Abstract
Purpose of review
Molecular imaging aims to illuminate vital molecular and cellular aspects of disease in vivo, and is rapidly translating into the clinical arena. Advantages of this field include enabling serial biological studies in living subjects, assessment of pharmaceutical efficacy, and in vivo characterization of clinical diseases. Here we present recent exciting advances in molecular imaging of atherosclerotic vascular disease.
Recent findings
Atherosclerosis molecular imaging approaches are now available for magnetic resonance, nuclear, computed tomography, ultrasound, and near-infrared fluorescence imaging. Advances in agent synthesis and detection technology are now enabling in vivo imaging of endothelial cell activation, macrophages, cellular metabolism, protease activity, apoptosis, and osteogenic activity. Several agents show clinical utility for the detection of high-risk plaques.
Summary
Molecular imaging is actively unraveling the biological basis of atherosclerosis in living subjects. In the near-term, molecular imaging will play an important role in assessing novel atherosclerosis pharmacotherapies in clinical trials. Longer term, molecular imaging should enable accurate identification of high-risk plaques responsible for myocardial infarction, stroke, and ischemic limbs.
Keywords: molecular imaging, atherosclerosis, imaging, myocardial infarction, stroke
Introduction
Molecular imaging aims to elucidate the in vivo biology of physiologically relevant cellular and molecular targets, and serves to complement current anatomic and physiologic imaging. Cardiovascular molecular imaging studies utilize MRI, nuclear (PET/SPECT), CT, ultrasound, and optical imaging, in stand-alone or integrated/hybrid systems. Through non-invasive assessment of important disease-specific markers, molecular imaging has the potential to transform clinical cardiovascular disease management by providing answers to unsolved questions in the diagnosis, risk stratification, selection and efficacy of drug therapy, and clinical testing of new pharmacological therapeutics. This review provides an update on recent advances in molecular imaging of atherosclerosis, with a specific focus on clinical applications of this promising field. For an in-depth discussion of the relevant biology and chemistry underpinning molecular imaging, the interested reader is referred to several recent reviews[1, 2, 3••, 4, 5].
Atherosclerosis
Atherosclerosis is a progressive inflammatory disease characterized by the accumulation of lipid-filled macrophages within the arterial intima[6]. Continued inflammation may promote atherosclerotic plaque rupture, thrombotic vessel occlusion, and death. Current clinical atherosclerosis imaging methods visualize vessel stenosis and plaque anatomy, but offer limited information regarding the underlying vessel biology. Can we utilize local plaque biological information to better identify high-risk plaques? Molecular imaging technology aims to address this question, and recent studies demonstrate in vivo detection of plaque macrophages, activated endothelial cells, inflammatory proteases, osteogenic activity, and apoptosis.
Monocytes/macrophages
Monocyte-derived macrophages produce cytokines, reactive oxygen species, and destabilizing proteases. Macrophages are critically involved in atheroma initiation, propagation, and rupture[6], and demarcate high-risk plaques[7, 8]. Therefore, specific of plaque macrophages may have important implications in the assessment of high-risk plaques and macrophage-modulating pharmacotherapies.
CT of plaque macrophages
A significant step towards enabling molecular CT imaging of atherosclerosis was recently achieved with the validation of a positive contrast, crystalline iodinated nanoparticle (N1177) for macrophage detection [9••]. In vitro uptake of N1177 by murine macrophages was demonstrated by optical microscopy of individual cells and inductively coupled–plasma mass spectrometry of cell pellets. Next, in vivo testing of N1177 was performed in atherosclerotic rabbits with serial aortic CT scans after either N1177 or control iodinated contrast agent injection (250 mg iodine/kg). Both agents initially provided a vascular angiogram on post-injection images. However, compared to the control agent, N1177 induced a 40% greater late-phase signal enhancement in atherosclerotic plaques (Figure 1). Areas of N1177-enhancement correlated with increased plaque macrophages in >90% of samples. Thus N1177 offers the ability to identify high-risk inflamed plaques in coronary-sized arteries, and could be readily integrated into a comprehensive CT study to assess coronary calcium, angiographic stenosis, and plaque macrophage content. Additional studies will determine N1177’s ability to resolve macrophages from calcification, and address the radiation and contrast risk of multiple CT scans required for this approach.
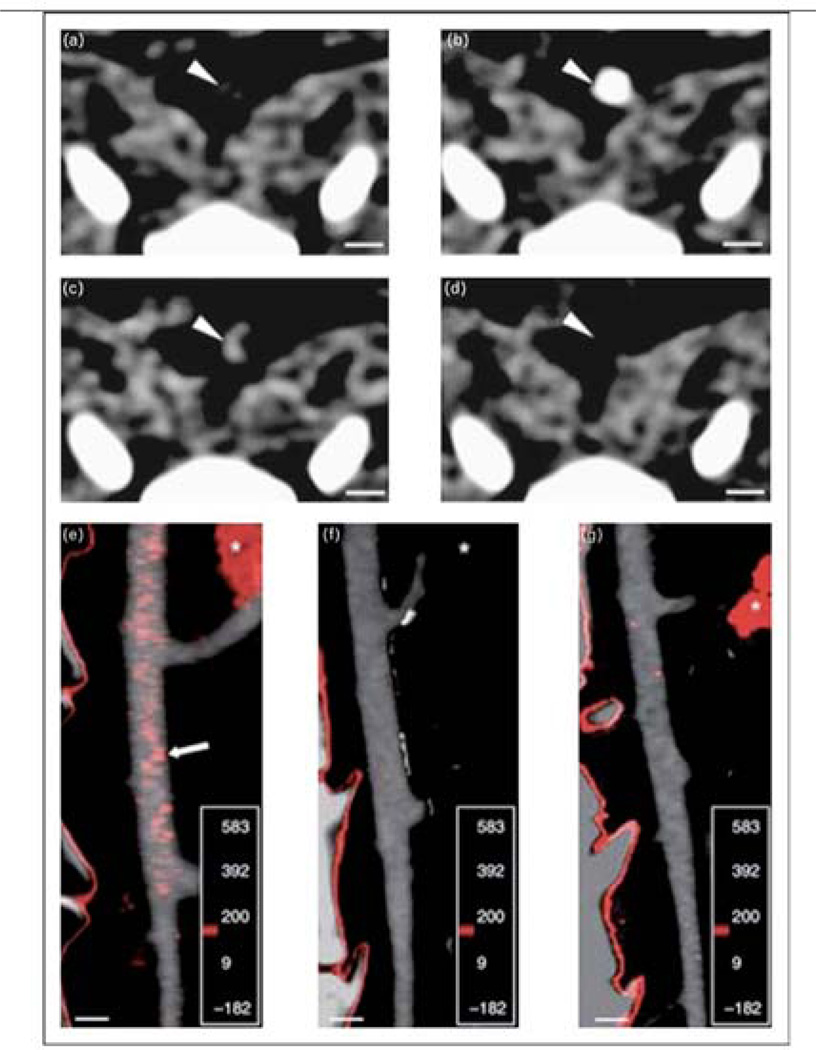
Figure 1.
Molecular CT imaging of atherosclerotic plaque inflammation with a macrophage specific nanoparticle, N1177, in hypercholesterolemic rabbits. Serial axial images of an aortic atheroma (arrowheads) before (a), immediately after (b), and 2 hours following N1177 injection (c). Initially, N1177 functions as an angiographic contrast agent. At 2 hours, compared to control injections (d), N1177 focally accumulates within the arterial wall plaque (arrowhead). Fusion images of angiograms and CT slices 2 hours post-N1177 injection reveal enhancement (red) at sites corresponding to atherosclerotic plaques (e), which were not visualized after injection of a control agent (f) or in normocholesterolemic rabbits injected with N1177 (g). White asterisk: spleen. Scale bar, 5 µm. Reproduced with permission from [9••].
MRI of plaque macrophages
Dextran-coated magnetic nanoparticles (MNP) or ultrasmall superparamagnetic iron oxide (USPIO) particles (30 nanometer diameter, blood half-life 30 hours) show clinical utility for detecting plaque macrophages in atherosclerosis[10–12]. As the MNP circulate, plaque macrophages (and other inflammatory cells) phagocytose the nanoparticles inducing strong signal loss on T2*-weighted MRI. Subjects are typically re-imaged 24–36 hours post-injection. MNP deposition can be detected histologically and correlates well with plaque macrophages on carotid endarterectomy specimens.
In a recent intriguing clinical study, Tang et al. found no significant relationship between MNP/USPIO-derived carotid plaque inflammation and luminal stenosis[13•], leading the authors to suggest that inflammation and vessel stenosis may be independent risk factors of carotid atherosclerosis. Future outcome studies will assess the clinical risk of moderately stenotic lesions based on their MNP level of plaque inflammation.
MNP can also assess changes in inflammation induced by atherosclerosis therapies. Preliminary clinical data suggests that only 12 weeks of high-dose statin therapy significantly reduces MNP-detected MRI plaque inflammation, as well as plaque emboli, compared to low-dose statin therapy[14]. In another recent study, MNP-enhanced MRI was employed to evaluate the putative anti-inflammatory effects of the novel p38 mitogen-activated protein kinase (p38 MAPK) inhibitor SB-239063 on atherosclerosis[15•]. In hypercholesterolemic apolipoprotein E knockout mice (ApoE−/−) prone to severe atheroma formation, T2*-weighted in vivo MRI demonstrated high levels of MNP uptake into atheromata that was nearly abolished by p38 MAPK inhibition (Figure 2). Intriguingly, histopathological analysis revealed minimal differences in plaque macrophage content between SB-239063 and control animals, but did show diminished iron oxide accumulation in SB-239063 treated mice, suggesting that p38 MAPK inhibition diminished macrophage phagocytic activity. Alternative positive contrast reporters for macrophages include a novel gadolinium-containing immunomicelle targeted to the macrophage scavenger receptor, which also appears promising for in vivo molecular MRI of plaque macrophages[16•].
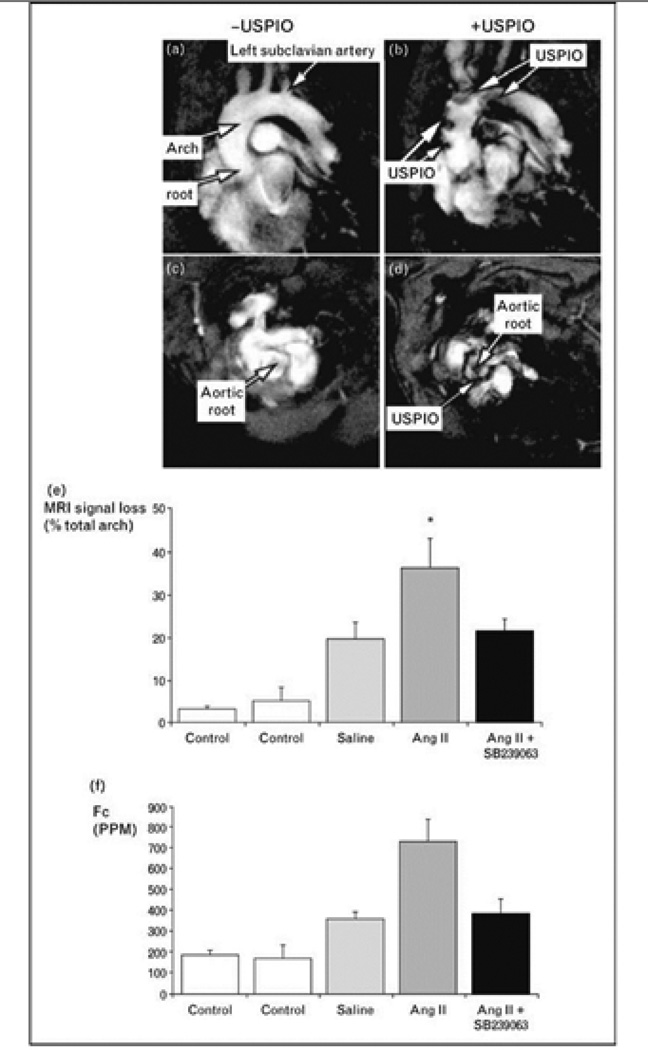
Figure 2.
Effect of p38 MAPK inhibition on atherosclerotic plaque inflammation with MRI molecular imaging in angiotensin II infused ApoE−/− mice. Oblique MRI slices through the aortic root and arch were obtained after injection of ultrasmall superparamagnetic iron oxide (USPIO) particles to target plaque macrophages, which can be identified as signal loss on T2*-weighted images. Control experiments were performed identically but without USPIO injection. USPIO revealed preferential uptake in the aortic arch and root (b, d) that was absent in non-injected control animals (a, c). p38 MAPK inhibition completely inhibited detectable atherosclerotic plaque USPIO accumulation (e, f). Reproduced and adapted with permission from [15•].
Multimodality PET-MRI-fluorescence imaging of plaque macrophages
The validation of a tri-modality nanoparticle (TNP) for PET, MRI, and near infrared fluorescence (NIRF) imaging represents a significant advance for imaging plaque macrophages[17••]. The TNP agent is based on an earlier macrophage-targeted MNP for atherosclerosis[18], and is derivatized with the radiotracer 64Cu for PET imaging. In testing of TNP in ApoE−/− mice, PET-based TNP detection provided a 50-fold improvement in sensitivity compared to MRI. By PET, TNP localized to anticipated regions of high plaque burden (aortic root and arch, carotids) with a 260–392% signal increase compared to wild-type mice, and correlated with T2-weighted MRI and ex vivo fluorescence imaging (Figure 3). Flow cytometry of digested aortas and fluorescence microscopy of plaque sections confirmed that TNP was highly restricted to biomarker-confirmed macrophages. Compared to PET imaging with 18F-2-fluoro-2-deoxy-D-glucose (18FDG), which identifies metabolically active tissue, TNP showed a higher and prolonged PET signal when evaluated in the same animal, presumably due to phagocytic uptake by resident atheroma macrophages. Using the TNP reporter, it may eventually be possible perform specific whole-body vascular surveys to quantify the extent of vessel inflammation and thus identify high-risk plaques.
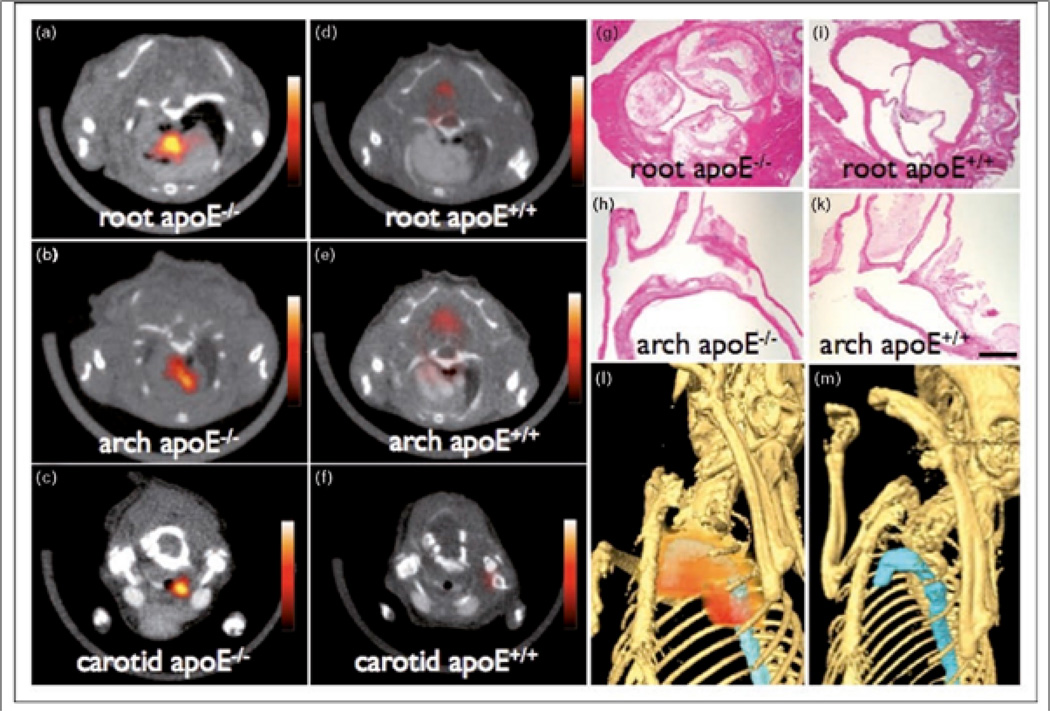
Figure 3.
Visualization of atherosclerotic plaque inflammation in hypercholesterolemic ApoE−/− mice with a tri-modality nanoparticle (TNP) for PET, MRI, and NIRF molecular imaging. PET-CT images of the aortic root (a) and arch (b), and carotid artery (c) revealed wall enhancement in areas of atheroma formation. Wild-type mouse aortas demonstrated minimal TNP uptake (d, e, f). Histopathology confirms atheroma formation at regions of TNP accumulation in ApoE−/− mice (g, h), but not in control ApoE+/+ mice (i, k). Three-dimensional hybrid reconstructions localize PET signal (red) primarily to sites in the proximal thoracic aorta (blue) in hypercholesterolemic ApoE−/− mice (l), but not wild-type controls (m). Magnification: 40x (g, i); 20x (h, k). Reproduced with permission from [17••].
SPECT imaging of monocyte trafficking
Non-invasive determination of monocyte trafficking to experimental atherosclerosis was evaluated with the FDA-approved nuclear agent 111In-oxine and integrated SPECT-CT imaging[19•]. 111In-oxine labeled monocytes were adoptively transferred to ApoE−/− mice followed by serial imaging from 30 min to 10 days with micro-single photon emission computed tomography (micro-SPECT) combined with CT. 111In-oxine labeled monocytes localized to ascending aortic plaques by 5 days as detected on in vivo SPECT-CT, and was confirmed by ex vivo autoradiography and histological methods. Using this method, the authors then tested whether statin therapy could rapidly modulate monocyte recruitment, and discovered that atorvastatin treatment (human equivalent dose 40 mg) significantly reduced plaque monocyte accumulation by 5-fold. These rapid, beneficial effects of statins are in line with recent clinical data from myocardial infarction patients [20]. This study highlights the ability of molecular imaging to illuminate the in vivo biology underlying clinical outcome studies.
PET imaging of plaque metabolism
Rapid growth in PET-based imaging of plaque metabolic activity has occurred in the past several years, in part due to greater accessibility to the radiotracer 18FDG. 18FDG is a glucose analog that incorporates into metabolically active cells via glucose transporters whose feasibility in atherosclerosis imaging was demonstrated several years ago[21]. Subsequent reports showed that 18FDG-PET signals correlate with atherosclerotic disease and macrophage content in excised plaques from humans and animal models[22], although additional studies are required to determine the precise relationship between 18FDG signal and inflammation. Recent clinical studies have revealed that augmented 18FDG carotid plaque signal is present in 30% of plaques[23•], as well as in patients with the metabolic syndrome[24•].
18FDG-PET imaging of atheromata appears to be highly reproducible[25•], a particularly favorable aspect for drug efficacy trials. Based on this study, future clinical studies will be well-powered to efficiently evaluate the metabolic effects of pharmacotherapies. This hypothesis was recently successfully tested when Tahara et al. demonstrated that statins reduced 18FDG uptake in the aorta and carotid arteries in a 43 patient study[26]. Additional PET studies of novel pharmacotherapies are underway[27]. 18FDG-PET phase II studies of new drugs will likely play an important role in determining whether a putative anti-inflammatory agent will be supported for costly phase III outcome studies.
Activated endothelial cells
Activation of endothelial cells promotes expression of pro-inflammatory surface markers that mediate recruitment of circulating leukocytes to evolving plaques[6]. A key adhesion molecule that fosters leukocyte-endothelial trafficking is vascular cell adhesion molecule (VCAM)-1, which is also present in very early stages of disease. While sensitive endothelial targeting is challenging given the thin monolayer and high shear stress environment, several innovative strategies have been developed to overcome these difficulties. In particular, two internalizing MNP have been synthesized for in vivo MRI of VCAM-1 in atherosclerosis[28, 29]. In addition, a new ultrasound approach for VCAM-1 imaging has been developed recently[30•].
Ultrasound imaging of VCAM-1
Kaufmann et al. utilized contrast-enhanced ultrasound with VCAM-1 coated microbubbles to study atherosclerosis[30•]. In cholesterol-fed ApoE−/− mice, VCAM-1 microbubbles targeted aortic plaque, but did not bind significantly in control mice or ApoE−/− on a normal diet. VCAM-1 microbubble induced changes in ultrasound signals that correlated with histological VCAM-1 expression. In the future, contrast-enhanced ultrasound with VCAM-1 microbubbles in easily accessible large vessels (e.g. carotids) may be clinically useful for vascular inflammation screening, early detection of subclinical atherosclerosis, and monitoring of anti-inflammatory therapy.
Proteases
Inflammatory proteases promote extracellular matrix protein digestion, plaque remodeling, and fibrous cap weakening, and are implicated in plaque destabilization and rupture[6]. Proteases thus represent attractive targets for molecular imaging.
Near-infrared fluorescence (NIRF) imaging of cathepsin K activity
An exciting NIRF molecular imaging approach has been developed to sense protease activity using protease-mediated cleavage of fluorescent substrates[31–34], and can now report on cathepsin K (CatK) activity in vivo[35••]. CatK is a potent cysteine protease that localizes to the shoulders of ruptured atherosclerotic plaques. Using a novel NIRF protease-activatable agent that is optically silent until cleaved by CatK, Jaffer et al. imaged CatK activity in vivo in mice and ex vivo in human carotid endarterectomy specimens[35••]. In ApoE−/− mice, intravital confocal fluorescence microscopy revealed augmented CatK activity in carotid atheroma, which was not seen in the control agent group (Figure 4). In addition, CatK activity was detected at the intima-media interface where elastin fibers were disrupted, further implicating CatK in extracellular matrix degradation and expansive plaque remodeling. As NIRF detection technology is translating into the clinical arena via catheter and tomographic approaches, imaging of CatK activity may offer a new method to detect high-risk plaques.
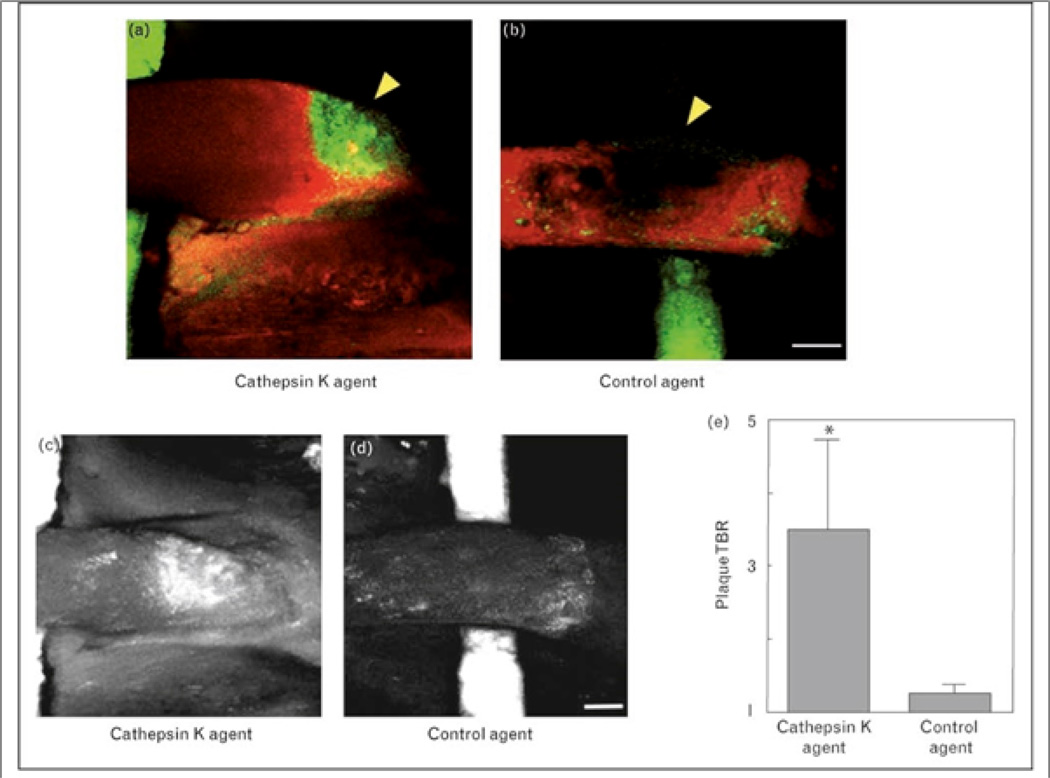
Figure 4.
Molecular intravital fluorescence imaging of cathepsin K (CatK) activity in atherosclerosis. CatK fluorescence liberated by activated endogenous enzyme was measured in surgically exposed carotid artery atheroma of ApoE−/− mice 24 hours after injection. Fusion image of a spectrally distinct intravascular near-infrared fluorochrome (red, ex/em 750/800nm) injected immediately prior to imaging localizes activated CatK signal (green, ex/em 680/700nm) to within the atherosclerotic plaque (arrowhead pointing to the region of signal void) rather than the vessel lumen (a). A control NIRF agent did not accumulate in the atheroma (b), with visualization aided by a fluorescent phantom (green) placed under the carotid artery. CatK (c) or control (d) projection images of carotid plaques were used to quantitate the significant CatK NIRF signal increase in carotid plaques compared to the control agent group (e). Scale bar, 250 µm. Reproduced with permission from [35••].
MRI of MMP expression
Lancelot et al. developed a novel gadolinium based agent (P947) to report on matrix metalloproteinase (MMP) presence using in vivo MRI[36•]. P947 is a gadolinium chelate linked to a broad spectrum MMP inhibitor that inhibits MMP-1, -2, -3, -8, -9, and -13 in vitro with micromolar affinity. In human carotid endarterectomy specimens, P947 delineated MMP-rich complex internal carotid atheroma with 2x-increased signal from that of MMP-poor external and common carotid arteries without significant disease. On in vivo testing, ApoE−/− mice injected with P947 showed strong and heterogeneous signal uptake in aortic plaques, and induced significantly greater and longer contrast effects than the control agent gadolinium-DOTA. P947-induced signal enhancement co-localized with the fibrous cap and plaque shoulders, but not with lipid cores. P947 may aide in the non-invasive determination of inflamed, high-risk plaques.
Apoptosis
Dying cells within the internal confines of the atheroma, typically composed of lipid-laden macrophages and smooth muscle cells, may structurally weaken the protective fibrous cap and expand the underlying necrotic core, facilitating plaque rupture[7, 8]. Imaging of apoptotic cells in atheromata may demarcate plaques at risk for future complications.
SPECT imaging of plaque apoptosis
Annexin A5 is a naturally occurring protein that binds with high-affinity to phosphatidylserine residues exposed on apoptotic cell surfaces. Radiolabeled annexin A5 has demonstrated feasibility to illuminate unstable carotid plaques in vivo using noninvasive SPECT imaging[37]. Recently, Sarai et al. utilized annexin A5 to image the effects of caspase inhibition on apoptosis in experimental atherosclerosis[38•]. In this well-designed study, cholesterol-fed rabbits with aortic atherosclerosis were treated with acute dosages of a nonselective caspase inhibitor or specific inhibitors to caspase-1, -3, -8, or -9, or no inhibitor. After administration of the caspase inhibitors at 6 hours and 1 hour prior to imaging, rabbits were injected with 99mTc-labeled human recombinant annexin A5 or mutant annexin A5, and then imaged with a micro-SPECT/CT hybrid system. Annexin A5 signal was 10x greater in atheromata of hypercholesterolemic rabbits compared to normals and was significantly reduced by broad caspase inhibition, and also to a lesser extent by selective inhibition of caspase-1, -3, or -9. Histology confirmed a marked decrease in macrophage apoptotic figures after broad caspase inhibitor treatment. Annexin A5-based SPECT imaging thus offers a highly translatable approach to assess alterations of apoptosis in vivo.
Osteogenesis / Calcification
Calcification occurs frequently in atherosclerosis, and calcific plaque burden is predictive of cardiovascular events. In addition, atheromata harboring a superficial calcified nodule demarcate a class of high-risk plaques[7]. A novel NIRF molecular imaging strategy has been recently developed to image fundamental events in plaque calcification.
NIRF imaging of osteogenic activity
To explore osteogenesis (bone formation, plaque calcification) in vivo and investigate its relationship with plaque inflammation, serial molecular imaging in ApoE−/− mice was performed with a bone-specific NIRF probe and macrophage-avid MRI magnetic nanoparticles coupled with intravital confocal fluorescence microscopy[39••]. Prior to intravital microscopic imaging, the carotid arteries were surgically exposed and a bisphosphonate-conjugated agent was injected to detect sites of new bone formation. Serial molecular imaging provided the first in vivo evidence that macrophage infiltration and plaque inflammation preceded osteogenesis. Interestingly, the sensitivity of the bisphosphonate probe allowed detection of osteogenesis in early plaques undetectable by histology or CT imaging. Early detection of plaque mineralization via NIRF molecular imaging could offer a novel approach to detect atherosclerotic lesions at risk.
CONCLUSIONS
The recent years has witnessed an exponential growth of molecular imaging applications for atherosclerosis. Novel molecular imaging agents are illuminating critical molecular and cellular aspects of atherosclerosis biology in vivo, and show utility for all major clinical cardiovascular imaging modalities. In the clinical arena, the next 3–5 years should solidify the role of molecular imaging in atherosclerosis drug efficacy evaluation. In addition, a number of molecular imaging strategies are strongly positioned to aid in the clinical detection of high-risk plaques.
ACKNOWLEDGMENTS
Supported by the Howard Hughes Medical Institute Early Career Award (FJ), American Heart Association Scientist Development Grant (FJ), and Donald W. Reynolds foundation (FJ).
REFERENCES
- 1.Jaffer FA, Libby P, Weissleder R. Molecular and cellular imaging of atherosclerosis: emerging applications. J Am Coll Cardiol. 2006;47:1328–1338. doi: 10.1016/j.jacc.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 2.Jaffer FA, Libby P, Weissleder R. Molecular imaging of cardiovascular disease. Circulation. 2007;116:1052–1061. doi: 10.1161/CIRCULATIONAHA.106.647164. [DOI] [PubMed] [Google Scholar]
- 3. Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451:953–957. doi: 10.1038/nature06803. •• This recent comprehensive review of atherosclerosis imaging summarizes the currently available state-of-the-art technologies for clinical and pre-clinical non-invasive evaluation of atherosclerotic cardiovascular disease.
- 4.Wickline SA, Neubauer AM, Winter P, et al. Applications of nanotechnology to atherosclerosis, thrombosis, and vascular biology. Arterioscler Thromb Vasc Biol. 2006;26:435–441. doi: 10.1161/01.ATV.0000201069.47550.8b. [DOI] [PubMed] [Google Scholar]
- 5.Wu JC, Bengel FM, Gambhir SS. Cardiovascular molecular imaging. Radiology. 2007;244:337–355. doi: 10.1148/radiol.2442060136. [DOI] [PubMed] [Google Scholar]
- 6.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 7.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 8.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation. 2003;108:1772–1778. doi: 10.1161/01.CIR.0000087481.55887.C9. [DOI] [PubMed] [Google Scholar]
- 9. Hyafil F, Cornily JC, Feig JE, et al. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat Med. 2007;13:636–641. doi: 10.1038/nm1571. •• With the development of a novel positive-contrast CT molecular imaging agent that targets atherosclerotic inflammation, this work represents a major advance towards the goal of in vivo coronary atherosclerotic plaque biological imaging. In concert with the anatomical and calcium scoring information already available by coronary CT clinical translation of this agent may provide additional important data for coronary plaque risk-stratification.
- 10.Kooi ME, Cappendijk VC, Cleutjens KB, et al. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation. 2003;107:2453–2458. doi: 10.1161/01.CIR.0000068315.98705.CC. [DOI] [PubMed] [Google Scholar]
- 11.Tang T, Howarth SP, Miller SR, et al. Assessment of inflammatory burden contralateral to the symptomatic carotid stenosis using high-resolution ultrasmall, superparamagnetic iron oxide-enhanced MRI. Stroke. 2006;37:2266–2270. doi: 10.1161/01.STR.0000236063.47539.99. [DOI] [PubMed] [Google Scholar]
- 12.Trivedi RA, JM UK-I, Graves MJ, et al. In vivo detection of macrophages in human carotid atheroma: temporal dependence of ultrasmall superparamagnetic particles of iron oxide-enhanced MRI. Stroke. 2004;35:1631–1635. doi: 10.1161/01.STR.0000131268.50418.b7. [DOI] [PubMed] [Google Scholar]
- 13. Tang TY, Howarth SP, Miller SR, et al. Correlation of Carotid Atheromatous Plaque Inflammation Using USPIO-Enhanced MR Imaging With Degree of Luminal Stenosis. Stroke. 2008 doi: 10.1161/STROKEAHA.107.504753. • This clinical study of patients with asymptomatic carotid stenosis who underwent USPIO-enhanced MRI reveals that the amount of carotid plaque inflammation appears to be independent of the severity of stenosis.
- 14.Tang TY, et al. American College of Cardiology March 29 – April 1. Chicago, IL: 2008. p. 6. http://acc08.acc.org/Documents/ACC%20D4.pdf. [Google Scholar]
- 15. Morris JB, Olzinski AR, Bernard RE, et al. p38 MAPK inhibition reduces aortic ultrasmall superparamagnetic iron oxide uptake in a mouse model of atherosclerosis: MRI assessment. Arterioscler Thromb Vasc Biol. 2008;28:265–271. doi: 10.1161/ATVBAHA.107.151175. • This study demonstrates that, in a murine atherosclerosis model, anti-inflammatory treatment with a p38 MAPK inhibitor abrogates evidence of atherosclerotic plaque inflammation.
- 16. Amirbekian V, Lipinski MJ, Briley-Saebo KC, et al. Detecting and assessing macrophages in vivo to evaluate atherosclerosis noninvasively using molecular MRI. Proc Natl Acad Sci U S A. 2007;104:961–966. doi: 10.1073/pnas.0606281104. • Development and testing of a novel gadolinium-containing immunomicelle that targets the macrophage scavenger receptor for MRI-based evaluation of atherosclerotic plaque inflammation.
- 17. Nahrendorf M, Zhang H, Hembrador S, et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. •• This significant work validates a versatile nanoparticle contrast agent for macrophage plaque detection that can be used with three imaging modalities (PET, MRI, NIRF).
- 18.Jaffer FA, Nahrendorf M, Sosnovik D, et al. Cellular imaging of inflammation in atherosclerosis using magnetofluorescent nanomaterials. Mol Imaging. 2006;5:85–92. [PubMed] [Google Scholar]
- 19. Kircher MF, Grimm J, Swirski FK, et al. Noninvasive in vivo imaging of monocyte trafficking to atherosclerotic lesions. Circulation. 2008;117:388–395. doi: 10.1161/CIRCULATIONAHA.107.719765. • Demonstrates use of an FDA-approved radiotracer in ApoE−/−mice to track monocyte recruitment to atherosclerotic plaques in vivo.
- 20.Patti G, Pasceri V, Colonna G, et al. Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention: results of the ARMYDA-ACS randomized trial. J Am Coll Cardiol. 2007;49:1272–1278. doi: 10.1016/j.jacc.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 21.Rudd JH, Warburton EA, Fryer TD, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105:2708–2711. doi: 10.1161/01.cir.0000020548.60110.76. [DOI] [PubMed] [Google Scholar]
- 22.Tawakol A, Migrino RQ, Bashian GG, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48:1818–1824. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 23. Tahara N, Kai H, Nakaura H, et al. The prevalence of inflammation in carotid atherosclerosis: analysis with fluorodeoxyglucose-positron emission tomography. Eur Heart J. 2007;28:2243–2248. doi: 10.1093/eurheartj/ehm245. • Clinical study of patients undergoing atherosclerosis screening to establish the burden of detectable inflammation in carotid atherosclerotic plaques by 18FDG-PET.
- 24. Tahara N, Kai H, Yamagishi S, et al. Vascular inflammation evaluated by [18F]-fluorodeoxyglucose positron emission tomography is associated with the metabolic syndrome. J Am Coll Cardiol. 2007;49:1533–1539. doi: 10.1016/j.jacc.2006.11.046. • In patients being screened for cancer with 18FDG-PET, radiotracer uptake in carotid atheromas positively correlates with components of the metabolic syndrome.
- 25. Rudd JH, Myers KS, Bansilal S, et al. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol. 2007;50:892–896. doi: 10.1016/j.jacc.2007.05.024. • This study establishes 18FDG-PET atherosclerosis imaging as a highly reproducible technique.
- 26.Tahara N, Kai H, Ishibashi M, et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006;48:1825–1831. doi: 10.1016/j.jacc.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 27.ClinicalTrials.gov on World Wide Web URL: http://clinicaltrials.gov/ct2/results?term=FDG+atherosclerosis
- 28.Kelly KA, Allport JR, Tsourkas A, et al. Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circ Res. 2005;96:327–336. doi: 10.1161/01.RES.0000155722.17881.dd. [DOI] [PubMed] [Google Scholar]
- 29.Nahrendorf M, Jaffer FA, Kelly KA, et al. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006;114:1504–1511. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 30. Kaufmann BA, Sanders JM, Davis C, et al. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation. 2007;116:276–284. doi: 10.1161/CIRCULATIONAHA.106.684738. • This work demonstrates the identification of early markers of endothelial activation in vivo with contrast-enhanced ultrasound providing a tool for noninvasive assessment of vascular inflammation.
- 31.Chen J, Tung CH, Mahmood U, et al. In vivo imaging of proteolytic activity in atherosclerosis. Circulation. 2002;105:2766–2771. doi: 10.1161/01.cir.0000017860.20619.23. [DOI] [PubMed] [Google Scholar]
- 32.Deguchi JO, Aikawa E, Libby P, et al. Matrix metalloproteinase-13/collagenase-3 deletion promotes collagen accumulation and organization in mouse atherosclerotic plaques. Circulation. 2005;112:2708–2715. doi: 10.1161/CIRCULATIONAHA.105.562041. [DOI] [PubMed] [Google Scholar]
- 33.Jaffer FA, Tung CH, Gerszten RE, et al. In vivo imaging of thrombin activity in experimental thrombi with thrombin-sensitive near-infrared molecular probe. Arterioscler Thromb Vasc Biol. 2002;22:1929–1935. doi: 10.1161/01.atv.0000033089.56970.2d. [DOI] [PubMed] [Google Scholar]
- 34.Weissleder R, Tung CH, Mahmood U, et al. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999;17:375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 35. Jaffer FA, Kim DE, Quinti L, et al. Optical visualization of cathepsin K activity in atherosclerosis with a novel, protease-activatable fluorescence sensor. Circulation. 2007;115:2292–2298. doi: 10.1161/CIRCULATIONAHA.106.660340. •• This study represents a major advance in the application of in vivo biological imaging with the development of a novel protease-activatable NIRF molecular agent able to identify potentially destabilizing enzyme activity in atherosclerotic plaques.
- 36. Lancelot E, Amirbekian V, Brigger I, et al. Evaluation of matrix metalloproteinases in atherosclerosis using a novel noninvasive imaging approach. Arterioscler Thromb Vasc Biol. 2008;28:425–432. doi: 10.1161/ATVBAHA.107.149666. • This article reports on a new gadolinium-based MRI contrast agent that has increased affinity for matrix metalloproteinase-rich atherosclerotic plaques.
- 37.Kietselaer BL, Reutelingsperger CP, Heidendal GA, et al. Noninvasive detection of plaque instability with use of radiolabeled annexin A5 in patients with carotid-artery atherosclerosis. N Engl J Med. 2004;350:1472–1473. doi: 10.1056/NEJM200404013501425. [DOI] [PubMed] [Google Scholar]
- 38. Sarai M, Hartung D, Petrov A, et al. Broad and specific caspase inhibitor-induced acute repression of apoptosis in atherosclerotic lesions evaluated by radiolabeled annexin A5 imaging. J Am Coll Cardiol. 2007;50:2305–2312. doi: 10.1016/j.jacc.2007.08.044. • This study demonstrates a novel nuclear imaging molecular probe for detection of macrophage apoptosis in hypercholesterolemic rabbits.
- 39. Aikawa E, Nahrendorf M, Figueiredo JL, et al. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. •• This interesting work elegantly defines a new NIRF approach that has the ability to demarcate early plaque osteogenesis before it becomes histologically evident, and provides compelling evidence that macrophage infiltration and inflammation are key predisposing factors in this process.