
- Rowlinson JM, Gering M. Hey2 acts upstream of Notch in hematopoietic stem cell specification in zebrafish embryos. Blood. 2010;116:2046–2056. doi: 10.1182/blood-2009-11-252635. [DOI] [PubMed] [Google Scholar]
- Rybtsov S, Sobiesiak M, Taoudi S, Souilhol C, Senserrich J, Liakhovitskaia A, Ivanovs A, Frampton J, Zhao S, Medvinsky A. Hierarchical organization and early hematopoietic specification of the developing HSC lineage in the AGM region. J Exp Med. 2011;208:1305–1315. doi: 10.1084/jem.20102419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Watanabe T, Saito D, Takahashi T, Yoshida S, Kohyama J, Ohata E, Okano H, Takahashi Y. Notch mediates the segmental specification of angioblasts in somites and their directed migration toward the dorsal aorta in avian embryos. Dev Cell. 2008;14:890–901. doi: 10.1016/j.devcel.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Taoudi S, Medvinsky A. Functional identification of the hematopoietic stem cell niche in the ventral domain of the embryonic dorsal aorta. Proc Natl Acad Sci U S A. 2007;104:9399–9403. doi: 10.1073/pnas.0700984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteson P, Johansson BR, Jukkola T, Breuer S, Akyurek LM, Partanen J, Lindahl P. Developmental origin of smooth muscle cells in the descending aorta in mice. Development. 2008;135:1823–1832. doi: 10.1242/dev.020958. [DOI] [PubMed] [Google Scholar]
- Wiegreffe C, Christ B, Huang R, Scaal M. Remodeling of aortic smooth muscle during avian embryonic development. Dev Dyn. 2009;238:624–631. doi: 10.1002/dvdy.21888. [DOI] [PubMed] [Google Scholar]
- Wilkinson RN, Pouget C, Gering M, Russell AJ, Davies SG, Kimelman D, Patient R. Hedgehog and Bmp polarize hematopoietic stem cell emergence in the zebrafish dorsal aorta. Dev Cell. 2009;16:909–916. doi: 10.1016/j.devcel.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilting J, Eichmann A, Christ B. Expression of the avian VEGF receptor homologues Quek1 and Quek2 in blood-vascular and lymphatic endothelial and non-endothelial cells during quail embryonic development. Cell Tissue Res. 1997;288:207–223. doi: 10.1007/s004410050807. [DOI] [PubMed] [Google Scholar]
- Yokomizo T, Dzierzak E. Three-dimensional cartography of hematopoietic clusters in the vasculature of whole mouse embryos. Development. 2010;137:3651–3661. doi: 10.1242/dev.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, Gasson JC, et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3:625–636. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.