SUMMARY
The hippocampus is critical for human episodic memory, but its role remains controversial. One fundamental question concerns whether the hippocampus represents specific objects or assigns context-dependent representations to objects. Here, we used multivoxel pattern similarity analysis of fMRI data during retrieval of learned object sequences to systematically investigate hippocampal coding of object and temporal context information. Hippocampal activity patterns carried information about the temporal positions of objects in learned sequences, but not about objects or temporal positions in random sequences. Hippocampal activity patterns differentiated between overlapping object sequences and between temporally adjacent objects that belonged to distinct sequence contexts. Parahippocampal and perirhinal cortex showed different pattern information profiles consistent with coding of temporal position and object information, respectively. These findings are consistent with models proposing that the hippocampus represents objects within specific temporal contexts, a capability that might explain its critical role in episodic memory.
INTRODUCTION
Episodic memories consist of temporally organized sequences of events that occur within a given context (Tulving, 1984). The neural mechanisms that support the temporal organization of episodic memories, however, remain largely unknown. Drawing on evidence that hippocampal damage leads to severe impairments in episodic memory, some models have proposed that the hippocampus may facilitate the binding of temporally contiguous events such that they can be linked as parts of a larger episodic memory (Rawlins, 1985; Levy, 1989; Wallenstein et al., 1998; Jensen and Lisman, 2005; Howard et al., 2005; see also Staresina and Davachi, 2009). Some of these models posit that the internal dynamics of hippocampal activity give rise to a temporally evolving context representation that is associated with incoming information during the experience of an event, thereby supporting the creation of an episodic memory and the disambiguation of memories that share common elements (Levy, 1996; Sohal and Hasselmo, 1998).
Although temporal context models can explain a great deal of behavioral data on temporal organization in memory (Sederberg et al., 2008; Polyn et al., 2009), it remains unclear whether or how these models correspond to computations carried out in the human hippocampus. Some recent studies in monkeys and rats have indicated that individual hippocampal neurons selectively respond at different times during repetitive event sequences such that hippocampal ensemble firing patterns change as time proceeds (Pastalkova et al., 2008; MacDonald et al., 2011; Naya and Suzuki, 2011). Furthermore, single-cell recordings in rats have reported that hippocampal activity patterns distinctly represent identical segments of a path common to different trajectories (Frank et al., 2000; Wood et al., 2000; Ferbinteanu and Shapiro, 2003; Ginther et al., 2011), indicating the sensitivity of hippocampal spatial coding to sequence contexts.
Other models do not incorporate a special role for the hippocampus in context representation. Rather, these models propose a general role for the hippocampus in the representation of stimulus attributes in declarative memory (McClelland, 1998; Frank et al., 2003; Wixted and Squire, 2011). According to this view, the hippocampus should represent information about specific items, such as objects, as well as other event attributes. Support for stimulus attribute models of hippocampal function comes from fMRI studies indicating that the hippocampus may be involved in “pattern separation” processes that differentiate between studied objects and highly similar but novel objects (e.g., Bakker et al., 2008).
A strong version of the view that the hippocampus represents stimulus attributes in memory would suggest that the hippocampus should assign similar representations to events that include the same objects. In contrast, a strong version of the context-based view would suggest that the hippocampus assigns distinct representations to multiple encounters with the same object in different temporal contexts. Thus, a fundamental, and currently unresolved, question is whether the hippocampus supports memory for temporal context, over and above memory for specific objects.
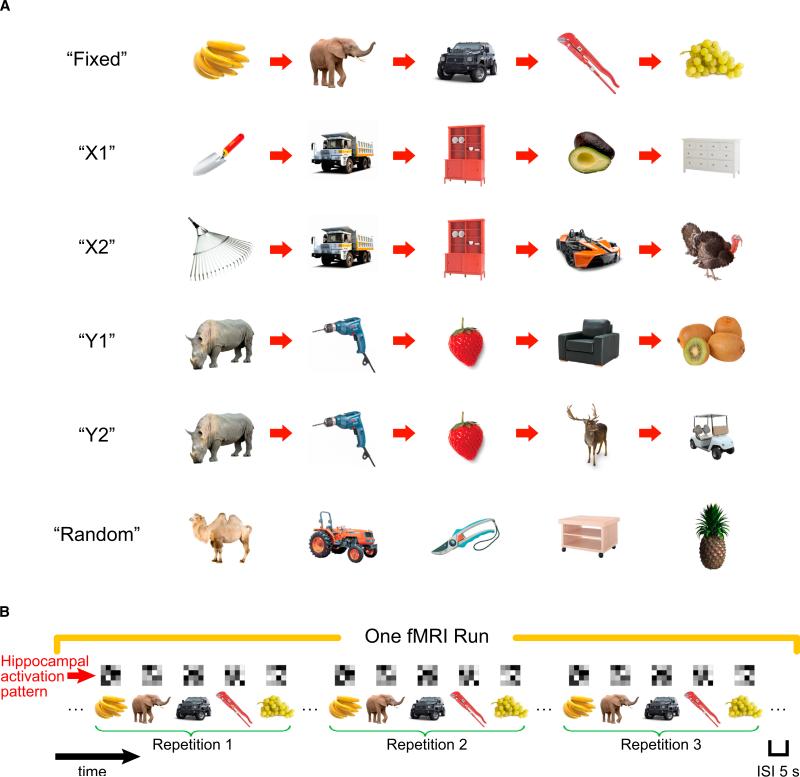
Here, we used fMRI, along with an application of multivoxel pattern similarity analysis (Kriegeskorte et al., 2008; Kriegeskorte, 2009), to address this question by characterizing hippocampal coding of object and temporal context information during retrieval of object sequences. Prior to scanning, each participant learned five sequences by making semantic decisions about each object in the sequence (see Figure 1A). One “Fixed” sequence consisted of five objects that did not overlap with objects in other sequences. Two pairs of sequences shared common objects—“X1” and “X2” sequences shared the same objects in positions 2 and 3 and “Y1” and “Y2” sequences shared common objects in the first three positions. These overlapping sequences were constructed to investigate the ability of the hippocampus to differentiate between occurrences of the same object in different temporal contexts. That is, we expected that participants could differentiate between the “X1” and “X2” sequences upon seeing the first object in the sequence, and this could lead to the development of different, context-specific representations of the overlapping objects. In contrast, we did not expect participants to differentiate between the “Y1” and “Y2” sequences until the fourth object was presented and therefore did not expect to see context-dependent representation of the overlapping objects in these sequences. Finally, to control for learning about specific objects, irrespective of temporal order, we also included a “Random” sequence, which always consisted of the same five objects presented in a random order.
Figure 1. Object Sequences and Schematic of Sequence Retrieval.
(A) Illustration of the six types of temporal sequences. On each repetition of the “Random” sequence, the five objects were presented in a different random order, in contrast to other sequences in which the temporal order between objects was always fixed. Participants learned these sequences to criteria before proceeding to sequence retrieval session (see also Supplemental Experimental Procedures for details).
(B) Schematic diagram of sequence retrieval in one fMRI run (five fMRI runs in total). Each type of temporal sequence was presented three times within an fMRI run, with the constraint that a specific sequence was not presented consecutively and all six sequences must have been presented before the second and the third repetitions. Although brackets are shown to denote each sequence in a run, there were no explicit cues to mark divisions between sequences and the interval between objects was constant across all trials. Above each trial, a matrix is shown depicting a hypothetical hippocampal voxel activation pattern. These voxel patterns were then used to estimate similarity in hippocampal ensemble activity across different pairs of trials.
Immediately after the learning session, participants completed an MRI scan session. During scanning, they made semantic decisions on a continuous stream of objects consisting of contiguous presentations of the five learned sequences and one “Random” sequence (see Figure 1B). Although there were no obvious boundaries between the object sequences during the scan session, we expected that participants’ semantic decisions would be faster for objects in learned sequences than for objects in the “Random” sequence.
Multivoxel pattern similarity analysis (Kriegeskorte et al., 2008; Kriegeskorte, 2009; see also Jenkins and Ranganath, 2010; Hannula et al., 2013) was used to characterize the extent to which the hippocampus codes for object and temporal information. This technique is analogous to neural population vector analyses in single-unit recording studies (Quian Quiroga and Panzeri, 2009; see e.g., Leutgeb et al., 2007), in that the similarity in population-level activity patterns is assessed across different experimental conditions. Voxel pattern similarity analysis is based on the idea that the relative pattern of activation among voxels in a given region is informative with regard to the kind of information that is processed by that brain region (Kriegeskorte et al., 2008). Accordingly, if a region codes for a particular kind of information, one should see correlations in voxel activity patterns between pairs of trials that share this information.
Using this approach, we tested the hypothesis that hippocampal activity patterns would carry information about the temporal order of objects in learned sequences, over and above information about objects in the “Random” sequence. We additionally tested whether hippocampal activity patterns could differentiate between processing of the same objects in distinct, but overlapping, sequences and between adjacent objects in different sequences. Finally, we investigated the roles of the parahippocampal and perirhinal cortex (PHc and PRc) in object and temporal processing and compared these profiles to what was observed for the hippocampus.
RESULTS
Behavioral Results during Sequence Retrieval
To the extent that participants utilized sequence knowledge to facilitate semantic judgments during the scan session, we would expect that accuracy, and especially reaction times (RTs), would be facilitated for objects from learned sequences (i.e., “Fixed,” “X1,” “X2,” “Y1,” and “Y2”), compared to objects from the “Random” sequence. Accuracy on semantic judgments (average across all five serial positions) during sequence retrieval indicated significant differences between the six temporal sequences (F5,90 = 2.498, p < 0.05). Follow-up analyses determined that averaged accuracy of semantic judgments for objects in learned sequences was significantly higher than for objects in the “Random” sequence (F1,18 = 5.635, p < 0.05), consistent with our prediction.
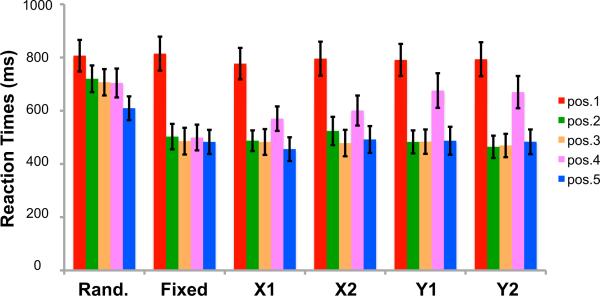
Consistent with the accuracy results, RTs on semantic judgments (average across the five serial positions) differed between the six temporal sequences (F2.085,37. 539 = 25.317, p < 0.001), and this effect was mainly due to slower RTs for the “Random” sequence (F1,18 = 36.018, p < 0.001; Figure 2). To further examine the extent to which sequence knowledge facilitates semantic judgments as a function of serial position, a two-way ANOVA was conducted, breaking down RT effects at each serial position for the six temporal sequences. The analysis indicated a main effect of serial position (F1. 338,24.084 = 40.969, p < 0.001), as well as a significant temporal sequence by serial position interaction (F9.608,172.936 = 7.450, p < 0.001). As is evident in Figure 2, RTs were slower for the first position in each of the six temporal sequences as compared to RTs for other serial positions (F1,18 = 46.075, p < 0.001), which reflects the fact that, during a sequence transition, participants could not predict the first object in an upcoming sequence.
Figure 2. Reaction Time Results Associated with Semantic Judgments during Sequence Retrieval.
Reaction times are separately plotted for each of the five temporal positions in each sequence. Error bars denote ±1 SEM.
To follow up on the temporal sequence by serial position interaction and to better characterize how different sequence contexts modulated behavioral performance, we examined RTs for each serial position in each sequence. In the “Fixed” sequence, RTs were slower for objects in the first serial position than for objects in subsequent serial positions (F1,18 = 52.014, p < 0.001), and RTs did not significantly differ between other serial positions (all p > 0.57). For the “Random” sequence, in addition to the initial increase in RT for objects in the first serial position (F1,18 = 45.170, p < 0.001), RTs were significantly faster for objects in the fifth serial position, relative to objects in other positions (F1,18 = 19.740, p < 0.001). The latter decrease in RT suggested that participants were able to anticipate the last object in the “Random” sequence.
Our next analyses turned to RTs for objects embedded in sequences with overlapping elements. We predicted that the overlap of objects across the “X1” and “X2” sequences and across the “Y1” and “Y2” sequences would impede the ability to predict objects that immediately followed the overlapping objects (i.e., slower RTs for the fourth position objects). Additionally, we predicted that the RT increment should be larger for the “Y” sequences than for the “X” sequences. This is because, in the “Y” sequences, they could not differentiate whether they were in the “Y1” or “Y2” sequence until the fourth object appeared in the sequence. In contrast, in the “X” sequences, participants could use the identity of the first object to immediately disambiguate whether they were presented with the “X1” or “X2” sequence. Consistent with our predictions, in addition to an initial drop in RT after the first serial position (all p < 0.001), there was an RT increase at the fourth serial position for both the “X” and “Y” sequences (all p < 0.001). Moreover, the increase in RT at the fourth serial position was significantly higher in the “Y” sequences than in the “X” sequences (F1,18 = 5.204, p < 0.05), indicating that the “X” sequences were successfully disambiguated from each other.
The above results demonstrate that learning of the object sequences facilitated participants’ semantic decisions during the scan session. Because participants performed different semantic tasks in each scanning run, the results suggest that the learning was not at the level of motor responses or of object-response associations, but rather driven by learning about the temporal relationships among the objects.
Hippocampal Multivoxel Activation Patterns Are Sensitive to Sequence Retrieval
To investigate whether hippocampal activity patterns carry information about temporal sequences, we examined voxel pattern similarity between repetitions of each of the five learned object sequences. Analyses were performed separately for the right and the left hippocampus. Additional analyses quantified pattern similarity effects separately for posterior and anterior segments of the left and right hippocampus, based on evidence suggesting functional differentiation along the longitudinal axis of the hippocampus (Fanselow and Dong, 2010; Poppenk et al., 2013). In general, the analyses revealed highly similar results for anterior and posterior regions of interest (ROIs), so except where the results deviated, we will only report the results for the aggregate ROIs.
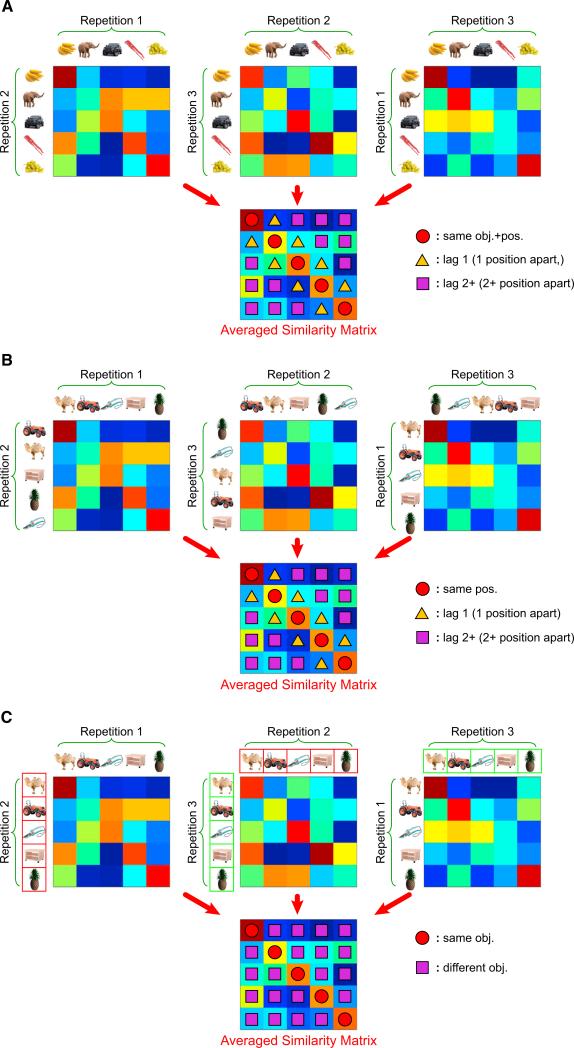
As depicted in Figure 3A, we quantified hippocampal activation pattern similarity across serial positions within each constant temporal sequence (i.e., “Fixed,” “X1,” “X2,” “Y1,” and “Y2”), which yielded a single 5 3 5 similarity matrix for each temporal sequence. The diagonal elements of the 5 3 5 similarity matrix index pattern similarity between pairs of trials that share the same object and position information. Off-diagonal elements, in turn, reflect pattern similarity between pairs of trials that are one or more than one position apart (i.e., “lag 1” or “lag 2+” elements) and have different object information. For both the right and the left hippocampus, similarity values were significantly higher for the diagonal elements of the similarity matrix than for off-diagonal elements corresponding to adjacent pairs of trials in a sequence (right: t17 = 4.073, p < 0.001; left: t17 = 3.112, p < 0.01), or off-diagonal elements corresponding to pairs of trials separated by two or more positions (right: t17 = 4.131, p < 0.001; left: t17 = 2.818, p < 0.05; see Figures 4 and S1 available online). The graded decrease in pattern similarity as a function of lag (i.e., “same obj.+pos.,” “lag 1,” and “lag 2+” trial pairs) was further confirmed by a significant linear trend for both the right (F1,17 = 17.064, p < 0.001) and the left (F1,17 = 7.944, p < 0.05) hippocampal ROIs. These results suggest that the pattern of activation in the hippocampus is more similar across pairs of trials that share the same object and serial position information (e.g., the retrieval of “banana” in the first and second repetition of a constant temporal sequence illustrated in Figure 1B) than for pairs of trials within the same sequence that did not share the same object and serial position information (e.g., low pattern similarity between the retrieval of “banana” in the first repetition and the retrieval of “elephant” in the second repetition). Importantly, these effects were not seen when the same analyses were conducted on “Random” sequence trials (Figure 4), indicating that the hippocampal pattern similarity effects observed for learned object sequences were not driven by artifactual temporal autocorrelation.
Figure 3. Schematic of Pattern Similarity Analyses Associated with Learned Sequences and the “Random” Sequence.
(A) Procedures on how to obtain the 5 × 5 pattern similarity matrix for one particular learned sequence in one fMRI run are illustrated. Pattern similarity was computed between every possible pair of trials between repetitions of a learned object sequence, and these data were organized into three 5 × 5 correlation matrices. Colors are used to visually depict the correlation magnitudes (note that these matrices were generated for explanatory purposes and that the real data are presented in subsequent figures). These pattern similarity matrices were then averaged into a single 5 × 5 pattern similarity matrix. The diagonal of the resulting matrix (denoted by red circles) reflected averaged pattern similarity across repetitions of the same object in the same temporal position, whereas the off-diagonal elements corresponded to averaged pattern similarity between adjacent objects in a sequence (yellow triangles) or between objects that were two or more positions apart in the same sequence (purple squares).
(B) Schematic depiction of procedures for computing pattern similarity across repetitions of the “Random” sequence, in order to quantify shared temporal position information.
(C) Depiction of procedures for sorting and computing pattern similarity across repetitions of the “Random” sequence in order to quantify shared object information. Note that data from different repetitions of the “Random” sequence were rearranged such that pattern similarity values along the diagonal elements were computed from repetitions of the same object but in different temporal positions. Color boxes around objects in Repetition 2 (red boxes) and 3 (green boxes) are to highlight the fact that data were rearranged for this analysis.
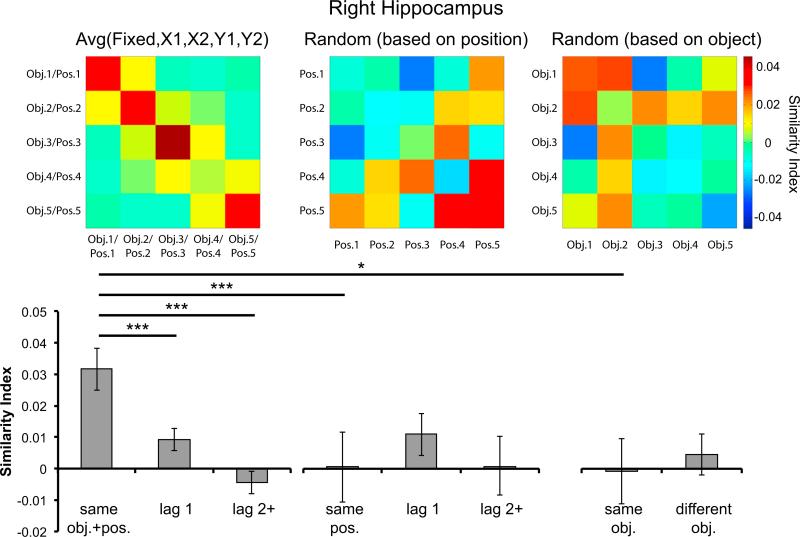
Figure 4. Right Hippocampal Activation Pattern Similarity across Sequence Repetitions.
At top, correlation matrices depict pattern similarity across repetitions of the object sequences (“hotter” colors denote higher pattern similarity). The “Avg(Fixed, X1, X2, Y1, Y2)” similarity matrix (left) was the average of the similarity matrices generated for each of the five constant temporal sequences (i.e., “Fixed,” “X1,” “X2,” “Y1,” and “Y2”). The middle and the right similarity matrices were associated with the “Random” sequence and were generated according to the procedures illustrated in Figures 3B and 3C, respectively. Bar graphs (bottom) quantify averages of the pattern similarity values. The leftmost bar graph illustrates mean pattern similarity across repetitions of the same object in the same position (“same obj.+pos.”), and across repetitions of objects separated by one (“lag 1”) or two or more (“lag 2+”) positions. The greater pattern similarity for “same obj.+pos.” than for “same pos.” and “same obj.” suggested that the enhanced hippocampal pattern similarity in the right hippocampus for “same obj.+pos.” pairs could not be explained by either object or position information alone. *p < 0.05; **p < 0.01; ***p < 0.001. Error bars denote ±1 SEM.
Hippocampal Voxel Patterns Specifically Carry Information about the Temporal Position of Objects in Learned Sequences
The above analyses demonstrated that hippocampal activation patterns reliably carry information about objects in learned sequences. Within each of the constant temporal sequences, each object always appeared at the same serial position across repetitions (see Figure 1B). Therefore, the increased pattern similarity along the diagonal elements in Figure 4 (i.e., “Avg(Fixed, X1, X2, Y1, Y2)” similarity matrix) could be due to the overlap of object (e.g., “banana”), position (e.g., the first object in the sequence), or object-position binding (e.g., “banana” at the first position) information between repetitions. To specify which of the three processes contributed to the lag-dependent pattern similarity effects depicted in Figure 4, we conducted a series of pattern similarity analyses on trial pairs from the “Random” sequence.
First, to examine whether the enhanced hippocampal pattern similarity along the diagonal elements were driven by objects that shared the same serial position information, we computed a similarity matrix across repetitions of the “Random” sequence (see Figure 3B). Constructing the similarity matrix in this way allowed us to estimate the contribution of serial position information to hippocampal pattern similarity. This is because, across repetitions of the “Random” sequence, different objects were associated with each serial position. Thus, if the enhanced hippocampal pattern similarity effects shown in Figure 4 (i.e., “same obj.+pos.” > “lag 1,” “lag 2+”) were solely driven by position information, we should expect that “same obj.+pos.” in Figure 4 should be similar to “same pos.” associated with the “Random” sequence after procedures illustrated in Figure 3B. In contrast, if the increased hippocampal pattern similarity was driven by the processing of information other than serial position (i.e., object or object-position binding information), then the “same obj.+pos.” in Figure 4 should be significantly greater than “same pos.” Results revealed a significant difference between “same obj.+pos.” and “same pos.” in the right hippocampus (t17 = 4.143, p < 0.001; see Figure 4), although this effect was not significant for the left (p > 0.24; see Figure S1), suggesting that hippocampal activation pattern similarity effects for learned sequences were not solely driven by serial position information. Furthermore, there was no evidence of purely temporal coding in the hippocampus, as pattern similarity did not significantly differ between “same pos.” pairs and either “lag 1” (right: p > 0.19; left: p > 0.43) or “lag 2+” (right: p > 0.49; left: p > 0.25) pairs in the “Random” sequence.
We next tested the extent to which hippocampal voxel patterns carry information about objects, irrespective of temporal position. That is, across repetitions of the “Random” sequence, we correlated voxel patterns between trials for which the same object was presented (i.e., at different serial positions on each repetition). As a result, correlating the same object across repetitions of the “Random” sequence yielded an estimate of hippocampal pattern similarity solely driven by object information (see Figure 3C for illustration). If pattern similarity values were higher for “same obj. + pos.” pairs than for “same obj.” pairs, it would support the hypothesis that hippocampal activation patterns carry information about object-position bindings, over and above information about individual objects. Indeed, the results showed that voxel pattern similarity was significantly higher for “same obj.+pos.” than for “same obj.” trial pairs in the right hippocampus (t17 = 2.575, p < 0.05; see Figure 4), although this effect was not significant for the left (p > 0.37; see Figure S1). Moreover, there was no evidence of any object-based information in hippocampal voxel patterns—pattern similarity in the hippocampus did not significantly differ between “same obj.” and “different obj.” pairs in the “Random” sequence (right hippocampus: p > 0.33; left hippocampus: p > 0.17). The results therefore clearly support the hypothesis that the hippocampus is specifically involved in the binding of object and position information during temporal sequence retrieval.
The above analyses were based on the average of the five constant sequences. To confirm that this effect was not driven by only one of the learned sequences, we repeated the same contrast separately for each of the five constant temporal sequences against the “Random” sequence. The results were similar for all five of the constant sequences (see Figure S2), suggesting that the binding of object and temporal position information is robust in the right hippocampus.
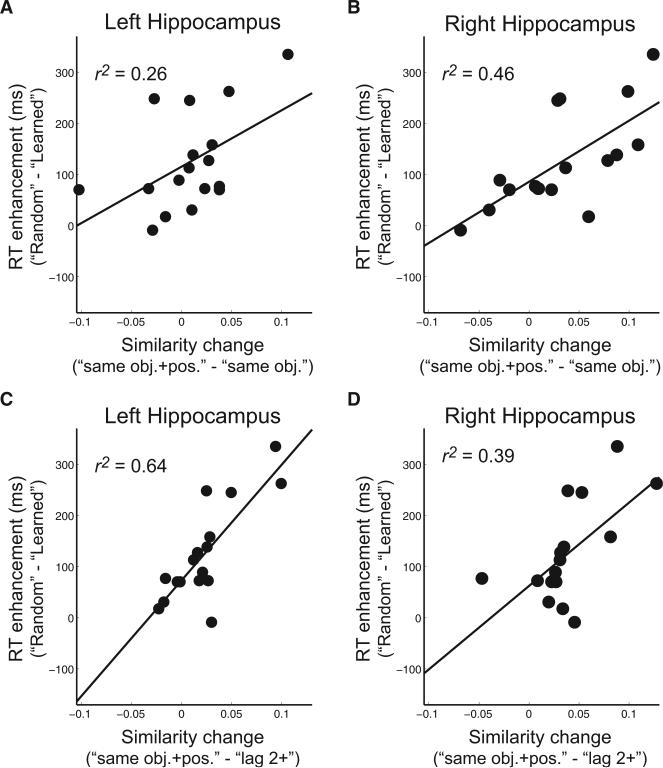
Hippocampal Pattern Similarity Effects Are Highly Correlated with Individual Differences in Sequence Learning
The behavioral results described above demonstrated robust learning of the object sequences, but there were substantial across-participant differences in the behavioral effects. We therefore tested whether the hippocampal pattern similarity effects were correlated with behavioral indices of sequence memory. Behavioral benefits of sequence learning were quantified by computing the RT difference between the average across all five constant temporal sequences versus the “Random” sequence. Results showed that participants who showed more behavioral enhancement for the learned sequences (i.e., faster RTs for the learned sequences than the “Random” sequence) also showed more of a hippocampal pattern similarity effect for learned, relative to random, sequences (i.e., larger difference between “same obj.+pos.” and “same obj.” pattern similarity values). The positive correlation was significant in both the left (r = 0.51, p < 0.05) and right (r = 0.68, p < 0.01) hippocampus (see Figures 5A and 5B). Similar results were found when RT differences between learned and random sequences were correlated with hippocampal pattern similarity differences between “same obj.+pos.” versus “lag 2+” trial pairs of learned sequences (left: r = 0.80, p < 0.001; right: r = 0.63, p < 0.01; see Figures 5C and 5D). These results demonstrate that information about temporal sequences carried by hippocampal activation patterns is highly correlated with behavioral indices of sequence memory (accounting for ~25%–65% of the behavioral variance), regardless of which pattern similarity matrix was used to index hippocampal object-position binding. It is also noteworthy that the correlations were robust for the left hippocampus, despite the fact that the group-averaged difference between “same obj.+pos.” versus “same obj.” in the left hippocampus was not statistically significant. This suggests that, when individual differences in sequence learning are taken into consideration, the left hippocampus also carries information about the serial positions associated with objects in temporal sequences.
Figure 5. Hippocampal Pattern Similarity Effects Are Highly Correlated with Behavioral Enhancement during Sequence Retrieval.
Both the left (A) and the right (B) hippocampus showed enhanced pattern similarity effects (i.e., larger “same obj.+pos.” > “same obj.”; shown on the x axis) as RT enhancement increased (i.e., larger RTRandom > RTLearned) during sequence retrieval. Similar results were obtained when hippocampal pattern similarity effects (C, left hippocampus; D, right hippocampus) were quantified by comparing “same obj.+pos.” versus “lag 2+” within learned sequences. Note that RTLearned was the average of RTs across all five constant temporal sequences (i.e., “Fixed,” “X1,” “X2,” “Y1,” and “Y2”).
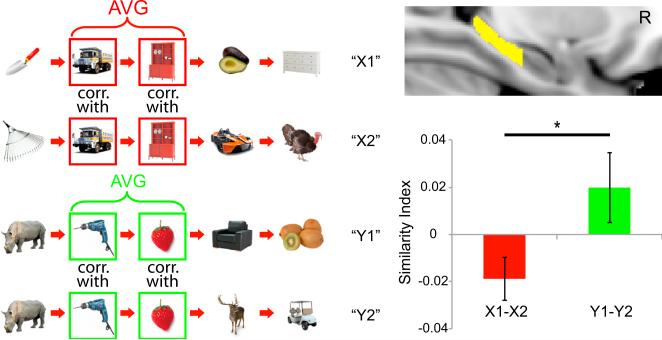
Hippocampal Activation Patterns Disambiguate Overlapping Sequences
If the hippocampus carries information about objects in temporal context rather than object information, one would expect that the same objects, but presented in different sequence contexts, would exhibit different activation patterns. Specifically, we hypothesized that items that are common in both the “X1” and “X2” sequences should be less similar to each other than repetitions of these shared items across repetitions of the same sequence (i.e., in Figure 6, “truck” in “X1” should be less similar to “truck” in “X2” as compared to “truck” in the first and second repetitions of “X1” or “X2” sequence). We restricted our analyses to objects in positions 2 and 3, as these objects were used in both the “X1” and “X2” sequences and occupied the same serial positions. Similarity values across repetitions of “X1” sequence (“X1-X1”) were combined with similarity values across repetitions of “X2” (“X2-X2”) sequence. The combined similarity values were then compared against pattern similarity between “X1” and “X2” sequences (“X1-X2”). Consistent with our predictions, pattern similarity was significantly higher for “X1-X1” and “X2-X2” trial pairs than for “X1-X2” trial pairs in the right hippocampus (t17 = 3.574, p < 0.005). Similar results were found in the left hippocampus, but this effect did not reach significance (p > 0.06).
Figure 6. Right Posterior Hippocampal Activation Patterns Can Disambiguate Overlapping Sequences.
“X1-X2” pattern similarity reflected the average of pattern similarity across repetitions of the same objects in positions 2 and 3 of the “X1” and “X2” sequences, and similar procedures were used to obtain the value for “Y1-Y2” (i.e., green bar in the bar graph) pattern similarity estimates. “Y1-Y2” pattern similarity was significantly higher than “X1-X2” in the right posterior hippocampus, consistent with behavioral results showing that “X” sequences were more psychologically separable from each other than “Y” sequences (i.e., slower RTs for the fourth position objects in the “Y” sequences than in the “X” sequences). *p < 0.05. Error bars denote ±1 SEM.
We next turned to comparisons between the “X” and “Y” sequences. We hypothesized that items that are common in both the “Y1” and “Y2” sequences should exhibit higher hippocampal pattern similarity than items that are shared between the “X1” and “X2” sequences. This is because, in the “X” sequences, participants could use the identity of the first object to immediately disambiguate whether they would encounter an “X1” or “X2” sequence. In contrast, for the “Y” sequences, they could not differentiate whether they were in the “Y1” or “Y2” sequence until the fourth object appeared in the sequence. We therefore predicted that the hippocampus should exhibit more distinctive activation patterns between overlapping objects in the “X1” and “X2” sequences than between the overlapping objects in the “Y1” and “Y2” sequences. To ensure comparability between the “X” and “Y” sequences, pattern similarity comparisons were restricted to objects in the second and third serial positions in the “X” and “Y” sequences. Results did not reveal significant pattern similarity differences between pairs of overlapping objects in the “Y” sequences and pairs of overlapping objects in the “X” sequences for either the right or left hippocampal ROIs (all p > 0.18). Some previous findings, however, indicate that the right posterior hippocampus might be particularly involved in sequence disambiguation (Kumaran and Maguire, 2006; Brown et al., 2010) and sequence learning (Schendan et al., 2003). Accordingly, we conducted further analyses separately for the anterior and the posterior hippocampus in sequence disambiguation. These analyses revealed that overlapping objects in the “Y” sequences elicited higher pattern similarity than the overlapping objects in the “X” sequence in the right posterior hippocampus (t17 = 2.198, p < 0.05; see Figure 6).
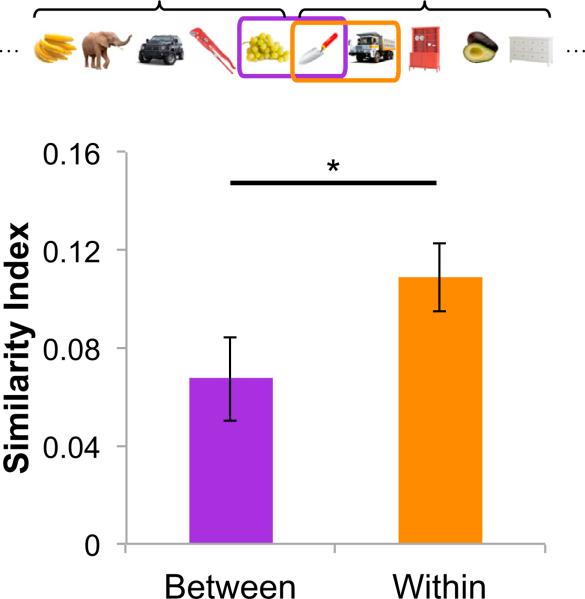
Hippocampal Activation Patterns Are Sensitive to Sequence Boundaries
Previous studies have indicated hippocampal involvement in the processing of boundaries in spatial contexts (Doeller et al., 2008; Doeller and Burgess, 2008; Bird et al., 2010) and during transitions between psychologically distinct events (Swallow et al., 2011). We therefore hypothesized that hippocampal activation patterns might also be sensitive to boundaries between temporal sequences. To test this hypothesis, we compared pattern similarity between the first and the second position objects of each learned sequence (hereafter referred to as “Within” pairs) versus object pairs that bridged the fifth position of a temporal sequence and the first position object of the temporal sequence that immediately followed (hereafter referred to as “Between” pairs; see Figure 7). The fact that a fixed interstimulus interval (ISI) was used throughout the entire sequence retrieval phase ensured that objects within the “Between” and “Within” pairs were matched for temporal distance. Trial pairs with “Random” sequence trials were excluded from this analysis, as we would not expect to see strong boundary effects for these trials, as compared with the constant temporal sequences. Consistent with our predictions, pattern similarity was higher for “Within” than for “Between” trial pairs in the left hippocampus (t17 = 2.147, p < 0.05). A similar effect was evident for the right hippocampus but did not reach significance (p > 0.08).
Figure 7. Left Hippocampal Activation Patterns Are Sensitive to Sequence Boundaries.
Pattern similarity was computed for pairs of adjacent trials that belonged to different object sequences (“Between”) and for pairs of adjacent trials that belonged to the same object sequence (“Within”). The higher pattern similarity for the “Within” than for the “Between” pairs suggest that activation patterns in the left hippocampus are sensitive to sequence boundaries. *p < 0.05. Error bars denote ±1 SEM.
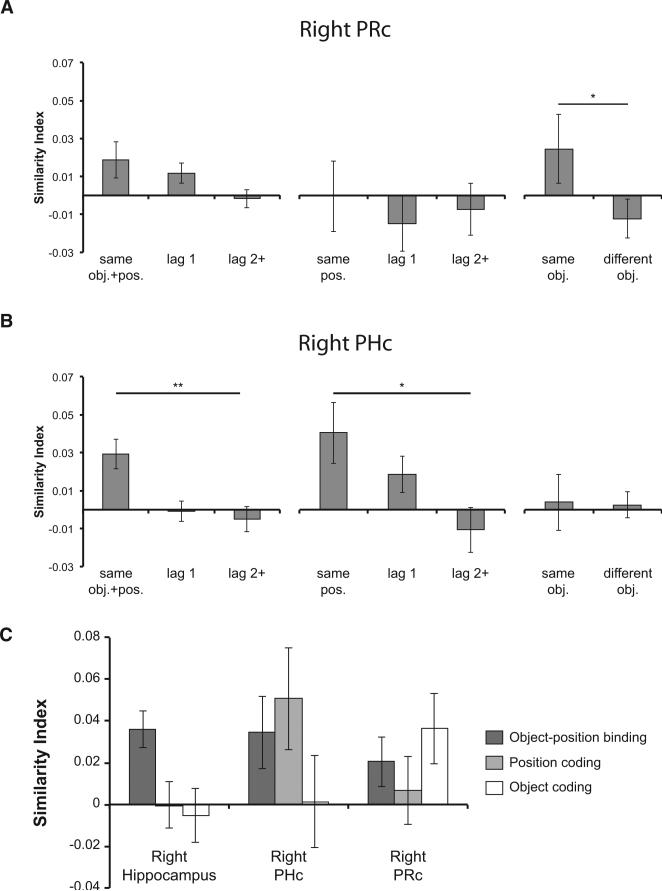
Position and Object Information in the PHc and PRc
Our next analyses addressed effects in other regions of the medial temporal lobe (MTL). It is well established that, in addition to the hippocampus, the PRc and PHc also contribute to episodic memory (Ranganath and Ritchey, 2012). For instance, according to the Binding of Items and Contexts (BIC) model, the PRc would be expected to carry information about objects, whereas the PHc would be expected to carry information about the context in which objects are encountered (Diana et al., 2007; Eichenbaum et al., 2007; Ranganath, 2010; see also Aminoff et al., 2007). Based on this model, we hypothesized that activation patterns in the PRc might carry information about object identity, whereas the PHc would carry information about temporal context. To test these hypotheses, multivoxel pattern analyses were conducted on brain voxels within the PRc and PHc ROIs. In contrast to pattern similarity results on the hippocampus, none of the comparisons between the constant sequences versus the “Random” sequence (i.e., “same obj.+pos.” versus “same obj.” and “same obj.+pos.” versus “same pos.”) were significant in either the right or the left PRc and PHc (all p > 0.10; Figures 8A and 8B). Consistent with our predictions, there was a significant effect of object coding (i.e., “same obj.” > “different obj.”) in the right PRc (t17 = 2.150, p < 0.05; Figure 8A), but not in the right PHc (p > 0.46). Moreover, there was a significant position coding in the right PHc (“same obj.+pos.” > “lag 2+,” t17 = 3.119, p < 0.005; “same pos.” > “lag 2+,” t17 = 2.063, p < 0.05; Figure 8B), but this effect was not observed in the right PRc (p > 0.38). Although object and temporal position coding effects were also evident in the left PRc and PHc, respectively, these effects did not reach significance (all p > 0.06).
Figure 8. Differential Information Coding of the Hippocampus, PHc, and PRc.
(A and B) Pattern analyses were conducted on brain voxels within the PRc and PHc ROIs following procedures illustrated in Figures 3A, 3B, and 3C. (A) Object coding in the right PRc. Pattern similarity was significantly higher across repetitions of the same object (“same obj.”) than between pairs of trials that corresponded to different objects (“different obj.”). (B) Position coding in the right PHc. Pattern similarity was significantly higher across trials that shared the same temporal position information (“same obj.+pos.” or “same pos.”) than across trials that were 2 or more than 2 positions apart (“lag 2+”).
(C) The hippocampus, PHc, and PRc encode different types of information. Indices of object-position binding, position coding, and object coding are plotted for each of the right hemisphere MTL ROIs. The three ROIs showed qualitatively different patterns of information coding. *p < 0.05; **p < 0.01. Error bars denote ±1 SEM.
The analyses described above demonstrated that the hippocampus carries information about object-position binding and that right PHc and right PRc activation patterns are sensitive to temporal position and object identity, respectively. To more directly test whether the three regions process different types of information, we conducted a two-way (3 3 3) ANOVA including brain regions (i.e., the right hippocampus, right PHc, and right PRc) as one factor, and similarity metrics that best captured object-position binding (i.e., “same obj.+pos.” – “lag 2+” in learned sequences), position (i.e., “same pos.” – “lag 2+” in the “Random” sequence), and object coding (i.e., “same obj.” – “different obj.” in the “Random” sequence), respectively, as three levels of the other factor. If the three brain structures process qualitatively different types of information, we would expect a significant interaction in the ANOVA analysis (i.e., each of the three brain regions is differentially sensitive to object-position, position, and object coding). Indeed, there was a significant interaction between the two factors (F3.372,53.317 = 2.798, p < 0.05; Figure 8C), further demonstrating that the activation patterns in the hippocampus, PHc, and PRc are sensitive to different types of information.
DISCUSSION
The present study used fMRI to examine how the hippocampus represents sequences of objects. We found that hippocampal activation patterns specifically carried information about objects in particular temporal positions (i.e., “object-position binding”), and this could not be explained by the processing of object or temporal position information alone. Moreover, individual differences in hippocampal voxel pattern information explained over one-third of the interindividual variance in reaction time indices of sequence learning. Individuals who exhibited more robust hippocampal object-position binding showed more behavioral facilitation during sequence retrieval. Using overlapping sequences, we also found that hippocampal activation patterns differentiate between different sequence contexts, even when the object and its temporal position within the sequence were identical. Finally, we found that hippocampal voxel pattern similarity was higher for pairs of adjacent trials that belonged to the same sequence context as compared to pairs of trials that bridged between different sequence contexts, despite identical temporal distance between the pairs of trials. Together, these results are consistent with the idea that the hippocampus represents information about the temporal context associated with specific items.
The present results are pertinent to a significant debate about the role of the hippocampus in memory. Several theories have proposed that the hippocampus is involved in integrating stimulus attributes, including object information (e.g., McClelland, 1998; Frank et al., 2003; Wixted and Squire, 2011). A strong version of this view would suggest that the hippocampus should assign similar mnemonic representations across multiple encounters with the same object. Other models propose a more specific role for the hippocampus in associating information about people, things, and situations to a representation of context (Wallenstein et al., 1998; Howard et al., 2005; Davachi, 2006; Ranganath, 2010; Nadel and Hardt, 2011; Howard and Eichenbaum, 2013). A strong version of this view would predict that the hippocampus should assign different representations to the same object in different contexts. Our findings are more consistent with the context-based view.
We found no evidence to support the idea that hippocampal activity patterns carry information about objects when the temporal order was random. This finding is consistent with results from a single-unit recording study showing minimal object coding in the monkey hippocampus (Naya and Suzuki, 2011). The lack of object coding in the hippocampus is striking and qualitatively different from right PRc, which showed reliable pattern similarity effects across repetitions of objects in random sequences. Additionally, right PHc showed evidence for coding of serial position, even in random sequences, for which the object information changed on each repetition. We also found that the right hippocampus, PRc, and PHc exhibited distinct pattern information profiles, confirming that these regions play different roles in the processing of object and temporal information (Figure 8C). The present findings fit with results from fMRI studies that have examined coding of category-level stimulus attributes in the MTL. These studies have generally failed to find evidence for category-level attribute coding in the hippocampus, whereas activity patterns in the PRc and PHc carry category-level information about visual stimuli (Diana et al., 2008; LaRocque et al., 2013; but see Liang et al., 2013). However, the present results go further by demonstrating that, even when the same object is repeated, hippocampal voxel patterns are dissimilar unless the temporal context is reinstated.
Our results complement and add to findings from previous fMRI studies that have examined the role of the hippocampus in memory. Several studies have reported that the magnitude of hippocampal activity is increased during successful encoding (e.g., Davachi et al., 2003; Ranganath et al., 2004; Kirwan and Stark 2004; Kensinger and Schacter, 2006; Staresina and Davachi, 2006) and retrieval (e.g., Cansino et al., 2002; Yonelinas et al., 2005; Hannula and Ranganath, 2009; Johnson et al., 2009; Diana et al., 2010, 2013; Duarte et al., 2011) of contextual information, including temporal context (Tubridy and Davachi, 2011; Jenkins and Ranganath, 2010; Ekstrom et al., 2011). These findings, however, could be explained in terms of a role for the hippocampus in encoding of very strong or detailed memories (but see Diana and Ranganath, 2011; Montaldi and Mayes, 2011). Furthermore, studies have reported evidence indicating that the hippocampus links successive elements of a film clip (Gelbard-Sagiv et al., 2008; Paz et al., 2010), sequences of auditory stimuli (Kalm et al., 2013), or temporally paired visual stimuli (e.g., Turk-Browne et al., 2010, 2012; Schapiro et al., 2012). These findings demonstrate a role for the hippocampus in linking items that are in close temporal proximity (consistent with our finding of lag-dependent similarity effects). The present results add to these findings by demonstrating that the hippocampus specifically codes for the positions of objects in learned sequences, over and above purely temporal or object-based coding.
We speculate that the capability of the hippocampus to encode objects in relation to a temporal context might relate to the ability to distinguish between temporally distinct events that share common elements. For instance, parking a car in the same parking structure on different days requires the formation of distinct memory representations in order to efficiently retrieve the car at a later time. Previous studies have implicated the hippocampus in this ability—lesions to the hippocampus in rats impaired the ability to disambiguate overlapping odor sequences (Agster et al., 2002), and neuroimaging studies of humans have reported stronger hippocampal activation during processing of overlapping as compared to nonoverlapping sequences (Kumaran and Maguire, 2006; Brown et al., 2010; Brown and Stern, 2013). The present results help to explain these findings by indicating that the hippocampus may assign distinct representations to overlapping but psychologically distinct events, as predicted by computational models of hippocampal sequence representation (Levy, 1989, 1996; Wallenstein et al., 1998). Specifically, we found that, even when comparing pairs of trials corresponding to the same object in the same temporal position, hippocampal pattern similarity was higher for pairs of trials in the same learned sequence (“X1-X1” or “X2-X2” pairs) than across pairs of trials in different sequences (“X1-X2” pairs). Furthermore, voxel pattern similarity in the right posterior hippocampus was lower for “X1-X2” pairs than for “Y1-Y2” pairs. This finding is notable, given that the “X1” and “X2” sequences could be differentiated during processing of the overlapping objects, whereas the “Y1” and “Y2” sequences could not be differentiated until presentation of the fourth (nonoverlapping) object. These results suggest that the hippocampus only differentiates between overlapping sequences that are psychologically distinct. This result parallels findings from studies that have found differences in hippocampal ensemble activity patterns as a rat traverses the common path of different trajectories (Frank et al., 2000; Wood et al., 2000; Ferbinteanu and Shapiro, 2003; Ginther et al., 2011). Taken together, the results are consistent with the idea that temporal context coding in the hippocampus may help to disambiguate overlapping events in episodic memory, thereby contributing to “pattern separation” (Kim and Yassa, 2013).
The present findings suggest parallels between human memory for temporal sequences and recent studies of hippocampal “time cells” in rats (MacDonald et al., 2011, 2013; Kraus et al., 2013; Pastalkova et al., 2008). For instance, MacDonald et al. (2011) conducted a study in which the rat learned object-odor associations separated by a temporal gap. They found that different hippocampal neurons fired at distinct segments of time within a trial such that the serial firing of hippocampal time cells filled the temporal gap between object sampling and presentation of the odor (see also Pastalkova et al., 2008). Additionally, hippocampal time cells elicited distinct context-specific firing patterns during identical blank intervals that corresponded to different object-odor sequences. Other studies have shown that ensemble activity in hippocampal subfield CA1 could support temporal coding across broader timescales, extending across tens of seconds (Manns et al., 2007) and even across hours and days (Mankin et al., 2012). A recent study in humans is also consistent with these results, demonstrating hippocampal context effects that operate across longer timescales (L.J.J. and C.R., unpublished data). Notably, some models, such as the model of Howard and Kahana (2002), can account for temporal context effects across short and long timescales.
A recent study in monkeys (Naya and Suzuki, 2011) also reported evidence for temporal coding in the hippocampus, although their results were somewhat different from the findings observed here. Naya and Suzuki (2011) recorded activity from the monkey temporal lobe during a task that required memory for the temporal order of two objects. Consistent with MacDonald et al. (2011), they found that hippocampal neurons fired at specific time points during the delay between each object, which they termed an “incremental timing signal.” Naya and Suzuki did not, however, report that the hippocampal incremental timing signal was modulated by different sequence contexts (i.e., different two-object sequences). Thus, hippocampal neurons encoded the temporal structure of trial events, irrespective of the currently relevant object sequence, a finding that contrasts with the current results and those reported in MacDonald et al. (2011). Naya and Suzuki (2011) also found that neurons in the PRc did not show the temporally graded “incremental timing” signal seen in the hippocampus, but rather they showed object-selective responses. Some of these cells integrated object information with information about the ordinal position of each object (first versus second) on each trial, however, which is seemingly at odds with the present study, which did not find evidence for object-position binding in the PRc.
We speculate that differences in results across studies might have to do with differences in task requirements. In our study, participants learned a small set of relatively unique object sequences, and these sequences remained consistent throughout the experiment. In MacDonald et al. (2011), the task also required learning of unique object-odor sequences. In Naya and Suzuki (2011)'s study, however, a pool of eight objects was used to generate different two-object sequences on each trial. We speculate that extensive training on the task, in which the stimulus pairs and temporal order relationships changed across trials, created conditions under which hippocampal neurons picked up on the temporal structure of each test trial as the salient contextual information remained consistent across sessions. It is also possible that, under these conditions, the PRc encoded serial position as a “semantic” feature attached to each object. Considered collectively, the evidence is consistent with the possibility that hippocampal neurons only retain associations between objects and temporal context if they remain consistent across learning events. If object-position associations are not reliable across learning events, however, then hippocampal neurons might show more purely temporal coding. This speculation can be tested in a future study.
Some temporal context models explicitly predict that contextual states are correlated across time (Howard and Kahana, 2002; Sederberg et al., 2008). This idea is consistent with the graded reduction in hippocampal pattern similarity that we observed across adjacent positions in learned sequences. More direct evidence for this idea has come from single-unit recording studies in rats (Manns et al., 2007) and humans (Howard et al., 2012) demonstrating that patterns in hippocampal ensemble activity change gradually over time. Howard et al. (2012) additionally found that, during memory retrieval, the pattern of activity in ensembles of hippocampal neurons resembled the activity pattern elicited before the item was first encountered. Manning et al. (2011) reported a similar finding, showing that recall of a previously studied item elicited patterns of field potentials that were similar to the activity pattern elicited during study of that item, and also similar to the pattern elicited during processing of temporally contiguous study items. This effect was maximal in temporal lobe electrodes, although Manning et al. could not localize it to the hippocampus.
In contrast to the graded similarity of hippocampal representations across adjacent positions within a learned sequence, we found that the left hippocampus shows disproportionate reductions in voxel similarity across adjacent trials that are in different sequences. It is likely that similar dynamics play a role in the segmentation of events in episodic memory. For instance, behavioral research indicates that, while processing continuous narrative text or movie stimuli, people tend to segment incoming information into distinct event representations, and this, in turn, affects how they will be remembered (Zacks et al., 2007; Ezzyat and Davachi, 2011). For instance, Ezzyat and Davachi (2011) reported reduced recall performance for sentences that immediately followed a boundary between two events. To the extent that the object sequences studied here are relevant to processing of more complex episodic materials, we would expect that hippocampal activity patterns should show sharp transitions following perception of an event boundary. To our knowledge, this prediction has not yet been tested, but, in a related study, Swallow et al. (2011) found that, with a short 5 s retention interval, hippocampal activation was increased during retrieval of objects across an event boundary.
It is also possible that hippocampal processing of abstract event boundaries is related to processing of physical boundaries in the environment. For instance, one study found that left hippocampal activation is modulated by the number of boundaries embedded in spatial contexts (Bird et al., 2010). Future work might therefore investigate the relationship between hippocampal coding of boundaries in spatial contexts and event boundaries during temporally extended cognitive processing.
In summary, the present results indicate that hippocampal activity patterns carry information about the temporal positions of objects in learned sequences. Although the results do not necessitate a hippocampal representation of temporal context that is analogous to those described in mathematical models (Howard and Kahana, 2002; Howard et al., 2005; Sederberg et al., 2008; Polyn et al., 2009), they do suggest that hippocampal representations incorporate more than simply the attributes of the currently processed item. The context-sensitive hippocampal activation patterns observed here might support a wide range of memory capacities, including the ability to learn spatial maps (O'Keefe and Nadel, 1978), differentiate highly similar, yet distinct memories (Levy, 1989, 1996; Wallenstein et al., 1998; Yassa and Stark, 2011; Kesner, 2013), and the ability to segment continuous incoming information into distinct episodic memories (Zacks et al., 2007; Ezzyat and Davachi, 2011). More generally, the results underscore the importance of temporal information in understanding hippocampal function, potentially explaining how the hippocampus supports the ability to remember what happened when.
EXPERIMENTAL PROCEDURES
Participants
Twenty individuals participated in the experiment, but due to technical difficulties, behavioral data from one participant and fMRI data from two participants were excluded. Thus, the reported behavioral analyses are based on results from 19 (10 females) participants, and group-averaged fMRI results are based on data from 18 (9 females) and correlations between behavioral and fMRI results are reported for 17 (8 female) participants. The study was approved by the Institutional Review Board at the University of California at Davis. Written informed consent was obtained from each participant before the experiment.
Task Procedures
The experimental procedures are summarized briefly in the Introduction and Figure 1 and are presented in detail in Supplemental Experimental Procedures.
fMRI Pattern Analysis
Analyses of fMRI data were performed by assessing patterns of activity across voxels within anatomically defined ROIs evoked during single trials. Parameter estimates (beta weights) indexing activity evoked by each trial were estimated with the Least-Square2 (LS2) method as described in Turner et al. (2012) (see also Supplemental Experimental Procedures for details). ROIs were manually traced using each participant's native-space MPRAGE structural image. The left and right hippocampus, PRc, and PHc cortex were identified according to a protocol based on structural MRI studies of the medial temporal lobe (MTL) (Insausti et al., 1998; Pruessner et al., 2002; Frankó et al., 2014). The hippocampal ROI was further segmented into the anterior and the posterior portions based on uncal apex landmark (Poppenk et al., 2013).
Pattern similarity between presented objects was estimated by computing the correlation coefficient between vectors of beta weights across pairs of trials. The resulting correlation coefficient was then Fisher transformed and averaged within particular bins prior to conducting parametric statistical tests. All reported parametric statistical tests for pattern analysis are one-tailed, as each of these tests was conducted with a clear directional prediction. Nonetheless, the overall pattern of results was essentially unchanged with two-tailed statistical tests.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grant R01 MH068721, a Guggenheim Fellowship, a Leverhulme Trust Visiting Professorship, and a Parke-Davis Exchange Fellowship from the University of Cambridge, UK, to C.R. We thank each of the reviewers for their helpful comments and suggestions. We also thank Drs. Russell Poldrack and Jeanette Mumford for providing their code for single trial modeling and Shao-Fang Wang for her help with brain tracing. L.-T.H. is a Howard Hughes Medical Institute International Student Research fellow.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures and two figures and can be found with this article online at http://dx.doi.org/10.1016/j.neuron.2014.01.015.
REFERENCES
- Agster KL, Fortin NJ, Eichenbaum H. The hippocampus and disambiguation of overlapping sequences. J. Neurosci. 2002;22:5760–5768. doi: 10.1523/JNEUROSCI.22-13-05760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff E, Gronau N, Bar M. The parahippocampal cortex mediates spatial and nonspatial associations. Cereb. Cortex. 2007;17:1493–1503. doi: 10.1093/cercor/bhl078. [DOI] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CEL. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird CM, Capponi C, King JA, Doeller CF, Burgess N. Establishing the boundaries: the hippocampal contribution to imagining scenes. J. Neurosci. 2010;30:11688–11695. doi: 10.1523/JNEUROSCI.0723-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TI, Stern CE. Contributions of medial temporal lobe and striatal memory systems to learning and retrieving overlapping spatial memories. Cereb. Cortex. 2013 doi: 10.1093/cercor/bht041. http://dx.doi.org/10.1093/cercor/bht041. [DOI] [PMC free article] [PubMed]
- Brown TI, Ross RS, Keller JB, Hasselmo ME, Stern CE. Which way was I going? Contextual retrieval supports the disambiguation of well learned overlapping navigational routes. J. Neurosci. 2010;30:7414–7422. doi: 10.1523/JNEUROSCI.6021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cansino S, Maquet P, Dolan RJ, Rugg MD. Brain activity underlying encoding and retrieval of source memory. Cereb. Cortex. 2002;12:1048–1056. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr. Opin. Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc. Natl. Acad. Sci. USA. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Ranganath C. Recollection, familiarity and memory strength: confusion about confounds. Trends Cogn. Sci. 2011;15:337–338. doi: 10.1016/j.tics.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn. Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. High-resolution multi-voxel pattern analysis of category selectivity in the medial temporal lobes. Hippocampus. 2008;18:536–541. doi: 10.1002/hipo.20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Medial temporal lobe activity during source retrieval reflects information type, not memory strength. J. Cogn. Neurosci. 2010;22:1808–1818. doi: 10.1162/jocn.2009.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Parahippocampal cortex activation during context reinstatement predicts item recollection. J. Exp. Psychol. Gen. 2013;142:1287–1297. doi: 10.1037/a0034029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeller CF, Burgess N. Distinct error-correcting and incidental learning of location relative to landmarks and boundaries. Proc. Natl. Acad. Sci. USA. 2008;105:5909–5914. doi: 10.1073/pnas.0711433105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeller CF, King JA, Burgess N. Parallel striatal and hippo-campal systems for landmarks and boundaries in spatial memory. Proc. Natl. Acad. Sci. USA. 2008;105:5915–5920. doi: 10.1073/pnas.0801489105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Henson RN, Graham KS. Stimulus content and the neural correlates of source memory. Brain Res. 2011;1373:110–123. doi: 10.1016/j.brainres.2010.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu. Rev. Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Copara MS, Isham EA, Wang W-C, Yonelinas AP. Dissociable networks involved in spatial and temporal order source retrieval. Neuroimage. 2011;56:1803–1813. doi: 10.1016/j.neuroimage.2011.02.033. [DOI] [PubMed] [Google Scholar]
- Ezzyat Y, Davachi L. What constitutes an episode in episodic memory? Psychol. Sci. 2011;22:243–252. doi: 10.1177/0956797610393742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong H-W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbinteanu J, Shapiro ML. Prospective and retrospective memory coding in the hippocampus. Neuron. 2003;40:1227–1239. doi: 10.1016/s0896-6273(03)00752-9. [DOI] [PubMed] [Google Scholar]
- Frank LM, Brown EN, Wilson M. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron. 2000;27:169–178. doi: 10.1016/s0896-6273(00)00018-0. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Rudy JW, O'Reilly RC. Transitivity, flexibility, conjunctive representations, and the hippocampus. II. A computational analysis. Hippocampus. 2003;13:341–354. doi: 10.1002/hipo.10084. [DOI] [PubMed] [Google Scholar]
- Frankó E, Insausti AM, Artacho-Pérula E, Insausti R, Chavoix C. Identification of the human medial temporal lobe regions on magnetic resonance images. Hum. Brain Mapp. 2014;35:248–256. doi: 10.1002/hbm.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbard-Sagiv H, Mukamel R, Harel M, Malach R, Fried I. Internally generated reactivation of single neurons in human hippocampus during free recall. Science. 2008;322:96–101. doi: 10.1126/science.1164685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginther MR, Walsh DF, Ramus SJ. Hippocampal neurons encode different episodes in an overlapping sequence of odors task. J. Neurosci. 2011;31:2706–2711. doi: 10.1523/JNEUROSCI.3413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C. The eyes have it: hippocampal activity predicts expression of memory in eye movements. Neuron. 2009;63:592–599. doi: 10.1016/j.neuron.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Libby LA, Yonelinas AP, Ranganath C. Medial temporal lobe contributions to cued retrieval of items and contexts. Neuropsychologia. 2013;51:2322–2332. doi: 10.1016/j.neuropsychologia.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MW, Eichenbaum H. The hippocampus, time, and memory across scales. J. Exp. Psychol. Gen. 2013;142:1211–1230. doi: 10.1037/a0033621. http://dx.doi.org/10.1037/a0033621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MW, Kahana MJ. A distributed representation of temporal context. J. Math. Psychol. 2002;46:269–299. [Google Scholar]
- Howard MW, Fotedar MS, Datey AV, Hasselmo ME. The temporal context model in spatial navigation and relational learning: toward a common explanation of medial temporal lobe function across domains. Psychol. Rev. 2005;112:75–116. doi: 10.1037/0033-295X.112.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MW, Viskontas IV, Shankar KH, Fried I. Ensembles of human MTL neurons “jump back in time” in response to a repeated stimulus. Hippocampus. 2012;22:1833–1847. doi: 10.1002/hipo.22018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkänen A. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR Am. J. Neuroradiol. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- Jenkins LJ, Ranganath C. Prefrontal and medial temporal lobe activity at encoding predicts temporal context memory. J. Neurosci. 2010;30:15558–15565. doi: 10.1523/JNEUROSCI.1337-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. Hippocampal sequence-encoding driven by a cortical multi-item working memory buffer. Trends Neurosci. 2005;28:67–72. doi: 10.1016/j.tins.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Johnson JD, McDuff SGR, Rugg MD, Norman KA. Recollection, familiarity, and cortical reinstatement: a multivoxel pattern analysis. Neuron. 2009;63:697–708. doi: 10.1016/j.neuron.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalm K, Davis MH, Norris D. Individual sequence representations in the medial temporal lobe. J. Cogn. Neurosci. 2013;25:1111–1121. doi: 10.1162/jocn_a_00378. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Amygdala activity is associated with the successful encoding of item, but not source, information for positive and negative stimuli. J. Neurosci. 2006;26:2564–2570. doi: 10.1523/JNEUROSCI.5241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP. An analysis of the dentate gyrus function. Behav. Brain Res. 2013;254:1–7. doi: 10.1016/j.bbr.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Kim J, Yassa MA. Assessing recollection and familiarity of similar lures in a behavioral pattern separation task. Hippocampus. 2013;23:287–294. doi: 10.1002/hipo.22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Stark CEL. Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus. 2004;14:919–930. doi: 10.1002/hipo.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus BJ, Robinson RJ, 2nd, White JA, Eichenbaum H, Hasselmo ME. Hippocampal “time cells”: time versus path integration. Neuron. 2013;78:1090–1101. doi: 10.1016/j.neuron.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N. Relating population-code representations between man, monkey, and computational models. Front. Neurosci. 2009;3:363–373. doi: 10.3389/neuro.01.035.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Mur M, Bandettini P. Representational similarity analysis - connecting the branches of systems neuroscience. Front. Syst. Neurosci. 2008;2:4. doi: 10.3389/neuro.06.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. The dynamics of hippocampal activation during encoding of overlapping sequences. Neuron. 2006;49:617–629. doi: 10.1016/j.neuron.2005.12.024. [DOI] [PubMed] [Google Scholar]
- LaRocque KF, Smith ME, Carr VA, Witthoft N, Grill-Spector K, Wagner AD. Global similarity and pattern separation in the human medial temporal lobe predict subsequent memory. J. Neurosci. 2013;33:5466–5474. doi: 10.1523/JNEUROSCI.4293-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Levy W. A computational approach to hippocampal function. In: Hawkins RD, Bowers GH, editors. Computational Models of Learning in Simple Neural Systems. Academic Press; San Diego: 1989. pp. 243–305. [Google Scholar]
- Levy WB. A sequence predicting CA3 is a flexible associator that learns and uses context to solve hippocampal-like tasks. Hippocampus. 1996;6:579–590. doi: 10.1002/(SICI)1098-1063(1996)6:6<579::AID-HIPO3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Liang JC, Wagner AD, Preston AR. Content representation in the human medial temporal lobe. Cereb. Cortex. 2013;23:80–96. doi: 10.1093/cercor/bhr379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71:737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald CJ, Carrow S, Place R, Eichenbaum H. Distinct hippocampal time cell sequences represent odor memories in immobilized rats. J. Neurosci. 2013;33:14607–14616. doi: 10.1523/JNEUROSCI.1537-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin EA, Sparks FT, Slayyeh B, Sutherland RJ, Leutgeb S, Leutgeb JK. Neuronal code for extended time in the hippocampus. Proc. Natl. Acad. Sci. USA. 2012;109:19462–19467. doi: 10.1073/pnas.1214107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning JR, Polyn SM, Baltuch GH, Litt B, Kahana MJ. Oscillatory patterns in temporal lobe reveal context reinstatement during memory search. Proc. Natl. Acad. Sci. USA. 2011;108:12893–12897. doi: 10.1073/pnas.1015174108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Howard MW, Eichenbaum H. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56:530–540. doi: 10.1016/j.neuron.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL. Complementary learning systems in the brain. A connectionist approach to explicit and implicit cognition and memory. Ann. N Y Acad. Sci. 1998;843:153–169. doi: 10.1111/j.1749-6632.1998.tb08212.x. [DOI] [PubMed] [Google Scholar]
- Montaldi D, Mayes AR. Familiarity, recollection and medial temporal lobe function: an unresolved issue. Trends Cogn. Sci. 2011;15:339–340. doi: 10.1016/j.tics.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Nadel L, Hardt O. Update on memory systems and processes. Neuropsychopharmacology. 2011;36:251–273. doi: 10.1038/npp.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya Y, Suzuki WA. Integrating what and when across the primate medial temporal lobe. Science. 2011;333:773–776. doi: 10.1126/science.1206773. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford University Press; Oxford: 1978. [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsáki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz R, Gelbard-Sagiv H, Mukamel R, Harel M, Malach R, Fried I. A neural substrate in the human hippocampus for linking successive events. Proc. Natl. Acad. Sci. USA. 2010;107:6046–6051. doi: 10.1073/pnas.0910834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyn SM, Norman KA, Kahana MJ. A context maintenance and retrieval model of organizational processes in free recall. Psychol. Rev. 2009;116:129–156. doi: 10.1037/a0014420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn. Sci. 2013;17:230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Köhler S, Crane J, Pruessner M, Lord C, Byrne A, Kabani N, Collins DL, Evans AC. Volumetry of temporopolar, perirhinal, entorhinal and parahippocampal cortex from high-resolution MR images: considering the variability of the collateral sulcus. Cereb. Cortex. 2002;12:1342–1353. doi: 10.1093/cercor/12.12.1342. [DOI] [PubMed] [Google Scholar]
- Quian Quiroga R, Panzeri S. Extracting information from neuronal populations: information theory and decoding approaches. Nat. Rev. Neurosci. 2009;10:173–185. doi: 10.1038/nrn2578. [DOI] [PubMed] [Google Scholar]
- Ranganath C. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus. 2010;20:1263–1290. doi: 10.1002/hipo.20852. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nat. Rev. Neurosci. 2012;13:713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D'Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Rawlins JNP. Associations across time: The hippocampus as a temporary memory store. Behav. Brain Sci. 1985;8:479–496. [Google Scholar]
- Schapiro AC, Kustner LV, Turk-Browne NB. Shaping of object representations in the human medial temporal lobe based on temporal regularities. Curr. Biol. 2012;22:1622–1627. doi: 10.1016/j.cub.2012.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendan HE, Searl MM, Melrose RJ, Stern CE. An FMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron. 2003;37:1013–1025. doi: 10.1016/s0896-6273(03)00123-5. [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Howard MW, Kahana MJ. A context-based theory of recency and contiguity in free recall. Psychol. Rev. 2008;115:893–912. doi: 10.1037/a0013396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Hasselmo ME. GABA(B) modulation improves sequence disambiguation in computational models of hippocampal region CA3. Hippocampus. 1998;8:171–193. doi: 10.1002/(SICI)1098-1063(1998)8:2<171::AID-HIPO9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. J. Neurosci. 2006;26:9162–9172. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Mind the gap: binding experiences across space and time in the human hippocampus. Neuron. 2009;63:267–276. doi: 10.1016/j.neuron.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow KM, Barch DM, Head D, Maley CJ, Holder D, Zacks JM. Changes in events alter how people remember recent information. J. Cogn. Neurosci. 2011;23:1052–1064. doi: 10.1162/jocn.2010.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubridy S, Davachi L. Medial temporal lobe contributions to episodic sequence encoding. Cereb. Cortex. 2011;21:272–280. doi: 10.1093/cercor/bhq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Précis of elements of episodic memory. Behav. Brain Sci. 1984;7:223–268. [Google Scholar]
- Turk-Browne NB, Scholl BJ, Johnson MK, Chun MM. Implicit perceptual anticipation triggered by statistical learning. J. Neurosci. 2010;30:11177–11187. doi: 10.1523/JNEUROSCI.0858-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk-Browne NB, Simon MG, Sederberg PB. Scene representations in parahippocampal cortex depend on temporal context. J. Neurosci. 2012;32:7202–7207. doi: 10.1523/JNEUROSCI.0942-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BO, Mumford JA, Poldrack RA, Ashby FG. Spatiotemporal activity estimation for multivoxel pattern analysis with rapid event-related designs. Neuroimage. 2012;62:1429–1438. doi: 10.1016/j.neuroimage.2012.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein GV, Eichenbaum H, Hasselmo ME. The hippo-campus as an associator of discontiguous events. Trends Neurosci. 1998;21:317–323. doi: 10.1016/s0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Squire LR. The medial temporal lobe and the attributes of memory. Trends Cogn. Sci. 2011;15:210–217. doi: 10.1016/j.tics.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. J. Neurosci. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks JM, Speer NK, Swallow KM, Braver TS, Reynolds JR. Event perception: a mind-brain perspective. Psychol. Bull. 2007;133:273–293. doi: 10.1037/0033-2909.133.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.