Abstract
Purpose
To design a computer-controlled, MR compatible foot pedal device that allows in vivo mapping of changes in morphology and in strain of different musculoskeletal components of the lower leg under passive, isometric, concentric and eccentric contractions.
Materials and Methods
A programmable servo-motor in the control room pumped hydraulic fluid to rotate a foot-pedal inside the magnet. Towards validating the performance of the device, six subjects were imaged with gated velocity-encoded phase-contrast (VE-PC) imaging to investigate dynamics of muscle and aponeurotic structures.
Results
Artifact-free VE-PC imaging clearly delineated different muscle compartments by differences in distribution of mechanical strains. High repeatability of contraction cycles allowed establishing that fascicles lengthened 6.1% more during passive compared to eccentric contractions. Aponeurosis separation during passive (range between three locations: −2.6~1.3 mm) and active (range: −2.4 ~1.6 mm) contractions were similar but significantly different from concentric (range: −0.9~3.3 mm), with proximal and distal regions showing mostly negative values for the first two modes, but positive for the last.
Conclusion
The device was sufficiently robust and artifact-free to accurately assess, using VE-PC imaging, physiologically important structure and dynamics of the musculo-tendon complex.
Keywords: Magnetic Resonance Imaging (MRI) compatible device, Foot Pedal, Cine Velocity Encoded Phase Contrast MRI, Muscle dynamics, Muscle Fiber Architecture
INTRODUCTION
An elucidation of the in vivo relationship between muscle geometry and function of whole muscle-tendon complex will lead to an improved geometric muscle model (1; 2) and yield better predictions of alterations in muscle function under various pathological conditions such as that of aging (3), training (4, 5), clinical intervention (6; 7), and surgical operations (8; 9). While B-mode ultrasonography has been successfully used to study the kinematics of the musculoskeletal (MSK) system in real time, it is limited in field of view (FOV) with visibility and contrast of different structures somewhat operator-dependent and relatively sub-optimal. MRI, with its high signal-to-noise ratio (SNR) and contrast, can image over large FOV’s allowing simultaneous study of the dynamics of all the muscles of the lower leg. However, such dynamic MRI scans, usually gated, are often degraded by artifacts from non-identical repetitions of movement for each phase encoding level. The presence of magnetic material within the imaging volume and electronic noise from equipment further degrade the MR images with artifacts.
Mechanical constraints on the lateral movement of the aponeuroses have been postulated as a mechanism for amplifying the effect of muscle fiber length change to the aponeurosis movement (10) with changes in the separation postulated to modify this amplification factor (2). While there seems to be a wide-spread assumption in literature that aponeurosis separation remains relatively constant, it has been recently suggested that the separation between the tibia and posterior aponeurosis may change as a consequence of contraction in the marginal soleus (11).
The main objective of this study was to design and develop an MR-compatible, computer controlled, foot-pedal device that could operate inside the bore of an MR scanner, at both 1.5 and 3 Tesla (T). This device would have to enable gated velocity-encoded phase-contrast (VE-PC) imaging of reproducible and consistent leg motion in order to map the changes in strain and shape during passive and neutral conditions and at different activation levels, load, and ankle joint angle under dynamic conditions of plantarflexion or dorsiflexion using. As a test of the validity of the equipment, we investigated the changes in fascicle lengths and in aponeurosis separation at different proximo-distal locations during joint rotation under the three contraction modes.
MATERIALS AND METHODS
Foot Pedal Device
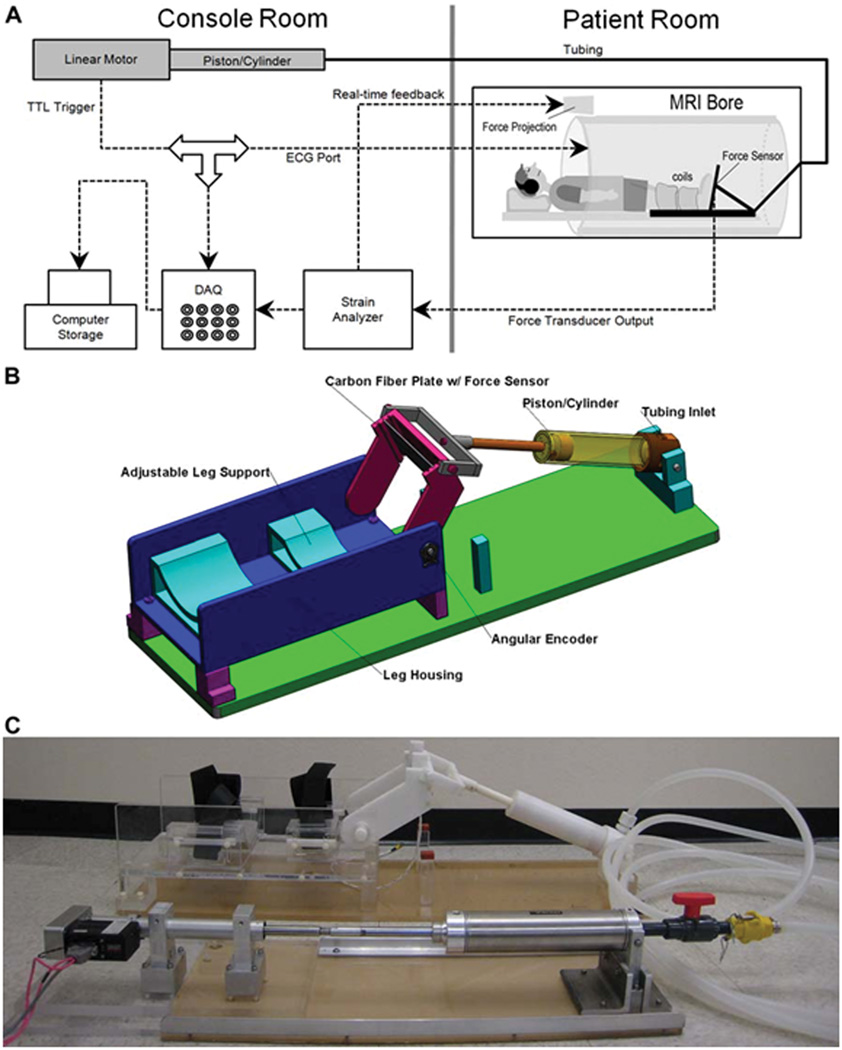
The device (Fig.1) was designed so that all parts that were to operate inside the magnet bore were MR compatible and fitted within it, with anatomy to be imaged at the isocenter. A computer controlled motor (Animatics, Santa Clara, CA), mounted on aluminum brackets fixed to a ½ inch thick acrylic base-board and programmed to provide a trigger signal at any point of its traversal cycle for a gated mode acquisition, drove a stainless-steel “driving” piston-and-cylinder (Parker Hannifin, Cleveland, OH). This pumped hydraulic fluid (water in this case) via a stop-valve and quick-connect through a 35’ length of 5/8” diameter reinforced flexible plastic tubing (McMaster-CARR, Santa Fe Springs, CA). The tubing was led through a wave-guide in the scanner room wall to the back of the magnet bore and via another quick-connect attached to a MR-compatible “slave” cylinder, machined out of Delrin using rubber O-rings for the cylinder seals, with a piston connected to the foot-pedal, all of which resided inside the magnet bore.
Fig. 1.
(A) Schematic diagram of the experimental setup. The experimental components are interconnected via coaxial cables. Work output of the motor was transmitted to the foot pedal via hydraulic fluid (water) in high-pressure tubing. The tube passed from the console room to the MRI scanning room through a waveguide in the wall. Force data from the foot pedal was projected onto the white front surface of the magnet through a projector for real-time feedback to the subject. An electronic trigger signal was transmitted directly from the motor to the ECG port of the scanner at a preselected point of each contraction cycle for gated image acquisition. (B) Schematic of the foot pedal apparatus – Assembly of all CAD parts. Different components are annotated. (C) Photo of the actual Foot-Pedal Device. The motor is shown in the lower left connected to the stainless steel piston-cylinder, hydraulic tubing coiled on the right, connected to the white, non-metallic piston-cylinder which goes inside the magnet bore. The piston can be seen connected to the foot-pedal in the upper middle part of the picture.
The foot-pedal (Fig. 1B, C) was mounted on a flat acrylic bed. The subject’s leg, in the curved holders shown in Fig. 1B and C, was immobilized by bandages inside a fiber-glass half-cast and secured with Velcro straps fastened to the leg-holders and slots in the leg holder device. The center of rotation of the ankle was aligned with the center of rotation of the foot-pedal bracket with adjustable spacers at the bottom of the cast for minute vertical direction adjustments. The ball of the foot rested against the pressure plate. The angular movement of the foot was initiated entirely by the motor and foot plate. To ensure that the foot did not disengage during the forward plantarflexion movement, a Velcro strap going around the top of the foot and the other side of the foot-plate, securely fastened the foot to plate. In this configuration, the subject was asked to either (i) exert force against the plate either during plantarflexion movement for concentric contraction or, (ii) dorsiflexion movement for eccentric contraction or (iii) exert no force at all for passive movement. For isometric contraction, the foot plate was anchored at a neutral 90° using pins inserted into corresponding holes between the arm carrying the foot plate and the outside frame. Cyclical isometric force could then be exerted by the foot against the now-fixed foot-plate following a computer-generated audio cue transmitted through head-phones.
The foot-plate was a carbon fiber reinforced polymer sheet with a Fabry-Perot optical strain gauge bonded to it (Luna Innovations, Roanoke, VA). The strain gauge was connected via optical fiber to a commercial measurement system (Fiberscan, Luna Innovations, Roanoke, VA). The output voltage from this device was proportional to the force exerted on the foot plate. This was sampled by the computer and projected in real time on the face of the scanner visible to the subject to provide a visual force feedback, to enable exertion of consistent force level cycles. It was also used to produce a threshold-voltage-activated trigger pulse mimicking the R component of the QRS complex of an electrocardiogram to gate the acquisition during isometric contractions, when the foot was stationary and the pump was not used. Finally, it was recorded in a digitized form for subsequent force-displacement analysis.
Predicted Excursions and Fluid Dynamics
The mean angles subtended by the longitudinal axis of the tibia and the foot at maximum dorsiflexion, rest, and maximum plantarflexion, were estimated in six subjects, using sagittal images in the body coil that showed both the foot and the tibia (12). They were found to be 75°, 115°, and 135° (±4° for all three) respectively. Therefore the device was designed to achieve an ankle rotation range of 65° to 135° by adjusting the displacement of the programmable linear motor. The diameters of the actuating and the driving cylinders were chosen to be relatively large at 5” and 2” respectively, to maintain a relatively low working pressure (~50 psi) for the hydraulic system and minimize the risk of fluid leakage. The diameter of the slave cylinder was chosen to provide the required stroke of 7.5” from the specified stroke of the linear motor (~5”) to produce the maximum ankle rotation cycle noted above. The period or duration of one cycle was determined by the rate of muscle contraction desired for a given experiment, typically 30 cycles per minute, with a fluid flow rate of ~22 in/s. Based on these numbers, the average speed of the motor was calculated to be 4.8”/s (4.8” × 2 / 2 sec) with the maximum force output achievable of ~150 lbs. Standard software (http://www.pressure-drop.com) was used to calculate the pressure drop through the 35’ length of tubing. A further consideration in the loading of the motor was the inertia of the water column, ~400 g (0.8 lbs), which limited the acceleration of the motor to 12 in/s2.
Human Subjects
Six healthy male subjects [age 30.1 ± 10.5 yr, height 182.5±8 cm, weight: 68.9±15.4 kg], with no history of orthopedic or neuromuscular abnormalities, were recruited after signing consent form approved by the Institutional Human Research Protections Program. These 6 subjects were used for (i) testing the device at the two magnetic fields of 1.5T and 3T and using various coil configurations described below and (ii) for the acquisition of the physiological measurements of the fascicle length changes and aponeurosis separation reported in this paper only at 3T. Similar and additional types of physiological imaging including Diffusion Tensor and Spin Tag imaging have been reported in prior publications (12; 13).
MR Imaging
The apparatus was designed to be used in two different magnetic field scanners, 1.5 and 3 Tesla (GE Medical Systems, Milwaukee, Wisconsin) and is compatible with various coils such as the phased array Cardiac and Spine for the lower leg and large field-of-view, knee coil for high SNR but small FOV, the Body coil for largest FOV but low SNR (e.g. for imaging the foot angle as explained above) and an custom-made 8-Channel high SNR phased array coil for diffusion tensor imaging of the leg (12).
The MRI experiments included VE-PCMR imaging of tissue displacement during various modes of contractions, in the gated mode, and with the velocity sensitized in any or all three directions, with a VENC value of 10 cm/s. Other sequence parameters included Repetition Time (TR)/ Echo Time (TE)/ Flip Angle (FA): 16.5ms/7.7 ms/20°; FOV: 16.5×30 cm, 141 × 256 matrix, Slice: 5 mm, Bandwidth (BW): ±15.6 kHz, views-per-segment (VPS): 4, averages (NEX): 2. With segmented acquisition and fractional FOV, a total of ~84 contractions with ~2.20 sec/cycle required a total scan time of ~2.4 min. With view sharing, 22 phases could be collected in each cycle, yielding an acquisition window of ~80 ms for each phase, with velocity encoded in one direction only. Forty percent maximum-voluntary-contraction (MVC) was used for the two active contraction modes.
Static morphologic axial images of the leg were acquired with Gradient Echo sequences (with TE/TR/FA of 2.65 ms/140 ms/45°) (13). Sufficient contrast to segment out different muscle compartments could be achieved to subsequently generate 3D volume rendered images of different compartments for volumetric measurements. In order to better visualize the fatty fascicular tissue, a Fast Spin-Echo (FSE) T1-weighted sequence was utilized in which signal from water protons were suppressed. Typical parameters used were TR/TE: 725 ms/12 ms; Echo Train Length = 7; 62.5 KHz BW. FOV, Slice Thickness, spacing and orientation remained the same as the VE-PC slice, in order to register the water-suppressed, fat (fascicle) enhanced FSE images on to the magnitude image of the VE-PC.
Analysis
(a) Fiber length changes
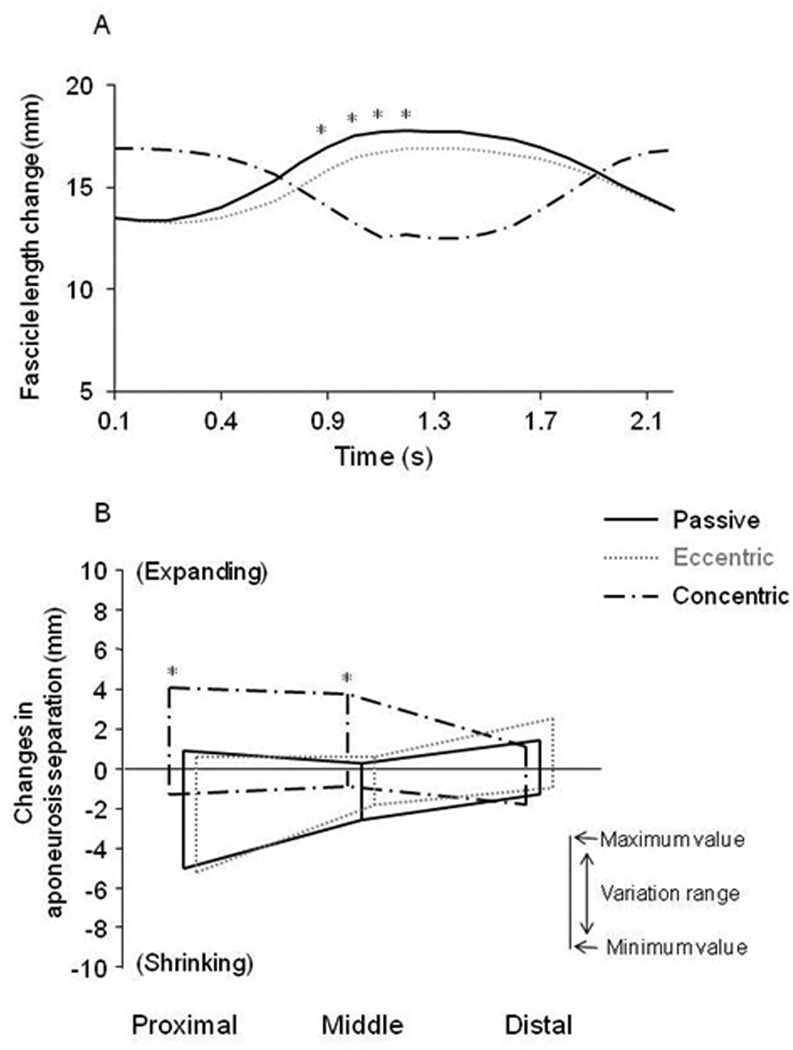
The ends of muscle fibers, oriented parallel to fatty fascicles, were first localized along the length of the MG using water-saturated FSE images. The end locations of these fascicles, one at its origin (superficial aponeurosis) and the other at its insertion (deep aponeurosis), were then tracked through time in the two-dimensional plane using the oblique sagittal VE-PC images, over all the phases of the contraction cycle (13, 14). When the displacement of one ROI was subtracted from the other at each phase, the change in length of the fiber was determined for that phase and is plotted in Fig. 4. A for all the phases, for the two different types of contractions, and averaged over 5 to 6 fascicles.
Fig. 4.
(A) Changes in fascicle length of the medial gastrocnemius at different time points during the three modes of muscle contraction with ankle rotation from images acquired at 3T. Points in each curve represent the mean lengths of all 5~6 fibers along the length of the MG muscle. It can be seen that the dynamic range of fiber stretching/shortening is much less in active eccentric contraction, which is consistent with the findings from Fig. 3 in which the dynamic range of velocity was less in active eccentric than in passive mode. (B) Changes in the separation between the superficial and deep aponeurosis of the Medial Gastrocnemius, during passive, eccentric and concentric contractions, as a function of position along the superior-inferior direction. The maximum range of values of this change is indicated as a function of the superior-inferior position.
(b) Aponeurosis separation
Aponeurosis separation was calculated as the horizontal distance between the two ends of each fascicle at the two aponeuroses. The aponeurosis separations were calculated throughout the ankle rotation cycle and averaged over 5 to 6 fascicles.
(c) Statistics
Values are presented as mean and SD. Differences in the muscle fiber length and aponeurosis separation among the three types of contractions were tested by one-way analysis of variance. Post hoc comparison (Fisher) was performed when significance was found. The level of statistical significance was set at P < 0.05.
RESULTS
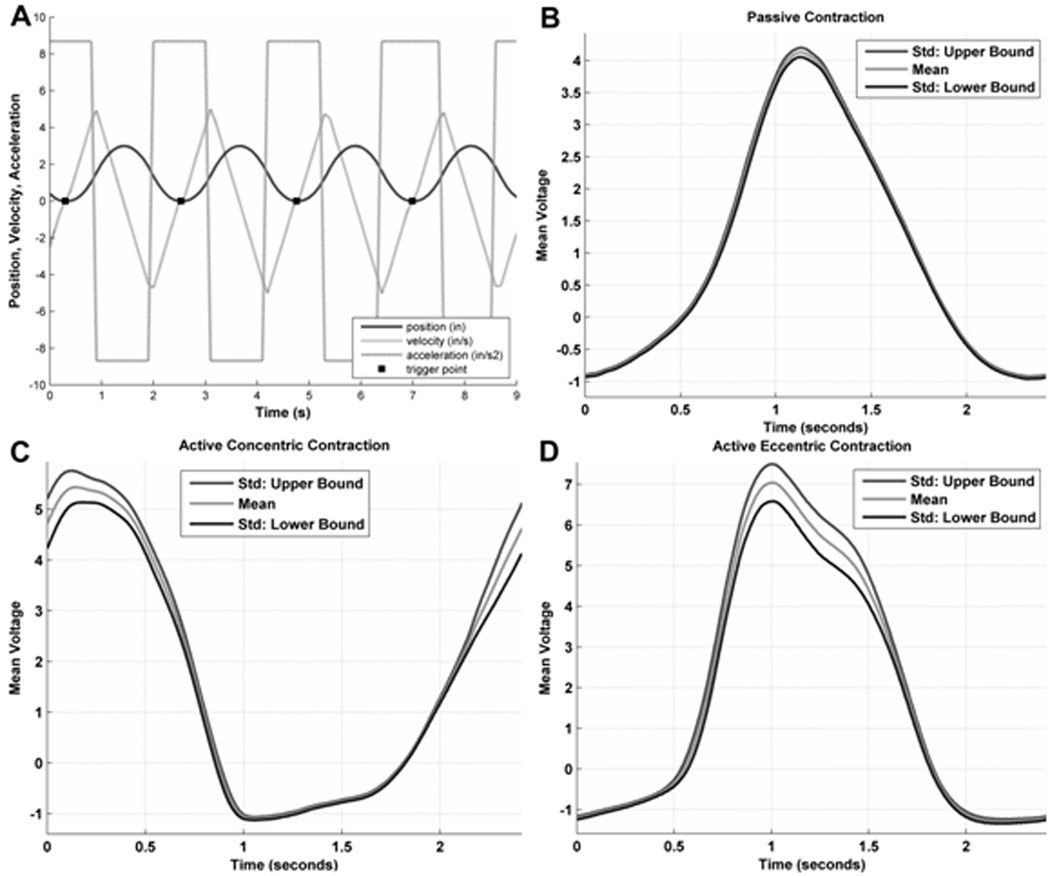
The entire apparatus took about 20 minutes to set up, including priming the pistons and tubing, leading the tubing through the waveguide hole, and connecting it at both ends to the two pistons. Fig. 2A shows the various outputs provided by the electronics driving the servo-motor including a constant maximum acceleration of 12 in/s2 for both plantar- and dorsiflexion phases of the movement. Higher values resulted in excessive loading on the motor. The velocity generated by such an acceleration function is the triangular waveform shown. Integrating the velocity over time yielded the approximately sinusoidal displacement of the piston at various time points. Triggering is shown in this figure to be in the maximally dorsiflexed position of the foot.
Fig. 2.
(A) Output from the motor electronics showing acceleration (rectangular), velocity (triangular) and displacement (sinusoidal) curves. The solid dots on the displacement curve represent points where the motor output was used to trigger the MR scanner. (B), (C) and (D) represent single-subject traces of the digitally recorded force output from the force transducer in the foot plate, with the subject instructed to exert passive, concentric and eccentric contractions respectively. The shaded curve in the middle of each represent the mean while the solid curves on either side represent +/− 1SD of the force produced. While these data were acquired at 3T, these are independent of field strength, and similar curves were observed at 1.5T.
Fig. 2-B, C and D show the force output from the force transducer for each of the passive, concentric and eccentric contractions mode respectively. The scatter was maximum at the peak force and minimum at the relaxed phase for each of the three different modes. It is also minimal for the passive mode and about the same for the concentric and eccentric modes.
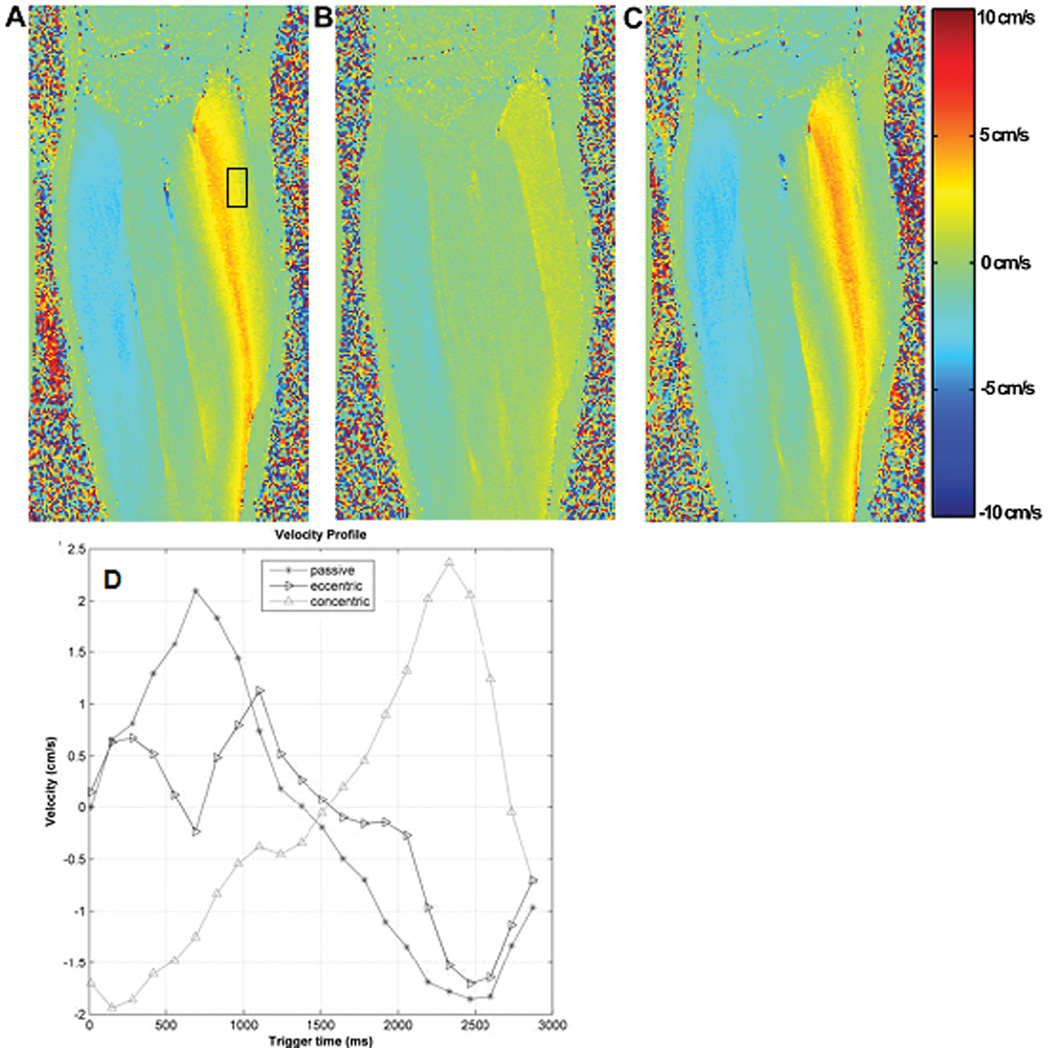
Fig. 3 A, B, C show the VE-PC MR images of the lower leg for each of the passive, active eccentric and active concentric contractions, with some typical results of analysis shown in Fig. 3-D.
Fig. 3.
Velocity encoded phase contrast images acquired at 1.5T with the foot pedal device, with the ankle undergoing three different modes of dynamic ankle rotation: passive (A, curve in Fig. 3.D connected with circles), active eccentric (B, inverted triangles), and active concentric (C, triangles) contractions. Pixel intensity of the images shown represents velocity encoded in superior-inferior direction (positive: up, negative: down), with the maximum and minimum range of ± 10 cm/s (VENC), as shown in the adjacent color code bar. Fig. 3-A and C were acquired during the plantarflexion phase of the rotation cycle while B was taken during the dorsiflexion phase and all three denote the phase in which maximum velocity occurs. Characteristic distribution patterns of velocity for the three different contraction mode are clearly demonstrated. (D) Changes in the velocity of the medial gastrocnemius within the rectangular ROI shown in (A) with time during the three modes of dynamic ankle rotation. The velocity within the ROI was averaged at each phase of the rotation cycle. It is to be noted that while these data are from images acquired at 1.5T, physiologically similar data are obtained at higher field of 3T, albeit with a higher signal-to-noise ratio.
The analysis presented here from images acquired at 3T demonstrates the difference in the extent of shortening of the muscle fascicles averaged across all fascicles within the MG muscle, when the foot is flexed passively, eccentrically and concentrically, through the contraction cycle. The average fascicle length stretches and shortens much more significantly (about 6%) during the passive mode than during the eccentric one (P < 0.05). Note that the active concentric contraction starts with the ankle joint in the dorsiflexed position and thus initial fascicle lengths are much longer than in the other two contractions, i.e. there is a phase lag of fiber length changes between the 3 modes.
Fig 4-B and Table 1 shows that changes in aponeurosis separation between superficial and deep aponeuroses through ankle plantar- and dorsi-flexed rotations were dependent on the location and contractile condition. The pattern of changes in the aponeurosis separation was similar between the passive and eccentric contractions at different sites, but different from the concentric contraction data. The proximal and middle regions showed mostly negative values during the passive (with minimum value of −4.6 ± 2.2 mm and maximum value of ~ 1.4 ± 1.3 mm) for the proximal and corresponding values of −2.4 ± 1.1 mm and ~ 0.6 ± 0.5 mm for the middle) and eccentric (−5.3 ± 0.5 mm, ~ 0.8 ± 1.1 mm for the proximal and −1.3 ± 0.9 mm, ~ 1.2 ± 1.0 mm for the middle) contractions whereas mostly positive values were observed during the concentric (−0.8 ± 0.9 mm, ~ 4.3 ± 2.5 mm for the proximal and −0.5 ± 0.7 mm, ~ 4.2 ± 1.7 mm for the middle, P < 0.05) contraction suggesting that inter-aponeurosis distances actually increases. In the distal region, aponeurosis separation remained constant or changed only slightly for all three contraction modes.
Table 1.
Change in Aponeurosis separation amoung passive, eccentric, and concentric contractions at three different locations.
| Proximal | Middle | Distal | ||||
|---|---|---|---|---|---|---|
| Range | Range | Range | ||||
| Minimum | Maximum | Minimum | Maximum | Minimum | Maximum | |
| Passive | −4.6 ± 2.2 | 1.4 ± 1.3 | −2.4 ± 1.1 | 0.6 ± 0.5 | −0.8 ± 1.0 | 1.8 ± 1.4 |
| Eccentric | −5.3 ± 0.5 | 0.8 ± 1.1 | −1.3 ± 0.9 | 1.2 ± 1.0 | −0.5 ± 0.5 | 2.8 ± 1.3 |
| Concentric | −0.8 ± 0.9* | 4.3 ± 2.5* | −0.5 ± 0.7* | 4.2 ± 1.7* | −1.3 ± 1.8 | 1.5 ± 1.4 |
Values are mean and SD.
P < 0.05 vs. passive and eccentric.
DISCUSSION
Device Operation
To date, we have had no adverse incident in any of the over twenty subjects (6 reported here), scanned at both 1.5T and 3T, other than occasional fatigue due to the prolonged and repetitive contractions. While there have been a few designs of MR compatible devices for investigating muscle contraction, they have generally focused on different muscles such as the biceps (1) or the quadriceps (15) or most recently for other applications such as fMRI for the wrist (16) and ankle (17). Typically the degree of control on the force was much less (15) with the use of bungee cords. The shortcomings of the spin tag technique including fading of tag lines, their spacing and finite widths can be overcome to a large extent by the VE-PC MRI technique. There is no fading so that motion can be followed over the entire contraction cycle of ~2 sec, and analysis of velocity/strain can be carried out at each pixel.
Care had to be taken in centering of the ankle rotation on the rotation center of the foot pedal. Importantly, most subjects needed clear instructions and training just once before the start of the imaging session to produce contractions of the desired type with the required consistence of force exerted. Some subjects however were simply unable to produce such consistency even after repeated instructions and were excluded from the study. Each of the contraction cycles were correctly and consistently synchronized by the device, with sufficiently low scatter of the force exerted (Fig. 2-B, C, D), producing artifact-free PC images in either the isometric, concentric or eccentric excursions, (Fig. 3-A, B, C).
Determination of Changes in Fascicle Length and Aponeurosis Separation with Velocity Encoded Phase Contrast Imaging
The VE-PC imaging technique in conjunction with the device demonstrated significant differences, with low standard deviations, between strains of fibers between passive plantar flexion compared to active eccentric motion (Fig. 4.A). During eccentric contraction, the shortening of muscle fibers required to produce force during the dorsiflexion phase decreases the lengthening of fibers that occurs from passive dorsiflexion, leading to less total change in fiber length. During concentric contraction however, the two shortening changes in fiber lengths, one from force production and the other from plantarflexion are additive, increasing the total fiber length change.
Our physiological observations (Table 1 and Fig. 4.B) demonstrate that the aponeurosis distance can alter during ankle joint rotation, and that this change can vary along the superior-inferior location and among contraction modes. Changes in aponeurosis separation can be attributed to dynamics of muscle fascicle as muscle length changes and to regional differences in the pennation angle, which has been verified by modeling studies (18). This amplification of muscle fiber length changes into aponeurosis displacement, termed the "gear ratio" (19) is due to the architectural mechanics of the muscle (10). The gear ratio is sensitive to slight changes in aponeurosis separation as well as the muscle fiber pennation angle (2). The heterogeneity and regional differences of changes in aponerurosis separation may indicate that intramuscular mechanics are considerably more complex than is generally assumed.
In conclusion, this paper reports the design, development and testing of a computer controlled foot pedal device compatible with 1.5T and 3T MR scanners that enables dynamic structural and function muscle imaging of the lower leg. Overall the device is sufficiently rugged and precise to enable accurate and consistent measurements of changes in fiber bundle lengths and aponeurosis seperation within a muscle.
Acknowledgments
Grant Support: National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant 2RO1AR053343-05A1.
REFERENCES
- 1.Pappas GP, Asakawa DS, Delp SL, Zajac FE, Drace JE. Nonuniform shortening in the biceps brachii during elbow flexion. J Appl Physiol. 2002;92:2381–2389. doi: 10.1152/japplphysiol.00843.2001. [DOI] [PubMed] [Google Scholar]
- 2.Hodgson JA, Finni T, Lai AM, Edgerton VR, Sinha S. Influence of structure on the tissue dynamics of the human soleus muscle observed in MRI studies during isometric contractions. J Morphol. 2006;267:584–601. doi: 10.1002/jmor.10421. [DOI] [PubMed] [Google Scholar]
- 3.Narici MV, Reeves ND, Morse CI, Maganaris CN. Muscular adaptations to resistance exercise in the elderly. J Musculoskelet Neuronal Interact. 2004;4:161–164. [PubMed] [Google Scholar]
- 4.Aagaard P, Andersen JL, Dyhre-Poulsen P, et al. A mechanism for increased contractile strength of human pennate muscle in response to strength training: changes in muscle architecture. J Physiol. 2001;534:613–623. doi: 10.1111/j.1469-7793.2001.t01-1-00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawakami Y, Abe T, Fukunaga T. Muscle-fiber pennation angles are greater in hypertrophied than in normal muscles. J Appl Physiol. 1993;74:2740–2744. doi: 10.1152/jappl.1993.74.6.2740. [DOI] [PubMed] [Google Scholar]
- 6.Akima H, Kawakami Y, Kubo K, et al. Effect of short-duration spaceflight on thigh and leg muscle volume. Med Sci Sports Exerc. 2000;32:1743–1747. doi: 10.1097/00005768-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Narici M, Cerretelli P. Changes in human muscle architecture in disuse-atrophy evaluated by ultrasound imaging. J Gravit Physiol. 1998;5:73–74. [PubMed] [Google Scholar]
- 8.Jaspers RT, Brunner R, Pel JJ, Huijing PA. Acute effects of intramuscular aponeurotomy on rat gastrocnemius medialis: force transmission, muscle force and sarcomere length. J Biomech. 1999;32:71–79. doi: 10.1016/s0021-9290(98)00159-6. [DOI] [PubMed] [Google Scholar]
- 9.Lieber RL. Skeletal muscle architecture: implications for muscle function and surgical tendon transfer. J Hand Ther. 1993;6:105–113. [PubMed] [Google Scholar]
- 10.Gans C, Gaunt AS. Muscle architecture in relation to function. J Biomech. 1991;24:53–65. doi: 10.1016/0021-9290(91)90377-y. [DOI] [PubMed] [Google Scholar]
- 11.Agur AM, Ng-Thow-Hing V, Ball KA, Fiume E, McKee NH. Documentation and three-dimensional modelling of human soleus muscle architecture. Clin Anat. 2003;16:285–293. doi: 10.1002/ca.10112. [DOI] [PubMed] [Google Scholar]
- 12.Sinha U, Sinha S, Hodgson JA, Edgerton VR. Human Soleus Muscle Architecture at Different Ankle Joint Angles from Magnetic Resonance Diffusion Tensor Imaging. J Appl Physiol. 2011;110:807–819. doi: 10.1152/japplphysiol.00923.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin D, Finni T, Ahn S, Hodgson JA, Lee HD, Edgerton VR, Sinha S. In-vivo estimation and repeatability of force-length relationship and stiffness of the human Achilles tendon using phase contrast MRI. J Magn Reson Imaging. 2008;28:1039–1045. doi: 10.1002/jmri.21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drace JE, Pelc NJ. Tracking the motion of skeletal muscle with velocity-encoded MR imaging. J Magn Reson Imaging. 1994;4:773–778. doi: 10.1002/jmri.1880040606. [DOI] [PubMed] [Google Scholar]
- 15.Finni T, Havu M, Sinha S, Usenius JP, Cheng S. Mechanical behavior of the quadriceps femoris muscle tendon unit during low-load contractions. J Appl Physiol. 2008;104:1320–1328. doi: 10.1152/japplphysiol.01069.2007. [DOI] [PubMed] [Google Scholar]
- 16.Khanicheh A, Mintzopoulos D, Weinberg B, Tzika AA, Mavroidis C. MR_CHIROD v.2: magnetic resonance compatible smart hand rehabilitation device for brain imaging. IEEE Trans Neural Syst Rehabil Eng. 2008;16:91–98. doi: 10.1109/TNSRE.2007.910286. [DOI] [PubMed] [Google Scholar]
- 17.Newton JM, Dong Y, Hidler J, et al. Reliable assessment of lower limb motor representations with fMRI: use of a novel MR compatible device for real-time monitoring of ankle, knee and hip torques. Neuroimage. 2008;43:136–146. doi: 10.1016/j.neuroimage.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chi SW, Hodgson J, Chen JS, et al. Finite element modeling reveals complex strain mechanics in the aponeuroses of contracting skeletal muscle. J Biomech. 2010;43:1243–1250. doi: 10.1016/j.jbiomech.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azizi E, Brainerd EL, Roberts TJ. Variable gearing in pinnate muscles. Proc Natl Acad Sci USA. 2008;105:1745–1750. doi: 10.1073/pnas.0709212105. [DOI] [PMC free article] [PubMed] [Google Scholar]