Summary
Perceptual decisions involve distributed cortical activity. Does information flow sequentially from one cortical area to another, or do networks of interconnected areas contribute at the same time? Here we delineate when and how activity in specific areas drives a whisker-based decision in mice. A short-term memory component temporally separated tactile “sensation” and “action” (licking). Using optogenetic inhibition (spatial resolution, 2 mm; temporal resolution, 100 ms) we surveyed the neocortex for regions driving behavior during specific behavioral epochs. Barrel cortex was critical for sensation. During the short-term memory, unilateral inhibition of anterior lateral motor cortex biased responses to the ipsilateral side. Consistently, barrel cortex showed stimulus-specific activity during sensation, whereas motor cortex showed choice-specific preparatory activity and movement-related activity, consistent with roles in motor planning and movement. These results suggest serial information flow from sensory to motor areas during perceptual decision making.
Keywords: optogenetics, somatosensory cortex, frontal cortex, persistent activity, short-term memory
Introduction
Perceptual decisions involve multiple, spatially distributed cortical areas (Felleman and Van Essen, 1991; Hernandez et al., 2010). Romo and colleagues recorded neuronal correlates of sensation, memory, and action in primates during passive tactile decision tasks. Their findings suggest a hierarchically organized cortical system, where sensory information in primary somatosensory cortex (Zainos et al., 1997) is gradually (Hernandez et al., 2010) transformed into choice in frontal cortical areas (de Lafuente and Romo, 2005) (reviewed in (Romo and de Lafuente, 2012)). Serial information flow has also been observed in visual perceptual decision tasks in primates (Bisley et al., 2001; Gold and Shadlen, 2002; Seidemann et al., 1998). Relating neuronal signals to behavior requires rapid and reversible (within a trial epoch) silencing of neuronal activity during behavior. Pharmacological silencing (Hikosaka and Wurtz, 1985) and cooling (Long and Fee, 2008; Ponce et al., 2008) are too slow to reveal the involvement of individual brain areas during specific behavioral epochs. Electrical microstimulation has the requisite temporal resolution (Bisley et al., 2001; de Lafuente and Romo, 2005; Seidemann et al., 1998), but all of these methods have low throughput, prohibiting comprehensive surveys of brain regions contributing to behavior.
The mouse is a genetically tractable organism (Luo et al., 2008; O’Connor et al., 2009), providing access to defined cell types for transgene expression. Its lissencephalic macrostructure allows access to a large fraction of the brain for functional analysis. The mouse is therefore a powerful model to examine the circuit mechanisms underlying behavior. Progress will require quantitative perceptual decision tasks and establishing causal relationships between cortical activity in specific brain regions and behavior. The flow of information through interconnected cortical areas underlying a perceptual decision has not been examined in mice. A key challenge is that decision tasks with the requisite behavioral components are not available.
Here we developed a tactile decision behavior in head-fixed mice to track the flow of information during perceptual decision making. Rodents use their whiskers to navigate tight spaces and explore objects (Knutsen et al., 2006; O’Connor et al., 2010a; Ritt et al., 2008; Voigts et al., 2008). In our task, head-fixed mice measured the location of a pole using their whiskers and reported their choice with licking. In contrast to previous versions (O’Connor et al., 2010a), a delay epoch separates “sensation” and “action”. We used optogenetic silencing to identify the cortical regions involved during any trial epoch.
The vibrissal primary somatosensory cortex (vS1, also called ‘barrel cortex’) receives whisker-related tactile input via the thalamus (Koralek et al., 1988; Lu and Lin, 1993; Petreanu et al., 2009; Woolsey and Van der Loos, 1970). vS1 is heavily connected with thalamic and other cortical areas via long range connections (Hoffer et al., 2005; Hooks et al., 2013; Mao et al., 2011). The vast majority of cortical areas remain unstudied in the context of tactile discrimination.
Inactivating vS1 caused deficits in object location discrimination (Hutson and Masterton, 1986; O’Connor et al., 2010a; O’Connor et al., 2013), mainly during sensation. Unilateral inactivation of a frontal cortical area (anterior lateral motor cortex, ALM) during the delay epoch, preceding the motor response, biased the upcoming choice in the ipsilateral direction. Neurons in vS1 showed stimulus specific activity during the sample epoch (Curtis and Kleinfeld, 2009; O’Connor et al., 2010b; von Heimendahl et al., 2007). Neurons in ALM showed choice specific preparatory activity and movement-related activity, consistent with roles in driving the response. Our results consistent with serial information flow, where information is passed from sensory areas to motor areas during perceptual decision making.
Results
Object location discrimination with a short-term memory epoch
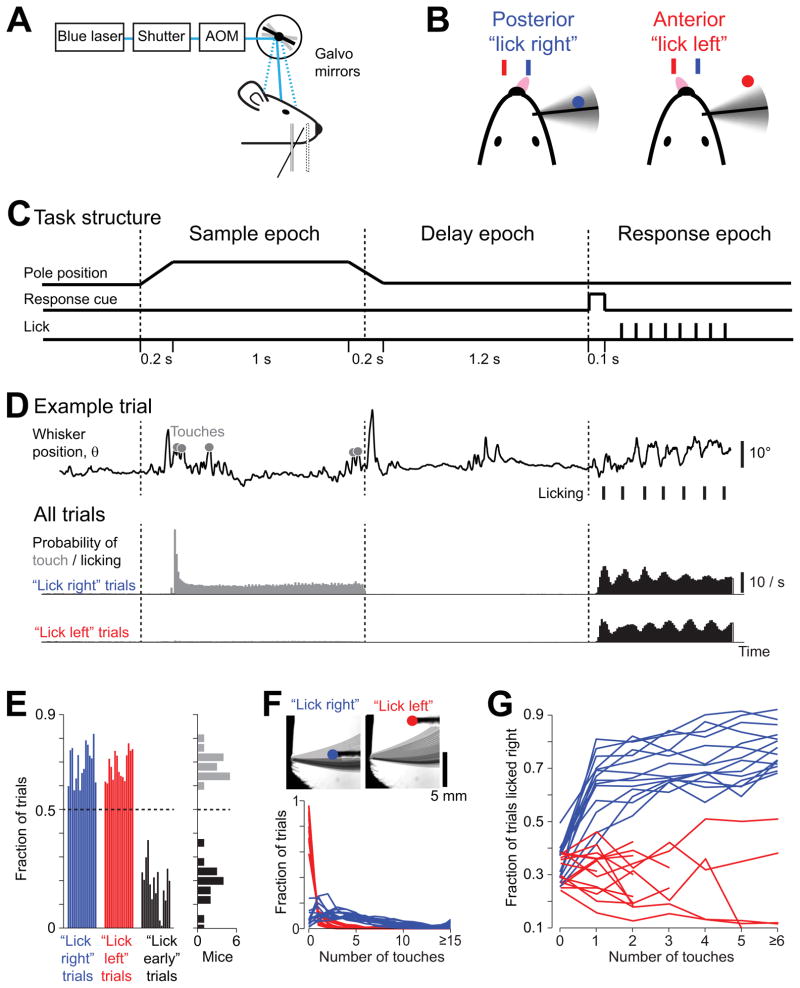
The behavioral task (Figure 1A) was adapted from a lick/no-lick object location discrimination task (O’Connor et al., 2010a) with two modifications: first, mice indicated their choice by symmetrically (Sanders and Kepecs, 2012; Schwarz et al., 2010) licking one of two lickports (“lick left/lick right”); second, mice withheld their response during a delay epoch. The symmetric response ensured that the expectation of reward is independent of choice, minimizing bias towards one of the two responses. In each trial, a vertical pole was presented in one of two positions (anterior or posterior). The mouse was trained to locate the pole with a single whisker (the C2 whisker) and report the perceived pole position by licking (Figure 1B). The standard contingency was: posterior → lick left and anterior → lick right (the contingency was reversed for the experiments of Figure 5). The task was divided into sample, delay and response epochs, thus separating “sensation” and “action” (Figure 1C) (Tanji and Evarts, 1976). At the beginning of the sample epoch, the pole moved quickly (0.2 s) into reach of the C2 whisker, whereupon the mouse whisked to touch the pole (Figure 1C, D). The pole was present during the sample epoch (1.3 s), which terminated when the pole moved out of reach. Following the sample epoch was the delay epoch (1.3 s), during which the mouse withheld the response (licking) while remembering its behavioral choice. An auditory cue (0.1 s) signaled the beginning of the response epoch and mice initiated licking (Figure 1D). Premature licking, during the sample or delay epochs, triggered an alarm sound and a brief timeout (“lick early” trials, Figure 1E).
Figure 1. Object location discrimination task with a delay epoch.
(A) Head-fixed mouse performing object location discrimination under optogenetic perturbation.
(B) A mouse producing “lick right” and “lick left” responses based on pole location.
(C) Task structure. The pole was within reach during the sample epoch. Mice responded with licking after an auditory response cue.
(D) Behavioral data. Top, one example trial. Whisker position (azimuthal angle, θ) for a representative “lick right” trial. Touches, grey circles; licks, black ticks. Middle, Summary data for “lick right” trials in 8 mice. Probability of touch, grey; licking, black (10 ms time bin). Bottom, same as middle for “lick left” trials.
(E) Behavioral performance across mice. Left, fraction correct “lick right” (blue), “lick left” (red), and “lick early” (black) trials. Each bar corresponds to one mouse (n = 15). Right panel, histogram of performance (grey) and fraction of “lick early” trials (black) across individual mice.
(F) Top, whisker density for representative “lick right” and “lick left” trials (overlaid whisker images). Bottom, distribution of the number of touches in “lick right” and “lick left” trials. Each line corresponds to one mouse (n = 14).
(G)The fraction of “lick right” responses as a function of number of touches for “lick right” (blue) and “lick left” (red) trials (n = 14). One mouse from (E) was excluded because there were not enough trials to sort by the number of touches (Table 1). See also Figure S1.
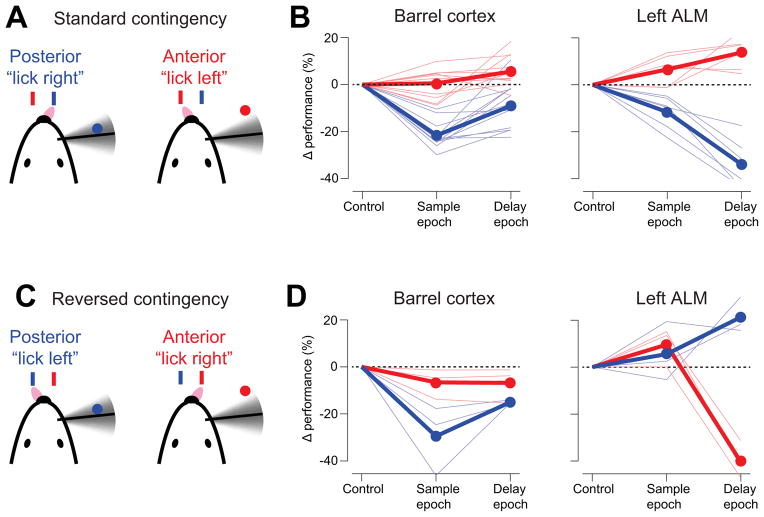
Figure 5. vS1 and ALM contribute differently to behavior.
(A) “Lick left/lick right” task with standard contingency, where mice learned to associate posterior pole position with licking right.
(B) Photoinhibition of vS1 and left ALM during the sample and delay epochs. Same as Figure 4C, D.
(C) “Lick left/lick right” task with reversed contingency, where mice learned to associate posterior pole position with licking left.
(D) Photoinhibition of vS1 and left ALM during the sample and delay epochs under the reversed contingency.
We trained a total of 22 mice in the object location discrimination task (Table 1; fraction correct, 69% ± 5%, mean ± SD; Figures 1E, S1A). Mice achieved criterion performance (70% correct) within three weeks (Figure S1B) with little bias between “lick right” and “lick left” trials (Figure S1C). Mice suppressed licking until the response epoch on a majority of the trials. “Lick early” trials (18% ± 9%, mean ± SD, Figure 1E, S1D) were excluded from our analyses. Rhythmic licking (7.6 ± 0.46 Hz, mean ± SD; Figure 1D) began immediately after the auditory cue (median reaction time post cue onset, 80.2 ± 10.5 ms, mean ± SD).
Table 1.
Mice appearing in this paper.
| Mouse | # sessions* | # trials | # recording sessions | # recorded neurons | Experiment types | Figures |
|---|---|---|---|---|---|---|
| JF140689 (female) | 12 | 4905 | 3 | 23 | vS1 spatial photoinhibition vS1 sample vs. delay photoinhibition S1 recording |
1D–G, 3C, 3E, 4C, 5B, 6C–D |
| JF138070 (male) | 17 | 6903 | 0 | 0 | vS1 spatial photoinhibition vS1 sample vs. delay photoinhibition |
1D–G, 3C, 3E, 4C, 5B |
| JF138072 (male) | 9 | 3191 | 1 | 3 | vS1 spatial photoinhibition vS1 sample vs. delay photoinhibition vS1 recording |
1D–G, 3C, 3E, 4C, 5B, 6C–D |
| JF147593 (male) | 9 | 3450 | 0 | 0 | vS1 sample vs. delay photoinhibition | 1D–E, 3C, 4C, 5B |
| JF147595 (male) | 18 | 6534 | 2 | 17 | vS1 laser power vs photoinhibition vS1 sample vs. delay photoinhibition ALM sample vs. delay photoinhibition Left ALM recording |
1D–G, 3C–D, 4C–D, 5B, 7C–G |
| JF160925 (male) | 24 | 9909 | 1 | 15 | vS1 laser power vs photoinhibition vS1 spatial photoinhibition vS1 sample vs. delay photoinhibition S1 recording |
1D–G, 3C–E, 4C, 5B, 6C–D |
| JF163936 (male) | 29 | 12645 | 3 | 13 | vS1 laser power vs photoinhibition vS1 spatial photoinhibition vS1 sample vs. delay photoinhibition vS1 recording |
1D–G, 3C–E, 4C, 5B, 6C–D |
| JF163938 (male) | 35 | 14564 | 5 | 61 | vS1 laser power vs photoinhibition Neocortex spatial photoinhibition vS1 sample vs. delay photoinhibition ALM sample vs. delay photoinhibition Left ALM recording Right ALM recording |
1D–G, 3C–D, 4B–E, 5B, 7C–G |
| JF166185 (female) | 29 | 11260 | 3 | 17 | vS1 laser power vs photoinhibition vS1 spatial photoinhibition vS1 sample vs. delay photoinhibition vS1 recording |
1D–G, 3C–E, 4C, 5B, 6C–D |
| JF147596 (male) | 57 | 22590 | 0 | 0 | Neocortex spatial photoinhibition | 1D–G, 3C, 4B, 5B |
| JF147594 (male) | 85 | 30990 | 5 | 39 | vS1 laser power vs photoinhibition vS1 sample vs. delay photoinhibition ALM sample vs. delay photoinhibition Neocortex spatial photoinhibition vS1 recording Left ALM recording |
1D–G, 3C–D, 4B–E, 5B, 6C–D, 7C–G |
| JF147599 (female) | 34 | 10099 | 0 | 0 | Neocortex spatial photoinhibition vS1 laser power vs photoinhibition vS1 sample vs. delay photoinhibition |
1D–G, 3C–D, 4B–C, 5B |
| JF166182 (male) | 69 | 36055 | 5 | 31 | vS1 laser power vs photoinhibition vS1 sample vs. delay photoinhibition vS1 spatial mapping ALM sample vs. delay photoinhibition Neocortex spatial photoinhibition vS1 recording Left ALM recording |
1D–G, 3C–E, 4B–E, 5B, 6C–D, 7C–G |
| JF167783 (male) | 26 | 12763 | 5 | 41 | vS1 laser power vs photoinhibition vS1 sample vs. delay photoinhibition vS1 spatial photoinhibition ALM sample vs. delay photoinhibition vS1 recording Left ALM recording |
1D–G, 3C–E, 4 C–E, 5B, 6C–D, 7C–G |
| JF167784 (male) | 39 | 21959 | 5 | 41 | vS1 laser power vs photoinhibition vS1 sample vs. delay photoinhibition vS1 spatial photoinhibition ALM sample vs. delay photoinhibition vS1 recording Left ALM recording |
1D–G, 3C–E, 4 C–E, 5B, 6C–D, 7C–G |
| JF190962(male) | 29 | 11744 | 0 | 0 | Neocortex spatial photoinhibition | 4B |
| JF173436 (male) | 35 | 13044 | 0 | 0 | vS1 reverse contingency ALM reverse contingency Targeted photoinhibition experiments |
5D, Supplemental |
| JF173437 (male) | 34 | 11918 | 0 | 0 | vS1 reverse contingency; ALM reverse contingency Targeted photoinhibition experiments |
5D, Supplemental |
| JF185608 (male) | 16 | 6036 | 0 | 0 | vS1 reverse contingency; ALM reverse contingency Targeted photoinhibition experiments |
5D, Supplemental |
| JF155551 (male) | 6 | 2172 | 0 | 0 | Wild type, photo-stimulation C2 whisker trimming |
Supplemental |
| JF155552 (male) | 4 | 1137 | 0 | 0 | Wild type, photo-stimulation C2 whisker trimming |
Supplemental |
| JF155553 (male) | 4 | 1013 | 0 | 0 | Wild type, photo-stimulation C2 whisker trimming |
Supplemental |
| JF152330 (male) | 3 | 20 | vS1 recording, non-behaving | 2 | ||
| JF159681 (male) | 1 | 4 | vS1 recording, non-behaving | 2 | ||
| JF159342 (male) | 3 | 26 | vS1 recording, non-behaving | 2 | ||
| JF152965 (female) | 4 | 17 | vS1 recording, non-behaving | 2 | ||
| JF152142 (male) | 4 | 28 | vS1 recording, non-behaving | 2 | ||
| JF152143 (male) | 2 | 4 | vS1 recording, non-behaving | 2 | ||
| JF152962 (male) | 2 | 8 | vS1 recording, non-behaving | 2 | ||
| JF161464 (male) | 4 | 31 | vS1 recording, non-behaving | 2 | ||
| JF175015 (male) | 3 | 24 | striatum recording, non-behaving | Supplemental | ||
| JF175016 (male) | 3 | 15 | striatum recording, non-behaving | Supplemental | ||
| JF211314 (female) | Clear-skull cap measurement | Supplemental | ||||
| JF179413 (female) | Clear-skull cap measurement | Supplemental | ||||
| JF212537 (female) | Photobleaching experiment | Supplemental | ||||
| JF212538 (female) | Photobleaching experiment | Supplemental |
including session in which neuronal recording was carried out during active behavior (column 4)
High-speed measurements of whisker movements (see Experimental Procedures, Figure 1F) (Clack et al., 2012; O’Connor et al., 2010a) revealed that the C2 whisker scanned across the posterior pole position (Figure 1F) (O’Connor et al., 2010a). Mice made significantly more touches in posterior trials (4.9 ± 1.2 contacts, mean ± SD) than in anterior trials (0.4 ± 0.4 contacts, mean ± SD, p < 0.001, t-test). Mice thus solved the task using a highly asymmetric whisking strategy. The preference for one pole position over the other (‘exploration bias’) (Figure S5B) was much higher than in previous experiments without delay epoch (O’Connor et al., 2010a; O’Connor et al., 2013). This extreme bias suggests that mice solve the task by detecting the pole in the posterior position, and ignore the pole in the anterior position. The majority of the touches occurred at the beginning of the sample epoch (Figure 1D). Mice used touch to solve the task: first, “lick right” response probability increased with larger numbers of touches per trial (Figure 1G); second, mice were unable to perform the task after the C2 whisker was trimmed (Figure S1E). Head-fixed mice can perform object location discrimination with a single whisker, and hold the decision in memory for a delay epoch.
Inactivating cortical activity
To probe the role of specific brain areas, we inactivated small volumes of cortical tissue by photostimulating channelrhodopsin-2 (ChR2) in GABAergic interneurons (VGAT-ChR2-EYFP) (Zhao et al., 2011) (Figure S2). Since GABAergic interneurons have dense local axonal arbors (Helmstaedter et al., 2008), this approach is expected to produce potent and local inhibition (Figure 2A).
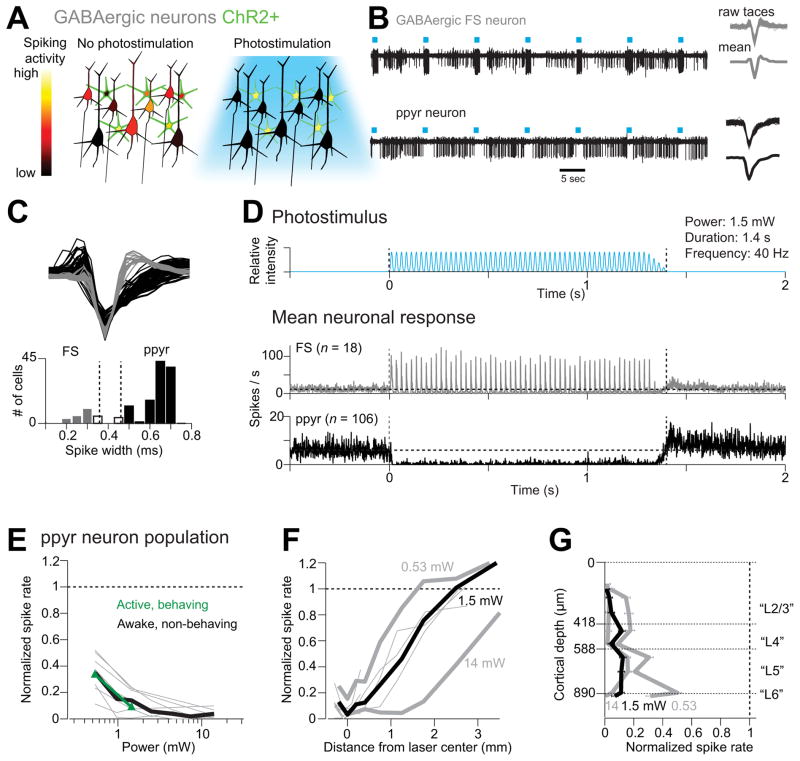
Figure 2. ChR2-assisted photoinhibition.
(A) Inactivation by photostimulating ChR2-positive GABAergic interneurons (green).
(B) Silicon probe recordings. Top, a GABAergic fast spiking (FS) neuron (other units with smaller spike amplitudes were also recorded on this electrode). Bottom, a putative pyramidal (ppyr) neuron. Right, corresponding spike waveforms.
(C) Spike classification. Top, spike waveforms for FS neurons (n = 18; grey) and ppyr neurons (n = 106; black). Bottom, histogram of spike durations. Neurons that could not be classified based on spike width were excluded from analysis (white bar, n = 9; see Experimental Procedures).
(D) Top, the photostimulus. Vertical dotted lines, start and stop of photostimulation. Bottom, mean peristimulus time histogram (PSTH, 1 ms bin) for FS neurons and ppyr neurons recorded under awake, non-behaving conditions. All neurons <0.25 mm from the laser center were pooled.
(E) Spike rate as a function of laser power (<1 mm from laser center, all cortical depths). Spike rates were normalized to baseline (dash line, see Experimental Procedures). Thick black line, mean for awake, non-behaving condition. Thin grey lines, individual mice (7 mice, 103 ppyr neurons; one mouse with only 3 ppyr neurons was excluded). Green line, mean for active behaving condition (35 neurons, 6 mice; error bars reflect s.e.m. over mice).
(F) Normalized spike rate versus distance from the photostimulus center (all cortical depths). Neurons were pooled across cortical depths. Thin lines, individual mice for the 1.5 mW condition.
(G)Normalized spike rate versus cortical depth (< 0.2 mm from laser center). Recording depths and cortical layers (“L”) are based on histology. Error bars indicate s.e.m. over neurons (n = 106). See also Figures S2, S3, S4.
We characterized “photoinhibition” in awake mice (Figure S3, see Experimental Procedures). A laser beam (wavelength, 473 nm; Figure 2D) was focused onto the surface of the brain (Figure 1A). Extracellular recordings were made in vS1 close to the center of the laser (n = 133 isolated single-units, see Experimental Procedures, Figure S3). The distribution of spike widths was bimodal (Figure 2B, C). Fast spiking (FS) neurons with narrow spikes were likely parvalbumin-positive interneurons (Kawaguchi, 1993; McCormick et al., 1985), and these neurons were activated by photostimulation, on average (Figure 2B, D). Neurons with wide spikes likely were mostly pyramidal neurons (putative pyramidal neurons, ppyr) and were inhibited by photostimulation (Figure 2B, D). To quantify inhibition of the ppyr neurons (see Experimental Procedures, n = 106), we normalized the firing rate during photostimulation to the baseline firing rate (“normalized spike rate”, see Experimental Procedures; range of baseline firing rates, 0.01–28.8 spikes/s; mean firing rate, 5.5 spikes/s). Near the center of the photostimulus (< 1 mm from the laser center), activity of the ppyr neurons was reduced over a wide range of power levels (Figure 2E). At moderate laser powers (1.5 mW; 87% ± 3% activity reduction, mean ± s.e.m.; 83/106 significantly inhibited, p < 0.05, t-test), photoinhibition was localized to a region with radius of approximately 1 mm (radius at half-max, Figure 2F). At higher powers (14 mW), neurons were inhibited further from the center of the photostimulus (Figure 2F). Photoinhibition was nearly uniform across cortical layers (Figure 2G, Figure S4J). ChR2-YFP expression was largely absent in the striatum beneath the cortex and recording experiments confirmed that inhibition directly caused by photostimulation was confined to neocortex (Figure S2). Photoinhibition reached steady state 17.3 ms after photostimulus onset with an offset time of 124 ms (see Experimental Procedures).
Inactivating vS1 during behavior
Studies using aspiration lesions and pharmacological silencing have implicated vS1 in object localization (Hutson and Masterton, 1986; O’Connor et al., 2010a). We thus photoinhibited cortical activity transiently during the sample epoch in the C2 column (Figures 1 and 3A). We obtained optical access to the neocortex (lateral ± 4 mm, bregma ± 3 mm) by outfitting mice with a clear-skull cap (Figure 3B, light transmission, approximately 50%; see Experimental Procedures). During behavior we therefore photostimulated directly through the clear-skull cap.
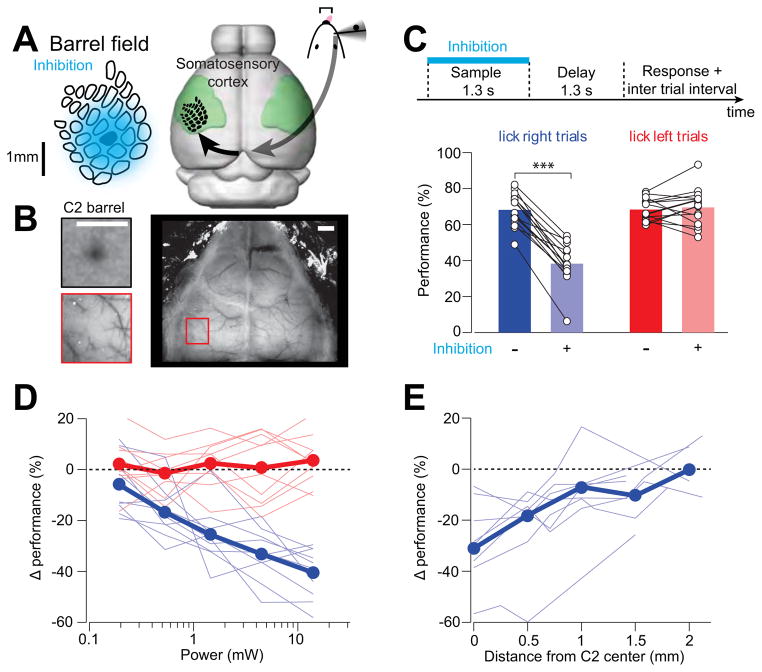
Figure 3. Photoinhibition of vS1 during object location discrimination.
(A) Approximate spatial extent of photoinhitibion under our standard condition (1.5 mW). Photoinhibition spans at least 10 barrel columns. Right, the primary somatosensory cortex (green), with the barrel field superposed.
(B) Mapping the C2 column with iIntrinsic signal imaging (top left) relative to vasculature landmarks (bottom left). Right, an example clear-skull cap. Scale bar, 1 mm.
(C) Photoinhibition of vS1 during the sample epoch. Top, time-line of photoinhibition. Bottom, effects of photoinhibition on behavior in “lick right” trials (blue) and “lick left” trials (red). Performance is the fraction of correct reports for each trial type (Experimental procedures). Thin lines, individual mice (n = 15). Data from different laser powers are pooled (range, 0.97 to 14 mW; mean, 3.94 mW). ***, p < 0.001, two-tailed t-test.
(D) Change in performance caused by photoinhibition versus laser power. Blue, “lick right” trials; red, “lick left” trials. Thick lines, mean performance; thin lines, individual mice (n = 10).
(E) Change in performance in “lick right” trials versus photostimulus location from C2 barrel (n = 8). Laser power, 1.5 mW. See also Figure S5.
C2 barrel columns were mapped with intrinsic signal imaging (Figure 3B) (Masino et al., 1993; O’Connor et al., 2010b). Photoinhibition at our standard photostimulation condition (1.5 mW) spanned multiple barrel columns, centered on the C2 column (Figure 3A, 85% reduction in spike rate at laser center; 0.9 mm radius at half-max; approximated from Figure 2E and 2F assuming 50% light attenuation). Photoinhibition in behaving mice was nearly as strong as that observed under non-behaving condition (Figure 2E and S4I; measured without the clear-skull cap to enable direct comparison between behaving and non-behaving condition: p = 0.33, t-test at 1.5 mW; 6 mice, 35 vS1 neurons, see Experimental Procedures).
We inactivated vS1 in 15 mice (Table 1) during behavior. Mice performed a large number of trials per session (422 ± 115 trials, mean ± SD, Table 1). Photostimuli were applied (Figure 1D) randomly in 25% of the trials. Because mice employed a whisking strategy that appeared to maximize touches for “lick right” trials (corresponding to the posterior pole position) and minimize touches in “lick left” trials (Figure 1F), a neuronal signal coding for touch was likely responsible for detection of the pole in “lick right” trials. A simple prediction is that inactivating vS1 reduces performance in “lick right” trials. Consistent with this hypothesis, photoinhibition decreased performance in “lick right” trials (performance reduction, 29.8 ± 9.4%, mean ± SD, p < 0.001, two-tailed t-test, Figure 3C). This deficit increased as a function of light intensity (Figure 3D), implying a relationship between vS1 activity and pole detection. We observed little effect in performance on “lick left” trials (Figure 3D). The behavioral change was not due to non-specific effects of photostimulation, since light itself, without the VGAT-ChR2 transgene, produced no effect (Figure S5A). Photoinhibition did not change whisking (Figure S5C, D) or the number of touches (paired t-test, p > 0.05; Figure S5E), despite a deficit in reporting pole location (Figure S5F). In addition, photoinhibition did not change the fraction of “lick early” trials and “no lick” trials (Figure S5A).
We next measured the spatial resolution of photoinhibition in behaving mice. The behavioral effect decreased rapidly with distance of the laser from C2, vanishing at 1.35 ± 0.6 mm (mean ± s.e.m.) from the C2 barrel center (p > 0.05, two-tailed t-test) (Figure 3E). The behavioral effect was thus caused by photoinhibition of vS1, and not the surrounding cortical areas. We conclude that touch-evoked activity in vS1 is critical for whisker-based object location discrimination.
Mapping cortical regions involved in object location discrimination
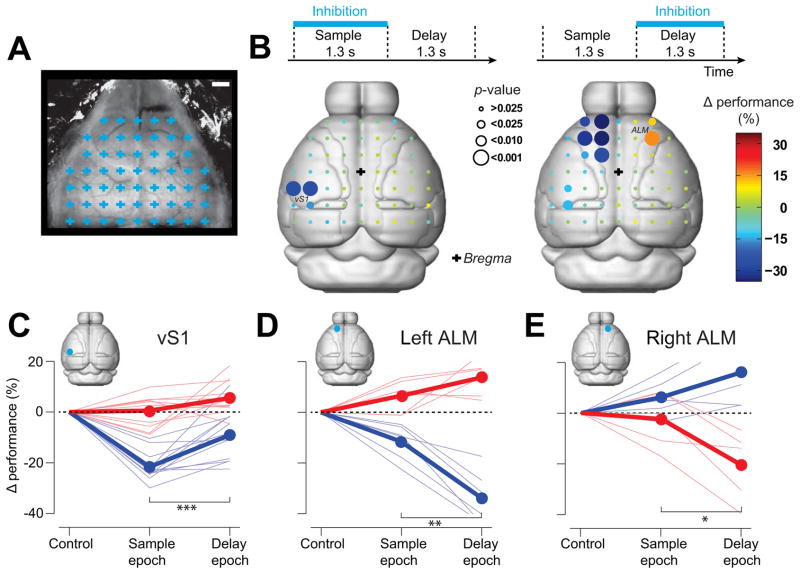
We next used photoinhibition to survey the dorsal neocortex during specific epochs of the object location discrimination behavior (Figure 1C). 55 evenly spaced cortical volumes were tested for their involvement in the behavior (Figure 4A). We tested 6 mice across 151 behavioral sessions (53190 trials). Photoinhibition was deployed in 75% of the trials at one of the 55 grid locations either during the sample or delay epochs (168 ± 11 “lick right” trials and 170 ± 11 “lick left” trials during sample epoch at each location; 150 ± 9 “lick right” trials and 150 ± 10 “lick left” trials during delay epoch at each location; mean ± SD; see Experimental Procedures). We measured the mean performance change in “lick right” trials caused by photoinhibition at particular locations (Figure 4B, see Figure S6 for raw performance numbers).
Figure 4. Cortical areas involved in object location discrimination revealed by photoinhibition.
(A) A grid of 55 photostimulus locations through the clear-skull cap (grid spacing, 1 mm). Each grid location was chosen randomly for photostimulation during either sample or delay epoch (see Experimental Procedures). Scale bar, 1 mm.
(B) Photoinhibition during different behavioral epochs. Top, photoinhibition during sample (left) and delay (right) epochs. Bottom, cortical regions involved in object location discrimination during sample (left) and delay (right) epochs in “lick right” trials. Color codes for the change in performance (%) under photoinhibition relative to control performance. Circle size codes for significance obtained from bootstrap (Experimental Procedures; from small to large; > 0.025, < 0.025, < 0.01, < 0.001). Effects on “lick left” trials are shown in Figure S6. Boundaries of cortical areas are from Allen Brain Atlas (Brain Expolorer 2, www.brain-map.org).
(C) Photoinhibition of vS1 during the sample epoch caused a larger behavioral deficit than during the delay epoch in “lick right” trials (***, blue, “lick right” trials, p < 0.001, n = 12, two tailed t-test). Thick lines, mean; thin lines, individual mice.
(D) Photoinhibition of the left ALM during the delay epoch caused a larger behavioral deficit than during the sample epoch in “lick right” trials (**, blue, “lick right” trials, p = 0.0016, n = 6; two tailed t-test; red, “no” trials, p = 0.09).
(E) Photoinhibition of the right ALM during the delay epoch caused a larger behavioral deficit than during the sample epoch in “no” trials (blue, “lick right” trials, p = 0.22, n = 5; *, red, “no” trials, p = 0.05, two tailed t-test). See also Figure S6.
Photoinhibition of most sites did not cause a detectable change in performance (Figure 4B, S6). During the sample epoch, photoinhibiting vS1 reduced performance (Figure 3B), consistent with the targeted vS1 experiments (Figure 3C). vS1 photoinhibition reduced performance significantly more during the sample epoch compared to the delay epoch (p < 0.001, two-tailed t-test, Figure 4C), suggesting that tactile information dissipates rapidly in vS1 after the sample epoch. However, photoinhibition of vS1 during the delay period produced a small but significant effect (p < 0.01; two-tailed t-test against 0; Fig 4C).
Photoinhibition during the delay epoch uncovered a frontal area (lateral ±1.5 mm, bregma +2.5mm;) (Figures 4, S6). This area was anterior and lateral to, and largely non-overlapping with the vibrissal primary motor cortex (vM1) (lateral 0.8mm, bregma +1 mm) (Huber et al., 2012). At the spatial resolution of photoinhibition this area was indistinguishable from the anterior lateral motor area (ALM) previously identified to play a role in high-level control of licking in mice (Komiyama et al., 2010) and rats (Travers et al., 1997). We thus refer to this region as ALM. Photoinhibition of ALM on either side of the midline perturbed the animal’s performance, with the strongest effects during the delay epoch (p ≤ 0.05, two-tailed t-test, Figure 4D–E). Photoinhibition of the left ALM biased the choice toward the left lickport, resulting in an increased performance in “lick left” trials and decreased performance in “lick right” trials (Figure 4D). Photoinhibition of the right ALM biased the choice toward the right lickport (Figure 4E, S6). Unilateral photoinhibition of each side of ALM therefore yielded the opposite pattern of behavioral bias (Figure 4D–E). This suggests that unilateral photoinhibition of ALM biased the upcoming choice to the ipsilateral direction. We did not observe any effect of ALM photoinhibition on the animals’ licking latencies (sample epoch inhibition vs. control trials, p = 0.86, paired t-test, n = 6 mice; delay epoch vs. control, p = 0.48, n = 6 mice).
Is ALM involved in motor preparation? We performed experiments in three new mice with the same pole locations but reversed motor choice (posterior → lick left; anterior → lick right) (Figure 5A, C). If a cortical area is involved in processing sensory information, the pattern of deficit caused by photoinhibition should be unchanged by this motor choice reversal. Indeed, the deficit caused by vS1 photoinhibition was similar as in the standard contingency, with a significant performance decrease in the “lick left” trials (Figure 5D). In contrast, if a cortical area is involved in determining the animal’s motor choice, the deficit caused by photoinhibition should be reversed. Consistent with this hypothesis, the bias in ALM photoinhibition was reversed by reversing the sensorimotor contingency (Figure 5D).
We validated the mapping experiments in two ways. First, we applied false discovery rate analyses to correct for multiple comparisons in our mapping results (Benjamini and Hochberg, 1995) (Experimental Procedures; Supplementary Table 1). vS1 and ALM remained significant after the correction. Second, in a separate group of three mice we targeted specific cortical regions (vS1, ALM, vM1, V1, and PPC; Figure S6). Only photoinhibition of vS1 and ALM produced significant behavioral changes (Figure S6). Even in experiments with bilateral photoinhibition of large areas overlapping PPC (simultaneous inhibition of 8 grid points, Figure S6H) performance was not significantly affected.
We thus identified two cortical regions involved in tactile object location discrimination. Inactivation of vS1 during the sample epoch caused a deficit in pole detection regardless of motor choice. Unilateral inactivation of ALM during the delay epoch biased choice to the ipsilateral direction.
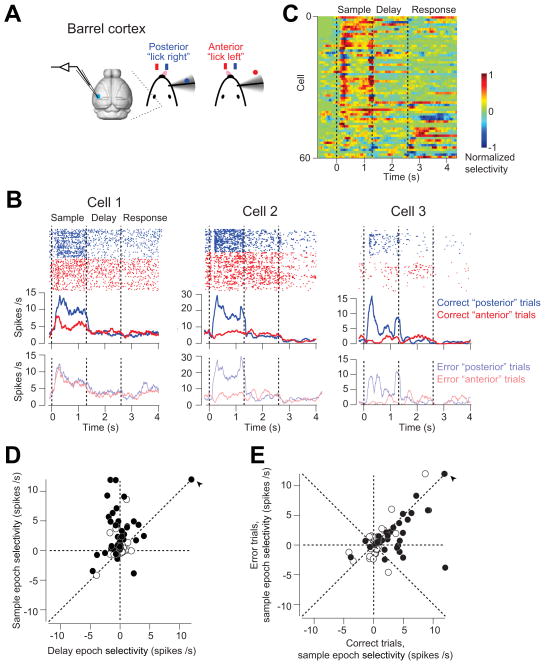
Neuronal selectivity in vS1 and ALM
We recorded single units from vS1 and ALM in mice performing object location discrimination. A large fraction of vS1 neurons differentiated trial types (Figure 6B), typically with higher spike rates in “posterior” trials compared to “anterior” trials (Figure 6B). We computed ‘selectivity’ as the difference in spike rates between the trial types (Figure 6C). The selectivity was largest at the beginning of the sample epoch (34/75 neurons significantly differentiated trial types in spike counts during the sample epoch, t-test, p < 0.05), likely reflecting active touch (Figure 1D). Indeed, aligning the response to the first touch revealed a peak of activity with a 10 ms delay (Figure S7), consistent with previously reported latencies in vS1 (Armstrong-James et al., 1992; Brecht and Sakmann, 2002; O’Connor et al., 2013; Simons, 1978). Selectivity in vS1 was much reduced during the delay epoch (Figure 6C, D; selectivity sample vs. delay: p < 0.001, paired t-test); 20/75 neurons significantly differentiated trial types in spike counts during the delay epoch (t-test, p < 0.05). Higher selectivity during the sample epoch compared to the delay epoch is consistent with the photoinhibition experiments (Figure 4C). The photoinhibition experiments also predicted that vS1 selectivity should be largely unaffected by the animals’ motor choice (Figure 5). Indeed, the selectivity was largely maintained on error trials (Figure 6E, slope = 0.47, r = 0.54, p < 0.001, also see examples in Figure 6B).
Figure 6. vS1 neurons show stimulus-specific activity.
(A) vS1 recording during behavior.
(B) Three example vS1 neurons during object location discrimination. Top, spike raster and PSTH for correct “posterior” (blue) and “anterior” (red) trials. Bottom, PSTH for error trials (transparent color). Averaging window, 200 ms. Dashed lines delineate behavioral epochs.
(C) vS1 population selectivity. Selectivity is the difference in spike rate between the “posterior” and “anterior” trials, normalized to the peak. Averaging window, 200 ms. 15/75 vS1 neurons did not show significant selectivity during any behavioral epoch, and they were excluded from the plot.
(D) vS1 neurons are mainly selective during the sample epoch. Circles correspond to individual neurons (n = 75). Selectivity is the firing rate (FR) difference between “posterior” and “anterior” trials during sample or delay epoch (FR “posterior” − FR “anterior”). Filled circles indicate neurons with significant selectivity during either the sample or delay epoch (p < 0.05, two-tailed t-test). Arrow, values are off scale; sample epoch selectivity, 19.4 spikes/s; delay epoch selectivity, 19 spikes/s.
(E) vS1 maintains selectivity on error trials. Selectivity on correct trials versus error trials, slope = 0.47, r = 0.54, p < 0.001. Filled circles indicate neurons with significant sample epoch selectivity on the correct trials (p < 0.05, two-tailed t-test). Arrow, the same outlier neuron as in (C). See also Figure S7.
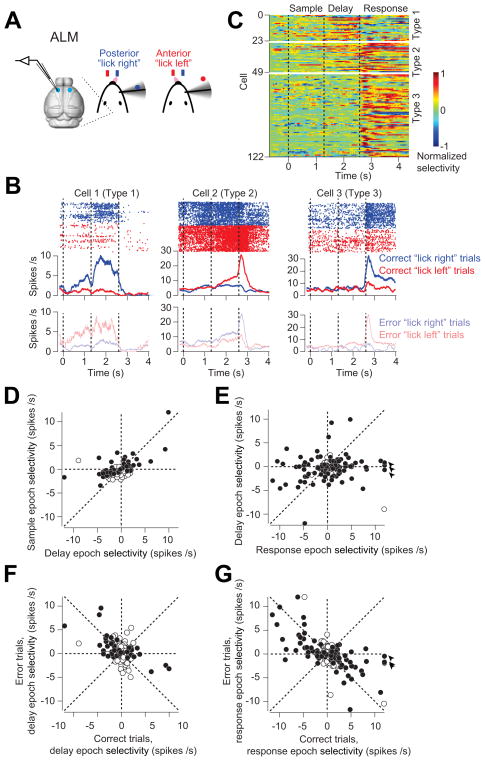
A large fraction of ALM neurons also differentiated between trial types (122/186; Fig 7A–C). ALM selectivity emerged late in the sample epoch (13/186 neurons), and ramped up throughout the delay epoch (43/186 neurons), often long before the response (Figure 7B–D response Type 1 and 2). Selectivity reached a maximum during the response epoch (99/186 neurons) (Figure 7E). In this respect, activity in ALM resembles preparatory activity previously seen in motor cortex in macaques (Tanji and Evarts, 1976). Immediately after the response cue, a subset of the neurons with preparatory activity became silent (23/186, response Type 1, see example neuron in Figure 7B), whereas other neurons showed enhanced activity and selectivity (26/186, response Type 2, Figure 7B). Another group of ALM neurons did not show preparatory activity, but became active immediately after the response cue (63/186, response Type 3, Figure 7B). This enhancement of selectivity immediately after the response cue is consistent with a motor command (movement-related activity, Figure 7C response Type 2 and 3). The preparatory and movement-related activity closely tracked the animals’ choice. In error trials neurons switched their trial type preference (Figure 7F–G; delay epoch: slope = −0.41, r = −0.46, p < 0.001; response epoch: slope = −0.48, r = −0.62, p < 0.001; see examples in Figure 7B). This choice-specific selectivity is consistent with the ALM photoinhibition experiments (Figure 5).
Figure 7. ALM neurons show choice-specific preparatory and movement-related activity.
(A) ALM recording during behavior.
(B) Three example ALM neurons during object location discrimination. Top, spike raster and PSTH for correct “lick right” (blue) and “lick left” (red) trials. Bottom, PSTH for error trials (transparent color). Averaging window, 200 ms. Dashed lines delineate behavioral epochs.
(C) ALM population selectivity. Selectivity is the difference in spike rate between the preferred and non-preferred trial-type, normalized to the peak. For each neuron, we defined its preferred trial-type (“lick right” or “lick left”) using spike counts from a subset of the trials (10 trials), and the remaining data was used to compute the selectivity. Averaging window, 200 ms. Six neurons of type 1 showed significant selectivity only during the sample epoch, thus only 43 neurons showed significant delay epoch selectivity.
(D) ALM neurons show choice-specific preparatory activity during the delay epoch. Selectivity is the firing rate (FR) difference between “lick right” and “lick left” trials during sample or delay epoch (FR”lick right” − FR”lick left”). Circles correspond to individual neurons (n = 186). Filled circles indicate neurons with significant selectivity during either the sample or delay epoch (p < 0.05, two-tailed t-test). Data from both left ALM and right ALM are shown.
(E) ALM neurons show movement-related selectivity during the response epoch. Circles correspond to individual neurons (n = 186). Filled circles indicate neurons with significant selectivity during either the delay or response epoch (p < 0.05, two-tailed t-test). Arrows, values are off scale; response epoch selectivity, 12.5 spikes/s; 12.2 spikes/s, 16 spikes/s.
(F) ALM preparatory activity during the delay epoch correlated with the animals’ behavioral choice. Selectivity on correct trials versus error trials, slope = −0.41, r = −0.46, p < 0.001. Filled circles indicate neurons with significant delay epoch selectivity on the correct trials (p < 0.05, two-tailed t-test).
(G)ALM motor-related activity during the response epoch correlated with the animals’ behavioral choice. Selectivity on correct trials versus error trials, slope = −0.48, r = −0.62, p < 0.001. Filled circles indicate neurons with significant response epoch selectivity on the correct trials (p < 0.05, two-tailed t-test). Arrow, the same outlier neuron as in (D).
In summary, neuronal selectivity in vS1 represents information about pole location independent of motor choice. Neuronal selectivity in ALM represents motor preparation and movement.
Discussion
Cortical information flow during tactile decision making
We developed a behavioral task for head-fixed mice that separated “sensation” and “action” in time (Figure 1). To map the cortical activity involved we transiently and reversibly inactivated pyramidal neurons using photoinhibition in mice expressing ChR2 in GABAergic interneurons (Zhao et al., 2011). The photoinhibition was potent (Figure 2E), spatially restricted (Figure 2F), and temporally precise (Figure 2D). Head-fixation, the clear-skull cap preparation, and the laser scanning system together provided access to most of the dorsal cortex for photoinhibition (Figure 4A). We show that sensory processing in vS1 contributes to behavior mainly during the sample epoch, whereas a frontal region (ALM) is mainly required during the delay and early response epochs (Figure 4). Single unit recordings supported these conclusions: a large fraction of neurons in vS1 show object location-dependent activity during the sample epoch (Figure 6), whereas the majority of neurons in ALM are choice-selective during the delay and response epochs (Figure 7).
Our study makes four contributions. First, we show that head-fixed mice can perform a symmetric-response perceptual decision behavior with a delay epoch. Translating perceptual decision behavioral paradigms to mice will facilitate understanding of the underlying neural circuit mechanisms. The delay epoch was critical, effectively boosting the time-resolution of optogenetic inhibition. Individual mice performed many thousands of trials with consistent performance. These features together allowed us to use focal photoinhibition to reveal the brain areas involved in specific task epochs.
Second, we outline a powerful method to inactivate small regions of cortex through the intact skull, by local photostimulation of ChR2-expressing GABAergic neurons. We characterized photoinhibition at unprecedented levels of detail (Figures 2, S4). Photoinhibition allowed us to survey dozens of cortical regions (about half of the cortex) in individual mice. The scanning laser system also allows near-simultaneous photoinhibition of multiple cortical regions (Figure S6H, Experimental Procedures). The high throughput of this technique allows comprehensive surveys of multiple cortical regions underlying behaviors in mice.
Third, we identified two cortical regions involved in specific aspects of tactile decisions. vS1 is critical for the perception of object location (Figures 3, 5, 6). Previous lesion and pharmacological inactivation studies of vS1 caused changes in motor strategies (Hutson and Masterton, 1986; O’Connor et al., 2010a), therefore the behavioral effect was confounded by motor deficits and possibly decreased levels of motivation (Kleinfeld and Deschenes, 2011). The motor strategies remained unchanged under transient photoinhibition (Figure S5), thus vS1 activity was critical for tactile sensation underlying object location discrimination. Lesion analysis of monkey somatosensation did not probe active sensation (Zainos et al., 1997). ALM is involved during the delay and response epochs (Figures 5, 7), consistent with a role in motor preparation and movement.
Fourth, the photoinhibition and recording experiments outline the information flow underlying a tactile decision in the mouse cortex. Our data is consistent with a serial scheme, where information is handed off from sensory to motor cortex, with little temporal overlap (Figures 6, 7). Cortical information flow has previously been examined in primates using extracellular recording and correlations with behavior (de Lafuente and Romo, 2005; Hernandez et al., 2010; Romo and de Lafuente, 2012), as well as electrical microstimulation (Bisley et al., 2001; de Lafuente and Romo, 2005; Seidemann et al., 1998). To our knowledge, ours is the first study to map task-relevant information flow on a cortex-wide scale using loss of function methods. It is possible that in more complex behavioral settings, or in difficult perceptual tasks, persistent reciprocal interactions may occur between sensory and motor areas. The neuronal selectivity in vS1 and ALM, and their contribution to behavior, provides hypotheses about neuronal coding and transformations between sensory and motor areas.
Relation to previous studies
Decision tasks with delay epochs have been widely used in non-human primates to study perception (Mountcastle et al., 1990; Seidemann et al., 1998), working memory (Fuster and Alexander, 1971; Goldman-Rakic, 1995; Romo et al., 1999), and motor preparation (Tanji and Evarts, 1976). The ability to separate behavioral events in time allowed for analyses and manipulations of specific behavioral components. Previously, Romo and colleague have used a comparison task to separate tactile-flutter discrimination into sensation, working memory, and choice. Neuronal correlates of sensation, working memory and movement were found across a hierarchy of cortical areas (Hernandez et al., 2010; Romo and de Lafuente, 2012). There is a surge of interest in developing similar behavioral tasks suitable for head-fixed mice (Harvey et al., 2012; Komiyama et al., 2010; O’Connor et al., 2010a; Pammer et al., 2013; Sanders and Kepecs, 2012). Our behavioral task design is similar to previous task designs in primates used to study how sensory information is evaluated for decision (de Lafuente and Romo, 2005; Seidemann et al., 1998); the key difference is that our mice were asked to actively move their whiskers for tactile sensation (O’Connor et al., 2010a; O’Connor et al., 2013; Pammer et al., 2013). The ability to carry out sophisticated behavioral tasks in mice opens up the possibility of investigating the circuits, cellular and synaptic mechanisms underlying perceptual decisions.
The involvement of vS1 in touch sensation (Figure 3) is consistent with previous studies (Hutson and Masterton, 1986; O’Connor et al., 2010a). Our data further show that vS1 is mainly involved during the sample epoch of the task. A partial deficit remained when vS1 was inactivated during the delay epoch, although this effect was small and heterogeneous across individual mice (Figure 4C). Consistently, our recording data from vS1 revealed that some cells signaled pole location throughout part of the delay epoch, after the pole was out of reach (Figure 6). Similarly, experiments in non-human primates showed that several visual areas contribute to visual decisions mainly during the sample epoch (Afraz et al., 2006; Bisley et al., 2001; Seidemann et al., 1998), with a small remaining effect during the delay epoch (Seidemann et al., 1998). A similar conclusion was inferred based on neuronal responses in somatosensory tasks (Hernandez et al., 2010; Romo and de Lafuente, 2012).
Photoinhibition of vS1 in a similar task without a delay epoch leads to an increase in performance in the “anterior”/“lick left” trials (O’Connor et al., 2013). Addition of the delay epoch abolished this effect (Fig 4D). The underlying reasons for this difference are currently not understood. Photoinhibition during the sample epoch could produce some rebound activity during the delay epoch and thus increase the “lick right” rate (Fig 2D). Explaining this discrepancy will require an understanding of how vS1 activity is interpreted by downstream areas during the delay epoch.
The dissociation of vS1 activity and whisking (Figure S5) is likely not a general phenomenon but task specific. Previous studies have reported that perturbation of vS1 activity in other tasks can affect whisking (Matyas et al., 2010). The lack of vS1 involvement in whisking dovetail with an earlier lesion experiment in primate which showed that S1 lesions do not impair motor responses (Zainos et al., 1997).
Both the temporal specificity of photoinhibition (Figures 4D–E, 5) and neuronal recording (Figure 7) support the idea that a dorsal anterior cortex (overlapping with ‘M2’ in Paxinos and Franklin, 2004) is involved in motor preparation. This region overlaps with a previously reported motor area involved in control of licking (ALM, Komiyama et al., 2010; Travers et al., 1997) and we adopted this term here. The ALM defined here by photoinhibtion (Fig 4B, centre 1.5mm lateral, 2.5 mm anterior to bregama) is slightly medial to the previously reported coordinates defined by microstimulation (Komiyama et al., 2010, centre, 2.0 mm lateral, 2.4 mm anterior) and extends into primary motor cortex (Fig 4B). The precise borders of ALM, if they exist, remain to be mapped.
Since the offset latency of photoinhibition was ~124 ms, ALM photoinhibition (Fig. 4) cannot delineate between effects during delay epoch related to motor preparation and suppression of motor output during early parts of the response epoch. ALM neurons developed their selectivity gradually during the sample and delay epochs, reaching a maximum at the beginning of the response epoch (Figure 7). Ramping activity was also recently reported in rats performing a memory-guided orienting task (Erlich et al., 2011) in a region of the motor cortex that overlaps with the classical vibrissal motor cortex (vM1) (Brecht, 2011; Huber et al., 2012). Unilateral muscimol inactivation of this region biased the rats’ choice in the ipsilateral direction. Our result is in broad agreement with this finding, in that unilateral inactivation of ALM biased licking in the ipsilateral direction (Figure 4D–E). This study also reported a mixture of neurons preferring either direction of orienting in each hemisphere (Erlich et al., 2011). Similarly, our recordings from ALM revealed a mixture of neurons preferring either direction of licking (Figure 7C). How these mixed, bilateral activity patterns are consistent with the lateralized inactivation behavioral effects (Figure 4) remains mysterious.
Inactivation of vM1 using muscimol causes behavioral deficits in mice trained in a “go/no-go” whisker-dependent object detection task (Huber et al., 2012). We did not observe consistent behavioral effects with vM1 inactivation in the “lick left/lick right” task (Figure 4B, S6). It is possible that differences in motor strategies could account for this discrepancy. In the “go/no-go” object detection task, poles were placed in one of multiple locations and the mouse was asked to report whether the pole was within reach or out of reach (Huber et al., 2012). Mice solved this task using large-amplitude, stereotyped whisking (peak to peak amplitude, > 40 degrees), presumably to search for the pole. In contrast, in our experiments mice adapted a strategy with remarkably little rhythmic whisking (peak to peak amplitude, < 20 degrees; Figure S5D).
Other brain areas in tactile decision making
In the cortical mapping experiment we photoinhibited one cortical region at a time (Figure 4B). Several cortical areas could participate in tactile perception in parallel in a redundant manner. Inactivation of these areas simultaneously might be necessary to reveal their function.
Active perceptual decisions also involve subcortical regions, including the superior colliculus (Horwitz and Newsome, 1999), the striatum (Ding and Gold, 2010), and possibly the thalamus (Sommer and Wurtz, 2006). Targeting these subcortical regions, as well as the ventral cortex, for inactivation may require different methods of photoinhibition, for example using implantable optical fibers (Aravanis et al., 2007). Alternatively, red-shifted opsins (Lin et al., 2012; Packer et al., 2012) may allow non-invasive inactivation of deep brain structures.
Our survey of cortical areas involved in tactile decisions identified cortical regions at the beginning and the end of cortical processing. vS1 is the main conduit of tactile information from the periphery to other cortical areas, including secondary somatosensory cortex (S2), and vM1 (Ferezou et al., 2007, Mao et al, 2011). vS1 also projects to thalamus and the dorsolateral striatum. ALM controls directional licking, the action indicating behavioral choice in our behavior. How does tactile information reach ALM? vS1 does not directly project to ALM (Mao et al., 2011), but at least three indirect pathways link vS1 and ALM. First, vS1 projects to S2, which projects to ALM (Allen brain institute for Brain Science, http://mouse.brain-map.org; ZG, NL, KS, unpublished observations). An analogous pathway has been posited to play a key role in primate somatosensory decision tasks (Romo and de Lafuente, 2012). Second, vS1 projects to the posterior nucleus of the thalamus (PO), which projects to ALM. Third, vS1 projects to the dorsolateral striatum, which might shape decision-related activity in ALM via the output nuclei of the basal ganglia and motor thalamus. The specific roles of these different pathways will be the subject of future studies.
Experimental Procedures
Animals & Surgical procedures
This study is based on data from 38 mice (Table 1) (30 males; 8 females; 2 months to 10 months old). Nineteen VGAT-ChR2-EYFP mice (Jackson laboratory, http://www.jax.org) were used for photoinhibition (Figures 3, 4, 5) and neuronal recordings (Figures 6, 7). Eight VGAT-ChR2-EYFP mice were used to characterize photoinhibition (Figure 2). Two VGAT-ChR2-EYFP mice were used for immunohistochemistry (Figure S2). Two VGAT-ChR2-EYFP mice were used to characterize photoinhibition in striatum (Figure S2). Three wild type mice (C57Bl/6Crl) were used for control behavioral testing (Figures S1E, S5). Four mice were used for measuring light transmission through the clear-skull cap (Figure S4, two VGAT-ChR2-EYFP mice and two transgenic mice in which Rosa-LSL-H2B-GFP, gift from Josh Huang, Cold Spring Harbor Laboratory, was crossed to PV-ires-cre, Hippenmeyer et al., 2005).
All procedures were in accordance with protocols approved by the Janelia Farm Institutional Animal Care and Use Committee. Mice were implanted with a clear-skull cap constructed from a thin layer of clear dental cement over intact skull (Figure 3B) and a headpost. For silicon probe recording, a small craniotomy was made through the clear-skull cap. Neuronal recordings and photoinhibition in vS1 were guided by intrinsic signal imaging (Figure 3B) (Masino et al., 1993). Detailed information on water restriction and surgical procedures is provided in Supplemental Experimental Procedures.
Behavior
Mice were trained to perform object location discrimination through operant conditioning (O’Connor et al., 2010a, Supplemental Experimental Procedures). The hardware and software used for behavioral control was largely as described in O’Connor et al., 2010a (see details in Supplemental Experimental Procedures). The stimulus was a pole (0.9 mm in diameter), presented at one of two possible positions (Figure 1). The two pole positions were 4.29 mm apart along the anterior-posterior axis (40 deg of whisking angle) and were constant across sessions. The posterior pole position was 5 mm from the whisker pad. A two-spout lickport (4.5 mm apart) was used to deliver water rewards and record licks (Supplemental Experimental Procedures). Mouth movements (reaction time) were monitored using a photodiode and an infrared laser diode (Thorlabs). High speed video was taken at 1 kHz using Mikrotron Eosens Camera (Norpix, MC1362) to track the C2 whisker (Supplemental Experimental Procedures).
At the beginning of each trial, the vertical pole moved into the plane within reach of the C2 whisker (0.2 s travel time). The sound produced by mechanically moving the pole triggered whisking before the pole was within reach (see Figure 1D example trial). The pole remained within reach for 1 second, after which it was retracted. The retraction time was 0.2 second, of which the pole remained within reach in the first 0.1 second. Thus, we define the sample epoch as the time from onset of pole movement to 0.1 s after the retraction onset of the pole (sample epoch, 1.3 s total, Figure 1C). The delay epoch lasted for another 1.2 seconds after the completion of pole retraction (delay epoch, 1.3 s total, Figure 1C). An auditory “response” cue indicated the end of the delay epoch (pure tone, 3.4 kHz, 0.1 s duration, DigiKey, 458-1088-ND). Licking early during the trial was punished by a loud “alarm” sound (siren buzzer, 0.05 s duration, RadioShack, 273-079), followed by a brief timeout (1–1.2 s). Continued licking triggered additional timeouts; these trials were excluded from the analyses (“lick early” trials, Figure 1E, black bars). Licking the correct lickport after the auditory “response” cue led to a small drop of liquid reward (3 μL). Licking the incorrect lickport triggered a timeout (2–5 s). Trials in which mice did not lick within a 1.5 second window after the “response” cue were rare and typically occurred at the end of a session. Sessions were terminated when signs of fatigue were observed (e.g. reduced whisking, occurrence of “no lick” trials). Typically, the last 20 trials within each session were excluded from analyses. All mice learned to perform this task with the C2 whisker. The total training time to criterion performance (> 70 % correct) was 3–4 weeks (Figure S1) (see details in Supplemental Experimental Procedures).
Photostimulation
Light from a 473 nm laser (DHOM-M-473-200, UltraLaser) was controlled by an acousto-optical modulator (AOM; MTS110-A3-VIS, Quanta Tech) and a shutter (Vincent Associates), coupled to a 2D scanning galvo system (GVSM002, Thorlabs), then focused onto the brain surface (Figure 1A, Supplemental Experimental Procedures). The laser at the brain surface had a Gaussian profile with a beam diameter of 400 μm at 4σ (Figure S4). The scanning galvo system (0.5–5 ms step time for step size 0.57–35mm) and AOM (extinction ratio 1:2000; 1μs rise time) allowed simultaneous targeting of multiple non-adjacent cortical regions for photostimulation (Figure S6H).
The standard photostimulus had a near sinusoidal temporal profile (40 Hz) with a linear attenuation in intensity over the last 100 ms (duration: 1.3 s + 0.1 s ramp, Figure 2D). The power values reported is the time-average. To prevent the mice from distinguishing photostimulation trials from control trials using visual cues, a “masking flash” (40 1ms pulses at 10 Hz) was delivered using a blue LED near the eyes (Supplemental Experimental Procedures). The masking flash began as the pole started to move and continued through the end of the epoch in which photostimulation could occur.
For photostimulation of vS1, the laser beam was positioned over the C2 barrel column. For photostimulation of multiple cortical locations (Figure 4), the laser beam was aligned to bregma. For sessions in which we targeted specific cortical regions, photostimulation was delivered on 25% of behavioral trials. Photostimulation locations were chosen randomly, but never twice in succession. For sessions in which we targeted 55 cortical locations (Figures 4B, S6), photostimulation was delivered on 75% of behavioral trials. Each cortical location was photostimulated once in random order over 55 stimulation trials.
For recording in awake non-behaving mice, photostimulation was delivered at ~7 s intervals (data in Figure 2). The power (0.53, 1.0, 1.5, 2.5, 4.5, 7.3, 14 mW) and locations of photostimulation (0.5, 0.75, 1.0, 2.0, 3.0 mm from the recording sites) were chosen randomly. In addition to vS1 (Figure 2) we tested photoinhibition in ALM during behavior and found identical effects (Figure S4C). Furthermore, photoinhibition was similar during the sample and delay epochs (Figure S4C).
We measured light transmission through the clear-skull cap (dental cement and skull) using two independent methods. First, we directly measured laser power before and after passing through the isolated clear-skull cap (Fig S4F). Second, we measured the rate of photobleaching in vivo in a transgenic mouse line expressing GFP in the nuclei of a subset of neurons (Rosa-LSL-H2B-GFP, crossed to PV-ires-cre). Photobleaching was induced by prolonged (10 min) illumination at different laser powers with and without the clear-skull cap. Nuclear fluorescence was measured in fixed tissue sections (Fig S4). Both methods gave light transmission of approximately 50%.
Neuronal recordings
Extracellular spikes were recorded using silicon probes (NeuroNexus) (for details see Supplemental Experimental Procedures). Under awake, non-behaving condition, mice remained idle while different photostimulation conditions were tested. For recordings during behavior, sessions started after setting up the recording apparatus. At the end of the recording sessions, the electrode was removed and the craniotomy was covered with Kwik-Sil (World Precision Instruments) and further reinforced with dental acrylic. On subsequent days, recordings were made through the same craniotomy. Due to deterioration of the neuronal tissue caused by electrode penetrations, only 1–3 recordings were made in each craniotomy. In an subset of mice, two craniotomies were made for recording from vS1 and ALM (Table 1). Silicon probes were painted with DiI and recording tracks were recovered to measure recording depth.
Data analysis
We separately computed the performance for “lick right” and “lick left” trials as the fraction of correct reports (Fig 1E, 3C). Chance performance was 50%. Behavioral effects of photoinhibition were quantified by comparing the performance under photostimulation with control performance (Figures 3, 4). Significance was determined using two tailed t-test (Figures 1–3, 4C–E, 6, 7) and bootstrap (Fig 4B).
We tested against the null hypothesis that each photoinhibition site did not cause a performance change. Performance changes must be interpreted against behavioral variability. We performed bootstrap to consider the variability across mice, sessions, and trials (Efron and Tibshirani, 1994). For each cycle of bootstrap, repeated 106 times, we randomly sampled with replacement 1) animals, 2) sessions performed by each animal, 3) trials within each session. We computed control performance and performance under photoinhibition for each condition (i.e. “lick right” and “lick left” trial performances for each cortical location). The p value for each photoinhibition condition was the fraction of times the performance change from the control condition changed sign (if photoinhibition showed a mean decrease in performance from the control, p value for this condition was the number of times it showed an increase in performance during bootstrap). This p value can be interpreted as follows: if we were to repeat this experiment, what is the chance that we would not see the performance change observed here? The bootstrap analysis determined the confidence interval around our originally observed performance values (the standard errors of performance were the standard deviations of the estimates from bootstrap; these were reported as error bars in Figure S6), and the duality of confidence intervals and hypotheses testing allowed us to report that confidence interval as a p value (Efron and Tibshirani, 1994). The threshold p value was α = 0.025 (for one tailed tests). To correct for multiple comparisons, we used the Benjamini–Hochberg procedure (Benjamini and Hochberg, 1995): We first sorted the p values corresponding to the 55 photoinhibition locations in ascending order (i.e. p(1) ≤ p(2)≤···p(i)··≤p(55)) and found the largest i such that p(i)≤α·i/55. The performance change for grid locations, 1, ···, i, was scored as significant (Supplementary Table 1). We performed power analyses to estimate the number of trials necessary to observe significant performance changes under normal behavioral variability. We randomly down-sampled the full dataset (53190 behavioral trials) and recomputed p-values to look for the minimal sample size needed to reach p < 0.025. The minimal sample size depended on the mean effect size. For ALM, (mean Δperformance, 26%, Figure 4B, “lick right” trials, delay epoch), a minimal of 75 photoinhibition trials were needed to observe a significant performance change (156 trials were collected). For vS1, (mean Δperformance, 23%, Figure 4B, “lick right” trials, sample epoch), a minimal of 91 photoinhibition trials were needed to observe a significant performance change (174 trials were collected). With the full dataset (53190 behavioral trials), we could reliably detect a minimal behavioral change of 10% (Fig 4B).
The extracellular recording traces were band-pass filtered (300–6k Hz). Events that exceeded an amplitude threshold (4 standard deviations of the background) were subjected to manual spike sorting to extract single units (see details in Supplemental Experimental Procedures). 133 single units were recorded under awake, non-behaving conditions (Table 1). For each unit, its spike width was computed as the trough to peak interval in the mean spike waveform (Figures 2C, S3). We defined units with spike width <0.35 ms as FS neurons (18/133) and units with spike width >0.45 ms as putative pyramidal neurons (ppyr, 106/133). Units with intermediate values (0.35–0.45 ms, 9/133) were excluded from our analyses.
Effect of photoinhibition on activity was quantified in “normalized spike rate” relative to the baseline (see Supplemental Experimental Procedures). The time course of photoinhibition (onset, 17.3 ± 1.4 ms, mean ± s.e.m.; offset 124 ± 9.4 ms) was computed from averaged PSTH (Figure 2D, see Supplemental Experimental Procedures). Bootstrap was performed over neurons to obtain the standard errors.
Under the active behaving condition, 261 neurons were isolated for >40 behavioral trials (>20 “lick right” trials and >20 “lick left” trials). 75 neurons were recorded from vS1 and 138 neurons were recorded from the left ALM and 48 neurons were recorded from the right ALM. These neurons were further screened for significant trial type selectivity using spike count during the sample, delay, or response epoch (two-tailed t-test, Figures 6, 7, filled symbols). For this analysis, only trials in which the mice correctly reported pole locations were included. To quantify the effect of photoinhibition during active behavior, we focused our analysis on 35/75 vS1 neurons (6 mice) that were classified as pyramidal neurons and tested for > 5 photostimulation trials (25% of the trials). These neurons were presented in Figure 2E. A few recording sessions were discarded due to excessive bleeding (7/25 sessions) over the craniotomy.
Supplementary Material
Highlights.
Head fixed mice perform a whisker-based tactile task with a memory component
Barrel cortex (vS1) is selectively involved during sensory exploration
The anterior motor cortex is involved after sensation, preceding the action
Decision-related information flows serially from vS1 to anterior motor cortex
Acknowledgments
We thank Carlos Brody, Daniel O’Connor, Misha Ahrens, Mac Hooks, Nick Sofroniew, Jianing Yu, Tsai-Wen Chen, Simon Peron, Dima Rinberg for comments on the manuscript, Susan Michael and Amy Hu for histology, S. Andrew Hires for help with whisker tracking, Daniel O’Connor for help with the laser scanning system, Tim Harris, Brian Barbarits, Anthony Leonardo for help with silicon probe recordings. This work was funded by Howard Hughes Medical Institute. N.L. is a Helen Hay Whitney Foundation postdoctoral fellow.
Footnotes
Author Contributions
ZVG, NL, KS conceived the project. ZVG and NL performed the experiments. ZVG, NL, and EO performed animal training. ZVG, NL, and KS analyzed the data. DH developed the clear skull cap preparation and performed pilot studies using the “lick left/lick right” task. DG developed the silicon probe recording system and the lickport system. JTT and GF provided the VGAT-ChR2-EYFP transgenic mice. ZVG, NL, KS wrote the paper with comments from other authors.
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afraz SR, Kiani R, Esteky H. Microstimulation of inferotemporal cortex influences face categorization. Nature. 2006;442:692–695. doi: 10.1038/nature04982. [DOI] [PubMed] [Google Scholar]
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng. 2007;4:S143–156. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M, Fox K, Das-Gupta A. Flow of excitation within rat barrel cortex on striking a single vibrissa. J Neurosci. 1992;68:1345–1354. doi: 10.1152/jn.1992.68.4.1345. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, series B. 1995;57:289–300. [Google Scholar]
- Bisley JW, Zaksas D, Pasternak T. Microstimulation of cortical area MT affects performance on a visual working memory task. Journal of neurophysiology. 2001;85:187–196. doi: 10.1152/jn.2001.85.1.187. [DOI] [PubMed] [Google Scholar]
- Brecht M. Movement, confusion, and orienting in frontal cortices. Neuron. 2011;72:193–196. doi: 10.1016/j.neuron.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Brecht M, Sakmann B. Dynamic representation of whisker deflection by synaptic potentials in spiny stellate and pyramidal cells in the barrels and septa of layer 4 rat somatosensory cortex. J Physiol. 2002;543:49–70. doi: 10.1113/jphysiol.2002.018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clack NG, O’Connor DH, Huber D, Petreanu L, Hires A, Peron S, Svoboda K, Myers EW. Automated tracking of whiskers in videos of head fixed rodents. PLoS computational biology. 2012;8:e1002591. doi: 10.1371/journal.pcbi.1002591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis JC, Kleinfeld D. Phase-to-rate transformations encode touch in cortical neurons of a scanning sensorimotor system. Nat Neurosci. 2009;12:492–501. doi: 10.1038/nn.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lafuente V, Romo R. Neuronal correlates of subjective sensory experience. Nat Neurosci. 2005;8:1698–1703. doi: 10.1038/nn1587. [DOI] [PubMed] [Google Scholar]
- Ding L, Gold JI. Caudate encodes multiple computations for perceptual decisions. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:15747–15759. doi: 10.1523/JNEUROSCI.2894-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. An Introduction to the Bootstrap. 1. Chapman and Hall/CRC; 1994. [Google Scholar]
- Erlich JC, Bialek M, Brody CD. A cortical substrate for memory-guided orienting in the rat. Neuron. 2011;72:330–343. doi: 10.1016/j.neuron.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Ferezou I, Haiss F, Gentet LJ, Aronoff R, Weber B, Petersen CC. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron. 2007;56:907–923. doi: 10.1016/j.neuron.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173:652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. Banburismus and the brain: decoding the relationship between sensory stimuli, decisions, and reward. Neuron. 2002;36:299–308. doi: 10.1016/s0896-6273(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. New York: John Wiley & Sons Ltd; 1966. [Google Scholar]
- Harvey CD, Coen P, Tank DW. Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature. 2012;484:62–68. doi: 10.1038/nature10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey MA, Sachdev RN, Zeigler HP. Cortical barrel field ablation and unconditioned whisking kinematics. Somatosens Mot Res. 2001;18:223–227. doi: 10.1080/01421590120072213. [DOI] [PubMed] [Google Scholar]
- Helmstaedter M, Sakmann B, Feldmeyer D. L2/3 Interneuron Groups Defined by Multiparameter Analysis of Axonal Projection, Dendritic Geometry, and Electrical Excitability. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn130. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Nacher V, Luna R, Zainos A, Lemus L, Alvarez M, Vazquez Y, Camarillo L, Romo R. Decoding a perceptual decision process across cortex. Neuron. 2010;66:300–314. doi: 10.1016/j.neuron.2010.03.031. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. J Neurophysiol. 1985;53:266–291. doi: 10.1152/jn.1985.53.1.266. [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffer ZS, Arantes HB, Roth RL, Alloway KD. Functional circuits mediating sensorimotor integration: quantitative comparisons of projections from rodent barrel cortex to primary motor cortex, neostriatum, superior colliculus, and the pons. J Comp Neurol. 2005;488:82–100. doi: 10.1002/cne.20579. [DOI] [PubMed] [Google Scholar]
- Hooks BM, Mao T, Gutnisky DA, Yamawaki N, Svoboda K, Shepherd GM. Organization of cortical and thalamic input to pyramidal neurons in mouse motor cortex. J Neurosci. 2013;33:748–760. doi: 10.1523/JNEUROSCI.4338-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz GD, Newsome WT. Separate signals for target selection and movement specification in the superior colliculus. Science. 1999;284:1158–1161. doi: 10.1126/science.284.5417.1158. [DOI] [PubMed] [Google Scholar]
- Huber D, Gutnisky DA, Peron S, O’Connor DH, Wiegert JS, Tian L, Oertner TG, Looger LL, Svoboda K. Multiple dynamic representations in the motor cortex during sensorimotor learning. Nature. 2012;484:473–478. doi: 10.1038/nature11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson KA, Masterton RB. The sensory contribution of a single vibrissa’s cortical barrel. J Neurophysiol. 1986;56:1196–1223. doi: 10.1152/jn.1986.56.4.1196. [DOI] [PubMed] [Google Scholar]
- Jackson N, Muthuswamy J. Artificial dural sealant that allows multiple penetrations of implantable brain probes. J Neurosci Methods. 2008;171:147–152. doi: 10.1016/j.jneumeth.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Groupings of nonpyramidal and pyramidal cells with specific physiological and morphological characteristics in rat frontal cortex. J Neurophysiol. 1993;69:416–431. doi: 10.1152/jn.1993.69.2.416. [DOI] [PubMed] [Google Scholar]
- Kiritani T, Wickersham IR, Seung HS, Shepherd GM. Hierarchical connectivity and connection-specific dynamics in the corticospinal-corticostriatal microcircuit in mouse motor cortex. J Neurosci. 2012;32:4992–5001. doi: 10.1523/JNEUROSCI.4759-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfeld D, Deschenes M. Neuronal basis for object location in the vibrissa scanning sensorimotor system. Neuron. 2011;72:455–468. doi: 10.1016/j.neuron.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsen PM, Pietr M, Ahissar E. Haptic object localization in the vibrissal system: behavior and performance. J Neurosci. 2006;26:8451–8464. doi: 10.1523/JNEUROSCI.1516-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama T, Sato TR, O’Connor DH, Zhang YX, Huber D, Hooks BM, Gabitto M, Svoboda K. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature. 2010;464:1182–1186. doi: 10.1038/nature08897. [DOI] [PubMed] [Google Scholar]
- Koralek KA, Jensen KF, Killackey HP. Evidence for two complementary patterns of thalamic input to the rat somatosensory cortex. Brain Res. 1988;463:346–351. doi: 10.1016/0006-8993(88)90408-8. [DOI] [PubMed] [Google Scholar]
- Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY. Transcranial optogenetic excitation with a novel red-shifted variant of channelrhodopsin. Paper presented at: Society for Neuroscience; New Orlean: Society for Neuroscience; 2012. [Google Scholar]
- Long MA, Fee MS. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature. 2008;456:189–194. doi: 10.1038/nature07448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SM, Lin RCS. Thalamic afferents of the rat barrel cortex: a light- and electron-microscopic study using Phaseolus vulgaris leucoagglutinin as an anterograde tracer. Somatosens Mot Res. 1993;10:1–16. doi: 10.3109/08990229309028819. [DOI] [PubMed] [Google Scholar]
- Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao T, Kusefoglu D, Hooks BM, Huber D, Petreanu L, Svoboda K. Long-range neuronal circuits underlying the interaction between sensory and motor cortex. Neuron. 2011;72:111–123. doi: 10.1016/j.neuron.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino SA, Kwon MC, Dory Y, Frostig RD. Characterization of functional organization within rat barrel cortex using intrinsic signal optical imaging through a thinned skull. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:9998–10002. doi: 10.1073/pnas.90.21.9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyas F, Sreenivasan V, Marbach F, Wacongne C, Barsy B, Mateo C, Aronoff R, Petersen CC. Motor control by sensory cortex. Science. 2010;330:1240–1243. doi: 10.1126/science.1195797. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. Journal of Neurophysiology. 1985;54:782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- Morishima M, Kawaguchi Y. Recurrent connection patterns of corticostriatal pyramidal cells in frontal cortex. J Neurosci. 2006;26:4394–4405. doi: 10.1523/JNEUROSCI.0252-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB, Steinmetz MA, Romo R. Frequency discrimination in the sense of flutter: psychophysical measurements correlated with postcentral events in behaving monkeys. J Neurosci. 1990;10:3032–3044. doi: 10.1523/JNEUROSCI.10-09-03032.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor DH, Clack NG, Huber D, Komiyama T, Myers EW, Svoboda K. Vibrissa-based object localization in head-fixed mice. J Neurosci. 2010a;30:1947–1967. doi: 10.1523/JNEUROSCI.3762-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor DH, Hires SA, Guo ZV, Li N, Yu J, Sun QQ, Huber D, Svoboda K. Neural coding during active somatosensation revealed using illusory touch. Nature neuroscience. 2013;16:958–965. doi: 10.1038/nn.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor DH, Huber D, Svoboda K. Reverse engineering the mouse brain. Nature. 2009;461:923–929. doi: 10.1038/nature08539. [DOI] [PubMed] [Google Scholar]
- O’Connor DH, Peron SP, Huber D, Svoboda K. Neural activity in barrel cortex underlying vibrissa-based object localization in mice. Neuron. 2010b;67:1048–1061. doi: 10.1016/j.neuron.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Packer AM, Peterka DS, Hirtz JJ, Prakash R, Deisseroth K, Yuste R. Two-photon optogenetics of dendritic spines and neural circuits. Nat Methods. 2012 doi: 10.1038/nmeth.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pammer L, O’Connor DH, Hires SA, Clack NG, Huber D, Myers EW, Svoboda K. The Mechanical Variables Underlying Object Localization along the Axis of the Whisker. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:6726–6741. doi: 10.1523/JNEUROSCI.4316-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin HBJ. The Mouse Brain in Stereotaxic Coordinates: Compact. 2. Amsterdam: Elsevier; 2004. [Google Scholar]
- Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce CR, Lomber SG, Born RT. Integrating motion and depth via parallel pathways. Nat Neurosci. 2008;11:216–223. doi: 10.1038/nn2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritt JT, Andermann ML, Moore CI. Embodied information processing: vibrissa mechanics and texture features shape micromotions in actively sensing rats. Neuron. 2008;57:599–613. doi: 10.1016/j.neuron.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo R, Brody CD, Hernandez A, Lemus L. Neuronal correlates of parametric working memory in the prefrontal cortex. Nature. 1999;399:470–473. doi: 10.1038/20939. [DOI] [PubMed] [Google Scholar]
- Romo R, de Lafuente V. Conversion of sensory signals into perceptual decisions. Prog Neurobiol. 2012 doi: 10.1016/j.pneurobio.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Sanders JI, Kepecs A. Choice ball: a response interface for two-choice psychometric discrimination in head-fixed mice. Journal of neurophysiology. 2012;108:3416–3423. doi: 10.1152/jn.00669.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz C, Hentschke H, Butovas S, Haiss F, Stuttgen MC, Gerdjikov TV, Bergner CG, Waiblinger C. The head-fixed behaving rat--procedures and pitfalls. Somatosensory & motor research. 2010;27:131–148. doi: 10.3109/08990220.2010.513111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidemann E, Zohary E, Newsome WT. Temporal gating of neural signals during performance of a visual discrimination task. Nature. 1998;394:72–75. doi: 10.1038/27906. [DOI] [PubMed] [Google Scholar]
- Shepherd GM. Corticostriatal connectivity and its role in disease. Nat Rev Neurosci. 2013;14:278–291. doi: 10.1038/nrn3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons DJ. Response properties of vibrissa units in rat SI somatosensory neocortex. J Neurophysiol. 1978;41:798–820. doi: 10.1152/jn.1978.41.3.798. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Influence of the thalamus on spatial visual processing in frontal cortex. Nature. 2006;444:374–377. doi: 10.1038/nature05279. [DOI] [PubMed] [Google Scholar]
- Tanji J, Evarts EV. Anticipatory activity of motor cortex neurons in relation to direction of an intended movement. J Neurophysiol. 1976;39:1062–1068. doi: 10.1152/jn.1976.39.5.1062. [DOI] [PubMed] [Google Scholar]
- Travers JB, Dinardo LA, Karimnamazi H. Motor and premotor mechanisms of licking. Neurosci Biobehav Rev. 1997;21:631–647. doi: 10.1016/s0149-7634(96)00045-0. [DOI] [PubMed] [Google Scholar]
- Voigts J, Sakmann B, Celikel T. Unsupervised whisker tracking in unrestrained behaving animals. J Neurophysiol. 2008;100:504–515. doi: 10.1152/jn.00012.2008. [DOI] [PubMed] [Google Scholar]
- von Heimendahl M, Itskov PM, Arabzadeh E, Diamond ME. Neuronal activity in rat barrel cortex underlying texture discrimination. PLoS Biol. 2007;5:e305. doi: 10.1371/journal.pbio.0050305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Zainos A, Merchant H, Hernandez A, Salinas E, Romo R. Role of primary somatic sensory cortex in the categorization of tactile stimuli: effects of lesions. Exp Brain Res. 1997;115:357–360. doi: 10.1007/pl00005704. [DOI] [PubMed] [Google Scholar]
- Zhao S, Ting JT, Atallah HE, Qiu L, Tan J, Gloss B, Augustine GJ, Deisseroth K, Luo M, Graybiel AM, et al. Cell type-specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nature methods. 2011;8:745–752. doi: 10.1038/nmeth.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.