Abstract
Fractional flow reserve (FFR) and intravascular imaging respectively provide hemodynamic and anatomical assessments of angiographic intermediate stenoses. Frequency domain optical coherence tomography (FD-OCT) is a promising high-resolution imaging modality, but its clinical use in determining severity of coronary disease has yet to be determined. There, we set out to determine the role of FD-OCT to complement FFR in the evaluation of intermediate coronary artery stenoses. FD-OCT was planned in 176 consecutive interventional procedures at our institution to delineate the proper use of FD-OCT in clinical practice. The decision to use other invasive assessments was at the discretion of the operator. This report describes an early series of the 14 patients who underwent FFR of 18 target stenoses in addition to FD-OCT. FD-OCT was successfully performed without complications in all cases. Fractional flow reserve was <0.80 in four patients, with minimal lumen areas and reference vessel diameters ranging from 1.03 to 3.47 mm2 and 2.60 to 2.94 mm by FD-OCT, respectively. FD-OCT was important to rule out plaque rupture, erosion and thrombosis and to help guide decision to defer PCI in six patients with acute coronary syndrome and FFR > 0.80. FD-OCT was also valuable to guide PCI strategy in tandem lesions with an FFR < 0.80. This initial experience with FD-OCT suggests a potential complementary role of physiological and anatomical assessment to guide decision making in complex clinical scenarios. Future investigations are warranted to validate these findings and define the role of FD-OCT in assessing intermediate lesions.
Keywords: Optical coherence tomography, Fractional flow reserve, Intermediate coronary stenosis
Introduction
Coronary angiography is a poor discriminator of coronary artery disease severity as confirmed by fractional flow reserve (FFR) and intravascular ultrasound (IVUS) investigations. These invasive hemodynamic (FFR) and morphologic (IVUS) diagnostic tools have become essential in the catheterization laboratory to assess intermediate coronary artery stenoses [1–6]. Frequency domain optical coherence tomography (FD-OCT) is a new promising intravascular imaging modality to assess coronary artery disease in the catheterization laboratory. FDOCT employs near-infrared light rather than ultra-sound to generate vascular images. Lumen and arterial wall images are acquired at extremely high speed (100 frames per second) and axial resolution (10–15 lm), a ten-fold improvement compared to IVUS [7]. This technology has been tested in vitro and has shown to provide more accurate and reproducible measurements than IVUS [8].
However, the clinical use of FD-OCT to evaluate plaque features and severity of lumen stenosis in conjunction with FFR has yet to be investigated. This report describes the first series of patients who underwent simultaneous FD-OCT and FFR evaluation for the assessment of intermediate coronary artery stenoses.
Methods
The University Hospitals Case Medical Center OCT Quality Initiative
FD-OCT has been used routinely at the University Hospitals Case Medical Center (UH-CMC) Catheterization Laboratories since the technology was approved for clinical use by the FDA in May 2010. Clinical indications and guidelines for use of this imaging tool were not well defined; thus, it remained unclear how to implement OCT into routine practice and whether this imaging tool would improve the overall quality of patient care. Therefore, The University Hospitals Case Medical Center OCT Quality Initiative (UH-OCT) was launched in September 2010 to collect data regarding implementation of OCT in routine interventional procedures. This program was approved by the UH-CMC Institutional Review Board (IRB). All patients undergoing interventional coronary procedures were considered for OCT imaging as part of their clinical care (i.e. 100% usage). There was no specific selection of patients representing an “all-comers, all-indications” initiative without comparative groups. Clinically relevant information was collected prospectively in a customized questionnaire, which was also reviewed and approved by the IRB. The goal of the quality initiative was to assess the use of the new FD-OCT technology in the cardiac catheterization laboratory.
The UH-OCT initiative was active for 60 days (September to November, 2010) and FD-OCT was considered in 176 consecutive angiography cases in which intravascular imaging was contemplated for either diagnostic assessment of an intermediate lesion or for guidance of percutaneous coronary intervention (PCI). The present report consists of 15 patients with 19 target moderate stenoses (40–70% stenosis on angiography) which were assessed by FFR per the operator's discretion. One patient with an FFR > 0.8 was excluded from the report due to uninterpretable FD-OCT data. This patient had baseline elevated creatinine and FD-OCT imaging was not repeated to avoid additional contrast. There were no specific guidelines for online interpretation of FD-OCT images and the decision to intervene on the lesion was at the discretion of the operator.
Angiographic assessment
All angiograms were performed in at least two orthogonal views after 100–200 μg intracoronary injection of nitroglycerin. Digital coronary angiograms were analyzed offline with a validated automated edge detection system (CAAS II, PIE Medical, Maastricht, The Netherlands). In brief, angiographic measurements were made during diastole and using the contrast-filled guiding-catheter for calibration. The entire segment of interest was selected for analysis. The location of the lesion in each vessel was classified by the Coronary Artery Surgery Study system [9]. Lesion morphology was assessed according to the modified American College of Cardiology/American Heart Association classification system [10]. The quantitative angiographic parameters evaluated were: (1) reference vessel diameter, (2) minimal luminal diameter, (3) lesion length, and (4) percent diameter stenosis, which was determined as: 100 × (1-[minimal luminal diameter/reference vessel diameter]).
OCT procedure
Frequency domain optical coherence tomography images were acquired with a commercially available system (C7-XR™ OCT Intravascular Imaging System, St. Jude Medical, St. Paul, Minnesota) after intracoronary injection of 200 μg of nitroglycerin through conventional 6F guiding-catheters. A 0.014-mm guidewire was positioned distally and the OCT catheter (C7 Dragonfly™, St. Jude Medical, St. Paul, Minnesota) was advanced to the distal end of the target lesion. The entire length of the region of interest was scanned using the integrated automated pullback device at 20 mm/s. During image acquisition, coronary blood flow was replaced by continuous flushing of contrast media with a power injector, in order to create a virtually blood-free environment. The flow rate was also decided by the operator, but ranged from 12 to 20 cc at 3.5–5 cc/s at 400 PSI. All FD-OCT measurements were performed using proprietary software (St. Jude Medical, St. Paul, Minnesota).
All images were recorded digitally, stored, and submitted to the Cardiovascular Imaging Core Laboratory (Harrington McLaughlin Heart and Vascular Institute, UH-CMC, Cleveland, Ohio) for offline evaluation and subsequent analysis by 2 independent investigators (D.C. and G.A.), who were blinded to the FFR values. When there was discordance between the observers, a consensus reading was obtained by a 3rd senior expert (H.G.B.). After confirming proper calibration settings of the Z-offset [7], the longitudinal view was used to identify the region of interest, and luminal areas and diameters were obtained at every frame interval (0.2 mm).
The proximal and distal reference luminal areas and diameters were taken in their respective locations relative to the lesion where the stenosis transitioned to a “normal” appearing vessel, before the origin of a side branch. The mean reference vessel luminal area (mRLA) and diameter (mRLD) was determined by averaging the proximal and distal measurements. In cases in which the proximal or distal reference could not be established due to the lesion being at an ostium or a stent edge, the mRLA/mRLD was taken as the proximal or distal available reference. The minimal luminal area (MLA) and the minimal luminal diameter (MLD) were identified within the lesion and the respective percent stenosis was defined by: [(mRLAMLA)/mRLA] × 100 and [(mRLD-MLD)/mRLD] × 100. Lesion length was measured as the distance between the segments taken for the proximal and distal references. Lesions were classified as fibrotic, calcified, mixed (features of both fibrotic and calcific plaque), or in-stent restenosis.
FFR procedure
Fractional flow reserve was measured with a coronary pressure guidewire (PressureWire™ Certus, St. Jude Medical, St. Paul, Minnesota) at maximal hyperemia induced by intravenous adenosine, which was administered at a rate of 140 μg per kilogram of body weight per minute. FFR was calculated as the mean distal coronary pressure (measured with the pressure wire) divided by the mean aortic pressure (measured simultaneously with the guiding catheter) after 2 min of adenosine infusion.
Statistical analysis
Continuous variables are presented as means ± standard deviation and categorical variables are presented as the percentage of the group total. Pearson correlation coefficients were employed to analyze correlations between FFR values and pertinent FD-OCT variables.
Results
Frequency domain optical coherence tomography and FFR procedures were performed in all attempted cases without complication. OCT provided optimal visualization in 95% (18/19) of target segments. The primary indications for lesion assessment were intermediate angiographic stenosis in patients with stable angina (47%) and acute coronary syndrome (ACS) (53%). One patient presenting with an NSTEMI was evaluated following intervention of the culprit lesion due to an intermediate lesion distal to the stented segment. Target vessels were left anterior descending artery (72%), left circumflex artery (11%), right coronary artery (11%), and left main (6%). There were 4 lesions (22%) which had an FFR < 0.80, all of which were intervened upon. The minimal lumen area and reference vessel luminal diameter ranged from 1.03 to 3.47 mm2 and 2.60 to 2.94 mm by FDOCT, respectively, in these patients.
Comprehensive data on clinical characteristics, physiology, FD-OCT, angiography and clinical management of each patient and target lesion are displayed in Table 1. There was no significant correlation between FFR and the FD-OCT variables of MLA, MLD, area stenosis, and diameter stenosis (Table 2).
Table 1.
Comprehensive data on clinical characteristics, FFR, FD-OCT, QCA and clinical decision making
| Patient Information |
Stenosis Data |
FFR | FD-OCT Data |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient # | Age/Sex | Past Medical History | Clinical Presentation | Vessel | CASS | Tandem Lesion | Proximal RLA | Distal RLA | mRLA | MLA | AS | Proximal RLD | Distal RLD | |
| 1* | 67/M | HTN, smoking, PAD, PCI | Stable angina | LAD | 14 | Yes | 0.68 | 7.27 | 3.64 | 5.46 | 1.03 | 81 | 3.04 | 2.15 |
| LAD | 12 | 0.85 | 7.35 | 7.04 | 7.2 | 4.30 | 40 | 3.01 | 2.99 | |||||
| 2† | 64/m | HTN, DM, DLD, smoking, MI, CVA, PCI | Staged PCI | LAD | 12 | No | 0.73 | 7.45 | 6.46 | 6.96 | 3.47 | 50 | 3.01 | 2.86 |
| 3* | 75/M | HTN, DLD, MI, PCI | UA | LAD | 14 | Yes | 0.76 | 7.54 | 4.73 | 6.14 | 1.86 | 70 | 3.09 | 2.45 |
| LAD | 12 | 0.85 | 7.78 | - | 7.78 | 5.75 | 26 | 3.09 | - | |||||
| 4 | 63/F | HTN, smoking | Stable angina | LAD | 13 | No | 0.78 | 7.25 | 6.17 | 6.71 | 3.35 | 50 | 3.02 | 2.8 |
| 5* | 55/M | HTN, DM, DLD, smoking, PCI | UA | LAD | 13 | Yes | n/a | 4.38 | 3.92 | 4.15 | 2.92 | 30 | 2.36 | 2.25 |
| LAD | 12 | 0.82 | 8.44 | 8.22 | 8.33 | 4.11 | 51 | 3.26 | 3.22 | |||||
| 6 | 57/M | HTN, DM, DLD, PAD | Stable angina | LAD | 12 | No | 0.82 | 9.55 | 8.86 | 9.21 | 3.25 | 65 | 3.47 | 3.35 |
| 7 | 71/F | HTN, DLD | UA | LAD | 13 | No | 0.83 | 4.09 | 5.26 | 4.68 | 2.59 | 45 | 2.27 | 2.58 |
| 8 | 79/M | HTN, DM, smoking, EF 35, MI, CABG | UA | LM | 11 | No | 0.85 | 19.23 | - | 19.23 | 9.04 | 53 | 4.93 | - |
| 2f | 64/M | HTN, DM, DLD, smoking, MI, CVA, PCI | NSTEMI: lesion post-PCI | LCx | 19 | No | 0.89 | - | 5.95 | 5.95 | 3.13 | 47 | - | 2.73 |
| 9 | 72/F | None | “NSTEMI”: ?? Takotsubo cardiomyopathy | LAD | 12 | No | 0.89 | - | 7.63 | 7.63 | 2.58 | 66 | 3.22 | 2.71 |
| 10 | 39/F | HTN, DLD, smoking | Stable angina | RCA | 1 | No | 0.93 | 8.39 | 8.68 | 8.54 | 5.96 | 30 | 3.26 | 3.32 |
| 11 | 58/M | HTN, DM, DLD, smoking, PCI | Stable angina | LCx | 19 | No | 0.94 | 6.46 | 6.86 | 6.66 | 4.42 | 34 | 2.85 | 2.95 |
| 12 | 72/M | HTN, DLD, smoking | Stable angina | LAD | 12 | No | 0.94 | 7.39 | 7.24 | 7.32 | 2.37 | 68 | 3.06 | 3.03 |
| 13 | 72/F | HTN, DM, DLD, smoking, PCI | UA | LAD | 12 | No | 0.96 | - | - | - | 2.19 | - | - | - |
| 14 | 55/F | HTN, smoking, prior MI, PCI | Stable angina | RCA | 2 | No | 0.96 | - | 5.03 | 5.03 | 1.95 | 61 | - | 2.53 |
| Patient Information |
FD-OCT Data |
QCA Data |
Management | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient # | Age/Sex | Past Medical History | Clinical Presentation | mRLD | MLD | DS | Lesion Length | Lesion characteristics | ACC/AHA classification | RVD | MLD | DS | Lesion Length | |
| 1* | 67/M | HTN, smoking, PAD, PCI | Stable angina | 2.6 | 1.03 | 60 | >45 | Calcified | C | 1.77 | 0.98 | 45 | 59.72 | PCI |
| 3 | 1.63 | 46 | 6.8 | Mixed | A | 2.62 | 1.56 | 41 | 4.63 | Defer | ||||
| 2 | 64/m | HTN, DM, DLD, smoking, MI, CVA, PCI | staged PCI | 2.94 | 1.8 | 39 | 10.7 | Fibrotic | A | 3.31 | 1.75 | 47 | 9.94 | PCI |
| 3* | 75/M | HTN, DLD, MI, PCI | UA | 2.77 | 1.31 | 53 | 13.2 | Calcified | B1 | 2.25 | 1.67 | 26 | 8.79 | PCI |
| 3.09 | 2.19 | 29 | 5.5 | Calcified | B1 | 2.65 | 1.88 | 29 | 8.04 | Defer | ||||
| 4 | 63/F | HTN, smoking | stable angina | 2.91 | 1.79 | 38 | 26.8 | Fibrotic | C | 2.5 | 1.64 | 35 | 27.6 | PCI |
| 5* | 55/M | HTN, DM, DLD, smoking, PCI | UA | 2.31 | 1.91 | 17 | 8.9 | Fibrotic | Bl | 2.14 | 1.34 | 37 | 12.93 | Defer |
| 3.24 | 2.22 | 31 | 7.7 | Mixed | Bl | 3 | 2.01 | 33 | 7.08 | Defer | ||||
| 6 | 57/M | HTN, DM, DLD, PAD | stable angina | 3.41 | 1.7 | 50 | 7.3 | Mixed | Bl | 2.43 | 1.81 | 25 | 5.34 | Defer |
| 7 | 71/F | HTN, DLD | UA | 2.43 | 1.43 | 41 | 8.5 | Calcified | Bl | 2.55 | 1.71 | 33 | 11.29 | Defer |
| 8 | 79/M | HTN, DM, smoking, EF 35, MI, CABG | UA | 4.93 | 2.46 | 50 | 5.8 | Fibrotic | B2 | 5.01 | 2.62 | 48 | 7.53 | Defer |
| 2† | 64/M | HTN, DM, DLD, smoking, MI, CVA, PCI | NSTEMI: lesion post-PCI | 2.73 | 1.43 | 48 | 3.6 | Fibrotic | C | 2.18 | 1.32 | 39 | 24.09 | Defer |
| 9 | 72/F | None | “NSTEMI”: ?? Takotsubo cardiomyopathy | 2.97 | 1.63 | 45 | 23.2 | Mixed | Bl | 2.55 | 1.61 | 37 | 10.18 | Defer |
| 10 | 39/F | HTN, DLD, smoking | stable angina | 3.29 | 2.6 | 21 | 4.8 | Fibrotic | A | 3.08 | 2.16 | 30 | 8.14 | Defer |
| 11 | 58/M | HTN, DM, DLD, smoking, PCI | stable angina | 2.9 | 2.26 | 22 | 4.4 | Fibrotic | Bl | 2.72 | 1.69 | 38 | 8.11 | Defer |
| 12 | 72/M | HTN, DLD, smoking | stable angina | 3.05 | 1.59 | 48 | 18.4 | Fibrotic | Bl | 2.24 | 0.94 | 58 | 17.58 | Defer |
| 13 | 72/F | HTN, DM, DLD, smoking, PCI | UA | - | 1.54 | - | 10.1 | Calcified | Bl | 2.26 | 1.39 | 38 | 8.37 | Defer |
| 14 | 55/F | HTN, smoking, prior MI, PCI | stable angina | 2.53 | 1.36 | 46 | 9.8 | ISR | Bl | 2.29 | 1.58 | 31 | 11.41 | PCI |
HTN hypertension, DM diabetes, DLD dyslipidemia, PAD peripheral arterial disease, MI myocardial infarction, CVA cerebrovascular accident, PCI percutaneous intervention, CABG prior bypass surgery, UA unstable angina, NSTEMI non-ST segment myocardial infarction, STEMI ST-segment elevation MI, LAD left anterior descending coronary artery, LCx left circumflex coronary artery, RCA right coronary artery, LM left main coronary artery, FFR fractional flow reserve, RLA reference vessel luminal area (mm2), mRLA mean reference vessel luminal area (mm2), MIA minimal luminal area (mm2), AS area stenosis (%), RLD reference vessel luminal diameter (mm), mRLD reference vessel luminal diameter (mm), MLD minimal lumen diameter (mm), DS diameter stenosis (%), lesion length (in mm), ISR in-stent restenosis, Defer: no PCI performed to lesion
patients with tandem lesions in vessel and hence two rows of data (one for each lesion) are listed
patient is listed on table twice as had lesions in separate vessels evaluated at different time points
Table 2.
Correlation between FFR values and lesion morphometric quantitative parameters by OCT and angiography
| Pearson correlation coefficient | 95% CI of correlation coefficient | P value to test correlation coefficient = 0 | |
|---|---|---|---|
| MLA (OCT) | 0.167 | (–0.378, 0.626) | 0.560 |
| AS (OCT) | –0.424 | (–0.779, 0.137) | 0.133 |
| MLD (OCT) | 0.293 | (–0.258, 0.700) | 0.297 |
| DS (OCT) | –0.430 | (–0.782, 0.131) | 0.128 |
| MLD (QCA) | 0.025 | (–0.493, 0.531) | 0.930 |
| DS (QCA) | 0.006 | (–0.508, 0.517) | 0.984 |
One patient presented with a chest pain syndrome in the setting of emotional distress and was found to have a mild troponin elevation along with apical ballooning on echocardiography suggestive of Takotsubo cardiomyopathy. She had an intermediate angiographic stenosis (50% on visual angiography, 37% on QCA) that was evaluated by FFR and FD-OCT. PCI was deferred based on an FFR of 0.89 and FD-OCT was performed to rule out plaque rupture and to better define anatomical stenosis. FD-OCT showed a stable mixed (predominantly fibrous) plaque without signs of rupture or erosion with an MLA of 2.58 mm2 (Fig. 1) and no intraluminal thrombus. Interestingly, there were no signs of plaque rupture, erosion, or thrombus in the target vessels of the 6 patients presenting with ACS, who also had FFR > 0.8.
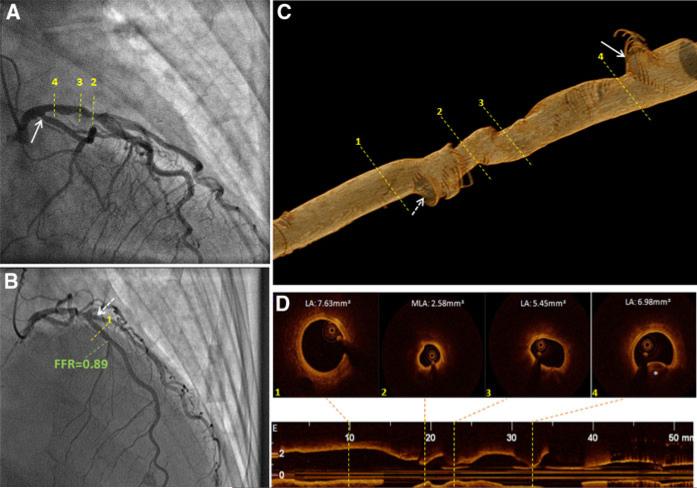
Fig. 1.
Representative case of a 72-year-old female who presented with a non-ST elevation myocardial infarction. Her presentation was initiated in the setting of emotional stress and her echocardiogram showed apical ballooning suggestive of Takotsubo cardiomyopathy. The angiogram (A, B) did not show significant disease in either the RCA (not shown) or LCx. C shows the 3-D reconstruction by FD-OCT of the region of interest, with the numbered dashed yellow lines representing the same regions identified in the angiograms. The origins of both the LCx (arrow) as well as the diagonal branch (dashed arrow) can be readily depicted. D, E show FD-OCT cross-sectional images of the points of interest with their respective lumen area values. Although FD-OCT revealed a relatively extensive diseased segment in the proximal LAD, and minimal lumen area of 2.58 mm2, no signs of plaque instability were noticeable. A variety of plaque composition was noticed, predominantly lipidic (D2, D3), and calcified (marked by asterisk in D4). Normal distal reference is also illustrated (D1). The FFR value obtained distal to this segment was 0.89. Based on the composite of the above results, percutaneous intervention was deferred. RCA right coronary artery, LCx left circumflex artery, LAD left anterior descending artery, FD-OCT frequency domain optical coherence tomography, FFR fractional flow reserve; LV left ventricle, MLA minimal lumen area (mm2), LA lumen area (mm2)
Three patients were noted to have angiographic multiple (tandem) lesions involving the proximal and mid left anterior descending artery. Two of these patients had an FFR < 0.80 across the distal lesion. The complementary use of physiological and anatomical assessment in one of these patients is illustrated in Fig. 2. In the other patient, FFR was 0.76 across the distal lesion and upon pullback increased to 0.85 at the proximal segment. FD-OCT revealed a large MLA of 5.75 mm2 of the proximal stenosis with an area stenosis of 26%. The distal lesion showed an MLA of 1.86 mm2 with an area stenosis of 70% and the decision was made to limit intervention to the distal lesion. The third patient had an FFR of 0.82 across the distal lesion. FD-OCT demonstrated an MLA of 4.11 mm2 with an area stenosis of 51% of the proximal lesion and an MLA of 2.92 mm2 with an area stenosis of 30% in the distal stenosis. Given the composite FFR and FD-OCT data, PCI was deferred in this patient.
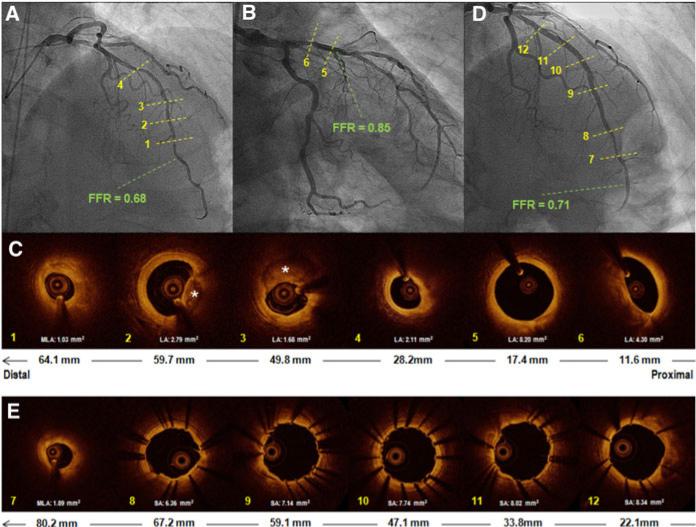
Fig. 2.
Representative case of a patient who presented with stable angina found to have tandem stenoses in the LAD. RAO cranial view (A) demonstrated diffuse disease in the mid to distal segments of the LAD and in the RAO caudal projection (B), one can identify a focal lesion in its proximal segment. C shows FD-OCT images of different points along the LAD in correspondence to the numbered dashed yellow lines from the angiographic pictures. The bottom arrow in the panel shows the distance from the LAD ostium. The MLA was 1.03 mm2 (C1). Images (C2) and (C3) demonstrate calcified (asterisk) lesions. In the proximal LAD segment, one can identify a “non-diseased” segment (C5) followed by the proximal stenosis, which presented a lumen area of 4.30 mm2. The FFR value distal to the whole segment of disease was 0.68. However, the FFR evaluation of the proximal lesion was 0.85. Based on these results, the decision was to limit intervention to the lesions located on the mid to distal portions of the LAD. Picture (D) exhibits the control angiography after the aforementioned treatment. E represents FD-OCT images post-treatment. In (E7) it was observed a significant stenosis distal to the stented segment (MLA 1.89 mm2). One can observe that along the entire treated segment (E8–12) that the stents are well expanded and apposed, including the overlapping regions (E9 and E11). Repeat FFR of the lesion distal to the stented segment was 0.71, which prompted subsequent intervention to that lesion (not shown). LAD left anterior descending artery, RAO right anterior oblique, FD-OCT frequency domain optical coherence tomography, MLA minimal lumen area, FFR fractional flow reserve, LA lumen area, SA stent area
Discussion
This report provides the first description of the use of FD-OCT to assess intermediate lesions in association with FFR. This early experience provides important clinical insights on the potential future use of FD-OCT to assess intermediate coronary stenoses and warrants investigations to define the precise role of this new imaging tool in guiding decision making in the catheterization laboratory.
The first observation that emerged was that FFR, in spite of the availability of FD-OCT imaging in all patients, was used for decision-making in all patients with angiographically intermediate stenoses, representing 10% of the procedures performed in our institution during the UH-OCT Quality Initiative. While these indications for use of FFR reflect current practice standards and are supported by extensive outcome data provided by the DEFER, FAME, and PHANTOM trials [1–3], it also reveals that morphological assessment, even if provided at 15-μm resolution with optimal visualization and quantification of minimal luminal area, does not eliminate the need for assessment of “physiology” at the present time.
The use of an IVUS MLA of 4.0 mm2 as a “cutoff” to perform or defer PCI has become common practice. The original study indeed reported that deferring intervention in an MLA > 4.0 mm2 had similar event rates as those who had PCI deferred based on physiological data [4]. However, this observation does not warrant intervening on stenoses with a MLA < 4.0 mm2. A composite of percent area stenosis > 70%, an MLD ≤ 1.8 mm, an MLA ≤ 4.0 mm2 and a lesion length > 10 mm on IVUS has been well correlated with functionally significant intermediate stenosis, but the use of an MLA ≤ 4.0 mm2 alone has a sensitivity of 92% and specificity of 56% to predict an FFR of < 0.75 [5]. This lack of specificity leads to over-performance of PCI when using anatomical parameters to assess an intermediate stenosis [6]. An MLA of < 3.0 mm2 can increase the specificity of IVUS to 92% to demonstrate a functionally significant lesion by FFR at the expense of sensitivity (83%) [11]. Furthermore, a fixed cutoff value for MLA by IVUS is not applicable for different vessel sizes, such as in the left main coronary artery or small vessels [3, 12].
It is also important to notice that luminal sizing between OCT and IVUS are not necessarily equivalent [13, 14]. Luminal areas measured by IVUS have been shown to be larger than those measured by previous generation time-domain OCT systems, even when a non-occlusive pullback technique was used [15]. Our data, by means of a well controlled phantom model, suggest that FD-OCT and IVUS have a good correlation for luminal area measurements, which also showed better reproducibility by FD-OCT measurements [8].
There were 11 patients with single stenosis with an FFR > 0.80. It should not be surprising that single stenoses with FFR > 0.80 had a wide range of MLA from 1.95 to 9.04 mm2 in our patients. Indeed, we could not observe any significant correlation between physiologic assessment by FFR and morphologic quantitative parameters by OCT.
Normal FFR values can also result from poor response to adenosine such as in patients with microvascular disease [16] despite the presence of anatomically significant disease. However, the treatment of these lesions are unlikely to improve myocardial perfusion. Whether FD-OCT and FFR can have a complimentary role in the diagnosis of microvascular disease and guidance of future therapeutic strategies remains to be investigated. There was only one case in which the physician decided to proceed with PCI based on the FD-OCT data and clinical grounds, despite an FFR of >0.80. This patient was admitted with chest pain and had in-stent restenosis with an MLA of 1.95 mm2.
Nonetheless, the availability of both physiology and anatomical data provided by FFR and FD-OCT was extremely valuable for decision making in this series. The adjunctive use of both technologies was most useful to guide intervention in patients with multiple stenoses because of the difficult in defining which lesion to treat based on FFR values alone, as illustrated in Fig. 2. Serial stenoses within a vessel have been shown to pose a physiologic challenge and FFR may underestimate the true functional significance of both the proximal and distal lesions [17]. There are no clear guidelines on how to manage multiple lesions with a positive FFR in the distal segment, particularly if a similar drop in pressure gradient is observed across both stenoses. In 2 cases in the present series, FD-OCT was important to identify the non-critical MLA in the proximal stenoses leading to selective intervention of the distal stenosis and optimization of the stent deployment procedure.
Frequency domain optical coherence tomography was also invaluable in the setting of ACS and a negative FFR to evaluate any feature of plaque instability (rupture, erosion, or presence of thrombus). OCT is superior to IVUS for detection of lipid plaque, intracoronary thrombus, plaque rupture, and fibrous cap erosion [18]. Somewhat surprising was the fact that none of the ACS patients had plaque rupture or erosion depicted by FD-OCT in the suspected “culprit” vessel. These findings suggest the importance of morphological evaluation when there is a high clinical suspicion for plaque rupture, particularly because the use of FFR in the acute phase of ACS is not well established [19].
Abbreviations
- FFR
Fractional flow reserve
- IVUS
Intravascular ultrasound
- FD-OCT
Frequency domain optical coherence tomography
- UH-CMC
University hospitals case medical center
- FDA
United States food and drug administration
- IRB
Institutional review board
- PCI
Percutaneous intervention
- PSI
Pounds per square inch
- QCA
Quantitative coronary angiography
- HTN
Hypertension
- DM
Diabetes
- DLD
Dyslipidemia
- PAD
Peripheral arterial disease
- MI
Myocardial infarction
- CVA
Cerebrovascular accident
- CABG
Coronary bypass surgery
- UA
Unstable angina
- NSTEMI
Non-ST segment myocardial infarction
- STEMI
ST-segment elevation myocardial infarction
- LAD
Left anterior descending coronary artery
- LCx
Left circumflex coronary artery
- RCA
Right coronary artery
- LM
Left main coronary
- RLA
Reference vessel luminal area (mm2)
- mRLA
Mean reference vessel luminal area (mm2)
- MLA
Minimal luminal area (mm2)
- AS
Area stenosis (%)
- RLD
Reference vessel luminal diameter (mm)
- mRLD
Mean reference vessel luminal diameter (mm)
- MLD
Minimal lumen diameter (mm)
- DS
Diameter stenosis (%) lesion length (in mm)
Footnotes
Conflict of interest Dr. Costa is on the Speaker Bureau and is a consultant for BSC, Sanofi/Aventis, Eli Lilly, and Medtronic and is on the Speaker Bureau and a member of the Scientific Advisory Board for Abbott, Cordis, St. Jude Medical and Scitech. Dr. Bezerra reports receiving honoraria/research grants from St. Jude Medical.
References
- 1.Pijls NH, van Schaardenburgh P, Manoharan G, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER study. J Am Coll Cardiol. 2007;49:2105–2111. doi: 10.1016/j.jacc.2007.01.087. [DOI] [PubMed] [Google Scholar]
- 2.Tonino PA, De Bruyne B, Pijls NH, on behalf of FAME Study Investigators et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 3.Costa MA, Sabate M, Staico R, et al. Anatomical and physiologic assessments in patients with small coronary artery disease: final results of the physiologic and anatomical evaluation prior to and after stent implantation in small coronary vessels (PHANTOM) trial. Am Heart J. 2007;153:296.e1–7. doi: 10.1016/j.ahj.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 4.Abizaid AS, Mintz GS, Mehran R, et al. Long-term follow-up after percutaneous transluminal coronary angioplasty was not performed based on intravascular ultra-sound findings: Importance of lumen dimensions. Circulation. 1999;100:256–261. doi: 10.1161/01.cir.100.3.256. [DOI] [PubMed] [Google Scholar]
- 5.Briguori C, Anzuini A, Airoldi F, et al. Intravascular ultrasound criteria for the assessment of the functional significance of intermediate coronary artery stenoses and comparison with fractional flow reserve. Am J Cardiol. 2001;87:136–141. doi: 10.1016/s0002-9149(00)01304-7. [DOI] [PubMed] [Google Scholar]
- 6.Nam CW, Yoon HJ, Cho YK, et al. Outcomes of percutaneous coronary intervention in intermediate coronary artery disease: fractional flow reserve-guided versus intravascular ultrasound-guided. J Am Coll Cardiol Intv. 2010;3:812–817. doi: 10.1016/j.jcin.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Bezerra HG, Costa MA, Guagliumi G, Rollins AM, Simon DI. Intracoronary optical coherence tomography: a comprehensive review. J Am Coll Cardiol Intv. 2009;2:1035–1046. doi: 10.1016/j.jcin.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tahara S, Bezerra HG, Baibars M, et al. In vitro validation of new fourier-domain optical coherence tomography. Eurointervention. 2011;6:875–882. doi: 10.4244/EIJV6I7A149. [DOI] [PubMed] [Google Scholar]
- 9.The Principal Investigators of CASS and Associates The National Heart, Lung and Blood Institute Coronary Artery Surgery Study (CASS). Circulation. 1981;63(Suppl I):1–81. [Google Scholar]
- 10.Ellis SG, Vandormael MG, Cowley MJ, et al. Multivessel angioplasty prognosis study group. Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease. Implications for patient selection. Circulation. 1990;82:1193–1202. doi: 10.1161/01.cir.82.4.1193. [DOI] [PubMed] [Google Scholar]
- 11.Takagi A, Tsurumi Y, Ishii Y, et al. Clinical potential of intravascular ultrasound for physiological assessment of coronary stenosis: relationship between quantitative ultra-sound tomography and pressure-derived fractional flow reserve. Circulation. 1999;100:250–255. doi: 10.1161/01.cir.100.3.250. [DOI] [PubMed] [Google Scholar]
- 12.Jasti V, Ivan E, Yalamanchili V, Wongpraparut N, Leesar MA. Correlations between fractional flow reserve and intravascular ultrasound in patients with an ambiguous left main coronary artery stenosis. Circulation. 2004;110:2831–2836. doi: 10.1161/01.CIR.0000146338.62813.E7. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi T, Terashima M, Akasaka T, et al. Safety and feasibility of an intravascular optical coherence tomography image wire system in the clinical setting. Am J Cardiol. 2008;101:562–567. doi: 10.1016/j.amjcard.2007.09.116. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki Y, Ikeno F, Koizumi T, et al. In vivo comparison between optical coherence tomography and intravascular ultrasound for detecting small degrees of in-stent neointima after stent implantation. J Am Coll Cardiol Intv. 2008;1:168–173. doi: 10.1016/j.jcin.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalo N, Serruys PW, García-García HM, et al. Quantitative ex vivo and in vivo comparison of lumen dimensions measured by optical coherence tomography and intravascular ultrasound in human coronary arteries. Rev Esp Cardiol. 2009;62:615–624. doi: 10.1016/s1885-5857(09)72225-x. [DOI] [PubMed] [Google Scholar]
- 16.Blows LJ, Redwood SR. The pressure wire in practice. Heart. 2007;93(4):419–422. doi: 10.1136/hrt.2005.066837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pijls NH, de Bruyne B, Bech JW, et al. Coronary pressure measurement to assess the hemodynamic significance of serial stenoses within one coronary artery. Validation in humans. Circulation. 2000;102:2371–2377. doi: 10.1161/01.cir.102.19.2371. [DOI] [PubMed] [Google Scholar]
- 18.Kubo T, Imanishi T, Takarada S, et al. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol. 2007;50:933–939. doi: 10.1016/j.jacc.2007.04.082. [DOI] [PubMed] [Google Scholar]
- 19.Kern MJ, Samady H. Current concepts of integrated coronary physiology in the catheterization laboratory. J Am Coll Cardiol. 2010;55:173–185. doi: 10.1016/j.jacc.2009.06.062. [DOI] [PubMed] [Google Scholar]